Abstract
Background
The longitudinal trajectories of cardiopulmonary abnormalities and symptoms following infection with coronavirus disease (COVID-19) are unclear. We sought to describe their natural history in previously hospitalised patients, compare this with controls, and assess the relationship between symptoms and cardiopulmonary impairment at 6 months post-COVID-19.
Methods
Fifty-eight patients and thirty matched controls (single visit), recruited between 14th March - 25th May 2020, underwent symptom-questionnaires, cardiac and lung magnetic resonance imaging (CMR), cardiopulmonary exercise test (CPET), and spirometry at 3 months following COVID-19. Of them, forty-six patients returned for follow-up assessments at 6 months.
Findings
At 2-3 months, 83% of patients had at least one cardiopulmonary symptom versus 33% of controls. Patients and controls had comparable biventricular volumes and function. Native cardiac T1 (marker of fibroinflammation) and late gadolinium enhancement (LGE, marker of focal fibrosis) were increased in patients at 2-3 months. Sixty percent of patients had lung parenchymal abnormalities on CMR and 55% had reduced peak oxygen consumption (pV̇O2) on CPET. By 6 months, 52% of patients remained symptomatic. On CMR, indexed right ventricular (RV) end-diastolic volume (-4·3 mls/m2, P=0·005) decreased and RV ejection fraction (+3·2%, P=0·0003) increased. Native T1 and LGE improved and was comparable to controls. Lung parenchymal abnormalities and peak V̇O2, although better, were abnormal in patients versus controls. 31% had reduced pV̇O2 secondary to symptomatic limitation and muscular impairment. Cardiopulmonary symptoms in patients did not associate with CMR, lung function, or CPET measures.
Interpretation
In patients, cardiopulmonary abnormalities improve over time, though some measures remain abnormal relative to controls. Persistent symptoms at 6 months post-COVID-19 did not associate with objective measures of cardiopulmonary health.
Funding
The authors’ work was supported by the NIHR Oxford Biomedical Research Centre, Oxford British Heart Foundation (BHF) Centre of Research Excellence (RE/18/3/34214), United Kingdom Research Innovation and Wellcome Trust. This project is part of a tier 3 study (C-MORE) within the collaborative research programme entitled PHOSP-COVID Post-hospitalization COVID-19 study: a national consortium to understand and improve long-term health outcomes, funded by the Medical Research Council and Department of Health and Social Care/National Institute for Health Research Grant (MR/V027859/1) ISRCTN number 10980107.
Keywords: CMR, COVID-19, CPET, long COVID, SARS-CoV-2
Research in context.
Evidence before this study
Several studies have shown that following hospitalisation with COVID-19, patients continue to experience a broad range of symptoms, together with evidence of cardiac and respiratory abnormalities accompanied by exercise limitations. However, research assessments have typically been undertaken at a single time point and do not reveal the natural history of cardiopulmonary pathology or how they relate with ongoing symptoms in patients.
Added value of this study
This study describes the longitudinal trajectories of cardiopulmonary symptoms and abnormalities in patients recovering from COVID-19. We demonstrate that among previously hospitalised patients both symptoms and early evidence of cardiopulmonary impairment improve over time from 3 to 6 months after the illness. However, some patients continue to have lung abnormalities and exercise limitation. Notably, more than half the patients continue to experience symptoms at 6 months and there was a dissociation between persistent symptoms and objective measures of cardiopulmonary health.
Implications of all the available evidence
Our findings suggest that contemporary tools that are used to assess cardiopulmonary health in the community remain poor at elucidating a cause for ongoing symptoms. Patients can have evidence of abnormalities on clinical tests and still be asymptomatic. The pathophysiological basis for cardiopulmonary symptoms is still unclear and alternative mechanisms for ongoing symptoms need to be explored.
Alt-text: Unlabelled box
1. Introduction
First described in December 2019 [1], severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), a beta coronavirus, is responsible for coronavirus disease (COVID-19). Our understanding of how this virus came to invade human cell lines has rapidly evolved, as the role of angiotensin-converting enzyme-2 receptors (ACE2) in facilitating viral entry into cells was elucidated [2]. ACE2 receptors are not only present in type II pneumocytes but are ubiquitously expressed by the vascular cells and other visceral organs [3]. The effect of SARS-CoV-2 on the heart is of particular importance, as it can cause a range of abnormalities including myocardial dysfunction, inflammation, and ischaemic damage via direct (cytotoxic) and indirect (dysregulated immune response, thrombo-inflammation) mechanisms [4]. Myocardial injury is more common in moderate to severe infections and predictive of poor clinical outcomes among those admitted to hospital [5]. A number of recent studies have highlighted the role of cardiac magnetic resonance imaging (CMR) and cardiopulmonary exercise testing (CPET) in evaluating the mechanisms and functional consequences of cardiopulmonary injury in COVID-19 survivors [5], [6], [7]. Detailed assessments have typically been undertaken at a single time point within weeks to months after infection and do not reveal the natural history of cardiopulmonary pathology. A high burden of cardiopulmonary symptoms has also been reported and the role of contemporaneous investigations in elucidating the underlying cause for symptoms is unknown.
Previously, we undertook a holistic assessment of COVID-19 patients at 2-3 months following moderate to severe infection using symptom-based questionnaires, multiorgan magnetic resonance imaging (MRI), spirometry, and CPET [8]. We observed a high prevalence of tissue abnormalities involving the heart (26%) and lungs (60%) on MRI, together with reduced forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) and marked exercise intolerance on CPET in patients. Here, we sought to describe the time course evolution of cardiopulmonary symptoms, CMR, pulmonary function and CPET abnormalities in these patients from 2-3 months to 6 months and evaluate the relationship between symptoms and objective measures of cardiopulmonary health at 6 months.
2. Methods
2.1. Study population
Fifty-eight patients with moderate to severe laboratory-confirmed (SARS-CoV-2 polymerase chain reaction positive) COVID-19, admitted for inpatient treatment at the Oxford University Hospitals National Health Service Foundation Trust between 14th March - 25th May 2020, and 30 SARS-CoV-2 immunoglobulin negative controls, group-matched for age, sex, body mass index and risk factors (smoking, diabetes, and hypertension) from the community (recruited during the same period) were prospectively enrolled in this observational cohort study as previously described. A flow chart for recruitment is listed in the Supplementary Material, p10.
This study was registered at ClinicalTrials.gov (NCT04510025) and approved in the United Kingdom by the North West Preston Research Ethics Committee (reference 20/NW/0235).
2.2. Study procedures
Informed consent was obtained from all patients. Patient health questionnaires, cardiopulmonary magnetic resonance imaging (MRI), spirometry, CPET, electrocardiogram (ECG) and blood tests were undertaken in patients at 2-3 months and 6 months post-infection and at a single time point in controls. Gas transfer assessments were undertaken in patients at 6 months alone.
Disease severity was graded using the World Health Organisation ordinal scale for clinical improvement [9]. Patients with severe illness were defined as those having a score of ≥5 (high flow oxygen, non-invasive and invasive ventilation).
An electrocardiogram (ECG) was performed for every participant and interpreted according to the Minnesota Code of Electrocardiographic Findings [10].
Patient health questionnaire-15 (PHQ-15) [11] was completed using an electronic data capture platform (CASTOR EDC, https://www.castoredc.com). The Medical Research Council (MRC) dyspnoea scale [12] and Fatigue Severity Scale (FSS) [13] were used to assess the prevalence and severity of breathlessness and fatigue, respectively (Supplementary material, p3).
CMR was carried out at 3 Tesla (Prisma, Siemens Healthineers, Erlangen, Germany) and included cine imaging to assess biventricular volumes, diastolic strain rate, T1 and T2 mapping to assess myocardial inflammation and oedema, and post-contrast T1 mapping and late gadolinium enhancement (LGE) imaging to assess diffuse and focal/patchy fibrosis. Lung abnormalities were assessed using Half‐Fourier‐acquisition single‐shot turbo spin‐echo (HASTE) MRI before the administration of contrast (Supplementary Material, p4).
CMR studies were analysed using CVI42 5.11.4 (Circle Cardiovascular Imaging, Calgary, Canada). All cardiac images were anonymised and analysed by CMR experts (BR, MC) (Supplementary Material, p4). Lung images were qualitatively assessed for parenchymal involvement by an expert radiologist (CX), with the extent of lung parenchymal opacities scored as 0 (0%), 1 (1-25%), 2 (26-50%), 3 (51-75%), or 4 (76-100%) [14]. BR, MC and CX were blinded to the subject group allocation during analysis.
Spirometry, including FVC and FEV1, was performed as per recommended guidance [15]. Diffusion capacity for carbon monoxide (DLCO) and alveolar volume (Va) were measured using a ten-second single breath-hold technique with methane as the tracer gas, and adjusted for haemoglobin [16].
Symptom-limited incremental CPET was undertaken using a cycle ergometer as previously described. Following two minutes of unloaded cycling, the work rate was increased to 20W, followed by a 10W/min ramp (Supplementary Material, p6) [17].
Blood-based testing consisted of complete blood count, biochemical analysis, coagulation testing, liver and renal function assessment, markers of cardiac injury (troponin T and N-terminal pro-brain natriuretic peptide/NT-proBNP), and measures of electrolytes, C-reactive protein (CRP), and procalcitonin.
Details on clinical symptoms, signs, vitals, and laboratory findings during admission were extracted from electronic medical records.
2.3. Statistics
Continuous variables were described using mean and standard deviation for variables with parametric data across all groups. When non-parametric data was present in one or more groups, median and interquartile range (IQR) were used to facilitate comparison. Normality was assessed by the Shapiro-Wilk test. Group differences were evaluated using Student's t-tests, Mann-Whitney U-tests, paired Student's t-tests, and Wilcoxon Signed Ranks tests as appropriate. Categorical variables were reported as frequency and percentages, with group differences evaluated using the Chi-square test, Fisher's exact test, Fisher-Freeman-Halton exact test, Stuart-Maxwell test, or McNemar test as appropriate. Spearman's correlation coefficients were used to describe the relationship between two variables where relevant. Univariate and multivariate binary logistic regression were used to assess the association between cardiopulmonary symptoms (chest pain, palpitations, syncope, dyspnoea, or dizziness) and objective measures of cardiopulmonary health. To maintain the absence of collinearity, NT-proBNP (<125 ng/L or ≥125 ng/L), ECG (normal or abnormal), left ventricular ejection fraction, right ventricular ejection fraction, mid myocardial T1, mid myocardial T2, volume of late gadolinium enhancement, left ventricular diastolic strain rate, FEV1, FVC, DLco, peak oxygen consumption and V̇E/V̇CO2 slope were included as independent variables in the multivariate analysis. The Box-Tidwell test was used to demonstrate maintenance of linearity in the logit.
