Abstract
The bacterial genus Methylococcus, which comprises aerobic thermotolerant methanotrophic cocci, was described half-a-century ago. Over the years, a member of this genus, Methylococcus capsulatus Bath, has become a major model organism to study genomic and metabolic basis of obligate methanotrophy. High biotechnological potential of fast-growing Methylococcus species, mainly as a promising source of feed protein, has also been recognized. Despite this big research attention, the currently cultured Methylococcus diversity is represented by members of the two species, M. capsulatus and M. geothermalis, while finished genome sequences are available only for two strains of these methanotrophs. This study extends the pool of phenotypically characterized Methylococcus strains with good-quality genome sequences by contributing four novel isolates of these bacteria from activated sludge, landfill cover soil, and freshwater sediments. The determined genome sizes of novel isolates varied between 3.2 and 4.0Mb. As revealed by the phylogenomic analysis, strains IO1, BH, and KN2 affiliate with M. capsulatus, while strain Mc7 may potentially represent a novel species. Highest temperature optima (45–50°C) and highest growth rates in bioreactor cultures (up to 0.3h−1) were recorded for strains obtained from activated sludge. The comparative analysis of all complete genomes of Methylococcus species revealed 4,485 gene clusters. Of these, pan-genome core comprised 2,331 genes (on average 51.9% of each genome), with the accessory genome containing 846 and 1,308 genes in the shell and the cloud, respectively. Independently of the isolation source, all strains of M. capsulatus displayed surprisingly high genome synteny and a striking similarity in gene content. Strain Mc7 from a landfill cover soil differed from other isolates by the high content of mobile genetic elements in the genome and a number of genome-encoded features missing in M. capsulatus, such as sucrose biosynthesis and the ability to scavenge phosphorus and sulfur from the environment.
Keywords: Methylococcus, methanotrophic bacteria, cultivation studies, comparative genomics, pan-genome analysis, growth on methane, phage-associated regions
Introduction
The genus Methylococcus was described in 1966 by Foster and Davis, who isolated a culture of coccus-shaped bacteria capable of growth in a mineral medium with methane (CH4) as the only carbon source from a sludge sample of the Austin municipal sewage plant, Texas (Foster and Davis, 1966). After description of “Pseudomonas methanica” (Dworkin and Foster, 1958; currently known as Methylomonas methanica), this was the second documented case of an organism, which consumes CH4 for growth. The original description of the genus Methylococcus characterized its members as non-motile, obligately aerobic, Gram-negative cocci, approximately 1μm in diameter, with a characteristic diplococcoid arrangement, which utilize methane or methanol as the only carbon sources. The species epithet capsulatus was assigned to these bacteria in order to denote the presence of capsules revealed by staining cell specimens with the India ink. The originally described isolate, strain Texas, was defined as the type strain of the species Methylococcus capsulatus. Several later proposed species of this genus, such as “M. bovis,” “M. chroococcus,” “M. luteus,” “M. vinelandii,” and “M. whittenburyi” (Romanovskaya et al., 1981), were transferred to the genus Methylobacter after detailed re-examination of their characteristics (Bowman et al., 1993). The species M. thermophilus (Malashenko et al., 1975) has been retained in the genus Methylococcus (Bowman et al., 1993), but the type strain of this species is no longer available from culture collections. The single strain making up the species Methylococcus mobilis (Hazeu et al., 1980) has also been lost. Apparently, all Methylococcus species are difficult to preserve (Bowman, 2015), which is the main reason behind poor availability of these bacteria from both public and laboratory culture collections. Recently described Methylococcus geothermalis (Awala et al., 2020) is the second described species of the genus, which is currently available in culture besides M. capsulatus.
Members of the genus Methylococcus are thermotolerant or moderately thermophilic bacteria, with optimal growth between 40–60°C (Bowman, 2015). They have been isolated from sewage sludge, sediments of rivers and ponds, wastewater of coal mines, and geothermal fields (Malashenko et al., 1975; Bowman et al., 1993). As suggested by culture-independent studies, Methylococcus-like methanotrophs can also be detected in landfill cover soils (Gebert et al., 2009), geothermal soils (Gagliano et al., 2014), and hot springs (Kizilova et al., 2014; Houghton and Stewart, 2020). Cells of these bacteria possess both known types of methane monooxygenase (MMO), which catalyzes the oxidation of methane to methanol, i.e., particulate (pMMO) and soluble (sMMO) forms of this enzyme. Taxonomically, the genus Methylococcus belongs to the gammaproteobacterial family Methylococcaceae, which accommodates the so-called type I methanotrophs. The latter utilize the ribulose monophosphate pathway (RuMP) for formaldehyde assimilation, while alphaproteobacterial type II methanotrophs employ the serine pathway. One specific feature of the metabolic organization of Methylococcus and Methylococcus-related methanotrophs is that, in addition to RuMP pathway, they also possess the Calvin–Benson–Bassham (CBB) cycle. This was one of the key reasons to consider these bacteria as representing a separate, type X, methanotrophs (Whittenbury, 1981; Whittenbury and Dalton, 1981). Most cultured representatives of the genus Methylococcus did not receive much research attention. One clear exception is M. capsulatus strain Bath, which was extensively studied and gradually became probably the best characterized of the aerobic methanotrophs in terms of genetics and physiology (Anthony, 1983; Dalton, 2005; Murrell, 2010). All major insights into MMO structure and function were made by using M. capsulatus Bath. Those include studies on resolving the structure of soluble and particulate MMO (Colby and Dalton, 1978; Rosenzweig et al., 1993; Zahn and DiSpirito, 1996; Müller et al., 2002; Chatwood et al., 2004; DiSpirito et al., 2004; Lieberman and Rosenzweig, 2005; Balasubramanian et al., 2010; Ross et al., 2019) as well as control of the MMOs expression based on copper-to-biomass ratios known as a “copper switch” (Stanley et al., 1983; Kao et al., 2004; Larsen and Karlsen, 2016). Methylococcus capulatus Bath was also the first methanotrophic organism for which a genome was published (Ward et al., 2004). This bacterium has a relatively small genome (3.3Mb) compared to other methanotrophs. The genome contains two copies of the gene clusters encoding pMMO and one copy of the sMMO gene cluster as well as mxa and xox operons encoding two alternative types of methanol dehydrogenases (MDHs). Primary route for carbon assimilation is the RuMP pathway although genes for the serine pathway, and the CBB cycle are also present. Routes for nitrogen assimilation include ammonia assimilation enzymes (glutamine synthetase, glutamate synthase, and alanine dehydrogenase) and nitrogenase. Genome contains genes encoding all tricarboxylic acid (TCA) cycle enzymes, but the absence of 2-oxoglutarate dehydrogenase activity in vitro still suggests that the Krebs cycle cannot operate in M. capsulatus Bath (Murrell, 2010). To date, two metabolic models were published based on the genome sequence of M. capsulatus Bath (Gupta et al., 2018; Lieven et al., 2018). Experimental verification of metabolic features of this methanotroph was performed in transcriptomic and proteomic studies (Kao et al., 2004; Larsen and Karlsen, 2016).
High biotechnological potential of fast-growing Methylococcus species has also been recognized. In particular, the conversion of methane to biomass by M. capsulatus has been exploited for large-scale commercial production of microbial proteins by fermentation (Skrede et al., 1998). The biomass of M. capsulatus Bath was approved as a promising source of protein based on criteria such as amino acid composition, digestibility, and animal performance and health (Skrede et al., 2009). Single-cell protein based on M. capsulatus is suitable for fish feeding as besides good nutritional value it was proved to prevent soybean meal-induced enteritis in Atlantic salmon (Romarheim et al., 2011). Methylococcus capsulatus Bath was also explored for the potential of generating electricity directly from methane (Jawaharraj et al., 2021).
Despite the big research interest in methanotrophs of the genus Methylococcus, the number of currently available good-quality genome assemblies of Methylococcus species is limited to those of M. capulatus Bath and M. geothermalis IM1T. Besides these two finished genomes, 24 draft genome assemblies for members of the genus Methylococcus have been deposited in GenBank. Of these, 21 assemblies represent metagenome-assembled genomes (MAGs). This lack of good-quality genome sequences limits the potential of comparative genomic studies as well as our understanding of the variability of genome-encoded features within the genus Methylococcus.
This study was initiated in order to expand the narrow range of currently available Methylococcus cultures by isolating novel strains of these bacteria from various habitats. Our work resulted in obtaining four new strains of the genus Methylococcus and the corresponding complete genomes. These newly obtained genomes as well as all available complete genome sequences of other Methylococcus strains were compared in order to examine variability of genome-encoded features within this genus. Despite being isolated from geographically remote habitats, all strains of the species M. capsulatus displayed surprisingly high genome synteny and a striking similarity in gene content. By contrast, one novel isolate from a landfill cover soil possessed a number of genome-encoded features missing in M. capsulatus, such as sucrose biosynthesis and the ability to scavenge phosphorus and sulfur from the environment. This isolate may potentially represent a novel species of the genus Methylococcus.
Materials and Methods
Isolation Procedures
In this study, activated sludge, freshwater sediment, and landfill cover soil were used as sources for isolation of new Methylococcus strains. Activated sludge samples were collected in bottles (500ml each) from two municipal wastewater treatment plants in Moscow, Russia. Freshwater sediment samples were collected in 50ml falcon tubes from beneath shallow water in an unnamed lake in Krasnodar region, Russia. Surface (depth of 0–5cm) soil samples were collected from a landfill site in Khanty-Mansiysk region. Aliquots of collected samples were used as inoculum to obtain enrichment cultures of methanotrophic bacteria. The latter were obtained using modified AMS medium (mAMS), containing (in grams per liter of distilled water) NH4Cl, 0.1; MgSO4×7H2O, 0.2; CaCl2×2H2O, 0.02; 100mM phosphate buffer, pH 5.8, 1% (vol/vol); and trace element solution 0.1% (vol/vol), containing the following (g/L): EDTA, (in grams per liter) EDTA, 5; FeSO4×7H2O, 2; ZnSO4×7H2O, 0.1; MnCl2×4H2O, 0.03; CoCl2×6H2O, 0.2; CuSO4×5H2O, 0.1; NiCl2×6H2O, 0.02; and Na2MoO4, 0.03. After inoculation, 500ml bottles were sealed with silicone rubber septa, and methane was added aseptically using a syringe equipped with a disposable filter (0.22μm) to achieve a 10–20% mixing ratio in the headspace. Bottles were incubated on a rotary shaker (150 r.p.m.) at 42°C. After 1week of incubation, the cultures enriched with methanotrophic bacteria were subjected to serial dilutions. After several serial dilution steps, cell suspensions were plated on agar-solidified mAMS medium. The plates were incubated at 42°C in desiccators containing approximately 30% methane in air. The colonies appearing on the plates were picked and restreaked on the same agar medium. The set of finally selected colonies was subjected to several additional serial dilution steps in a liquid mAMS medium at 42°C until isolates of methanotrophic bacteria were obtained. Culture purity was verified by examination using phase-contrast microscopy and by plating on 10-fold diluted Luria–Bertani agar (1.0% tryptone, 0.5% yeast extract, 1.0% NaCl).
Morphological Characterization and Growth Tests
Prior to cell size measurements and growth experiments, the isolates were maintained in liquid mAMS medium with 20% (v/v) CH4 at 42°C for 10days, with regular transfers each 2–3days. Methylococcus capulatus Bath was used as a reference organism in all experiments. Morphological observations and cell-size measurements were made with a Zeiss Axioplan 2 microscope and Axiovision 4.2 software (Zeiss). Cell sizes were measured for 20 randomly selected cells of all strains. Comparative analysis of growth characteristics of the isolates was performed by monitoring their growth dynamics in liquid mineral medium mAMS with 20% methane in the headspace within the temperature range of 25–55°C. Variations in the pH were achieved by mixing 0.1 M solutions of H3PO4, KH2PO4, and K2HPO4. All incubations were performed in triplicate. Growth dynamics was determined by measuring OD600 of the cultures on an Eppendorf Biophotometer AG spectrophotometer. The ability to grow on methanol was tested in mAMS medium containing 0.01–3% (v/v) CH3OH. Nitrogen sources were tested by replacing NH4Cl in mAMS with 0.01% (w/v) NaNO3, NaNO2, urea, methylamine, glutamine, glycine, alanine, peptone, and yeast extract. Growth was examined after 3days of incubation. Salt tolerance was examined by adding NaCl to mAMS medium in concentrations of 0–2% (w/v).
