Abstract
Human granulocytotropic ehrlichias are tick-borne bacterial pathogens that cause an acute, life-threatening illness, human granulocytic ehrlichiosis (HGE). Ehrlichias within neutrophil granulocytes that invade tick bite sites are likely ingested by the vector, to be transmitted to another mammalian host during the tick’s next blood meal. Thus, the cycle of replication and development in the vector is prerequisite to mammalian infection, and yet these events have not been described. We report tick cell culture isolation of two strains of the HGE agent directly from an infected horse and a dog and have also established a human isolate from HL60 culture in tick cells, proving that the blood stages of the HGE agent are infectious for tick cells, as are those replicating in the human cell line HL60. This required changes to the culture system, including a new tick cell line. In tick cell layers, the HGE agent induced foci of infection that caused necrotic plaques and eventual destruction of the culture. Using the human isolate and electron microscopy, we monitored adhesion, internalization, and replication in vector tick cells. Both electron-lucent and -dense forms adhered to and entered cells by a mechanism reminiscent of phagocytosis. Ehrlichial cell division was initiated soon after, resulting in endosomes filled with numerous ehrlichias. During early development, pale ehrlichias with a tight cell wall dominated, but by day 2, individual bacteria condensed into dark forms with a rippled membrane. These may become compacted into clumps where individual organisms are barely discernible. Whether these are part of an ehrlichia life cycle or are degenerating is unknown.
Human granulocytic ehrlichiosis (HGE) agents are obligate intracellular prokaryotes that are transmitted to mammalian hosts via the bite of an infected tick. They cause an emerging disease, HGE (1, 9), that is characterized by an acute, sometimes fatal febrile syndrome most commonly accompanied by malaise, headache, myalgia, and arthralgia. Laboratory findings include leukopenia, thrombocytopenia, anemia, and elevated serum transaminases (1, 17). Stained blood films reveal the presence of groups of organisms, called morulae, enclosed in a common vacuole within neutrophil granulocytes. Besides humans, horses and dogs also appear to be susceptible to the HGE agent (2, 13, 14, 18, 20), and white-footed mice (8, 30, 40) and possibly white-tailed deer (4, 24) may act as a reservoir in nature. In North America, the HGE agent is transmitted by the ticks Ixodes scapularis (12, 31, 40) and Ixodes pacificus (34, 36), and in Europe, the closely related tick Ixodes ricinus appears to be the main vector (16, 32). Serologic studies place the HGE agent within the same geographic range as the Lyme disease spirochete, Borrelia burgdorferi, which shares the same vector tick (18, 37).
Demonstration of the agent in its tick vector has been achieved by PCR amplification of HGE agent-specific nucleic acid sequences based on 16S ribosomal DNA (rDNA) (7, 10, 31) and visualization by light microscopy in salivary glands stained with the Feulgen technique (40). It appears that levels of infection in ticks are either extremely low or that, in the vector, the HGE agent exists in a form that is not easily recognizable by cytologic or serologic methods. We previously reported culture isolation of a closely related tick-borne pathogen of cattle, Anaplasma marginale, and the MRK strain of Ehrlichia equi in cell line IDE8 (27, 29). Here, we describe direct cultivation from blood of two new primary isolates of the HGE agent from a horse and a dog and describe invasion of tick cells in culture by a human patient isolate, as well as its intracellular morphogenesis. To achieve this required changes to the culture system, including the use of a new cell line.
MATERIALS AND METHODS
Tick cell culture.
Cell line ISE6 (26) from the tick I. scapularis was used. Stock cultures were maintained as described elsewhere (28), except that they were grown at 34°C. Also, the L-15B culture medium was modified by addition of one-fourth water by volume, to lower the osmotic pressure from approximately 420 mosM/liter to approximately 315 mosM/liter (L-15B300). Fetal bovine serum (5%; Sigma, St. Louis, Mo.), tryptose phosphate broth (10%; Difco, Detroit, Mich.), and lipoprotein concentrate (0.1%; ICN, Irvine, Calif.) were added, and the pH was adjusted to 7.0 to 7.2. Medium was changed once a week.
Ehrlichia culture.
Primary isolates from a dog and a horse were obtained from animals naturally infected in Minnesota. Blood from the horse was brought to the laboratory the same day as collected, and dog blood was taken from a patient presenting to the small animal clinic in St. Paul, Minn. Blood was drawn into EDTA as anticoagulant. Buffy coat cells were harvested after centrifugation for 10 min at 500 × g at room temperature and washed once in L-15B300. Buffy coat and contaminating erythrocytes were layered onto a 25-cm2 (Sarstedt, Newton, N.C.; vented plug cap) culture of ISE6 cells with 5 ml of L-15B300 supplemented as for routine cell stock maintenance, plus 0.25% NaHCO3 and 25 mM HEPES, pH 7.5, referred to as ehrlichia medium. Cultures were incubated in a candle jar at 34°C as described elsewhere (29), and medium was changed twice weekly, taking care not to remove the erythrocytes. If erythrocytes became depleted due to lysis during the first 1 to 2 months, fresh, washed human erythrocytes were added (approximately 108 per 5 ml of culture).
To transfer a human patient-derived HGE isolate from HL60 to tick cell culture, HL60 cells infected 30 to 90% with isolate HGE-MN1 (17) in the fifth passage in vitro were added to ISE6 cells at a ratio of 1:20. Washed human erythrocytes were added, and cultures were incubated as described above.
