Abstract
The Ca2+-sensing receptor (CaSR) drives essential Ca2+ and E-cadherin-mediated processes in the epidermis, including differentiation (Komuves et al., 2002), cell-to-cell adhesion (Tu et al., 2008) and epidermal barrier homeostasis in cells and young adult mice (Tunggal et al., 2005; Tu et al., 2012; Tu et al., 2019). We now report that decreased CaSR expression leads to impaired Ca2+ signal propagation in aged (>22 months) mouse epidermis and (>79 years donor age) human keratinocytes. Baseline cytosolic Ca2+ concentrations were higher, and capacitive Ca2+ entry was lower in aged vs. young keratinocytes, even though endoplasmic reticulum (ER) stores were largely intact. As in CaSR knockout mice (EpidCaSR−/−), decreased CaSR expression led to decreased E-cadherin, PLC γ expression and a compensatory upregulation of Stromal Interaction Molecule 1 (STIM1). Pretreatment with the CaSR agonist NPS-R568 normalized Ca2+ propagation and E-cadherin organization after experimental wounding. These results suggest that age-related defects in CaSR expression dysregulate normal keratinocyte and epidermal Ca2+ signaling, leading to impaired E-cadherin expression, organization and function. These findings illustrate a innovative mechanism whereby Ca2+ and E-cadherin-dependent functions are impaired in aging epidermis and suggest a new therapeutic approach by restoring CaSR function.
Keywords: Aging, calcium, calcium-sensing receptor, E-cadherin, keratinocyte
Introduction
In this study, we report that loss of the CaSR leads to impaired Ca2+ and E-cadherin signaling in aged human keratinocytes and epidermis. Ca2+ is essential for normal keratinocyte proliferation, differentiation, migration and wound repair (Cordeiro and Jacinto, 2013). Raised extracellular Ca2+ , epidermal barrier perturbation and mechanical or laser stimulation all act via intracellular Ca2+ release and subsequent store-operated or voltage-sensitive Ca2+ entry (Tu et al., 2005; Numaga-Tomita and Putney, 2013) that propagates Ca2+ signaling to neighboring keratinocytes both laterally and vertically (Tsutsumi et al., 2013; Kumamoto et al., 2017). Epidermal Ca2+ signaling is driven by a marked Ca2+ gradient (Menon and Elias, 1991; Forslind et al., 1999), with Ca2+ concentrations approximately 4-fold higher in the uppermost viable keratinocytes relative to the basal cells (Elias et al., 1998; Mauro et al., 1998). Much of this Ca2+ gradient and resulting Ca2+ signaling depends on Ca2+ sequestered within the endoplasmic reticulum by SERCA (Celli et al., 2016). While raising extracellular Ca2+ increases keratinocyte differentiation, it also decreases lipid secretion and barrier repair in terminally differentiated stratum granulosum keratinocytes (Lee and Lee, 2018). Thus, an approach that enhances keratinocyte sensitivity to Ca2+ could optimize differentiation, migration and barrier homeostasis, especially in aging epidermis.
The G-protein associated Ca2+ sensing receptor (CaSR) senses Ca2+ concentrations in the micromolar to millimolar range, making it particularly useful in sensing changes in extracellular or organelle Ca2+ concentrations. In keratinocytes, CaSR signaling activates Phospholipase C beta (PLC β) via Gq and leads to inositol triphosphate (IP3) mediated acute release of calcium from intracellular calcium stores. CaSR expression is essential for epidermal differentiation and barrier function (Tu et al., 2012), controlling both keratinocytes’ ability to take up and store Ca2+ in the endoplasmic reticulum (Tu et al., 2007). CaSR mediates the formation and stabilization of the E-cadherin signaling complex, leading to E-cadherin-mediated adherens junction (AJ) and cell-to-cell adhesion (Tu et al., 2008). Mice with conditional knock out of the CaSR in the epidermis (EpidCaSR−/−) display loss of the epidermal Ca2+ gradient, impaired keratinocyte differentiation, and defective permeability barrier (Tu et al., 2012). Conversely, experimental CaSR overexpression accelerates epidermal differentiation and permeability barrier formation (Turksen and Troy, 2003). Combined Vitamin D receptor and CaSR deletion delays wound re-epithelization (Oda et al., 2017), and deleting CaSR from young adult mice epidermis decreases E-cadherin expression and impairs Ca2+ signal propagation (Tu et al., 2019). Aged human epidermis and keratinocytes demonstrate similar defects in Ca2+ signaling, expression and re-epithelialization as those seen in mice in which CaSR was experimentally ablated. These studies suggest that CaSR may be a relevant target for improving Ca2+ and E-cadherin-mediated processes in aged epidermis.
Results
Epidermal Ca2+ Signaling After Laser Stimulation is Blunted in Aged Mouse Epidermis and Aged Human Keratinocyte Monolayers
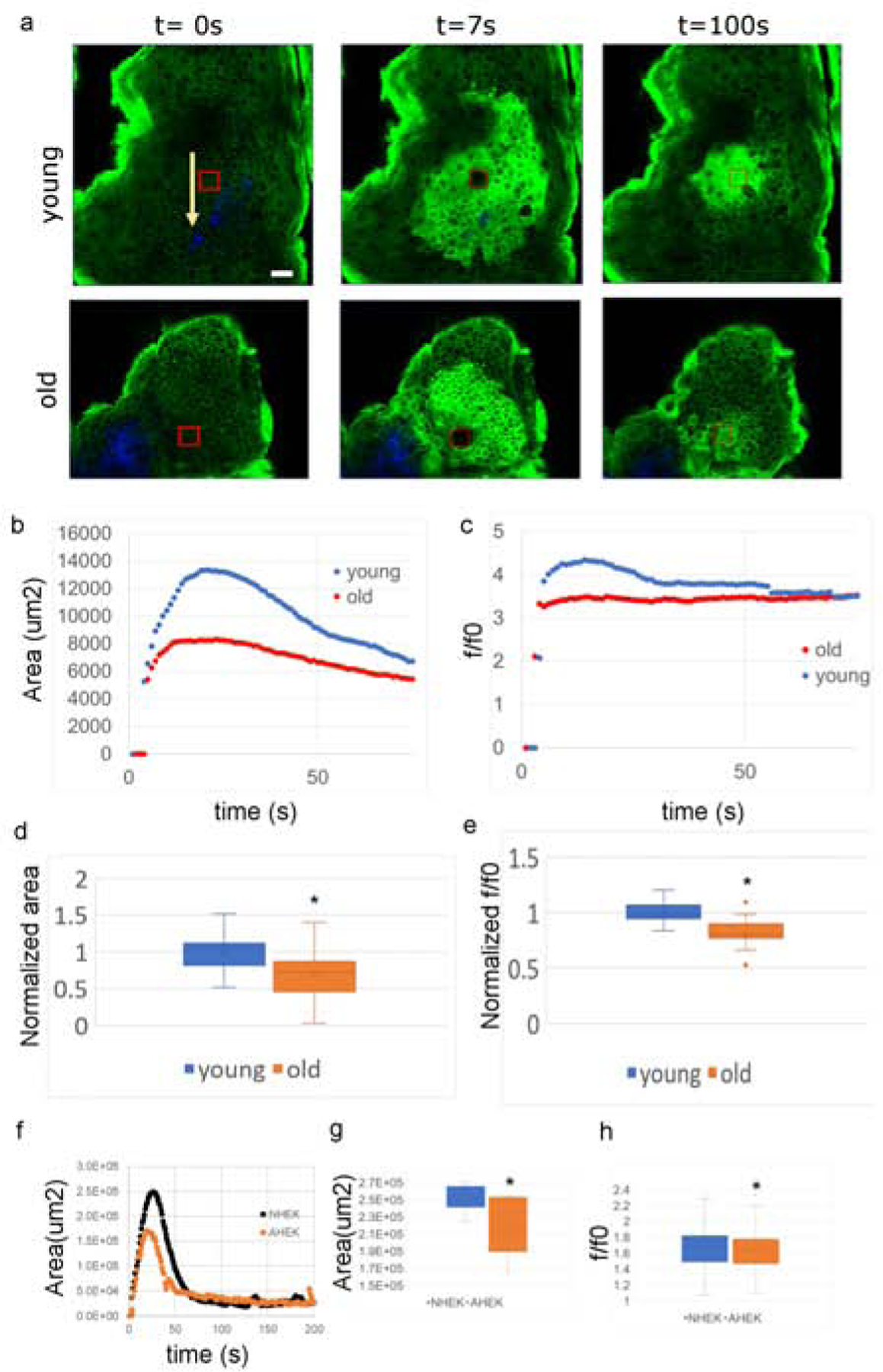
Previous studies demonstrated that aged human keratinocytes respond sluggishly to mechanical stimulation (Denda et al., 2017). To assess lateral calcium signaling in aged vs. young epidermis, we used a multiphoton excitation microscopy-based laser stimulation assay previously developed for EpidCaSR−/− studies (Tu et al., 2019). This experimental approach selectively perturbs a selected area of 20×20 μm2 (corresponding roughly to one or two cells) in the stratum basale of the epidermis of mice expressing the fluorescent Ca2+ reporter GCaMP3 under the k14 promoter. After laser stimulation, we monitored Ca2+ propagation by tracking epidermal fluorescence using time lapse imaging (Tu et al., 2019) (Fig. 1A). Ca2+ propagation spread to a significantly smaller area in aged (>22mo) vs. young (6–8 wo) mice (Figure 1 B, and D, and S1). The keratinocyte cytosolic Ca2+ ([Ca2+]i) response after perturbation also was lower in aged vs. young mice (Figure 1c and e and Supplementary Figure S1).
