Abstract
Background and Aims
Neuroendocrine neoplasms (NENs) of the presacral space are an extremely rare disease entity with largely unknown outcome and no established standard of care treatment. Therefore, we wanted to analyze clinical presentation, histopathological findings, treatment outcomes, and prognosis in a multicentric patient cohort.
Methods
We searched local databases of six German NEN centers for patients with presacral NEN. Retrospective descriptive analyses of age, sex, stage at diagnosis, symptoms, grade, immunohistochemical investigations, biomarkers, treatment, and treatment outcome were performed. Kaplan–Meier analysis was used to determine median overall survival.
Results
We identified 17 patients (11 female, 6 male) with a median age of 50 years (range, 35–66) at diagnosis. Twelve cases presented initially with distant metastases including bone metastases in nine cases. On pathological review the majority of patients had well-differentiated G2 tumors. Immunohistochemical profile resembled rectal NENs. All but one patient had non-functioning tumors. Somatostatin receptor imaging was positive in 14 of 15 investigated cases. Eight patients were treated surgically including palliative resections; 14 patients received somatostatin analogs with limited efficacy. With 14 PRRTs completed, 79% showed clinical benefit, whereas only one patient with neuroendocrine carcinoma (NEC) responded to chemotherapy. Treatment with everolimus in three patients was not successful, whereas cabozantinib resulted in a disease stabilization in a heavily pretreated patient. During a median observation period of 44.5 months, 6 patients died. Median overall survival was not reached.
Conclusion
Presacral NEN are histopathologically similar to rectal NENs. Presacral NEN should be considered as possible primary in NEN of unknown primary. The majority of tumors is non-functioning and somatostatin receptor positive. PRRT demonstrated promising activity; tyrosine kinase inhibitors warrant further investigations. Further molecular characterization and prospective evaluation of this rare tumor entity are needed.
Keywords: presacral, retrorectal, CUP-NET, neuroendocrine tumor, neuroendocrine carcinoma, carcinoid, PRRT, prognosis
Introduction
Neuroendocrine neoplasms (NENs) are heterogeneous neoplasms originating from the diffuse neuroendocrine cell system. They are defined by their endocrine phenotype, which is verified by immunohistochemical staining for the small synaptic vesicle-analogue protein synaptophysin and the large dense core-vesicle protein chromogranin A (1). They may originate nearly everywhere in the body, but most often, the primary tumor is located in the gastroenteropancreatic system or in the lung (2). For treatment planning, the knowledge of the primary and the differentiation between primary and metastatic lesion is important. Despite improvement of diagnostic techniques in 8–12% of the NEN patients, the primary remains undetected (CUP-NEN; cancer of unknown primary) (2–5). Somatostatin receptor (SSTR) expression is characteristic for neuroendocrine tumors (NETs) and allows detection of SSTR-expressing NETs by scintigraphy or specific SSTR-PET/CT (68Ga DOTATOC- or DOATATATE-PET-CT) (6). 68Ga-DOTA PET/CTs are particularly important for primary tumor search in CUP-NET, as their sensitivity is superior to other imaging modalities (7).
Specific immunohistochemical stainings including the transcription factors CDX-2 (intestinal primary), TTF-1 (lung/thyroid gland), Islet-1 (pancreas), PDX1 (duodenum, pancreas) (8), and specific hormones may help to identity the primary and are therefore recommended in CUP-NET patients (9). These markers are of very limited use in NEC (9). Prostate-specific acid phosphatase (PSAP) is a glycoprotein-enzyme produced in prostate carcinomas, particularly indicative of its spread beyond the prostate but also characteristic of hindgut NETs (10). In patients with hindgut NETs staining for chromogranin A often is only weakly positive or may even be negative (9, 11).
The presacral space lies between the rectum anteriorly, the sacrum posteriorly, and the endopelvic fascia laterally. It contains embryological remnants of different tissues. Tumors of this presacral space are rare, mostly benign, but several malignant tumors have also been reported (12), including NEN. Immunohistochemistry is important for the differentiation of NEN from other primary tumors or metastases of the presacral region (9, 13). Presacral NENs are extremely rare; to the best of our knowledge, about 70 cases have been reported so far mainly in single case reports (14–70) or small series (15, 17, 20, 26, 39, 42, 55, 71, 72). The majority of presacral NEN was diagnosed in female individuals of younger age compared to the median age of diagnosis in other gastroenteropancreatic NEN. According to the literature, presacral NENs are usually well-differentiated tumors with local involvement, but cases with distant metastases have also been reported (20, 72).