Once the ehrlichias from animal blood or HL60 cultures became established in ISE6 cells, usually by the third to fourth passage, cultures were incubated in tightly capped flasks (i.e., without CO2). During the early passages, 1/5 to 1/10 of an infected cell layer was transferred to a new culture of ISE6 cells. Later, the inoculum for pathogen maintenance was reduced to 1 to 5%, and transfers were made once every 7 to 10 days. Addition of fresh tick cells during that time was not needed. Progress of infection in ISE6 cells was monitored by phase-contrast microscopy or by examination of Giemsa-stained cell spreads.
To test animal infectivity of HGE isolates grown in ISE6 cells, cultures in which at least 70% of the cells were infected were resuspended in their medium, and 0.5 ml of the suspension was inoculated intraperitoneally into 3-week-old hamsters (Mesocricetus auratus) or 4-week-old mice (C3/HeJ). From day 7 postinoculation (p.i.), tail blood smears were prepared and stained with Giemsa’s stain to detect infected neutrophil granulocytes by light microscopy. Animals were killed by exposure to CO2 gas at 2 weeks p.i. and exsanguinated by cardiac puncture. DNA from EDTA-blood was purified with the PureGene reagent kit (Gentra Systems, Inc., Minneapolis, Minn.). Animals were cared for and used in accordance with the rules and regulations established by the Institutional Animal Care and Use Committee of the University of Minnesota.
PCR and sequencing.
To verify the identity of the new equine and canine isolates, DNA was purified from infected ISE6 cultures with either ISOQuick (Orca Industries, Bothell, Wash.) or PureGene (Gentra). Ehrlichia DNA was amplified with the genus-specific primers PER1 and -2 and a species-specific primer pair, GER3 and -4, and electrophoresed, stained, and visualized as described elsewhere (17). For comparison, DNA extracted from HL60 cells infected with HGE-MN1 and -2 (17), IDE8 cells infected with Ehrlichia canis (15), DH82 cells infected with Ehrlichia chaffeensis (courtesy of J. E. Dawson, Centers for Disease Control and Prevention, Atlanta, Ga. [11]), and uninfected ISE6 cells were also subjected to PCR under the same conditions.
16S rRNA gene sequence analysis.
16S rDNA to be sequenced was PCR amplified with primers PER3 (5′ ATG CAT TAC TCA CCC CTC TG 3′) and GER4 (5′ AAG TGC CCG GCT TAA CCC GCT GGC 3′), which span bases 1 to 20 and 1077 to 1101, respectively (17), of the reported sequence for the agent of HGE (GenBank accession no. U02521). Both strands of the amplification product were sequenced with the oligonucleotide primers PER1 (bp 187 to 211), PER2 (bp 616 to 638), PER3 (bp 1 to 20), PER4 (bp 92 to 111), PER5 (bp 818 to 837), PER6 (bp 925 to 943), GER3 (bp 950 to 973), and GER4 (bp 1077 to 1101) (17). Sequencing was carried out in a 377XL DNA sequencer (Applied Biosystems GmbH, Darmstadt, Germany) by the Taq DyeDideoxy terminator method. Sequence analyses were done with DNAMAN for Windows 95 (Lynnon BioSoft, Quebec, Quebec, Canada). Sequence data for comparison were obtained from GenBank.
Time course of infection.
HGE-MN1-infected ISE6 cultures (90 to 100% of cells infected, <10 passages in ISE6 tick cells) were resuspended and passed five times through a 25-gauge needle by means of a 5-ml LuerLock syringe to disrupt the cells and liberate the ehrlichias. After low-speed centrifugation of the resulting suspension to remove large debris and intact cells (175 × g, 10 min at room temperature), ehrlichias in the supernatant were sedimented for 15 min at 13,000 × g. Pellets were resuspended in ehrlichia medium, mixed with approximately 2.5 × 107 ISE6 cells at a ratio of one infected to one uninfected cell, and seeded into a tissue culture plate (24 well; Nunc, Roskilde, Denmark), 0.5 ml per well. The plate was incubated at 34°C in a candle jar. After 30 min, the plate was gently rocked, the supernatant was discarded, and cell layers were rinsed once with unsupplemented L-15B300 to remove nonadherent ehrlichias. Wells were refilled with 0.5 ml of ehrlichia medium. At 1, 2, 4, 8, 12, 24, 48, and 96 h p.i., cells from two wells were resuspended and pipetted into 1 ml of fixative each (see below). The suspension was centrifuged for 5 min at 175 × g, and the supernatant was replaced with fresh fixative. Cells from a third well were spun onto microscope slides, fixed in absolute methanol, and stained with Giemsa’s stain. The experiment was repeated twice, with ehrlichias of the same isolate and at equivalent passage levels.
Electron microscopy.
Cultures were processed for electron microscopy as outlined elsewhere for E. equi (29). Briefly, cells were fixed in modified Ito’s fixative (19, 22) and treated with 0.1% tannic acid in water to enhance demonstration of ehrlichial membranes. Postfixation was done in 0.5% osmium tetroxide with 0.8% potassium-ferrocyanide as a reducing agent. Cells were soaked in 0.1% uranyl acetate overnight, dehydrated in ascending concentrations of ethanol, and embedded in Spurr’s low-viscosity resin (EM Sciences, Cherry Hill, N.J.). Thin sections were stained with lead citrate (35, 38) and examined on a Philips EM12 or a Hitachi HU-11E-1 electron microscope.
RESULTS
Primary isolation and transfer from HL60 cells.