Figure 1: Calcium response to laser perturbation in aged versus young epGCaMP mice and human keratinocytes monolayers.
(a) Calcium response to laser perturbation to a 20×20μm2 SB region (red box) of young and old epGCamP mouse epidermis. Arrows indicate dermal collagen (blue). (b) Time-traces of response area and (c) [Ca2+]i increase in young (blue) and aged (red) mice. (d) Distribution of maximum response area and e) maximum [Ca2+]i increase over baseline for young and aged mice. Data normalized to young mice mean value. N=18 (aged mice) and 19 (young mice) traces from two biopsies per mouse, from three separate mouse pairs. (f) Time-traces of calcium response area after laser perturbation in NHEK (black) vs. AHEK (orange). (g) Distribution of maximum calcium response area (N=15 traces from 3 separate experiments), and h) single cell maximum [Ca2+]i increase (N=1370–1552 from 3 experiments). NHEK (blue), AHEK (orange). Asterisks indicate p<0.05 by a two tailed t-test.
We next examined Ca2+ signaling in response to laser stimulation in aged (>79yo) human (AHEKs) vs. neonatal (NHEKs) keratinocyte monolayers, using the cell permeant, calcium sensitive fluorescent probe Calcium Green 1-AM (Thermo Fisher). In three separate experiments conducted on cells from three separate pairs of donors, we found that aged human keratinocytes monolayers responded with blunted calcium propagation (Figure 1f and g) and a lower average increase in the aged single cells’ cytosolic calcium concentration (Figure 1h).
Ca2+ Signaling in Aged vs. Young Keratinocytes
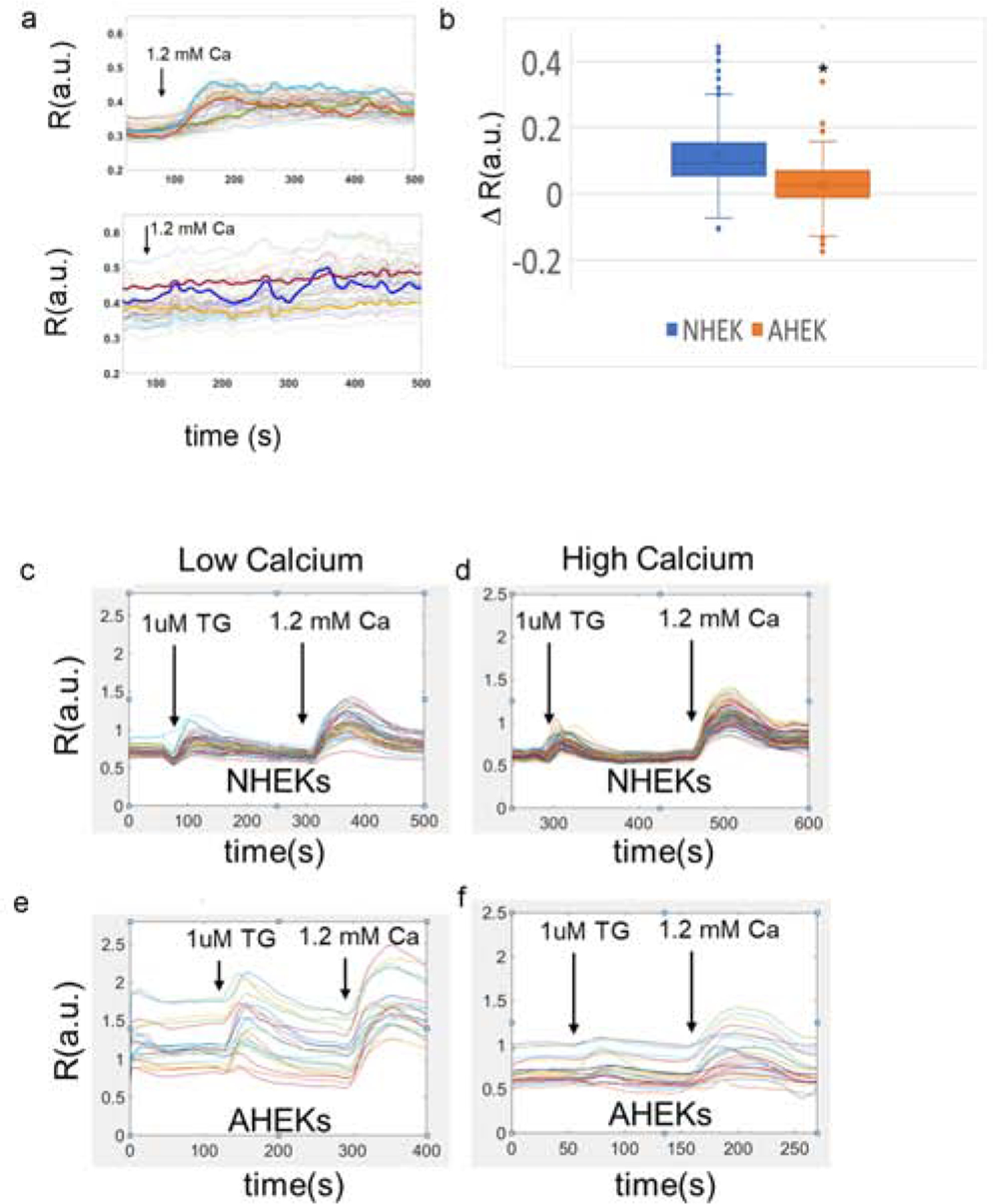
We next compared the response to extracellular Ca2+, the intracellular Ca2+ stores and the capacitive [Ca2+]i response in neonatal vs. keratinocytes obtained from aged (>79 yo) humans (Figure 2a and b, respectively). Fura2 loaded keratinocytes were exposed to 1.2mM extracellular calcium. Traces representative of 6 (AHEK)-7 (NHEK) experiments on cells from 3 separate donors are shown in Figure 2a, top panel (NHEK) and bottom panel (AHEK). While NHEKs responded to raised extracellular calcium with a robust and rapid increase in [Ca2+]i, most AHEKs had a much more limited and slower response during the experiment time frame. Overall, AHEKs [Ca2+]i response to increased extracellular calcium was significantly less pronounced than in NHEKs (Figure 2b).