In a separate analysis, determinants of breathlessness were also ascertained (Supplementary Material, p11). The conventional level of statistical significance of 5% was used. Statistical analyses were performed using SPSS Version 27.0 (IBM, Armonk, NY, USA).
3. Role of Funding
The sponsors played no role in the design of the study; collection, analysis and interpretation of data; in writing the manuscript, and in the decision to submit the paper for publication.
4. Results
Baseline characteristics of all patients and controls are listed in Table 1.
Table 1.
Demographics and baseline characteristics of COVID-19 patients who underwent single assessment, serial assessments (2-3 months & 6 months) and controls.
| COVID-19, 2-3m (N=58) | COVID-19, 6m (N=46) | Controls (N=30) |
P-values |
|||
|---|---|---|---|---|---|---|
| 2-3m vs Controls | 6m vs Controls | 2-3m vs 6m | ||||
| General demographics | ||||||
| Age, years | 55·4 (13·2) | 55·2 (13·3) | 53·9 (12·3) | 0·62 | 0·67 | 0·96 |
| Gender | 1·00a | 0·81a | 0·69a | |||
| Female | 24/58 (41·4%) | 17/46 (37·0%) | 12/30 (40·0%) | |||
| Male | 34/58 (58·6%) | 29/46 (63·0%%) | 18/30 (60·0%) | |||
| BMI, kg/m2 | 30·8 (26·2 - 36·4) | 30·6 (26·6 - 35·6) | 27·3 (23·1 - 35·1) | 0·17b | 0·19b | 0·91b |
| Black/Asian and minority ethnic groups | 13/58 (22·4%) | 10/46 (21·7%) | 1/30 (3·3 %) | 0·03c | 0·04c | 1·00a |
| Current/Ex-smoker | 20/58 (34·5%) | 17/46 (37·0%) | 7/30 (23·3%) | 0·34c | 0·31c | 0·84a |
| Type 1 Diabetes | 1/58 (1·7%) | 1/46 (2·2%) | 0/30 (0·0%) | 1·00c | 1·00c | 1·00c |
| Type 2 Diabetes | 8/58 (13·8%) | 7/46 (15·2%) | 3/30 (10·0%) | 0·74c | 0·73c | 1·00a |
| Hypertension | 22/58 (37·9%) | 17/46 (37·0%) | 9/30 (30·0%) | 0·49c | 0·62c | 1·00a |
| Coronary artery disease | 2/58 (3·4%) | 1/46 (2·2%) | 0/30 (0·0%) | 0·55c | 1·00c | 1·00c |
| Cerebrovascular Disease | 1/58 (1·7%) | 0/46 (0·0%) | 0/30 (0·0%) | 1·00c | 1·00c | 1·00c |
| Asthma | 20/58 (34·5%) | 17/46 (37·0%) | 6/30 (20·0%) | 0·22c | 0·13c | 0·84a |
| COPD | 3/58 (5·2%) | 2/46 (4·3%) | 0/30 (0·0%) | 0·55c | 0·51c | 1·00c |
| Previous cancer | 2/58 (3·4%) | 2/46 (4·3%) | 3/30 (10·0%) | 0·33c | 0·38c | 1·00c |
| Depression | 3/58 (5·2%) | 3/46 (6·5%) | 1/30 (3·3%) | 1·00c | 1·00c | 1·00c |
| Admission details | ||||||
| Median length of stay, days | 8·5 (5·0 - 17·0) | 9·0 (5·0 - 17·5) | ·· | ·· | ·· | 0·85b |
| Readmitted | 10/58 (17·2%) | 9/46 (19·6%) | ·· | ·· | ·· | 0·48a |
| Required ITU admission | 21/58 (36·2%) | 17/46 (37·0%) | ·· | ·· | ·· | 0·55a |
| qSOFA | ||||||
| 0 | 17/58 (29·3%) | 15/46 (32·6%) | ·· | ·· | ·· | 0·94d |
| 1 | 38/58 (65·5%) | 29/46 (63·0%) | ·· | ·· | ·· | |
| 2 | 3/58 (5·2%) | 2/46 (4·3%) | ·· | ·· | ·· | |
| 3 | 0/58 (0·0%) | 0/46 (0·0%) | ·· | ·· | ·· | |
| Ordinal scale for clinical improvement (WHO) | ||||||
| 1 | 0/58 (0·0%) | 0/46 (0.0%) | ·· | ·· | ·· | 1·00d |
| 2 | 4/58 (6·9%) | 3/46 (6·5%) | ·· | ·· | ·· | |
| 3 | 22/58 (37·9%) | 16/46 (34·8%) | ·· | ·· | ·· | |
| 4 | 5/58 (8·6%) | 4/46 (8·7%) | ·· | ·· | ·· | |
| 5 | 15/58 (25·9%) | 12/46 (26·1%) | ·· | ·· | ·· | |
| 6 | 7/58 (12·1%) | 6/46 (13·0%) | ·· | ·· | ·· | |
| 7 | 5/58 (8·6%) | 5/46 (10·9%) | ·· | ·· | ·· | |
| Signs and symptoms on admission | ||||||
| Fever | 51/58 (87·9%) | 40/46 (87·0%) | ·· | ·· | ·· | 0·56a |
| Malaise | 51/58 (87·9%) | 41/46 (89·1%) | ·· | ·· | ·· | 0·55a |
| Shortness of breath | 51/58 (87·9%) | 41/46 (89·1%) | ·· | ·· | ·· | 0·55a |
| Cough | 35/58 (60·3%) | 26/46 (56·5%) | ·· | ·· | ·· | 0·42a |
| Dysgeusia | 29/58 (50·0%) | 21/46 (45·7%) | ·· | ·· | ·· | 0·70a |
| Anosmia | 26/58 (44·8%) | 20/46 (43·5%) | ·· | ·· | ·· | 1·00a |
| Diarrhoea | 17/58 (29·3%) | 13/46 (28·3%) | ·· | ·· | ·· | 1·00a |
| Chest pain | 16/58 (27·6%) | 13/46 (28·3%) | ·· | ·· | ·· | 1·00a |
| Headache | 13/58 (22·4%) | 12/46 (26·1%) | ·· | ·· | ·· | 0·82a |
| Vomiting | 9/58 (15·5%) | 6/46 (13·0%) | ·· | ·· | ·· | 0·79a |
| Treatment | ||||||
| Oxygen replacement | 54/58 (93·1%) | 43/46 (93·5%) | ·· | ·· | ·· | 1·00c |
| Nasal cannula | 14/58 (24·1%) | 10/46 (21·7%) | ·· | ·· | ·· | 1·00d |
| Simple face mask | 7/58 (12·1%) | 5/46 (10·9%) | ·· | ·· | ·· | |
| Venturi face mask | 6/58 (10·3%) | 5/46 (10·9%) | ·· | ·· | ·· | |
| High flow oxygen delivery | 7/58 (12·1%) | 5/46 (10·9%) | ·· | ·· | ·· | |
| CPAP | 8/58 (13·8%) | 7/46 (15·2%) | ·· | ·· | ·· | |
| Intubation | 12/58 (20·7%) | 11/46 (23·9%) | ·· | ·· | ·· | |
| ECMO | 0/58 (0%) | 0/46 (0.0%) | ·· | ·· | ·· | |
| Inotropic support | 4/58 (6·9%) | 4/46 (8·7%) | ·· | ·· | ·· | 0·73c |
| Renal replacement therapy | 2/58 (3·4%) | 2/46 (4·3%) | ·· | ·· | ·· | 1·00c |
| Antibiotics | 57/58 (98·3%) | 45/46 (97·8%) | ·· | ·· | ·· | 1·00c |
| Antivirals | 4/58 (6·9%) | 2/46 (4·3%) | ·· | ·· | ·· | 0·69c |
| Steroids | 16/58 (27·6%) | 14/46 (30·4%) | ·· | ·· | ·· | 0·83a |
| Acute organ injury | ||||||
| Acute liver injurye | 18/58 (31·0%) | 18/46 (39·1%) | ·· | ·· | ·· | 0·41a |
| Acute kidney injuryf | 6/58 (10·3%) | 6/46 (13·0%) | ·· | ·· | ·· | 0·76a |
| Acute cardiac injuryg | 3/58 (5·2%) | 0/46 (0.0%) | ·· | ·· | ·· | 0·25c |
| Pulmonary embolism | 7/58 (12·1%) | 6/46 (13·0%) | ·· | ·· | ·· | 1·00a |
| Central | 1/58 (1·7%) | 0/46 (0.0% | ·· | ·· | ·· | 1·00c |
| Peripheral | 6/58 (10·3%) | 6/46 (13·0%) | ·· | ·· | ·· | 0·76a |
Data are mean (SD), median (IQR) and n/N (%), where N is the total number of participants with available data. P-values from independent Student's t-test, Chi-square (a), Mann-Whitney U test (b), Fisher's exact test (c), or Fisher-Freeman-Halton exact test (d), with bold values highlighting statistical significance. 2-3m = Two to three months. 6m = Six months. COPD = Chronic obstructive pulmonary disease. ITU = Intensive treatment unit. qSOFA = Quick sequential organ failure assessment. CPAP = Continuous positive airway pressure. ECMO = Extracorporeal membrane oxygenation. WHO = World health organization. e defined as blood levels of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) above 3x the upper reference limit (>135 IU/L or >126 IU/L, respectively), alkaline phosphatase or gamma-glutamyltransferase above 2x the upper reference limit (>260 IU/L or >80 IU/L, respectively). f defined as an increase in serum creatinine of at least 26 umol/L within 48 hours, or 1·5 to 2-fold increase from baseline. g defined as an acute rise in hypersensitive troponin I above the 99th percentile upper reference limit (>34 ng/L). Control subjects were matched for co-morbidities as closely as possible.
Of the 58 patients recruited, 46 (79%) returned for follow-up assessments. The mean age of patients was 55±13 years. Thirty-four (59%) were men (Table 1). Thirteen (22%) belonged to Black (7/13) and Asian (6/13) ethnic groups. Twenty (34%) patients required non-invasive ventilation or intubation, and 16 (28%) received steroids as part of their care (median duration 5 days, IQR 4-10 days). The median duration of hospitalization was 9 days (IQR 5-17). In all patients, readmission was due to increased breathlessness secondary to progression of COVID-19, within a week of the initial admission. The first assessment took place at a median interval of 2·3 months (IQR 2·1–2·5) from disease onset and second took place at 6·0 months (IQR 6·0 – 6·8).