The behavior of ehrlichias from infected animal blood and that of ehrlichias from HL60 cells were similar and are described together here. Successful isolation of the HGE agent, whether from infected blood or from HL60 culture, required alteration of the culture conditions and the use of a different tick cell line. Initially, we used the cell line (IDE8) and culture conditions (i.e., undiluted L-15B, no blood supplementation) that led to the successful isolation of the MRK strain of E. equi. Every time, infected tick cells were seen in Giemsa-stained smears by 1 week p.i., but by 3 weeks, infected cells could no longer be demonstrated. Despite repeated attempts, no isolates were obtained in IDE8 cells or when undiluted L-15B was used. We then replaced cell line IDE8 with cell line ISE6, lowered the medium osmolarity, and included erythrocytes in the cultures. Under those circumstances, the ehrlichias successfully became established in tick cell culture, continued to multiply, and efficiently spread through the cultures, eventually infecting 90% or more of the cells. During the first 4 to 8 weeks p.i., ehrlichial growth was erratic. Addition of erythrocytes was beneficial and stimulated development of ehrlichias. Once regular subcultures could be made, an atmosphere reduced in O2 and enriched in CO2 was no longer required, cultures could be maintained outside the candle jar, and blood supplementation was unnecessary. HGE isolates propagated in tick cell culture retained infectivity for hamsters or mice until at least the 28th passage in tick cells and caused infection of peripheral blood neutrophil granulocytes as demonstrated in Giemsa-stained blood smears and by PCR. In ISE6 cells, the appearance of the ehrlichial inclusions was similar to that of the E. equi inclusions in IDE8 cells. Notably, single colonies of ehrlichias were much larger than morulae in granulocytes or HL60 cells, and pathogen morphology was more variable, ranging from masses of highly pleomorphic, bluish-staining organisms to tiny coccoid, magenta forms and deep inky blue compacted bodies probably representing the dense colonies described below.
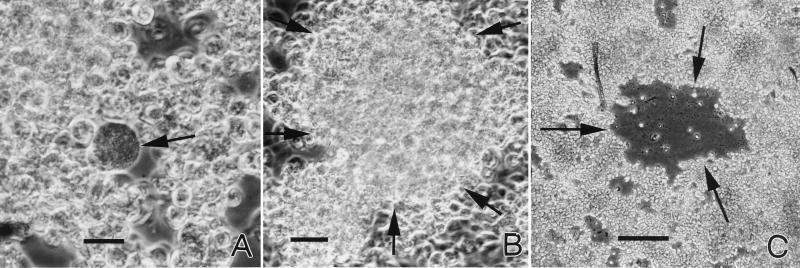
With regular subculturing and at higher dilutions of the inoculum (1:50 to 1:100), distinct foci of ehrlichial multiplication were demonstrable in the cell layer by phase-contrast microscopy. Single infected cells (Fig. 1A) lysed, releasing the organisms, which in turn infected other neighboring cells, enlarging the plaque (Fig. 1B). Dead cells in the center detached and disintegrated, causing the appearance of holes in the cell layer (Fig. 1C). Such cytopathic changes were not seen in uninfected cultures.
FIG. 1.
Phase-contrast microscopy of plaque formation by the HGE agent in cultured tick cells. (A) Single infected cell (arrow). Note distention of the cell membrane and granular cell contents representing ehrlichias. Bar, 40 μm. (B) Small infected focus of highly granular cells (arrows). Bar, 40 μm. (C) Plaque that has formed by lysis and detachment of infected cells (arrows). Bar, 100 μm.
PCR and nucleotide sequence analysis.
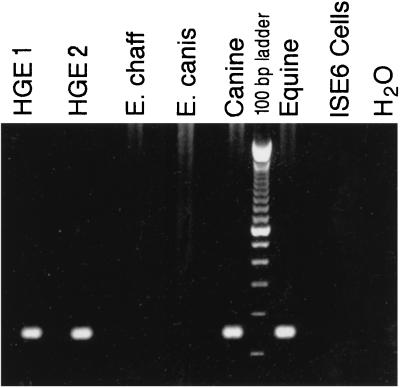
Primers PER1 and -2 mediated amplification of DNA of the appropriate size from all ehrlichia sources but not from tick host cell DNA (data not shown), while GER3 and -4 produced a 150-bp fragment in the PCR only when template DNA was from granulocytic ehrlichia-infected cultures (i.e., isolates HGE-MN1 and -2, as well as the canine and the equine HGE isolates [Fig. 2]). This established and confirmed that all three strains growing in ISE6 tick cells were HGE isolates. Analysis of a 1,098-bp stretch (bp 1 to 1098) of 16S rDNA additionally revealed 100% identity of the two new animal isolates with the first described isolate (17).
FIG. 2.
Agarose gel electrophoresis of PCR products amplified with primers GER1 and GER3, specific for 16S rDNA of ehrlichias in the E. phagocytophila group. Shown is a 1.75% agarose gel stained with ethidium bromide. Target DNA was purified from different materials as follows: lanes HGE1 and HGE2, I. scapularis tick cell culture, line ISE6, infected with the human isolate HGE-MN1 and HGE-MN2 (17), respectively; lane E. chaff, DH82 cells infected with E. chaffeensis (11); lane E. canis, I. scapularis tick cell cultures, line IDE8, infected with E. canis (15); lane Canine, I. scapularis tick cell culture, line ISE6, infected with the canine HGE isolate; lane Equine, I. scapularis tick cell culture, line ISE6, infected with the equine HGE isolate; lane ISE6 Cells, I. scapularis tick cell cultures, line ISE6, uninfected host cell control; lane H2O, no target control.
Invasion and morphogenesis in ISE6 cells.