Figure 2: Calcium signaling is impaired in aged human keratinocytes.
(a) Response to high extracellular calcium of FURA2 labelled NHEK (top panel) and AHEK (bottom panel) monolayers. Representative traces of 6–7 separate experiments on cells cultured from 3 neonatal and 3 aged donors. (b) distribution of single cell [Ca2+]i variation after calcium switch reported as ΔR. N=220–410 cells per group from 6 (AHEK)-7 (NHEK) separate experiments on cells cultured from 3 neonatal and 3 aged donors. Asterisk denotes p<0.05. (c-f) Representative traces of cytosolic Ca2+ concentrations in keratinocytes at baseline and in response to 1uM thapsigargin followed by 1.2 mM [Ca2+]. NHEK (c) and AHEK (d) in 0.07 mM Ca2+. NHEK (e) vs. AHEK (f) cultured in 1.2 mM [Ca2+] for 24 hours. Data are reported as the ratio R of the fluorescence intensity at 340nm excitation (fbound) over the fluorescence intensity at 390nm excitation (ffree). N=102–380 cells per group from 3–7 separate experiments on cells cultured from 3 neonatal and 3 aged donors. Results are summarized in Table 1.
Next, we compared intracellular Ca2+ stores and cytosolic [Ca2+]i capacitive influx in NHEKs (Figure 2c, d) vs AHEKs (Figure 2e and f) in keratinocytes monolayers cultured in low (0.07 mM) and high (1.2mM) calcium containing medium for 24 hours (Figure 2c–f). Keratinocytes first were placed in 0 mM extracellular Ca2+. After a brief period of equilibration, 1μM thapsigargin (TG), a concentration that releases both the ER and Golgi Ca2+ stores, was added to the medium to assess intracellular Ca2+ stores. Extracellular Ca2+ then was raised to a final concentration of 1.2 mM to quantify capacitive Ca2+ entry (Figure 2, Table 1).
Table 1: Response to thapsigargin and high calcium in NHEKs and AHEKs.
Capacitive calcium entry traces (See Figure 2) were analyzed to determine baseline intracellular calcium levels (baseline), peak calcium release from stores after exposure to 1μM thapsigargin (P1), and peak capacitive calcium entry after medium supplementation with 1.2 mM [Ca2+] (P2). Data are reported as the ratio R of the fluorescence intensity at 340nm excitation (fbound) over the fluorescence intensity at 390nm excitation (ffree). The percentage of AHEKs responding to thapsigargin is reported in the last row of the table. Asterisks denote a statistically significant difference between the NHEK and AHEK values determined via two tailed t-test with p<0.05. N= 102–380 single cell traces from 3–7 experiments per group from 3 aged and 3 neonatal donors.
| Low Calcium | High Calcium | |||
|---|---|---|---|---|
| AHEK | AHEK | |||
| Baseline | 0.644±0.005 | 1.06±0.05* | 0.704±0.006 | 0.97±0.05* |
| P1 | 0.33±0.01 | 0.22±0.04* | 0.232±0.007 | 0.25±0.02 |
| P2 | 0.45±0.02 | 0.26±0.02* | 0.44±0.01 | 0.34±0.02* |
| Percentage of cells responding to 1uM thapsigargin | 83±8 | 44±8* | 92±3 | 53±12* |
First, we found that baseline cytosolic Ca2+ concentrations were markedly elevated and heterogeneous in aged keratinocytes. Responses of aged keratinocytes to both thapsigargin and calcium switch were notably more variable than young keratinocytes. While 82% of all NHEK cells cultured in low calcium and 93% of NHEK cells cultured in high calcium responded to both thapsigargin and calcium switch, only 44% and 53% of AHEK in cultured in low and high calcium respectively responded to thapsigargin or raised extracellular Ca2+.
CaSR and E-cadherin Protein Expression is Downregulated in Aged Human Epidermis
In non-excitable cells such as keratinocytes, Ca2+ influx often is regulated by Store-Operated Ca2+ Entry (SOCE) (Tu et al., 2005; Numaga-Tomita and Putney, 2013; Vandenberghe et al., 2013), which requires phospholipase C (PLC) -mediated release of intracellular Ca2+ stores from the endoplasmic reticulum or Golgi and refill via migration to the plasma membrane of the Stromal Interaction Molecule 1 (STIM1) (Nomaga-Tomita and Putney 2013). These processes lead to adherens junction and desmosome reorganization, mediated by E-cadherin.
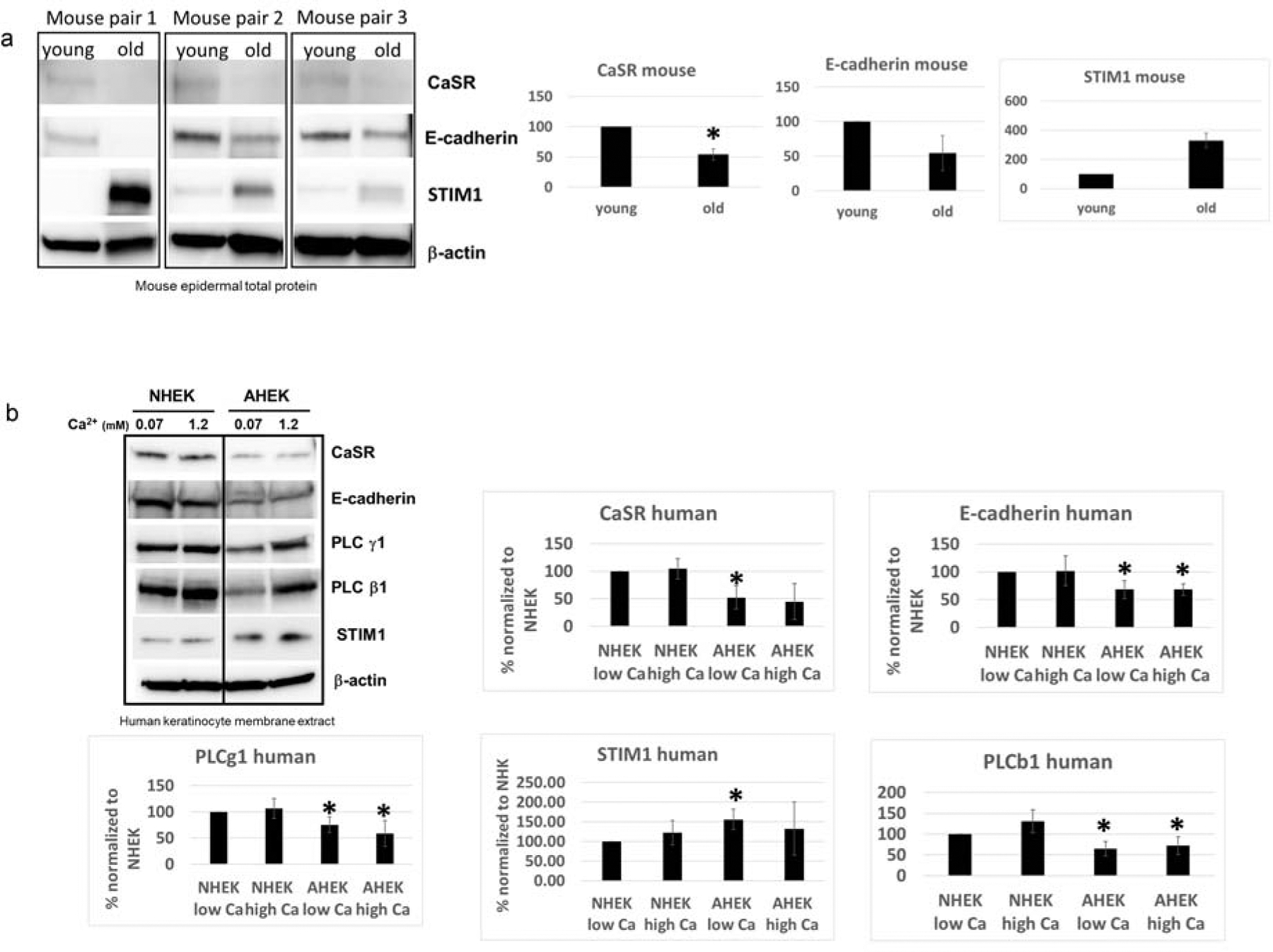
To define changes in the CaSR dependent signaling pathway, we first compared CaSR, E-cadherin, and STIM1 protein expression in total epidermal lysate from aged vs. young mice (Figure 3a). We found that both CaSR and E-cadherin protein expression was consistently decreased, while STIM1 levels were elevated in aged mice. A similar pattern of E-cadherin down regulation was seen in young mice in which CaSR was experimentally ablated (Tu et al., 2019).