Therapeutic options of metastatic NEN include somatostatin analogs (SSA), chemotherapy, peptide receptor radionuclide therapy (PRRT), everolimus, and tyrosine kinase inhibitors (TKIs) (73). Even in more common NENs like pancreatic NEN, data of comparative treatment trials or on best sequence of treatments are not available at the moment. In rare subtypes like presacral NEN, data on treatment outcome are lacking. The aim of our study is to describe clinical, histopathological, therapeutic, and prognostic features of patients with presacral NEN who presented at one of five contributing NEN referral centers within the last 10 years. We were particularly interested in the number of patients we could collect in the participating NEN referral centers as a surrogate for the frequency of this disease, in the percentage of patients who were initially diagnosed as CUP-NEN, to analyze whether all had differentiated tumors and get a hint which therapeutic option may be of benefit in this extremely rare subgroup.
Patients and Methods
All patients with neuroendocrine neoplasm and suspected primary tumor within the presacral space were included in this retrospective multicenter evaluation. In the case of initial presentation as NEN with unknown primary, investigations to detect the primary/exclude another primary tumor localization included gastroscopy, colonoscopy, CT or MRI, SSTR imaging [scintigraphy or specific positron emission tomography (PET)] and in some cases fluorodeoxyglucose (FDG)-PET-CT and endoscopic ultrasound. For the vast majority of patients (14 of 17), SSTR-based PET-CT was available during follow-up. In addition to the standard immunohistochemical stainings, such as synaptophysin, chromogranin A, and Ki67, further specific stainings were done according to local practice at the centers, e.g., for the transcriptional factors CDX-2 and TTF-1 in the majority of cases, and ISLET-1, prostate-specific acid phosphatase (PSAP), vimentin, CD56, and somatostatin receptor subtype 2 (SSTR2) in some cases. Several patients received molecular diagnostics via “next generation sequencing” panels or whole exome/genome sequencing as part of the German Cancer Consortium (DKTK) Molecularly Aided Stratification for Tumor Eradication Research Trial (MASTER) (74–76).
The patients were identified via center-based databases or personal knowledge, and the available essential information was extracted and evaluated across centers. The following German centers have participated: Dresden, Essen, Halle (Saale), Hamburg, Heidelberg, and Marburg. Collected data included date of diagnosis, date of birth, sex, histology, stage, functionality, symptoms of tumor disease, localization of metastases, date of diagnosis of metastases, somatostatin receptor status, treatments with outcome, and date and cause of death or date of last contact. This study was conducted in accordance with the Declaration of Helsinki. All patients were included in the local disease databases conducted with approval of the local ethics committees at the respective sites. Written informed patient consent and approval for data collection and analysis were obtained upon admission to our institutions. For the use of the images, an additional consent was obtained in the selected cases.
Statistical analysis was performed IBM® SPSS® Statistics 27.0 (IBM, Armonk, NY, USA). Descriptive statistical analysis was performed for most parameters. Kaplan–Meier analyses of median duration of observation and overall survival were investigated.
Results
Patient Characteristics and Clinical Presentation
We identified 17 patients (n = 17) in the databases of six German centers for neuroendocrine neoplasms [Dresden, Essen, Hamburg, Halle (Saale), Heidelberg and Marburg], who were diagnosed with a primary presacral NEN. Most patients were referred to one of our centers with the diagnosis of cancer of unknown primary (CUP) NET. Presacral NENs were diagnosed more frequently in women (n = 11; 64.7%) than in men (n = 6; 35.3%). In our study population, the initial diagnosis occurred at an age between 35 and 66 years. The patients had a median age of 50 years (mean, 50.3 years).
