Abstract
Background
Young (≤40 years) breast cancers (YBC) are uncommon, inadequately represented in trials and have unique concerns and merit studying.
Methods
The YBC treated with a curative intent between 2015 and 2016 at our institute were analysed.
Results
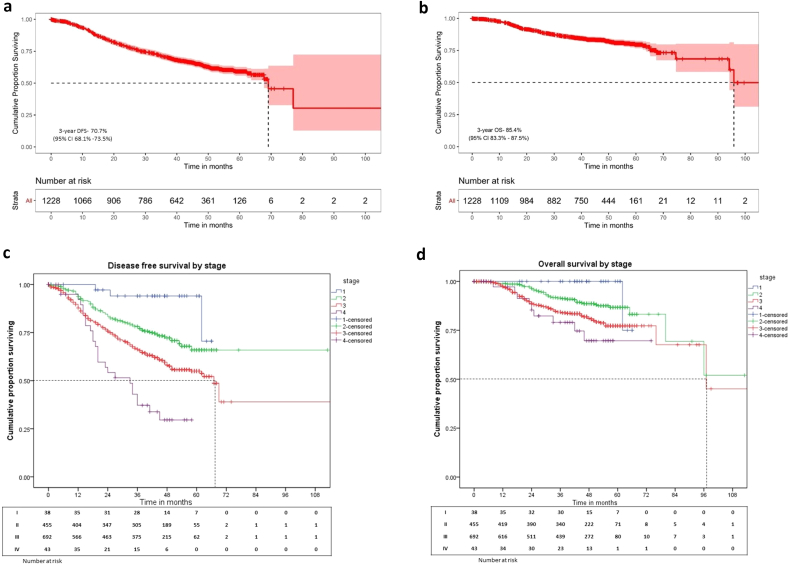
There were 1228 patients with a median age of 36 (12–40) years; 38 (3.1%) had Stage I, 455 (37.1%) - II, 692 (56.3%) –III, and remaining 43 (3.5%) Stage IV (oligo-metastatic) disease; 927 (75.5%) were node positive; 422 (34.4%) were Triple negatives (TNBC), 331 (27%) were HER-2 positive. There were 549 (48.2%) breast conservations and 591 (51.8%) mastectomies of which 62 (10.4%) underwent breast reconstruction. 1143 women received chemotherapy, 617 (53.9%) received as neoadjuvant and 142 (23.1%) had pathological complete response; 934 (81.9%) received adjuvant radiotherapy. At the median follow-up of 48 (0–131) months, 5-year overall and disease-free survival was 79.6% (76.8–82.5) and 59.1% (55.8–62.6). For stage I, II, III and IV, the 5-year overall-survival was 100%, 86.7% (82.8–90.6), 77.3% (73.4–81.2), 69.7% (52.5–86.9) and disease-free survival was 94% (85.9–100), 65.9% (60.3–71.5), 55% (50.5–59.5), and 29.6% (14–45.2) respectively. On multivariate analysis, TNBC and HER-2+ subgroups had poorer survival (p = 0.0035). 25 patients had BRCA mutations with a 5-year DFS of 65.1% (95% CI:43.6–86.6). Fertility preservation was administered in 104 (8.5%) patients; seven women conceived and 5 had live births. Significant postmenopausal symptoms were present in 153 (13%) patients.
Conclusion
More than half of the YBC in India were diagnosed at an advanced stage with aggressive features leading to suboptimal outcomes. Awareness via national registry and early diagnosis is highly warranted. Menopausal symptoms and fertility issues are prevalent and demand special focus.
Keywords: Young breast cancer, Chemotherapy, Outcomes, Fertility, Quality of life
Highlights
-
•
Largest data of young breast cancer from India analysing demographics, outcomes and fertility.
-
•
Higher proportion of YBC with advanced stage and aggressive features compared to western data.
-
•
Inferior survival in HER-2+ not receiving Trastuzumab, need access of life saving drugs to all.
-
•
Scope of improvement and collaboration in challenges like fertility, pregnancy and quality of life.
1. Introduction
Young women with breast cancer (YBC) face unique challenges in the management of their cancer. The most widely accepted definition of YBC are women diagnosed with breast cancer at ≤ 40 years of age although some consider it to be ≤ 45 years [1,2]. Among them is a small population of very young women (<35 years) with breast cancer (very YBC) [3,4]. These women merit attention to their specific needs pertaining to body image, quality of life (QOL), sexual health and fertility preservation, premature menopause, pregnancy and lactation among others. Research into their poor outcomes has revealed a distinct biology with adverse clinico-pathological risk factors [5].
The National Cancer registry Programme (NCRP) of India has estimated that by the year 2025 there will be 2,30,000 breast cancer patients annually with a significant increase in YBC [6]. Despite their growing numbers there are very few reports on the outcomes and management of these patients from India [6,7]. Our institute is a major tertiary cancer care centre in India and as per the NCRP report of 2012–2016, it has registered the highest number of cancer patients out of all 57 Hospital Based Cancer registries [6]. Therefore, we wanted to study the outcomes of such patients in the real world setting of LMIC.1
2. Materials and methods
2.1. Study population
The study included patients aged ≤40 years with histologically proven invasive breast cancer consecutively, treated with curative intent registered at our institute between January 2015–December 2016. Among these, patients ≤35 years were categorised as very YBC.
2.2. Data collection and management
The details of patient demographics and treatment was collected prospectively using the hospital electronic medical records (EMR) system, personal files and further updated by telephonic follow up as required. The AJCC 7th edition was used for staging. The management for all the patients is decided in a multidisciplinary joint clinic as per standard institutional guidelines summarized in Supplementary table 4. To define pCR (pathological complete response), no evidence of invasive residual disease in both the breast and the nodes was required. The data was locked on October 8, 2020 for analysis.