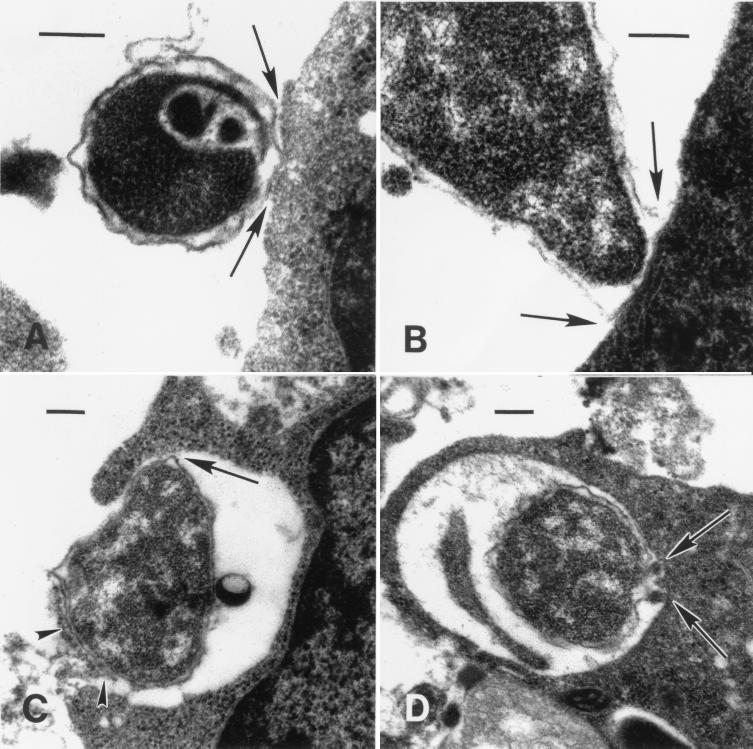
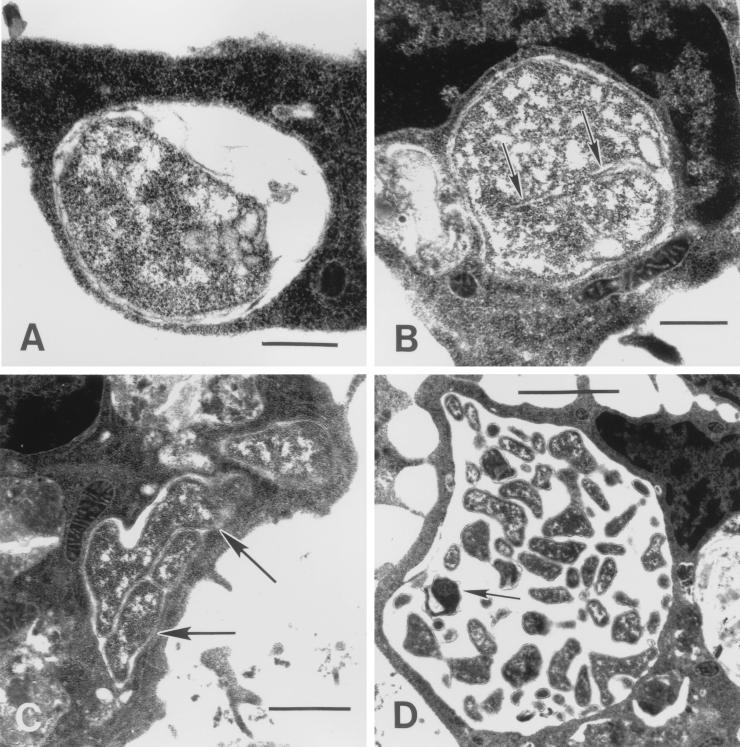
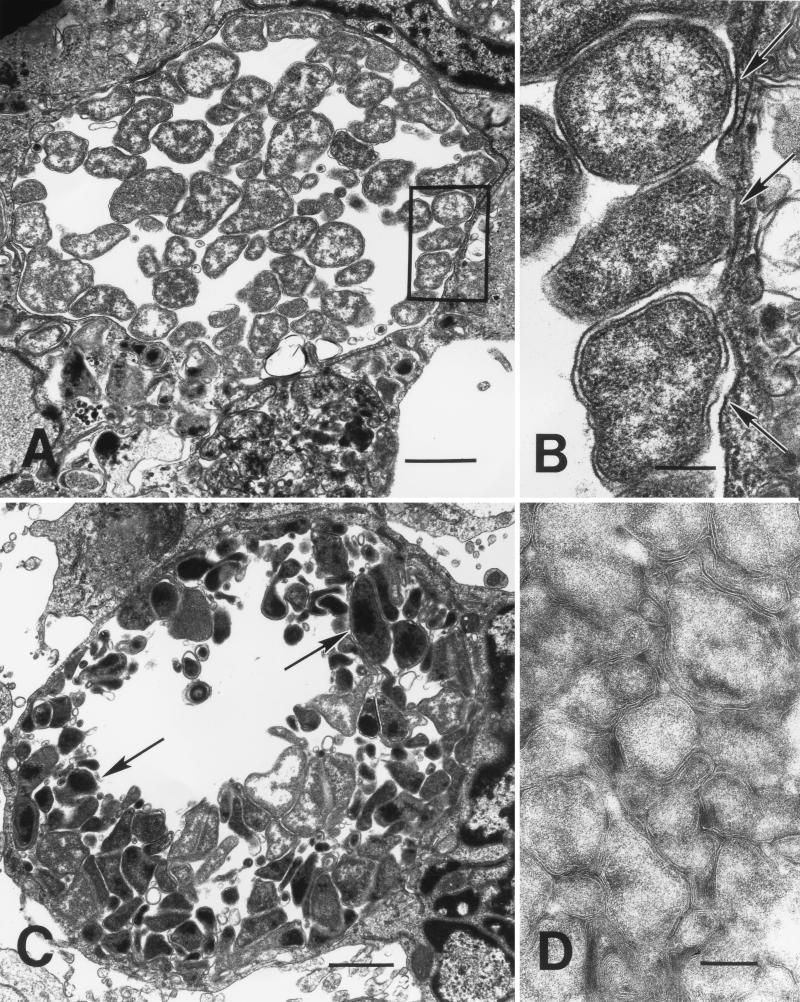
For each time point p.i., several sections from the two experiments were examined to ensure that the phenomena observed were representative of ehrlichial behavior. Within 1 h p.i., ehrlichias were found attached to tick cells (Fig. 3A and B). Attached and invading organisms were condensed to a varying degree as indicated by a loose, rippled membrane and included very dense, dark forms (Fig. 3A) as well as paler, mottled organisms. By 2 h p.i., spreading of the ehrlichial membrane at the tick cell attachment site was noticeable (Fig. 3B, arrows), and at 4 h p.i., host cells had become actively engaged in taking up ehrlichias by a process that resembled phagocytosis (Fig. 3C). Organisms at the cell surface caused protrusion of a cytoplasmic lip that eventually enclosed and internalized the bacterium (Fig. 3D). There was indication that focal points of adhesion between the cell and ehrlichial membrane (Fig. 3C, arrow and arrowheads) migrated and shifted position around the bacterial body until the lips of the phagocytic pit met, enclosing it. These points of attachment appeared preserved within the early endosome (Fig. 3D, arrows), suggesting a receptor-mediated interaction. Between 4 and 8 h p.i., ehrlichias that were initially resting singly in the endosome (Fig. 4A) were observed to show initial evidence of cell division (Fig. 4B), leading to the appearance of small morulae comprising just a few organisms as early as 12 h p.i. (Fig. 4C). Thereafter, ehrlichial cell division and development proceeded rapidly, and by 48 h p.i. distended endosomes harbored large numbers of bacteria, including the first condensed, electron-dense forms (Fig. 4D). After that time, development became asynchronous, as ehrlichias liberated from tick host cells reinvaded and initiated new infections. An examination of ehrlichias near the endosomal periphery showed close apposition of bacterial and host endosomal membranes (Fig. 5A and B, arrowheads), possibly indicative of parasite-host metabolic interaction. In older (96 h p.i.) cultures, ehrlichial colonies comprising only pale, electron-lucent forms coexisted with those harboring appreciable numbers (≥50% of bacteria in the section) of condensing organisms (Fig. 5C). In some cells, these became compacted into masses of bacteria in which individual organisms could just barely be delineated by their membranes (Fig. 5D).
FIG. 3.
Sequence of adhesion to and invasion of cultured vector tick cells by the HGE agent. (A) One hour p.i.; condensed ehrlichia with rippled membrane, attached to a tick cell. Note intimate contact with the tick host cell membrane as indicated by the arrows. (B) Two hours p.i.; the membrane of an attached ehrlichia has spread out along the tick host cell membrane (arrows). (C) Four hours p.i.; uptake of ehrlichia into host cells by a process resembling phagocytosis. Note protrusion of a cytoplasmic lip in preparation of internalization of the ehrlichia. Arrow and arrowheads indicate focal points of adhesion between the cell and ehrlichial membrane. (D) Completely internalized ehrlichia. Arrows indicate points of initial attachment that were apparently preserved within the early endosome. Bars, 0.2 μm.