Experiments on continuous cultivation were conducted in a 1.5L bioreactor filled with 1L of mineral medium. The inlet gases were methane with a flow rate of 100mlmin−1 and air pumped in with an air compressor with a flow rate of 500mlmin−1. The pH level of 5.6 was controlled by titration with 0.8% NH4OH solution. Agitation was kept constant at 1,000 r.p.m. All gases were filtered with a 0.22μm sterile membrane. Each of the growth experiments started as a batch cultivation. When the culture reached exponential phase, bioreactor was switched to a continuous mode at a dilution rate of 0.05h−1. The later was increased with an increment of 0.05h−1 every 1–2days until a culture washout was observed. After that, the dilution rate was decreased by 0.05h−1 and bioreactor was operated in a continuous mode for the next 5days.
Hydrogen Utilization
Cells of Methylococcus strains were pre-grown to the exponential phase in 120-ml serum bottles containing 20ml of mAMS medium. Hydrogen utilization was tested with and without the addition of methane under fully aerobic conditions. Headspace of experimental bottles was filled with either 2% CO2, 2% H2 and 20% CH4 or only 2% H2 and 2% CO2. Strains were cultivated for 24h using the same cultivation conditions as described above. Control treatments contained 2% CO2 or 20% CH4 and 2% CO2 in the headspace. Methane and hydrogen were measured by gas chromatography at the beginning of the experiment and after 24h of cultivation. The optical density was measured at 600nm wave length (OD600) using Eppendorf Biophotometer UV/Vis spectrophotometer (Eppendorf, Germany).
DNA Extraction
Cultures of new isolates were grown in the liquid mAMS as described above. The cells were harvested after incubation at 42°C on a rotary shaker at 150rpm for 2days. Genomic DNA extraction was done using the standard CTAB and phenol-chloroform protocol (Wilson, 2001).
Genome Sequencing and Annotation
Genomic libraries suitable for MiSeq sequencing were prepared with a NEBNext ultra II DNA Library kit (New England Biolabs). On average, a total of 1.68 million paired-end reads (2×300; 250nt) were obtained for each genome. Nanopore sequencing library was prepared using the 1D ligation sequencing kit (SQK-LSK108, Oxford Nanopore, United Kingdom). Sequencing was performed on an R9.4 flow cell (FLO-MIN106) using MinION device. Hybrid assembly of short and long reads was performed using Unicycler v.0.4.8 (Wick et al., 2017). Assemblies were evaluated with Quast 5.0 (Gurevich et al., 2013) and Busco 5.1.2 (Simão et al., 2015).
Annotation was performed using the RAST server (Aziz et al., 2008) and Prokka (Seemann, 2014). In addition, the presence of genes encoding enzymes of the primary and central metabolism as well as some secondary metabolic pathways discussed below was verified manually by web version of NCBI blastp using 35% identity and 50% coverage of amino acid sequence as a cut-off. The same criteria were used for searching genes of interest in the genomes of other methylotrophs. Assembled genomes have been deposited in NCBI GenBank under the accession numbers CP079095–CP079098.
Phylogenomic Analysis
A genome-based tree for members of the Methylococcus group was reconstructed using the Genome Taxonomy Database and GTDB-toolkit,1 release 04-RS89. The maximum likelihood phylogenetic tree was constructed using MegaX software (Kumar et al., 2018). The Pyani program was used to estimate average nucleotide identities across Methylococcus genomes (Pritchard et al., 2016). The resulting distance matrices were further visualized as a heatmap in R (R Core Team, 2020).
Pan-Genome Analysis
The pan-genome was reconstructed using microbial pangenomics workflow in Anvi’o (Eren et al., 2015). The genomes were annotated in Prokka, after which the genes were organized using MCL algorithm into core, shell, and singleton clusters (Distance: Euclidean; Linkage: Ward). The core, shell, and singleton genes were separately annotated by BLASTp against the NCBI COG database using eggNOG-mapper (Cantalapiedra et al., 2021). Heatmap based on the annotated COG functions of the core and singleton gene clusters was then plotted in R. The Tettelin best-fit curves of the core- and pan-genomes were constructed using OMCL v1.4 implemented in GET_HOMOLOGUES pipeline (Contreras-Moreira and Vinuesa, 2013).
Whole-genome synteny was computed using Sibelia (Minkin et al., 2013). Synteny blocks were visualized using Circos (Krzywinski et al., 2009).
Identification of the Mobile Genetic Elements and Prophage Regions
Insertion sequences (IS) were identified and classified into IS families using ISsaga pipeline (Varani et al., 2011) with IS finder database (Siguier et al., 2006) and ISEScaner pipeline (Xie and Tang, 2017). ICEfinder and ICEBerg v2.0 were used for IME detection (Liu et al., 2018b). Integron Finder (Cury et al., 2016) was used to identify regions containing integrons. Potential prophage regions were searched with PHASTER server2 using the settings described in Arndt et al. (2016). Gene functions were determined by homology with known viral proteins in the NCBI GenBank database and the VirFam package3 using the settings described in Lopes et al. (2014).
Results and Discussion
Identification and Characterization of New Methylococcus Strains
In total, four novel isolates of Methylococcus-like methanotrophs were obtained and characterized in this study. Strains IO1 and KN2 were isolated from activated sludge samples of the Moscow municipal sewage plant (Table 1). These isolates displayed nearly identical 16S rRNA gene sequences and were closely related to M. capsulatus Bath (99.93–99.97% 16S rRNA gene sequence similarity). Strain BH was obtained from a sediment of an unnamed freshwater lake in Krasnodar region, South Russia and was also closely related to M. capsulatus Bath (99.02% 16S rRNA gene sequence similarity). Finally, strain Mc7 was isolated from a landfill cover soil in Khanty-Mansiysk region, West Siberia, Russia. In contrast to other three isolates, strain Mc7 displayed highest 16S rRNA gene sequence similarity (98.56%) to M. geothermalis IM1T.
Table 1.
Isolation sources and some characteristics of new Methylococcus isolates.
| Strain | Isolation source | Cell size, μm | Growth temperature range (optimum), °C | Growth on methanol, % CH3OH | NaCl tolerance, % | pH growth range (optimum) | Growth rate in batch culture/bioreactor, h−1 |
|---|---|---|---|---|---|---|---|
| IO1 | Activated sludge, Moscow, Russia | 1.14±0.02 | 28–52 (48) | 0.05–0.1 (trace) | 0.75 | 5.5–7.5 (6.5–7.0) | 0.19/0.26 |
| KN2 | Activated sludge, Moscow, Russia | 1.12±0.03 | 25–53 (48–50) | 0.05–3.0 | 0.75 | 4.6–8.5 (6.5–8.0) | 0.22/0.30 |
| BH | Lake sediment, Krasnodar region, Russia | 1.12±0.02 | 25–52 (42) | 0.05–1.5 | 0.75 | 5.5–8.0 (6.5–7.2) | 0.18/0.28 |
| Mc7 | Landfill cover soil, Khanty-Mansiysk, Russia | 1.59±0.03 | 28–53 (40) | 0.05–0.1 (trace) | 1.0 | 5.5–7.5 (6.5–7.0) | 0.23/0.27 |
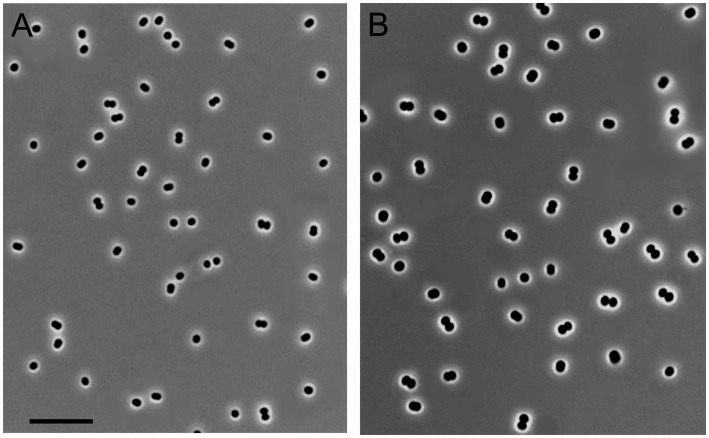
Four novel isolates were represented by non-motile, Gram-negative cocci, with a characteristic diplococcoid arrangement (Figure 1). Cell sizes of strains IO1, KN2, and BH were the same as those reported for M. capsulatus, approximately 1μm in diameter (Table 1; Figure 1A). Cell sizes of strain Mc7 (1.6μm in diameter; Figure 1B) were larger than those of other three strains. The colonies formed by these isolates on agar mineral medium after 2weeks of incubation with 20% (v/v) methane were small (1–2mm in diameter), round, milk-white (for strain Mc7) or cream colored (for strains IO1, KN2, and BH).
Figure 1.
Cell morphology of strains KN2 (A) and Mc7 (B) grown for 1day in liquid medium mAMS with methane. Marker, 10μm.
Methane and methanol were used as growth substrates, although the isolates differed in their ability to grow on methanol. Similar to M. capsulatus Bath, strains IO1 and Mc7 displayed only a trace growth (up to OD600 0.10–0.12) in a relatively narrow range of CH3OH concentrations (Table 1). By contrast, strains KN2 and BH were capable of a relatively good growth (up to OD600 0.3) on methanol in a wide range of concentrations. The isolates were quite uniform with regard to their pH preferences (Table 1). Most strains grew in the pH range of 5.5–8.0. Strain KN2 demonstrated slightly better adaptation to moderately acidic conditions growing between pH 4.6 and 8.5. All strains used nitrate and ammonium as nitrogen sources. In addition, strains BH and KN2 were able to utilize glutamine, while strain IO1 showed weak growth on peptone as a nitrogen source. Strain Mc7 displayed highest tolerance to NaCl, up to 1% (w/v), while other isolates tolerated up to 0.75% (w/v) NaCl. For comparison, NaCl tolerance determined in our experiments for M. capsulatus Bath was 0.5% (w/v).
The ability to utilize hydrogen was tested using incubations with either H2 and CO2 or H2, CO2 and CH4 in the headspace (see Materials and Methods). No growth as well as no H2 consumption were observed in the absence of CH4, with H2 as the only energy source. With the only exception of strain KN2, the presence of H2 in addition to CH4 increased the growth yield of all strains examined in this study by 2–20% (Supplementary Figure S1A) as well as the corresponding CH4 consumption (Supplementary Figure S1B). H2 utilization in the presence of CH4 was observed for all studied strains, including strain KN2 (Supplementary Figure S1C). These results agree well with the previous report of the absence of autotrophic growth of M. capsulatus Bath in liquid medium with H2 and CO2 (Baxter et al., 2002; Henard et al., 2021). Apparently, Methylococcus strains are capable of using H2 as an alternative energy source during their growth on methane. Recently, the ability to grow mixotrophically on H2 and CH4 was also reported for verrucomicrobial (Methylacidiphilum sp. RTK17.1) and proteobacterial (Methylocystis sp. strain SC2) methanotrophs (Carere et al., 2017; Mohammadi et al., 2019; Hakobyan et al., 2020).
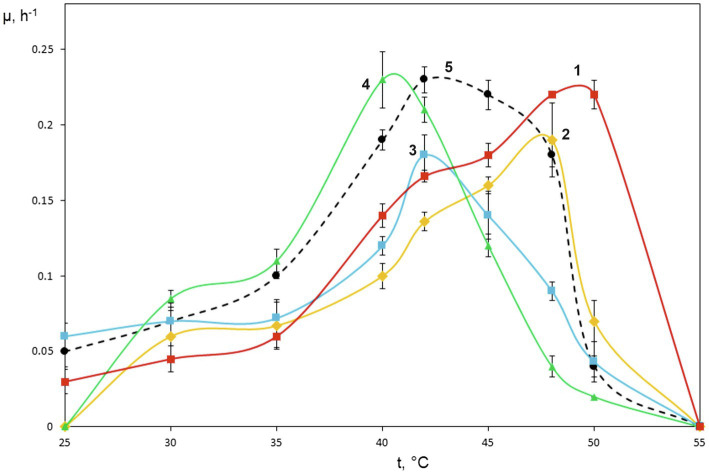
The special attention in our study was given to assessing growth characteristics of novel Methylococcus isolates. The results of measuring specific growth rates of novel isolates and M. capsulatus Bath within the temperature range of 25–55°C are shown in Figure 2. The two strains obtained from sludge samples, IO1 and KN2, were clearly more thermotolerant than M. capsulatus Bath and displayed growth optima at 48–52°C. The lowest temperature growth optimum of 40°C was revealed for strain Mc7, which is reasonable given that it was isolated from a landfill soil in West Siberia. Highest specific growth rates, 0.22–0.23h−1, which were comparable to that of M. capsulatus Bath, were recorded for strains KN2 and Mc7. We also performed additional experiments in order to examine specific growth rates of novel isolates during continuous cultivation on methane in 1.5L bioreactor. The highest specific growth rate in a fermenter culture (0.3h−1) was recorded for strain KN2. Other strains also demonstrated stable growth under conditions of continuous cultivation. Their growth rates, however, were below that demonstrated by strain KN2 (Table 1).