On admission, all patients had a raised CRP (>10mg/L), 47% had lymphopenia, and 21% were anaemic. By 6 months, CRP was raised in 13%, compared to none in controls (P=0·076), lymphocyte count normalized, and the proportion of those with anaemia was comparable to controls (11% versus 13%, P=1·0) (Table 2).
Table 2.
Blood test results and symptom prevalence for patients with COVID-19 and controls.
| COVID-19 (admission) (N=58) | COVID-19, 2-3m (N=58) | COVID-19, 6m (N=46) | Controls (N=30) |
P-values |
|||
|---|---|---|---|---|---|---|---|
| 2-3m vs Controls | 6m vs Controls | 2-3m vs 6m | |||||
| Haematology and Coagulation | |||||||
| White cell count, x109 / L | 6·5 (5·0 - 8·1) | 6·5 (1·8) | 6·4 (2·1) | 6·7 (1·6) | 0·72 | 0·24a | 0·072b |
| <4 | 6/58 (10·3%) | 5/57 (8·8%) | 5/46 (10·9%) | 0/30 (0·0%) | 0·16c | 0·054d | 0·73e |
| 4-11 | 45/58 (77·6%) | 52/57 (91·2%) | 39/46 (84·8%) | 30/30 (100%) | |||
| >11 | 7/58 (12·1%) | 0/57 (0·0%) | 2/46 (4·3%) | 0/30 (0·0%) | |||
| Neutrophil count, x109 / L | 5·2 (3·5 - 6·6) | 3·6 (2·9 - 4·6) | 3·4 (2·8 - 4·5) | 3·9 (2·8 - 4·3) | 0·65a | 0·50a | 0·70b |
| Lymphocyte count, x109 / L | 0·9 (0·7 - 1·3) | 1·8 (1·6 - 2·3) | 1·7 (1·4 - 2) | 1·9 (1·6 - 2·5) | 0·91a | 0·016 | 0·002b |
| <1·0 | 27/58 (46·6%) | 0/57 (0·0%) | 0/46 (0·0%) | 0/30 (0·0%) | |||
| Haemoglobin, g/L | 141·0 (125·5 - 150·5) | 135·4 (13·2) | 140·2 (14·7) | 139·0 (14·4) | 0·25 | 0·65 | 0·008f |
| <120 (females)/<130 (males) | 12/58 (20·6%) | 8/57 (14·0%) | 5/46 (10·9%) | 4/30 (13·3%) | 1·00c | 0·73c | 1·00g |
| Platelet count, x109 / L | 207·5 (168·8 - 259·5) | 261·0 (213·5 - 285·5) | 243·5 (213·0 - 267·3) | 269·0 (220·0 - 292·0) | 0·63a | 0·24 | 0·0002b |
| <100 | 1/58 (1·7%) | 0/57 (0·0%) | 0/46 (0·0%) | 0/30 (0·0%) | |||
| D-dimer, µg/L | 780·0 (636·0 - 1490·0) | 418·0 (253·8 - 829·3) | 390·0 (255·0 - 625·0) | 337·0 (227·0 - 498·75) | 0·054a | 0·23a | 0·003b |
| Hepatic panel | |||||||
| Total bilirubin, mmol/L | 10·0 (7·0 - 13·8) | 10·0 (6·8 - 14·0) | 10·5 (7·0 - 14·3) | 8·0 (7·0 - 11·5) | 0·51a | 0·17a | 0·17b |
| ALT, IU/L | 34·0 (22·3 - 62·8) | 23·5 (18·8 - 39·0) | 24·0 (18·8 - 37·0) | 23·5 (16·0 - 28·0) | 0·19a | 0·20a | 0·63b |
| >135 IU/L (>3xULN) | 4/56 (7·1%) | 1/58 (1·7%) | 0/46 (0·0%) | 0/30 (0·0%) | |||
| Alk Phos, IU/L | ·· | 72·0 (60·0 - 85·5) | 69·0 (54·8 - 83·0) | 65·5 (55·8 - 80·3) | 0·21a | 0·46a | 0·20b |
| >260 IU/L (>2xULN) | ·· | 0/58 (0·0%) | 0/46 (0·0%) | 0/30 (0·0%) | |||
| AST, IU/L | ·· | 23·0 (18·0 - 28·0) | 21·0 (18·0 - 26·0) | 21·0 (18·0 - 27·0) | 0·36a | 0·87a | 0·07b |
| >126 IU/L (>3xULN) | ·· | 0/55 (0·0%) | 0/46 (0·0%) | 0/25 (0·0%) | |||
| GGT, IU/L | ·· | 33·0 (21·8 - 52·3) | 30·5 (22·0 - 42·3) | 29·0 (18·5 - 47·5) | 0·25a | 0·74a | 0·002b |
| >80 IU/L (>2xULN) | ·· | 6/54 (11·1%) | 1/46 (2·2%) | 1/25 (4·0%) | 0·42c | ||
| Renal function and electrolytes | |||||||
| Potassium, mmol/L | 3·8 (3·7 - 4·1) | 3·9 (0·3) | 3·9 (0·3) | 3·9 (0·3) | 0·92 | 0·23 | 0·55f |
| Sodium, mmol/L | 136·0 (2·9) | 141·0 (139·0 - 141·3) | 141·0 (139·0 - 142·0) | 140·0 (139·0 - 141·0) | 0·12a | 0·050a | 0·11b |
| Creatinine, umol/L | 75·5 (69·0 - 91·0) | 69·5 (60·0 - 79·3) | 74·5 (64·8 - 86·0) | 79·0 (63·0 - 89·0) | 0·16a | 0·64a | 0·012b |
| ≤133 | 55/58 (94·8%) | 57/58 (98·3%) | 44/46 (95·7%) | 30/30 (100%) | |||
| >133 | 3/58 (5·2%) | 1/58 (1·7%) | 2/46 (4·3%) | 0/30 (0%) | |||
| eGFR, ml/min/1·73m2 | |||||||
| ≥90 | 31/58 (53·4%) | 38/58 (65·5%) | 26/46 (56·5%) | 17/30 (56·7%) | 0·53d | 0·74d | 0·22e |
| 60-89 | 21/58 (36·2%) | 17/58 (29·3%) | 18/46 (39·1%) | 13/30 (43·3%) | |||
| 45-59 | 3/58 (5·2%) | 1/58 (1·7%) | 0/46 (0·0%) | 0/30 (0·0%) | |||
| 30-44 | 2/58 (3·4%) | 2/58 (3·4%) | 2/46 (4·3%) | 0/30 (0·0%) | |||
| 15-29 | 1/58 (1·7%) | 0/58 (0·0%) | 0/46 (0·0%) | 0/30 (0·0%) | |||
| <15 | 0/58 (0·0%) | 0/58 (0·0%) | 0/46 (0·0%) | 0/30 (0·0%) | |||
| Inflammatory markers | |||||||
| C-reactive protein, mg/L | 119·1 (75·9 - 185·5) | 2·0 (0·9 - 5·0) | 1·7 (0·9 - 5·6) | 1·2 (0·7 - 2·6) | 0·058a | 0·23a | 0·98b |
| >10 | 58/58 (100%) | 4/58 (6·9%) | 6/46 (13·0%) | 0/30 (0·0%) | 0·29c | 0·076c | 0·45g |
| Procalcitonin, ug/L | ·· | 0·020 (0·020 - 0·040) | 0·020 (0·010 - 0·030) | 0·02 (0·020 - 0·030) | 0·80a | 0·22a | 0·083b |
| Heart failure, cardiac injury | |||||||
| NT-proBNP, ng/L | ·· | 56·8 (32·3 - 113·6) | 56·3 (31·2 - 98·3) | 48·1 (23·0 - 88·4) | 0·22a | 0·50a | 0·20b |
| ≥125 | 11/56 (19·6%) | 8/46 (17·4%) | 3/28 (10·7%) | 0·37c | 0·52c | 0·75g | |
| Troponin I, ng/L | ·· | 2·0 (2·0 - 3·0) | 2·0 (2·0 - 4·0) | 2·0 (2·0 - 3·0) | 0·49a | 0·27a | 0·14b |
| >34 | 0/58 (0·0%) | 0/46 (0·0%) | 0/27 (0·0%) | ||||
Data are median (IQR) for non-parametric data and mean (SD) for parametric data, and n/N (%), where N is the total number of participants with available data. P-values comparing COVID-19 groups (post-discharge) and control group are from independent t-test, Mann-Whitney U test (a), Wilcoxon Signed Ranks test (b), Fisher's exact test (c), Fisher-Freeman-Halton exact test (d), Stuart Maxwell test (e), paired t-test (f) or McNemar (⁎⁎) test, with bold values highlighting statistical significance. 2-3m = Two to three months. 6m = Six months. ALT = Alanine aminotransferase. Alk Phos = Alkaline phosphatase. AST = Aspartate aminotransferase. GGT = Gamma-glutamyl transferase. eGFR = Estimated Glomerular Filtration Rate. CRP = C-reactive protein. NT-proBNP = N-terminal pro-brain natriuretic peptide.
As previously reported, troponin on admission (measured in 38 patients) was abnormal in three (5%) patients. By 2-3 and 6 months, all patients had troponin measured and none had elevated high-sensitivity troponin levels (>34ng/L).
Only four patients had NT-proBNP measured during admission. At 2-3 months, all patients had NT proBNP measured and NT proBNP was elevated in 11 (20%), reducing to eight (17%) patients at 6 months versus 11% in controls (P=0·52).
4.1. Electrocardiography
ECG analysis revealed atrial fibrillation in one patient at both assessments (2-3 months and 6 months), with all other study participants (both patients and controls) demonstrating sinus rhythm. The prevalence of bundle branch block, ST-segment elevation/depression and T wave inversion did not differ between patients (on both visits) and controls (P>0·05 for all variables).
4.2. Symptom burden
Symptom prevalence in patients and controls are listed in Table 3. As a whole, 98% had one or more symptoms (cardiopulmonary and non-cardiopulmonary) at 2-3 months from infection, reducing to 89% by 6 months. The prevalence of cardiopulmonary symptoms (chest pain, palpitations, syncope, dyspnoea or dizziness) in patients was 83% at 2-3 months and dropped to 52% at 6 months (P=0·0001). At 6 months, symptoms of breathlessness (MRC) and fatigue (FSS) were worse in patients than controls (MRC grade ≥2: 57% vs 10%, P<0·0001; Mean FSS ≥4: 44% vs 17%, P=0·023, Table 3); statistical significance was maintained after adjusting for a history of mild chronic lung disease.
