Abstract
Lenadogene nolparvovec (Lumevoq) gene therapy was developed to treat Leber hereditary optic neuropathy (LHON) caused by the m.11778G > A in MT-ND4 that affects complex I of the mitochondrial respiratory chain. Lenadogene nolparvovec is a replication-defective, single-stranded DNA recombinant adeno-associated virus vector 2 serotype 2, containing a codon-optimized complementary DNA encoding the human wild-type MT-ND4 subunit protein. Lenadogene nolparvovec was administered by unilateral intravitreal injection in MT-ND4 LHON patients in two randomized, double-masked, and sham-controlled phase III clinical trials (REVERSE and RESCUE), resulting in bilateral improvement of visual acuity. These and other earlier results suggest that lenadogene nolparvovec may travel from the treated to the untreated eye. To investigate this possibility further, lenadogene nolparvovec was unilaterally injected into the vitreous body of the right eye of healthy, nonhuman primates. Viral vector DNA was quantifiable in all eye and optic nerve tissues of the injected eye and was detected at lower levels in some tissues of the contralateral, noninjected eye, and optic projections, at 3 and 6 months after injection. The results suggest that lenadogene nolparvovec transfers from the injected to the noninjected eye, thus providing a potential explanation for the bilateral improvement of visual function observed in the LHON patients.
Keywords: viral vector, transduction, recombinant adeno-associated virus vector 2 serotype 2, ND4, qPCR assay, biodistribution, lenadogene nolparvovec, Lumevoq, Leber hereditary optic neuropathy
Graphical abstract
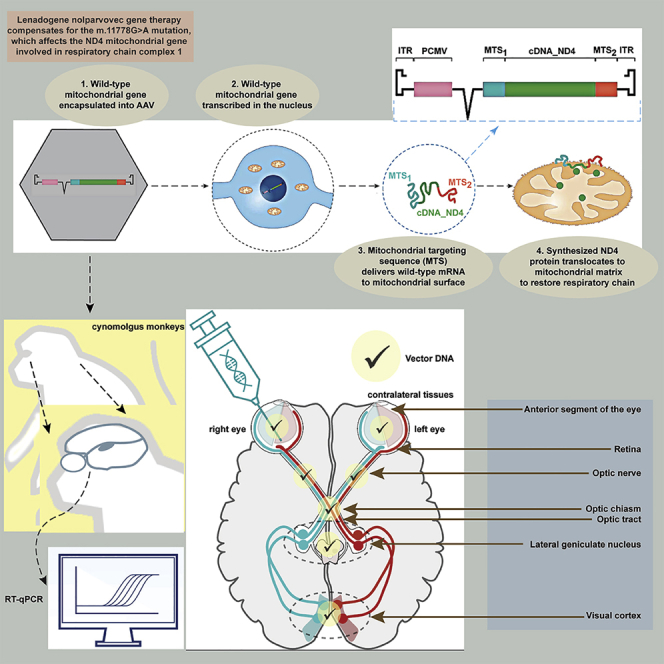
Lenadogene nolparvovec is a gene therapy for LHON, and bilateral improvement of visual acuity was observed after unilateral intravitreal injection in clinical trials. NHPs were therefore treated by unilateral injection, and viral vector DNA was detected in the contralateral eye, thus providing a potential explanation for the observed bilateral improvement.
Introduction
Lenadogene nolparvovec gene therapy was developed as a treatment for patients with vision loss due to Leber hereditary optic neuropathy (LHON) caused by the most common m.11778G > A mutation affecting the nicotinamide adenine dinucleotide hydride (NADH) dehydrogenase (complex I) subunit 4 (MT-ND4) gene. The ND4 gene is encoded by the mitochondrial genome (deoxyribonucleic acid [mtDNA]), and it is expressed and translated locally within the mitochondria to be assembled into complex I, which is the first entry site of the respiratory chain. Lenadogene nolparvovec is a recombinant replication-defective, single-stranded DNA adeno-associated viral vector 2 serotype 2 (AAV2/2). The treatment strategy is based on allotopic expression of the recoded wild-type ND4 gene in the nucleus of the retinal ganglion cell (RGC), in which axons in the optic nerve degenerate in LHON, thereby causing vision loss. Lenadogene nolparvovec contains a complementary DNA (cDNA) sequence that encodes the human wild-type ND4 protein under the control of a cytomegalovirus (CMV) immediate early promoter in an intron-containing expression cassette (beta globin intron, HBB2) flanked by the viral inverted terminal repeats from the vector. The construct includes the cis-acting elements of human cytochrome c oxidase 10 (COX10) mitochondrial ribonucleic acid (mtRNA), thus ensuring efficient delivery of the corresponding hybrid mRNA to the mitochondrial surface and the translocation of the translated human ND4 protein into the mitochondria. This allotopic expression strategy efficiently complements the mtDNA mutation in cell and animal models, allowing rescue of the mitochondrial respiratory chain defect and improved RGC survival.1, 2, 3, 4, 5, 6, 7
Two randomized, double-masked, sham-controlled phase 3 clinical trials (REVERSE, ClinicalTrials.gov: NCT02652780, and RESCUE, ClinicalTrials.gov: NCT02652767) and their long-term follow-up study (RESTORE, ClinicalTrials.gov: NCT03406104) in LHON patients harboring the m.11778G > A mutation (hereafter named MT-ND4 patients) who received a unilateral intravitreal (IVT) injection of lenadogene nolparvovec showed improvement of best-corrected visual acuity (BCVA) in both treated and untreated eyes.8, 9, 10, 11 Two other gene-therapy trials using a viral vector containing a cDNA coding the human wild-type mitochondrial ND4 protein have shown similar bilateral improvement after unilateral IVT of a similar allotopic expression-based gene therapy (AAV2-ND4) in LHON MT-ND4 patients.12, 13, 14, 15, 16 This visual improvement is beyond what would be expected from the natural history of visual function in MT-ND4 LHON patients.10,17,18
In a preliminary study in which lenadogene nolparvovec was administered sequentially by IVT injection in both eyes of nonhuman primates (NHPs), viral vector DNA was detected and quantified from tissue samples of the optic nerve, optic chiasm, and optic tract 3 months after the second IVT administration without local or systemic toxicity.
Taken together, these findings suggest that lenadogene nolparvovec travels from the injected eye, prompting us to assess this further in NHPs, in which the visual system is comparable to that of humans. We evaluated the biodistribution of lenadogene nolparvovec DNA following single unilateral IVT treatment in cynomolgus monkeys. After treatment, the animals were kept for an observation period of either 3 or 6 months to evaluate the presence of the vector in an ocular, visual pathway and associated brain tissues (Table 1). Biodistribution of lenadogene nolparvovec viral genome (vg) DNA and corresponding RNA transcripts were determined using validated qPCR and qRT (reverse transcriptase)-PCR assays. The qPCR results at 3 months were already released, combined with REVERSE clinical study results.8 Here, we compare the full results of this NHP study, including qPCR and qRT-PCR results at both time points.
Table 1.
Single unilateral IVT injection in the right eye with 3- and 6-month observation periods
| Groups | Number of animals kept for 3 months | Number of animals kept for 6 months | Concentration (copies/eye) | Volume (μL/eye) | |
|---|---|---|---|---|---|
| Control (BSSS)a | 1 | 1 | 0 | 90 | |
| Lenadogene nolparvovec | 3 | 3 | 4.30 × 1010 | 90 |
Supplemented with 0.001% Pluronic F68.
Results
Experimental in vivo phase
There were no deaths, adverse clinical signs, or effects on body weight or food consumption related to lenadogene nolparvovec treatment. The conjunctiva of two of the treated animals and one control animal was red during the first week and occasionally during the study. This was transient, observed with and without lenadogene nolparvovec treatment, and considered to be related to the injection. No ophthalmologic abnormalities were observed in any animal following IVT administration and during the observation periods. Tonometry values for intraocular pressure (IOP) of the injected eye were above the normal range in some treated (4 of 6) and control (1 of 2) animals immediately after IVT administration and rapidly came back to normal range. This was expected and related to the introduction of fluid volume into the vitreous. All animals were healthy prior to termination of the investigation at 3 and 6 months.