2.3. Statistical analysis
Patient, tumour and treatment related characteristics were reported using descriptive statistics. The differences in proportions of categorical variables were calculated using the Chi-square and Fisher's exact test. Univariate analysis was performed on factors affecting the outcomes as shown in supplementary table 1. Factors which were significant on univariate analysis were analysed by the Cox regression model for multivariate analysis. For all results, p-values were two-sided with an alpha error of 0.05. SPSS® (IBM, California) version 25 was used for performing the statistical analysis.
Kaplan Meier method was used for survival analysis and log rank test was used for comparison. Disease free survival (DFS) was defined as the time from diagnosis to the occurrence of any event (local/regional or distant relapse) or death from any cause. Overall survival (OS) was calculated from the date of diagnosis to the date of death due to any cause and those alive were censored at their last follow up. The study was approved by Institutional Ethics Committee and registered to clinical trials registry India (CTRI/2021/01/030325) and conducted as per good clinical practise guidelines.
3. Results
3.1. Patient characteristics
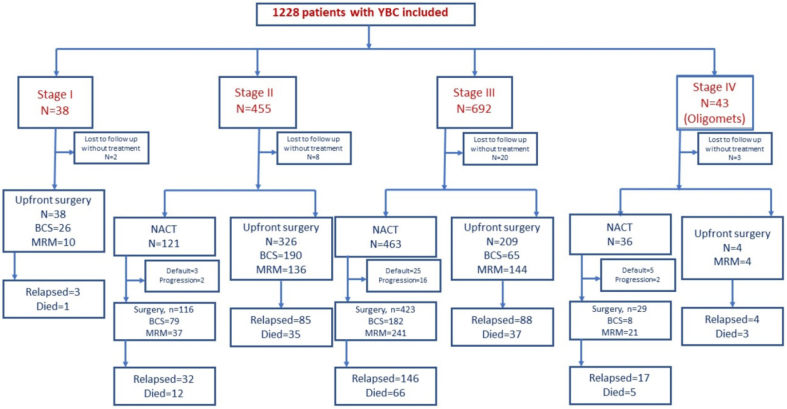
Of the 8634 breast cancers registered to our centre, 1445 (16.7%) were YBC. Among them 1228 patients aged ≤40 years with a median age of 36 years (IQR:32–38 years) treated with a curative intent were included in this study. There were 217 (15%) upfront metastatic women with YBC and those will be separately reported. Almost half of them (n = 608,49.6%) were aged ≤35 years (very YBC) and 39 (3.2%) patients ≤25 years. Majority had grade-3 (n = 1068, 86.9%) and stage-3 disease (n = 692,53.7%). 422 (34.4%) patients had TNBC and 331 (27%) had HER-2 positive disease. Patient and treatment characteristics are summarized in Table 1.
Table 1.
Baseline patient and treatment characteristics.
| Age group (n = 1228) | No of Patients (%) |
|---|---|
| •<25 years | 39 (3.2) |
| •25–29 years | 129 (10.5) |
| •30–35 years | 440 (35.8) |
| •36–40 years | 620 (50.4) |
| Family History of malignancy (n = 1183) | 242 (20.4) |
| Nulliparous (n = 1159) | 157 (13.5) |
| Single live child (n = 1159) | 281 (24.2) |
| Associated with pregnancy | 24 (1.9%) |
| T stage | |
| •T1 | 63 (5.1%) |
| •T2 | 575 (46.8%) |
| •T3 | 298 (24.3%) |
| •T4 | 292 (23.8%) |
| N stage | |
| •N0 | 301 (24.5%) |
| •N1 | 552 (45%) |
| •N2 | 256 (20.8%) |
| •N3 | 119 (9.7%) |
| Clinical Stage | |
| •I | 38 (3.1%) |
| •II | 455 (37.6%) |
| •III | 692 (56.3%) |
| •IV | 43 (3.5%) |
| Grade | |
| •I | 3 (0.2%) |
| •II | 157 (12.7%) |
| •III | 1068 (86.9%) |
| Hormone/HER-2 receptor status | |
| •HR +/HER-2- | 475 (38.7%) |
| •HR+/HER-2+ | 174 (14.2%) |
| •HR-/HER-2+ | 157 (12.8%) |
| •TNBC | 422 (34.4%) |
| Surgery (n = 1140) | |
| •Breast conservation surgery | 549 (48.2%) |
| •Modified radical mastectomy | 591 (51.8%) |
| •Breast Reconstruction | 62 (10.4%) |
| Chemotherapy (n = 1143) | |
| •NACT alone | 243 (21.2%) |
| •ACT alone | 526 (46%) |
| •Both NACT and ACT (sandwich approach) | 374 (32.7%) |
| Neoadjuvant Chemotherapy type | |
| •Anthracycline + taxane | 203 (32.9%) |
| •Only anthracyclines | 371 (60.1%) |
| •Only taxane w/o anthracyclines | 43 (6.9%) |
| •Platinum based | 52 (8.4%) |
| Trastuzumab use (n = 174) | |
| •Neoadjuvant | 72 (21.7%) |
| •Adjuvant | 102 (30.8%) |
| •Maintenance | 90 (27.1%) |
| •Short course (3 months) | 84 (25.3%) |
HR: Hormone receptor; +: positive, -: negative; TNBC: Triple negative breast cancer; BCS: Breast Conservation Surgery; MRM: Modified Radical Mastectomy; NACT: Neoadjuvant Chemotherapy; ACT: Adjuvant chemotherapy; CR: complete response.
4. Treatment characteristics
4.1. Surgery
549 (48.2%) underwent breast conservation surgery (BCS), 591 (51.8%) underwent modified radical mastectomy (MRM). The option of whole breast reconstruction was discussed with most patients who were not suitable for BCS and needed MRM. However, only 10.4% patients finally received post mastectomy reconstruction due to various reasons including patient reluctance, irrational fears of delay in treatment, additional cost, and other logistic issues. All 62 patients underwent autologous microvascular reconstruction using DIEP (deep inferior epigastric perforator), ALT (Anterolateral thigh), Gracilis and extended LD (Latissimus Dorsi) flaps. Upfront surgery was performed in 359 (72.8%) patients with early breast cancer (EBC: Stage I/II; n = 493) where 216 (60.1%) patients underwent BCS. For those undergoing surgery first and with clinically node negative axilla, axillary staging was done with low axillary sampling (LAS) (n = 406). We previously validated the procedure of LAS to be equivalent to sentinel node biopsy in N0 axilla [8]. All patients post-chemotherapy and those with positive lymph nodes either clinically or on intra-operative frozen section analysis had a complete axillary lymph nodal dissection (ALND) (n = 910). Out of the patients who underwent NACT (n = 617), 269 (43.6%) underwent BCS and 299 (48.5%) underwent MRM.
