Abstract
Objectives
To report the spectrum and trends of isolated microorganisms and their antibiotic susceptibility profile in patients with infectious keratitis in a 6-year period at a referral centre in Tehran.
Methods
The microbiology records of all corneal scrapings with a diagnosis of infectious keratitis were reviewed.
Results
A total of 6282 corneal scrapings were performed during the study period, of which 2479 (39.5%) samples were culture positive. Pseudomonas aeruginosa was found to be the most common causative agent in patients with keratitis, although Streptococcus pneumonia was the most prevalently isolated microorganism in patients older than 50 years. Fusarium sp. was the most common responsible pathogen in patients with fungal keratitis. The prevalence of bacterial keratitis due to gram positive microorganisms increased over time, however the number of Pseudomonas keratitis decreased in the second half of the study. Gram negative organisms showed a good sensitivity to levofloxacin, however, 34.1% of S. aureus isolates and 29.7% of coagulase negative staphylococci were resistant to this antibiotic. The odds of ciprofloxacin and levofloxacin resistance increased 1.25 and 1.15 for each 1-year increase in culture date, respectively (P < 0.001, P = 0.004).
Conclusions
We documented an increasing trend in the percentage of gram positive bacteria. Levofloxacin monotherapy might still be a good option in patients with gram negative bacterial keratitis, however owing to increasing resistance of staphylococci to fluoroquinolones, a regimen consisting of a combination of fortified antibiotics may be more effective in staphylococcal keratitis.
Subject terms: Corneal diseases, Fungal infection
Introduction
Microbial keratitis is a common ophthalmic emergency that can lead to progressive tissue destruction and loss of sight. Prompt diagnosis and treatment are of utmost importance to prevent a poor visual outcome [1, 2]. The clinical management of infectious keratitis often starts with sample collection from the cornea for culture and stains. Empiric antibiotic treatment is often initiated before the identification of causative organism. Once a specific microorganism is isolated it is possible to adjust the treatment based on the results of culture and antibiogram. The spectrum of pathogens in each area is influenced by its geographical location, the occupation of its residents and the presence of risk factors such the prevalence of contact lens wear [3]. The widespread use of antibiotics for the treatment of ocular infections and prophylaxis in ophthalmic procedures has led to the emergence of antibiotic resistant bacterial isolates. It is the responsibility of the clinician to be updated about the data from local microbiologic laboratories and their antibiotic susceptibility profile in this ever-shifting battle to preserve the sight of the patient.
A smaller study identified P. aeruginosa and S. pneumonia as the most common causative pathogens in patients with bacterial keratitis in our area. The isolated organisms were highly sensitive to amikacin (94%) and ceftazidime (94%) [4]. However, considering this ever changing treatment battle, larger studies examining the updated local microbiology data are required. Herein, we report the spectrum of pathogens isolated from corneal cultures and the antibiotic resistance patterns in a tertiary eye care centre in Iran.
Materials and methods
This study was approved by ethics committee and institutional review board of Tehran University of Medical Sciences and it followed the tenets of Declaration of Helsinki. A retrospective analysis of all patients with a diagnosis of corneal ulcer in Farabi Eye Hospital between September 2012 and October 2018 was performed. Corneal scraping was performed for all corneal ulcers except for peripheral infiltrates, smaller than 3 mm and with no evidence of corneal thinning on slit lamp examination. The corneal scrapes were obtained after instillation of topical anaesthesia. A sterile scalpel blade was used under the visualization of slit lamp biomicroscope to provide corneal samples. The corneal ulcer material was placed on two microscopy slides for Gram and Giemsa stains after which fresh scalpel blades were used to directly inoculate three culture media (sheep blood agar, chocolate agar and Sabouraud’s dextrose agar). The plates were then incubated in an atmosphere containing 5% carbon dioxide under the temperature of 37 °C for 96 h. The culture was considered positive by the laboratory staff if there was discrete colonies of the same organism on two solid media or confluent growth of organism was observed along the site of inoculation on one culture plate. Antibiotic susceptibility of microbial isolates was determined using the disk diffusion method. Susceptibility testing was performed for the following antibiotics: Carbenicillin, Cefalexin, Cefixime, Cefotaxime, Amikacin, Ceftazidime, Gentamicin, Chloramphenicol, Cefazolin, Ciprofloxacin, Trimethoprim/sulfamethoxazole, Vancomycin, Erythromycin, Oxacillin, Ampicillin, Cephalothin, Penicillin G, Rifampicin, Tetracycline, Imipenem, Ceftriaxone, Ofloxacin, PolymyxinB, Norfloxacin, Azithromycin, Doxycycline, Cefoxitin, Levofloxacin.