Biodistribution in NHPs
Biodistribution of lenadogene nolparvovec was evaluated in all animals. Most samples had DNA and RNA concentration and purity ratios above or close to the recommended thresholds (2.0 and 1.8, respectively). All analyzed RNA samples had RNA integrity number (no.; RIN) values between 4.4 and 9.4, indicating that the RNA quality was sufficient for qRT-PCR analysis. All DNA samples were analyzed first by the CMV qPCR assay and then reanalyzed by the ND4 qPCR assay, specific for the plasmid AAV2/2-ND4 (pAAV2/2-ND4) portion of the construct. The results from the two qPCR assays were similar. Differences in results between the 2 assays occurred in <8% of analyzed samples and were considered due mainly to the presence of vg quantities close to the lower limit of quantification (LLOQ) and to the lower limit of detection (LOD) of the ND4 assay compared to the CMV assay (6.25 versus 25 vg/well). ND4 qPCR assay results are presented in Table 2, and the CMV assay results are available in Table S1. qRT-PCR assay results are presented in Table 3.
Table 2.
qPCR detection of lenadogene nolparvovec in DNA from NHP fluid and tissue samples using the ND4 assay
| Control animals n = 1 | Treated animals n = 3 (at 3 and 6 months) | |||||||
|---|---|---|---|---|---|---|---|---|
| At 3 months after IVT injection | ||||||||
| 1 sample per eye | 3 samples of left and right eyes | |||||||
| BLD | BLQ | Contralateral left eye | Injected right eye | |||||
| BLD | BLQ | Quantified copiesa | BLD | BLQ | Quantified copiesa | |||
| Tears1 | ||||||||
| Lachrymal gland | 2 | − | 2 | 1 | − | − | 1 | 2 (4.35 × 102) |
| Aqueous humor | 2 | − | 3 | − | − | − | 1 | 2 (1.65 × 103) |
| Vitreous humor | 2 | − | 3 | − | − | − | − | 3 (1.99 × 105) |
| Anterior segment of the eye | 2 | − | − | − | 3 (3.39 × 103) | − | − | 3 (3.21 × 106) |
| Retina | 2 | − | − | 1 | 2 (5.99 × 103) | − | 1 | 2 (2.70 × 106) |
| Optic nerve | 2 | − | − | − | 3 (1.00 × 104) | − | 1 | 2 (1.45 × 103) |
| Visual pathway and associated brain tissues (2 samples collected per animal) | ||||||||
| BLD | BLQ | BLD | BLQ | Quantified copiesa | ||||
| Optic chiasm | 2 | − | − | 4 | 2 (3.28 × 104) | |||
| Optic tract | 2 | − | 1 | 5 | − | |||
| Visual cortex | 2 | − | 3 | 2 | 1 (2.05 × 103) | |||
| Lateral geniculate nucleus | 2 | − | 1 | 4 | 1 (2.84 × 102) | |||
| Cerebellum | 2 | − | 6 | − | − | |||
| Thalamus | 2 | − | 6 | − | − | |||
| Auricular lymph node | 2 | − | 5 | 1 | − | |||
| At 6 months after IVT injection | ||||||||
| 1 sample per eye | 3 samples per eye | |||||||
| BLD | BLQ | Contralateral left eye | Injected right eye | |||||
| BLD | BLQ | Quantified copiesa | BLD | BLQ | Quantified copiesa | |||
| Tears | 2 | − | 3 | − | − | 3 | − | − |
| Lachrymal gland | 2 | − | 3 | − | − | 2 | − | 1 (6.18 × 103) |
| Aqueous humor | 2 | − | 2 | 1 | − | − | − | 2 (4.48 × 102)b |
| Vitreous humor | 2 | − | 3 | − | − | − | − | 3 (1.13 × 106) |
| Anterior segment of the eye | 2 | − | 2 | 1 | − | − | − | 3 (2.65 × 106) |
| Retina | 2 | − | 3 | − | − | − | − | 3 (8.48 × 106) |
| Optic nerve | 2 | − | 2 | − | 1 (3.19 × 102) | − | 2 | 1 (5.18 × 102) |
| Visual pathway and associated brain tissues (2 samples collected per animal) | ||||||||
| BLD | BLQ | BLD | BLQ | Quantified copiesa | ||||
| Optic chiasm | 1 | 1 | 3 | 2 | 1 (7.68 × 102) | |||
| Optic tract | 2 | − | 3 | 3 | − | |||
| Visual cortex | 2 | − | 4 | 2 | − | |||
| Lateral geniculate nucleus | 2 | − | 1 | 3 | 2 (5.73 × 103) | |||
| Cerebellum | 2 | − | 3 | 2 | 1 (2.69 × 102) | |||
| Thalamus | 2 | − | 4 | 2 | − | |||
| Auricular lymph node | 2 | − | 3 | 3 | − | |||
BLD, below the limit of detection (15.625 copies/μg DNA); BLQ, below the limit of quantification (<250 copies/μg DNA); −, no sample gave this result; Tears,1 insufficient sample available for analysis.
Quantity of copies per microgram of DNA in tissues or copies per microliter of DNA in humor; values are shown as mean when >1 sample had quantifiable results.
One sample was excluded, as the result was equivalent to that of the blank control sample.
Table 3.
qRT-PCR detection of lenadogene nolparvovec in RNA from NHP fluid and tissue samples
| Control animals n = 1 | Treated animals n = 3 (at 3 and 6 months) | |||||||
| At 3 months after IVT injection | ||||||||
| 1 sample per eye | 3 samples of left and right eyes | |||||||
| BLD | BLQ | Contralateral left eye | Injected right eye | |||||
| BLD | BLQ | Quantified copiesa | BLD | BLQ | Quantified copiesa | |||
| Tears | 2 | − | 3 | − | − | 3 | − | − |
| Lachrymal gland | 2 | − | 3 | − | − | 3 | − | − |
| Aqueous humor | 2 | − | 3 | − | − | 2 | 1 | − |
| Vitreous humor | 2 | − | 1 | b | − | 2 | b | − |
| Anterior segment of the eye | 2 | − | 1 | 2 | − | − | − | 3 (2.28 × 105) |
| Retina | 2 | − | 3 | − | − | 1 | − | 2 (5.96 × 105) |
| Optic nerve | 2 | − | 3 | − | − | 2 | 1 | − |
| Visual pathway and associated brain tissues (2 samples collected per animal) | ||||||||
| BLD | BLQ | BLD | BLQ | Quantified copiesa | ||||
| Optic chiasm | 2 | − | 5 | 1 | − | |||
| Optic tract | 2 | − | 6 | − | − | |||
| Visual cortex | 2 | − | 6 | − | − | |||
| Lateral geniculate nucleus | 2 | − | 5 | 1 | − | |||
| Cerebellum | 2 | − | 6 | − | − | |||
| Thalamus | 2 | − | 6 | − | − | |||
| Auricular lymph node | 2 | − | 6 | − | − | |||
| At 6 months after IVT injection | ||||||||
| 1 sample per eye | 3 samples per eye | |||||||
| BLD | BLQ | Contralateral left eye | Injected right eye | |||||
| BLD | BLQ | Quantified copiesa | BLD | BLQ | Quantified copiesa | |||
| Tears | 2 | − | 3 | − | − | 3 | − | − |
| Lachrymal gland | 2 | − | 3 | − | − | 3 | − | − |
| Aqueous humor | 2 | − | 3 | − | − | 2 | 1 | − |
| Vitreous humor | 2 | − | 3 | − | − | − | 1 | 1 (5.51 × 102)c |
| Anterior segment of the eye | 2 | − | 3 | − | − | − | − | 3 (1.26 × 106) |
| Retina | 2 | − | 3 | − | − | − | − | 3 (7.47 × 105) |
| Optic nerve | 2 | − | 3 | − | − | 2 | 1 | − |
| Visual pathway and associated brain tissues (2 samples collected per animal) | ||||||||
| BLD | BLQ | BLD | BLQ | Quantified copiesa | ||||
| Optic chiasm | 2 | − | 5 | 1 | − | |||
| Optic tract | 2 | − | 6 | − | − | |||
| Visual cortex | 2 | − | 6 | − | − | |||
| Lateral geniculate nucleus | 2 | − | 3 | 3 | − | |||
| Cerebellum | 2 | − | 6 | − | − | |||
| Thalamus | 2 | − | 6 | − | − | |||
| Auricular lymph node | 2 | − | 6 | − | − | |||
BLD, below the limit of determination (25 copies/μg RNA); BLQ, below the limit of quantitation (1,000 copies/μg RNA).