Figure 2.
Specific growth rates of novel isolates on methane as dependent on incubation temperature. Methylococcus capsulatus Bath was used as a reference organism. 1, strain KN2, 2, strain IO1, 3, BH, 4, Mc7, and 5, Methylococcus capsulatus Bath.
Genome Sequencing and Assembly
The genomes of four novel isolates were sequenced using a hybrid approach. Oxford Nanopore sequencing yielded 192,565–278,437 reads with a total length of 1.05–1.59 Gb (Supplementary Table S1). Sequencing on Illumina MiSeq platform generated a total of 415,244–3,477,384 paired-end reads, with a mean read length of 250bp. Both short and long reads were combined to perform a hybrid assembly using Unicycler, resulting in circular genomes. Genome characteristics of new isolates are summarized in Table 2.
Table 2.
General genome features of new isolates and publicly available complete Methylococcus genomes.
| Strain KN2 | Strain BH | Strain Mc7 | Strain IO1 | Methylococcus capsulatus Bath | Methylococcus geothermalis IM1T | |
|---|---|---|---|---|---|---|
| Genome size (Mb) | 3.6 | 3.2 | 4.0 | 3.3 | 3.3 | 3.4 |
| Contigs | 1 | 1 | 1 | 1 | 1 | 1 |
| G+C content (mol %) | 63.49 | 63.52 | 63.44 | 63.49 | 63.60 | 63.25 |
| CDS | 3,289 | 2,912 | 3,752 | 3,008 | 3,043 | 3,091 |
| Repeat region | 2 | 2 | 6 | 2 | 2 | 3 |
| tRNA | 48 | 50 | 48 | 50 | 49 | 48 |
| 5S, 16S, 23S | 2, 2, 2 | 2, 2, 2 | 2, 2, 2 | 2, 2, 2 | 2, 2, 2 | 2, 2, 2 |
| pMMO operon | 2 | 2 | 2 | 2 | 2 | 2 |
| sMMO operon | 1 | 1 | 1 | 1 | 1 | 1 |
| Complete IS elements | 17 | 23 | 78 | 26 | 33 | 45 |
Genome sizes varied from 3.2Mb in strain BH to 4.0Mb in strain Mc7. The DNA G+C content was highly similar in all examined genomes and constituted 63.44–63.52%. Each genome contained two copies of rRNA operon, two copies of pMMO, and one copy of sMMO. The number of protein-coding genes varied between 2,912 and 3,752. No plasmids were detected. Lowest number (17) of insertion (IS) elements was detected in strain KN2, while substantially higher number of IS elements (78) was observed in strain Mc7.
Genome-Based Phylogeny and Genome-to-Genome Comparison
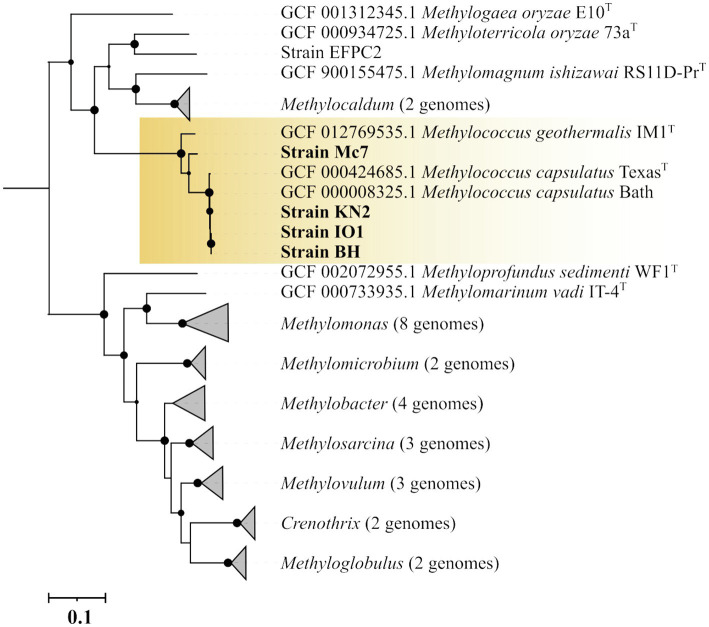
The genome-based phylogeny of four novel Methylococcus-affiliated isolates was determined using the comparative sequence analysis of 120 ubiquitous single-copy proteins (Figure 3). Three isolates (strains IO1, KN2, BH) were clustered together with two strains of M. capsulatus, TexasT, and Bath, whereas strain Mc7 and M. geothermalis IM1T constituted two separate branches within the genus Methylococcus. The complete genome of strain EFPC2, which is annotated in the GenBank as belonging to Methylococcus species, was also included in the analysis. However, as seen from Figure 3, strain EFPC2 clustered together with Methyloterricola oryzae 73aT and did not affiliate with Methylococcus clade. The genome of strain EFPC2, therefore, was excluded from further comparative analysis of finished genomes available for Methylococcus species.
Figure 3.
Genome-based phylogeny showing the position of new isolates in relation to other gammaproteobacterial methanotrophs based on the comparative sequence analysis of 120 ubiquitous single-copy proteins. The clade of Methylococcus methanotrophs is highlighted by orange. The tree was constructed using the Genome Taxonomy Database toolkit (Parks et al., 2018), release 04-RS89. The significance levels of interior branch points obtained in maximum-likelihood analysis were determined by bootstrap analysis (100 data re-samplings). Bootstrap values of >70% are shown. The root (not shown) is composed of all genomes available in GTDB for methanotrophs of the genera Methylosinus and Methylocystis. Bar, 0.1 substitutions per amino acid position.
The average nucleotide identity (ANI) values were estimated for each pair of genomes (Supplementary Figure S2). ANI values calculated for isolates IO, KN2, BH, and M. capsulatus Bath were within a range of 98.75–99.73% which indicates that these organisms belong to the same species as intra-species level is defined at ≥95% ANI (Konstantinidis and Tiedje, 2005; Goris et al., 2007). ANI value determined for separately clustered strain Mc7 and M. geothermalis IM1T was 88.56%. Thus, strain Mc7 may potentially represent a novel species of the genus Methylococcus.
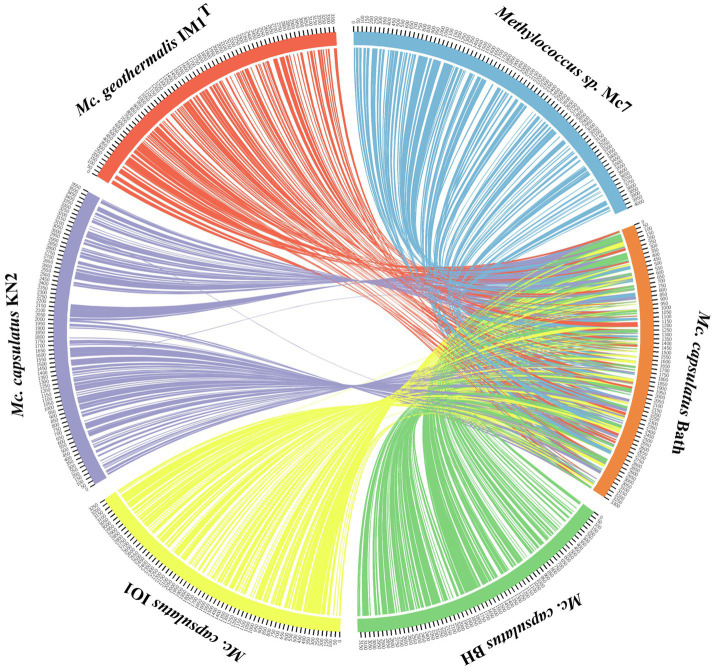
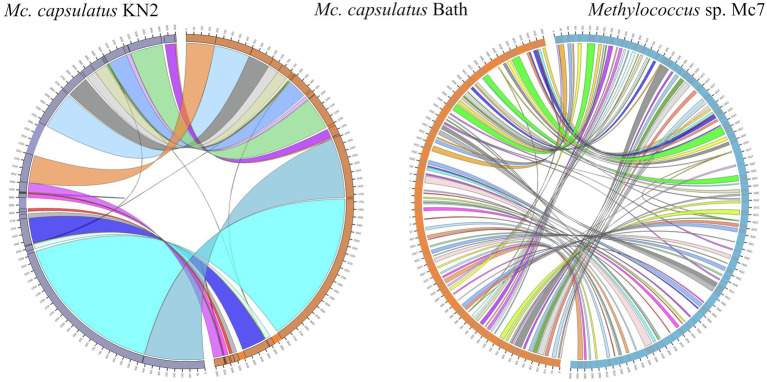
The genomes of novel isolates, M. capsulatus Bath and M. geothermalis IM1T, were included in the whole-genome synteny analysis in order to explore the evolution of genome structure. Overall, 216 shared synteny regions were identified. Among those, 86 regions were common for all six genomes. Figure 4 depicts only the synteny blocks affiliated with M. capsulatus Bath.
Figure 4.
Visualization of synteny blocks between Methylococcus capsualtus Bath and other examined Methylococcus genomes. Each link represents a single synteny block. Circle is divided into the individual arcs representing genomes of Methylococcus capsulatus Bath (orange), Methylococcus capsulatus BH (green), Methylococcus capsulatus IO1 (yellow), Methylococcus capsulatus KN2 (purple), Methylococcus geothermalis IM1T (red), and Methylococcus sp. Mc7 (blue).
The number of synteny blocks revealed between M. capsulatus Bath and each of the isolates IO1, KN2, and BH was 102–103. Strain Mc7 and M. geothermalis IM1T exhibited slightly lower levels of synteny to M. capsulatus Bath by displaying 97 and 93 synteny blocks, respectively. One of M. capsulatus-related isolates, strain KN2, and Methylococcus sp. Mc7 were chosen for more detailed analysis of whole-genome synteny patterns (Figure 5). As expected, the calculations made for two genomes resulted in lower number of synteny blocks, which were larger in size. Synteny regions shared between strain KN2 and M. capsulatus Bath covered 88.9 and 94.9% genomes of these bacteria, respectively. The largest synteny block in the genome of strain KN2 spanned about 1.1Mb and contained 978 genes. Two relatively large synteny break regions in the genome of strain KN2 spanned 224 and 76kb. Synteny breaks are caused by rearrangements, the insertion of novel genes, or the presence of genes that are too diverged to establish an orthologous relationship or have undergone expansion or loss (Liu et al., 2018a). Of 224 genes in the first synteny break region, 187 genes encoded hypothetical proteins. This region also included several genes coding for transposases, formate dehydrogenase displaying 95.5% amino acid sequence identity to NAD-dependent formate dehydrogenase from Methylocaldum marinum, flagellar transcriptional regulator FlhC, lysozyme RrrD, several helicases, and endonucleases. Of 84 genes in the second synteny break region in the genome of strain KN2, 76 genes encoded hypothetical proteins, while others coded for transport proteins. Comparison of the genomes of strain Mc7 and M. capsulatus Bath revealed 81 synteny blocks, which corresponded to nearly 50% of their genome length (Figure 5). The largest synteny break regions spanned 98.9, 98.5, 79.7, and 74.4kb. Approximately half of the genes within these regions encoded hypothetical proteins. Interestingly, 98.9kb region contained genes encoding small and large subunits of ribulose bisphosphate carboxylase.
Figure 5.
Visualization of synteny blocks between Methylococcus capsulatus Bath, Methylococcus capsualtus KN2, and Methylococcus sp. Mc7. Each link represents a single synteny block. Each circle is divided into two parts, which represent two compared genomes. The genome of Methylococcus capsulatus Bath is shown in orange, while the genomes of Methylococcus capsulatus KN2 and Methylococcus sp. Mc7 are displayed in purple and blue, respectively.
