Abstract
Cost-effective exogenous application of some antioxidant, viz. salicylic acid (SA) and ascorbic acid (AA), and essential micronutrient elements like Zn might alleviate the harmful impacts of drought stress. Here, we evaluated the interaction of foliar-sprayed SA (1 mM), AA (10 mM), and Zn (3 g L–1) and irrigation regime (normal irrigation, moderate water stress, and severe water stress) by assaying an array of agronomic, physiological, analytical and biochemical parameters of Moldavian balm (Dracocephalum moldavica L.). Accordingly, the SA and AA treatments reduced the harmful effects of moderate and severe drought stress. Well-watered plants applied with Zn had the highest biomass yield (4642.5 kg ha–1). Severe water stress decreased plant biomass, essential oil (EO) content, EO yield, relative water content, and chlorophyll a content by 37.6%, 23.3%, 47.5%, 35.3%, and 53%, respectively, relative to normal irrigation. Plants treated with Zn under moderate drought stress had the highest EO content. Moderate and severe water stress increased enzymatic antioxidant (catalase, superoxide dismutase, and peroxidase) activities and total soluble sugars and proline contents. In terms of EO composition, SA-treated plants under moderate water stress contained the most geraniol (22.8%) and geranial (26.3%), while Zn-treated plants under severe water stress contained the most geranyl acetate (48.2%). This study demonstrated that foliar application of Zn and SA significantly improves EO productivity and quality in Moldavian balm under moderate water stress. The relevant findings were supported by heatmap clustering, revealing that irrigation regime had main effect on the essential oil compounds and biochemical and physiological parameters.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-021-01084-1.
Keywords: Biomass yield, Geranyl acetate, Secondary metabolites, Soluble sugars, Superoxide dismutase, Water deficit
Introduction
Moldavian balm or dragonhead (Dracocephalum moldavica L., Lamiaceae) is an annual, aromatic plant that produces a substantial amount of essential oil (EO) (Dastmalchi et al. 2007). The plant is widely used in the food, medicinal, and health industries (Dmitruk and Weryszko-Chmielewska 2010; Sabzi-Mehrabad et al., 2017). Although Moldavian balm is native to Siberia and Central Asia, it has been cultivated widely in eastern and central parts of Europe.
This species is cultivated in the northwest of Iran, particularly Tabriz, Urmia, Mazandaran, and Alborz mountainous areas (Dastmalchi et al. 2007). Dragonhead EO contents range from 0.06–0.92%, with the highest content present during flowering (Kakasy et al. 2006), and the major components are neral, geranial, geraniol, and geranyl acetate (Amani Machiani et al. 2019). The EO of Moldavian balm has antibacterial and antimicrobial properties (Gilling et al. 2014), antioxidant properties (Fallah et al. 2018), antinociceptive properties (Maham et al. 2013), and a sedative effect (Martínez-Vázquez et al. 2012). However, Moldavian balm’s EO content and composition vary under drought stress conditions (Kulak 2020).
Various strategies, including foliar treatments, can minimize the devastating effects of drought stress (Askari and Ehsanzadeh 2015). Salicylic acid (SA) is a phenolic substance, considered a phytohormone, that has a significant role in modulating plant responses to various abiotic stresses (Pandey and Chakraborty 2015). It acts as an antioxidant in plants and scavenges free radicals (Miura and Tada 2014). Furthermore, SA protects plants against drought stress by regulating photosynthesis, proline metabolism, and water relations (Khan et al. 2015).
Exogenous application of antioxidants can alleviate the pressure of adverse environmental conditions (Noman et al. 2015; Mohammadi et al. 2019; Rabêlo et al. 2019). Ascorbic acid (AA) is an antioxidant compound that improves drought stress tolerance through cellular signaling (Khazaei et al. 2020), enhancing photosynthesis, transpiration, and oxidative defense potential. In addition, ascorbic acid can eliminate reactive oxygen species (ROS) and increase photosynthetic pigment contents to improve plant growth (Amira and Qados 2014; Xu et al. 2015; Akram and Ashraf 2017).
Zinc (Zn) deficiency is a major nutritional problem in soils worldwide (Sadeghzadeh 2013). As a vital micronutrient and constituent of several plant enzymes and proteins, Zn affects plant water status and protects plants from light-dependent oxidative stress (Sofy 2015; Ma et al. 2017). Foliar application of Zn increased enzyme activity and cell defense against ROS attack in drought-stressed wheat (Yavas and Unay 2016).
The impact of water stress has been investigated extensively for crop plants but not for medicinal and aromatic herbs, particularly in arid and semi-arid areas. Exogenous treatments are often used to cope with water stress. This study investigated the effect of foliar applications of SA, AA, and Zn on physiological and agronomic traits and EO yield and components. As per previous studies and the role of antioxidants, we hypothesized that: i) exogenous application of antioxidants improves dry matter yield, EO, EO yield, and EO constituents in Moldavian balm exposed to water stress; ii) exogenous application of antioxidants increases antioxidant activities in Moldavian balm exposed to water stress, and (iii) exogenous application of SA increases some physiological activities in Moldavian balm exposed to water stress.
Materials and methods
Research site and experimental design
A two-year field trial was conducted in 2016 and 2017 at a research farm in Naqadeh, West Azerbaijan Province, Iran (36°57′ N, 45°24′ W, 1330 m a.s.l.). The experimental protocol used a randomized complete block design with a factorial combination of three irrigation regimes [normal irrigation (irrigation following 60 mm evaporation from Class A pan), moderate water stress (irrigation following 110 mm evaporation from Class A pan), and severe water stress (irrigation following 160 mm evaporation from Class A pan)] and four antioxidant treatments (1 mM SA, 10 mM AA and 3 g L–1 Zn, plus a water spraying control) with three replications.
The experiment was carried out in a silty-clay soil with average pH 7.4, 9.5 g kg–1 organic carbon content, 0.36 dS m–1 EC, 27% field capacity (FC), 0.8 g kg–1 total N, 11.14 mg kg–1 available P, and 252 mg kg–1 available K.
Monthly average temperature and precipitation of the relevant experimental field in 2016 and 2017 are given in Fig. 1.
Fig. 1.
Monthly average temperature and precipitation in 2016 and 2017 in the experimental fields
The seeds of local landraces of Moldavian balm were provided by Zarrin‐Giah Medicine Plant Co. Ltd., Urmia, Iran. The soil was prepared by plowing, disking, and leveling. Each experimental plot (9.6 m2) comprised eight 3 m long rows spaced 40 cm apart. Each plot and block was spaced 2 m and 3 m from each other, respectively, to prevent water runoff to adjacent plots. Seeds were sown 20 cm apart within each row on 10 May 2016 and 2017. Based on the soil analysis, the experimental field was fertilized with 100 kg ha–1 urea (before sowing and topdressing), 80 kg ha–1 triple superphosphate, and 200 kg ha–1 sulfur bentonite per ha (before sowing). Invasive plants were manually removed during the growing season when required. The first irrigation was done immediately after planting to facilitate seedling emergence, with the subsequent irrigations according to the treatments.