Quantity of copies per microgram of RNA in tissues or copies per microliter of RNA in humor; values are shown as mean when >1 sample had quantifiable levels.
3 samples with RNA detection were exposed to DNA contamination (at similar levels), and the results are not presented in the summary table (vitreous humor at 3 months: animals 2002 and 2003 [samples from the contralateral eye] and animal 2001 [sample from the injected eye]).
1 sample with RNA quantification was exposed due to DNA contamination, and the result of this sample was not reported in the summary table (vitreous humor at 6 months: animal 2006 [sample from the injected eye: 1.54 × 101]).
Control animals
In the ND4 qPCR assay, lenadogene nolparvovec DNA was undetectable in all control animal samples 3 and 6 months after unilateral IVT injection of vehicle, with the exception of one optic chiasm sample from one control animal at 6 months, which was below the limit of quantification (BLQ), defined as 250 vg/μg DNA. Five sentinel control samples included in the DNA extraction batches and PCR plates were below the limit of detection (BLD) in the assay, indicating a lack of cross contamination in the DNA extraction and qPCR processing. In addition, this single BLQ result was not replicated in the duplicate optic chiasm sample from the same animal. The RNA transcript of lenadogene nolparvovec was BLD, defined as 25 copies per microgram of RNA in all control animal samples at 3 or 6 months.
Lenadogene nolparvovec-treated animals
ND4 qPCR results at 3 and 6 months in samples from injected right eyes
At 3 months after injection, lenadogene nolparvovec DNA was quantifiable in all ipsilateral anterior segment and vitreous humor samples from the 3 treated NHPs and in the lacrimal gland, aqueous humor, retina, and optic nerve samples in 2 of 3 animals. At 6 months after injection, all ipsilateral anterior segment, vitreous humor, and retina samples and 2 of 3 aqueous humor samples were quantifiable from all 3 treated NHPs. Vector DNA was also quantifiable in lacrimal gland and optic nerve samples in 1 of 3 individuals and detectable in the optic nerve in 2 of 3 individuals. Lenadogene nolparvovec was BLD in all tear samples and in 2 of 3 lacrimal gland samples.
ND4 qPCR results at 3 and 6 months in samples from untreated left eyes
At 3 months after injection, lenadogene nolparvovec DNA was quantifiable in the anterior segment and optic nerve samples of all 3 animals, detected or quantified in all retina samples (2 quantified and 1 detected), and detected in one sample of the lacrimal gland. At 6 months after injection, lenadogene nolparvovec DNA was quantifiable in the optic nerve (animal 2006) and detected in aqueous humor (animal 2007) and anterior segment (animal 2006) samples. Vector DNA was BLD in all remaining samples from the noninjected eye.
ND4 qPCR results at 3 and 6 months in samples from visual pathway and brain tissues
At 3 months after injection, lenadogene nolparvovec DNA was quantifiable in the optic chiasm of 2 animals and was detected in the remaining four samples from the 3 animals. Lenadogene nolparvovec was quantifiable in the visual cortex and lateral geniculate nucleus of 1 animal, and it was detected in 2 of 5 and 4 of 5 remaining samples from these tissues, respectively. Lenadogene nolparvovec was detected in 5 of 6 optic tract samples and in 1 of 6 auricular lymph node samples, but it was not detected or quantified in the cerebellum or thalamus at 3 months.
At 6 months after injection, lenadogene nolparvovec DNA was quantified in 1 and detected in 2 samples from the optic chiasm. In the optic tract, lateral geniculate nucleus, and visual cortex, there was quantification or detection in 3, 5, and 2 samples out of 6, respectively. Lenadogene nolparvovec DNA was quantified (1 sample) or detected (2 samples) in the cerebellum and detected in 2 thalamus and 3 auricular lymph node samples.
qRT-PCR results at 3 and 6 months in samples from the injected right eye
At 3 months after injection, lenadogene nolparvovec RNA transcript was quantified in all anterior segment samples and in 2 of 3 retina samples from the injected eye. The RNA transcript was also detected in the aqueous humor (animal 2002) and the optic nerve (animal 2001) at 3 months.
At 6 months after injection, lenadogene nolparvovec RNA was quantified in all samples from the anterior segment and retina and in 1 of 3 vitreous humor samples from the injected eye. Lenadogene nolparvovec RNA transcript was detected in the aqueous humor, vitreous humor, and optic nerve in 1 of 3 animals at 6 months. No PCR inhibition was detected in any sample.
qRT-PCR assay results at 3 and 6 months in samples from the untreated left eyes
At 3 months after injection, lenadogene nolparvovec transcript RNA was detected in anterior segment samples from the untreated eye in 2 of 3 animals (animals 2001 and 2003). Of note, there was DNA contamination in 2 vitreous humor samples, which were disqualified. At 6 months after injection, lenadogene nolparvovec RNA was neither quantified nor detected in samples from untreated left eyes. No PCR inhibition was detected in any sample.
qRT-PCR results at 3 and 6 months in samples from visual pathway and brain tissues
At 3 months after injection, lenadogene nolparvovec transcript RNA was detected in the optic chiasm and lateral geniculate nucleus of 1 sample (animal 2001), but it was neither detected nor quantified in the optic tract, visual cortex, cerebellum, thalamus, and auricular lymph nodes.
At 6 months after injection, lenadogene nolparvovec RNA was detected in 1 optic chiasm sample and in 3 lateral geniculate nucleus samples, but it was neither detected nor quantified in the optic tract, visual cortex, cerebellum, thalamus, and auricular lymph nodes.
Discussion
In four separate clinical trials, including one phase 1/2 study (REVEAL), two pivotal trials (REVERSE and RESCUE), and one long-term follow-up study (RESTORE), MT-ND4 LHON patients unilaterally injected with lenadogene nolparvovec demonstrated bilateral visual improvement beyond the expectations of the natural history of the disease.8, 9, 10, 11,17, 18, 19 A similar contralateral effect was reported in two other comparable gene-therapy trials developed for MT-ND4 LHON subjects in the United States20,21 and in China;12,13 each used a viral vector containing a cDNA coding the human wild-type mitochondrial ND4 protein (AAV2-ND4), the route of administration was IVT, and MT-ND4 subjects were injected in only one eye in all studies. This contralateral effect has not been described in gene therapies with subretinal administration targeting other ophthalmic genetic diseases, specifically in patients with RPE65-mediated inherited retinal dystrophy who participated in a randomized, controlled, open-label, phase 3 trial.22 Both the nature of the treated disease (neuro-ophthalmic mitochondrial disease) and the IVT route of administration may play a role in the development and mechanism of the contralateral effect.
These convergent findings prompted us to assess the ability of lenadogene nolparvovec to transfer from the injected eye to tissues of the contralateral eye in healthy NHPs. A mechanistic and biodistribution study using qPCR in NHPs (whose visual system, optic nerve fiber decussation, and brain projections are comparable to humans) was conducted to better understand the biodistribution of lenadogene nolparvovec DNA. We tested tissues of the injected and noninjected eyes, the visual pathway, and associated brain tissues to explore the in vivo transfer of the lenadogene nolparvovec active substance following single unilateral IVT administration in cynomolgus monkeys. Six healthy male NHPs received a unilateral IVT in the right eye followed by an observation period of 3 or 6 months prior to evaluation of biodistribution. We demonstrated the presence of viral vector DNA in not only the injected eyes but also in tissues of the noninjected eyes. Lenadogene nolparvovec DNA was quantified or detected in the injected eyes and ipsilateral optic nerves 3 and 6 months after injection. Vector DNA was also quantified or detected at lower levels in most of the eye tissues and optic nerves of the noninjected eyes at 3 months. Lenadogene nolparvovec DNA was still quantifiable in the optic nerve and detected in the aqueous humor and anterior segment of the noninjected eyes at 6 months.