Table 3.
Symptom prevalence, Fatigue Severity Score and MRC dyspnoea scale in patients at follow-up and controls.
| COVID-19, 2-3m | COVID-19, 6m | Controls |
P-values |
|||
|---|---|---|---|---|---|---|
| 2-3m vs Controls | 6m vs Controls | 2-3m vs 6m | ||||
| Symptoms at follow-up | ||||||
| Stomach Pain | 12/57 (21·1%) | 12/46 (26·1%) | 5/30 (16·7%) | 0·78a | 0·41a | 1·00b |
| Back Pain | 38/57 (66·7%) | 24/46 (52·2%) | 11/30 (36·7%) | 0·012a | 0·24a | 0·33b |
| Pain in the arms, legs or joints | 45/57 (78·9%) | 27/46 (58·7%) | 17/30 (56·7%) | 0·045a | 1·00a | 0·077b |
| Feeling tired or too little energy | 49/57 (86·0%) | 28/46 (60·9%) | 16/30 (53·3%) | 0·002a | 0·64a | 0·004b |
| Trouble falling asleep or sleeping too much | 42/57 (73·7%) | 29/46 (63·0%) | 16/30 (53·3%) | 0·093a | 0·48a | 0·29b |
| Headaches | 24/57 (42·1%) | 16/46 (34·8%) | 13/30 (43·3%) | 1·00a | 0·48a | 0·63b |
| Constipation or diarrhoea | 17/57 (29·8%) | 12/46 (26·1%) | 6/30 (20·0%) | 0·44a | 0·59a | 1·00b |
| Chest pain | 18/57 (31·6%) | 8/46 (17·4%) | 1/30 (3·3%) | 0·002c | 0·079c | 0·11b |
| Dizziness | 19/57 (33·3%) | 13/46 (28·3%) | 5/30 (16·7%) | 0·13a | 0·283a | 1·00b |
| Syncope | 5/57 (8·8%) | 1/46 (2·2%) | 1/30 (3·3%) | 0·66c | 1·00c | 0·13b |
| Palpitations | 23/57 (40·4%) | 13/46 (28·3%) | 6/30 (20·0%) | 0·093a | 0·59a | 0·092b |
| Shortness of breath | 45/57 (78·9%) | 20/46 (43·5%) | 3/30 (10·0%) | <0·0001c | 0·002c | <0·0001b |
| Any of the above | 56/57 (98·2%) | 41/46 (89·1%) | 26/30 (86·7%) | 0·031c | 0·73c | 0·063b |
| Presence of cardiopulmonary symptoms | 47/57 (82·5%) | 24/46(52·2%) | 10/30 (33·3%) | <0·0001c | 0·16c | 0·0001b |
| Fatigue Severity Scale12 | ||||||
| Median (IQR) | 34·0 (18·0-49·0) | 29·0 (14·0- 44·5) | 17·0 (11·0-24·0) | 0·001d | 0·035d | 0·001e |
| Mean FSS ≥4 | 30/55 (54·5%) | 20/45 (44·4%) | 5/29 (17·2%) | 0·001c | 0·023c | 0·34b |
| Medical Research Council Dyspnoea Scale11 | ||||||
| MRC grade 2 - 5 | 36/56 (64·3%) | 26/46 (56·5%) | 3/29 (10·3%) | <0·0001c | <0·0001c | 0·42b |
Data are n/N (%), where N is the total number of participants with available data. P-values are from Chi-square (a), McNemar (b) test, Fisher's exact test (c), Mann-Whitney U test (d) or Wilcoxon Signed Ranks test (e), with bold values highlighting statistical significance. Cardiopulmonary symptoms defined as any of chest pain, dizziness, syncope, palpitations or shortness of breath. 2-3m = Two to three months. 6m = Six months. MRC = Medical research council. FSS = Fatigue severity scale.
4.3. Serial Cardiac Imaging
Left ventricular (LV) volumes, mass, and function (including diastolic strain rate) were not different between patients (at 2-3 months and 6 months) and controls (Table 4). At 6 months, two (4·5%) patients had an LV ejection fraction (LVEF) just below the cut-off of 50% (49·6 and 49·8%). Those with severe illness had lower LVEF at 6 months than other patients (60·8±6·6% vs 64·8±6·5%, P=0·049). None of the patients had a history of pre-existing cardiac failure.
Table 4.
Cardiopulmonary MRI parameters in patients and controls.
| COVID-19, 2-3m | COVID-19, 6m | Controls |
P-values |
|||
|---|---|---|---|---|---|---|
| 2-3m vs Controls | 6m vs Controls | 2-3m vs 6m | ||||
| Lung MRI | ||||||
| Lung parenchymal abnormalities, % | 32/53 (60·4%) | 30/44 (68·2%) | 3/28 (10·7%) | <0·0001a | <0·0001a | 0·344b |
| 0% | 21/53 (39·6%) | 14/44 (31·8%) | 25/28 (89·3%) | 0·0003c | <0·0001c | 0·005d |
| 1-25% | 3/53 (5·7%) | 21/44 (47·7%) | 0/28 (0·0%) | |||
| 26 - 50% | 8/53 (15·1%) | 5/44 (11·4%) | 2/28(7·1%) | |||
| 51 - 75% | 9/53 (17·0%) | 4/44 (9·1%) | 0/28 (0·0%) | |||
| >75% | 12/53 (22·6%) | 0/44 (0·0%) | 1/28 (3·6%) | |||
| Cardiac MRI | ||||||
| Left ventricular cine analysis | ||||||
| End-diastolic volume, mls | 143·8 (127·3 - 165·9) | 151·1 (125·0 - 183·4) | 153·3 (124·5 - 178·5) | 0·59e | 0·78 | 0·21f |
| End-diastolic volume (indexed), mls/m2 | 73·3 (64·5 - 83·5) | 76·7 (66·4 - 86·6) | 75·6 (63·4 - 87·5) | 0·51e | 0·90 | 0·59f |
| End-systolic volume, mls | 53·1 (41·5 - 71·7) | 54·6 (44·3 - 71·0) | 53·1 (47·7 - 70·3) | 0·81e | 0·90e | 0·31f |
| Mass (diastole), g | 116·1 (100·1 - 135·1) | 119·5 (98·8 - 134·0) | 107·3 (84·3 - 138·3) | 0·39e | 0·25 | 0·25f |
| Mass (indexed), g/m2 | 58·9 (49·8 - 66·2) | 57·0 (50·2 - 65·2) | 53·8 (48·6 - 63·6) | 0·21e | 0·37e | 0·15f |
| Stroke volume, mls | 89·6 (79·5 - 104·7) | 94·2 (80·5 - 109·1) | 95·0 (78·4 - 116·5) | 0·59e | 1·00 | 0·058g |
| Ejection fraction, % | 63·0 (7·7) | 62·7 (6·8) | 63·6 (6·32) | 0·70 | 0·58 | 0·27g |
| Left Ventricular Diastolic Strain Analysis | ||||||
| Global Longitudinal Strain Rate | 0·83 (0·21) | 0·81 (0·16) | 0·78 (0·15) | 0·30 | 0·53 | 0·24aa |
| Right ventricular cine analysis | ||||||
| End-diastolic volume, mls | 164·4 (36·6) | 160·1 (40·4) | 169·3 (46·5) | 0·61 | 0·38 | 0·023g |
| End-diastolic volume (indexed), mls/m2 | 81·8 (14·0) | 78·8 (15·8) | 84·3 (18·5) | 0·51 | 0·18 | 0·005g |
| End-systolic volume, mls | 70·4 (23·6) | 65·1 (23·0) | 72·7 (24·2) | 0·69 | 0·19 | 0·0001g |
| Mass, g | 28·8 (25·8 - 35·5) | 32·6 (28·8 - 39·8) | 33·2 (23·7 - 41·8) | 0·26e | 0·88e | 0·13f |
| Mass (indexed), g/m2 | 14·4 (12·6 - 17·2) | 16·4 (14·4 - 19·1) | 16·7 (13·9 - 19·3) | 0·19e | 0·90e | 0·31f |
| Stroke volume, mls | 94·0 (19·3) | 95·1 (20·9) | 96·6 (25·6) | 0·61 | 0·78 | 0·68g |
| Ejection fraction, % | 57·9 (7·8) | 60·2 (6·2) | 57·6 (6·0) | 0·85 | 0·085 | 0·0003 |
| T1 and T2 map analysis | ||||||
| Native T1 (basal myocardium), ms | 1179·7 (34·4) | 1152·6 (37·3) | 1149·3 (24) | 0·0001 | 0·65 | <0·0001g |
| >1197 ms (>2SD from control mean) | 13/50 (26·0%) | 4/44 (9·1%) | 1/28 (3·6%) | 0·015a | 0·64a | 0·065b |
| Native T1 (mid myocardium), ms | 1173·1 (33·6) | 1145·6 (41·2) | 1150·2 (32·4) | 0·004 | 0·62 | <0·0001g |
| >1215 ms (>2SD from control mean) | 4/51 (7·8%) | 1/43 (2·3%) | 0/28 (0%) | 0·29a | 1·00a | 0·38b |
| Native T1 (apical myocardium), ms | 1177·4 (44·7) | 1153·8 (45·5) | 1168·3 (53·2) | 0·42 | 0·22 | 0·001g |
| >1275 ms (>2SD from control mean) | 1/50 (2·0%) | 1/43 (2·3%) | 1/28 (3·6%) | 1·00a | 1·00a | 1·00b |
| ECV (basal myocardium), % | 30·4 (28·3 - 31·3) | 27·4 (25·9 - 30·0) | 28·3 (26·8 - 31·5) | 0·12 | 0·19e | 0·001f |
| >34.52% (>2SD from control mean) | 1/35 (2·9%) | 2/36 (5·6%) | 0/21 (0·0%) | 1·00a | 0·53a | 1·00b |
| ECV (mid myocardium), % | 30·1 (27·2 - 31·4) | 27·8 (26·1 - 30·8) | 29·4 (27·1 - 30·7) | 0·41e | 0·35 | 0·030f |
| >35.87% (>2SD from control mean) | 0/37 (0·0%) | 0/42 (0·0%) | 1/23 (4·3%) | 0·38a | 0·35a | |
| ECV (apical myocardium), % | 28·7 (27·0 - 31·6) | 28·8 (27·0 - 30·5) | 29·7 (27·2 - 31·5) | 0·51e | 0·24e | 0·32g |
| >37.87% (>2SD from control mean) | 1/40 (2·5%) | 0/36 (0·0%) | 1/23 (4·3%) | 1·00a | 0·39a | 1·00b |
| T2 (basal myocardium), ms | 41·7 (2·2) | 41·4 (2·1) | 41·6 (2·2) | 0·80 | 0·80 | 0·71g |
| >46 ms (>2SD from control mean) | 3/50 (6·0%) | 1/43 (2·3%) | 1/28 (3·6%) | 1·00a | 1·00a | 1·00b |
| T2 (mid myocardium), ms | 41·8 (2·2) | 41·4 (1·8) | 41·1 (2·3) | 0·21 | 0·53 | 0·50g |
| >46 ms (>2SD from control mean) | 1/50 (2·0%) | 1/42 (2·4%) | 1/28 (3·6%) | 1·00a | 1·00a | 1·00b |
| T2 (apical myocardium), ms | 43·5 (3·0) | 42·9 (2·4) | 43·7 (3·5) | 0·81e | 0·33 | 0·51f |
| >51ms (>2SD from control mean) | 1/50 (2·0%) | 0/43 (0·0%) | 1/28 (3·6%) | 1·00a | 0·39a | 1·00b |
| Late gadolinium enhancement analysis | ||||||
| % LGE volume enhancement | 0·8 (0·5 - 1·9) | 0·7 (0·1 - 2·2) | 0·6 (0·3 - 1) | 0·023e | 0·62e | 0·91f |
| Myocarditis pattern | 6/52 (11·5%) | 5/43 (11·6%) | 2/28 (7·1%) | |||
| Myocardial infarction | 1/52 (1·9%) | 0/43 (0·0%) | 0/28 (0·0%) | |||
| LV/RV insertion point | 7/52 (13·5%) | 5/43 (11·6%) | 1/28 (3·6%) | |||
| Mixed | 0/52 (0·0%) | 0/43 (0·0%) | 0/28 (0·0%) | |||
| Other | 0/52 (0·0%) | 0/43 (0·0%) | 0/28 (0·0%) | |||
| Pericardial effusion >10mm | 1/52 (1·9%) | 0/43 (0·0%) | 0/52 (0·0%) | |||
Data are median (IQR) for non-parametric data and mean (SD) for parametric data, and n/N (%), where N is the total number of participants with available data. P-values are from independent t-test, Fisher's exact test (a), McNemar (b) test, Fisher-Freeman-Halton exact test (c), Stuart-Maxwell test (d), Mann-Whitney U test (e), Wilcoxon Signed Ranks test (f), or paired t-test (aa), with bold values highlighting statistical significance. 2-3m = Two to three months. 6m = Six months. MRI = Magnetic resonance imaging. ECV = Extracellular volume. LGE = Late gadolinium enhancement.