4.2. Systemic therapy (ST)
4.2.1. Chemotherapy
A large majority of our patients received chemotherapy (n = 1143, 93.16%): Neoadjuvant [NACT (n = 243,21.2%)], adjuvant chemotherapy [ACT, n = 526 (46%)] or both NACT and ACT (n = 374, 32.7%). Overall, 889 (77.7%) patients received both anthracyclines and taxanes. In total, 617 patients underwent neoadjuvant chemotherapy of which majority had locally advanced disease with biologically aggressive tumours; 463 (75%) were stage 3, 33 (5.3%) were stage 4, 239 (38.7%) were TNBC, 168 (27.2%) were HER-2 positive.
4.2.2. Targeted therapy
Out of the 331 HER-2+ patients, only 174 (54.5%) patients could receive trastuzumab and among those, 84 (25.3%) received 3 months of trastuzumab (short course); 90 (27.1%) patients received full course of adjuvant trastuzumab for 1 year (Table 1).
4.2.3. Pathological complete response (pCR)
Out of the 617 patients who received NACT, 221 received both anthracyclines and taxanes as NACT and 67 of them (30.3%) achieved a pCR. It was highest in the TNBC subgroup (37.6%, n = 90/239) and lowest in the HR+/HER-2- subgroup (10%, 21/210). Some 21% (48/239) TNBC patients received additional platinums with NACT. In HER-2+ patients, the pCR rate was 32% (24/75) with and 8.4% (8/93) without the addition of Trastuzumab to chemotherapy. (Supplementary table 3).
4.2.4. Toxicity
There were few grade III/IV adverse events: febrile neutropenia (FN) (n = 57, 4.9%), thrombocytopenia (n = 15, 1.3%), peripheral neuropathy (n = 13, 1.1%) and vomiting (n = 8, 0.6%). Three patients, with no comorbidities, died due to treatment related infective complications (2 post-chemotherapy and 1 post-surgery).
4.3. Hormone therapy (HT)
All 650 patients with HR + tumours were recommended adjuvant HT but only 613 (94.3%) received it and 37 (5.7%) defaulted. In HR + patients, tamoxifen was administered as adjuvant HT of which 87 (14.1%) also received ovarian suppression.
4.4. Radiotherapy (RT)
Out of 1140 patients who underwent curative surgery, 934 (81.9%) patients also received adjuvant RT (910 with Field-in-field technique, 24 with intensity modulated RT) to a dose of 40Gy in 15 fractions over 3 weeks, additionally tumour bed boost was administered in all patients receiving whole breast irradiation. The supraclavicular nodal region was irradiated in 508 (54.5%) patients, and axillary and internal mammary lymph nodal regions were rarely irradiated (12 patients each) as axilla was adequately addressed surgically. Very few patients developed Grade 3 toxicities, e.g., skin reactions (n = 14,1.49%).
4.5. Postmenopausal symptoms, fertility preservation and pregnancy
Since 1143 (93.5%) received CT and 650 received HT, several of them experienced postmenopausal symptoms (PMS) and fertility issues. The details on the fertility issues and pregnancy were available for 351 patients in the EMR or were gathered during the telephonic follow-up. Among them 341 patients experienced post-chemotherapy amenorrhoea which produced transient symptoms in 148 (43.4%) patients whereas 153 (44.8%) patients experienced significantly distressing PMS like sleep disturbances (n = 72, 47.3%), memory problems and mental exhaustion (n = 57,37.2%), mood swings (n = 54,35.2%) followed by hot flushes (n = 37,24.1%). A very small number of patients reported vaginal dryness (n = 23,6.7%) or adverse impact on sexual quality of life (n = 12,3.5%) (Supplementary Table 2).
Majority of the patients received fertility counselling, however there were gaps in communication and documentation and its record was available for 278/341 (81.5%) patients. Only 105 (8.5% of overall population) of those (35 nulliparous and 85 desirous for 2nd child) opted for fertility preservation predominantly with LHRH agonists due to various factors including logistic reasons as well as majority having children already with less motivation. Seven (38.8%) patients conceived post treatment completion, and 5 (27.7%) patients had live births between 2 and 7 years after treatment.
4.6. Survival analysis
A total of 407 events and 175 deaths were recorded after a median follow up of 48 (range:0–131; IQR: 25–54) months. The median DFS and OS were 61 (95% CI: 60.1 – NR) months and 94 (95% CI: 80.1–NR) months (Fig. 1, Fig. 2a and b) and the 5-year DFS and OS were 59.1% (55.8–62.6) and 79.6% (76.8–82.5) respectively (Table 2).
Fig. 1.
CONSORT diagram.
Fig. 2.
a: Overall cohort (n = 1228): Disease free survival curve, b: Overall cohort (n = 1228): Overall survival curve, c: Stage wise survival: DFS, d: Stage wise survival: OS.
Table 2.
