Abstract
Introduction:
Evaluate outcomes and radiation exposure across different splenic artery embolization (SAE) techniques for splenic injuries secondary to blunt trauma.
Methods:
This retrospective cohort study included patients 18 years of age or older who underwent SAE for splenic injury after blunt trauma from January 2011 to June 2019.
Results:
Sixty patients underwent angiography for splenic injury after blunt traumatic injury. Forty-four patients were embolized. Seventeen patients underwent proximal SAE, and 23 underwent distal SAE. Four patients had a combination of proximal and distal SAE. Eleven patients had subsequent major complications requiring splenectomy. There was no significant difference in major complication rate when comparing proximal SAE 29.4% versus distal SAE 21.7%. No significant difference was noted across the two groups with respect to age or grade of injury. There was a statistically significant difference (P = 0.004) in fluoroscopy time between the proximal 10.1 ± 4.2 min and distal group 17.8 ± 8.7 min. No statically significant difference was found in major complications when comparing coil versus gel foam embolization.
Conclusion:
Proximal SAE is associated with a significantly lower fluoroscopy time (P = 0.004). Complication rates are similar after proximal and distal SAE. No significant difference was found in major complication rates comparing coil versus gel foam embolization. Minor complications more commonly occurred after proximal embolization with gel-foam.
Keywords: Splenic artery embolization, splenic laceration, splenic trauma
INTRODUCTION
The spleen is the most commonly injured solid organ during a blunt traumatic injury.[1] Over the past few decades, nonoperative management has become the mainstay of treatment for lower grade injuries based on the American Association for the Surgery of Trauma grading system.[2] Splenic artery embolization (SAE) is a key tool in nonoperative management. SAE provides patients with a quick, minimally invasive treatment for acute splenic hemorrhage.[3,4] However, there is varying data on the outcomes of these patients with regard to technical factors. There are several important technical considerations. The most controversial is the location of embolization proximal or main artery embolization versus distal selective embolization. In addition, there is conflicting data on the use of different embolic agents and their respective complication rates.[5] The purpose of the review was to evaluate radiation dose and complication rates after SAE for blunt splenic trauma. A major aim was to determine if there is a difference in complication rate or fluoroscopy times between proximal and distal SAE s. In addition, this study examined potential differences in efficacy and complication rates across different embolic agents, including coils, gel foam, and vascular plugs.
METHODS
The Research Subjects Review Board approved this retrospective cohort study. Visceral Angiograms performed at a level 1 trauma center from January 2011 to June 2019 were evaluated. Patients over the age of 18 years who underwent catheter-based angiogram for splenic injury were identified. All angiograms were performed by a radiology resident or fellow under the direct supervision of an interventional radiology attending. Cases were excluded if performed for penetrating trauma or iatrogenic injury. The patient's age, body mass index (BMI), mechanism of injury, concomitant injuries, the grade of injury based on imaging, time of angiography, angiographic findings, fluoroscopy time, radiation dose, contrast administered, vessel embolized, and embolic used were recorded. Angiographic findings were defined as positive if there was active extravasation, contrast blush, or pseudoaneurysm. Radiation dose was recorded as cumulative air kerma (AK) in Gy and incident Kerma Area Product (KAP) in Gy x cm2. The effective dose was calculated using a conversion factor E/KAP of 0.14.[6] KAP/BMI was also used as a surrogate marker to account for differences in body habitus. Proximal embolization was defined as occlusion of the main trunk of the splenic artery distal to the great pancreatic artery [Figure 1]. Distal embolization was defined as the embolization of one or more of the terminal splenic artery branches [Figure 2]. Additionally, patient charts were examined in the 90 days following intervention to identify major complications. Major complications included nontarget embolization, splenic necrosis, or continued bleeding requiring splenectomy. Minor complications not requiring further intervention were also recorded. Minor complications included infarct, infection, or bleeding.
Figure 1.
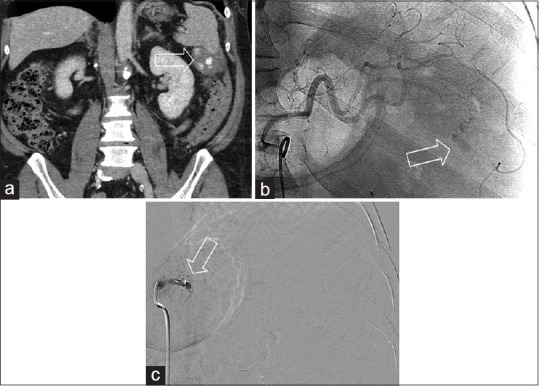
Proximal plug embolization. A patient struck while riding a bicycle. (a) Coronal contrast-enhanced computed tomography demonstrates active bleeding and pseudoaneurysm formation at the inferior aspect of the spleen (white arrow). (b) The initial angiographic image demonstrates extravasation (white arrow) (c) angiogram after proximal deployment of an 8 mm Amplatzer plug demonstrates no further flow to the spleen
Figure 2.