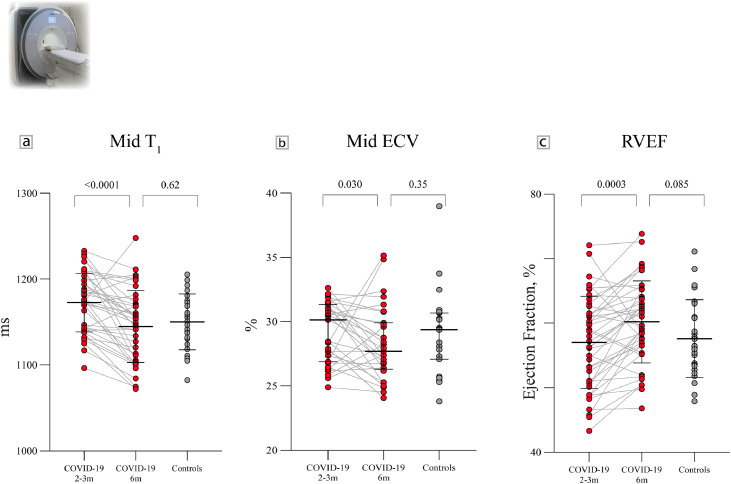
Right ventricular (RV) volumes, mass and function did not differ between patients (at 2-3 months and 6 months) and controls (Table 4). In patients, indexed RV end-diastolic volume decreased (mean difference -4·3 mls/m2, P=0·005) and function (RVEF) increased (mean difference +3·2%, P=0·0003) from 2-3 months to 6 months (Figure 1). At 6 months, RVEF tended to be lower in patients with severe illness (58·5±5·1% vs 62·1±6·9%, P=0·055).
Figure 1.
Serial CMR findings in previously hospitalised COVID-19 patients and controls. A: Mid ventricular native T1 (mean + SD) in patients at 2-3 months was higher than controls, and normalized by 6 months. B: Mid ventricular extracellular volume fraction (ECV, median + IQR) in patients at 2-3 months was comparable to controls, but decreased in patients by 6 months. C: Right ventricular ejection fraction (mean + SD) in patients at 2-3 months was comparable to controls, and increased by 6 months. P-values are for group differences (COVID-19 2-3 months vs COVID-19 6 months and COVID-19 6 months vs controls).
Basal and mid-ventricular native T1 (a biomarker sensitive to inflammation) values were higher in patients than controls (Table 4). By 6 months, myocardial native T1 decreased and was no longer statistically different from control T1 (Table 4; Figure 1). Native T2 (a biomarker sensitive to oedema) was not significantly different between patients and controls at both time points.
Extracellular volume fraction (ECV, a biomarker sensitive to diffuse fibrosis) did not differ between patients and controls. In patients, slice-averaged ECV decreased (mean difference -1·13%, P=0·005) from 2-3 months to 6 months post-infection.
LGE (measured as % of myocardial volume, a biomarker of focal fibrosis) was slightly higher in patients than controls at 2-3 months (P=0·023). By 6 months, this did not differ from controls (P=0·62). There were six patients with LGE in a myocarditis pattern and one with evidence of a subendocardial infarction (elevated troponin during admission). None of the patients satisfied the updated Lake Louise criteria [18] for active myocarditis (increased native T1/LGE and increased native T2) at 6 months.
4.4. Lung imaging and functional assessment
At 2-3 months, 60% of patients had lung parenchymal abnormalities, becoming less extensive (Table 4) with time, but were still more common compared to controls at 6 months (P<0·0001). Forty percent of patients had lung parenchymal abnormalities involving more than half the lungs at 2-3 months. This reduced to 9% by 6 months.
At 2-3 months, patients had lower FEV1 and FVC compared to controls but most values remained within the normal range (Table 5). At 6 months, FEV1 was no longer different from controls (P=0·10), whereas FVC remained slightly lower (P=0·024). Reduced gas transfer (DLCO <80% predicted) and reduced accessible lung volume (VA) were seen in 24 patients (52%). Reduced transfer coefficient for carbon monoxide (Kco) was present in six patients (13%). Patients with parenchymal abnormalities had lower DLco compared to those without (77% vs 91%, P=0·009). DLco was not significantly different in patients with severe illness at admission versus non-severe patients (77·4% vs 84·5%, P=0·15).
Table 5.
Spirometry and gas transfer testing results in patients at follow-up and controls.
| COVID-19, 2-3m | COVID-19, 6m | Controls |
P-values |
|||
|---|---|---|---|---|---|---|
| 2-3m vs Controls | 6m vs Controls | 2-3m vs 6m | ||||
| Spirometry | ||||||
| FVC, % predicted | 108·3 (22·8) | 119·2 (22·0) | 131·4 (21·8) | <0·0001 | 0·024 | <0·0001a |
| <80% | 7/56 (12·5%) | 0/46 (0·0%) | 0/28 (0·0%) | 0·090b | ·· | 0·016c |
| FEV 1, % predicted | 101·4 (19·7) | 110·7 (18·6) | 118·7 (22·1) | 0·0004 | 0·10 | <0·0001a |
| <80% | 6/56 (10·7%) | 1/46 (2·2%) | 1/28 (3·6%) | 0·42b | 1·00b | 0·063c |
| FEV1/FVC | 0·77 (0·73 - 0·80) | 0·76 (0·73 - 0·80) | 0·75 (0·70 - 0·78) | 0·027d | 0·24 | 0·051a |
| Peak expiratory flow, % predicted | 105·7 (27·7) | 108·8 (21·7) | 114·5 (24·7) | 0·16 | 0·31 | 0·74a |
| Gas Transfer | ||||||
| DLCO, % of predicted | ·· | 80·9 (16·9) | ·· | ·· | ·· | ·· |
| <80% | ·· | 24/46 (52·2%) | ·· | ·· | ·· | ·· |
| KCO, % of predicted | ·· | 101·8 (18·2) | ·· | ·· | ·· | ·· |
| <80% | ·· | 6/46 (13·0%) | ·· | ·· | ·· | ·· |
| Va, % of predicted | ·· | 79·9 (14·7) | ·· | ·· | ·· | ·· |
| <80% | ·· | 24/46 (52·2%) | ·· | ·· | ·· | ·· |
Data are median (IQR) for non-parametric data, mean (SD) for parametric data, and n/N (%), where N is the total number of participants with available data. P-values from independent t-test, paired t-test (a), Fisher's exact test (b), McNemar test (c) or Mann-Whitney U test (d), with bold values highlighting statistical significance. 2-3m = Two to three months. 6m = Six months. FVC = Forced vital capacity. FEV1 = Forced expiratory volume in 1 second. DLCO = Diffusion capacity for carbon monoxide. KCO = Transfer coefficient for carbon monoxide. Va = Alveolar volume.
4.5. Serial Cardiopulmonary Exercise Testing
As previously reported, patients had reduced peak oxygen consumption (V̇O2) at 2-3 months. By 6 months, this improved but was still reduced relative to controls (Table 6, Figure 2).
Table 6.