Statistical analysis was performed in SPSS (Version 23 SPSS, IBM Inc., Chicago, IL, USA). Descriptive statistics, means and SD were used for continuous variables. Percentages were used for categorical variables. One way analysis of variance (ANOVA) accompanied by Tukey’s post hoc test was performed to compare the mean age of the patients between the most common isolated microorganisms. To evaluate the prevalence of isolated microorganisms over time, we arbitrarily divided the study period into 2 periods. Chi square test was used to compare the prevalence of microbial isolates in the first half (2012–2015) and the second half (2015–2018). A logistic regression model was used to assess the trend of antibiotic resistance over time. P < 0.05 was considered statistically significant.
Results
A total of 6282 corneal cultures were performed during a 6-year time period from 2012 to 2018 at Farabi Eye Hospital. The mean age of the patients was 54.6 ± 23.3 and 3957 (63%) patients were male. Of total corneal specimens, 2479 isolates (39.5%) were culture positive. Among culture positive isolates 1064 (42.9%) were gram positive bacteria, 891 isolates (35.9%) were gram negative bacteria and 383 isolates (15.4%) were fungal organisms. The most common gram positive bacteria was streptococcus pneumonia (347 of 1064 [32.6%]). Pseudomonas aeruginosa was found to be the most common gram negative bacteria (641 of 891 [71.9%]). Among fungal organisms, fusarium sp. represented 199 of 383 (60.0%) cultures, making it the most common fungal pathogen in this study.
In patients younger than 19 years old and patients between 19 and 50 years, P. aeroginosa was the most frequently isolated organism, however streptococcus pneumonia was the most common causative agent of keratitis in patients older than 50 years. Among the most common etiologic agents for microbial keratitis, the patients who were diagnosed with streptococcus pneumonia keratitis were older (P < 0.001). The average patient’s age in P. aeroginosa keratitis, S.aureus keratitis, CoNS keratitis and S.peneumonia keratitis was 45.4 ± 23.9, 50.2 ± 22.6, 54.5 ± 23.3 and 64.7 ± 23.0, respectively.
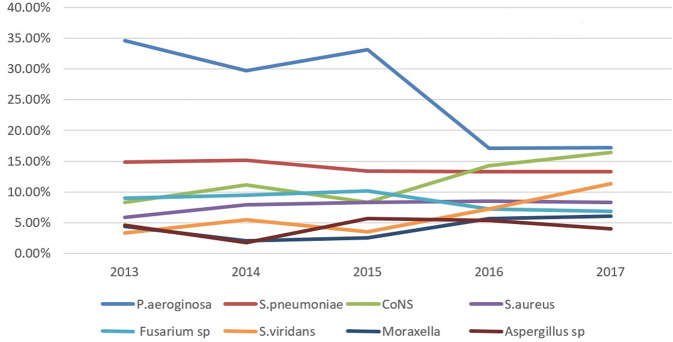
An increase in the prevalence of bacterial keratitis due to gram positive bacteria was observed after 2015 [specifically S. aureus (P = 0.04), CoNS (P < 0.001) and S. viridans (P < 0.001)], however the number of Pseudmonas keratitis decreased after 2015 (P < 0.001). Table 1 shows the number of isolated microorganisms during the two study periods. Figure 1 illustrates the percentage of most common isolated microorganisms per year.