Figure 3: Calcium signaling molecules expression in aged and young mice and neonatal and aged human keratinocytes.
(a) epidermal lysate from 3 aged and 3 young mice was probed for levels of CaSR, e-cadherin, and STIM1 using western blotting and differences in expression levels were quantified (bar graphs). (b) Crude membrane extract of NHEK and AHEK cultured in low or high calcium for 24 hours were probed for CaSR, E-cadherin, PLC γ1, PLC β1, and STIM1 using Western blotting and expression levels were quantified (bar graphs). Data are representative of 3–4 different sets of aged and neonatal cells. Asterisks denote p<0.05 via a two tailed t-test.
We then compared the expression levels of CaSR, E-cadherin, STIM1, PLCγ1 and PLCβ1 in NHEKs and AHEKs from 3–4 separate donors per group. We found that CaSR and e-cadherin levels were consistently down regulated in AHEKs from 4 separate donors (Figure 3b and Supplementary Figure S2a) compared to NHEKs from 4 separate donors. PLCγ1 and PLCβ1 levels were also consistently decreased while STIM1 levels were increased in AHEKs respect to NHEKs. Exposure to high calcium appeared to reduce the difference in STIM1 level between NHEKs and AHEKs, but it did not normalize expression levels of the other proteins. We observed a similar pattern in the epidCaSR−/− mouse, where PLC γ1 levels were downregulated and STIM1 levels upregulated (Fig. S2b) compared to wild type animals. PLCβ1 was upregulated in epidCaSR−/− mice.
Aged Keratinocytes Display Defective E-Cadherin Staining, Slower Migration and Impaired Cell-to-Cell Adhesion
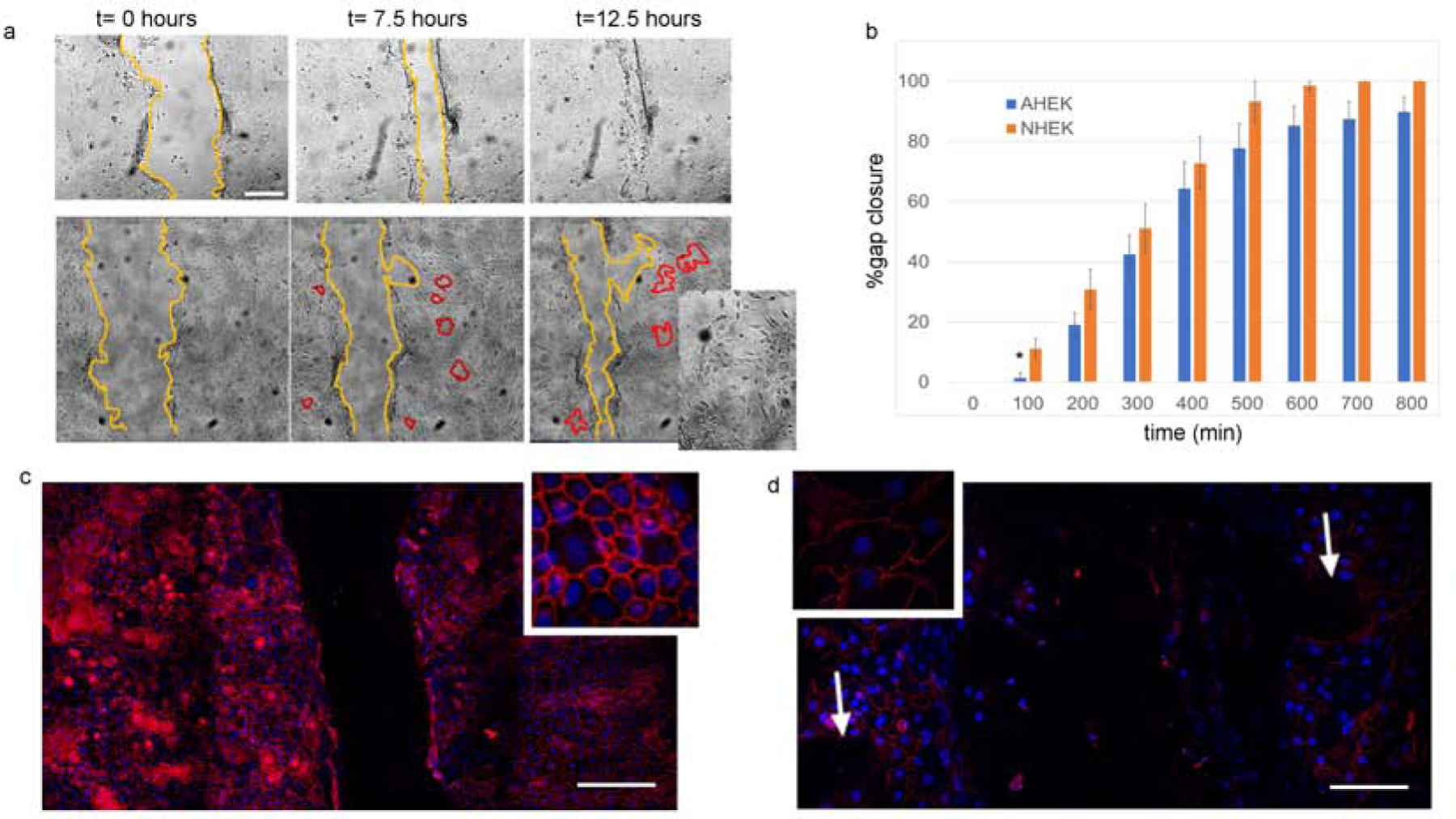
Previous studies (Tu et al., 2019) showed that decreased or absent epithelial CaSR levels lead to delayed re-epithelialization both in mice and in scratch assays using human keratinocytes via defective E-cadherin reorganization or CaSR mediated [Ca2+]i, increases. Passage 2 keratinocytes from aged or neonatal donors were plated in low calcium on plastic dishes for time lapse imaging or multichambered glass slides for IF microscopy until 80% confluent. Extracellular calcium levels were then raised to 1.2 mM to promote cell-to-cell adhesion and E-cadherin expression. After 24 hours in high calcium, the epithelial sheets were perturbed with a scratch assay and imaged at 100-minute intervals for 12–24 hours (Figure 4a). Time lapse images revealed that epithelial sheets from aged keratinocytes were slower on average than NHEKs at closing the defect (Fig. 4b), due to an initial delay at 100 minutes (asterisk, Figure 4b). Moreover, while NHEKs migrated as a sheet, aged cells appeared not to migrate in unison, but instead lost cell-to-cell adhesion and developed gaps as the sheets migrated (Figure 4a). E-cadherin IF staining was performed 6 hours after the scratch assay and revealed decreased and irregular E-cadherin plasma membrane staining in aged (Figure 4d) vs. young (Figure 4c) keratinocyte monolayers. Gaps in keratinocyte cell-to-cell adhesion were co-located with absent E cadherin staining.