Antioxidant treatments were sprayed onto plant leaves on 5 June 2016 and 2017 (30 days after sowing, DAS) and repeated three weeks (stem elongation) after applying the water stress treatments to ensure their effectiveness.
Harvesting of moldavian balm
At the 50% lowering stage, plant samples (shoots) were harvested from a 2 m2 area in each plot. The aerial plant parts were air-dried in the laboratory at room temperature (25 °C and dry shade) for ten days, with the dry weights reported as biomass.
Essential oil (EO) extraction and EO yield
The air-dried parts of Moldavian balm (50 g dry material, including leaves and flowers) were hydro-distilled with a Clevenger-type apparatus with 500 mL deionized water for 3 h. Water was removed from the essential oil over anhydrous sodium sulfate and transferred into a dark, capped bottle stored at 4 °C until GC–MS analysis. The EO content (%) and EO yield (kg ha–1) were calculated as follows (Rezaei-Chiyaneh et al. 2020a):
Essential oil analysis
Gas chromatography–mass spectrometry analysis was undertaken using an Agilent 7890/5975C (Santa Clara, California, USA) GC/MSD. An HP-5 MS capillary column (5% phenyl methyl polysiloxane, 30 m length, 0.25 mm i.d., 0.25 μm film thickness) was used to separate the EO components. The following oven temperature was applied: 3 min at 80 °C, before increasing by 8 °C min−1 to 180 °C, and held for 10 min at 180 °C. Helium was used as the carrier gas at a flow rate of 1 ml min–1. The sample was injected (1 μL) in split mode (1:50). The EI mode was 70 Ev. Mass range was set from 40 to 550 m/z. The components were recognized by comparing the calculated Kovats retention indices with a mixture of n-alkane series (C8–C30, Supelco, Bellefonte, CA) and mass spectra (Adams 2007; Rezaei-Chiyaneh et al. 2020b). GC-FID analysis was undertaken using an Agilent 7890 A instrument. The separation was performed in an HP-5 capillary column. The analytical conditions were the same as above. Quantification methods were the same as those reported by Faridvand et al. (2021).
Physiological parameters
At the flower initiation stage (early flowering), fresh leaf samples were collected on 22 July 2016 and 2017, covered in aluminum foil in a Petri dish and stored at –80 °C for the analysis of physiological parameters.
Chlorophylls and carotenoids
Leaf tissue was extracted with acetone (80%) to calculate chlorophyll a (Chl-a), chlorophyll b (Chl-b), and carotenoid (CX+C) concentration according to Lichtenthaler (1987):
‘A’ refers to the absorbance.
Relative water content (RWC)
The RWC content of the topmost fully expanded leaf of the main shoot was measured at the flower initiation stage (early flowering). Fresh leaf samples (0.2 g) were placed in 50 mL of distilled water for 4 h to measure turgid leaf weight (Xu et al. 2006), before drying the leaf samples at 70 °C for 48 h to measure dry weight. The RWC was determined as follows:
Osmolyte measurements
Proline
Leaf proline content was estimated according to the method of Bates et al. (1973). Briefly, 0.5 g fresh leaf was homogenized in 5 mL of 3% sulfosalicylic acid and centrifuged to remove leaf tissue. A proline:ninhydrin acid:glacial acetic (1:1:1) containing solution was added to 2 mL leaf extract, heated at 100 °C for 1 h, before adding 4 mL toluene. After phase separation, absorbance was read at 520 nm.
Total soluble sugars
The phenol sulfuric acid method was used to estimate total soluble sugars (TSS) in leaf samples (Irigoyen et al. 1992). Briefly, 0.5 g fresh leaf was homogenized with ethanol and filtered before adding 5% phenol and 98% sulfuric acid. After cooling, absorbance was read at 485 nm.
Antioxidant enzymes
Fresh leaf (200 mg) was ground to powder in a mortar and pestle with liquid nitrogen. The powdered samples were extracted with 4 mL of 0.05 M Na2HPO4/NaH2PO4 (pH 7.0) buffer containing 0.2 mM ethylene-diamine tetra acetic acid and 1% polyvinylpyrrolidone. After centrifuging at 15,000g for 20 min at 4 °C, the supernatant was used to determine enzymatic activity.
Catalase (CAT) activity was estimated using the disappearance rate of hydrogen peroxide (H2O2) (Aebi 1984). The reaction mixture contained 50 mL enzyme extract, 0.1 mL H2O2, and 50 mM phosphate buffer (pH 7.0). CAT activity was estimated according to recorded absorbance at 240 nm.
Superoxide dismutase (SOD) activity was estimated using the photochemical reduction of nitro blue tetrazolium (Beauchamp and Fridovich 1971). The reaction mixture contained 390 mM methionine, 2.25 mM NBT, 3 mM EDTA, 1.5 M Na2CO3, and 150 μL crude enzyme extract. Absorbance was read at 560 nm.
Peroxidase (POX) activity was estimated using the guaiacol oxidation method (Gueta-Dahan et al. 1997). The reaction mixture containing 1 mL of 1% guaiacol, 1 mL of 1% H2O2, 2.5 mL of 50 mM potassium phosphate buffer (pH 7.0), and 0.3 mL enzyme extract. Absorbance was read at 470 nm.
Statistical analysis
A combined analysis of variance across two years of data was conducted using a mixed linear model with PROC MIXED procedure in SAS 9.4 software (SAS Institute Inc., Cary, NC, USA). Irrigation regime and antioxidant treatment were considered fixed effects, and block, year, and their interactions were considered random effects. The means were compared using Duncan’s multiple range test, with differences between individual means considered significant at P < 0.05.
Results
Biomass yield
Irrigation regime, antioxidant treatment, and their interaction significantly affected biomass yield of Moldavian balm (P < 0.01) (Table 1). Biomass yield in the water-sprayed control plants decreased by 11.65% and 37.60% under moderate and severe water stress, respectively, relative to normal irrigation. In contrast, the Zn and SA foliar treatments increased biomass yield under all irrigation regimes. Normal irrigation with foliar Zn had the highest biomass yield (4642.50 kg ha–1), while water-sprayed plants under severe water stress had the lowest biomass yield (2590.50 kg ha–1). Under severe water stress, SA, AA, and Zn application increased biomass yield by 18.95%, 14.57%, and 17.94%, respectively, relative to the water-sprayed control plants (Table 2).
Table 1.
Effect of irrigation regime and antioxidant.
Source: treatments on agronomic and physiological traits and antioxidant enzyme activities in Moldavian balm
| SOV | Biomass yield | Essential oil content | Yield oil | RWC | Chlorophyll a | Chlorophyll b | Carotenoid content | Proline content | Soluble sugar content | CAT | POX | SOD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year (Y) | ** | NS | NS | NS | ** | NS | NS | * | ** | ** | NS | NS |
| Irrigation regime (I) | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| Y × I | NS | NS | NS | NS | ** | NS | NS | NS | NS | NS | NS | NS |
| Antioxidant source (A) | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| Y × A | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| I × A | ** | ** | ** | ** | ** | ** | NS | NS | * | ** | ** | ** |
| Y × I × A | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
SOV source of variation, NS non-significant, *Significant at 5% probability level (P < 0.05); **Significant at 1% probability level (P < 0.01). The same letters in each column indicate non-significant differences at the 5% probability level
Table 2.