Disease Free Survival and Overall Survival rates.
| Time | DFS (95% CI) | OS (95% CI) |
|---|---|---|
| 2-year | 78.8% (76.4–81.2) | 90.1% (88.4–91.9) |
| 3-year | 70.7% (68.1–73.5) | 85.4% (83.3–87.5) |
| 4-year | 63.6% (60.6–66.5) | 84.2% (81.9–86.5) |
| 5-year | 59.1% (55.8–62.6) | 79.6% (76.8–82.5) |
| Stage wise 3 year and 5-year survival rates | ||
| Stage | 3-year DFS (95% CI) | 3-year OS (95% CI) |
| I | 94.4% (87–100) | 100% |
| II | 77.2% (73.4–81.3) | 90.4% (87.7–93.3) |
| III | 66.5% (62.8–70.5) | 81.9% (78.7–85.1) |
| IV | 43.1% (30.5–60.8) | 79.1% (65.1–93) |
| 5-year DFS (95% CI) | 5-year OS (95% CI) | |
| I | 94% (85.9–100) | 100% |
| II | 65.9% (60.3–71.5) | 86.7% (82.8–90.6) |
| III | 55% (50.5–59.5) | 77.3% (73.4–81.2) |
| IV | 29.6% (14–45.2) | 69.7% (52.5–86.9) |
DFS: Disease free survival; OS: Overall Survival; CI: Confidence Interval.
4.7. Prognostic factors
There was a significant difference in the 5 year OS between tumour subtypes which is highest in HR+/HER-2- subgroup and least in the TNBC subgroup [84.3% (95% CI:80.2–88.4) vs 77.1% (95% CI: 71.8–82.4); p = 0.019]. Although, there was not a significant difference in the DFS among these subtypes. Furthermore, in the HR + group; when compared with stage I/II tumours, Stage III had significantly inferior 5- year DFS [72% (95% CI:64.9–79.1) vs 54.4% (48.1–60.7); p = 0.0] and OS [88.6% (95% CI: 83.3–93.9) vs 81.8% (95% CI:76.9–86.7); p = 0.025). Stagewise survival outcomes are represented in Table 2, Fig. 2c and d. Tumour subtype wise survival outcomes are represented in Supplementary Tables 5 and 6
Among the HER-2+ patients, there was a significant difference in the 5 year DFS (73.6% vs 44.6%; HR = 0.42, 95% CI: 0.28–0.63, p = 0.0) and OS (92.1% vs 71.8%; HR = 0.32; 95% CI: 0.16–0.62, p = 0.001) between patients who received trastuzumab and who did not receive respectively. On multivariate analysis (Table 3), older age, lower T/N-stage, absence of LVI and pCR were associated with superior DFS and OS while HR + status was associated with superior OS alone (p = 0.0035).
Table 3.
Multivariate analysis for Disease Free Survival and Overall Survival.
| Variables | Disease Free Survival HR (95% CI); p value | Overall Survival HR (95% CI); p value |
|---|---|---|
| Age | ||
| <25 years | ||
| 25–29 years | 0.72 (0.42–1.24); 0.241 | 0.31 (0.14–0.66); 0.002 |
| 30–35 years | 0.64 (0.40–1.04); 0.070 | 0.45 (0.25–0.83); 0.011 |
| 36–40 years | 0.54 (0.34–0.87); 0.012 | 0.40 (0.22–0.73); 0.003 |
| T size | ||
| T1 | 0.32 (0.13–0.75); 0.009 | 0.39 (0.13–1.15); 0.088 |
| T2 | 0.56 (0.40–0.80); 0.001 | 0.41 (0.25–0.67); 0.000 |
| T3 | 0.96 (0.73–1.27); 0.787 | 0.77 (0.52–1.14); 0.186 |
| T4 | ||
| Node status | ||
| N0 | 0.33 (0.22–0.50); 0.000 | 0.39 (0.22–0.69); 0.001 |
| N1 | 0.35 (0.25–0.48); 0.000 | 0.36 (0.23–0.58); 0.000 |
| N2 | 0.76 (0.55–1.05); 0.094 | 0.78 (0.49–1.24); 0.300 |
| N3 | ||
| Receptor subtypes | ||
| TNBC | 0.96 (0.63–1.47); 0.862 | |
| HR+/HER-2+ | 0.40 (0.22–0.73); 0.003 | |
| HR+/HER-2- | 0.62 (0.40–0.95); 0.028 | |
| Other pathological features | ||
| LVI | ||
| Negative | 0.72 (0.56–0.92); 0.009 | 0.68 (0.47–0.98); 0.039 |
| Positive | ||
| pCR | ||
| No | ||
| Yes | 0.32 (0.20–0.53); 0.000 | 0.23 (0.10–0.50); 0.000 |
DFS: Disease free survival; OS: Overall survival; HR: Hazard ratio; CI: Confidence interval; T: Tumour; TNBC: Triple negative breast cancer; LVI: lymphovascular invasion; pCR: Pathological complete response.
4.8. Very young breast cancer
The patient and treatment characteristics of very YBC were compared with the YBC patients in Table 4. A significantly lower PR expression (38.4% vs 44.03%, p = 0.048), higher NACT use (54.6% vs 46.3%, p = 0.009) and LHRH agonists for fertility preservation (13.3% vs 3.7%, p = 0.0) were noted in the very YBC subgroup. Rest of the characteristics were comparable. (Table 4). There was a significant difference in 5-year DFS of VYBC vs YBC [53.5% (95% CI: 48.4–58.6) vs 65.3% (95% CI: 60.8–69.8), p = 0.002], however, no significant difference was found in 5-year OS [79% (95% CI: 74.9–83.1) vs 83.9% (95% CI: 80.3–87.3), p = 0.145].
Table 4.