Figure 4: Impaired cell-to-cell adhesion in aged keratinocytes monolayers.
(a) Brightfield time-lapse images of scratch assay of second passage keratinocytes monolayers from neonatal (top row) and aged (bottom row) donors in high calcium. Yellow lines highlight the gap area at different timepoints, while red lines highlight gaps occurring in the AHEK monolayers during sheet migration. Inset shows gaps in aged epidermal keratinocytes sheet. (b) Mean percentage gap closure as a function of time. AHEK blue bars, NHEK orange bars. Error bars represent the standard error of the mean. N=4–8 wells from two experiments on cells from 3–4 donors per condition. (c-d) E-cadherin staining (red) of NHEK (c) and AHEK (d) monolayers 6 hours after scratching. Nuclear DAPI counterstain (blue). Insets show higher detail of e-cadherin staining. White arrows in d show gaps in cell-to-cell adhesion.
The CaSR agonist NPS R-568 Rescues Ca2+ wave propagation, [Ca2+], Response to increased extracellular calcium, and E-cadherin Translocation in Aged Human Epidermal Keratinocytes
N-(3-[2-chlorophenyl]propyl)-(R)-alpha-methyl-3-methoxybenzylamine (NPS R-568) selectively (Nemeth et al., 1998; Tang et al., 2018) binds to the transmembrane domain of the CaSR and increases its stability (Huang et al., 2011), thereby increasing its Ca2+ sensitivity and enhancing the effects of extracellular Ca2+ on CaSR receptors (Fox et al., 1999). To test whether enhancing the CaSR response also would normalize Ca2+ wave propagation, signaling, and E-cadherin translocation to the plasma membrane, we pre-treated aged keratinocytes with 0.5 or 1μM NPS R-568 for 24 hours. Vehicle-treated aged keratinocytes and neonatal foreskin keratinocytes were used as controls.
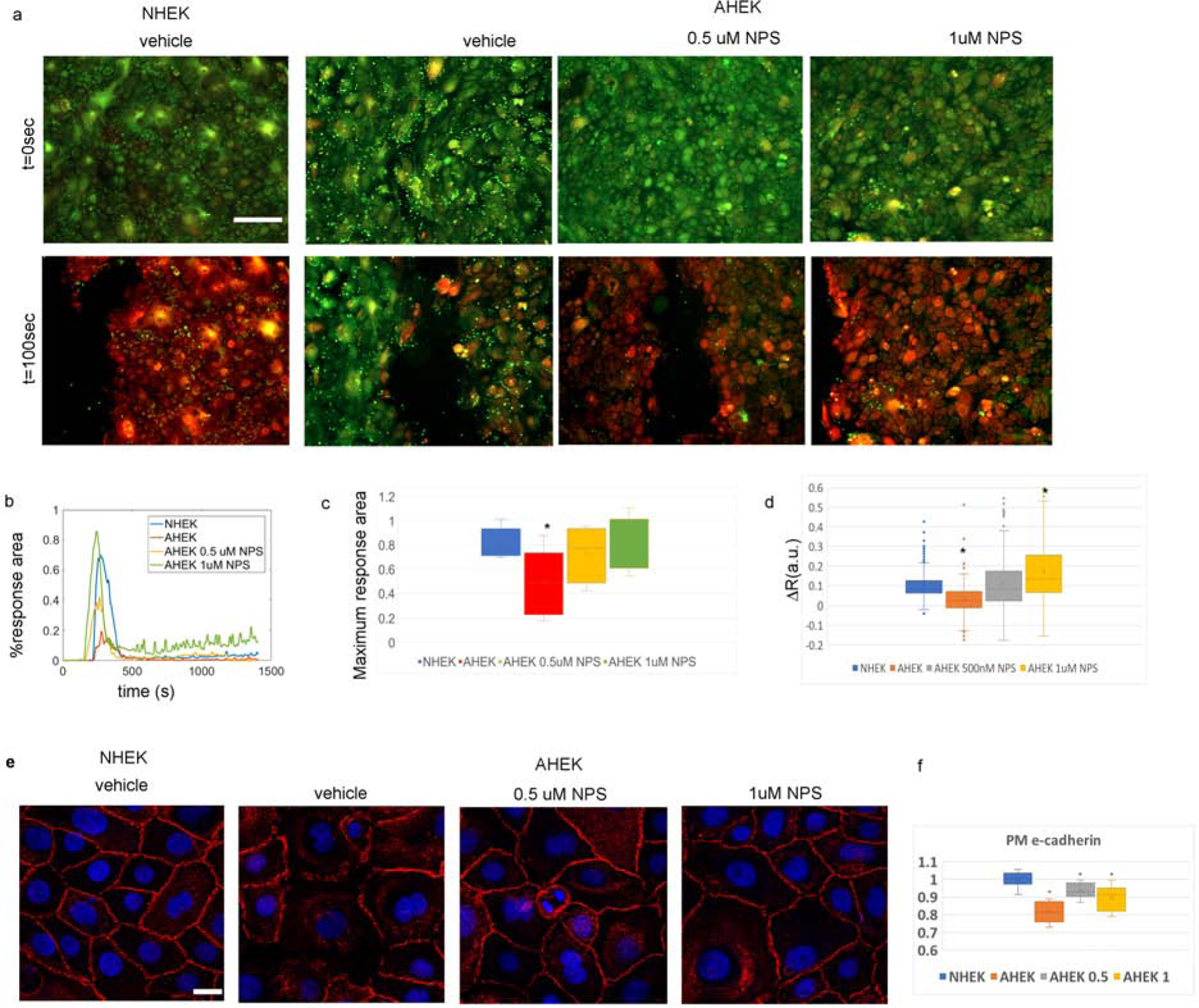
We first found that, like the epidermal response to laser stimulation, vehicle treated aged keratinocytes displayed markedly diminished propagation of calcium response to mechanical ablation (Figure 5a–c). However, pretreatment with NPS R-568 restored the Ca2+ wave propagation, in a dose-dependent fashion (Figure 5a–c). Pretreatment with NPS R-568 for 24 hours also restored the AHEKs [Ca2+]i response to increased extracellular calcium in a dose dependent manner (Figure 5d). CaSR stimulation with NPS R-568 also partially rescued E-cadherin plasma membrane translocation (Figure 5e and f). These findings demonstrate that enhancing CaSR activity through a pharmacological activator such as NPS R-568 can rescue the abnormal Ca2+ signaling and E-cadherin organization seen in aged keratinocytes.
Figure 5: NPS-R568 normalizes e-cadherin and calcium response after scratch wounding).