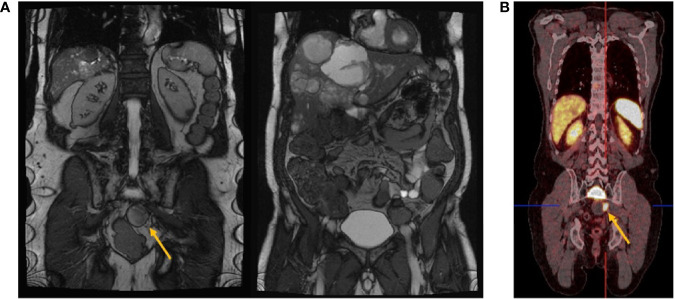
As mentioned above, an association with various anomalies such as tailgut cysts is frequently described in the literature (72). In our database, an association to an anomaly was detected in only one patient and suspected in another one. One patient showed a presacral localized histologically confirmed teratoma in addition to her primary presacral NEN G2. In another patient, a paraganglioma in the pterygopalatine fossa was suspected but could not be clearly distinguished from osseous metastasis due to a lack of histopathological confirmation. Even though there was no direct association with tailgut cysts, cystic portions of otherwise solid presacral NET could be detected on imaging in some cases ( Figure 1 ).
Figure 1.
(A) Magnetic resonance imaging. Coronal view demonstrating primary presacral neuroendocrine neoplasm (yellow arrow) and liver metastases. (B) Coronal view of a 68Ga-DOTATOC-PET/CT scan showing SSTR expression of the whole body. While the liver metastases showed a homogeneous SSTR expression, only a part of the presacral lesion showed a homogeneous SSTR expression (yellow arrow), suggesting a SSTR-negative/cystic portion besides the SSTR-positive solid presacral NEN. MRI and 68-DOTATOC-PET/CT are from the same patient (study-ID III) at different time points.
According to our database analysis, presacral NENs are predominantly non-functioning. Only one patient had a functionally active presacral NET producing parathyroid hormone-related peptide (PTHrP). This patient developed a seizure due to paraneoplastic hypercalcemia. No patient suffered from carcinoid syndrome.
Most of the patients (14/17) presented clinically with locoregional symptoms caused by the space-occupying process of presacral NEN. Primary presacral NEN predominantly caused symptoms such as pain of the lower abdomen, pelvis, sacral region, perineum, or lower back (12/17); unilateral paresthesia of the lower limb (2/17); and defecation disorders, e.g., chronic constipation (4/11) or urination disorders (1/17) due to their mass effect. Systemic symptoms showed a minor role in presacral NEN. Only two patients presented with b-symptoms at initial diagnosis.
Patients with presacral NEN were often diagnosed at an advanced stage. At the time of diagnosis, most primary tumors showed a pronounced local extension with a size of 3–9 cm in diameter and frequently an infiltration of the sacrum and the coccyx. In our cohort, most patients (12/17) had distant metastases at the time of diagnosis. Only five patients had a localized tumor stage. However, all patients except one with presacral NEN developed distant metastasis during the course of their disease in our series. Interestingly, one of the most frequent metastatic site in our cohort of patients with presacral NEN was the skeleton. Bone metastases were detected in 11 of 17 patients. Likewise, the liver was a common metastatic site (11/17). Furthermore, locoregional lymph node metastases occurred more frequently (10/17), whereas diffuse lymphatic metastasis to para-aortic and mesenteric lymph nodes and pulmonary metastases occurred less frequently (3/17). Metastases to the adrenal gland and peritoneum were diagnosed in two cases each (2/17). Brain metastases were not diagnosed in our cohort.
Patients characteristics are summarized in Table 1 .
Table 1.