Effect of irrigation regime and antioxidant
source treatments on agronomic and physiological traits and antioxidant enzyme activities in Moldavian balm
| Stress | Antioxidant | Biomass yield (kg ha–1) | Essential oil content (%) | Essential oil yield (kg ha–1) | Relative water content (%) | Chlorophyll a (mg g–1 FW) | Chlorophyll b (mg g–1 FW) | Total soluble sugars content (mg g–1 FW) | Catalase (Unit mg g–1 min–1) | Superoxide dismutase (Unit mg g–1 min–1) | Peroxidase (Unit mg g–1 min–1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal irrigation | Control | 4151.17 c | 0.463 c | 19.23 c | 88.63 b | 2.02 cd | 1.00 d | 1.87 e | 1.51 d | 1.05 e | 1.37 g |
| Salicylic acid | 4198.83 bc | 0.463 c | 19.45 c | 95.51 a | 2.30 a | 0.89 f | 1.88 e | 1.72 d | 1.17 e | 1.42 fg | |
| Ascorbic acid | 4164.50 bc | 0.470 c | 19.57 c | 95.50 a | 2.21 ab | 0.90 f | 1.88 e | 1.77 d | 1.18 e | 1.49 fg | |
| Zinc | 4642.50 a | 0.485 c | 22.51 b | 90.44 b | 1.98 cd | 0.85 g | 1.99 e | 1.73 d | 1.17 e | 1.38 g | |
| Moderate stress | Control | 3667.50 e | 0.533 b | 19.54 c | 63.80 ef | 1.30 g | 0.64 h | 2.52 d | 1.80 d | 1.56 d | 1.53 ef |
| Salicylic acid | 4183.33 bc | 0.596 a | 24.96 a | 77.51 c | 2.12 bc | 1.17 a | 3.04 c | 2.08 c | 1.93 c | 1.73 b-d | |
| Ascorbic acid | 4003.17 d | 0.575 a | 23.02 b | 75.16 c | 1.90 de | 1.04 cd | 3.25 c | 2.30 c | 1.97 c | 1.73 b-d | |
| Zinc | 4270.33 b | 0.593 a | 25.34 a | 69.61 d | 1.94 de | 1.15 ab | 3.34 c | 2.61 b | 2.10 c | 1.63 de | |
| Severe stress | Control | 2590.50 g | 0.320 e | 8.28 e | 50.81 h | 1.08 h | 0.52 i | 3.72 b | 2.19 c | 2.09 c | 1.68 cd |
| Salicylic acid | 3081.17 f | 0.381 d | 11.75 d | 66.18 e | 1.82 e | 1.11 b | 4.40 a | 2.75 ab | 3.08 a | 2.14 a | |
| Ascorbic acid | 2968.17 f | 0.366 d | 10.87 d | 62.20 fg | 1.65 f | 0.95 e | 4.51 a | 2.79 ab | 2.66 b | 1.85 b | |
| Zinc | 3055.33 f | 0.375 d | 11.46 d | 60.46 g | 1.66 f | 1.05 c | 4.57 a | 2.89 a | 3.07 a | 1.84 b |
Means within each column followed by at least one letter in common do not significantly differ at the 5% probability level (P < 0.05) using Duncan’s multiple range test
Essential oil content and EO yield
The interaction between irrigation regime and antioxidant treatment was significant (P < 0.01) for EO content and EO yield (Table 1). The highest EO content occurred under moderate water stress. Antioxidant application under normal irrigation did not significantly change EO content. Antioxidant application under moderate and severe water stress increased EO content, but it did not significantly differ between antioxidant treatments. The EO content increased by 22.18% under moderate water stress but decreased by 23.3% under severe water stress, relative to normal irrigation. Under moderate water stress, SA, AA, and Zn application increased EO contents by 11.81%, 7.88%, and 11.25%, respectively, relative to the water-sprayed control plants.
For all irrigation regimes, the antioxidant treatments increased EO yield, more so for Zn-treated plants. The Zn-treated plants under moderate water stress had the highest EO yield (25.34 kg ha–1), while the water-sprayed plants under severe water stress had the lowest EO yield (8.28 kg ha–1). Under severe water stress, EO yield decreased by 47.54%, relative to normal irrigation; however, the application of SA, AA, and Zn increased EO yield by 41.90%, 25%, and 38.40%, respectively, relative to the water-sprayed control plants (Table 2).
Essential oil composition
Nineteen EO compounds were identified in Moldavian balm (Supplemental Table 1), with neral (8.51–12.14%), geraniol (15.24–22.77%), geranial (18.47–26.29%), and geranyl acetate (33.33–48.15%) the principal components. The contents of these compounds varied with irrigation regime and antioxidant source. The most identified compounds were obtained in the AA treatment (99.86%) under severe water and followed by SA under moderate water (99.81%) and well-watered conditions (99.58%). The Zn-treated plants had the highest neral content for all irrigation regimes. The SA-treated plants under moderate water stress had the most geranial and geraniol (26.29% and 22.77%). The Zn-treated plants under severe water stress had the most geranyl acetate (48.15%), (Supplemental Table 1).
The heatmap clustering classified the EO compounds into three main groups (Fig. 2). The first group contained geraniol, geranial, thymol, methyl geranate, verbenol, cis chrysanthenol, and benzene acetaldehyde, which significantly decreased under moderate and severe water stress. However, the irrigation treatments and other treatments did not affect the other EO components. The heatmap (Fig. 2) shows that severe water stress have the highest impact on essential oil metabolism.
Fig. 2.
Heatmap clustering of irrigation regime and antioxidant
source treatments based on essential oil constituents in Moldavian balm. Control: water spraying control, SA: 1 mM salicylic acid, AA: 10 mM ascorbic acid, Zn: 3 g L–1 Zinc. Normal irrigation: 60 mm evaporation from Class A pan, Moderate stress: 110 mm evaporation from Class A pan, severe stress: 160 mm evaporation from Class A pan. The key color bar indicates standardized mean values (dark red indicates relatively lower mean values; dark blue indicates relatively higher mean values)
Relative water content
The interaction between irrigation regime and antioxidant treatment was significant for leaf RWC (P < 0.01) (Table 1). The RWC of Moldavian balm leaves significantly decreased by 22.70% and 35.24% under moderate and severe water stress, respectively, relative to normal irrigation (Table 2). The SA -treated plants under normal irrigation had the highest RWC (95.51%), while the water-sprayed plants under severe water stress had the lowest RWC (50.81%). The antioxidant treatments increased RWC under all irrigation regimes, especially moderate and severe water stress. The SA and AA treatments increased RWC more than the Zn treatment, with no statistical differences noted between SA and AA applications under normal irrigation and moderate water stress (Table 2).