(a) NHEK and AHEKs before and 100 seconds after scratch. Red hue denotes higher [Ca2+]i. Scale-bar = 200 μm. (b) Time-traces of percentage area with increased [Ca2+]i after scratch . Representative of 5–8 experiments on cells from 3 neonatal and 3 aged donors per group. (c) Distribution of maximum calcium response area after scratch. NHEK (blue) and AHEK (red) treated with vehicle, 0.5 μM NPSR-568 (yellow), or 1 μM NPS R-568 (green). (d) Distribution of single cell [Ca2+]i response to increased extracellular calcium. Asterisks indicate statistical significance with p<0.05. N=192–409 cells per group from 6–10 experiments on cells from 3 neonatal and 3 aged donors. (e) Immunofluorescence E-cadherin staining (red) of NHEK and AHEK monolayers switched to 1.2mM [Ca2+] media containing 0 (DMSO vehicle), 0.5, or 1μM NPS-R568 for 15 minutes. Images are representative of 3 separate experiments on cells from 3 neonatal and 3 aged donors. Scale-bar = 20 μm. (f) Quantification of e-cadherin levels at the plasma membrane. Asterisks denote statistically significant difference from NHEKs levels determined via a two tailed t-test with p<0.05.
Discussion
These results show that normal Ca2+ signaling and Ca2+-signaling protein expression are impaired in aged epidermis and keratinocytes. Similar to CaSR KO cells and epidermis (Tu et al., 2019), Ca2+ propagation after perturbation in murine epidermis and cell monolayers from aged donors was significantly reduced compared to young controls. Moreover, CaSR expression was consistently downregulated in aged keratinocytes. CaSR acts to release Ca2+ from the endoplasmic reticulum and Golgi via PLC generated IP3, which then binds to IP3R on the endoplasmic reticulum (Tu et al., 2008). Knock down of CaSR in human cells causes reduction in both Gq mediated activation of PLC β and e-cadherin mediated activation of PLC γ (Tu et al., 2005), which are in turn necessary for the acute and sustained keratinocytes response to elevated extracellular calcium. Both PLC β and γ expression levels were consistently reduced in keratinocytes from aged donors.
CaSR expression also stabilizes the E-cadherin complex, which in turn regulates cell-to-cell adhesion and cell migration and enables the sustained intracellular calcium increase needed for keratinocyte differentiation through recruitment of PLC γ. Decreased E-cadherin expression levels and translocation to the plasma membrane were associated with decreased CaSR expression in aged human keratinocytes, consistent with previous reports in mice (Tu et al., 2008) and human cells (Tu et al., 2011). Our data also suggest that decreased E-cadherin levels result in loss of cell-to-cell junction stability and concerted epithelial sheet migration during a scratch assay. More differentiated keratinocytes tend to have a blunted Ca2+ response, and if aged keratinocytes are more differentiated that young keratinocytes this might furnish alternative mechanism to explain our findings. However, past reports demonstrate that aged keratinocytes and skin are actually less differentiated, both in mice (Bourguignon et al., 2013) and humans (Berge et al., 2008; Dos Santos et al., 2015; Diekmann et al., 2016).
Taken together, these results demonstrate that decreased CaSR expression and function contributes significantly to the impaired Ca2+ signaling response seen in aging. Although changes in each of the Ca2+ signaling components could also be expected to modify Ca2+ signaling, our finding that treatment with the CaSR agonist NPS-R568 restored normal Ca2+ signaling and E-cadherin distribution strongly suggests that decreased CaSR expression in aging drives both impaired Ca2+ signaling and downstream changes in Ca2+ signaling proteins. While decreases in CaSR protein expression might be expected to impair NPS-R568 efficacy, this agent has been shown to increase CaSR function on mutant CaSR receptors as well (Rus et al., 2008).
STIM1 expression also increased in both aged mouse epidermis and aged human keratinocytes, likely as a compensatory response. STIM1 expression was found to increase to a lesser extent in a previous report (Takei et al., 2016), although this report examined younger subjects (maximum 70 years of age) vs. our older subjects (>79 years of age).
Several questions remain regarding CaSR-mediated Ca2+ signaling in aged keratinocytes. First, while raised cytosolic baseline Ca2+ is consistent with STIM1 upregulation, it also could be explained by impaired Ca2+ uptake or extrusion mechanisms, including functional defects in organelle and plasma membrane Ca2+ ATPases, or defects in plasma membrane Na+/Ca2+ antiporters. We do not see consistent differences in expression of these proteins. However, more subtle differences in these proteins’ functions, along with elevated STIM levels, may become apparent in subsequent investigations. Second, while increased STIM1 levels would suggest increased SOCE, similar to what observed in CaSR KO cells (Tu et al., 2008), we observe a significant defect in calcium entry after exposure to high (1.2mM) extracellular calcium in aged versus young cells in both proliferative and differentiative conditions. Further investigation into the calcium entry mechanisms, such as STIM1 translocation to the plasma membrane and Orai1 interactions will be needed to address this defect Moreover, keratinocytes have also been shown to express molecules involved in non-capacitive calcium entry such as voltage sensitive calcium channels (Lee et al., 1994, Denda et al., 2006), Transient Receptor Potential (TRP) channels (Peier et al., 2002), non-selective cation channels in undifferentiated keratinocytes (Fatherazi et al., 2004), which could also play a role in the decreased response to raised extracellular calcium we observe in aged keratinocytes. Finally, it is not clear what mechanisms underlie the increased variability seen in the aged keratinocyte response to extracellular Ca2+, thapsigargin or mechanical stimulation. Previous studies demonstrate that more substantial increases in cytosolic Ca2+ are seen in less differentiated keratinocytes, both in response to extracellular Ca2+ (Kruszewski et al., 1991) and to mechanical stimulation (Dube et al., 2012). In addition, basal cytosolic Ca2+ concentrations are variable within the same colonies, depending on cell size (Pillai et al., 1993). Likewise, CaSR expression and function decrease as gingival keratinocytes terminally differentiate (Fatherazi et al., 2004). Variations in aged keratinocyte response therefore could be due to exaggerated intrinsic aging processes, mutations in response to environmental agents such as UV, or a combination of intrinsic and extrinsic processes.
These results suggest that the CaSR plays an essential role in mediating Ca2+ signaling and E-cadherin-mediated processes in the epidermis. Moreover, decreased CaSR expression and function contributes to impaired keratinocyte signaling and E cadherin expression. These studies also suggest that CaSR may be a different target in improving E-cadherin mediated processes in aged epidermis.
Material and Methods (please see Suppl Material for more information)
Laser perturbation assay
All animal procedures were approved by the Animal Studies Subcommittee (IACUC) of the San Francisco Veterans Administration Medical Center and were performed in accordance with their guidelines. Live epidermal explants from GCaMP3+/+ - expressing young (6–8 weeks) vs. old (22 months) mice placed dermis side down on a 3% agar gel were secured on the heated stage of an upright Zeiss LSM 780 confocal microscope coupled to a Ti:Saph laser (Chameleon Ultra II, Coherent). Ca2+ signaling in the epidermis was stimulated by irradiating a spatially defined 20X 20 um2 region on the basal layer of the epidermis with 800 nm (~140mW). Irradiation parameters (laser intensity, scanning speed, number of iterations) were kept constant for all experiments and were optimized to consistently elicit a [Ca2+]i response without permanent cell damage. The resulting GCaMP fluorescence was imaged with two-photon excitation microscopy. The excitation wavelength was 900nm (~15mW). Two spectral windows of 550/50nm and 445/25nm were used to visualize GCaMP fluorescence in the epidermis and the second harmonic generation signal respectively. Dermal collagen, identified with second harmonic signal, was used as a spatial reference. Time series were analyzed in Fiji (Schindelin et al., 2012) and Matlab (Mathworks). The change in GCaMP fluorescence was expressed as the ratio of change with respect to the baseline fluorescence (f/f0), while the response area was measured as the area with significantly increased [Ca2+]i (f/f0>1.2) was reported in um2. A total of 3 mice per age group was used for these experiments. Two separate skin biopsies (one per flank) per mouse were used to collect 18-19 time resolved [Ca2+]i traces per experimental group.