Pan-Genome Analysis
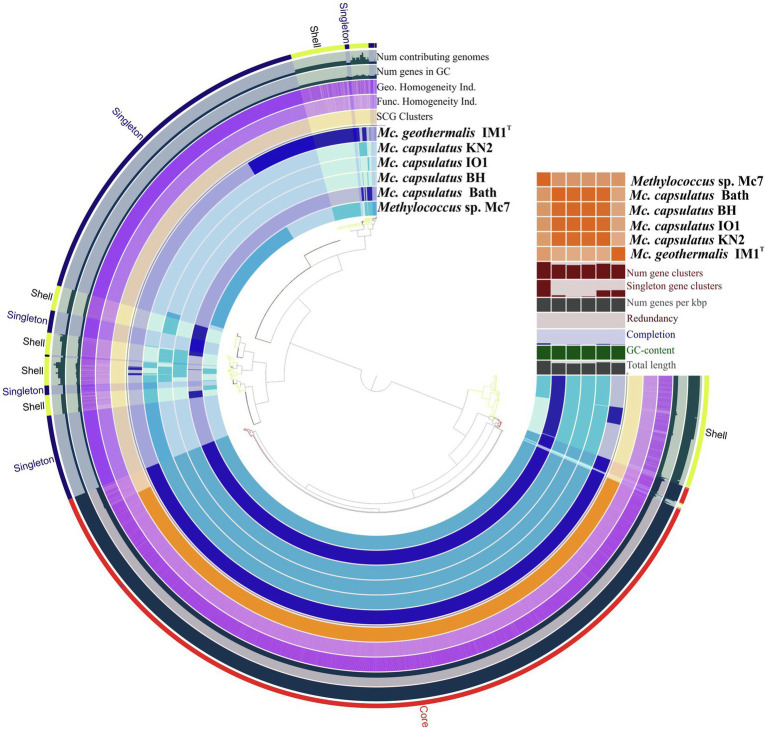
The Anvi’o pangenomics workflow was used to cluster protein-coding sequences into core, shell, and singleton genomes. Of 4,485 identified gene clusters, the Methylococcus pan-genome core comprised 2,331 genes (on average 51.9% of each genome), with the shell genome containing 846 gene clusters (18.9% of total gene clusters) and the cloud containing 1,308 gene clusters (29.2% of total gene clusters; Figure 6).
Figure 6.
Pan-genome analysis. Clustering of genomes based on the presence/absence patterns of 4,485 pan-genomic clusters. The genomes are organized in radial layers as core, singleton, and shell gene clusters (Euclidean distance; Ward linkage) which are defined by the gene tree in the center. Genomes of new isolates are colored by blue. Genomes of Methylococcus capsulatus Bath and Methylococcus geothermalis IM1T are colored by dark blue. Line segments indicate single gene clusters. The outermost circles carry the information regarding the number of genomes containing specific gene cluster, number of genes in gene clusters (gray), functional, geometric, and combined homogeneity indexes (purple), distribution of single-copy gene clusters (orange circle). The layers below the heatmap display from the bottom to top: total length of the genomes, GC content, genomes completion, redundancy of each genome, number of genes per kbp, number of singleton gene clusters, total number of gene clusters. Heatmap denotes correlation between the genomes based on average nucleotide identity values calculated using pyANI.
Pan-genome of the genus Methylococcus is open. Supplementary Figure S3 displays the number of core genes (A) and the pan-genome size (B) as a function of the number of included genomes. Fitting the curve in Supplementary Figure S3B to a power law (3,146.3n0.171) allows extrapolating calculations to n genomes. According to approximation, sequencing of one additional genome would provide 100 new genes to pan-genome. If the number of available genomes constituted 50 or 100 genomes, sequencing of one additional genome would contribute 21 and 12 genes, respectively. The core, shell, and cloud gene clusters were further annotated into COG classes (Supplementary Figure S4). The core genome was mostly conserved in the following: energy production and conversion (11.5% of total core gene clusters); translation, ribosomal structure, and biogenesis (8.6%); cell wall/membrane/envelope biogenesis (8.3%); coenzyme transport and metabolism (7.3%); inorganic ion transport and metabolism (6.3%); amino acid transport and metabolism (5.3%); posttranslational modification; protein turnover, chaperones (4.9%). However, the most abundant category was the one with no functional prediction (19.9%). The most abundantly represented functional categories in shell genome were replication, recombination, and repair, coenzyme transport and metabolism, signal transduction mechanisms, transcription, inorganic ion transport and metabolism, cell wall/membrane/envelope biogenesis, energy production and conversion. The majority of cloud genes were concentrated in the genomes of strain Mc7, M. geothermalis IM1T, and M. capsulatus KN2 and represented replication, transcription, cell wall/membrane/envelope biogenesis, and signal transduction mechanisms. These functional categories were also affiliated with few cloud genes found in M. capsulatus Bath and strain BH. A number of studies demonstrated that core genes systematically represented the smallest fraction of pan-genome (Bazinet, 2017; Livingstone et al., 2018; Kumar et al., 2020; Oshkin et al., 2020). The large proportion of core genes in Methylococcus pan-genome may be explained by both the small genome size of individual Methylococcus strains and the low number of available genomes that could be taken for analysis. However, approximation for core- and pan-genome (Supplementary Figure S3) suggests that even if 30 genomes were included into pan-genome analysis, the fraction of core genes would still be high (41.1% of the total gene clusters).
Methane Oxidation Enzymes
Two complete copies of the pmoCAB gene cluster encoding pMMO and one additional copy of the pmoC (pmoC3) gene were revealed in the genomes of all novel Methylococcus strains. The corresponding pmoA sequences were nearly identical to each other and to the pmoA sequences from M. capsulatus Bath and M. geothermalis IM1T. The sMMO-encoding gene cluster mmoXYBZDC was also present in all examined genomes, with MmoX sequences exhibiting high level of homology (100% identity between MmoX from M. capsulatus Bath and strains KN2, IO1, BH, and 97% identity with that from strain Mc7). This chromosomal locus in M. capsulatus Bath and strain IO1 contained also one gene encoding a hypothetic protein between mmoB and mmoZ genes. The gene cluster mmoGQSR coding for the large subunit of bacterial chaperonin GroEL (mmoG), the two-component sensory/regulatory system (mmoQ and mmoS), and the transcription activator (mmoR; Csáki et al., 2003) was found in all examined genomes.
Two PQQ-dependent MDHs catalyze the second stage of C1-oxidation. The structural components of heterodimeric Ca2+-dependent MDH (MxaFI type) and the proteins required for its catalytic activity are encoded by the mxaFJGIRACKLD cluster with gene organization completely identical in all Methylococcus strains. Namely, the mxaG gene encodes the specific electron acceptor cytochrome cL, the mxaACKL gene cluster encodes the proteins required for Ca2+ incorporation into the active site, while the mxaRS gene cluster encodes two proteins of unknown functions (Chistoserdova, 2011; Vuilleumier et al., 2012). The four strains harbor gene cluster xoxFJ, which encodes an alternative MDH (XoxF) containing a rare earth element in the active site (Hibi et al., 2011), and a periplasmic solute-binding protein (XoxJ). No other genes relevant to methanol oxidation were present in this locus. The xoxG gene coding for a putative electron acceptor from XoxF and being only distantly related to the mxaG gene (Keltjens et al., 2014; Yu et al., 2017; Zheng et al., 2018) was found in all genomes and was located separately from xoxFJ.
Oxidative transformations of formaldehyde are mediated by the enzymes of the tetrahydrofolate (THF)-based pathway whose amino acid sequences in the novel isolates are almost identical to those in M. capsulatus Bath. The complete sets of genes for the tetrahydromethanopterin (THMP)-dependent pathway of formaldehyde oxidation are present in all Methylococcus genomes. Each strain possesses the three-subunit formate dehydrogenase, which catalyzes the last stage of methane oxidation, and a five-gene operon encoding the cytoplasmic NAD+-dependent formate dehydrogenase composed of (abc)2 subunits that catalyze the reversible reaction of formate oxidation to CO2 (Hartmann and Leimkühler, 2013). All strains of M. capsulatus (Bath, KN2, IO1, and BH) possess also the two-subunit formate dehydrogenase, whereas this enzyme is missing in strain Mc7 and M. geotermalis IM1T. The genomes of three strains, Mc7, KN2, and M. geothermalis IM1T, encode the one-subunit formate dehydrogenase, which is not present in M. capsulatus Bath, IO1 and BH. Thus, strain KN2 possesses four formate dehydrogenases in comparison with other Methylococcus strains possessing three orthologs each. Since no other evident differences in the arrays of C1 oxidizing enzymes of these strains were found, one may hypothesize that the extended array of formate dehydrogenases helps increasing removal of C1 metabolites via their oxidation to CO2. However, the exact role of different formate dehydrogenases in metabolism of methanotrophic bacteria remains to be elucidated.
Carbon Assimilation Pathways
Ribulose Monophosphate Cycle
Genes encoding enzymes of the RuMP cycle are linearly duplicated in all studied Methylococcus genomes. The central part of this duplicated DNA locus (~5,000bp) contains the transaldolase gene, which is surrounded by the genes of phosphohexulose isomerase (phi), hexulose phosphate synthase (hps), fructose bisphosphate aldolase and transketolase (Ward et al., 2004). Each of the studied strains has a gene encoding the fusion protein hexulose phosphate synthase/isomerase. Further research is needed to clarify if this gene multiplicity for the RuMP cycle affects growth characteristics of methanotrophs.
Serine Pathway
All examined Methylococcus genomes contain the sga–hpr–gck3 gene cluster coding for the serine-glyoxylate aminotransferase, hydroxypyruvate reductase, and 3-glycerate kinase, which are the key enzymes of the serine pathway. They also harbor genes for malyl-CoA lyase and malate thiokinase responsible for glyoxylate biosynthesis but lack PEP carboxylase. Pyruvate carboxylase that can fulfill the function of replenishing C4 TCA intermediates is present only in strain Mc7. Although this function can be assigned to oxaloacetate decarboxylase (Ward et al., 2004) or to malic enzyme, these enzymes catalyze predominantly irreversible decarboxylation reactions (Rozova et al., 2019; Xu et al., 2020). The gene for PPi-dependent PEP carboxykinase was not detected in the examined Methylococcus genomes. The absence of genes coding for any of the currently known C3 carboxylation enzymes in most Methylococcus representatives requires further efforts to decipher mechanisms for replenishing TCA cycle intermediates. In addition, glyoxylate can be generated from phosphoglycolate, which is the product of the oxygenase reaction of ribulose bisphosphate carboxylase (RuBisCo) as previously evidenced for M. capsulatus Bath (Taylor et al., 1981; Baxter et al., 2002). The genes for phosphoglycolate phosphatase and glycolate oxidase are present in the genomes of all Methylococcus representatives.
Calvin Cycle
Genomes of all Methylococcus strains possess cbbL and cbbS genes for the large and the small subunits of RuBisCo, as well as the cbbQ gene encoding a polypeptide putatively acting as a posttranslational RuBisCo activator (Baxter et al., 2002). Additional copies of these genes are present only in the genome of strain Mc7. Six genes encoding carbonic anhydrases were found in strain KN2, five genes in other M. capsulatus strains, and only three enzymes are encoded by Mc7 and IM1. Recent study suggests that RuBisCO is essential for M. capsulatus Bath growth and central metabolites derived from CO2 enter core intermediary metabolic pathways, including the Embden–Meyerhof–Parnas (EMP) glycolytic pathway, the pentose phosphate pathway, and the TCA cycle (Henard et al., 2021).
Central Metabolism
Several pathways for C6-phosphosugars degradation are encoded in the genomes of four novel Methylococcus isolates. These include the modified Embden–Meyerhof–Parnas pathway, where PPi-dependent phosphofructokinase catalyzes the reaction of fructose-1,6-phosphate synthesis from fructose-6-phosphate (Reshetnikov et al., 2008), the Entner–Doudoroff pathway, and the phosphoketolase pathway. The genomes of strain Mc7 and M. geothermalis IM1T encode four isozymes of glucose-6-phosphate dehydrogenase, while all strains of M. capsulatus (Bath, KN2, IO1, BH) possess only two of these isozymes. The genomes of novel isolates encode phosphoketolase catalyzing cleavage of fructose-6-phosphate or xylulose-5-phosphate into glyceraldehyde-3-phosphate/erythrose-4-phosphate and acetyl phosphate; the genes coding for phosphoketolase and acetate kinase comprise a single cluster. These strains, therefore, are predicted to be able to convert acetylphosphate to acetate and to produce ATP as previously suggested for other gammaproteobacterial methanotrophs (Rozova et al., 2015; Henard et al., 2017). In strain Mc7 and M. geothermalis IM1T, acetyl phosphate can be directly converted into acetyl-CoA without ATP consumption, while strains of M. capsulatus lack the respective acetyl phosphotransferase. In all Methylococcus species, the synthesis of acetyl-CoA can also proceed from acetate by ATP-dependent acetyl-CoA synthetase forming AMP. Interestingly, two strains, KN2 and Bath, code for two enolases, while other strains possess only one enzyme. The relative excess of enolase, which directs the primary C3 intermediates of the Calvin cycle and the serine pathway to the central metabolism, most likely, can increase the impact of these “minor” pathways in overall carbon assimilation of these methanotrophs.
It is known that gammaproteobacterial methanotrophs are capable of fermentation under micro-oxic conditions (Kalyuzhnaya et al., 2013). The genomes of novel Methylococcus isolates contain the same genes potentially involved in fermentation (pyruvate formate lyase, alcohol dehydrogenase, acetate kinase) as M. capsulatus Bath (Ward et al., 2004). However, similar to strain Bath, these methanotrophs lack the lactate dehydrogenase gene.