Table 1.
Comparison of isolated microorganisms between two time periods in patients with keratitis.
| Organism | No. of positive isolates before year 2015 | No. of positive isolates after year 2015 | P value |
|---|---|---|---|
| P. aeroginosa | 422 | 219 | <0.001 |
| S.pneumoniae | 187 | 160 | 0.78 |
| CoNS | 119 | 190 | <0.001 |
| S. aureus | 92 | 108 | 0.04 |
| Fusarium sp. | 117 | 82 | 0.06 |
| S. viridans | 55 | 109 | <0.001 |
| Moraxella sp. | 42 | 64 | 0.006 |
| Aspergillus sp. | 54 | 47 | 0.53 |
| Candida sp. | 35 | 40 | 0.295 |
| Klebsiella sp. | 22 | 20 | 0.52 |
| Serratia sp. | 19 | 14 | 0.37 |
| Citrobacter | 13 | 18 | 0.28 |
| Hemophilus sp. | 9 | 7 | 0.50 |
| Other | 106 | 94 |
CoNS Coagulase negative staphylococci
Statistically significant are highlighted in bold letters and numbers.
Fig. 1. Percentage of the most common isolated microorganisms in patients with infectious keratitis per year during the 5 years of the study.
Each line represents the changing prevalence of isolated pathogens over time.
Gram positive organisms
Table 2 outlines the most common isolated gram positive organisms and their antibiotic susceptibility profiles. Streptococcus pneumonia was the most commonly isolated gram positive organism (N = 347). This pathogen showed excellent sensitivity to vancomycin (100%) cefazolin (98.8%), levofloxacin (97.8%) and ciprofloxacin (93.6%). However, it was fairly resistant to trimethoprim-sulfamethoxazole (with a sensitivity of 45.2%). Oxacillin resistant S.aureus comprised 20% of all isolated S.aureus organisms. No vancomycin resistant strains were identified among S.aureus isolates. S.aureus showed good sensitivity to cefazolin (94.4%) and variable sensitivity to ciprofloxacin (69.5%) and levofloxacin (65.9%). Coagulase negative staphylococcus (CoNS) was found to be a frequent pathogen in patients with keratitis (N = 309) of which 71.1% were oxacillin-resistant coagulase-negative staphylococcus. Vancomycin susceptibility of CoNS was 100.0%. CoNS showed good sensitivity to cefazolin (94.7%) and variable sensitivity to ciprofloxacin (57.5%) and levofloxacin (70.3%).
Table 2.
Antibiotic sensitivity of gram positive organisms.
| Organism | Amikacin | Cefazolin | Chloramphenicol | Ciprofloxacin | Levofloxacin | Oxacillin | Vancomycin |
|---|---|---|---|---|---|---|---|
| S.pneumoniae | 28.2% (93/329) | 98.8% (334/338) | 79.7% (263/330) | 93.6% (321/343) | 97.8% (134/137) | 42.8% (24/56) | 100.0% (336/336) |
| S.aureus | 70.2% (132/188) | 94.4% (187/198) | 68.6% (127/185) | 69.5% (137/197) | 65.9% (60/91) | 80.0% (68/85) | 100.0% (192/192) |
| CoNS | 87.5% (259/296) | 94.7% (291/307) | 65.9% (191/290) | 58.1% (178/306) | 70.3% (110/156) | 46.6% (62/133) | 100.0% (307/307) |
| S.viridans | 24.1% (38/158) | 93.6% (146/156) | 89.7% (140/156) | 68.3% (110/161) | 90.9% (80/88) | 16.7% (3/18) | 99.3% (154/155) |
(CoNS coagulase negative staphylococci).