Ca2+i Imaging in Keratinocyte Monolayers and Cultured Keratinocyte Sheets
Second to third passage keratinocytes from newborns vs. aged subjects (NHEK and AHEK respectively) were cultured as described above to 70–90% confluence for single keratinocyte Ca2+ imaging and 100% confluence for scratch assays and laser perturbation. Keratinocytes were loaded with 10μM Calcium Green-1AM (Life Technologies) for keratinocyte Ca2+ response to laser perturbation. Cells were placed on the heated stage of an upright Zeiss 780 two-photon confocal microscope and calcium recordings before and after laser perturbation were acquired as described above using a dipping 20X lens with NA=1. For response to calcium switch, scratch assays and capacitive calcium entry after stores depletion, keratinocytes were loaded with 7.5 uM Fura-2 AM (Sigma-Aldrich). Dyes were loaded for 45 min at 37C and washed three times with HBSS. Phenol red free HBSS containing the appropriate [Ca2+]e (0, 0.07, or 1.2 mM) was used during imaging. Fura2-loaded cells were secured on a Zeiss Axio Imager 2 inverted fluorescence microscope and alternately illuminated with 340 and 390 nm wavelengths. The fluorescence at emission wavelength 510 nm was recorded. Scratches were made to keratinocyte sheets with a 23-gauge needle. Changes in Cai2+ levels in cells neighboring the scratched area were imaged before and for 50–200 sec after wounding. For response to high extracellular calcium switch experiments, after a period of equilibration to establish baseline of 60–120 sec, high calcium medium was added to the wells to a final concentration of 1.2 mM. Cells were imaged every second for additional 5–15 minutesCa2+. Response is expressed in um2 for area; R(a.u.)=f390nm/f340nm, where f390nm and f340nm are the fluorescence intensities generated by excitation at 390nm and 340nm respectively, corresponding to calcium bound and free FURA2, for single keratinocyte cytosolic Ca2+ responses; and ΔR(a.u.)=RhiCa-Rbl where RhiCa is the average R value between 2 and 4 minutes after calcium switch, and Rbl is the average baseline R before switch to high calcium. For capacitive calcium entry after store depletion, 1uM thapsigargin was added to the culture well during ratiometric imaging. After the ratiometric signal returned to baseline, cells were exposed to 1.2 mM calcium containing media. Cell migration was assessed using bright-field time lapse imaging. Cells were plated on 24 well plates and switched to 1.2 mM [Ca2+]e for 24 hours prior to imaging on a Zeiss Cell Observer (Carl Zeiss Microscopy Inc., New York, NY) with full environmental control (37 C and 5% CO2). A 10 μl pipette tip was used to scratch the cultures.
NPS-R 568 treatment
For calcium imaging experiments, keratinocytes monolayers were switched to 0.07 mM or 1.2 mM calcium and 0 (1:1000 dilution of DMSO), 0.5, or 1 μM NPS R 568 containing medium 24 hours prior to imaging. For e-cadherin IF staining, cells were exposed to 1.2 mM calcium and NPS R 568 (0, 0.5,1 μM) containing media 15 minutes prior to fixation.
Supplementary Material
Acknowledgements
This work was supported by the National Institute of Arthritis, Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers R01 AR051930 and R01 AR061106 (PI, Mauro), administered by the Northern California Institute for Research and Education, with additional resources provided by the Veterans Affairs Medical Center (Award Number IO1 BX003814 [PI, Bikle]), San Francisco, CA, and grant LF16028 from LEO Foundation, Denmark. We gratefully acknowledge Joan Wakefield for her editing assistance, the contribution of the physicians and nurses at the UCSF Benioff Children’s Hospital Newborn Nursery and Drs. Arron, Saylor and Yu at the San Francisco Veterans Affairs Medical Center, as well as The Laboratory for Cell Analysis Core Facility at the Hellen Diller Family Comprehensive Cancer Research Center (NIH grant P30CA082103). This content is solely the responsibility of the authors and does not necessarily represent the official views of either the National Institutes of Health or the Department of Veterans Affairs.
Abbreviations:
- AHEK
Aged Human Epidermal Keratinocytes
- AJ
mediated adherens junction
- Cai
cytosolic Ca2+
- CaSR
Ca2+ sensing receptor
- ER
endoplasmic reticulum
- IP3R
inositol 3 phosphate receptor
- KO
knock out
- MAPK
mitogen activated kinase
- NHEK
Neonatal Human Epidermal Keratinocytes
- NPPS R-568
N-(3-[2-chlorophenyl]propyl)-(R)-alpha-methyl-3-methoxybenzylamine
- PLC β
Phospholipase C beta
- (PLC γ
Phospholipase C gamma
- SERCA2
Sarco-endoplasmic Reticulum ATPase 2
- SOCE
Store Operated Calcium Entry
- SPCA1
Calcium Transporting ATPase2 C1
- STIM1
stromal interacting molecule 1
- TG
thapsigargin
- VDR
Vitamin D receptor
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availablity - The data that support the findings of this study are available at http://dx.doi.org/10.17632/s5mk3nd495.1 and from the corresponding author upon reasonable request.