Comparison of patient, tumor and treatment characteristics between Very young (≤35 years) and young (36–40 years) subgroups.
| ≤35 YEARS (n = 608) | 36-40 YEARS (n = 620) | p value | |
|---|---|---|---|
| Grade | 0.505 | ||
| I | 1 (0.001%) | 2 (0.003%) | |
| II | 69 (11.3%) | 87 (14%) | |
| III | 536 (88.1%) | 529 (85.3%) | |
| PNE | 26/605 (4.2%) | 37/612 (6%) | 0.220 |
| LVI | 167/605 (27.6%) | 180/612 (29.4%) | 0.390 |
| ER+ | 311/608 (51.1%) | 332/620 (53.5%) | 0.400 |
| PR+ | 234/608 (38.4%) | 273/620 (44.03%) | 0.048 |
| HER-2+ | 166/608 (27.3%) | 165/620 (26.6%) | 0.233 |
| Phenotype | 0.919 | ||
| HR+/HER-2- | 198/571 (34.6%) | 207/568 (37.5%) | |
| HR+/HER-2+ | 89/571 (15.5%) | 89/568 (15.3%) | |
| HR-/HER-2+ | 77/571 (13.4%) | 76/568 (13.1%) | |
| TNBC | 207/571 (36.25%) | 196/568 (33.9%) | |
| Positive LN | 455/608 (74.8%) | 472/620 (76.1%) | 0.707 |
| Stage | 0.637 | ||
| I | 16/608 (2.6%) | 24/620 (3.8%) | |
| II | 242/608 (39.8%) | 244/620 (39.3%) | |
| III | 330/608 (54.2%) | 329/620 (53%) | |
| IV | 20/608 (3.2%) | 23/620 (3.7%) | |
| Received Neoadjuvant chemotherapy | 332/608 (54.6%) | 293/620 (47.2%) | 0.009 |
| Underwent curative surgery | n = 566 | n = 574 | 0.318 |
| MRM | n = 285 (50.3%) | n = 306 (53.3%) | |
| BCS | n = 281 (49.7%) | n = 268 (46.7%) | |
| Pathological Complete Remission rates | 79/332 (23.7%) | 68/293 (23.2%) | 0.919 |
| Received fertility preservation | 81/608 (13.3%) | 23/620 (3.7%) | 0.000 |
PNE: Perineural extension; LVI: Lymphovascular invasion; ER: Estrogen receptor; PR: Progesterone receptor; ‘+’: positive; ‘-’: negative; HR: Hormone receptor; TNBC: Triple negative Breast cancer LN: lymph node; NACT: Neoadjuvant chemotherapy; MRM: Modified radical mastectomy; BCS: Breast conservation surgery; pCR: Pathological complete response.
4.9. Familial and hereditary cancers
Significant family history was noted in 242 patients (19.7%). A dedicated cancer genetics clinic associated with laboratory at our hospital undertakes genetic testing for research purposes for patients unable to afford genetic testing at a private laboratory. 170 patients were tested in the cancer genetic clinic of which reports were available for 132 patients. 30 patients tested positive for germline mutations of which 25 patients had mutations in BRCA gene (21 pathogenic mutations; 4 variant of uncertain significance). 2 patients had germline mutations in APC gene and 1 each had mutations in FANCD, P53 and MSH-2 genes. Six (30%) and 7 (35%) patients respectively opted for prophylactic contralateral mastectomy and risk reducing bilateral salpingo-oophorectomy. The rest were on intensified follow-up for early detection of breast cancer. The 5-year DFS and OS in patients with germline mutations was 67.2% (95% CI:48.2–86.2) and 96.6% (95% CI:90–100) respectively. The 5-year DFS and OS in patients with BRCA mutations was 65.1% (95% CI:43.6–86.6) and 95.8% (95% CI:87.8–100) respectively.
4.10. Breast cancer during pregnancy (PrBC) or during 1-year postpartum period (PPBC)
There were 24 (1.9%) patients with PrBC(13; 54.1%) or PPBC(11; 45.8%) with a median age of 31 years (IQ range: 27–33). Five (38.4%) patients opted to interrupt their pregnancy. The actuarial 5-year DFS and OS in the PABC cohort was 58.5% (95% CI: 23.1%–93.9%) and 81.3% (95% CI:64.3%–98.3%) respectively.
5. Discussion
Our comprehensive study represents the largest cohort of YBC patients from India. Biological age can't be measured accurately and thus we used the ESO-ESMO guidelines age cut off as <40 years for YBC to determine our study participants [1]. Furthermore based on the studies by Liukkonen et al. and Fabiano et al. we classified patients aged ≤35 years in the “very young” subgroup (Very YBC) [3,4].
The incidence of YBC varies from 2 to 6% in west to 10–20% in Asia [2,9,10]. It was interesting to note that nearly half of our study patients belonged to the 36–40 years age group and may represent the age structure bias from a relatively younger Indian population. Yet, the scant literature reporting on Indian YBC is ironical. The incidence of YBC in India has been reported as 8% of all breast cancers (7). Contrary to their findings, a SEER data analysis showed a higher frequency of YBC (<40 years) among Asian women compared to the Caucasian women, 16.2% vs 6.23% (p < 0.0001) [11]. In our study, the proportion of YBC was 16.7% which is similar to the SEER data and reflects on the age distribution and possibly poor access to healthcare for older women [[12], [13], [14]].
Only half of the HER-2 positive patients were able to receive trastuzumab (54.5%).This indicates the real world scenarios wherein there is a constraint to offer standard of care targeted therapy even in curative setting and in YBCs [12,[15], [16], [17]]. As expected, the outcomes were significantly better among those who received trastuzumab versus those who could not. Health sector in India receives <2% of India's GDP/national budget allocation and the insurance coverage is negligible especially in the remote areas forcing most patients to spend out of pocket for health care unlike developed countries [18,19]. Our study sheds light on the impact of this disparity and more national and global efforts are required to increase the access of life saving drugs like trastuzumab to the majority.
Though BCS is preferable for YBC patients, mastectomy rates were higher (51.8%) due to the higher proportion of LABCs, multicentricity, poor access to radiation facilities [20,21] and patient preference. Compliance to RT was high possibly reflecting the effect of improved access from adoption of hypofractionated protocol at ours and many other centres across the country.
A greater proportion of our study patients had adverse prognostic factors such as Grade-III tumours (86%), node positivity (75%), HER-2+ (27%) and TNBC phenotype (34%) among others. Similar results have been reported in studies from India [7], Korea and Mexico [5,10,22,23]. When compared to studies from western countries, the proportion of HR+(52%) were lesser whereas, TNBC (34%) were higher. In a study from the California Cancer registry [24], 67% were HR+, 27% were HER-2+ and 10.8% were TNBC. Similar results were reported from the UK POSH study [25], where the proportion of HR+ were 65%, HER-2+ were 24.3% and TNBC were 19.9%.