Chlorophyll and carotenoids
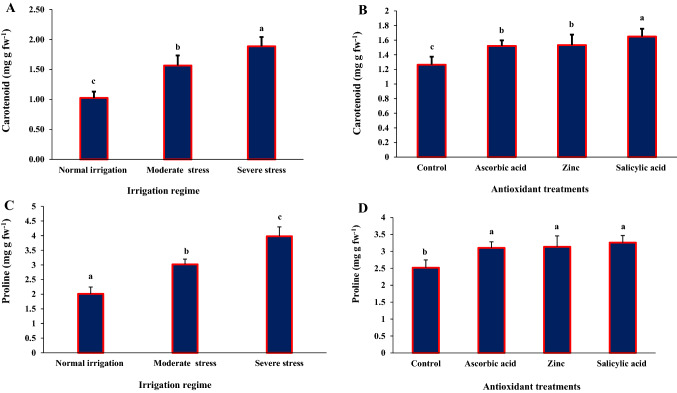
The interaction between irrigation regime and antioxidant treatment was significant (P < 0.01) for Chl-a and Chl-b concentrations (Table 1). Chlorophyll concentration decreased under moderate and severe water stress. The SA-treated plants under normal irrigation had the highest leaf Chl-a concentration (2.30 mg g–1 FW), while the water-sprayed plants under severe water stress had the lowest Chl-a concentration (1.08 mg g–1 FW). Indeed, SA application significantly increased leaf Chl-a concentration under all irrigation regimes (Table 2). The SA-treated plants under moderate water stress had the highest Chl-b concentration (1.17 mg g–1 FW), while the water-sprayed plants under severe water stress had the lowest Chl-b concentration (0.52 mg g–1 FW), (Table 2). Water-stressed plants had higher carotenoid concentration than those under normal irrigation (Fig. 3A), which further increased with foliar application of AA, SA, or Zn. There were no significant differences in carotenoid concentration for AA- and Zn-treated plants. The SA-treated plants had 30% higher carotenoid concentration than the water-sprayed control plants (Fig. 3B).
Fig. 3.
Mean comparisons of main effects of irrigation regime and antioxidant treatments on carotenoids (A, B) and proline contents (C, D). Means with different letters for each variable differ significantly based on Duncan’s multiple range test P < 0.05
Osmotic adjustment
The interaction between irrigation regime and antioxidant treatment was significant (P < 0.05) for TSS (Table 1). Zn application under severe water stress had the highest leaf TSS content (4.57 mg g FW–1), while the water-sprayed plants under normal irrigation produced the lowest leaf TSS content (1.87 mg g FW–1), (Table 2). The antioxidant treatments enhanced TSS content under moderate and severe water stress. Under severe water stress, leaf TSS content increased by 18%, 21%, and 23% with SA, AA, and Zn application, respectively, relative to the water-sprayed control plants (Table 2).
Irrigation regime and antioxidant treatment had a significant effect on proline (P < 0.01) (Table 1). Proline content increased under moderate and severe water stress (Fig. 3C). SA-sprayed plants grown under severe water stress had the highest proline content (3.97 mg g FW–1), while untreated plants under normal irrigation had the lowest proline content (2.01 mg g FW–1). Foliar SA, AA, and Zn applications increased leaf proline contents, with no significant differences between antioxidant treatments (Fig. 3D).
Antioxidant activities
The interaction between irrigation regime and antioxidant treatment was significant for antioxidant enzyme activities (Table 1). Increasing severity of water stress significantly increased POX, CAT, and SOD activities, being the highest and lowest under severe water stress and normal irrigation, respectively (Table 2).
In well-watered plants, the antioxidant treatments had no significant effect on antioxidant enzyme activity (Table 1). Under moderate and severe water stress, the foliar treatments increased CAT activity, more so in Zn-treated plants than SA- and AA-treated plants (Table 2). Zn application under severe water stress had the highest CAT activity (2.89 Unit mg g–1 min–1), while untreated plants under normal irrigation produced the lowest CAT activity (1.51 Unit mg g–1 min–1).
Under moderate water stress, the antioxidant treatments increased SOD activity, relative to the water-sprayed control plants, but the antioxidant treatments did not significantly differ (Table 2). Under severe water stress, the SA and Zn treatments had higher SOD activities than the AA treatment. The SA-treated plants under severe water stress had the highest SOD activities (3.08 Unit mg g–1 min–1), while the water-sprayed plants under normal irrigation had the lowest SOD activities (1.05 Unit mg g–1 min–1).
Under moderate and severe water stress, the foliar treatments increased POX activity. Under severe water stress, the SA treatment increased POX activity more than the Zn and AA treatments (Table 2). SA-sprayed plants grown under severe water stress had the highest POX activity (2.14 Unit mg g–1 min–1), while the water-sprayed plants under severe water stress had the lowest POX activity (1.37 Unit mg g–1 min–1), (Table 2).
The heatmap clustering classified the irrigation regime and antioxidant treatment combinations into three main groups (Fig. 4). Interestingly, normal irrigation, moderate water stress, and severe water stress were clearly separated, suggesting the high impact of irrigation level on the measured variables. In contrast, the measured variables were classified into two major groups, with TSS content and SOD, CAT, and POX activities in one group, and EO, EO yield, biomass yield, RWC, Chl-a, and Chl-b in the other group. The variables in the first group had the lowest values under normal irrigation, which increased with increasing severity of water stress. In the second group, the variables decreased with increasing water stress.
Fig. 4.
Heatmap clustering of irrigation regime and antioxidant
source treatments based on morpho-physiological characteristics in Moldavian balm. Control: water spraying control, SA: 1 mM salicylic acid, AA: 10 mM ascorbic acid, Zn: 3 g L–1 Zinc. Normal irrigation: 60 mm evaporation from Class A pan, Moderate stress: 110 mm evaporation from Class A pan, severe stress: 160 mm evaporation from Class A pan. The key color bar indicates standardized mean values (dark red indicates relatively lower mean values; dark blue indicates relatively higher mean values)
The correlation analysis revealed significant positive correlations between dry matter yield and variables in the second group, including EO yield (r = 0.92***), RWC (r = 0.86***), EO (r = 0.78***), Chl-b (r = 0.77***), and Chl-a (r = 0.76***). Conversely, significant negative correlations occurred between dry matter yield and TSS (r = –0.79***), SOD (r = –0.74***), carotenoids (r = –0.63**), proline (r = –0.61**), POX (r = –0.58**), and CAT (r = –0.56**) (Fig. 5).
Fig. 5.
Correlation coefficients of morpho-physiological characteristics in Moldavian balm
Discussion
Water stress is a major abiotic stress that hinders and shifts plant metabolism, affecting plant growth, development, yield, and quality (Osakabe et al. 2014; Rezaei-Chiyaneh et al. 2018). In response to water stress, plants have evolved physiological and biochemical strategies to survive and alleviate damage from various stresses. Water stress tolerance has been associated with the accumulation of osmolytes (e.g.proline and total soluble sugars), which improve antioxidative guard systems (Masoudi Sadaghiani et al. 2011; Rahimzadeh and Pirzad 2017). We found that severe water stress increased proline and TSS contents but decreased RWC and chlorophyll content, consistent with other studies (Chen et al. 2016; Khazaei et al. 2020). A reduction in chlorophyll content could be attributed to stress-mediated damage to plant pigment biosynthesis (Khazaei et al. 2020; Rahmani et al. 2019) or increased proline synthesis, as both are produced from similar precursors (Le Dily et al. 1993).