CPET parameters in patients at follow-up and controls.
| COVID-19, 2-3m | COVID-19, 6m | Controls |
P-values |
|||
|---|---|---|---|---|---|---|
| 2-3m vs Controls | 6m vs Controls | 2-3m vs 6m | ||||
| Cardiopulmonary exercise testing | ||||||
| Maximal tests performed | 26/51 (51·0%) | 31/42 (73·8%) | 23/27 (85·2%) | 0·003a | 0·37a | 0·057b |
| SpO2 at peak exercise, % | 95·0 (93·8 - 97·0) | 96·0 (95·0 - 97·0) | 96·0 (95·0 - 98·0) | 0·003c | 0·10c | 0·002d |
| <94% | 12/51 (23·5%) | 3/41 (7·3%) | 1/27 (3·7%) | 0·028a | 1·00a | 0·016b |
| V̇O2peak (all tests), mls/kg/min | 18·0 (14·4 – 21·9) | 20·5 (17·5 - 26·1) | 28·1 (22·1 – 34·0) | <0·001c | 0·001 | 0·001d |
| V̇O2max (maximal tests), mls/kg/min | 21·1 (16·1 – 27·9) | 22·7 (19·4 - 27·1) | 28·1 (22·1 – 34·5) | 0·012c | 0·044c | 0·006d |
| Anaerobic threshold, mls/kg/min | 9·7 (8·3 - 10·7) | 10·4 (9·0 - 12·2) | 11·9 (9·3 - 13·9) | 0·001c | 0·023c | 0·018d |
| V̇O2peak (all tests), % of predicted V̇O2max | 80·5 (23·1) | 93·3 (29·3) | 112·7 (27·0) | <0·0001 | 0·007 | 0·0001e |
| < 80% | 28/51 (54·9%) | 13/42 (31·0%) | 2/27(7·4%) | <0·0001a | 0·034a | 0·012b |
| V̇O2max (maximal tests), % of predicted | 95·5 (19·9) | 100·7 (27·1) | 112·3 (27·0) | 0·016 | 0·12 | 0·003e |
| <80% | 5/26 (19·2%) | 6/31 (19·4%) | 1/23 (4·3%) | 0·13a | 0·22a | 0·63b |
| Anaerobic threshold (% of predicted V̇O2max) | 40·7 (36·2 - 47·5) | 42·0 (39·0 - 51·6) | 46·8 (43·3 - 51·3) | 0·0005c | 0·041c | 0·030d |
| <40% of predicted V̇O2max | 20/48 (41·7%) | 14/40 (35·0%) | 0/27 (0·0%) | <0·0001a | 0·0004a | 0·55 |
| O2pulse, % of predicted max | 81·8 (18·2) | 90·2 (28·3) | 102·8 (20·8) | <0·0001 | 0·020c | 0·003d |
| O2pulse (maximal tests), % of predicted max | 91·4 (18·3) | 95·2 (2 6·5) | 103·3 (20·9) | 0·039 | 0·13c | 0·011e |
| Breathing reserve, % of predicted V̇Emax | 44·8 (15·3) | 42·4 (15·5) | 40·7 (11·0) | 0·22 | 0·62 | 0·71e |
| <20% | 3/51 (5·9%) | 2/42 (4·8%) | 1/27 (3·7%) | 1·00a | 1·00a | 1·00b |
| Breathing reserve (maximal tests), % of predicted V̇Emax | 34·9 (12·1) | 38·1 (12·6) | 38·9 (9·9) | 0·21 | 0·80 | 0·79e |
| HR recovery slope (maximal tests), bpm | 16·6 (7·1) | 22·2 (11·1) | 21·9 (7·5) | 0·018 | 0·67c | 0·001d |
| V̇E/V̇CO2 Slope | 33·4 (29·2 - 40·3) | 31·3 (28·6 - 34·5) | 28·2 (26·7 - 30·0) | <0·0001c | 0·002c | 0·033d |
| Oxygen Uptake Efficiency Slope | 1·9 (1·6 - 2·4) | 2·1 (1·7 - 2·8) | 2·7 (2·0 - 3·2) | 0·001c | 0·065c | 0·11d |
Data are median (IQR) for non-parametric data, mean (SD) for parametric data, and n/N (%), where N is the total number of participants with available data. P-values are from independent t-test, Fisher's exact test (a), McNemar (b) test, Mann-Whitney U test (c), Wilcoxon Signed Ranks test (d) or paired t-test (e), with bold values highlighting statistical significance. 2-3m = Two to three months. 6m = Six months. V̇O2 = oxygen consumption. V̇E/V̇CO2 = Ventilatory equivalent for carbon dioxide.
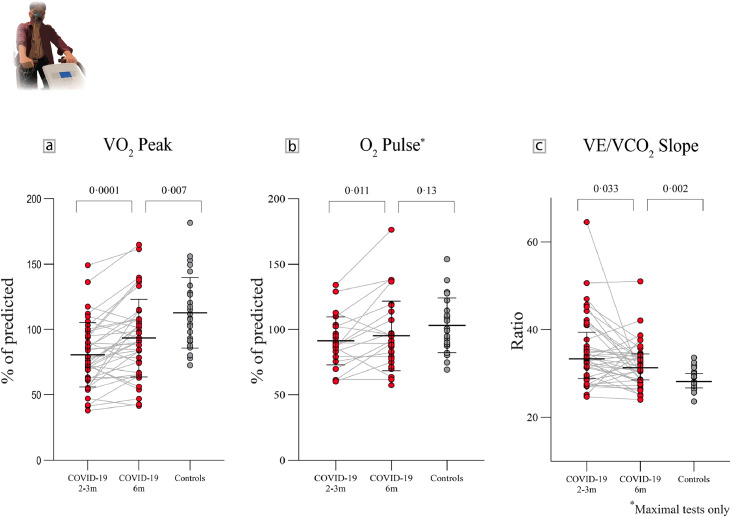
Figure 2.
Serial CPET assessments in previously hospitalised COVID-19 patients and controls. A: Peak oxygen consumption (V̇O2 peak, mean + SD) in patients improved from 2-3 months to 6 months, but remained lower than controls. B: Peak oxygen pulse (O2 pulse, mean + SD) in patients with maximal tests at 2-3 months was lower compared to controls. By 6 months, this improved and became comparable to controls. C: The ventilatory equivalent for carbon dioxide (V̇E/V̇CO2, median + IQR) slope in patients improved from 2-3 months to 6 months, but remained high versus controls. P-values are for group differences (COVID-19 2-3 months vs COVID-19 6 months and COVID-19 6 months vs controls).
Maximal test criteria consisted of a respiratory exchange ratio ≥ 1·1 and plateau in oxygen uptake [19]. At 2-3 months, 49% of patients had submaximal tests (versus 15% of controls, P=0·003). By 6 months, this prevalence reduced to 26% (P=0·37 for comparison with controls).
In those with a maximal test, maximal V̇O2 was lower in patients at 2-3 months but was no longer so by 6 months (P=0·12 for comparison with controls).
The ventilatory equivalent for carbon dioxide (V̇E/V̇CO2) slope, a marker of ventilatory efficiency, was abnormal in patients at 2-3 months and improved by 6 months (P=0·033). In spite of this, the V̇E/V̇CO2 slope remained borderline abnormal (median 31·3 (IQR 28·6-34·5)) versus controls (median 28·2 (IQR 26·7-30·0, P=0·002)). Reduced ventilatory efficiency had little effect on exercise capacity, with respiratory limitation (defined as a breathing reserve of less than 20% at peak exertion) only occurring in 6% and 5% of patients at 2-3 and 6 months, respectively. This did not differ from controls (4%, P=1·0).
At 2-3 months, oxygen (O2) pulse in maximal tests (a surrogate marker of exercise stroke volume, oxygen delivery and tissue oxygen extraction) was lower in patients versus controls and was accompanied by earlier attainment of the anaerobic threshold (AT). By 6 months, O2 pulse improved and became comparable to controls (95% of predicted vs 103% of predicted, P=0·13). Despite improvement in the AT, occurring later during exercise, it remained different from controls (42% of predicted V̇O2max vs 47% of predicted V̇O2max, P=0·041, Table 6).
The 13 patients with reduced V̇O2peak, 6 months post-infection, had lower serum creatine kinase levels (75 IU/L [47·5 – 133] vs 133 IU/L [70-210], P=0·039) and a shallower V̇O2/Work rate (WR) relationship (10·8 mls/min/watt [9·9 - 11·6] vs 11·6 mls/min/watt [11·0 – 12·4], P=0·035) compared to patients with normal oxygen consumption. Seven terminated exercise in the absence of any cardiorespiratory limitation (submaximal tests) due to fatigue, breathlessness and lower back/lower limb pain. Of the six patients with impaired exercise tolerance and a maximal test, despite reduced oxygen pulse seen in five patients and four having an early AT, none had significant anaemia, cardiac impairment on MRI, elevated NT-proBNP or reduced breathing reserve at peak exercise.
Heart rate recovery (HRR) in the first minute following exercise cessation was slower in patients compared to controls (16·6 vs 21·9 beats, P=0·018). By 6 months, HRR improved significantly (22·2 beats, P=0·001), and became comparable to controls (P=0·67). The severity of illness during admission was not associated with a reduction in peak or maximal oxygen consumption at 2-3 months and 6 months (P>0·20 for all comparisons).
4.6. Relationship between symptoms and cardiopulmonary health
At 6 months from infection, bivariate analysis and multivariate modelling showed that neither CMR (including diastolic strain rate) nor pulmonary function parameters, NT-proBNP, ECG abnormalities or CPET measures associated with cardiopulmonary symptoms (Figure 3) or breathlessness (Supplementary Material, p11). Longitudinal improvement in CMR and CPET parameters did not associate with improvement in cardiopulmonary symptoms from 2-3 months to 6 months (P>0·05). There was no correlation between the extent of lung abnormalities on MRI, lung function parameters (FEV1, FVC, FEV1/FVC, DLco) and breathlessness scores (Supplementary Material, p8). The dissociation between physiological measurements and symptoms were further highlighted by the fact that of the twenty patients who did not report significant breathlessness (MRC grade <2) at 6 months, 55% had abnormal gas transfer (DLco <80% predicted).
Figure 3.