Overall, the data from individual animals were consistent, as lenadogene nolparvovec DNA was recovered in contralateral tissues of the noninjected eye in 3 out of 3 animals at 3 months and in 2 out of 3 animals at 6 months following unilateral IVT. Lenadogene nolparvovec was quantified or detected in the visual pathway and associated brain tissues, including optic chiasm, optic tract, visual cortex, lateral geniculate nucleus, and sporadically in the cerebellum, thalamus, and auricular lymph node at 3 and 6 months. Because lenadogene nolparvovec DNA was detected and quantified in the optic chiasm, a possible anatomic route for the transfer of viral vector DNA from the treated eye to the nontreated eye was via the optic nerve and chiasm, through anterograde and subsequent retrograde movement along the anterior visual pathways. A systemic transfer of lenadogene nolparvovec cannot be excluded, even though it is unlikely given that biodistribution studies have shown only limited and transient presence of lenadogene nolparvovec in blood.8,9,19
The RNA transcript of lenadogene nolparvovec was quantified in all anterior segments and most retina samples and sporadically in the aqueous humor, vitreous humor, and optic nerve from the injected eye at 3 and 6 months. The RNA transcript was weakly distributed away from the injected eye compared to the vector DNA, being found only sporadically at detectable levels in the optic chiasm and lateral geniculate nucleus at 3 and 6 months and in the anterior segment only at 3 months in the noninjected eye.
Importantly, the reliability of the results from this study is high, due to the replication in 2 qPCR assays for the analysis of DNA samples, consistent with earlier NHP biodistribution studies and robust validation parameters for the qPCR and qRT-PCR assays. The CMV and ND4 qPCR assays gave similar results in >92% of analyzed samples. Of the 214 analyzed samples, only 17 (<8%) had a different result. Among these, 9 samples were BLD in the CMV assay and BLQ in the ND4 assay, whereas 2 were BLQ in the CMV assay and BLD in the ND4 assay. This could be explained by the LOD value in the ND4 assay (6.25 instead of 25 vg/well). The remaining 6 samples were BLQ in one assay and quantified in the other, which was likely to have been related to the samples having quantities of vgs close to the LLOQ, from 1.01 × 102 to 1.28 × 102 vg/well.
In this the study, there were no effects on mortality or clinical condition, clinical signs, body weight, food consumption, or ophthalmological examinations, including pupillary light reflex, slit lamp biomicroscopy, IOP, and indirect ophthalmoscopy, confirming the expected safety profile of lenadogene nolparvovec at 4.3 × 1010 vg/eye, the dose corresponding to a previously established no observable adverse effect level (NOAEL) in monkeys and a clinically relevant dose level in humans. Furthermore, at this same dose level per eye, NHPs had no evidence of histopathological abnormalities in the brain nor any evidence of systemic or local adverse effects on extensive ophthalmological examinations of qualitative (standard eye exams, OCT (Optical Coherence Tomography), tonometry), functional (ERG: Electroretinogram), and behavioral (reflexes) endpoints in a previous study in bilaterally injected NHPs.
Several mechanisms have been considered to explain the apparent clinical contralateral effect of unilateral IVT gene therapy for LHON. Despite the plausibility of some of these mechanisms, the detailed roles of these respective processes involved in this phenomenon remain unclear. The following mechanistic hypotheses behind the contralateral effect observed in lenadogene nolparvovec clinical studies have been proposed: (1) transfer of active substance (vector particles, DNA) between eyes via inter-orbital communication or via systemic transfer and (2) transfer of mitochondrial material (RNA, protein) between eyes. An examination of these two hypotheses follows. Other less-likely explanations include an underappreciation of the natural history of visual improvement in the disease, which is not supported by recent meta-analyses,10,17,18 involvement of inflammatory mediators or other signaling pathways triggered by the injections that activate cell survival in general,23,24 or brain plasticity with plastic changes in higher visual centers to restore the original visual modality, leading to improved visual performance in both eyes.25, 26, 27
Transfer of viral vector DNA between eyes
Systemic transfer of anti-vascular endothelial growth factor (VEGF) therapies injected IVT in conditions such as neovascular age-related macular degeneration (AMD) and cystoid macular edema (CME) has been demonstrated. Several publications report a clinical improvement in the contralateral eye after unilateral injection of bevacizumab or ranibizumab for the treatment of AMD or CME.28,29 Hanhart and colleagues30 conducted a retrospective study on 35 patients with bilateral diabetic macular edema to evaluate the effect of unilateral bevacizumab IVT on contralateral noninjected eyes. Improved BCVA was detected in 14 (40%) injected eyes and 15 (43%) noninjected eyes, associated with an average reduction in thickness of the macular edema similar between injected and noninjected eyes. Indeed, the mechanism of lenadogene nolparvovec transfer via the systemic circulation cannot be excluded, but the limited and transient biodistribution of lenadogene nolparvovec in blood argues against this route of transfer. At 3 and 6 months post-unilateral IVT, lenadogene nolparvovec DNA was found in the optic chiasm and optic nerves of both eyes of NHPs. Individual axons from RGCs use anterograde (from retina to brain) and retrograde (from brain to retina) transport for shuttling of signaling factors.31 In particular, growth factors are uploaded to ganglion cell axon terminals near LGN (Lateral Geniculate Nucleus) relay neurons for transport to the retina and to LGN neuronal axons from the primary visual cortex to the LGN and then to the retina.32 Recently, Calkins and colleagues33 presented a study of interactions between the two optic projections in an inducible model of glaucoma. Elevation of IOP in one eye was sufficient to cause the transfer of radiolabeled glucose to that eye via the optic chiasm following injection of the label in the contralateral eye. Elimination of transfer was also achieved by cutting the optic nerve of the eye receiving the injection of radiolabeled glucose in a way that did not disrupt the eye’s vascular supply, indicating that transfer in that model must have occurred via the optic chiasm.
Transfer of mitochondrial material (RNA, protein) between eyes
Importantly, the mitochondrial basis of LHON may play a role in the mechanism of the contralateral effect. Damaged or depleted mitochondria from the RGC axon terminal in the LGN are shuttled back to the RGC soma.34 As mitochondria move along the axon in both directions, they are in constant contact with one another, often fusing and thereby sharing complementary genetic material in the form of mtRNA or even proteins. Astrocyte networks play several possible roles in white matter tracts, similar to the optic projections,35 where axons propagate energy demanding action potentials at great distances from their nuclei. In particular, astrocytes interact intimately with axons along the entirety of their length. One purpose of this interaction is to engulf mitochondria, in a process called mitophagy. This is of particular importance in the optic nerve, which is highly, metabolically active.36 In this way, mitochondria from RGCs and their axons can make their way into the astrocyte plexus of the optic nerve. The content of these mitochondria includes mRNA and proteins, which are absorbed into endosomes within the astrocytes.37 Importantly, astrocytes not only internalize material from RGC axons but also transfer material back to them, including mitochondria, mRNA, and other cytoplasmic proteins.38 The highly energy-dependent RGCs with axons that form the optic nerves, optic chiasm, and optic tracts are enriched with mitochondria, which are transported along the axons of the RGCs to deliver energy where it is needed, in a highly regulated process coordinated by the nuclear and mitochondrial genomes.39 Although this mitochondrial mechanism was not studied in our NHP experiment, it is possible that “healthy” mitochondrial RNA or other pro-survival factors from RGC axons of the treated eye traverse the two nerves via astrocyte coupling following axonal mitophagy, thereby inducing the contralateral improvement seen in MT-ND4 LHON gene-therapy trials.