Plants treated with SA and AA increased RWC and chlorophyll, indicating enhanced resistance to drought. Antioxidant treatments can overcome the adverse effects of water stress. In our study, drought-stressed and well-watered Moldavian balm plants treated with SA had the highest values for photosynthetic pigments and RWC, which is consistent with other studies on peppermint (Elhakem 2019), pepper (Khazaei et al. 2020), and basil (Said-Al Ahl and Mahmoud 2010) sprayed with SA, AA, and Zn, respectively.
Proline and TSS are considered major osmolytes for regulating cell turgor pressure (Sharma et al. 2019). Water stress significantly increased proline and TSS contents in Moldavian balm, which increased plant performance, as reported in other studies on basil (Damalas 2019) and Moldavian balm (Kabiri et al. 2018). Foliar spraying of antioxidants generally increases leaf TSS and proline contents by regulating plant cell water content (Rady and Hemida 2016). Increasing the activity of proline biosynthetic enzymes, reducing the activity of proline oxidase, and inhibiting the activity of polysaccharide hydrolyzing enzymes enhanced proline biosynthesis and soluble sugar content in SA-treated plants under water stress (Sharma et al. 2019; Abd Allah et al. 2015). Increased proline content due to foliar Zn application maintained RWC, prevented membrane deformation, and acted as a radical scavenger (Rahmani et al. 2019). Moreover, increased proline biosynthesis after spraying AA can lead to ROS scavenging (Agami 2014).
In the present study, water stress increased SOD, CAT, and POX activities in Moldavian balm, more so under severe water stress and Zn and SA application (Table 2). Studies have revealed that increased antioxidant activities increase tolerance to oxidative stress (Askari and Ehsanzadeh 2015; Khazaei et al. 2020; Gholinezhad et al. 2020). Increased antioxidant activities with Zn and AA application neutralize the harmful effects of water stress by eliminating active oxygen species (Khazaei et al. 2020; Pourghasemian et al. 2020). Similar studies have revealed that Zn mitigates oxidative stress by enhancing SOD, CAT, and POX activities and protecting against oxidative membrane damage (Sofy 2015; Mohammadi et al. 2019). Zinc is a regulator co-factor of many enzymes (Marschner 1995), playing a catalytic role in Cu/Zn-SOD to reduce the harmful effects of ROS. Furthermore, Zn limits ROS accumulation by binding to cysteine and histidine (Wu et al. 2015).
In the present study, the SA foliar spray increased SOD and POX activities under severe water stress. Similarly, Askari and Ehsanzadeh (2015) reported that SA increased antioxidant enzyme activities in fennel plants, reducing lipid peroxidation and decreasing oxidative stress. Presumably, SA is one of the mediators providing pre-adaptive responses to protect photosynthetic machinery and membrane integrity and improve plant growth and development (Farhangi-Abriz and Ghassemi-Golezani 2018).
In this study, severe water stress reduced the biomass yield of Moldavian balm by 37%, relative to normal irrigation, possibly due to reductions in water uptake and transpiration, and stomatal closure due to lower osmotic potential in the soil (Shahhoseini et al. 2020). In contrast, zinc and, to a lesser extent, SA and AA significantly increased biomass in Moldavian balm under all irrigation regimes. In general, the antioxidant treatments dramatically improved plant physiological responses, thus increasing biomass by alleviating the adverse effects of water stress. Water stress significantly increased leaf TSS and proline contents in Moldavian balm, both of which had positive correlations with dry matter yield. Under adverse conditions such as water stress, the application of Zn—well-known for being a catalytic component of proteins and enzymes and a co-factor for pigment biosynthesis—encourages plant growth by improving photosynthetic characteristics (Said-Al Ahl and Mahmoud 2010; Ma et al. 2017).
Our results showed that moderate water stress increased the EO content in Moldavian balm; however, this did not occur under severe water stress because plants allocated more photosynthates to synthesize osmotic compounds (e.g., proline and glycine betaine) and sugars (e.g., sucrose, fructose, and fructan) to reduce cell water potential (Mahmoud et al. 2017). Plants treated with Zn had the highest EO contents, which could be associated with its critical role in developing and dividing new EO-containing cells, EO channels, secretion tubes, and tuber hairs (Butnariu and Ioan 2018). Significant increases in EO content in response to water stress have been reported for many medicinal plants (Askari and Ehsanzadeh 2015; Tátrai et al. 2016; García-Caparrós et al. 2019). In thyme, water stress reduced leaf area (Tátrai et al. 2016). As reported for numerous plant species, changes in essential oil content could be due to alterations in gland density, gland number per leaf, leaf characters such as leaf area, thickness, and mass per area, and trichome density and size under stress conditions (King et al. 2006; Karray-Bouraoui et al. 2009; Yadav et al. 2014; Olfa et al. 2016; Zhou et al. 2018).
The antioxidant treatments did not significantly alter EO content under normal irrigation; however, they increased EO accumulation under moderate and severe water stress to similar levels. Mohammadi et al. (2019) reported that foliar SA application to water-stressed thyme increased EO quantity and quality, with similar findings reported for fennel (Askari and Ehsanzadeh 2015) and lemon balm (Ghasemi Pirbalouti et al. 2019). Foliar SA application could change leaf oil gland number and monoterpene biosynthesis (Mohammadi et al. 2019). As a co-factor to enzymatic reactions, AA application could enhance the capacity of meristematic cells and synthesis of vital substrates for EO biosynthesis (Idrees et al. 2010). Considering that nutrients are a source of energy for terpenoid biosynthesis, Zn has an important role in EO production and accumulation (Said-Al Ahl and Mahmoud 2010). Neral, geraniol, geranial, and geranyl acetate, as the main EO compounds, accounted for 85.01–94.93% of the total EO components in the various treatments. Under moderate water stress, the SA-treated and water-sprayed control plants had the highest and lowest percentages of these components, respectively, confirming the findings of another study on Moldavian balm (Said-Al Ahl et al. 2015). As previously reported, relative to control plants, higher levels of geranyl acetate content were observed with foliar applications of Zn (Hegazy et al. 2016) and SA and AA (Said-Al Ahl et al. 2014) in Moldavian balm under severe water stress, suggesting that the concentration of individual volatile compounds can change with the selection of appropriate modes of application, timing, and concentration (Hegazy et al. 2016; Mohammadi et al. 2019; Ghasemi Pirbalouti et al. 2019).