Prevalence and determinants of cardiopulmonary symptoms (chest pain, palpitations, syncope, dyspnoea, or dizziness) among previously hospitalised COVID-19 patients. A: At 2-3 months, 83% of patients experienced at least one cardiopulmonary symptom. By 6 months, this improved to 52% and was comparable to controls. B: Forest plot depicts the odds ratio and 95% confidence intervals of having any cardiopulmonary symptom at 6 months given the changes on ECG, CMR, PFT, and CPET measures. An abnormal ECG was defined as rhythm abnormalities and/or the presence of bundle branch block, ST-segment elevation/depression or T wave inversion. Elevated NT-proBNP was defined as ≥125 ng/L. (OR - Odds ratio. CI - Confidence interval. ECG – Electrocardiogram. NT-proBNP - N-terminal pro b-type natriuretic peptide. LVEDVi - Left ventricular end-diastolic volume (indexed), mls/m2. LVESVi - Left ventricular end-systolic volume (indexed). LVSVi - Left ventricular stroke volume (indexed), mls/m2. RVEDVi - Right ventricular end-diastolic volume (indexed), mls/m2. RVESVi - Right ventricular end-systolic volume (indexed), mls/m2. RVSVi - Right ventricular stroke volume (indexed), mls/m2. LGE - Late gadolinium enhancement, %. FEV1 – Forced expiratory volume in 1 second, % of predicted. FVC – Forced vital capacity, % of predicted. DLco - Diffusing capacity for carbon monoxide, % of predicted. pVO2 - Peak oxygen consumption, % of predicted. VE/VCO2 - Ventilatory equivalent for carbon dioxide. O2 pulse - Oxygen pulse, % of predicted.)
5. Discussion
The main findings from our study are as follows: First, serial measures of cardiopulmonary health on CMR in moderate to severe COVID-19 improve over time. Second, exercise tolerance in patients improves at 6 months post-infection but remains abnormal in some when compared to controls, potentially due to symptomatic limitation and muscular fatigue. Third, by 6 months, more than half the patients remain symptomatic, and neither CMR nor pulmonary function or CPET measures associate with persistent symptom burden.
Since the start of the pandemic, several studies have harnessed the power of CMR to better understand the mechanisms underlying myocardial injury associated with COVID-19 [6,20]. Prevalence estimates of injury have varied due to differences in cohort characteristics and methodologies used. In the largest CMR follow-up study of patients with elevated troponin, Kotecha and colleagues observed that up to 49% of patients have evidence of either myocarditis or myocardial ischemia/infarction [20]. In contrast, similar-sized studies of younger athletes [21] and older individuals [6] with milder infections (predominantly non-hospitalised) have reported variable estimates of myocardial injury (ranging from 1·5% to 70%). The present study is unique to others in the literature, as we prospectively recruited hospitalised COVID-19 patients and risk factor matched controls (who served as our reference) and longitudinally evaluated changes in CMR myocardial tissue characteristics in patients. Here, we show that whilst there were some patients with abnormal myocardial native T1 (a marker of oedema and inflammation) at 2-3 months, native T1 normalized in the majority by 6 months and was accompanied by a decrease in extracellular volume. These findings highlight two important points. The first is that early tissue abnormalities on CMR are likely due to dynamic alterations in the extracellular environment (hyperaemia [22] or changes in extracellular proteins/matrix) influenced by circulating cytokines and importantly, not explained by comorbidities alone. This is in line with recent studies that have also demonstrated temporal improvement in inflammatory cytokines (IL-1, IL-2, IL-6, IL-18, TNF, IFNL1) in COVID-19 patients on serial assessments [23,24]. The second is that cardiac health is restored in the majority of patients by 6 months. Only two patients had borderline low LV function, RV parameters were normal, and there were no cases of active myocarditis (as per the updated Lake Louise criteria [18]). These findings are in keeping with the low prevalence (7%) of cardiac dysfunction (defined by levels of NT-proBNP) reported by a large UK-wide prospective follow-up study of post-hospitalised COVID-19 patients by Evans and colleagues [25].
A number of studies have also described diastolic dysfunction following COVID-19, both during admission and at follow-up [26], [27], [28]. However, patients with pre-existing cardiac conditions were included in these studies which makes it difficult to ascertain if diastolic dysfunction was specific to COVID-19 or an indicator of co-morbid status. In our study, only patients with mild co-morbidities were included and compared to an age, sex and risk-factor matched control group, and we did not see a significant difference in diastolic strain rate.
Six months following symptom onset, impaired gas transfer (as measured by DLCO) was the predominant abnormality seen on lung function testing. A high burden of gas transfer impairment accompanied by improvements on spirometry have been documented by others [5,29] and may be potentially secondary to abnormalities in pulmonary vascular homeostasis (dysfunctional pulmonary vasoconstriction [30] or thrombosis [31]) and persistent injury to the alveolar-capillary barrier [32]. Further studies are required to investigate whether such abnormalities will persist, together with their long-term impact on symptom burden in patients.
Exercise intolerance is common among patients recovering from coronavirus infections (SARS, MERS, and COVID-19) [7,8,33,34]. We had previously shown that at 2-3 months [8], CPET revealed a number of abnormalities in patients. By 6 months, many of these parameters improved, though a proportion of patients (31%) still had a reduction in peak oxygen consumption. Of importance, the majority of these patients with limited exercise tolerance on CPET terminated exercise due to fatigue, breathlessness and musculoskeletal symptoms in the absence of physiological limitation. Of the six patients in our study with impaired exercise capacity despite maximal effort, no limitations in cardiorespiratory function or oxygen-carrying capacity were seen. These findings, together with the lower levels of serum creatine kinase and stunted V̇O2-WR relationship observed in patients with impaired exercise capacity, suggest that reduced muscle mass and alterations in skeletal muscle metabolism are likely contributors to exercise limitation [35,36]. This is in line with other studies that have attributed exercise limitation to muscular deconditioning [7,37,38]. Early AT and reduced oxygen pulse despite the absence of cardiorespiratory abnormalities were commonly reported in these studies in support of this hypothesis. Taken together, these findings highlight the role of dedicated rehabilitation in augmenting recovery.
Postural orthostatic tachycardia and other manifestations of dysautonomia have frequently been described among patients post-COVID-19 [39,40]. Here, we showed that at 2-3 months, heart rate recovery on CPET, an indirect measure of autonomic health, was impaired in patients compared to controls [41]. By six months, heart rate recovery improved, implying that dysautonomia may be transient and does spontaneously recover in some patients.
As the COVID-19 pandemic has progressed, our understanding of the long-term effects of SARS-CoV-2 infection has evolved [42], [43], [44]. Multiple studies [5,25] have demonstrated that some patients recovering from COVID-19 experience a diverse range of persistent symptoms months beyond infection, commonly referred to as “long haul COVID” or “post-COVID-19 syndrome” [44,45]. In the present study, 1 in 2 patients reported persistent cardiopulmonary symptoms (chest pain, palpitations, syncope, dyspnoea, or dizziness) at 6 months, despite an improvement in symptoms from 3 months. Neither CMR nor CPET or pulmonary function measures were associated with enduring symptoms. These findings highlight the reduced yield of standard clinical investigations in elucidating a cause for persistent symptoms and the need to explore other mechanisms (sarcopenia, muscle weakness, neurohormonal factors, autoantibodies, nociceptive alterations, mast cell activation syndrome) that may be relevant [46], [47], [48], [49], [50]. Another important finding from this study is that more than half the patients who were asymptomatic had impaired DLco at 6 months, implying that physiological recovery may not be reliably captured by subjective measures of cardiopulmonary health (e.g. symptom questionnaires). Further efforts are needed to better understand the determinants of impaired DLco and persistent parenchymal abnormalities associated with COVID-19, as we seek to develop effective treatments that could potentially reverse the long-term sequelae of COVID-19.
The small sample size, lack of generalizability and the potential for residual confounders are some limitations of this study. However, to our knowledge, this is the first study to comprehensively (cardiopulmonary imaging, static physiology, whole-body exercise testing, patient health questionnaires) evaluate the longitudinal trajectory of cardiopulmonary abnormalities on CMR and CPET in patients at 3 and 6 months post-infection. From a diagnostic perspective, our study provides important insights into the lack of association between symptoms and results from standard clinical investigations. The longitudinal design and incorporation of a risk-factor matched control group clarified the relevance of some early abnormalities.
Patients were enrolled from the first wave only, at a time where the evidence in support of steroid use was limited. While this could, in theory, affect prevalence estimates of symptoms, a recent large follow-up study of hospitalised patients did not see an association between steroid use and ongoing symptom burden [25]. Ethno-racial differences between enrolled controls and patients were also present. However, even after relevant adjustments (Supplementary Material, p8), previously observed associations and differences in multiple parameters remained. Another important limitation was the lack of arterial blood gas sampling or echocardiography during CPET, which did not permit assessment of tissue oxygen extraction, cardiac output during exercise and pulmonary dead space. The use of patient health questionnaires may have introduced self-reporting bias. Finally, not all the patients came back for follow-up assessments (due to work commitments or having moved abroad; see supplement for details). While this could have inflated prevalence estimates of symptom burden in this study, it would not be expected to affect the relationship between symptoms and objective measures of cardiopulmonary health.
Our study provides novel insights into the trajectory of cardiopulmonary symptoms and abnormalities on serial CMR, spirometry and CPET in patients. At 6 months, cardiac abnormalities on CMR improved in the majority of patients and were not different to matched controls. Parenchymal abnormalities, lung function impairment and CPET improved but were still abnormal relative to controls. Nearly half the patients continue to experience symptoms at 6 months. There was a surprising dissociation between persistent cardiopulmonary symptoms and CMR/CPET parameters, underscoring the need to examine alternative mechanisms for symptom persistence in patients.
Author Contributions
SN and BR contributed to the conception of this study. MC, EMT, AJL, MM, NPT, DH, SN and BR contributed to its design. MC, MM, AHAS and BR contributed to data acquisition. MC, EMT, CX and BR contributed to the analysis of data. MC, EMT, NP, AJL, RAE, CEB, LH, SKP, NPT, DH, VMF, SN and BR contributed to interpretation of data. All authors contributed to drafting the article, and had full access to all the data in the study and accept responsibility to submit for publication.
Funding
The authors’ work was supported by the NIHR Oxford Biomedical Research Centre, Oxford British Heart Foundation (BHF) Centre of Research Excellence (RE/18/3/34214), United Kingdom Research Innovation and Wellcome Trust. This project is part of a tier 3 study (C-MORE) within the collaborative research programme entitled PHOSP-COVID Post-hospitalization COVID-19 study: a national consortium to understand and improve long-term health outcomes, funded by the Medical Research Council and Department of Health and Social Care/National Institute for Health Research Grant (MR/V027859/1) ISRCTN number 10980107.
This work also arises from one of the national "COVID-19 Cardiovascular Disease Flagship Projects" designated by the NIHR-BHF Cardiovascular Partnership.
The views expressed are those of the authors and not necessarily those of the National Health Service, NIHR, or the United Kingdom Department of Health.