Our NHP study presents some limitations. The study aimed to provide a better understanding of the biodistribution of lenadogene nolparvovec DNA in the injected and noninjected eyes, the visual pathway, and associated brain tissues to explore the possibility of in vivo migration of the lenadogene nolparvovec active substance following single unilateral IVT administration to cynomolgus monkeys. Our results do not provide the necessary data to formally describe or confirm the route of lenadogene nolparvovec transfer and its mechanism. Although we provide evidence that transfer of lenadogene nolparvovec DNA to the contralateral eye may have occurred, further work using more sensitive assays is needed to determine if transgene mRNA in the contralateral eye could have been produced in situ or transported post-transcription from the injected eye.
The demonstration of a transfer of the lenadogene nolparvovec active substance between eyes has major consequences for designing gene-therapy clinical trials and should lead to prioritizing interpatient comparisons whenever possible.
The presence of viral vector DNA in the contralateral noninjected eye has been demonstrated in NHPs following a unilateral IVT of lenadogene nolparvovec. As viral vector DNA was consistently detected or quantified in the optic chiasm for up to 6 months after unilateral IVT, we propose a potential anatomic route from the treated eye to the nontreated eye via the optic chiasm through anterograde and subsequent retrograde transport along the optic projections to the untreated eye. The current NHP study provides a possible explanation for the bilateral improvement of visual function in LHON patients treated with a single unilateral IVT of lenadogene nolparvovec gene therapy. Further studies are needed to validate these observations and, importantly, to evaluate the mechanisms that facilitate the interocular transfer of vector DNA and/or mRNA transcripts and how specific expression levels of the transgene translate to corresponding improvements in visual function.
Materials and methods
Biodistribution following unilateral IVT administration in NHPs
The objective of the study was to investigate the potential propagation of the lenadogene nolparvovec vector from the treated eye to the contralateral untreated eye by evaluating biodistribution in ocular, visual pathway, and associated brain tissues at 3 and 6 months after a single IVT injection in cynomolgus monkeys.
Experimental in vivo phase
Animals
This NHP was used because its visual system, optic nerve fiber decussations, and brain projections are similar to that of humans. Purpose-bred male cynomolgus monkeys were obtained from Noveprim Europe (Ebene, Mauritius). On the day of treatment, the animals were at least 28 months old and had a mean body weight of 3.73 kg (range: 3.46 to 4.18 kg). The animals were quarantined for 4 weeks on arrival in Europe, during which time, tuberculin testing, treatment against parasites, and a coprology test were performed. Tuberculin testing and antiparasitic treatment were also performed regularly at the test facility. The NHPs were acclimatized to study conditions for at least 42 days before treatment. They were group housed in ETS 123 (European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes) and Directive 2012/63/EU-compliant cages located in a dedicated primate unit. The animals received enrichment rewards on each handling occasion, free access to water, and 180 g Primate Leaf-Eater vegetarian diet (SSNIFF Spezialdiaten, Germany) and fruit by daily distribution.
Test item, control item, and analytes
The test item, lenadogene nolparvovec (AAV2/2-ND4), was obtained as a clear, colorless viral suspension formulated in balanced sterile saline solution (BSSS) buffer supplemented with 0.001% Pluronic F68 from GenSight Biologics (Paris, France). The control item was BSSS supplemented with 0.001% Pluronic F68, prepared at Charles River Laboratories. The reference analyte used for qPCR calibration standards and quality control (QC) samples, pAAV2/2-ND4, was obtained from Aldevron with a certificate of analysis from Novasep (Lyon, France). The reference analyte used for qRT-PCR, lenadogene nolparvovec RNA (AAV2/2-RNA), a single-stranded RNA encompassing pAAV2/2-ND4, was prepared at Charles River Laboratories (Evreux, France) by in vitro transcription from an ND4 PCR amplicon containing a T7 RNA polymerase promoter.
Administration and animal care
On the day of treatment, lenadogene nolparvovec was thawed, re-homogenized, and diluted with BSSS vehicle formulation supplemented with 0.001% Pluronic F68 before being administered as a single unilateral IVT injection in the right eye of 6 male NHPs at 4.3 × 1010 vg in a dose volume of 90 μL. The 4.3 × 1010 vg/eye dose corresponded to the NOAEL dose in monkeys, determined in a preliminary study, and it is allometrically equivalent (based on vitreous volumes) to the human clinical dose of 9 × 1010 vg/eye used in the REVERSE (Yu-Wai-Man et al.8), RESCUE (Newman et al.9), and REFLECT pivotal clinical studies. A control group of 2 NHPs received 90 μL BSSS, supplemented with 0.001% Pluronic F68 in the right eye by single IVT injection. The animals were then observed for a period of 3 or 6 months (Table 1). Observation-period monitoring included assessment of body weight and food consumption, ophthalmologic exams, local tolerance, and general clinical condition.
Prior to IVT injection, animals were placed under general anesthesia with ketamine hydrochloride, and pupils were dilated with tropicamide. Animals were sedated with xylazine and given a local anesthetic (tetracaine). Analgesics (buprenorphine and meloxicam) and preventive antibiotics (tobramycin) were administered before and after the injection. Immediately following, the eye was examined by indirect ophthalmoscopy and slit lamp biomicroscopy to confirm the location of the injected drug in the vitreous body and any abnormalities caused by the procedure.
Ophthalmological examinations were performed regularly on both eyes including pupillary light reflex, indirect ophthalmoscopy, and slit lamp biomicroscopy under sedation with ketamine and xylazine. IOP was measured by tonometry using a TonoPen under tranquilization and local anesthesia before and after the injection of lenadogene nolparvovec or BSSS. At the end of the observation periods of 3 and 6 months, the animals were tranquilized by an intramuscular injection of ketamine hydrochloride, then deeply anesthetized by intravenous injection of pentobarbital sodium, and humanely euthanized by exsanguination. Tissues and fluids were analyzed for biodistribution using validated qPCR and qRT-PCR assays to determine vector DNA and transgene transcript levels.
NHP biodistribution study sample analysis
Tissue collection
The following tissues and fluids were collected from both eyes using sterile sets of disposable instruments and preserved separately: tears, lachrymal gland, aqueous humor, anterior segment of the eye (including cornea, iris, and lens), vitreous humor, retina (dissection centered on the fovea whenever possible), optic nerve, optic chiasm, optic tract, lateral geniculate nucleus, visual cortex, cerebellum, thalamus, and mandibular and auricular lymph nodes. The time of euthanasia of the animals and of snap freezing of the tissues was recorded in the raw data. Care was taken during sample collection and preservation to avoid cross contamination. Tissue and fluid samples were collected from control NHPs (group 1) before collections from lenadogene nolparvovec-treated NHPs (group 2) and from the untreated left eye before collections from the treated right eye. A new set of disposable sterile instruments was used for each animal and for each organ, tissue, or fluid collected. Tissues were rinsed with cold PBS at 4°C, and organs were split into 2 pieces, put into separate cryotubes, and snap frozen in liquid nitrogen. Samples were stored at −80°C pending nucleic acid extractions.
Extraction of nucleic acids
DNA for the quantification of lenadogene nolparvovec vgs
Tissue and fluid samples were homogenized, and DNA was extracted using the NucleoSpin Tissue Kit (Macherey Nagel). DNA was extracted from tears using the QIAamp Viral Mini Kit (QIAGEN). DNA concentration and purity were determined by UV spectrophotometry using the Nanodrop 8000 apparatus (Thermo Fisher). Sentinel control samples (extraction buffer) were included every 10 samples within each DNA extraction batch to monitor any cross contamination between samples. DNA samples were stored at −20°C pending qPCR analysis.
RNA for the quantification of lenadogene nolparvovec transgene transcripts
RNA was extracted from tissues using a combined Trizol/RNeasy Kit (QIAGEN) and from biological fluids using the QIAamp Viral RNA Mini Kit (QIAGEN), according to validated in-house procedures.