FIG. 4.
Replication of the HGE agent in tick cells in vitro. (A) Single ehrlichia enclosed with endosome, 4 h p.i. Bar, 0.4 μm. (B) Ehrlichia with enfolding membrane (arrows), possibly the first evidence of division by 8 h p.i. Bar, 0.4 μm. (C) At 12 h p.i., replication has been initiated, leading to appearance of small morulae (arrows). Bar, 1 μm. (D) By 48 h p.i., distended endosomes harbor large numbers of bacteria, including the first electron-dense forms (arrow). Bar, 2 μm.
FIG. 5.
Late developmental forms of the HGE agent in tick cell culture. (A) From 72 h p.i., tick cells contained large endosomes more or less densely filled with highly pleomorphic ehrlichias. Bar, 1 μm. (B) Inset from panel A, enlarged. Ehrlichias near the endosomal periphery showed close contact between bacterial and host endosomal membranes (arrows). Bar, 0.2 μm. (C) In older (96 h p.i.) cultures, darker-staining, condensing organisms (arrows) coexisted with pale forms inside ehrlichial colonies. Bar, 1 μm. (D) In some cells, these colonies became compacted into masses of bacteria in which individual organisms could barely be delineated by their membranes. Bar, 0.2 μm.
DISCUSSION
In recent years, a number of previously unknown pathogens have been linked to emergence of new human disease entities. It is noteworthy that several of these are arthropod-borne, in particular tick-borne; have a feral animal reservoir; or are closely related to animal pathogens. Ticks that feed on a wide range of hosts may be vehicles for the transfer of pathogens to new host species (41), aiding the emergence of new diseases. One such group of emerging pathogens are the human ehrlichiosis agents, E. chaffeensis (11) and the recently discovered HGE agent (1, 9, 17). This is the newest member of the Ehrlichia phagocytophila group that also includes pathogens of ruminants, horses (E. equi) (25), and dogs (18) and appears to have acquired the ability to infect humans. Pathogens in that group are so closely related that they cannot be distinguished by 16S rDNA nucleotide sequence analysis (12, 16) and could conceivably be considered strains of one species. Nevertheless, the behavior of the HGE agent compared with that of E. equi in tick cell culture indicated that biological differences exist between the two and might be responsible for their seemingly divergent host spectrum in nature. For example, despite repeated attempts, we have not been able to transfer the HGE-MN1 isolate from ISE6 to IDE8 cells, and while the HGE isolates are infectious for small laboratory rodents, E. equi is not (data not shown).