Nocardia keratitis was reported in 12 of our patients and was 5.0 times more common in men. The mean age of the patients was 39.2 ± 16.3. In vitro susceptibility testing showed that nocardia was highly sensitive to amikacin (100.0%) and variably sensitive to ciprofloxacin (63.6%), gentamicin (63.6%) and trimethoprim-sulfamethoxazole (36.4%).
Gram negative organisms
Table 3 shows the most common gram negative bacteria in patients with keratitis and their antibiotic susceptibility profiles. As stated earlier, P. aeruginosa was the most common bacterial pathogen in patients with keratits. This organism showed high susceptibility to amikacin (98.1%), gentamicin (97.0%) and ceftazidim (95.9%). Fluoroquinolones were also highly effective against this bacterial pathogen (ciprofloxacin 98.1% and levofloxacin 94.0%).
Table 3.
Antibiotic sensitivity of gram negative organisms.
| Organism | Amikacin | Gentamicin | Chloramphenicol | Ciprofloxacin | Levofloxacin | Ceftazidim | TMP-SMX |
|---|---|---|---|---|---|---|---|
| Pseudmonas sp | 98.0% (626/639) | 96.8% (609/629) | 1.1% (7/614) | 98.0% (629/640) | 93.6% (189/202) | 95.9% (606/632) | 2.2% (11/509) |
| Moraxella sp | 96.1% (98/102) | 99.0% (102/103) | 94.9% (94/99) | 96.1% (98/102) | 100.0% (59/59) | 92.1% (93/101) | 38.2% (26/68) |
| Citrobacter sp | 93.1% (27/29) | 96.8% (30/31) | 66.7% (20/30) | 93.3% (28/30) | 86.7% (13/15) | 96.7% (29/30) | 64.0% (16/25) |
| Serattia sp | 93.9% (31/33) | 93.8% (30/32) | 93.3% (28/30) | 100.0% (33/33) | 100.0% (14/14) | 96.9% (31/32) | 95.6% (22/23) |
| Klebsiella sp | 90.5% (38/42) | 97.5% (39/40) | 97.4% (38/39) | 95.1% (38/41) | 100.0% (16/16) | 89.2% (36/41) | 55.2% (16/29) |
| H. influenzae | 60% (6/10) | 92.8% (13/14) | 78.6% (11/14) | 93.3% (14/15) | 100.0% (3/3) | 54.5% (6/11) | 33.3% (5/15) |
(TMP-SMX trimethoprim-sulfamethoxazole)
Moraxella spp. was the second most common gram negative bacterial pathogen in patients with keratitis (N = 106). The mean age of the patients was 58.6 ± 20.8 and it was 4.6 times more common in males. Amikacin (96.1%), ceftazidim (93.1%), gentamicin (99.0%), chloramphenicol (94.9%), ciprofloxacin (96.1%) and levofloxacin (100.0%) were highly effective against this bacterial agent.
Citrobacter spp. is a rare cause of keratitis, with a prevalence of 0.5% in this study (N = 31). The mean age of the patients with citrobacter keratitis was 57.9 ± 23.2 and it was 2.4 time more common in males. This bacterial agent was highly sensitive to antibiotics that are commonly prescribed for gram negative bacterial keratitis (amikacin 93.1%, gentamicin 96.8%, ceftazidim 96.7%, ciprofloxacin 93.3% and levofloxacin 86.7%).
Trends of antibiotic resistance over time
Risk of ciprofloxacin resistance among bacterial agents causing keratitis increased over time. There was 1.25 increased odds of culturing an organism resistant to ciprofloxacin each year, even after controlling for type of bacterial pathogen (P < 0.001). Testing for levofloxacin resistance started in our laboratory since 2015. The odds of levofloxacin resistance also increased 1.15 for each 1-year increase in culture date after controlling for type of organism (P = 0.004).