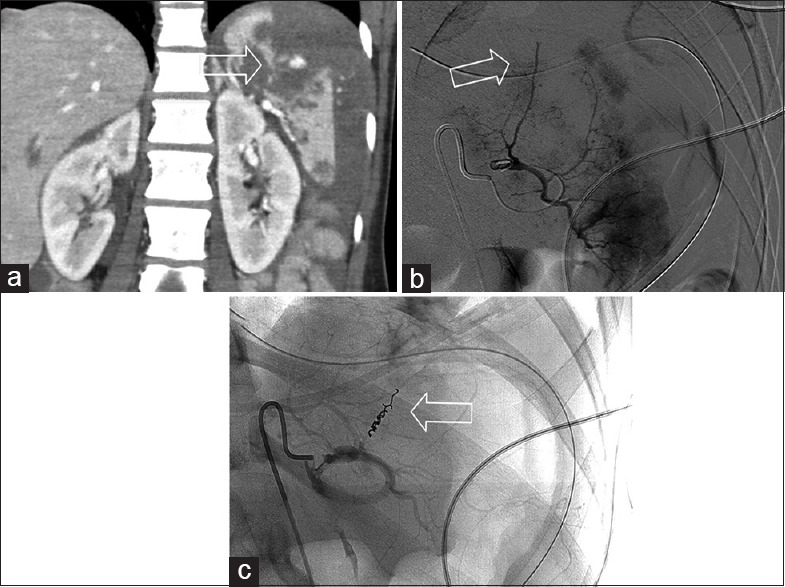
Distal micro coil embolization. An unrestrained driver during a motor vehicle collision. (a) Coronal contrast-enhanced computed tomography demonstrates multiple splenic lacerations with a pseudoaneurysm formation at the superior aspect. (b) Catheter angiography demonstrates an abnormal truncated vessel in the upper pole of spleen consistent with the known injury. (c) Post distal micro coil embolization of the superior branch
Statistical methods
Comparisons were made using Unpaired Students t-test across continuous variables including age, fluoroscopy time, BMI, AK, KAP, KAP/BMI, and contrast administered assuming equal variances. Fischer's exact test was used to compare groups across positive findings and major complications. A P value of 0.05 or less was considered statistically significant.
RESULTS
A total of 60 angiograms performed for splenic injury following blunt trauma were identified. Out of these, a total of 44 patients underwent embolization. Seventeen patients underwent proximal embolization, and 23 underwent distal embolization [Table 1]. Four patients had a combination of proximal and distal embolization, and these were not included in the analysis. The majority of injuries involved occupants of motor vehicles in a collision. Seventy percent of patients were male. There were similar rates of concomitant injuries in both groups [Table 1]. There was a significantly larger percentage of patients with active extravasation or pseudoaneurysm on angiogram in the distal group, 91% versus 47% in the proximal group. Among these, there were similar rates of patients with pseudoaneurysms in both groups. There was no significant difference between the two groups with respect to major complications, 29.4% in the proximal group and 21.7% in the distal group. In the distal group, four patients required splenectomy due to concern for re-bleeding. Of these two were suspected grade 5 injuries in the distal group, one required splenectomy due to persistent bleeding. In the proximal group, five patients required splenectomy, two patients secondary to persistent bleeding, including one grade five injury. Two patients had splenic necrosis, and one developed an abscess. All these patients underwent splenectomy. There were no cases of nontarget embolization. No patients underwent repeat embolization. Among patients with subsequent cross-sectional imaging, one demonstrated evidence of partial infarct after distal SAE (n = 5, follow-up time: 0.12–4.6 years) and two with partial infarcts after proximal SAE (n = 5, follow-up time: 0.03–4.9 years). No infarcts required subsequent intervention.
Table 1.