Events which take place in the tick are of importance with respect to their transmission to mammals and contribution to or influence on pathogenicity. Unfortunately, the portion of the life cycle which the HGE agent spends inside the tick is an enigma and has barely been investigated. A single publication shows the ultrastructure of E. canis in the tick (39). It clearly demonstrates morphological differences between the blood and the tick stages of this species, including condensation of ehrlichias residing in the salivary glands. It is reasonable to assume that the granulocytotropic ehrlichias, too, differ in their appearance in the tick versus in the mammal. E. canis in tick cell culture resembles the forms found in the tick (15), and the HGE agent in I. scapularis cells might be representative of its arthropod phase. In any case, the appearance of the HGE agent in tick cells is very different from that in mammalian cells, the neutrophil granulocyte as well as HL60 cells, hinting at specific adaptations to these divergent hosts. Moreover, and as previously shown with E. equi in the I. scapularis cell line IDE8 (29), granulocytotropic ehrlichias in ISE6 cells display a striking array of morphologic forms. Many host cells become completely filled with ehrlichias and are enlarged to several times their original size. In addition, ISE6 cells are cultured at more than 10 times the density of HL60 cells, i.e., 2 × 106 to 3 × 106/ml for ISE6 versus 1 × 105/ml for HL60. In conjunction with the higher pathogen load per cell, this results in a larger yield of antigen per milliliter of culture in a medium supplemented with half the amount of fetal bovine serum used for HL60.
As a first step in the investigation of the cellular interaction between pathogen and vector cell, we have analyzed the agent’s morphogenesis in this culture system under environmental conditions of temperature (34°C) and gaseous atmosphere (low O2 and high CO2) that mimic the tick’s internal environment during a blood meal (23). Presumably, infection of the arthropod vector results when the tick ingests ehrlichia-infected neutrophil granulocytes that enter the tick bite site. Peripheral blood neutrophils most likely were also the source of ehrlichias that invaded the tick cells in vitro. Our ability to transfer HGE isolates from the human cell line HL60 to tick vector cells further suggests that these cultured organisms are equivalent, at least in this aspect, to the granulocyte forms. Both primary isolates, and the human isolate which had been passaged in HL60 cells six times, noticeably benefited from the addition of erythrocytes to the culture. Iron presented in a biologically active form, e.g., bound to transferrin, has been shown to be utilized by E. chaffeensis (3) and generally plays a role as a virulence factor in pathogenic bacteria.
Some aspects of ehrlichial entry into ISE6 tick cells appeared similar to that described for A. marginale in IDE8 tick cell culture (5), but there were also differences including a much higher degree of pleomorphism in the ehrlichias as well as the fact that they did not grow in IDE8 cells. Although the two pathogens are closely related by 16S rDNA analysis (6), they differ markedly in their host cell tropism in the mammal (the erythrocyte is the only known target cell of A. marginale) and behavior in the vector. Such traits are likely the product of genes that evolve rapidly to allow divergence into new ecological niches and may not be reflected in the much more stable ribosomal genes.
Unlike the granulocytotropic ehrlichias, A. marginale often causes heavy infections in the tick. This facilitated light and electron microscopic analyses of its development in that host (21) and demonstrated that morphogenesis in tick cell culture is comparable to the situation in vivo (5, 27). Therefore, tick cell culture may also be a valid in vitro model for the tick phase of the ehrlichial life cycle. While there were similarities in the in vitro development of the two pathogens, the differences included a much higher degree of pleomorphism in the ehrlichias as well as the fact that they did not grow in IDE8 cells. Various morphological forms of both A. marginale and the HGE agent adhered to and were taken into the cells. These appeared more electron dense than the actively dividing intracellular organisms seen at 24 to 48 h p.i. and might be undergoing development toward the electron-dense forms present later. Highly pleomorphic, densely compacted E. chaffeensis in mammalian cell culture has been described elsewhere as aberrant and degenerating (33). However, in tick cells, ehrlichias were never found in association with debris typically present in lysosomes, such as fragments of cellular organelles or membrane whorls, suggesting that they were not being degraded.
ACKNOWLEDGMENTS
This study was supported in part by the following sources: grants from the National Institutes of Health, no. 1R29AI42792 (to U.G.M.) and no. 1RO1AI40952 (to J.L.G.), and the University of Minnesota Experiment Station (to T.J.K.).
We are grateful to Ann T. Palmer (Department of Entomology, University of Minnesota) for expert help in preparing the electron micrographs.