Nitrogen Metabolism
All Methylococcus strains possess the structural genes for nitrogenase (nifHDK) apparently constituting an operon together with nifENX; the latter being involved in synthesis of the nitrogenase iron-molybdenum cofactor. One gene of unknown function is present between nifK and nifE in strains Bath, BH, KN2, and IO1, whereas the same locus in strain Mc7 contains three genes. All strains possess the gene cluster responsible for nitrate reduction, i.e., nitrate reductase (NasA), nitrite reductase (NirBD), protein kinase and nitrate transporter. All strains contain the genes haoAB for putative hydroxylamine dehydrogenase that transforms hydroxylamine to nitric oxide. One potential source of hydroxylamine is ammonium oxidation by pMMO since these methanotrophs lack ammonia monooxygenase. The gene cluster norBC responsible for reduction in nitric oxide to nitrous oxide is also present in the genomes. Like M. capsulatus Bath (Ward et al., 2004), novel strains encode three predicted hydrogenases: a multisubunit formate hydrogen lyase, most likely involved in the conversion of formate to dihydrogen and carbon dioxide; a soluble cytoplasmic NAD-reducing hydrogenase, which transfers electrons to NAD; and a membrane-bound Ni-Fe hydrogenase.
Secondary Metabolism
The genomes of novel isolates as well as the genome of M. capsulatus Bath encode enzymes of glycogen synthesis, i.e., 4-α-glucanotransferase (amylomaltase), glucose-1-phosphate adenylyltransferase, 1,4-α-glucan-branching enzyme GlgB, and glycogen synthase. The corresponding genes are organized in one cluster similar to that in M. capsulatus Bath. All strains, including M. capsulatus Bath, also harbor an additional gene cluster, which encodes glycogen synthase 2 and α-amylase. Among examined Methylococcus representatives, only strain Mc7 possesses genes coding for sucrose biosynthesis and degradation. The genes for sucrose phosphate synthase, sucrose phosphate phosphatase, and sucrose synthase are assembled in one gene cluster in strain Mc7 similar to that in Methylocaldum szegediense O12 (But et al., 2018). Like the latter, strain Mc7 possesses the gene coding for mannose-1-phosphate guanyltransferase; however, it lacks fructokinase encoding gene. The ability to synthesize sucrose may be one of the reasons behind the enhanced salt tolerance observed for strain Mc7 in comparison with other Methylococcus isolates (Table 1).
The genomes of the studied methanotrophs encode enzymes homologous (40–42% sequence identity) to the previously characterized acetolactate decarboxylase from Klebsiella aerogenes (Blomqvist et al., 1993). Interestingly, in Methylococcus species, the acetolactate decarboxylase gene forms a cluster together with genes of the malic enzyme and acetolactate synthase; the latter known to produce acetolactate from two molecules of pyruvate with CO2 release (Blomqvist et al., 1993). Given that acetolactate decarboxylase degrades acetolactate into acetoin and CO2, functioning of a fermentation pathway that converts malate to acetoin through pyruvate and acetolactate appears to be possible. The BLAST search revealed that, besides methanotrophs of the genus Methylococcus, acetolactate decarboxylase is also encoded in the genomes of Methyloterricola oryzae, Methylogaea oryzae as well as in Methylovulum and Methyloprofundus representatives. In microorganisms, the pathway of acetoin synthesis is regarded as mechanism preventing over-acidification of the intracellular environment, and also as an energy-storing strategy (Xiao and Xu, 2007).
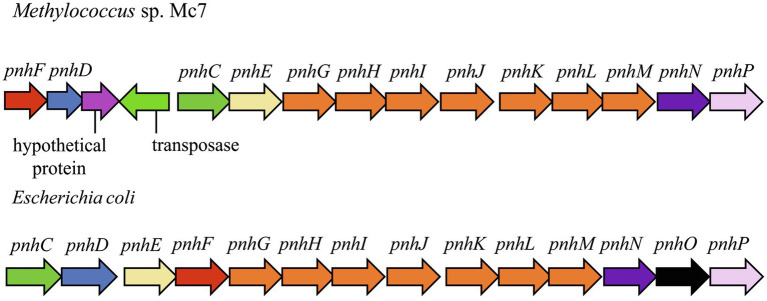
In strain Mc7, DNA locus of ~14,000bp is represented by the 13-gene cluster phnFFCEGHIJKLMNP, which displays high structural and sequence similarity (27–54% amino acid sequence identity) to the Escherichia coli operon encoding the carbon-phosphorus (CP) lyase (Figure 7). In E. coli, this gene cluster was found to determine degradation of organophosphonates (Adams et al., 2008; Jochimsen et al., 2011) and we may assume the same function of the corresponding genes in strain Mc7. BLAST search revealed that, among gammaproteobacterial methanotrophs, these genes are present only in the genome of marine methanotroph Methyloprofundus sedimenti WF1T. Organophosphonates are widely distributed in nature and are represented by various naturally occurring compounds (phosphonopyruvate, 2-aminoethylphosphonate, and phosphonoacetate; Ternan et al., 1998; Seto and Kuzuyama, 1999) as well as by synthetic xenobiotics such as herbicide glyphosate ([N-(phosphonomethyl) glycine]). Obviously, the potential substrates of CP lyase from strain Mc7 and M. sedimenti WF1T are naturally occurring organophosphonates. The ability to decompose a highly stable carbon-phosphorous bond allows survival in habitats with low contents of phosphates.
Figure 7.
The gene clusters carrying genes of pathway for organophosphonates degradation in methylotrophs and Escherichia coli. The products of the phn genes were proposed based on the comparison with the respective sequences in the Escherichia coli operon (Jochimsen et al., 2011): phosphonate ABC transporter ATP binding subunit (phnC); phosphonate ABC transporter periplasmic binding protein (phnD); phosphonate transport system permease protein (phnE); putative transcriptional regulator (phnF); carbon-phosphorus lyase core complex subunits (phnGHIJK); methylphosphonate degradation complex subunit (phnL); RPnTP hydrolase (phnM); ribose-1,5-bisphosphate phosphokinase (phnN); aminoalkylphosphonate N-acetyltransferase (phnO); 5-phospho-alpha-D-ribosyl-1,2-cyclic phosphate phosphodiesterase (phnP).
The genomes of strain Mc7 and M. geothermalis IM1T contain the genes for haloalkane dehalogenase, which catalyzes the hydrolytic cleavage of carbon–halogen bonds (EC 3.8.1.5). It is absent in M. capsulatus strains, whereas its homologues were found in the genomes of Methylocaldum spp. (with ~80% identity), Methylobacter tundripaludum and Methylovulum myaconense (<70%) as well as in alphaproteobacterial methanotrophs (<50%). The corresponding protein shares 51% identity with haloalkane dehalogenase characterized from Bradyrhizobium japonicum (Sato et al., 2005).
The genome of strain Mc7 contains the gene for putative methanethiol S-methyltransferase, which catalyzes the transmethylation between methanethiol (MeSH) and S-adenosyl-l-methionine into dimethylsulfide (DMS) and S-adenosyl-l-homocysteine as well as the genes for FMNH2-dependent dimethylsulfone monooxygenase and FMNH2-dependent monooxygenase SfnG. MeSH can be formed by sulfide methylation in anaerobic environments or by degradation of sulfur-containing amino acids, via the cleavage of dimethylsulfopropionate, which is osmoprotector in marine microalgae, or by demethiolation of sulfhydryl groups (Lomans et al., 2001, 2002; Bentley and Chasteen, 2004). Further metabolism of DMS can proceed via chemical or biochemical oxidation into dimethylsulfone. The latter compound, being substrate for FMNH2-dependent monooxygenases, is converted into inorganic sulfite and formaldehyde. Obviously, this mechanism of scavenging sulfur from organosulfur compounds enables bacterial survival in sulfate-depleted environments (Boden et al., 2011; Carrión et al., 2015). All strains under study possess genes for the sulfur oxidizing (Sox) system that allows utilization of inorganic sulfur compounds in energy metabolism. In these methanotrophs, the Sox system is represented by the SoxYZ complex that presumably carries the intermediates of the pathway on a cysteine residue near the C terminus of SoxY as well as by sulfane dehydrogenase SoxCD; the latter has been found to catalyze a six-electron oxidation reaction (Friedrich et al., 2005). However, all Methylococcus strains lack SoxB, SoxA, and SoxX components needed for the full Sox system. Functionality and role of the truncated pathway of sulfur oxidative metabolism in the methanotrophs is not clear.
Integrons
Integrons are gene-capturing platforms that play a significant role in generating phenotypic diversity and shaping adaptive responses in microorganisms, including the spread of antibiotic resistance genes (Mazel, 2006; Gillings, 2014). The integron structure includes variable gene cassette array (recombination site attC with corresponding genes) and a stable platform containing integrase (IntI), recombination site (attI) and promoter (Boucher et al., 2007). Of all novel Methylococcus isolates, integron-like elements were detected only in the genome of strain Mc7. This strain contains one complete integron with one attC site, one separate attC site, large attC-cassette (nine sites), and stand-alone integrase. The gene encoding 6-phosphogluconate phosphatase was found in a large attC-cassette; the functions of other genes near attC sites could not be predicted. A complete integron containing three attC sites was also revealed in the genome of M. geothermalis IM1T.
Integrative and Conjugative Elements/Integrated Mobilizable Elements
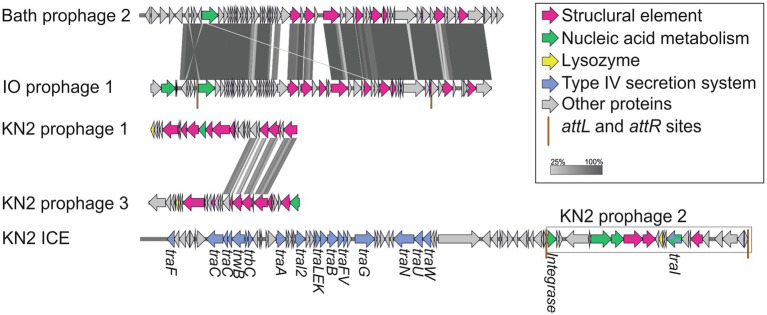
Integrative and conjugative elements (ICEs) are widespread mobile DNA that transmit both vertically, in a host-integrated state, and horizontally, through excision and transfer to new recipients. Recent findings indicated that the main actors of conjugative transfer are not the well- known conjugative or mobilizable plasmids but the integrated mobilizable elements (IMEs; Delavat et al., 2017; Guédon et al., 2017). IMEs encode their own excision and integration and use the conjugation machinery of unrelated co-resident conjugative element for their own transfer (Guédon et al., 2017). One putative IME was found in the genome of strain Mc7; unfortunately, the ends of the element could not be identified. A putative IСE region of 123,456bp was revealed in the genome of strain KN2. This region contained the genes of the type IV secretion system, which plays a key role in conjugation (Lawley et al., 2003). Interestingly, some fragments of a prophage were also found in this ICE region (Figure 8).
Figure 8.
The structure of integrative and conjugative element (ICE) region revealed in the genome of strain KN2 and prophage-associated regions (≥20Kb) detected in the examined Methylococcus genomes.
Phages
One to six prophage regions were found in the genomes of the examined Methylococcus strains (Table 3). Potentially complete prophages were detected in the genomes of strains IO1 and KN2. In the prophage region of strain IO1, the attL and attR sites were displaced, and the lysozyme genes were also absent (Figure 7). This prophage is structurally highly similar to the prophage 2 from M. capsulatus Bath. The prophage region in the genome of strain KN2 was, most likely, divided into large fragments as a result of integrase activity. This suggests that the conversion of these prophages to the lytic cycle is unlikely.
Table 3.