Sentinel control samples (extraction buffer) were included every 10 samples in each batch to monitor potential cross contamination. RNA concentration and purity were determined by UV spectrophotometry. RNA QC was performed by microcapillary electrophoresis (Agilent 2100 Bioanalyzer). RNA samples were stored at −80°C pending qRT-PCR analysis. RNA QC (except for sentinel control samples and RNA extracts from fluid samples) was performed by microcapillary electrophoresis using an Agilent 2100 Bioanalyzer.
qPCR analysis
Careful consideration was given to find the most robust method to measure viral vector genome quantities while limiting the risk of intersample cross contamination and ensuring qPCR assay specificity for the detection and quantification of lenadogene nolparvovec vgs in NHP tissues and biological fluids. vgs were first analyzed using a validated qPCR assay targeting the promoter region of the transgene, and results were confirmed with a second qPCR assay targeting the ND4 transcript region. The first assay targeted the CMV promoter of the vector and was validated for the quantitation of lenadogene nolparvovec vgs in NHP tissues and human samples. The second assay targeted the codon-optimized ND4 sequence and was validated for the quantitation of lenadogene nolparvovec vgs in human blood.
For qPCR assays, the following assay validation parameters were determined: matrix equivalence between NHP or human DNA and a surrogate matrix (herring sperm DNA), dynamic range, detection limit, quantification limits, within-run and between-run precision and accuracy, dilution integrity, matrix effect, and selectivity and specificity in monkey and human genomic DNA. The DNA extraction recovery yield from NHP tissues or human blood after spiking with known amounts of lenadogene nolparvovec was determined, and the stability of lenadogene nolparvovec DNA in these samples after storage at −20°C was evaluated.
Assay 1 (CMV)
The following primers and probe were used:
-
⋅
forward primer: 151CMV-F 5′-CATCAATGGGCGTGGATAGC-3′,
-
⋅
reverse primer: 274CMV-R 5′-GGAGTTGTTACGACATTTTGGAAA-3′, and
-
⋅
internal TaqMan probe: 187CMV-P 6FAM (6-Carboxyfluorescein) 5′-ATTTCCAAGTCTCCACCC-3′ MGB (Minor Groove Binder).
Assay 2 (ND4)
The following primers and probe were used:
-
⋅
forward primer: ND4-F 5′-TCCTGAAGCTGGGTGGTTATG-3′,
-
⋅
reverse primer: ND4-R 5′-GGCTCTTGAGGTCAGTCTGCC-3′, and
-
⋅
internal TaqMan probe: ND4b1_MGB.P FAM 5′-CATGGCTTACCCTTTC-3′ MGB.
For assay 2, primers were blasted against Macaca fascicularis sequences to ensure qPCR assay specificity.
For both assays, PCR reactions were performed using 400 ng of DNA as a template (primers and probe at 300 nM/150 nM for assay 1 and 200 nM/250 nM for assay 2, respectively) and TaqMan Gene Expression Master Mix 1×. All samples were analyzed in duplicate using an Applied Biosystems 7900HT (assay 1) or an Applied Biosystems Quantstudio 5 (assay 2) real-time PCR instrument. Study samples (including sentinel controls) were run in 96-well plates in parallel with the following:
-
⋅
a calibration curve containing 8 calibration standards ranging from 100 to 107 copies of a reference analyte containing the amplicon sequence, prepared in 400 ng of a surrogate DNA matrix (herring sperm DNA),
-
⋅
2 sets of QCs at the low, mid, and high levels,
-
⋅
no DNA controls (ultrapure nuclease-free water), and
-
⋅
no template controls (corresponding to PCR reactions without DNA).
For all samples, Ct (Cycle threshold) values were plotted according to the base 10 logarithms of the calibration point copy numbers, and the resulting curve was linear fitted using Microsoft Excel software. Sample test item DNA copy numbers in QC, study, and control samples were then interpolated from the standard curve.
Potential PCR inhibition was monitored in all samples by spiking a known quantity (2.5 × 103 copies) of an exogenous DNA (Drosophila melanogaster antp gene). The spiked samples were then assayed by qPCR, using a qPCR assay developed and validated in house. PCR was considered as inhibited when the recovery percentage of the measured antp copy number was lower than 50% of the nominal value. Acceptance criteria were set for all qPCR runs.
Assay 1: Validation of the CMV qPCR method for quantification of AAV2/2-ND4 vg in NHP samples
The qPCR method was validated for the determination of AAV2/2-ND4 copies with a dynamic range of 250 to 2.5 × 107 copies/μg of DNA and a detection limit of 31.25 copies/μg of DNA. The qPCR detection assay was found to be specific in DNA extracted from primate tissues, blood, and fluids.
Assay 2: Validation of the ND4 qPCR method for quantification of AAV2/2-ND4 vg in NHP samples
The ND4 qPCR method was validated for the determination of AAV2/2-ND4 copies with a dynamic range of 250 to 2.5 × 107 copies/μg of DNA and a detection limit of 15.625 copies/μg of DNA. No significant matrix effect was identified in any of the tested NHP samples. The qPCR detection assay was found to be specific in genomic DNA extracted from the liver in NHPs.
qRT-PCR analysis
A one-step qRT-PCR strategy was used, combining first-strand cDNA synthesis (RT) and PCR reaction in the same tube. The qRT-PCR assay for the determination of ND4 transcript quantities in total RNA used the same primers and probe as the corresponding qPCR assay. The following qRT-PCR assay validation parameters were determined: matrix equivalence between NHP tissue RNA from all tissues and surrogate matrix (yeast RNA), dynamic range, quantification limits, detection limit, within-run and between-run precision and accuracy, dilution integrity, matrix effect, selectivity, and specificity. The qRT-PCR assay specificity was evaluated by analyzing RNA samples from all tested tissues extracted from 6 NHP individuals. The RNA extraction recovery yield from NHP tissues after spiking with known amounts of an in vitro-transcribed ND4 RNA was determined, and the stability of the ND4 transcript in these samples was evaluated after storage at −80°C.
qRT-PCR analysis of RNA from study samples (100 or 500 ng of total RNA/qRT-PCR reaction) was done using the QuantiTect Probe RT-PCR Kit (QIAGEN). The qRT-PCR plates were run using an Applied Biosystems 7900HT instrument. Samples were run in duplicate in 96-well plates in parallel with the following:
-
⋅
a calibration curve containing 8 calibration standards ranging from 100 to 107 copies of a reference analyte containing the amplicon sequence, prepared in 100 ng of a surrogate RNA matrix (yeast RNA),
-
⋅
2 sets of QCs at the low, mid, and high levels,
-
⋅
no RNA controls,
-
⋅
no RT controls, and
-
⋅
no template controls (corresponding to PCR reactions without RNA).
For all samples, Ct values were plotted according to the base 10 logarithms of the calibration point copy numbers, and the resulting curve was linear fitted using Microsoft Excel software. ND4 transcript quantity in QC, study, and control samples was then interpolated from the standard curve.
Validation of the qRT-PCR method for quantification of pAAV2/2-ND4 copies in NHP samples
Lenadogene nolparvovec RNA was synthesized by in vitro transcription from a PCR product of the pAAV2/2-ND4 using T7 RNA polymerase and characterized. The qRT-PCR method was validated for the quantification of ND4 RNA in NHP tissues and fluids. The qRT-PCR assay was found to be specific in all primate RNA tissues and fluids, and no significant matrix effect was identified. The qRT-PCR assay was linear, with RNA quantities ranging from 29.5 to 60.0 ng for cerebellum or 100 ng for the other tested tissues and fluids. The dynamic range was from 1.00 × 103 to 1.00 × 108, and the limit of detection was 25.0 copies/μg of RNA.
Analyzed tissues and fluids
The following tissues and fluids were analyzed: tears, lacrimal gland, aqueous humor, vitreous humor, anterior segment, retina, optic nerve, optic chiasm, optic tract, visual cortex, lateral geniculate nucleus, cerebellum, thalamus, and auricular lymph node.
Tears, lacrimal gland, aqueous humor, vitreous humor, anterior segment, retina, and optic nerve were analyzed separately for the ipsilateral and contralateral eyes. As RGC axons from both eyes converge in the optic chiasm and beyond at approximately the same ratio, analyses of tissues posterior to the optic nerve, including the optic chiasm, optic tract, lateral geniculate nucleus, brain tissues (visual cortex, cerebellum, thalamus), and auricular lymph node, represent bilateral contributions.