REFERENCES
- 1.Bakken J S, Dumler J S, Chen S-M, Eckman M R, Van Etta L L, Walker D H. Human granulocytic ehrlichiosis in the upper midwest United States: a new species emerging? JAMA. 1994;272:212–218. [PubMed] [Google Scholar]
- 2.Barlough J E, Madigan J E, deRock E, Dumler J S, Bakken J S. Protection against Ehrlichia equi is conferred by prior infection with the human granulocytotropic ehrlichia (HGE agent) J Clin Microbiol. 1995;33:3333–3334. doi: 10.1128/jcm.33.12.3333-3334.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnewall R E, Rikihisa Y. Abrogation of gamma interferon-induced inhibition of Ehrlichia chaffeensis infection in human monocytes with iron transferrin. Infect Immun. 1994;62:4804–4810. doi: 10.1128/iai.62.11.4804-4810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belongia E A, Reed K D, Mitchell P D, Kolbert C P, Persing D H, Gill J S, Kazmierczak J J. Prevalence of granulocytic Ehrlichia infection among white-tailed deer in Wisconsin. J Clin Microbiol. 1997;35:1465–1468. doi: 10.1128/jcm.35.6.1465-1468.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blouin E F, Kocan K M. Morphology and development of Anaplasma marginale (Rickettsiales: Anaplasmataceae) in cultured Ixodes scapularis (Acari: Ixodidae) cells. J Med Entomol. 1998;35:788–797. doi: 10.1093/jmedent/35.5.788. [DOI] [PubMed] [Google Scholar]
- 6.Brenner D J, O’Connor S P, Winkler H H, Steigerwalt A G. Proposals to unify the genera Bartonella and Rochalimaea, with descriptions of Bartonella quintana comb. nov., Bartonella vinsonii comb. nov., Bartonella henselae comb. nov., and Bartonella elizabethae comb. nov., and to remove the family Bartonellaceae from the order Rickettsiales. Int J Syst Bacteriol. 1993;43:777–786. doi: 10.1099/00207713-43-4-777. [DOI] [PubMed] [Google Scholar]
- 7.Chang Y F, Novosel V, Chang C F, Kim J B, Shin S J, Lein D H. Detection of human granulocytic ehrlichiosis agent and Borrelia burgdorferi in ticks by polymerase chain reaction. J Vet Diagn Investig. 1998;10:56–59. doi: 10.1177/104063879801000110. [DOI] [PubMed] [Google Scholar]
- 8.Chang Y F, Novosel V, Dubovi E, Wong S J, Chu F K, Chang C F, Del Piero F, Shin S, Lein D H. Experimental infection of the human granulocytic ehrlichiosis agent in horses. Vet Parasitol. 1998;78:137–145. doi: 10.1016/s0304-4017(98)00133-2. [DOI] [PubMed] [Google Scholar]
- 9.Chen S-M, Dumler J S, Bakken J S, Walker D H. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cinco M, Padovan D, Murgia R, Maroli M, Frusteri L, Heldtander M, Johansson K E, Engvall E O. Coexistence of Ehrlichia phagocytophila and Borrelia burgdorferi sensu lato in Ixodes ricinus ticks from Italy as determined by 16S rRNA gene sequencing. J Clin Microbiol. 1997;35:3365–3366. doi: 10.1128/jcm.35.12.3365-3366.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawson J E, Anderson B E, Fishbein D B, Sanchez J L, Goldsmith C S, Wilson K H, Duntley C W. Isolation and identification of an Ehrlichia sp. from a patient diagnosed with human ehrlichiosis. J Clin Microbiol. 1991;29:2741–2745. doi: 10.1128/jcm.29.12.2741-2745.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Des Vignes F, Fish D. Transmission of the agent of human granulocytic ehrlichiosis by host-seeking Ixodes scapularis (Acari:Ixodidae) in southern New York state. J Med Entomol. 1997;34:379–382. doi: 10.1093/jmedent/34.4.379. [DOI] [PubMed] [Google Scholar]
- 13.Engvall E O, Pettersson B, Persson M, Artursson K, Johansson K E. A 16S rRNA-based PCR assay for detection and identification of granulocytic Ehrlichia species in dogs, horses, and cattle. J Clin Microbiol. 1996;34:2170–2174. doi: 10.1128/jcm.34.9.2170-2174.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ewing S A, Dawson J E, Panciera R J, Mathew J S, Pratt K W, Katavolos P, Telford S R., III Dogs infected with a human granulocytotropic Ehrlichia spp. (Rickettsiales:Ehrlichieae) J Med Entomol. 1997;34:710–718. doi: 10.1093/jmedent/34.6.710. [DOI] [PubMed] [Google Scholar]
- 15.Ewing S A, Munderloh U G, Blouin E F, Kocan K M, Kurtti T J. Presented at the 76th Conference of Research Workers in Animal Diseases, Chicago, Ill. 1995. [Google Scholar]
- 16.Fingerle V, Goodman J L, Johnson R C, Kurtti T J, Munderloh U G, Wilske B. Human granulocytic ehrlichiosis in southern Germany: increased seroprevalence in high-risk groups. J Clin Microbiol. 1997;35:3244–3247. doi: 10.1128/jcm.35.12.3244-3247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodman J L, Nelson C M, Vitale B, Madigan J E, Dumler J S, Kurtti T J, Munderloh U G. Direct cultivation of the causative agent from patients with human granulocytic ehrlichiosis. N Engl J Med. 1996;334:209–215. doi: 10.1056/NEJM199601253340401. [DOI] [PubMed] [Google Scholar]
- 18.Greig B, Asanovich K M, Armstrong P J, Dumler J S. Geographic, clinical, serologic and molecular evidence of granulocytic ehrlichiosis, a likely zoonotic disease, in Minnesota and Wisconsin dogs. J Clin Microbiol. 1996;34:44–48. doi: 10.1128/jcm.34.1.44-48.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito S, Vinson W, McGuire T J., Jr Murine typhus rickettsiae in the oriental rat flea. Ann N Y Acad Sci. 1975;266:35–60. doi: 10.1111/j.1749-6632.1975.tb35087.x. [DOI] [PubMed] [Google Scholar]
- 20.Johansson K-E, Pettersson B, Uhlén M, Gunnarsson A, Malmqvist M, Olsson E. Identification of the causative agent of granulocytic ehrlichiosis in Swedish dogs and horses by direct solid-phase sequencing of PCR products from the 16S ribosomal-RNA gene. Res Vet Sci. 1995;58:109–112. doi: 10.1016/0034-5288(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 21.Kocan K M, Yelling T N, Ewing S A, Hair J A, Barron S J. Morphology of colonies of Anaplasma marginale in nymphal Dermacentor andersoni. Am J Vet Res. 1984;45:1434–1440. [PubMed] [Google Scholar]
- 22.Kurtti T J, Munderloh U G, Hayes S F, Krueger D E, Ahlstrand G G. Ultrastructural analysis of the invasion of tick cells by Lyme disease spirochetes (Borrelia burgdorferi) in vitro. Can J Zool. 1994;72:977–994. [Google Scholar]
- 23.Lighton J R B, Fielden L J, Rechav Y. Discontinuous ventilation in a non-insect, the tick Amblyomma marmoreum (Acari, Ixodidae): characterization and metabolic modulation. J Exp Biol. 1993;180:229–245. [Google Scholar]
- 24.Little S E, Dawson J E, Lockhart J M, Stallknecht D E, Warner C K, Davidson W R. Development and use of specific polymerase reaction for the detection of an organism resembling Ehrlichia sp. in white-tailed deer. J Wild Dis. 1997;33:246–253. doi: 10.7589/0090-3558-33.2.246. [DOI] [PubMed] [Google Scholar]
- 25.Madigan J E, Gribble D. Equine ehrlichiosis in northern California: 49 cases (1968–1981) J Am Vet Med Assoc. 1987;190:445–448. [PubMed] [Google Scholar]
- 26.Munderloh, U. G. Unpublished results.