Discussion
In this study we report the spectrum of pathogens causing bacterial and fungal keratitis and antibiotic sensitivity patterns in patients with bacterial keratitis in a tertiary eye hospital in Tehran, Iran. We found that P. aeroginosa was the most common agent in young patients with bacterial keratitis, however in patients older than 50 years, S.pneumoniae was most frequently isolated. This is consistent with reports from Taiwan [5, 6] that identified P. aeroginosa as the most common pathogen in that area and in contrast with reports from the USA [7], UK [8–10] and Brazil [11] that identified Staphylocci as the most common cause of bacterial keratitis. Corneal ulcers caused by P. aeroginosa are often associated with contact lens wear [12]. The recent interest for contact lens wear especially for cosmetic reasons among youngsters might explain the prevalence of Pseudomonas keratitis in this study. Fungal keratitis represented 6.1% of all keratitis cases, with Fusarium sp being the most common fungal pathogen. The prevalence of fungal keratitis in different regions of the world is influenced by its local environment and the occupation of people of that region, therefore the epidemiology of fungal keratitis varies widely across the world [13]. The proportion of corneal ulcers caused by fungal organisms is reported to be as high as 36.5% in south India [14] to 1.2% in USA [15].
The early use of empiric topical antibiotic drops is of utmost importance to stop the corneal damage caused by infectious keratitis and minimize visual disability. The choice of antibiotics should be based upon the local prevalence of pathogens and information about local resistance patterns to antibiotics that retain widespread use is critical in this ever evolving treatment battle [16].
Fluoroquinolones are popular choices to treat bacterial keratitis owing to their high efficacy and broad spectrum activity against gram positive and gram negative ocular pathogens [17, 18]. Most of the isolated gram negative bacteria in this study were sensitive to levofloxacin and ciprofloxacin, however, a high proportion of staphylococci were resistant to these fluoroquinolones. These results mirror the previous reports about increasing resistance to fluoroquinolones, especially in gram positive bacterial keratitis [19, 20]. Given these results, levofloxacin monotherapy might be an appropriate option in patients with gram negative bacterial keratitis, the same recommendation, however, cannot be made in corneal ulcers due to gram positive bacteria. We also found an increasing resistance to ciprofloxacin and levofloxacin during the study period. This observation highlights the importance of close follow-up after initiating the empiric therapy and maintaining a low threshold for choosing an alternative therapy when the clinical response is not favourable. The high rate of resistance to levofloxacin among gram positive bacteria in our study compared to reports from other regions of the world [9] might be due to injudicious use of topical antibiotics in our country especially for prophylaxis in cataract surgery. The referral of corneal ulcers that were unresponsive to medical treatment in a primary care setting may also explain this finding.
Aminoglycosides such as amikacin are often combined with cephalosporins to treat patients with bacterial corneal ulcers. Most of the gram negative bacteria remained highly susceptible to amikacin and gentamicin. However, a high proportion of streptococci were resistant to this class of antibiotics, implying the decreased usefulness of aminoglycosides when streptococci are responsible for corneal ulcers. The rate of resistance to amikacin and gentamicin did not change significantly over time, a finding consistent with reports from other countries [8].
Topical preparations of chloramphenicol are commonly prescribed as an over-the-counter drug in Iran and its frequent application by primary care physicians may select for bacteria that are resistant to this antibiotic. Nevertheless, no trend for increased resistance to chloramphenicol was observed during the course of this study. Apart from Pseudomonas sp., gram negative organisms retained high sensitivity to chloramphenicol. The proportion of gram negative organisms resistant to chloramphenicol were lower compared to other studies [8, 20], this may be due to different antibiotic prescribing habits in other regions. Gram positive organisms also showed variable sensitivity to chloramphenicol.