Regulatory compliance
The in vivo phase plan was reviewed and approved by the Charles River Laboratories Evreux Ethics Committee. The study plan, together with available information concerning the test item, was reviewed and approved by the Citoxlab France Biosafety Committee. The test item, AAV2/2-ND4, is categorized by national authorities (Haut Conseil des Biotechnologies) and by the Citoxlab France Biosafety Committee as follows: class 2, biosafety level 2 (A2, L2; approval number 153). Appropriate precautions were followed in the performance of the study. The experimental work was performed according to the relevant current authority regulations.
Acknowledgments
We extend our thanks to Joanna Moore, ELS (Charles River Laboratories, Evreux, France), for help in the preparation of this manuscript. N.J.N. is supported in part by an Ophthalmology Department core grant from the NIH/NEI (P30 EY006360). P.Y.-W.-M. is supported by a Clinician Scientist Fellowship Award (G1002570) from the Medical Research Council (UK) and also receives funding from Fight for Sight (UK), the Isaac Newton Trust (UK), Moorfields Eye Charity, the Addenbrooke's Charitable Trust, the National Eye Research Centre (UK), the International Foundation for Optic Nerve Disease (IFOND), the UK National Institute of Health Research (NIHR) as part of the Rare Diseases Translational Research Collaboration, the NIHR Cambridge Biomedical Research Centre (BRC-1215-20014), and the NIHR Biomedical Research Centre based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology. V.C. is supported by grants from the Italian Ministry of Health (RF-2018-12366703), the Italian Ministry of Research (20172T2MHH), and Telethon-Italy (GUP15016). V.C. is also supported by patients’ organizations MITOCON and IFOND and patients’ donations. J.A.S. is supported by the Agence Nationale de la Recherche (France) within the Programme Investissements d’Avenir, Institut Hospitalo Universitaire FOReSIGHT (ANR-18-IAHU 0001), and LabEx LIFESENSES (ANR-10-LABX-65).
Author contributions
Writing and editing of the manuscript, all authors; the in vivo study was performed under the supervision of C.C. and P.S.; analysis of samples and interpretation of analysis results, A.R. and P.A.
Declaration of interests
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. M.T. is employed by GenSight Biologics, the sponsor of these studies. P.S., C.C., A.R., and P.A. are employees of Charles River Laboratories, Evreux, France, which received payment for the conduct of this research from GenSight Biologics. V.C. is a consultant for GenSight Biologics, Santhera Pharmaceuticals, and Stealth BioTherapeutics and has received research support from Santhera Pharmaceuticals and Stealth BioTherapeutics. D.J.C. is a consultant for GenSight Biologics and Stuart Therapeutics. P.Y.-W.-M. is a consultant for GenSight Biologics and Stealth BioTherapeutics and has received research support from GenSight Biologics and Santhera Pharmaceuticals. N.J.N. is a consultant for GenSight Biologics, Santhera Pharmaceuticals, and Stealth BioTherapeutics; has received research support from GenSight Biologics and Santhera Pharmaceuticals; served on the Data Safety Monitoring Board for Quark NAION study; and is a medical legal consultant. J.A.S. is the cofounder and shareholder of GenSight Biologics and the patent coauthor on allotopic transport.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtm.2021.09.013.
Supplemental information
References
- 1.Gray R.E., Law R.H., Devenish R.J., Nagley P. Allotopic expression of mitochondrial ATP synthase genes in nucleus of Saccharomyces cerevisiae. Methods Enzymol. 1996;264:369–389. doi: 10.1016/s0076-6879(96)64035-x. [DOI] [PubMed] [Google Scholar]
- 2.Guy J., Qi X., Pallotti F., Schon E.A., Manfredi G., Carelli V., Martinuzzi A., Hauswirth W.W., Lewin A.S. Rescue of a mitochondrial deficiency causing Leber Hereditary Optic Neuropathy. Ann. Neurol. 2002;52:534–542. doi: 10.1002/ana.10354. [DOI] [PubMed] [Google Scholar]
- 3.Koilkonda R.D., Chou T.H., Porciatti V., Hauswirth W.W., Guy J. Induction of rapid and highly efficient expression of the human ND4 complex I subunit in the mouse visual system by self-complementary adeno-associated virus. Arch. Ophthalmol. 2010;128:876–883. doi: 10.1001/archophthalmol.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koilkonda R., Yu H., Talla V., Porciatti V., Feuer W.J., Hauswirth W.W., Chiodo V., Erger K.E., Boye S.L., Lewin A.S. LHON gene therapy vector prevents visual loss and optic neuropathy induced by G11778A mutant mitochondrial DNA: biodistribution and toxicology profile. Invest. Ophthalmol. Vis. Sci. 2014;55:7739–7753. doi: 10.1167/iovs.14-15388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cwerman-Thibault H., Augustin S., Lechauve C., Ayache J., Ellouze S., Sahel J.A., Corral-Debrinski M. Nuclear expression of mitochondrial ND4 leads to the protein assembling in complex I and prevents optic atrophy and visual loss. Mol. Ther. Methods Clin. Dev. 2015;2:15003. doi: 10.1038/mtm.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnet C., Kaltimbacher V., Ellouze S., Augustin S., Bénit P., Forster V., Rustin P., Sahel J.A., Corral-Debrinski M. Allotopic mRNA localization to the mitochondrial surface rescues respiratory chain defects in fibroblasts harboring mitochondrial DNA mutations affecting complex I or v subunits. Rejuvenation Res. 2007;10:127–144. doi: 10.1089/rej.2006.0526. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet C., Augustin S., Ellouze S., Bénit P., Bouaita A., Rustin P., Sahel J.A., Corral-Debrinski M. The optimized allotopic expression of ND1 or ND4 genes restores respiratory chain complex I activity in fibroblasts harboring mutations in these genes. Biochim. Biophys. Acta. 2008;1783:1707–1717. doi: 10.1016/j.bbamcr.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Yu-Wai-Man P., Newman N.J., Carelli V., Moster M.L., Biousse V., Sadun A.A., Klopstock T., Vignal-Clermont C., Sergott R.C., Rudolph G. Bilateral visual improvement with unilateral gene therapy injection for Leber hereditary optic neuropathy. Sci. Transl. Med. 2020;12:eaaz7423. doi: 10.1126/scitranslmed.aaz7423. [DOI] [PubMed] [Google Scholar]
- 9.Newman N.J., Yu-Wai-Man P., Carelli V., Moster M.L., Biousse V., Vignal-Clermont C., Sergott R.C., Klopstock T., Sadun A.A., Barboni P., LHON Study Group Efficacy and Safety of Intravitreal Gene Therapy for Leber Hereditary Optic Neuropathy Treated within 6 Months of Disease Onset. Ophthalmology. 2021;128:649–660. doi: 10.1016/j.ophtha.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Newman N.J., Yu-Wai-Man P., Carelli V., Biousse V., Moster M.L., Vignal-Clermont C., Sergott R.C., Klopstock T., Sadun A.A., Girmens J.-F. Intravitreal Gene Therapy vs. Natural History in Patients With Leber Hereditary Optic Neuropathy Carrying the m.11778G>A ND4 Mutation: Systematic Review and Indirect Comparison. Front. Neurol. 2021;12:662838. doi: 10.3389/fneur.2021.662838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biousse V., Newman N.J., Yu-Wai-Man P., Carelli V., Moster M.L., Vignal-Clermont C., Klopstock T., Sadun A.A., Sergott R.C., Hage R. Long-term follow-up after unilateral intravitreal gene therapy for Leber hereditary optic neuropathy: the RESTORE study. J. Neuroophthalmol. 2021;41:309–315. doi: 10.1097/WNO.0000000000001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang S., Ma S.Q., Wan X., He H., Pei H., Zhao M.J., Chen C., Wang D.W., Dong X.Y., Yuan J.J., Li B. Long-term outcomes of gene therapy for the treatment of Leber’s hereditary optic neuropathy. EBioMedicine. 2016;10:258–268. doi: 10.1016/j.ebiom.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan X., Pei H., Zhao M.-j., Yang S., Hu W.-k., He H., Ma S.-q., Zhang G., Dong X.-y., Chen C. Efficacy and Safety of rAAV2-ND4 Treatment for Leber’s Hereditary Optic Neuropathy. Sci. Rep. 2016;6:21587. doi: 10.1038/srep21587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan J.J., Zhang Y., Wang L.L., Cheng M.S., Ma S.Q., Gao Q., Li B. Visual Field Variability after Gene Therapy for Leber’s Hereditary Optic Neuropathy. Ophthalmic Res. 2018;60:176–184. doi: 10.1159/000487485. [DOI] [PubMed] [Google Scholar]