Nearly half of the population in current study fall under the category of very YBC. The differences in PR expression, NACT use and fertility preservation and similarities in their stage distribution, tumour grade and HR/Her 2 expression are similar to a study from Argentina [3], but contrasting to the studies by Collins [26] and Dubsky [27] who did not find such differences. The reasons for this disparity can be due to geo-ethnic and healthcare access differences. Though no differences were found in the tumour characteristics, a poorer DFS was noted in very YBC group which suggests the adverse impact of very young age on survival.
Previous studies have reported young age to be a poor prognostic factor for survival. In the metanalysis by Maajani et al. [28], 776,431 women with breast cancer were analysed and the pooled 5-year OS of 73% was for the overall study population. When developing and developed countries were separately analysed, the 5-year OS was 69% in the developing countries and 76% in the developed countries which was similar to our study. But a recent study [29] of around 1.5 lakh non-metastatic YBC from the SEER database reported a 5 year-OS of 85.6% (95%CI: 84.2–86.8) and 87.9% (95%CI: 87.5–88.3) in the 20–29 and 30–39 year age group which is higher than our study. But it should be noted that the proportion of Stage III at diagnosis was <25% compared to 53% in our study, and additionally we had oligometastatic patients as well. Also, the stage matched outcomes were comparable to ours. Another large Indian multicentric study [30]of all breast cancers treated with curative surgery also reported a higher 5 year-OS of 94.1% (93.25–94.98). In this study, the median age was 53 and only 20% were stage III, which might be the cause for the survival disparity. Since a significantly higher advanced disease appears to be the cause of inferior overall outcomes, it is alarming to note that, even in the current era, more than half of the YBC present at an advanced stage in our country which calls for an urgent need to escalate cancer awareness and screening programmes in this subgroup. Comparable stage matched outcomes (28,29) may be attributed to higher use of effective chemotherapy (93%) including both anthracyclines and taxanes in 77% patients. This multi-drug chemotherapy was well tolerated with grade-III toxicity similar to that reported in major studies despite the high prevalence of malnutrition among multiparous Indian women [[31], [32], [33], [34]]. However, caution must be exercised to avoid intensification of treatment just based on the age as suggested by the international guidelines [1,9].The outcomes of the small subset of PABC patients in our study were similar to that seen in a larger study of 104 PABC patients published from our institute had showed comparable stage matched OS and DFS with non PABC patients [35].
Due to the small number of patients with germline genetic testing reports, the prevalence of pathogenic variants in this cohort of patients could not be determined and is among the major limitation of our study [36]. We are developing care pathways to improve referral for genetic counselling and provide financial aid for testing along with capacity and infrastructure building to improve the access to genetic testing across the country.
Chemotherapy can accelerate menopause by a decade [37] and there is a 70% lower chance of conception post breast cancer therapy compared to women of the same age [38]. Still, fertility counselling takes a backseat since priority is often given to initiating cancer treatments. We were able to evaluate the outcomes in only a third of our study population due to the retrospective nature of this study and forms a major limitation due to loss of follow-up; however, it was encouraging that 81.5% of these patients received some form of fertility counselling. Even though there were 13% nulliparous and 24% women with single child, only 8.5% of the patients opted for fertility preservation predominantly with LHRH agonists. This could be due to multiple reasons, especially financial (lack of insurance/reimbursement) and logistic issues. Still, 57% of the patients who desired to conceive and received LHRH agonists could do it successfully post treatment (4/7). Marklund et al.[39], reported a 25% improvement in 10-year cumulative childbirth incidence and Vriens et al.[40] reported an improvement in the 5-year live birth rate of 10.4% among those who opted for fertility preservation. This subgroup of patients is small and possibly reflects younger age at child bearing in India leading to most women being diagnosed with breast cancer after completion of family.
6. Conclusion
The proportion of YBC appears to be higher in India and represent a distinct subgroup. It is alarming to note that even in the current era, more than half of the patients present at an advanced stage which calls for an escalation of cancer awareness and screening programmes in this group. The unique problems of the YBC like fertility preservation and QOL issues, need to be addressed appropriately and the data recorded prospectively in future studies.
Funding source
No funding required.
The study was approved by Institutional Ethics Committee and registered to clinical trials registry India (CTRI/2021/01/030325) and conducted as per good clinical practise guidelines.
Declaration of competing interest
There are no conflicts of interest relevant to this material for any of the authors.
Acknowledgements
we are grateful to entire breast disease management group for their active contribution in patients management, Ms Smruti Mokal, for help in statistical analysis and patients and their families for their trust and support.
Footnotes
LMIC: Low and Middle Income Countries.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.09.008.