Conclusion
Water deficiency negatively affected the performance of Moldavian balm plants. Increasing oxidative and osmotic injuries under water stress reduced chlorophyll content, RWC, and plant biomass. Foliar application of SA, AA, or Zn significantly affected EO yield and EO accumulation of Moldavian balm by alleviating the adverse effects of water stress through improved enzymatic activity and increased photosynthetic pigments and proline and TSS contents. In particular, plants treated with SA and Zn significantly increased EO yield under moderate water stress, which could be economically crucial for Moldavian balm products in regions with limited water supplies. The foliar treatments with SA, AA, and Zn significantly affected the main EO components, including neral, geranial, geraniol, and geranyl acetate. This study showed that plant biomass and EO content—the most important components of medicinal plants—significantly increased with exogenous application of Zn to water-stressed Moldavian balm.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human and animal participants
This article does not contain any studies with human participants (or animals) performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Esmaeil Rezaei-Chiyaneh, Email: e.rezaeichiyaneh@urmia.ac.ir.
Hassan Mahdavikia, Email: h.mahdavikia@urmia.ac.ir.
Hashem Hadi, Email: h.hadi@urmia.ac.ir.
Hadi Alipour, Email: ha.alipour@urmia.ac.ir.
Muhittin Kulak, Email: muhyttynx@gmail.com.
Gianluca Caruso, Email: gcaruso@unina.it.
Kadambot H. M. Siddique, Email: kadambot.siddique@uwa.edu.au
References
- Abd Allah MMS, El-Bassiouny HMS, Elewa TAE, El-Sebai TN. Effect of salicylic acid and benzoic acid on growth, yield and some biochemical aspects of quinoa plant grown in sandy soil. Int J ChemTech Res. 2015;8:216–225. [Google Scholar]
- Adams RP. Identifications of essential oil components by gas chromatography/Mass Spectroscopy. Carol Stream: Allured Publishing Crop; 2007. [Google Scholar]
- Aebi H (1984) Catalase in vitro. Methods Enzymol Elsevier, Amsterdam 105:121–12610.1016/S0076-6879(84)05016-3 [DOI] [PubMed]
- Agami RA. Applications of ascorbic acid or proline increase resistance to salt stress in barley seedlings. Biol Plant. 2014;58:341–347. doi: 10.1007/s10535-014-0392-y. [DOI] [Google Scholar]
- AkramNA SF, Ashraf M. Ascorbic acid—a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front Plant Sci. 2017;8:613. doi: 10.3389/fpls.2017.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amani Machiani M, Rezaei Chiyaneh E, Javanmard A, Maggi F, Morshedloo MR. Evaluation of common bean (Phaseolus vulgaris L.) seed yield and quali-quantitative production of the essential oils from fennel (Foeniculum vulgare) and dragonhead (Dracocephalum moldavica) in intercropping system under humic acid application. J Clean Prod. 2019;235:112–122. doi: 10.1016/j.jclepro.2019.06.241. [DOI] [Google Scholar]
- Amira MS, Qados A (2014) Effect of ascorbic acid antioxidant on soybean (Glycine max L.) plants grown under water stress conditions. Int J Adv Res Biol Sci 1(6):189–205
- Askari E, Ehsanzadeh P (2015) Drought stress mitigation by foliar application of salicylic acid and their interactive effects on physiological characteristics of fennel (Foeniculum vulgare Mill.) genotypes. Acta Physiol Plant 37:4. 10.1007/s11738-014-1762-y
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–207. doi: 10.1007/bf00018060. [DOI] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assay and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Butnariu M, Sarac I. Essential oils from plants. J Biotech Biomed Sci. 2018;1(4):35–43. doi: 10.1302/issn.2576-6694.jbbs-18-2489. [DOI] [Google Scholar]
- Chen D, Wang S, Cao B, Cao D, Leng G, Li H, Yin L, Shan L, Deng X. Genotypic variation in growth and physiological response to drought stress and re-watering reveals the critical role of recovery in drought adaptation in maize seedlings. Front Plant Sci. 2016;6:1241. doi: 10.3389/fpls.2015.01241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damalas CA. Improving drought tolerance in sweet basil (Ocimum basilicum) with salicylic acid. Sci Hortic. 2019;246:360–365. doi: 10.1016/j.scienta.2018.11.005. [DOI] [Google Scholar]
- Dastmalchi K, Damien Dorman HJ, Koşar M, Hiltunen R (2007) Chemical composition and in vitro antioxidant evaluation of a water-soluble Moldavian balm (Dracocephalum moldavica L.) extract. LWT—Food Sci Technol 40(2):239–248. 10.1016/j.lwt.2005.09.019
- Dmitruk M, Weryszko-Chmielewska E. Morphological differentiation and distribution of non-glandular and glandular trichomes on dracocephalum moldavicum L. Shoots Acta Agrobot. 2010;63(1):11–22. doi: 10.5586/aa.2010.002. [DOI] [Google Scholar]
- Elhakem AH. Impact of salicylic acid application on growth, photosynthetic pigments and organic osmolytes response in Mentha arvensis under drought stress. J Biol Sci. 2019;19:372–380. doi: 10.3923/jbs.2019.372.380. [DOI] [Google Scholar]
- Fallah S, Rostaei M, Lorigooini Z, Surki AA. Chemical compositions of essential oil and antioxidant activity of dragonhead (Dracocephalum moldavica) in sole crop and dragonhead-soybean (Glycine max) intercropping system under organic manure and chemical fertilizers. Ind Crops Prod. 2018;115:158–165. doi: 10.1016/j.indcrop.2018.02.003. [DOI] [Google Scholar]
- Farhangi-Abriz S, Ghassemi-Golezani K. How can salicylic acid and jasmonic acid mitigate salt toxicity in soybean plants? Ecotoxicol Environ Saf. 2018;147:1010–1016. doi: 10.1016/j.ecoenv.2017.09.070. [DOI] [PubMed] [Google Scholar]
- Faridvand Sh, Rezaei-Chiyaneh E, Battaglia ML, Gitari HI, Raza MA, Siddique KHM (2021) Application of bio and chemical fertilizers improves yield, and essential oil quantity and quality of Moldavian balm (Dracocephalum moldavica L.) intercropped with mung bean (Vigna radiata L.). Food Energy Secur 00:e319. https:// doi.org/10.1002/fes3.319
- García-Caparrós P, Romero M, Llanderal A, Cermeño P, Lao M, Segura M. Effects of drought stress on biomass, essential oil content, nutritional parameters, and costs of production in six lamiaceae species. Water. 2019;11(3):573. doi: 10.3390/w11030573. [DOI] [Google Scholar]