Prophage regions revealed in the examined Methylococcus genomes.
| Strain | Region no. | Region length, kb | Region position | tRNA | Total proteins | Number of phage protein hits | att_site present |
|---|---|---|---|---|---|---|---|
| Bath | 1 | 36.1 | 2,819,640–2,855,795 | 0 | 40 | 20 | No |
| 2 | 51.7 | 3,091,049–3,142,838 | 0 | 58 | 44 | Yes | |
| IO1 | 1 | 48.4 | 2,508,110–2,556,599 | 2 | 64 | 45 | Yes |
| KN2 | 1 | 20.8 | 1,847,198–1,868,022 | 0 | 26 | 19 | No |
| 2 | 29 | 2,258,604–2,287,649 | 0 | 18 | 12 | Yes | |
| 3 | 21.4 | 2,313,376–2,334,848 | 0 | 27 | 16 | No | |
| 4 | 15.9 | 3,029,181–3,045,103 | 0 | 21 | 12 | No | |
| Mc7 | 1 | 13 | 1,444,044–1,457,136 | 0 | 8 | 6 | Yes |
| 2 | 7.4 | 3,652,375–3,659,823 | 0 | 7 | 6 | No | |
| IM1T | 1 | 11.6 | 672,463–684,108 | 1 | 12 | 7 | No |
| 2 | 11.3 | 2,433,262–2,444,593 | 0 | 8 | 7 | No | |
| BH | 1 | 11.3 | 501,048–512,403 | 0 | 8 | 7 | No |
| 2 | 7.8 | 650,531–658,368 | 0 | 8 | 6 | No | |
| 3 | 8.5 | 1,088,481–1,096,982 | 0 | 10 | 6 | No | |
| 4 | 4.1 | 1,607,407–1,611,579 | 0 | 8 | 7 | No | |
| 5 | 7.7 | 2,024,931–2,032,721 | 0 | 9 | 6 | No | |
| 6 | 8.1 | 2,164,268–2,172,463 | 0 | 8 | 6 | No |
Insertion Sequences
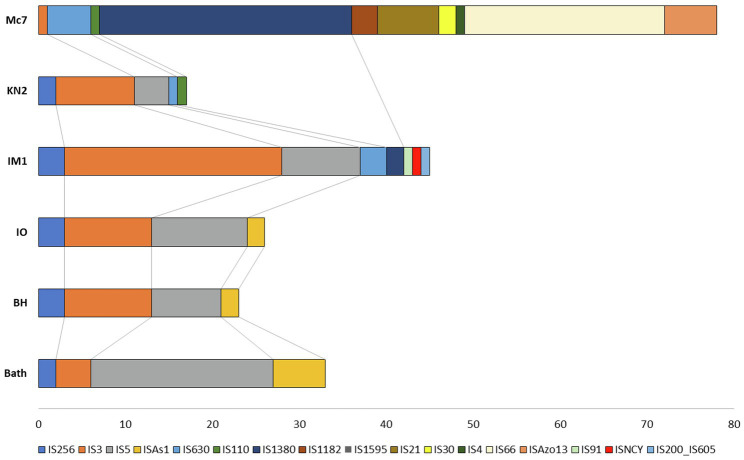
Insertion sequences are transposable DNA segments ranging in length from 0.7 to 3.5kb, generally including a transposase gene encoding the enzyme that catalyzes IS movement. The number of complete IS in the genomes of studied strains varied from 17 in KN2 to 78 in Mc7 (Figure 9). Similar to M. capsulatus Bath, the genomes of strains IO1 and BH contained IS elements belonging to IS256, IS3, IS5, and ISAs1 families (Siguier et al., 2014). The total number of complete IS elements in these three strains was also similar. By contrast, the genomes of strains IO1 and IM1T did not contain IS elements of the ISAs1 family but contained one IS of the IS110 and IS630 families. Strain IM1T also contained IS of IS91, ISNCY, IS200_IS605 families, which were not revealed in other studied genomes. Among the studied strains, the Mc7 genome was noticeably distinguished by both the total number and the variety of IS elements. IS massive expansion is accompanied by gene inactivation and decay, genome rearrangement, and genome reduction (Siguier et al., 2014). Some IS elements, for example, IS30 (Dalrymple and Arber, 1985) are capable of reactivating the expression of nearby genes. Therefore, IS elements can play an important role in the adaptation of the host cell to new lifestyle, such as continuous cultivation for the needs of industry (Gaffé et al., 2011). High similarity of the IS family composition between strains Bath, IO1, and BH suggests low influence of a lateral gene transfer on the evolution of their genomes. The change in the IS composition in other strains may be associated with the activity of other mobile elements, i.e., integrons in the genomes of strains Mc7 and M. geothermalis IM1T, and ICE in the genome of strain KN2.
Figure 9.
Distribution of insertion sequences (IS) element families in Methylococcus genomes.
In summary, this study expanded the pool of phenotypically characterized Methylococcus strains with good-quality genome sequences by contributing four novel isolates of these bacteria from various habitats. Three novel isolates, strains KN2, IO1, and BH, were assigned to the species M. capsulatus and displayed surprisingly high similarity in gene content to that in M. capsulatus Bath. As evidenced by the original study of Foster and Davis (1966) and our work, methanotrophs of this species tend to inhabit nutrient-rich freshwater habitats, such as wastewater, sewage or sediments. They possess relatively small (3.2–3.6Mb) genomes and display high growth rates on methane in the temperature range between 42 and 52°C. The ability to grow on methanol, however, may vary in different members of this species. Thus, M. capsulatus Bath displayed only a trace growth on methanol. The same was true for one of the isolates obtained in our study, strain IO1, which showed poor growth only at low methanol concentrations (0.05–0.1%, v/v). By contrast, two other strains, KN2 and BH, were capable of growth in a wide range of methanol concentrations, up to 3.0% CH3OH in case of strain KN2. Notably, high resistance of strain KN2 to methanol correlates with the occurrence of four different isozymes of formate dehydrogenase in this methanotroph. This array of formate dehydrogenases may potentially be responsible for the efficient oxidation of С1 metabolites to CO2, thus determining high resistance to methanol. However, the exact role of different formate dehydrogenases in metabolism of methanotrophic bacteria remains to be clarified. Strain KN2 was also distinct with regard to its temperature optimum (48–52°C), which was higher than that in other isolates and strain Bath. Finally, strain KN2 showed the best performance during its continuous cultivation in a bioreactor. Taken together, M. capsulatus KN2 has the highest potential for the biotechnologies implying high growth rates on methane and/or abilities to grow on methanol.
The fourth isolate obtained in our study from a landfill cover soil, strain Mc7, may potentially represent a novel species of the genus Methylococcus. This is suggested by the results of phylogenomic analysis, comparative genome analysis as well as by the phenotypic difference of strain Mc7 and two previously described Methylococcus species. Thus, cells of strain Mc7 (~1.6μm in diameter) were larger that cells of M. capsulatus (1.0–1.1μm) or M. geothermalis (0.7–1.0μm; Awala et al., 2020). The genome size of strain Mc7 (4.0Mb) also exceeded that in other described Methylococcus species. Notably, the genome of this methanotroph from a landfill cover soil contained high number and variety of mobile elements, which may have played an important role in the adaptation of strain Mc7 to highly variable conditions of its natural habitat. Thus, strain Mc7 possessed a number of genome-encoded features, which were absent in strains of M. capsulatus, such as sucrose biosynthesis and the ability to scavenge phosphorus and sulfur from the environment. These metabolic capabilities may represent important components of the genome-determined environmental adaptations of this methanotroph. Further examination of phenotypic and chemotaxonomic features is needed to establish the taxonomic position of strain Mc7.
Thus, our study contributes to the current knowledge of Methylococcus-like methanotrophs, the bacteria of high environmental and biotechnological relevance. As suggested by the pangenome analysis, the metabolic diversity within this genus remains underestimated and calls for further cultivation- and genome-based studies.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/genbank/, CP079095–CP079098.
Author Contributions
SD and NP obtained funding and designed the study. OD and IO isolated novel Methylococcus strains. IO obtained and annotated the genome sequences. IO, KM, SBu, and VK performed the comparative genome analysis. IO, RS, KM, ET, and SBe conducted physiology and growth tests. ET and NK assisted with experiments on continuous cultivation in bioreactor. IO, KM, VK, and SD wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The article was made with support of the Ministry of Science and Higher Education of the Russian Federation in accordance with agreement no. 075-15-2020-907, date November 16, 2020 on providing a grant in the form of subsidies from the Federal budget of Russian Federation. The grant was provided for state support for the creation and development of a World-Class Scientific Center “Agrotechnologies for the Future”.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.756830/full#supplementary-material
References
- Adams M. A., Luo Y., Hove-Jensen B., He S. M., Van Staalduinen L. M., Zechel D. L., et al. (2008). Crystal structure of PhnH: an essential component of carbon-phosphorus lyase in Escherichia coli. J. Bacteriol. 190, 1072–1083. doi: 10.1128/JB.01274-07, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony C. (1983). The Biochemistry of Methylotrophs. London, New York: Academic Press. doi: 10.1016/0968-0004(83)90116-0 [DOI] [Google Scholar]
- Arndt D., Grant J. R., Marcu A., Sajed T., Pon A., Liang Y., et al. (2016). PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res. 44, W16–W21. doi: 10.1093/nar/gkw387, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awala S. I., Bellosillo L. A., Gwak J.-H., Nguyen N.-L., Kim S.-J., Lee B.-H., et al. (2020). Methylococcus geothermalis sp. nov., a methanotroph isolated from a geothermal field in the Republic of Korea. Int. J. Syst. Evol. Microbiol. 70, 5520–5530. doi: 10.1099/ijsem.0.004442, PMID: [DOI] [PubMed] [Google Scholar]
- Aziz R. K., Bartels D., Best A. A., DeJongh M., Disz T., Edwards R. A., et al. (2008). The RAST server: rapid annotations using subsystems technology. BMC Genomics 9:75. doi: 10.1186/1471-2164-9-75, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian R., Smith S. M., Rawat S., Yatsunyk L. A., Stemmler T. L., Rosenzweig A. C. (2010). Oxidation of methane by a biological dicopper Centre. Nature 465, 115–119. doi: 10.1038/nature08992, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter N. J., Hirt R. P., Bodrossy L., Kovacs K. L., Embley M. T., Prosser J. I., et al. (2002). The ribulose-1,5-bisphosphate carboxylase/oxygenase gene cluster of Methylococcus capsulatus (Bath). Arch. Microbiol. 177, 279–289. doi: 10.1007/s00203-001-0387-x, PMID: [DOI] [PubMed] [Google Scholar]
- Bazinet A. L. (2017). Pan-genome and phylogeny of Bacillus cereus sensu lato. BMC Evol. Biol. 17:176. doi: 10.1186/s12862-017-1020-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley R., Chasteen T. G. (2004). Environmental VOSCs–formation and degradation of dimethyl sulfide, methanethiol and related materials. Chemosphere 55, 291–317. doi: 10.1016/j.chemosphere.2003.12.017, PMID: [DOI] [PubMed] [Google Scholar]
- Blomqvist K., Nikkola M., Lehtovaara P., Suihko M. L., Airaksinen U., Stråby K. B., et al. (1993). Characterization of the genes of the 2,3-butanediol operons from Klebsiella terrigena and Enterobacter aerogenes. J. Bacteriol. 175, 1392–1404. doi: 10.1128/jb.175.5.1392-1404.1993, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden R., Borodina E., Wood A. P., Kelly D. P., Murrell J. C., Schäfer H. (2011). Purification and characterization of dimethylsulfide monooxygenase from Hyphomicrobium sulfonivorans. J. Bacteriol. 193, 1250–1258. doi: 10.1128/JB.00977-10, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher Y., Labbate M., Koenig J. E., Stokes H. W. (2007). Integrons: mobilizable platforms that promote genetic diversity in bacteria. Trends Microbiol. 15, 301–309. doi: 10.1016/j.tim.2007.05.004, PMID: [DOI] [PubMed] [Google Scholar]
- Bowman J. P. (2015). “Methylococcus,” in Bergey’s Manual of Systematics of Archaea and Bacteria. eds. Trujillo M. E., Dedysh S., DeVos P., Hedlund B., Kampfer P., Rainey F. A., et al. (John Wiley & Sons, Inc.). [Google Scholar]
- Bowman J. P., Sly L. T., Nichols P. D., Hayward A. C. (1993). Erratum: revised taxonomy of the methanotrophs: description of Methylobacter gen. Nov., emendation of Methylococcus, validation of Methylosinus and Methylocystis species, and a proposal that the family Methylococcaceae includes only the group I methanotrophs. Int. J. Syst. Bacteriol. 43, 735–753. doi: 10.1099/00207713-44-2-375 [DOI] [Google Scholar]
- But S. Y., Solntseva N. P., Egorova S. V., Mustakhimov I. I., Khmelenina V. N., Reshetnikov A., et al. (2018). The genes and enzymes of sucrose metabolism in moderately thermophilic methanotroph Methylocaldum szegediense O12. Extremophiles 22, 433–445. doi: 10.1007/s00792-018-1006-y, PMID: [DOI] [PubMed] [Google Scholar]