Authors contribution statement
Jyoti Bajpai (JB): Conception, design, patient recruitment, management, follow-up, analysis,supervision, administration, first and final manuscript Pradeep Ventrapati (PV)- Patient recruitment, management, data entry, first, final manuscript. Sushmita Rath, Ravindra Nandhana, Samarpita Mohanty, Qurratulain Chougle, Mitchelle Engineer -Patient recruitment, management, data entry, final manuscript. Tabassum Wadasadawala, Rima Pathak, Shalaka Joshi - Patient recruitment, management, first and final manuscript. Jaya Ghosh, Nita Nair, Seem Gulia, Vani Parmar- Patient recruitment, management, final manuscript. Asawari Patil, Tanuja sheth, Sangeeta Desai, Meenakshi Thakur, Palak Popat, Venkatesh Rangrajan, - Lab reports interpretation, final manuscript Sudeep Gupta, RA Badwe, Rajiv Sarin - Patient recruitment, management, final manuscript, administration, supervision. Nissie Abraham (NA): recruitment, follow-up, final manuscript All others: recruitment, management, final manuscript.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Paluch-Shimon S., Cardoso F., Partridge A.H., Abulkhair O., Azim H.A., Bianchi-Micheli G. ESO–ESMO 4th international consensus guidelines for breast cancer in young women (BCY4) Ann Oncol. 2020;31(6):674–696. doi: 10.1016/j.annonc.2020.03.284. [DOI] [PubMed] [Google Scholar]
- 2.Villarreal-Garza C., Platas A., Miaja M., Fonseca A., Mesa-Chavez F., Garcia-Garcia M. Young women with breast cancer in Mexico: results of the Pilot Phase of the Joven & Fuerte prospective cohort. JCO Glob Oncol. 2020;6:395–406. doi: 10.1200/JGO.19.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fabiano V., Mandó P., Rizzo M., Ponce C., Coló F., Loza M. Breast cancer in young women presents with more aggressive pathologic characteristics: retrospective analysis from an Argentine national database. JCO Glob Oncol. 2020 Apr;6:639–646. doi: 10.1200/JGO.19.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liukkonen S., Leidenius M., Saarto T., Sjöström-Mattson J. Breast cancer in very young women. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2011 Dec;37(12):1030–1037. doi: 10.1016/j.ejso.2011.08.133. [DOI] [PubMed] [Google Scholar]
- 5.Azim H.A., Partridge A.H. Biology of breast cancer in young women. Breast Cancer Res. 2014;16(4):427. doi: 10.1186/s13058-014-0427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Report of national cancer registry programme. 2020. https://www.ncdirindia.org/All_Reports/Report_2020/default.aspx [Internet]. [cited 2021 Feb 14]. Available from: [Google Scholar]
- 7.Gogia A., Raina V., Deo S.V.S., Shukla N.K., Mohanti B.K. Young breast cancer: a single center experience. Indian J Cancer. 2014;51(4):604. doi: 10.4103/0019-509X.175332. [DOI] [PubMed] [Google Scholar]
- 8.Parmar V., Hawaldar R., Nadkarni M.S., Badwe R.A. Low axillary sampling in clinically node-negative operable breast cancer. Natl Med J India. 2009;22(5):234–236. [PubMed] [Google Scholar]
- 9.Tfayli A., Temraz S., Abou Mrad R., Shamseddine A. Breast cancer in low- and middle-income countries: an emerging and challenging epidemic. J Oncol. 2010;2010:490631. doi: 10.1155/2010/490631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryu J.M., Yu J., Kim S.I., Kim K.S., Moon H.-G., Choi J.E. Different prognosis of young breast cancer patients in their 20s and 30s depending on subtype: a nationwide study from the Korean Breast Cancer Society. Breast Cancer Res Treat. 2017;166(3):833–842. doi: 10.1007/s10549-017-4472-5. [DOI] [PubMed] [Google Scholar]
- 11.Kakarala M., Rozek L., Cote M., Liyanage S., Brenner D.E. Breast cancer histology and receptor status characterization in Asian Indian and Pakistani women in the U.S. - a SEER analysis. BMC Cancer. 2010;10(1):191. doi: 10.1186/1471-2407-10-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nair N., Shet T., Parmar V., Havaldar R., Gupta S., Budrukkar A. Breast cancer in a tertiary cancer center in India - an audit, with outcome analysis. Indian J Cancer. 2018;55(1):16–22. doi: 10.4103/ijc.IJC_484_17. [DOI] [PubMed] [Google Scholar]
- 13.Bhan N., Madhira P., Muralidharan A., Kulkarni B., Murthy G., Basu S. Health needs, access to healthcare, and perceptions of ageing in an urbanizing community in India: a qualitative study. BMC Geriatr. 2017;17(1):156. doi: 10.1186/s12877-017-0544-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dey S., Nambiar D., Lakshmi J.K., Sheikh K., Reddy K.S. Aging in Asia: findings from new and emerging data initiatives. National Academies Press (US); 2012. Health of the elderly in India: challenges of access and affordability [internet]https://www.ncbi.nlm.nih.gov/books/NBK109208/ [cited 2021 Sep 26]. Available from: [PubMed] [Google Scholar]
- 15.Li J., Wang S., Wang Y., Wang X., Wang H., Feng J. Disparities of trastuzumab use in resource-limited or resource-abundant regions and its survival benefit on HER2 positive breast cancer: a real-world study from China. Oncol. 2017;22(11):1333–1338. doi: 10.1634/theoncologist.2017-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adusumilli P., Konatam M.L., Gundeti S., Bala S., Maddali L.S. Treatment challenges and survival analysis of human epidermal growth factor receptor 2-positive breast cancer in real world. Indian J Med Paediatr Oncol Off J Indian Soc Med Paediatr Oncol. 2017;38(1):22–27. doi: 10.4103/0971-5851.203511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta N., Verma R.K., Gupta S., Prinja S. Cost effectiveness of trastuzumab for management of breast cancer in India. JCO Glob Oncol. 2020;6:205–216. doi: 10.1200/JGO.19.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasthuri A. Challenges to healthcare in India - the five A's. Indian J Community Med Off Publ Indian Assoc Prev Soc Med. 2018;43(3):141–143. doi: 10.4103/ijcm.IJCM_194_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahlin T., Nichter M., Pillai G. Health insurance in India: what do we know and why is ethnographic research needed. Anthropol Med. 2016;23(1):102–124. doi: 10.1080/13648470.2015.1135787. [DOI] [PubMed] [Google Scholar]