Patient demographics and complication rates
| Embolization | Proximal, n (%) | Distal, n (%) | P |
|---|---|---|---|
| Total patients | 17 (42.5) | 23 (57.5) | |
| Male | 11 (64.7) | 17 (73.9) | |
| Age (years)±SD | 45.2±18.1 | 42.3±13.0 | 0.563 |
| Mechanism | |||
| Fall | 1 (5.9) | 6 (26) | |
| Motor vehicle collision (occupant) | 8 (47) | 10 (43.4) | |
| Motorcycle collision (rider or passenger) | 2 (11.8) | 4 (17.4) | |
| Pedestrian struck | 2 (11.8) | 0 | |
| Assault | 0 | 2 (8.7) | |
| Concomitant injury | |||
| Intracranial hemorrhage | 5 (29.4) | 1 (4.3) | |
| Fracture | 10 (58.8) | 17 (73.9) | |
| Solid organ injury | 1 (5.9) | 5 (21.3) | |
| AAST grade of injury | |||
| Uncertain | 0 | 1 (4.3) | |
| II | 3 (17.6) | 5 (21.7) | |
| III | 8 (47.1) | 11 (47.8) | |
| IV | 4 (23.5) | 5 (21.7) | |
| V | 1 (5.9) | 2 (8.7) | |
| Angiographic findings | |||
| Positive | 8 (47) | 21 (91) | 0.0034* |
| Pseudoaneurysm | 2 (11.7) | 4 (19) | |
| Complication requiring splenectomy | |||
| Total | 5 (29.4) | 4 (17.4) | 0.456 |
| Rebleeding | 2 (11.8) | 4 (17.4) | |
| Abscess | 1 (5.9) | 0 | |
| Necrosis | 2 (11.7) | 0 |
*Fischer’s extact test demonstrated a significant difference in positive angiographic findings across both groups. AAST: American Association for the Surgery of Trauma, SD: Standard deviation
Comparisons related to radiation dose were compared across the two groups for patients whose data were available. There was a significant difference in fluoroscopy time 10.1 (±4.2) min in the proximal group and 17.8 (±8.7) min in the distal group (P = 0.004) [Table 2]. In addition, the AK, KAP, and KAP/BMI were compared between the two groups. This difference was not significant.
Table 2.
Comparision of radiation, exposure, contrast and body mass index across proximal and distal embolizations
| Radiation and contrast | Proximal (n=11) | Distal (n=16) | P |
|---|---|---|---|
| Fluoroscopy time, min (SD) | 10.14±4.2 | 17.88±8.7 | 0.004** |
| Cummulative AK, Gy (SD) | 1.89±1.35 | 1.24±0.86 | 0.011 |
| Air KAP, Gy*cm2 (SD) | 376.6±287.3 | 295.5±176.3 | 0.39 |
| Effective dose (mSv/Gy*cm2) | 52.7 | 41.3 | |
| BMI (kg/m2) | 32.5±5.9 | 30.2±6.4 | 0.35 |
| KAP/BMI | 11.1±7.8 | 9.45±4.8 | 0.51 |
| Contrast administered (cc) (SD) | 75.4±35 | 97.3±40 | 0.11 |
**Two-tailed t-test assuming equal variances demonstrated a significant difference across fluoroscopy time. SD: Standard deviation, AK: Air Kerma, KAP: Kerma area product, BMI: Body mass index
When comparing fluoroscopy time across location and embolic used proximal embolization with gel foam was the shortest 7.8 min (±2.9 min) followed by proximal embolization with coils 10.8 min (±4.2 min). Distal embolization with gel foam required more fluoroscopy time with gel foam 20.3 min (±13.6) than with coils 16.7 min (±9.9). As a whole coil embolization required on average 14.2 min (±8.8 min) fluoroscopy time compared with 16.5 min (±12.8 min) for gel foam. Proximal occlusion with vascular plugs averaged 11.2 min (±5.45) Statistical analysis was not performed on these metrics due to the small sample sizes and inherent variability of the data.
The average contrast used during proximal embolization was lower compared with distal embolization, although this difference was not significant. No significant difference was found in major complications when comparing coil versus gel foam embolization [Table 3]. No complications occurred after proximal SAE with vascular occlusion plugs.
Table 3.