- Cantalapiedra C. P., Hernández-Plaza A., Letunic I., Bork P., Huerta-Cepas J. (2021). eggNOG-mapper v2: functional annotation, orthology assignments, and domain prediction at the metagenomic scale. bioRxiv [Preprint]. doi: 10.1101/2021.06.03.446934 [DOI] [PMC free article] [PubMed]
- Carere C. R., Hards K., Houghton K. M., Power J. F., McDonald B., Collet C., et al. (2017). Mixotrophy drives niche expansion of verrucomicrobial methanotrophs. ISME J. 11, 2599–2610. doi: 10.1038/ismej.2017.112, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrión O., Curson A. R. J., Kumaresan D., Fu Y., Lang A. S., Mercadé E., et al. (2015). A novel pathway producing dimethylsulphide in bacteria is widespread in soil environments. Nat. Commun. 6:6579. doi: 10.1038/ncomms7579, PMID: [DOI] [PubMed] [Google Scholar]
- Chatwood L. L., Müller J., Gross J. D., Wagner G., Lippard S. J. (2004). NMR structure of the flavin domain from soluble methane monooxygenase reductase from Methylococcus capsulatus (Bath). Biochemistry 43, 11983–11991. doi: 10.1021/bi049066n, PMID: [DOI] [PubMed] [Google Scholar]
- Chistoserdova L. (2011). Modularity of methylotrophy, revisited. Environ. Microbiol. 13, 2603–2622. doi: 10.1111/j.1462-2920.2011.02464.x, PMID: [DOI] [PubMed] [Google Scholar]
- Colby J., Dalton H. (1978). Resolution of the methane mono-oxygenase of Methylococcus capsulatus (Bath) into three components. Purification and properties of component C, a flavoprotein. Biochem. J. 171, 461–468. doi: 10.1042/bj1710461, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Moreira B., Vinuesa P. (2013). GET_HOMOLOGUES, a versatile software package for scalable and robust microbial pangenome analysis. Appl. Environ. Microbiol. 79, 7696–7701. doi: 10.1128/AEM.02411-13, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csáki R., Bodrossy L., Klem J., Murrell J. C., Kovács K. L. (2003). Genes involved in the copper-dependent regulation of soluble methane monooxygenase of Methylococcus capsulatus (Bath): cloning, sequencing and mutational analysis. Microbiology 149, 1785–1795. doi: 10.1099/mic.0.26061-0 [DOI] [PubMed] [Google Scholar]
- Cury J., Jové T., Touchon M., Néron B., Rocha E. P. (2016). Identification and analysis of integrons and cassette arrays in bacterial genomes. Nucleic Acids Res. 44, 4539–4550. doi: 10.1093/nar/gkw319, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple B., Arber W. (1985). Promotion of RNA transcription on the insertion element IS30 of E. coli K12. EMBO J. 4, 2687–2693. doi: 10.1002/j.1460-2075.1985.tb03988.x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton H. (2005). The Leeuwenhoek lecture 2000 The natural and unnatural history of methane-oxidizing bacteria. Philos. Trans. R. Soc. B Biol. Sci. 360, 1207–1222. doi: 10.1098/rstb.2005.1657, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delavat F., Miyazaki R., Carraro N., Pradervand N., van der Meer J. R. (2017). The hidden life of integrative and conjugative elements. FEMS Microbiol. Rev. 41, 512–537. doi: 10.1093/femsre/fux008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSpirito A. A., Kunz R. C., Choi D.-W., Zahn J. A. (2004). Chapter 7: respiration in methanotrophs. 149–168. doi: 10.1007/978-1-4020-3163-2_7 [DOI]
- Dworkin M., Foster J. W. (1958). Experiments with some microorganisms which utilize ethane and hydrogen. J. Bacteriol. 75, 592–603. doi: 10.1128/jb.75.5.592-603.1958, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren A. M., Esen O. C., Quince C., Vineis J. H., Morrison H. G., Sogin M. L., et al. (2015). Anvi’o: an advanced analysis and visualization platformfor’omics data. PeerJ 2015, 1–29. doi: 10.7717/peerj.1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Davis R. H. (1966). A methane-dependent coccus, with notes on classification and nomenclature of obligate, methane-utilizing bacteria. J. Bacteriol. 91, 1924–1931. doi: 10.1128/jb.91.5.1924-1931.1966, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich C. G., Bardischewsky F., Rother D., Quentmeier A., Fischer J. (2005). Prokaryotic sulfur oxidation. Curr. Opin. Microbiol. 8, 253–259. doi: 10.1016/j.mib.2005.04.005, PMID: [DOI] [PubMed] [Google Scholar]
- Gaffé J., McKenzie C., Maharjan R. P., Coursange E., Ferenci T., Schneider D. (2011). Insertion sequence-driven evolution of Escherichia coli in chemostats. J. Mol. Evol. 72, 398–412. doi: 10.1007/s00239-011-9439-2, PMID: [DOI] [PubMed] [Google Scholar]
- Gagliano A. L., D’Alessandro W., Tagliavia M., Parello F., Quatrini P. (2014). Methanotrophic activity and diversity of methanotrophs in volcanic geothermal soils at Pantelleria (Italy). Biogeosciences 11, 5865–5875. doi: 10.5194/bg-11-5865-2014 [DOI] [Google Scholar]
- Gebert J., Singh B. K., Pan Y., Bodrossy L. (2009). Activity and structure of methanotrophic communities in landfill cover soils. Environ. Microbiol. Rep. 1, 414–423. doi: 10.1111/j.1758-2229.2009.00061.x, PMID: [DOI] [PubMed] [Google Scholar]
- Gillings M. R. (2014). Integrons: past, present, and future. Microbiol. Mol. Biol. Rev. 78, 257–277. doi: 10.1128/MMBR.00056-13, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goris J., Konstantinidis K. T., Klappenbach J. A., Coenye T., Vandamme P., Tiedje J. M. (2007). DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int. J. Syst. Evol. Microbiol. 57, 81–91. doi: 10.1099/ijs.0.64483-0, PMID: [DOI] [PubMed] [Google Scholar]
- Guédon G., Libante V., Coluzzi C., Payot S., Leblond-Bourget N. (2017). The obscure world of integrative and mobilizable elements, highly widespread elements that pirate bacterial conjugative systems. Genes 8:337. doi: 10.3390/genes8110337, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Ahmad A., Chothwe D., Madhu M. K., Srivastava S., Sharma V. K. (2018). Genome-scale metabolic reconstruction and metabolic versatility of an obligate methanotroph Methylococcus capsulatus str. Bath. PeerJ 7:e6685. doi: 10.7717/peerj.6685, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich A., Saveliev V., Vyahhi N., Tesler G. (2013). QUAST: quality assessment tool for genome assemblies. Bioinformatics 29, 1072–1075. doi: 10.1093/bioinformatics/btt086, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakobyan A., Zhu J., Glatter T., Paczia N., Liesack W. (2020). Hydrogen utilization by Methylocystis sp. strain SC2 expands the known metabolic versatility of type IIa methanotrophs. Metab. Eng. 61, 181–196. doi: 10.1016/j.ymben.2020.05.003, PMID: [DOI] [PubMed] [Google Scholar]
- Hartmann T., Leimkühler S. (2013). The oxygen-tolerant and NAD+-dependent formate dehydrogenase from Rhodobacter capsulatus is able to catalyze the reduction of CO2 to formate. FEBS J. 280, 6083–6096. doi: 10.1111/febs.12528, PMID: [DOI] [PubMed] [Google Scholar]
- Hazeu W., Batenburg-van der Vegte W. H., de Bruyn J. C. (1980). Some characteristics of Methylococcus mobilis sp. nov. Arch. Microbiol. 124, 211–220. doi: 10.1007/BF00427729 [DOI] [Google Scholar]
- Henard C. A., Smith H. K., Guarnieri M. T. (2017). Phosphoketolase overexpression increases biomass and lipid yield from methane in an obligate methanotrophic biocatalyst. Metab. Eng. 41, 152–158. doi: 10.1016/j.ymben.2017.03.007, PMID: [DOI] [PubMed] [Google Scholar]
- Henard C. A., Wu C., Xiong W., Henard J. M., Davidheiser-Kroll B., Orata F. D., et al. (2021). Ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO) is essential for growth of the methanotroph Methylococcus capsulatus strain Bath. Appl. Environ. Microbiol. 87:e00881-21. doi: 10.1128/AEM.00881-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi Y., Asai K., Arafuka H., Hamajima M., Iwama T., Kawai K. (2011). Molecular structure of La3+-induced methanol dehydrogenase-like protein in Methylobacterium radiotolerans. J. Biosci. Bioeng. 111, 547–549. doi: 10.1016/j.jbiosc.2010.12.017, PMID: [DOI] [PubMed] [Google Scholar]
- Houghton K. M., Stewart L. C. (2020). Temperature-gradient incubation isolates multiple competitive species from a single environmental sample. Access Microbiol. 2:acmi000081. doi: 10.1099/acmi.0.000081, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawaharraj K., Sudha Dhiman S., Bedwell S., Vemuri B., Islam J., Sani R. K., et al. (2021). Electricity from methane by Methylococcus capsulatus (Bath) and Methylosinus trichosporium OB3b. Bioresour. Technol. 321:124398. doi: 10.1016/j.biortech.2020.124398, PMID: [DOI] [PubMed] [Google Scholar]
- Jochimsen B., Lolle S., McSorley F. R., Nabi M., Stougaard J., Zechel D. L., et al. (2011). Five phosphonate operon gene products as components of a multi-subunit complex of the carbon-phosphorus lyase pathway. Proc. Natl. Acad. Sci. U. S. A. 108, 11393–11398. doi: 10.1073/pnas.1104922108, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyuzhnaya M. G., Yang S., Rozova O. N., Smalley N. E., Clubb J., Lamb A., et al. (2013). Highly efficient methane biocatalysis revealed in a methanotrophic bacterium. Nat. Commun. 4:2785. doi: 10.1038/ncomms3785, PMID: [DOI] [PubMed] [Google Scholar]
- Kao W. C., Chen Y. R., Yi E. C., Lee H., Tian Q., Wu K. M., et al. (2004). Quantitative proteomic analysis of metabolic regulation by copper ions in Methylococcus capsulatus (Bath). J. Biol. Chem. 279, 51554–51560. doi: 10.1074/jbc.M408013200, PMID: [DOI] [PubMed] [Google Scholar]
- Keltjens J. T., Pol A., Reimann J., Op Den Camp H. J. M. (2014). PQQ-dependent methanol dehydrogenases: rare-earth elements make a difference. Appl. Microbiol. Biotechnol. 98, 6163–6183. doi: 10.1007/s00253-014-5766-8, PMID: [DOI] [PubMed] [Google Scholar]
- Kizilova A. K., Sukhacheva M. V., Pimenov N. V., Yurkov A. M., Kravchenko I. K. (2014). Methane oxidation activity and diversity of aerobic methanotrophs in pH-neutral and semi-neutral thermal springs of the Kunashir Island, Russian Far East. Extremophiles 18, 207–218. doi: 10.1007/s00792-013-0603-z, PMID: [DOI] [PubMed] [Google Scholar]
- Konstantinidis K. T., Tiedje J. M. (2005). Genomic insights that advance the species definition for prokaryotes Konstantinos. Proc. Natl. Acad. Sci. U. S. A. 102, 2567–2572. doi: 10.1016/S0040-4020(01)97190-X, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzywinski M., Schein J., Birol I., Connors J., Gascoyne R., Horsman D., et al. (2009). Circos: an information aesthetic for comparative genomics. Genome Res. 19, 1639–1645. doi: 10.1101/gr.092759.109, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Bröms J. E., Sjöstedt A. (2020). Exploring the diversity within the genus Francisella – an integrated pan-genome and genome-mining approach. Front. Microbiol. 11:1928. doi: 10.3389/fmicb.2020.01928, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. doi: 10.1093/molbev/msy096, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen Ø., Karlsen O. A. (2016). Transcriptomic profiling of Methylococcus capsulatus (Bath) during growth with two different methane monooxygenases. Microbiol. Open 5, 254–267. doi: 10.1002/mbo3.324, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley T. D., Klimke W. A., Gubbins M. J., Frost L. S. (2003). F factor conjugation is a true type IV secretion system. FEMS Microbiol. Lett. 224, 1–15. doi: 10.1016/S0378-1097(03)00430-0, PMID: [DOI] [PubMed] [Google Scholar]
- Lieberman R. L., Rosenzweig A. C. (2005). Crystal structure of a membrane-bound metalloenzyme that catalyses the biological oxidation of methane. Nature 434, 177–182. doi: 10.1038/nature03311, PMID: [DOI] [PubMed] [Google Scholar]
- Lieven C., Petersen L. A. H., Jørgensen S. B., Gernaey K. V., Herrgard M. J., Sonnenschein N. (2018). A genome-scale metabolic model for Methylococcus capsulatus predicts reduced efficiency uphill electron transfer to pMMO. bioRxiv, 329714 [Preprint]. doi: 10.1101/329714 [DOI] [PMC free article] [PubMed]