- Ghasemi Pirbalouti A, Nekoei M, Rahimmalek M, Malekpoor F (2019) Chemical composition and yield of essential oil from lemon balm (Melissa officinalis L.) under foliar applications of jasmonic and salicylic acids. Biocatal Agric Biotechnol 101144. 10.1016/j.bcab.2019.101144
- Gholinezhad E, Darvishzadeh R, Siavash Moghaddam S, Popović-Djordjević J (2020) Effect of mycorrhizal inoculation in reducing water stress in sesame (Sesamum indicum L.): The assessment of agrobiochemical traits and enzymatic antioxidant activity. Agric Water Manag 238:106234. 10.1016/j.agwat.106234
- Gilling DH, Kitajima M, Torrey JR, Bright KR. Mechanisms of antiviral action of plant antimicrobials against murine norovirus. Appl Environ Microbiol. 2014;80(16):4898–4910. doi: 10.1128/AEM.00402-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueta-Dahan Y, Yaniv Z, Zilinskas B, Ben-Hayyim G. Salt and oxidative stress: similar and specific responses and their relation to salt tolerance in Citrus. Planta. 1997;203:460–469. doi: 10.1007/s004250050215. [DOI] [PubMed] [Google Scholar]
- Hegazy MH, Alzuaibr FMA, Mahmoud AA, Mohamed HFY, Said-Al Ahl HAH. The effects of zinc application and cutting on growth, herb, essential oil and flavonoids in three medicinal lamiaceae plants. Eur J Med Plants. 2016;12(3):1–12. doi: 10.9734/EJMP/2016/23589. [DOI] [Google Scholar]
- Idrees M, Khan MM, Aftab T, Naeem M, Hashmi N. Salicylic acid-induced physiological and biochemical changes in lemongrass varieties under water stress. J Plant Interact. 2010;5(4):293–303. doi: 10.1080/17429145.2010.508566. [DOI] [Google Scholar]
- Irigoyen JJ, Emerich DW, Sanchez-Diaz M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago Sativa) plants. Physiol Plant. 1992;84:67–72. doi: 10.1111/j.1399-3054.1992.tb08764.x. [DOI] [Google Scholar]
- Kabiri R, Hatami A, Oloumi H, Naghizadeh M, Nasibi F, Tahmasebi Z. Foliar application of melatonin induces tolerance to drought stress in Moldavian balm plants (Dracocephalum moldavica) through regulating the antioxidant system. Folia Hortic. 2018;30:155–167. doi: 10.2478/fhort-2018-0016. [DOI] [Google Scholar]
- Kakasy Z, Lemberkovics É, Simándi B, Lelik L, Héthelyi É, Antal I, Szöke É. Comparative study of traditional essential oil and supercritical fluid extracts of Moldavian dragonhead (Dracocephalum moldavica L.) Flavour Fragr J. 2006;21:598–603. doi: 10.1002/ffj.1569. [DOI] [Google Scholar]
- Karray-Bouraoui N, Rabhi M, Neffati M, Baldan B, Ranieri A, Marzouk B, Smaoui A. Salt effect on yield and composition of shoot essential oil and trichome morphology and density on leaves of Mentha pulegium. Ind Crops Prod. 2009;30(3):338–343. doi: 10.1016/j.indcrop.2009.06.003. [DOI] [Google Scholar]
- Khan MIR, Fatma M, Per TS, Anjum NA, Khan NA. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci. 2015;6:1–17. doi: 10.3389/fpls.2015.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazaei Z, Esmaielpour B, Estaji A. Ameliorative effects of ascorbic acid on tolerance to drought stress on pepper (Capsicum annuum L) plants. Physiol Mol Biol Plants. 2020 doi: 10.1007/s12298-020-00846-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DJ, Gleadow RM, Woodrow IE (2006). Regulation of oil accumulation in single glands of Eucalyptus polybractea. New phytol 172(3):440–451. 10.1111/j.1469-8137.2006.01842.x [DOI] [PubMed]
- Kulak M. Recurrent drought stress effects on essential oil profile of Lamiaceae plants: an approach regarding stress memory. Ind Crops Prod. 2020;154:112695. doi: 10.1016/j.indcrop.2020.112695. [DOI] [Google Scholar]
- Le Dily F, Billard JP, Saos JLE, Huault C. Effect of NaCl and gabaculine on chlorophyll and proline levels during growth of radish cotyledons. Plant Physiol Biochem. 1993;31:303–310. [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. doi: 10.1016/0076-6879(87)48036-1. [DOI] [Google Scholar]
- Ma D, Sun D, Wang C, Ding H, Qin H, Hou J, Huang X, Xie Y, Guo T. Physiological responses and yield of wheat plants in zinc-mediated alleviation of drought stress. Front Plant Sci. 2017;8:860. doi: 10.3389/fpls.2017.00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maham M, Akbari H, Delazar A. Chemical composition and antinociceptive effect of the essential oil of Dracocephalum moldavica L. Pharm Sci. 2013;18(4):187–192. [Google Scholar]
- Mahmoud MA, Shala AY, Rashed MN (2017) The mutual effect of irrigation scheduling and foliar spray of silica nanoparticles on basil plant. J Plant Production 8 (12):1303–1313. 10.21608/jpp.2017.41984
- Marschner H. Mineral nutrition of higher plants. 2. London: Academic Press; 1995. [Google Scholar]
- Martínez-Vázquez M, Estrada-Reyes R, Martínez-Laurrabaquio A, López-Rubalcava C, Heinze G. Neuropharmacological study of Dracocephalum moldavica L. (Lamiaceae) in mice: sedative effect and chemical analysis of an aqueous extract. J Ethnopharmacol. 2012;141:908–917. doi: 10.1016/j.jep.2012.03.028. [DOI] [PubMed] [Google Scholar]
- Masoudi Sadaghiani F, Abdollahi B, Zardoshti MR, Rasouli Sadaghiani H, Tavakoli A (2011) Response of proline, soluble sugars, photosynthetic pigments and antioxidant enzymes in potato (Solanum tuberosum L.) to different irrigation regimes in greenhouse condition. Aust J Crop Sci 5(1):55–60
- Miura K, Tada Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front Plant Sci. 2014;5:4. doi: 10.3389/fpls.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi H, Amirikia F, Ghorbanpour M, Fatehi F, Hashempour H. Salicylic acid induced changes in physiological traits and essential oil constituents in different ecotypes of Thymus kotschyanus and Thymus vulgaris under well-watered and water stress conditions. Ind Crops Prod. 2019;129:561–574. doi: 10.1016/j.indcrop.2018.12.046. [DOI] [Google Scholar]
- Noman A, Ali S, Naheed F, Ali Q, Farid M, Rizwan M, Irshad MK (2015) Foliar application of ascorbate enhances the physiological and biochemical attributes of maize (Zea mays L.) cultivars under drought stress. Arch Agron Soil Sci 61(12):1659–1672. 10.1080/03650340.2015.1028379
- Olfa B, Imen T, Mohamed C (2016). Essential oil and trichome density from Origanum majorana L. shoots affected by leaf age and salinity. Biosci J 32(1):238–245. 10.14393/BJ-v32n1a2016-30323