Major complications across embolic device
| Major complication | Yes | No | Total |
|---|---|---|---|
| Coil | 5 | 8 | 13 |
| Gel foam | 5 | 19 | 24 |
| Vascular plug | 0 | 3 | 3 |
Fischer exact test did not demonstrate a significant difference in major complication between coil and gel foam embolization (P=0.275)
DISCUSSION
Splenic arterial embolization has been a mainstay of treating blunt traumatic injuries for over 30 years. There have been varying data on proximal versus distal embolization. Prior studies have not demonstrated significant differences with regards to re-bleeding when comparing proximal to distal embolization. However, complications not requiring splenectomy were more common after distal embolization.[5] This is likely due to the lack of collateral flow after distal embolization resulting in segmental infarction.[7]
Our results were similar to prior studies with regard to complication rate [Table 3].[4,7] However, there were slightly higher rates of postprocedural necrosis and abscess after proximal embolization in our cohort when compared to distal embolization. This is likely due to the small sample size of our cohort. Furthermore, the embolic used in all three of these cases with subsequent complications was gel-foam. It is hypothesized that the gel form may have migrated distal to the site of embolization resulting in segmental infarction.[4] In addition, due to the preparation of the gel foam slurry in room air, there is a higher possibility of introducing infectious pathogens.[8,9] Prior studies have demonstrated higher complication rates with gel foam embolization compared with coils.[8,9] This was suggested by our data set, but when comparing embolic choice in aggregate, there was no significant difference. This is likely due to a lack of power [Table 2]. The complication rates after plug occlusion could not be statistically evaluated due to the limited number of cases (n = 3).
When comparing fluoroscopy time across the two groups, proximal embolization required shorter fluoroscopy time, on average, approximately 10 min compared with 17 min in the distal group [Table 2]. This was a meaningful difference for two main reasons. First, this serves as a proxy for reduced procedure time, which is especially useful in trauma patients who often have concomitant injuries.[10] Second, there is reduced radiation exposure to both the patient and providers. However, this difference was not statistically different in our cohort, even when accounting for differences in BMI. Interestingly, the BMI in the proximal groups was larger than the distal group and is a likely confounder.
When comparing fluoroscopy time across location and embolic used, proximal embolization with gel foam was the shortest 7.8 min (±2.9 min) followed by proximal embolization with coils 10.8 min (±4.2 min). In contrast, distal embolization required greater fluoroscopy time with gel foam 20.3 min (±13.6) than with coils 16.7 min (±9.9), although these cases were very variable. These data suggest that location plays a greater role than embolic with regard to fluoroscopy time. In aggregate, coil embolization required, on average, 14.2 min (±8.8 min) fluoroscopy time compared with 16.5 min (±12.8 min) for gel foam regardless of location. This contrasts prior work suggesting that gel foam requires shorter procedure times. However, our data were variable, and prior work by Rasuli did not stratify the data across embolization locations.[10]
Beyond location and embolic, several other possible confounding variables, including provider experience, suite characteristics, anatomic differences, etc., can affect fluoroscopy time and dose. These factors limit an accurate assessment and may explain the slightly higher averages noted in the proximal group. A more comprehensive control study would be needed to assess radiation doses across embolism techniques definitively. In addition, our analysis of radiation was limited to a smaller sample due to incomplete records.
On average, there was a lower amount of contrast used in proximal embolization relative to distal embolization. This is expected as it usually requires fewer angiographic runs to obtain distal access. This difference may have beneficial implicants for postcontrast acute kidney injury. As a whole, these findings support proximal embolization instead of distal embolization. These recommendations are in line with several contemporary papers examining splenic embolization techniques.[4,7,8]
There are several important caveats to consider. In this review, a larger portion of patients in the proximal group underwent embolization despite a lack of positive angiographic findings. This may potentially confound results as these patients may have had a lower propensity to re-bleed. Prior work has shown a significant portion of 14.7% of patients with negative angiograms have delayed bleeding requiring re-intervention, including laparotomy or embolization.[11] Lower rates have been seen in studies that employed empiric embolization.[4] In addition, there may have been several clinical and patient-specific reasons to perform empiric embolization in both groups, such as dropping hematocrit and prior computed tomography findings. The rationale for empiric embolization is to reduce intrasplenic blood pressure, which would, in turn, facilitate further clot formation and splenic healing even in cases where continued bleeding was not angiographically visualized.
Aside from this difference, there were several additional limitations to this review. The retrospective approach does not account for several other types of bias, as there may have been underlying variables not accounted for leading to proximal or distal embolization, including provider experience, provider preference, patient-based factors, among others. In addition, other factors, including varied postprocedural patient management and patient records, limited a more comprehensive analysis. For example, postoperative antibiotic therapy was not evaluated nor standardized in this study and may have played a role in the rates of postprocedural complication. This review was limited to a tertiary level one trauma care center. As such, the findings here may not be generalizable to centers with a different case-mix and provider experience. Additionally, the majority of embolics used were coils and gel foam. This limits the applicability of these results at other centers where other embolic, such as glue and particles, may be used.