- 15.Yuan J., Zhang Y., Liu H., Wang D., Du Y., Tian Z., Li X., Yang S., Pei H., Wan X. Seven-Year Follow-up of Gene Therapy for Leber’s Hereditary Optic Neuropathy. Ophthalmology. 2020;127:1125–1127. doi: 10.1016/j.ophtha.2020.02.023. [DOI] [PubMed] [Google Scholar]
- 16.Liu H.L., Yuan J.J., Zhang Y., Tian Z., Li X., Wang D., Du Y.Y., Song L., Li B. Factors associated with rapid improvement in visual acuity in patients with Leber’s hereditary optic neuropathy after gene therapy. Acta Ophthalmol. 2020;98:e730–e733. doi: 10.1111/aos.14379. [DOI] [PubMed] [Google Scholar]
- 17.Newman N.J., Carelli V., Taiel M., Yu-Wai-Man P. Visual Outcomes in Leber Hereditary Optic Neuropathy Patients With the m.11778G>A (MTND4) Mitochondrial DNA Mutation. J. Neuroophthalmol. 2020;40:547–557. doi: 10.1097/WNO.0000000000001045. [DOI] [PubMed] [Google Scholar]
- 18.Yu-Wai-Man, P., Newman, N.J., Carelli, V., La Morgia, C., Biousse, V., Bandello, F.M., Clermont, C.V., Campillo, L.C., Leruez, S., Moster, M.L., et al.; LHON REALITY Study Group (2021). Natural history of patients with Leber hereditary optic neuropathy-results from the REALITY study. Eye (Lond.). [DOI] [PMC free article] [PubMed]
- 19.Vignal-Clermont C., Girmens J.F., Audo I., Said S.M., Errera M.H., Plaine L., O’Shaughnessy D., Taiel M., Sahel J.A. Safety of Intravitreal Gene Therapy for Treatment of Subjects with Leber Hereditary Optic Neuropathy due to Mutations in the Mitochondrial ND4 Gene: The REVEAL Study. BioDrugs. 2021;35:201–214. doi: 10.1007/s40259-021-00468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feuer W.J., Schiffman J.C., Davis J.L., Porciatti V., Gonzalez P., Koilkonda R.D., Yuan H., Lalwani A., Lam B.L., Guy J. Gene Therapy for Leber Hereditary Optic Neuropathy: Initial Results. Ophthalmology. 2016;123:558–570. doi: 10.1016/j.ophtha.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guy J., Feuer W.J., Davis J.L., Porciatti V., Gonzalez P.J., Koilkonda R.D., Yuan H., Hauswirth W.W., Lam B.L. Gene Therapy for Leber Hereditary Optic Neuropathy: Low- and Medium-Dose Visual Results. Ophthalmology. 2017;124:1621–1634. doi: 10.1016/j.ophtha.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell S., Bennett J., Wellman J.A., Chung D.C., Yu Z.F., Tillman A., Wittes J., Pappas J., Elci O., McCague S. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–860. doi: 10.1016/S0140-6736(17)31868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giordano C., Iommarini L., Giordano L., Maresca A., Pisano A., Valentino M.L., Caporali L., Liguori R., Deceglie S., Roberti M. Efficient mitochondrial biogenesis drives incomplete penetrance in Leber’s hereditary optic neuropathy. Brain. 2014;137:335–353. doi: 10.1093/brain/awt343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cherry A.D., Piantadosi C.A. Regulation of mitochondrial biogenesis and its intersection with inflammatory responses. Antioxid. Redox Signal. 2015;22:965–976. doi: 10.1089/ars.2014.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hrvatin S., Hochbaum D.R., Nagy M.A., Cicconet M., Robertson K., Cheadle L., Zilionis R., Ratner A., Borges-Monroy R., Klein A.M. Single-cell analysis of experience-dependent transcriptomic states in the mouse visual cortex. Nat. Neurosci. 2018;21:120–129. doi: 10.1038/s41593-017-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabel B.A., Flammer J., Merabet L.B. Residual vision activation and the brain-eye-vascular triad: Dysregulation, plasticity and restoration in low vision and blindness - a review. Restor. Neurol. Neurosci. 2018;36:767–791. doi: 10.3233/RNN-180880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castaldi E., Cicchini G.M., Cinelli L., Biagi L., Rizzo S., Morrone M.C. Visual BOLD Response in Late Blind Subjects with Argus II Retinal Prosthesis. PLoS Biol. 2016;14:e1002569. doi: 10.1371/journal.pbio.1002569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Z., Sadda S.R. Effects on the contralateral eye after intravitreal bevacizumab and ranibizumab injections: a case report. Ann. Acad. Med. Singap. 2008;37:591–593. [PubMed] [Google Scholar]
- 29.Al-Dhibi H., Khan A.O. Bilateral response following unilateral intravitreal bevacizumab injection in a child with uveitic cystoid macular edema. J. AAPOS. 2009;13:400–402. doi: 10.1016/j.jaapos.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Hanhart J., Tiosano L., Averbukh E., Banin E., Hemo I., Chowers I., Medscape Fellow eye effect of unilateral intravitreal bevacizumab injection in eyes with diabetic macular edema. Eye (Lond.) 2014;28:646–653. doi: 10.1038/eye.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zingg B., Chou X.L., Zhang Z.G., Mesik L., Liang F., Tao H.W., Zhang L.I. AAV-Mediated Anterograde Transsynaptic Tagging: Mapping Corticocollicular Input-Defined Neural Pathways for Defense Behaviors. Neuron. 2017;93:33–47. doi: 10.1016/j.neuron.2016.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weber A.J., Viswanáthan S., Ramanathan C., Harman C.D. Combined application of BDNF to the eye and brain enhances ganglion cell survival and function in the cat after optic nerve injury. Invest. Ophthalmol. Vis. Sci. 2010;51:327–334. doi: 10.1167/iovs.09-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper M.L., Pasini S., Lambert W.S., D’Alessandro K.B., Yao V., Risner M.L., Calkins D.J. Redistribution of metabolic resources through astrocyte networks mitigates neurodegenerative stress. Proc. Natl. Acad. Sci. USA. 2020;117:18810–18821. doi: 10.1073/pnas.2009425117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnhart E.L. Mechanics of mitochondrial motility in neurons. Curr. Opin. Cell Biol. 2016;38:90–99. doi: 10.1016/j.ceb.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 35.Cooper M.L., Collyer J.W., Calkins D.J. Astrocyte remodeling without gliosis precedes optic nerve Axonopathy. Acta Neuropathol. Commun. 2018;6:38. doi: 10.1186/s40478-018-0542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calkins D.J. Adaptive responses to neurodegenerative stress in glaucoma. Prog. Retin. Eye Res. 2021;84:100953. doi: 10.1016/j.preteyeres.2021.100953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burdett T.C., Freeman M.R. Neuroscience. Astrocytes eyeball axonal mitochondria. Science. 2014;345:385–386. doi: 10.1126/science.1258295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayakawa K., Esposito E., Wang X., Terasaki Y., Liu Y., Xing C., Ji X., Lo E.H. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535:551–555. doi: 10.1038/nature18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carelli V., Ross-Cisneros F.N., Sadun A.A. Mitochondrial dysfunction as a cause of optic neuropathies. Prog. Retin. Eye Res. 2004;23:53–89. doi: 10.1016/j.preteyeres.2003.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