The strength of this study is that we report the corneal culture results on a large number of specimens performed at an ophthalmic microbiology laboratory. Although in vitro studies are currently the standard method for determining resistance to antibiotics, it should be appreciated that they are interpreted based on serum level of the drug. When translated into treatment for keratitis, in vitro resistance does not always correlate with poor clinical efficacy. In vitro analysis of antibiotic resistance patterns may underestimate the clinical efficacy of that drug, as the ophthalmic concentration of a pharmaceutical compound may reach much higher levels compared to what is used for in vitro susceptibility testing [21]. Additional limitations include that as a retrospective study, it is subject to biases inherent in such studies. In vitro analysis of antibiotic sensitivity does not take into account clinical findings and patients’ compliance; however, it is an important guide to initiate empiric therapy. Although Farabi Eye Hospital is a referral centre and many patients with corneal ulcers are referred from all over Iran, the antibiotic sensitivity profile cannot be extrapolated in other regions. Moreover, the lack of information on aetiology and clinical presentation of microbial keratitis further limits the results of this study.
In conclusion, this study found P. aeroginosa as the most common organism responsible for bacterial keratitis, however an increased prevalence of gram positive organisms was observed over the course of the study. Fusarium sp. was the most common fungal organism. We documented reducing ciprofloxacin and levofloxacin sensitivity over the study period. Given the results of this study, levofloxacin monotherapy might still be a good option in gram negative bacterial keratitis, however, owing to increased resistance of staphylococci to fluoroquinolones, topical administration of levofloxacin may not be effective when these germs are responsible for corneal ulcer.
Summary
What was known before
Monotherapy with fluoroquinolones may be an appropriate option in some patients with bacterial keratitis.
Increasing resistance to topical antibiotics might limit the armamentarium of the clinician to treat infectious keratitis in the future.
What this study adds
Gram negative organisms are still highly susceptible to fluoroquinolones.
There is an increasing resistance to flouroquinolones in patients with bacterial keratitis that might limit their use in the future.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kaye S, Tuft S, Neal T, Tole D, Leeming J, Figueiredo F, et al. Bacterial susceptibility to topical antimicrobials and clinical outcome in bacterial keratitis. Investig Ophthalmol Vis Sci. 2010;51:362–8. doi: 10.1167/iovs.09-3933. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez-Camarena JC, Graue-Hernandez EO, Ortiz-Casas M, Ramirez-Miranda A, Navas A, Pedro-Aguilar L, et al. Trends in microbiological and antibiotic sensitivity patterns in infectious keratitis: 10-year experience in Mexico City. Cornea. 2015;34:778–85. doi: 10.1097/ICO.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 3.Peng MY, Cevallos V, McLeod SD, Lietman TM, Rose-Nussbaumer J. Bacterial keratitis: isolated organisms and antibiotic resistance patterns in San Francisco. Cornea. 2018;37:84. doi: 10.1097/ICO.0000000000001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahimi F, Hashemian MN, Khosravi A, Moradi G, Bamdad S. Bacterial keratitis in a tertiary eye centre in Iran: a retrospective study. Middle East Afr J Ophthalmol. 2015;22:238. doi: 10.4103/0974-9233.151870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsiao C-H, Sun C-C, Yeh L-K, Ma DH, Chen PY, Lin H-C, et al. Shifting trends in bacterial keratitis in Taiwan: a 10-year review in a tertiary-care hospital. Cornea. 2016;35:313–7. doi: 10.1097/ICO.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 6.Liu H-Y, Chu H-S, Wang I-J, Chen W-L, Hu F-R. Microbial keratitis in Taiwan: a 20-year update. Am J Ophthalmol. 2019;205:74–81. doi: 10.1016/j.ajo.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 7.Jeng BH, Gritz DC, Kumar AB, Holsclaw DS, Porco TC, Smith SD, et al. Epidemiology of ulcerative keratitis in Northern California. Arch Ophthalmol. 2010;128:1022–8. doi: 10.1001/archophthalmol.2010.144. [DOI] [PubMed] [Google Scholar]