As a whole, the data support the use of proximal embolization over distal embolization, but the benefits of lower procedure time and radiation dose may be less clinically relevant in some cases. Performing distal embolization may be warranted in specific cases, for example, distal arteriovenous fistula, isolated pseudoaneurysm, extracapsular bleeding, etc. These considerations may provide increased splenic salvage, despite the risk of segmental infarction relative to proximal embolization depending on the clinical scenario and lead to improved outcomes.[12] Additionally, proximal embolization may limit access to distal vasculature and prevents reintervention, for example, in cases of persistent collateral bleeding, and distal pseudoaneurysm. However, no patients in our cohort required re-embolization. Patients with persistent bleeding were treated conservatively or went on to splenectomy. As in all interventional cases, the choice of embolic location and device should be decided on after weighing a number of important factors.
CONCLUSION
In this study, major complications requiring splenectomy were similar between proximal and distal splenic embolization. Proximal SAE was associated with a significantly lower fluoroscopy time. No significant difference was found between coil or gel foam embolization with regard to major complications. Minor complications, including abscess and necrosis, were noted in patients who underwent proximal embolization with gel foam. As a whole, the data support the use of proximal splenic embolization over distal embolization when clinically appropriate, as it results in reduced fluoroscopy time and does not impact complication rate.
Research quality and ethics statement
This study was approved by the Institutional Review Board IRB number #STUDY00004069. The authors followed applicable EQUATOR Network (http://www.equator-network.org/) guidelines during the conduct of this research project.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank David Conover and Dr. Thomas H. Foster for their assistance.
REFERENCES
- 1.Martin JG, Shah J, Robinson C, Dariushnia S. Evaluation and management of blunt solid organ trauma. Tech Vasc Interv Radiol. 2017;20:230–6. doi: 10.1053/j.tvir.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Fodor M, Primavesi F, Morell-Hofert D, Kranebitter V, Palaver A, Braunwarth E, et al. Non-operative management of blunt hepatic and splenic injury: A time-trend and outcome analysis over a period of 17 years. World J Emerg Surg. 2019;14:29. doi: 10.1186/s13017-019-0249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imbrogno BF, Ray CE. Splenic artery embolization in blunt trauma. Semin Intervent Radiol. 2012;29:147–9. doi: 10.1055/s-0032-1312577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahuja C, Farsad K, Chadha M. An overview of splenic embolization. AJR Am J Roentgenol. 2015;205:720–5. doi: 10.2214/AJR.15.14637. [DOI] [PubMed] [Google Scholar]
- 5.Schnüriger B, Inaba K, Konstantinidis A, Lustenberger T, Chan LS, Demetriades D. Outcomes of proximal versus distal splenic artery embolization after trauma: A systematic review and meta-analysis. J Trauma. 2011;70:252–60. doi: 10.1097/TA.0b013e3181f2a92e. [DOI] [PubMed] [Google Scholar]
- 6.Compagnone G, Giampalma E, Domenichelli S, Renzulli M, Golfieri R. Calculation of conversion factors for effective dose for various interventional radiology procedures. Med Phys. 2012;39:2491–8. doi: 10.1118/1.3702457. [DOI] [PubMed] [Google Scholar]
- 7.Quencer KB, Smith TA. Review of proximal splenic artery embolization in blunt abdominal trauma. CVIR Endovasc. 2019;2:11. doi: 10.1186/s42155-019-0055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rong JJ, Liu D, Liang M, Wang QH, Sun JY, Zhang QY, et al. The impacts of different embolization techniques on splenic artery embolization for blunt splenic injury: A systematic review and meta-analysis. Mil Med Res. 2017;4:17. doi: 10.1186/s40779-017-0125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayes A, Laroia S, Becker R, Guan J, Morales S, Laroia A. Perisplenic abscess formation after splenic artery embolization with gelfoam versus coil embolization. J Vasc Interv Radiol. 2016;27:S105. [Google Scholar]
- 10.Rasuli P, Moosavi B, French GJ, Petrcich W, Hammond I. Splenic artery embolization in blunt trauma: A Single-center retrospective comparison of the use of gelatin sponge versus coils. AJR Am J Roentgenol. 2017;209:W382–7. doi: 10.2214/AJR.17.18005. [DOI] [PubMed] [Google Scholar]
- 11.Haan J, Scott J, Boyd-Kranis RL, Ho S, Kramer M, Scalea TM. Admission angiography for blunt splenic injury: Advantages and pitfalls. J Trauma. 2001;51:1161–5. doi: 10.1097/00005373-200112000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Gauer JM, Gerber-Paulet S, Seiler C, Schweizer WP. Twenty years of splenic preservation in trauma: Lower early infection rate than in splenectomy. World J Surg. 2008;32:2730–5. doi: 10.1007/s00268-008-9733-3. [DOI] [PubMed] [Google Scholar]