- Osakabe Y, Osakabe K, Shinozaki K, Tran LP. Response of plants to water stress. Front Plant Sci. 2014;5:86. doi: 10.3389/fpls.2014.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S, Chakraborty D (2015) Salicylic acid and drought stress response: biochemical to molecular crosstalk. In: Tripathi B, Müller M (eds) Stress responses in plants. Springer, Cham, pp 247–265. 10.1007/978-3-319-13368-3-10
- Pourghasemian N, Moradi R, Naghizadeh M, Landberg T. Mitigating drought stress in sesame by foliar application of salicylic acid, beeswax waste and licorice extract. Agric Water Manag. 2020;231:105997. doi: 10.1016/j.agwat.2019.105997. [DOI] [Google Scholar]
- Rabêlo VM, Magalhães PC, Bressanin LA, Carvalho DT, Reis CO, dos Karam D, Doriguetto AC, Santos MH, Filho PRS, Souza TCD. The foliar application of a mixture of semisynthetic chitosan derivatives induces tolerance to water deficit in maize, improving the antioxidant system and increasing photosynthesis and grain yield. Sci Rep. 2019;9:8164. doi: 10.1038/s41598-019-44649-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rady MM, Hemida KA. Sequenced application of ascorbate-proline-glutathione improves salt tolerance in maize seedlings. Ecotoxicol Environ Safe. 2016;133:252–259. doi: 10.1016/j.ecoenv.2016.07.028. [DOI] [PubMed] [Google Scholar]
- Rahimzadeh S, Pirzad A (2017) Arbuscular mycorrhizal fungi and Pseudomonas in reduce drought stress damage in flax (Linum usitatissimum L.): a field study. Mycorrhiza 27(6):537–552. 10.1007/s00572-017-0775-y [DOI] [PubMed]
- Rahmani F, Sayfzadeh S, Jabbari H, Valadabadi SA, Hadidi Masouleh E. Alleviation of drought stress effects on safflower yield by foliar application of zinc. Int J Plant Prod. 2019;13:297–308. doi: 10.1007/s42106-019-00055-7. [DOI] [Google Scholar]
- Rezaei-Chiyaneh E, Seyyedi SM, Ebrahimian E, Siavash Moghaddama S, Damalasd CA. Exogenous application of gamma-aminobutyric acid (GABA) alleviates the effect of water deficit stress in black cumin (Nigella sativa L.) Ind Crops Prod. 2018;112:741–748. doi: 10.1016/j.indcrop.2017.12.067. [DOI] [Google Scholar]
- Rezaei-Chiyaneh E, Amani Machiani M, Javanmard A, Maggi F, Morshedloo M. Vermicompost application in different intercropping patterns improves the mineral nutrient uptake and essential oil compositions of sweet basil (Ocimum basilicum L.) J Soil Sci Plant Nutr. 2020;21(1):450–466. doi: 10.1007/s42729-020-00373-0. [DOI] [Google Scholar]
- Rezaei-Chiyaneh E, Amirnia R, Amani Machiani M, Javanmard A, Maggi F, Morshedloo MR (2020b) Intercropping fennel (Foeniculum vulgare L.) with common bean (Phaseolus vulgaris L.) as affected by PGPR inoculation: A strategy for improving yield, essential oil and fatty acid composition. Sci Hortic 261:10895. 10.1016/j.scienta.2019.108951
- Sabzi-Mehrabad Z, Lotfi R, Pessarakli M, Yarnia M (2017) Changes in essential oil accumulation of Moldavian balm (Dracocephalum moldavica L.) in response to phosphate biological and chemical fertilizer. J Plant Nutr 41(3):348–357. 10.1080/01904167.2017.1385802
- Sadeghzadeh B. A review of zinc nutrition and plant breeding. J Soil Sci Plant Nutr. 2013;13(4):905–927. doi: 10.4067/s0718-95162013005000072. [DOI] [Google Scholar]
- Said-Al Ahl HAH, Mahmoud AA (2010) Effect of zinc and/or iron foliar application on growth and essential oil of sweet basil (Ocimum basilicum L.) under salt stress. Ocean J Appl Sci 3(1):97–111
- Said-Al Ahl HAH, El Gendy AG, Omer EA. Effect of ascorbic acid, salicylic acid on coriander productivity and essential oil cultivated in two different locations. Adv Environ Biol. 2014;8(7):2236–2250. [Google Scholar]
- Said-Al Ahl HAH, Sabra AS, El Gendy ANG, Aziz EE, Tkachenko KG (2015) Changes in content and chemical composition of Dracocephalum moldavica L. essential oil at different harvest dates. J Med Plants Stud 3(2):61–64
- Shahhoseini R, Azizi M, Asili J, Moshtaghi N, Samiei L (2020) Effects of zinc oxide nanoelicitors on yield, secondary metabolites, zinc and iron absorption of Feverfew [Tanacetum parthenium (L.) Schultz Bip.]. Acta Physiol Plant 42:52. 10.1007/s11738-020-03043-x
- Sharma A, Shahzad B, Kumar V, Kohli SK, Sidhu GPS, Bali AS, Handa N, Kapoor D, Bhardwaj R, Zheng B. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules. 2019;9(7):285. doi: 10.3390/biom9070285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofy MR (2015) Application of salicylic acid and zinc improves wheat yield through physiological processes under different levels of irrigation intervals. Int J Plant Res 5(5):136–156. 10.5923/j.plant.20150505.06
- Tátrai ZA, Sanoubar R, Pluhár Z, Mancarella S, Orsini F, Gianquinto G. Morphological and physiological plant responses to drought stress in Thymus citriodorus. Int J Agron. 2016 doi: 10.1155/2016/4165750. [DOI] [Google Scholar]
- Wu H, Jiang H, Liu C, Deng Y. Growth, pigment composition, chlorophyll fluorescence and antioxidant defenses in the red alga Gracilaria lemaneiformis (Gracilariales, Rhodophyta) under light stress. S Afr J Bot. 2015;100:27–32. doi: 10.1016/j.sajb.2015.05.017. [DOI] [Google Scholar]
- Xu B, Li F, Shan L, Ma Y, Ichizen N, Huang J. Gas exchange, biomass partition, and water relationships of three grass seedlings under water stress. Weed Biol Manag. 2006;6(2):79–88. doi: 10.1111/j.1445-6664.2006.00197.x. [DOI] [Google Scholar]
- Xu Y, Xu Q, Huang B. Ascorbic acid mitigation of water stress inhibition of root growth in association with oxidative defense in tall fescue (Festuca arundinacea Schreb.) Front Plant Sci. 2015;6:1–14. doi: 10.3389/fpls.2015.00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav RK, Sangwan RS, Sabir F, Srivastava AK, Sangwan NS (2014) Effect of prolonged water stress on specialized secondary metabolites, peltate glandular trichomes, and pathway gene expression in Artemisia annua L. Plant Physiol Biochem 74:70–83. 10.1016/J.PLAPHY.2013.10.023 [DOI] [PubMed]
- Yavas I, Unay A. Effects of zinc and salicylic acid on wheat under drought stress. J Anim Plant Sci. 2016;26(4):1012–1018. [Google Scholar]
- Zhou Y, Tang N, Huang L, Zhao Y, Tang X, Wang K. Effects of salt stress on plant growth, antioxidant capacity, glandular trichome density, and volatile exudates of Schizonepeta tenuifolia Briq. Int J Mol Sci. 2018;19(1):252. doi: 10.3390/ijms19010252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.