- 8.Orlans H, Hornby S, Bowler I. In vitro antibiotic susceptibility patterns of bacterial keratitis isolates in Oxford, UK: a 10-year review. Eye. 2011;25:489–93. doi: 10.1038/eye.2010.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tavassoli S, Nayar G, Darcy K, Grzeda M, Luck J, Williams OM, et al. An 11-year analysis of microbial keratitis in the South West of England using brain–heart infusion broth. Eye. 2019;33:1619–25. doi: 10.1038/s41433-019-0463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan S, Walkden A, Au L, Fullwood C, Hamilton A, Qamruddin A, et al. Twelve-year analysis of microbial keratitis trends at a UK tertiary hospital. Eye. 2017;31:1229. doi: 10.1038/eye.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Passos RM, Cariello AJ, Yu MCZ, Höfling-Lima AL. Microbial keratitis in the elderly: a 32-year review. Arq bras oftalmol. 2010;73:315–9. doi: 10.1590/S0004-27492010000400002. [DOI] [PubMed] [Google Scholar]
- 12.Alfonso E, Mandelbaum S, Fox MJ, Forster RK. Ulcerative keratitis associated with contact lens wear. Am J Ophthalmol. 1986;101:429–33. doi: 10.1016/0002-9394(86)90641-0. [DOI] [PubMed] [Google Scholar]
- 13.Garg P, Roy A, Roy S. Update on fungal keratitis. Curr Opin Ophthalmol. 2016;27:333–9. doi: 10.1097/ICU.0000000000000272. [DOI] [PubMed] [Google Scholar]
- 14.Lin CC, Prajna L, Srinivasan M, Prajna NV, McLeod SD, Acharya NR, et al. Seasonal trends of microbial keratitis in South India. Cornea. 2012;31:1123. doi: 10.1097/ICO.0b013e31825694d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ritterband DC, Seedor JA, Shah MK, Koplin RS, McCormick SA. Fungal keratitis at the New York eye and ear infirmary. Cornea. 2006;25:264–7. doi: 10.1097/01.ico.0000177423.77648.8d. [DOI] [PubMed] [Google Scholar]
- 16.Austin A, Lietman T, Rose-Nussbaumer J. Update on the management of infectious keratitis. Ophthalmology. 2017;124:1678–89. doi: 10.1016/j.ophtha.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pavesio C, Morlet N, Allan B, El Kassaby H, DeCock R, Butcher J, et al. Ofloxacin monotherapy for the primary treatment of microbial keratitis: a double-masked, randomized, controlled trial with conventional dual therapy. Ophthalmology. 1997;104:1902–9. doi: 10.1016/S0161-6420(97)30009-8. [DOI] [PubMed] [Google Scholar]
- 18.Durrani AF, Atta S, Bhat A, Mammen A, Dhaliwal D, Kowalski RP, et al. Methicillin-resistant Staphylococcal aureus keratitis: initial treatment, risk factors, clinical features, and treatment outcomes. Am J Ophthal. 2020;214:119–26. doi: 10.1016/j.ajo.2020.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Afshari NA, Ma JJ, Duncan SM, Pineda R, Starr CE, DeCroos FC, et al. Trends in resistance to ciprofloxacin, cefazolin, and gentamicin in the treatment of bacterial keratitis. J Ocul Pharmacol therapeutics. 2008;24:217–23. doi: 10.1089/jop.2007.0085. [DOI] [PubMed] [Google Scholar]
- 20.Shalchi Z, Gurbaxani A, Baker M, Nash J. Antibiotic resistance in microbial keratitis: ten-year experience of corneal scrapes in the United Kingdom. Ophthalmology. 2011;118:2161–5. doi: 10.1016/j.ophtha.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Chang VS, Dhaliwal DK, Raju L, Kowalski RP. Antibiotic resistance in the treatment of Staphylococcus aureus keratitis: a 20-year review. Cornea. 2015;34:698. doi: 10.1097/ICO.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]