- 20.Munshi A., Ganesh T., Mohanti B.K. Radiotherapy in India: history, current scenario and proposed solutions. Indian J Cancer. 2019;56(4):359–363. doi: 10.4103/ijc.IJC_82_19. [DOI] [PubMed] [Google Scholar]
- 21.Datta N.R., Samiei M., Bodis S. Radiation therapy infrastructure and human resources in low- and middle-income countries: present status and projections for 2020. Int J Radiat Oncol Biol Phys. 2014;89(3):448–457. doi: 10.1016/j.ijrobp.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Villarreal-Garza C., Mohar A., Bargallo-Rocha J.E., Lasa-Gonsebatt F., Reynoso-Noverón N., Matus-Santos J. Molecular subtypes and prognosis in young Mexican women with breast cancer. Clin Breast Cancer. 2017;17(3) doi: 10.1016/j.clbc.2016.11.007. e95–102. [DOI] [PubMed] [Google Scholar]
- 23.Radecka B., Litwiniuk M. Breast cancer in young women. Ginekol Pol. 2016;87(9):659–663. doi: 10.5603/GP.2016.0062. [DOI] [PubMed] [Google Scholar]
- 24.Keegan T.H., DeRouen M.C., Press D.J., Kurian A.W., Clarke C.A. Occurrence of breast cancer subtypes in adolescent and young adult women. Breast Cancer Res. 2012;14(2):R55. doi: 10.1186/bcr3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Copson E., Eccles B., Maishman T., Gerty S., Stanton L., Cutress R.I. Prospective observational study of breast cancer treatment outcomes for UK women aged 18-40 years at diagnosis: the POSH study. J Natl Cancer Inst. 2013;105(13):978–988. doi: 10.1093/jnci/djt134. [DOI] [PubMed] [Google Scholar]
- 26.Collins L.C., Marotti J.D., Gelber S., Cole K., Ruddy K., Kereakoglow S. Pathologic features and molecular phenotype by patient age in a large cohort of young women with breast cancer. Breast Cancer Res Treat. 2012;131(3):1061–1066. doi: 10.1007/s10549-011-1872-9. [DOI] [PubMed] [Google Scholar]
- 27.Dubsky P.C., Gnant M.F.X., Taucher S., Roka S., Kandioler D., Pichler-Gebhard B. Young age as an independent adverse prognostic factor in premenopausal patients with breast cancer. Clin Breast Cancer. 2002;3(1):65–72. doi: 10.3816/CBC.2002.n.013. [DOI] [PubMed] [Google Scholar]
- 28.Maajani K., Jalali A., Alipour S., Khodadost M., Tohidinik H.R., Yazdani K. The global and regional survival rate of women with breast cancer: a systematic review and meta-analysis. Clin Breast Cancer. 2019;19(3):165–177. doi: 10.1016/j.clbc.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Thomas A., Rhoads A., Pinkerton E., Schroeder M.C., Conway K.M., Hundley W.G. Incidence and survival among young women with stage I–III breast cancer: SEER 2000–2015. JNCI Cancer Spectr. 2019;3(3):pkz040. doi: 10.1093/jncics/pkz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doval D.C., Radhakrishna S., Tripathi R., Kashinath R.I., Talwar V., Batra U. A multi-institutional real world data study from India of 3453 non-metastatic breast cancer patients undergoing upfront surgery. Sci Rep. 2020;10:5886. doi: 10.1038/s41598-020-62618-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin M., Pienkowski T., Mackey J., Pawlicki M., Guastalla J.-P., Weaver C. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352(22):2302–2313. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 32.Gianni L., Baselga J., Eiermann W., Porta V.G., Semiglazov V., Lluch A. Phase III trial evaluating the addition of Paclitaxel to doxorubicin followed by cyclophosphamide, methotrexate, and fluorouracil, as adjuvant or Primary systemic therapy: European cooperative trial in operable breast cancer. J Clin Oncol. 2009;27(15):2474–2481. doi: 10.1200/JCO.2008.19.2567. [DOI] [PubMed] [Google Scholar]
- 33.Mamounas E.P., Bryant J., Lembersky B., Fehrenbacher L., Sedlacek S.M., Fisher B. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(16):3686–3696. doi: 10.1200/JCO.2005.10.517. [DOI] [PubMed] [Google Scholar]
- 34.Bear H.D., Anderson S., Smith R.E., Geyer C.E., Mamounas E.P., Fisher B. Sequential Preoperative or Postoperative docetaxel added to Preoperative doxorubicin plus cyclophosphamide for operable breast cancer: national surgical adjuvant breast and bowel Project protocol B-27. J Clin Oncol. 2006;24(13):2019–2027. doi: 10.1200/JCO.2005.04.1665. [DOI] [PubMed] [Google Scholar]
- 35.Bajpai J., Simha V., Shylasree T.S., Sarin R., Pathak R., Popat P. Pregnancy associated breast cancer (PABC): report from a gestational cancer registry from a tertiary cancer care centre, India. Breast Edinb Scotl. 2021;56:88–95. doi: 10.1016/j.breast.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armstrong N., Ryder S., Forbes C., Ross J., Quek R.G. A systematic review of the international prevalence of BRCA mutation in breast cancer. Clin Epidemiol. 2019;11:543–561. doi: 10.2147/CLEP.S206949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wenners A., Grambach J., Koss J., Maass N., Jonat W., Schmutzler A. Reduced ovarian reserve in young early breast cancer patients: preliminary data from a prospective cohort trial. BMC Cancer. 2017;17(1):632. doi: 10.1186/s12885-017-3593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peccatori F.A., Azim H.A., Orecchia R., Hoekstra H.J., Pavlidis N., Kesic V. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. 2013;24(Suppl 6) doi: 10.1093/annonc/mdt199. vi160–170. [DOI] [PubMed] [Google Scholar]
- 39.Marklund A., Lundberg F.E., Eloranta S., Hedayati E., Pettersson K., Rodriguez-Wallberg K.A. Reproductive outcomes after breast cancer in women with vs without fertility preservation. JAMA Oncol. 2021;7(1):1–6. doi: 10.1001/jamaoncol.2020.5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vriens I.J.H., Ter Welle-Butalid E.M., de Boer M., de Die-Smulders C.E.M., Derhaag J.G., Geurts S.M.E. Preserving fertility in young women undergoing chemotherapy for early breast cancer; the Maastricht experience. Breast Cancer Res Treat. 2020;181(1):77–86. doi: 10.1007/s10549-020-05598-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.