Conflict of Interest: None
References
- Berge U, Kristensen P, Rattan SI. Hormetic modulation of differentiation of normal human epidermal keratinocytes undergoing replicative senescence in vitro. Exp Gerontol 2008; 43:658–62. [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Wong G, Xia W, Man MQ, Holleran WM, Elias PM. Selective matrix (hyaluronan) interaction with CD44 and RhoGTPase signaling promotes keratinocyte functions and overcomes age-related epidermal dysfunction. J Dermatol Sci 2013; 72:32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli A, Crumrine D, Meyer JM, Mauro TM. Endoplasmic Reticulum Calcium Regulates Epidermal Barrier Response and Desmosomal Structure. J Invest Dermatol 2016; 136:1840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro JV, Jacinto A. The role of transcription-independent damage signals in the initiation of epithelial wound healing. Nat Rev Mol Cell Biol 2013; 14:249–62. [DOI] [PubMed] [Google Scholar]
- Denda M, Fujiwara S, Hibino T. Expression of voltage-gated calcium channel subunit alpha1C in epidermal keratinocytes and effects of agonist and antagonists of the channel on skin barrier homeostasis. Exp Dermatol 2006; 15:455–60. [DOI] [PubMed] [Google Scholar]
- Denda S, Takei K, Kumamoto J, Goto M, Denda M. Expression level of Orai3 correlates with aging-related changes in mechanical stimulation-induced calcium signalling in keratinocytes. Exp Dermatol 2017; 26:276–278. [DOI] [PubMed] [Google Scholar]
- Diekmann J, Alili L, Scholz O, Giesen M, Holtkotter O, Brenneisen P. A three-dimensional skin equivalent reflecting some aspects of in vivo aged skin. Exp Dermatol 2016; 25:56–61. [DOI] [PubMed] [Google Scholar]
- Dos Santos M, Metral E, Boher A, Rousselle P, Thepot A, Damour O. In vitro 3-D model based on extending time of culture for studying chronological epidermis aging. Matrix Biol 2015; 47:85–97. [DOI] [PubMed] [Google Scholar]
- Dube J, Rochette-Drouin O, Levesque P, Gauvin R, Roberge CJ, Auger FA, et al. Human keratinocytes respond to direct current stimulation by increasing intracellular calcium: preferential response of poorly differentiated cells. J Cell Physiol 2012; 227:2660–7. [DOI] [PubMed] [Google Scholar]
- Elias PM, Nau P, Hanley K, Cullander C, Crumrine D, Bench G, et al. Formation of the epidermal calcium gradient coincides with key milestones of barrier ontogenesis in the rodent. J Invest Dermatol 1998; 110:399–404. [DOI] [PubMed] [Google Scholar]
- Fatherazi S, Belton CM, Cai S, Zarif S, Goodwin PC, Lamont RJ, et al. Calcium receptor message, expression and function decrease in differentiating keratinocytes. Pflugers Arch 2004; 448:93–104. [DOI] [PubMed] [Google Scholar]
- Forslind B, Werner-Linde Y, Lindberg M, Pallon J. Elemental analysis mirrors epidermal differentiation. Acta Derm Venereol 1999; 79:12–7. [DOI] [PubMed] [Google Scholar]
- Fox J, Lowe SH, Conklin RL, Nemeth EF. The calcimimetic NPS R-568 decreases plasma PTH in rats with mild and severe renal or dietary secondary hyperparathyroidism. Endocrine 1999; 10:97–103. [DOI] [PubMed] [Google Scholar]
- Huang Y, Cavanaugh A, Breitwieser GE. Regulation of stability and trafficking of calcium-sensing receptors by pharmacologic chaperones. Adv Pharmacol 2011; 62:143–73. [DOI] [PubMed] [Google Scholar]
- Komuves L, Oda Y, Tu CL, Chang WH, Ho-Pao CL, Mauro T, et al. Epidermal expression of the full-length extracellular calcium-sensing receptor is required for normal keratinocyte differentiation. J Cell Physiol 2002; 192:45–54. [DOI] [PubMed] [Google Scholar]
- Kruszewski FH, Hennings H, Yuspa SH, Tucker RW. Regulation of intracellular free calcium in normal murine keratinocytes. Am J Physiol 1991; 261:C767–73. [DOI] [PubMed] [Google Scholar]
- Kumamoto J, Goto M, Nagayama M, Denda M. Real-time imaging of human epidermal calcium dynamics in response to point laser stimulation. J Dermatol Sci 2017; 86:13–20. [DOI] [PubMed] [Google Scholar]
- Lee SE, Lee SH. Skin Barrier and Calcium. Ann Dermatol 2018; 30:265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Elias PM, Feingold KR, Mauro T. A role for ions in barrier recovery after acute perturbation. J Invest Dermatol 1994; 102:976–9. [DOI] [PubMed] [Google Scholar]
- Mauro T, Bench G, Sidderas-Haddad E, Feingold K, Elias P, Cullander C. Acute barrier perturbation abolishes the Ca2+ and K+ gradients in murine epidermis: quantitative measurement using PIXE. J Invest Dermatol 1998; 111:1198–201. [DOI] [PubMed] [Google Scholar]
- Menon GK, Elias PM. Ultrastructural localization of calcium in psoriatic and normal human epidermis. Arch Dermatol 1991; 127:57–63. [PubMed] [Google Scholar]
- Nemeth EF, Steffey ME, Hammerland LG, Hung BC, Van Wagenen BC, DelMar EG, et al. Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci U S A 1998; 95:4040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numaga-Tomita T, Putney JW. Role of STIM1- and Orai1-mediated Ca2+ entry in Ca2+-induced epidermal keratinocyte differentiation. J Cell Sci 2013; 126:605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Hu L, Nguyen T, Fong C, Tu CL, Bikle DD. Combined Deletion of the Vitamin D Receptor and Calcium-Sensing Receptor Delays Wound Re-epithelialization. Endocrinology 2017; 158:1929–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, et al. A heat-sensitive TRP channel expressed in keratinocytes. Science 2002; 296:2046–9. [DOI] [PubMed] [Google Scholar]
- Periasamy M, Kalyanasundaram A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve 2007; 35:430–42. [DOI] [PubMed] [Google Scholar]
- Pillai S, Menon GK, Bikle DD, Elias PM. Localization and quantitation of calcium pools and calcium binding sites in cultured human keratinocytes. J Cell Physiol 1993; 154:101–12. [DOI] [PubMed] [Google Scholar]
- Rus R, Haag C, Bumke-Vogt C, Bahr V, Mayr B, Mohlig M, et al. Novel inactivating mutations of the calcium-sensing receptor: the calcimimetic NPS R-568 improves signal transduction of mutant receptors. J Clin Endocrinol Metab 2008; 93:4797–803. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an opensource platform for biological-image analysis. Nat Methods 2012; 9:676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei K, Denda S, Nagayama M, Denda M. Role of STIM1-Orai1 system in intra-cellular calcium elevation induced by ATP in cultured human keratinocytes. Exp Dermatol 2016; 25:323–5. [DOI] [PubMed] [Google Scholar]
- Tang L, Jiang L, McIntyre ME, Petrova E, Cheng SX. Calcimimetic acts on enteric neuronal CaSR to reverse cholera toxin-induced intestinal electrolyte secretion. Sci Rep 2018; 8:7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi M, Goto M, Denda M. Dynamics of intracellular calcium in cultured human keratinocytes after localized cell damage. Exp Dermatol 2013; 22:367–9. [DOI] [PubMed] [Google Scholar]
- Tu CL, Celli A, Mauro T, Chang W. Calcium-Sensing Receptor Regulates Epidermal Intracellular Ca(2+) Signaling and Re-Epithelialization after Wounding. J Invest Dermatol 2019; 139:919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu CL, Chang W, Bikle DD. Phospholipase cgamma1 is required for activation of store-operated channels in human keratinocytes. J Invest Dermatol 2005; 124:187–97. [DOI] [PubMed] [Google Scholar]
- Tu CL, Chang W, Bikle DD. The role of the calcium sensing receptor in regulating intracellular calcium handling in human epidermal keratinocytes. J Invest Dermatol 2007; 127:1074–83. [DOI] [PubMed] [Google Scholar]
- Tu CL, Chang W, Bikle DD. The calcium-sensing receptor-dependent regulation of cell-cell adhesion and keratinocyte differentiation requires Rho and filamin A. J Invest Dermatol 2011; 131:1119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu CL, Chang W, Xie Z, Bikle DD. Inactivation of the calcium sensing receptor inhibits E-cadherin-mediated cell-cell adhesion and calcium-induced differentiation in human epidermal keratinocytes. J Biol Chem 2008; 283:3519–28. [DOI] [PubMed] [Google Scholar]
- Tu CL, Crumrine DA, Man MQ, Chang W, Elalieh H, You M, et al. Ablation of the calcium-sensing receptor in keratinocytes impairs epidermal differentiation and barrier function. J Invest Dermatol 2012; 132:2350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunggal JA, Helfrich I, Schmitz A, Schwarz H, Gunzel D, Fromm M, et al. E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J 2005; 24:1146–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turksen K, Troy TC. Overexpression of the calcium sensing receptor accelerates epidermal differentiation and permeability barrier formation in vivo. Mech Dev 2003; 120:733–44. [DOI] [PubMed] [Google Scholar]
- Vandenberghe M, Raphael M, Lehen’kyi V, Gordienko D, Hastie R, Oddos T, et al. ORAI1 calcium channel orchestrates skin homeostasis. Proc Natl Acad Sci U S A 2013; 110:E4839–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