- Liu D., Hunt M., Tsai I. J. (2018a). Inferring synteny between genome assemblies: a systematic evaluation. BMC Bioinformatics 19:26. doi: 10.1186/s12859-018-2026-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Li X., Xie Y., Bi D., Sun J., Li J., et al. (2018b). ICEberg 2.0: an updated database of bacterial integrative and conjugative elements. Nucleic Acids Res. 47, D660–D665. doi: 10.1093/nar/gky1123, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone P. G., Morphew R. M., Whitworth D. E. (2018). Genome sequencing and pan-genome analysis of 23 Corallococcus spp. strains reveal unexpected diversity, with particular plasticity of predatory gene sets. Front. Microbiol. 9:3187. doi: 10.3389/fmicb.2018.03187, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomans B. P., Leijdekkers P., Wesselink J. J., Bakkes P., Pol A., van der Drift C., et al. (2001). Obligate sulfide-dependent degradation of methoxylated aromatic compounds and formation of methanethiol and dimethyl sulfide by a freshwater sediment isolate, Parasporobacterium paucivorans gen. Nov., sp. nov. Appl. Environ. Microbiol. 67, 4017–4023. doi: 10.1128/AEM.67.9.4017-4023.2001, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomans B. P., van der Drift C., Pol A., Op den Camp H. J. M. (2002). Microbial cycling of volatile organic sulfur compounds. Cell. Mol. Life Sci. 59, 575–588. doi: 10.1007/s00018-002-8450-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes A., Tavares P., Petit M. A., Guérois R., Zinn-Justin S. (2014). Automated classification of tailed bacteriophages according to their neck organization. BMC Genomics 15:1027. doi: 10.1186/1471-2164-15-1027, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malashenko I. R., Romanovskaia V. A., Bogachenko V. N., Shved A. D. (1975). Thermophilic and thermotolerant bacteria that assimilate methane. Mikrobiologiia 44, 855–862. PMID: [PubMed] [Google Scholar]
- Mazel D. (2006). Integrons: agents of bacterial evolution. Nat. Rev. Microbiol. 4, 608–620. doi: 10.1038/nrmicro1462, PMID: [DOI] [PubMed] [Google Scholar]
- Minkin I., Patel A., Kolmogorov M., Vyahhi N., Pham S. (2013). “Sibelia: a scalable and comprehensive synteny block generation tool for closely related microbial genomes,” in Algorithms in Bioinformatics. eds. Darling A., Stoye J. (Berlin, Heidelberg: Springer Berlin Heidelberg; ), 215–229. [Google Scholar]
- Mohammadi S. S., Schmitz R. A., Pol A., Berben T., Jetten M. S. M., Op Den Camp H. J. M. (2019). The acidophilic methanotroph Methylacidimicrobium tartarophylax 4ac grows as autotroph on h2 under microoxic conditions. Front. Microbiol. 10:2352. doi: 10.3389/fmicb.2019.02352, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J., Lugovskoy A. A., Wagner G., Lippard S. J. (2002). NMR structure of the [2Fe-2S] ferredoxin domain from soluble methane monooxygenase reductase and interaction with its hydroxylase. Biochemistry 41, 42–51. doi: 10.1021/bi015668k, PMID: [DOI] [PubMed] [Google Scholar]
- Murrell J. C. (2010). “Genomics of Methylococcus capsulatus,” in Handbook of Hydrocarbon and Lipid Microbiology. ed. Timmis K. N. (Berlin, Heidelberg: Springer; ). [Google Scholar]
- Oshkin I. Y., Miroshnikov K. K., Grouzdev D. S., Dedysh S. N. (2020). Pan-genome-based analysis as a framework for demarcating two closely related methanotroph genera Methylocystis and Methylosinus. Microorganisms 8:768. doi: 10.3390/microorganisms8050768, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks D. H., Chuvochina M., Waite D. W., Rinke C., Skarshewski A., Chaumeil P. A., et al. (2018). A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat. Biotechnol. 36:996. doi: 10.1038/nbt.4229, PMID: [DOI] [PubMed] [Google Scholar]
- Pritchard L., Glover R. H., Humphris S., Elphinstone J. G., Toth I. K. (2016). Genomics and taxonomy in diagnostics for food security: soft-rotting enterobacterial plant pathogens. Anal. Methods 8, 12–24. doi: 10.1039/C5AY02550H [DOI] [Google Scholar]
- R Core Team (2020). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: https://www.r-project.org/
- Reshetnikov A. S., Rozova O. N., Khmelenina V. N., Mustakhimov I. I., Beschastny A. P., Murrell J. C., et al. (2008). Characterization of the pyrophosphate-dependent 6-phosphofructokinase from Methylococcus capsulatus Bath. FEMS Microbiol. Lett. 288, 202–210. doi: 10.1111/j.1574-6968.2008.01366.x, PMID: [DOI] [PubMed] [Google Scholar]
- Romanovskaya V. A., Malashenko Y. R., GrishChenko N. I. (1981). Diagnosis of methane-oxidizing bacteria by numerical methods based on cell fatty acid composition. Mikrobiologiya 49, 762–764. [PubMed] [Google Scholar]
- Romarheim O. H., Øverland M., Mydland L. T., Skrede A., Landsverk T. (2011). Bacteria grown on natural gas prevent soybean meal-induced enteritis in Atlantic salmon1-3. J. Nutr. 141, 124–130. doi: 10.3945/jn.110.128900, PMID: [DOI] [PubMed] [Google Scholar]
- Rosenzweig A. C., Frederick C. A., Lippard S. J., Nordlund P. (1993). Crystal structure of a bacterial non-haem iron hydroxylase that catalyses the biological oxidation of methane. Nature 366, 537–543. doi: 10.1038/366537a0, PMID: [DOI] [PubMed] [Google Scholar]
- Ross M. O., MacMillan F., Wang J., Nisthal A., Lawton T. J., Olafson B. D., et al. (2019). Particulate methane monooxygenase contains only mononuclear copper centers. Science 364, 566–570. doi: 10.1126/science.aav2572, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozova O. N., Khmelenina V. N., Gavletdinova J. Z., Mustakhimov I. I., Trotsenko Y. A. (2015). Acetate kinase-an enzyme of the postulated phosphoketolase pathway in Methylomicrobium alcaliphilum 20Z. Antonie Van Leeuwenhoek 108, 965–974. doi: 10.1007/s10482-015-0549-5, PMID: [DOI] [PubMed] [Google Scholar]
- Rozova O. N., Mustakhimov I. I., But S. Y., Reshetnikov A. S., Khmelenina V. N. (2019). Role of the malic enzyme in metabolism of the halotolerant methanotroph Methylotuvimicrobium alcaliphilum 20Z. PLoS One 14:e0225054. doi: 10.1371/journal.pone.0225054, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Monincová M., Chaloupková R., Prokop Z., Ohtsubo Y., Minamisawa K., et al. (2005). Two rhizobial strains, Mesorhizobium loti MAFF303099 and Bradyrhizobium japonicum USDA110, encode haloalkane dehalogenases with novel structures and substrate specificities. Appl. Environ. Microbiol. 71, 4372–4379. doi: 10.1128/AEM.71.8.4372-4379.2005, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann T. (2014). Prokka: rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069. doi: 10.1093/bioinformatics/btu153, PMID: [DOI] [PubMed] [Google Scholar]
- Seto H., Kuzuyama T. (1999). Bioactive natural products with carbon-phosphorus bonds and their biosynthesis. Nat. Prod. Rep. 16, 589–596. doi: 10.1039/a809398i, PMID: [DOI] [PubMed] [Google Scholar]
- Siguier P., Gourbeyre E., Chandler M. (2014). Bacterial insertion sequences: their genomic impact and diversity. FEMS Microbiol. Rev. 38, 865–891. doi: 10.1111/1574-6976.12067, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siguier P., Perochon J., Lestrade L., Mahillon J., Chandler M. (2006). ISfinder: the reference Centre for bacterial insertion sequences. Nucleic Acids Res. 34, D32–D36. doi: 10.1093/nar/gkj014, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simão F. A., Waterhouse R. M., Ioannidis P., Kriventseva E. V., Zdobnov E. M. (2015). BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31, 3210–3212. doi: 10.1093/bioinformatics/btv351, PMID: [DOI] [PubMed] [Google Scholar]
- Skrede A., Berge G. M., Storebakken T., Herstad O., Aarstad K. G., Sundstøl F. (1998). Digestibility of bacterial protein grown on natural gas in mink, pigs, chicken and Atlantic salmon. Anim. Feed Sci. Technol. 76, 103–116. doi: 10.1016/S0377-8401(98)00208-9 [DOI] [Google Scholar]
- Skrede A., Mydland L. T., Øverland M. (2009). Effects of growth substrate and partial removal of nucleic acids in the production of bacterial protein meal on amino acid profile and digestibility in mink. J. Anim. Feed Sci. 18, 689–698. doi: 10.22358/jafs/66442/2009 [DOI] [Google Scholar]
- Stanley S. H., Prior S. D., Leak D. J., Dalton H. (1983). Copper stress underlies the fundamental change in intracellular location of methane mono-oxygenase in methane-oxidizing organisms: studies in batch and continuous cultures. Biotechnol. Lett. 5, 487–492. doi: 10.1007/BF00132233 [DOI] [Google Scholar]
- Taylor S. C., Dalton H., Dow C. S. (1981). Ribulose-1,5-bisphosphate carboxylase/oxygenase and carbon assimilation in Methylococcus capsulatus (Bath). Microbiology 122, 89–94. doi: 10.1099/00221287-122-1-89 [DOI] [Google Scholar]
- Ternan N. G., McGrath J. W., Quinn J. P. (1998). Phosphoenolpyruvate phosphomutase activity in an L-phosphonoalanine- mineralizing strain of Burkholderia cepacia. Appl. Environ. Microbiol. 64, 2291–2294. doi: 10.1128/AEM.64.6.2291-2294.1998, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani A. M., Siguier P., Gourbeyre E., Charneau V., Chandler M. (2011). ISsaga is an ensemble of web-based methods for high throughput identification and semi-automatic annotation of insertion sequences in prokaryotic genomes. Genome Biol. 12:R30. doi: 10.1186/gb-2011-12-3-r30, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier S., Khmelenina V. N., Bringel F., Reshetnikov A. S., Lajus A., Mangenot S., et al. (2012). Genome sequence of the haloalkaliphilic methanotrophic bacterium Methylomicrobium alcaliphilum 20Z. J. Bacteriol. 194, 551–552. doi: 10.1128/JB.06392-11, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward N., Larsen Ø., Sakwa J., Bruseth L., Khouri H., Durkin A. S., et al. (2004). Genomic insights into methanotrophy: the complete genome sequence of Methylococcus capsulatus (Bath). PLoS Biol. 2:e303. doi: 10.1371/journal.pbio.0020303, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittenbury R. (1981). “The interrelationship of autotrophy and methylotrophy as seen in Methylococcus capsulatus (Bath),” in Microbiol Growth on C1 Compounds. ed. Dalton H. (London, UK: Heyden & Son Ltd.), 181–190. [Google Scholar]
- Whittenbury R., Dalton H. (1981). “The methylotrophic bacteria,” in The Prokaryotes: A Handbook on Habitats, Isolation, and Identification of Bacteria. eds. Starr M. P., Stolp H., Trüper H. G., Balows A., Schlegel H. G. (Berlin, Heidelberg: Springer Berlin Heidelberg; ), 894–902. [Google Scholar]
- Wick R. R., Judd L. M., Gorrie C. L., Holt K. E. (2017). Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 13:e1005595. doi: 10.1371/journal.pcbi.1005595, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. (2001). Preparation of genomic DNA from bacteria. Curr. Protoc. Mol. Biol. 56, 2–4. doi: 10.1016/B978-0-12-418687-3.00011-2, PMID: [DOI] [PubMed] [Google Scholar]
- Xiao Z., Xu P. (2007). Acetoin metabolism in bacteria. Crit. Rev. Microbiol. 33, 127–140. doi: 10.1080/10408410701364604 [DOI] [PubMed] [Google Scholar]
- Xie Z., Tang H. (2017). ISEScan: automated identification of insertion sequence elements in prokaryotic genomes. Bioinformatics 33, 3340–3347. doi: 10.1093/bioinformatics/btx433, PMID: [DOI] [PubMed] [Google Scholar]
- Xu X., Shi H., Gong X., Chen P., Gao Y., Zhang X., et al. (2020). Structural insights into sodium transport by the oxaloacetate decarboxylase sodium pump. eLife 9:e53853. doi: 10.7554/eLife.53853, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z., Beck D. A. C., Chistoserdova L. (2017). Natural selection in synthetic communities highlights the roles of Methylococcaceae and Methylophilaceae and suggests differential roles for alternative methanol dehydrogenases in methane consumption. Front. Microbiol. 8:2392. doi: 10.3389/fmicb.2017.02392, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn J. A., DiSpirito A. A. (1996). Membrane-associated methane monooxygenase from Methylococcus capsulatus (Bath). J. Bacteriol. 178, 1018–1029. doi: 10.1128/jb.178.4.1018-1029.1996, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Huang J., Zhao F., Chistoserdova L., Giovannoni S. (2018). Physiological effect of XoxG(4) on lanthanide-dependent methanotrophy. mBio 9:e02430-17. doi: 10.1128/mBio.02430-17, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/genbank/, CP079095–CP079098.