Declaration of Competing Interest
MC reports a grant from the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre. EMT reports a grant from the NIHR Oxford Biomedical Research Centre and is a shareholder in Perspectum. AL is a shareholder in Perspectum. SKP acknowledges support from the British Heart Foundation (BHF) Centre of Research Excellence and the NIHR Oxford Biomedical Research Centre at the Oxford University Hospitals, University of Oxford, UK. SKP has a US patent 61/387,591 licensed to Siemens and US patents 61/630,508 and 61-630,510 licensed to Perspectum. RAE reports a grant from the NIHR/Medical Research Council (MRC) for the PHOSP-COVID study. VMF reports grants from the BHF and the NIHR Oxford Biomedical Research Centre. SN reports grants from the NIHR Oxford Biomedical Research Centre, UK Research and Innovation, BHF and is a shareholder in Perspectum. SN was a board member and consultant to Perspectum until 2019. SN has patents ‘Multi-parametric magnetic resonance diagnosis & staging of liver disease’ and ‘System and methods for gated mapping of T1 values in abdominal visceral organs’ licensed to Perspectum. BR reports grants from the Oxford BHF Centre for Research Excellence (RE/18/3/34214), the NIHR Oxford Biomedical Research Centre and the United Kingdom Research Innovation Award. All other authors do not have relationships with industry or funding sources to declare.
Acknowledgements
We thank our participants and their families who have given their time to help others understand the medium to long-term effects of COVID-19. We are grateful to the University of Oxford, Oxford University Hospitals Trust and the OUH Clinical Data Warehouse for their support of this study. We would like to acknowledge OCMR staff, Dr Hanan Lamlum, Ms Rebecca Mills, Ms Polly Whitworth, Ms Claudia Nunes, Ms Harriet Nixon, Ms Liliana Da Silva Rodrigues, Ms Kinga A Várnai, Professor Jim Davies, Mr Hizni Salih and Ms Catherine Krasopoulos for their help with this work. We acknowledge the support of Siemens in providing WIP 1048 for cardiac T1 mapping.
Data sharing statement
The data underlying this article will be shared on reasonable request to the corresponding author, subject to institutional and ethical committee approvals.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101159.
Appendix. Supplementary materials
References
- 1.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271-80 e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashraf UM, Abokor AA, Edwards JM, et al. SARS-CoV-2, ACE2 expression, and systemic organ invasion. Physiol Genomics. 2021;53(2):51–60. doi: 10.1152/physiolgenomics.00087.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranard LS, Fried JA, Abdalla M, et al. Approach to Acute Cardiovascular Complications in COVID-19 Infection. Circ Heart Fail. 2020;13(7) doi: 10.1161/CIRCHEARTFAILURE.120.007220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19) JAMA Cardiology. 2020 doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clavario P, De Marzo V, Lotti R, et al. Assessment of functional capacity with cardiopulmonary exercise testing in non-severe COVID-19 patients at three months follow-up. medRxiv. 2020 [Google Scholar]
- 8.Raman B, Cassar MP, Tunnicliffe EM, et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31 doi: 10.1016/j.eclinm.2020.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organisation. COVID-19 Therapeutic Trial Synopsis. https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf (accessed 02/08/2020).
- 10.Prineas RJ, Crow RS, Zhang Z-M. The Minnesota code manual of electrocardiographic findings: including measurement and comparison with the Novacode; standards and procedures for ECG measurement in epidemiologic and clinical trials. 2nd ed. /Ronald J. Prineas, Richard S. Crow, Zhu-Ming Zhang. ed. London: Springer; 2010.
- 11.Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64(2):258–266. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 14.Li K, Fang Y, Li W, et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur Radiol. 2020;30(8):4407–4416. doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham BL, Steenbruggen I, Miller MR, et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham BL, Brusasco V, Burgos F, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 2017;49(1) doi: 10.1183/13993003.00016-2016. [DOI] [PubMed] [Google Scholar]
- 17.Mezzani A. Cardiopulmonary Exercise Testing: Basics of Methodology and Measurements. Ann Am Thorac Soc. 2017;14(Supplement_1):S3–S11. doi: 10.1513/AnnalsATS.201612-997FR. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J Am Coll Cardiol. 2018;72(24):3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 19.Levett DZH, Jack S, Swart M, et al. Perioperative cardiopulmonary exercise testing (CPET): consensus clinical guidelines on indications, organization, conduct, and physiological interpretation. Br J Anaesth. 2018;120(3):484–500. doi: 10.1016/j.bja.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Kotecha T, Knight DS, Razvi Y, et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. European Heart Journal. 2021 doi: 10.1093/eurheartj/ehab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Starekova J, Bluemke DA, Bradham WS, et al. Evaluation for Myocarditis in Competitive Student Athletes Recovering From Coronavirus Disease 2019 With Cardiac Magnetic Resonance Imaging. JAMA cardiology. 2021 doi: 10.1001/jamacardio.2020.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahmod M, Piechnik SK, Levelt E, et al. Adenosine stress native T1 mapping in severe aortic stenosis: evidence for a role of the intravascular compartment on myocardial T1 values. Journal of Cardiovascular Magnetic Resonance. 2014;16(1):1–8. doi: 10.1186/s12968-014-0092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talla A, Vasaikar SV, Lemos MP, et al. Longitudinal immune dynamics of mild COVID-19 define signatures of recovery and persistence. bioRxiv. 2021 [Google Scholar]
- 25.Group P-CC, Evans RA, McAuley H, et al. Physical, cognitive and mental health impacts of COVID-19 following hospitalisation – a multi-centre prospective cohort study. medRxiv. 2021 doi: 10.1016/S2213-2600(21)00383-0. 2021.03.22.21254057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonnweber T, Sahanic S, Pizzini A, et al. Cardiopulmonary recovery after COVID-19: an observational prospective multicentre trial. Eur Respir J. 2021;57(4) doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giustino G, Croft LB, Stefanini GG, et al. Characterization of Myocardial Injury in Patients With COVID-19. J Am Coll Cardiol. 2020;76(18):2043–2055. doi: 10.1016/j.jacc.2020.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szekely Y, Lichter Y, Taieb P, et al. Spectrum of Cardiac Manifestations in COVID-19: A Systematic Echocardiographic Study. Circulation. 2020;142(4):342–353. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu X, Liu X, Zhou Y, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021;9(7):747–754. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang M, Som A, Mendoza DP, et al. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis. 2020;20(12):1365–1366. doi: 10.1016/S1473-3099(20)30367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakr Y, Giovini M, Leone M, et al. Pulmonary embolism in patients with coronavirus disease-2019 (COVID-19) pneumonia: a narrative review. Ann Intensive Care. 2020;10:124. doi: 10.1186/s13613-020-00741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borczuk AC, Salvatore SP, Seshan SV, et al. COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City. Mod Pathol. 2020;33(11):2156–2168. doi: 10.1038/s41379-020-00661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohr A, Dannerbeck L, Lange TJ, et al. Cardiopulmonary exercise pattern in patients with persistent dyspnoea after recovery from COVID-19. Multidisciplinary Respiratory Medicine. 2021;16(1) doi: 10.4081/mrm.2021.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ong KC, Ng A-K, Lee L-U, et al. Pulmonary function and exercise capacity in survivors of severe acute respiratory syndrome. European Respiratory Journal. 2004;24(3):436–442. doi: 10.1183/09031936.04.00007104. [DOI] [PubMed] [Google Scholar]
- 35.Task Force of the Italian Working Group on Cardiac R, Prevention Working Group on Cardiac R, Exercise Physiology of the European Society of C. Statement on cardiopulmonary exercise testing in chronic heart failure due to left ventricular dysfunction: recommendations for performance and interpretation Part III: Interpretation of cardiopulmonary exercise testing in chronic heart failure and future applications. Eur J Cardiovasc Prev Rehabil. 2006;13(4):485–494. doi: 10.1097/01.hjr.0000201518.43837.bc. [DOI] [PubMed] [Google Scholar]
- 36.Rosalki SB. Low serum creatine kinase activity. Clin Chem. 1998;44(5):905. [PubMed] [Google Scholar]
- 37.Baratto C, Caravita S, Faini A, et al. Impact of COVID-19 on exercise pathophysiology. A combined cardiopulmonary and echocardiographic exercise study. J Appl Physiol. 1985:2021. doi: 10.1152/japplphysiol.00710.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rinaldo RF, Mondoni M, Parazzini EM, et al. Deconditioning as main mechanism of impaired exercise response in COVID-19 survivors. Eur Respir J. 2021 doi: 10.1183/13993003.00870-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodman BP, Khoury JA, Blair JE, Grill MF. COVID-19 Dysautonomia. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.624968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barizien N, Le Guen M, Russel S, Touche P, Huang F, Vallee A. Clinical characterization of dysautonomia in long COVID-19 patients. Sci Rep. 2021;11(1):14042. doi: 10.1038/s41598-021-93546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pierpont GL, Voth EJ. Assessing autonomic function by analysis of heart rate recovery from exercise in healthy subjects. Am J Cardiol. 2004;94(1):64–68. doi: 10.1016/j.amjcard.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 42.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. Jama. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nature Medicine. 2021:1–6. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis HE, Assaf GS, McCorkell L, et al. Characterizing Long COVID in an International Cohort: 7 Months of Symptoms and Their Impact. medRxiv. 2020 doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amenta EM, Spallone A, Rodriguez-Barradas MC, El Sahly HM, Atmar RL, Kulkarni PA. Postacute COVID-19: An Overview and Approach to Classification. Open Forum Infect Dis. 2020;7(12):ofaa509. doi: 10.1093/ofid/ofaa509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cox LS, Bellantuono I, Lord JM, et al. Tackling immunosenescence to improve COVID-19 outcomes and vaccine response in older adults. Lancet Healthy Longev. 2020;1(2):e55–ee7. doi: 10.1016/S2666-7568(20)30011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doykov I, Hallqvist J, Gilmour KC, Grandjean L, Mills K, Heywood WE 'The long tail of Covid-19′ - The detection of a prolonged inflammatory response after a SARS-CoV-2 infection in asymptomatic and mildly affected patients. F1000Res. 2020;9:1349. doi: 10.12688/f1000research.27287.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McFarland AJ, Yousuf MS, Shiers S, Price TJ. Neurobiology of SARS-CoV-2 interactions with the peripheral nervous system: implications for COVID-19 and pain. Pain Rep. 2021;6(1):e885. doi: 10.1097/PR9.0000000000000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang EY, Mao T, Klein J, et al. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021;595(7866):283–288. doi: 10.1038/s41586-021-03631-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.