- 27.Munderloh U G, Blouin E F, Kocan K M, Ge N-L, Edwards W L, Kurtti T J. Establishment of the tick (Acari: Ixodidae)-borne cattle pathogen Anaplasma marginale (Rickettsiales: Anaplasmataceae) in tick cell culture. J Med Entomol. 1996;33:656–664. doi: 10.1093/jmedent/33.4.656. [DOI] [PubMed] [Google Scholar]
- 28.Munderloh U G, Liu Y, Wang M, Chen C, Kurtti T J. Establishment, maintenance and description of cell lines from the tick Ixodes scapularis. J Parasitol. 1994;80:533–543. [PubMed] [Google Scholar]
- 29.Munderloh U G, Madigan J E, Dumler J S, Goodman J L, Hayes S F, Barlough J E, Nelson C M, Kurtti T J. Isolation of the equine granulocytic ehrlichiosis agent, Ehrlichia equi, in tick cell culture. J Clin Microbiol. 1996;34:664–670. doi: 10.1128/jcm.34.3.664-670.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholson W L, Muir S, Sumner J W, Childs J E. Serologic evidence of infection with Ehrlichia spp. in wild rodents (Muridae: Sigmodontinae) in the United States. J Clin Microbiol. 1998;36:695–700. doi: 10.1128/jcm.36.3.695-700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pancholi P, Kolbert C P, Mitchell P, Reed K D, Dumler J S, Bakken J S, Telford III S R, Persing D H. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1007–1212. doi: 10.1093/infdis/172.4.1007. [DOI] [PubMed] [Google Scholar]
- 32.Parola P, Beati L, Cambon M, Brouqui P, Raoult D. Ehrlichial DNA amplified from Ixodes ricinus (Acari: Ixodidae) in France. J Med Entomol. 1998;35:180–183. doi: 10.1093/jmedent/35.2.180. [DOI] [PubMed] [Google Scholar]
- 33.Popov V L, Chen S-M, Feng H-M, Walker D H. Ultrastructural variation of cultured Ehrlichia chaffeensis. J Med Microbiol. 1995;43:411–421. doi: 10.1099/00222615-43-6-411. [DOI] [PubMed] [Google Scholar]
- 34.Reubel G H, Kimsey R B, Barlough J E, Madigan J E. Experimental transmission of Ehrlichia equi to horses through naturally infected ticks (Ixodes pacificus) from northern California. J Clin Microbiol. 1998;36:2131–2134. doi: 10.1128/jcm.36.7.2131-2134.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reynolds E A. The use of lead citrate at a high pH as an electron-opaque stain for cell microscopy. J Cell Biol. 1963;1:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richter P J, Jr, Kimsey R B, Madigan J E, Barlough J E, Dumler J S, Brooks D L. Ixodes pacificus (Acari: Ixodidae) as a vector of Ehrlichia equi (Rickettsiales: Ehrlichieae) J Med Entomol. 1996;33:1–5. doi: 10.1093/jmedent/33.1.1. [DOI] [PubMed] [Google Scholar]
- 37.Rodgers S A, Morton J, Baldwin C. A serological survey of Ehrlichia canis, Ehrlichia equi and Borrelia burgdorferi in dogs in Oklahoma. J Vet Diagn Investig. 1989;1:154–159. doi: 10.1177/104063878900100212. [DOI] [PubMed] [Google Scholar]
- 38.Sato T. A modified method for lead staining of thin sections. J Electron Microsc. 1968;17:158–159. [PubMed] [Google Scholar]
- 39.Smith R D, Sells D M, Stephenson E H, Ristic M, Huxsoll D L. Development of Ehrlichia canis, causative agent of canine ehrlichiosis, in the tick Rhipicephalus sanguineus and its differentiation from a symbiotic rickettsia. Am J Vet Res. 1976;37:119–126. [PubMed] [Google Scholar]
- 40.Telford S R, Dawson J E, Katavolos P, Warner C K, Kolbert C P, Persing D H. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci USA. 1996;93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weller S J, Baldridge G D, Munderloh U G, Noda H, Simser J, Kurtti T J. Phylogenetic placement of rickettsiae from the ticks Amblyomma americanum and Ixodes scapularis. J Clin Microbiol. 1998;36:1305–1317. doi: 10.1128/jcm.36.5.1305-1317.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]