Patient characteristics.
| Study ID | Sex | Age at diagnosis | Grading | Stage at diagnosis | Endocrine function | SSTR imaging | Symptoms related to presacral NEN | Associated anomalies |
|---|---|---|---|---|---|---|---|---|
| I | M | 48 | G2 | IV | n.a. | Positive | Perineal pain | – |
| II | F | 35 | G2 | IV | Non-functional | Positive | Defecation disorder | – |
| III | M | 65 | G2 | IV | Non-functional | Positive | Asymptomatic | – |
| IV | F | 46 | G2 | II | Non-functional | Positive | Abdominal and pelvic pain | – |
| V | F | 66 | G2 | IV | Non-functional | Positive | Chronic obstipation | – |
| VI | M | 53 | G2 | IV | Non-functional | Positive | Defecation disorder, perineal pain | – |
| VII | F | 52 | G3 | IV | Non-functional | Positive | Abdominal pain | – |
| VIII | F | 40 | G3 | III | Non-functional | Positive | Asymptomatic | – |
| IX | M | 60 | G3/LCNEC | III | Non-functional | n.a. | Pain in the sacral region, paresthesia right lower limb, chronic obstipation | – |
| X | F | 44 | G2 | IV | Non-functional | Positive | Pain in the sacral region | – |
| XI | F | 65 | G2 | IV | Non-functional | Positive | Abdominal pain, diffuse backpain | – |
| XII | F | 33 | G2 | IV | Non-functional | Positive | Asymptomatic | – |
| XIII | M | 62 | G3 | III | Non-functional | Positive | Low backpain, paresthesia of the right lower limb, foot drop | – |
| XIV | F | 41 | G1 | III or IV | Non-functional | n.a. | Pelvic pain | Paraganglioma, DD: bone metastasis |
| XV | M | 50 | G2 | IV | Non-functional | Positive | Pelvic pain and swelling of the right hip | – |
| XVI | F | 58 | G2 | IV | Parathyroid hormone-related peptide | n.a. | Pelvic pain, urinary tract obstruction, seizure due to paraneoplastic hypercalcemia | – |
| XVII | F | 37 | G2 | IV | Non-functional | Positive | Pain in the sacral region | Teratoma |
n.a., not assessed.
Histopathological Features
Predominantly, presacral NENs were histologically well differentiated. Most presacral NETs were classified as G2 tumors based on their Ki67 index. Only one presacral NET corresponded to a G1 NET with a Ki67 index of <2%, and three patients had a G3 presacral NET. Poorly differentiated presacral NEC turned out to be extremely rare. In our databases, there was only one presacral NEN that was histopathologically classified as large-cell neuroendocrine carcinoma (LCNEC) and had a Ki67 index of 80%.
Synaptophysin was strongly positive by immunohistochemistry in all samples of presacral NEN. In contrast, chromogranin A was only weakly positive in the majority of cases and even negative in two cases ( Table 2 ).
Table 2.
Immunohistochemical features of patients with primary presacral neuroendocrine neoplasms.
| Study ID | Grading | Ki67 | Chromogranin A | Synaptophysin | CD56 | PSAP | Vimentin | TTF-1 | CDX2 | CK-7 | CK-18 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | G2 | 5% | Negative | Positive | Positive | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| II | G2 | 12% | Positive | Positive | n.a. | Positive | Negative | Negative | Negative | Negative | n.a. |
| III | G2 | 7% | Negative | Positive | n.a. | n.a. | Negative | n.a. | Negative | n.a. | n.a. |
| IV | G2 | −20% | Weak positive | Positive | n.a. | Positive | n.a. | Negative | Negative | n.a. | n.a. |
| V | G2 | 10% | Weak positive | Positive | n.a. | Positive | n.a. | Negative | Negative | Negative | Positive |
| VI | G2 | 5% | Weak positive | Positive | n.a. | Positive | Positive | Negative | Negative | n.a. | n.a. |
| VII | G3 | 30% | n.a. | Positive | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| VIII | G3 | 30% | Weak positive | Positive | n.a. | n.a. | n.a. | n.a. | n.a. | Negative | Positive |
| IX | G3/LCNEC | 80% | n.a. | Positive | Negative | n.a. | Positive | Positive | Negative | Negative | n.a. |
| X | G2 | −20% | Positive | Positive | n.a. | n.a. | Positive | n.a. | Negative | Negative | n.a. |
| XI | G2 | −15% | Positive | Positive | Positive | n.a. | Positive | Negative | Negative | Negative | Positive |
| XII | G2 | −15% | Weak positive | Positive | n.a. | n.a. | n.a. | Negative | Negative | n.a. | n.a. |
| XIII | G3 | 30% | Dot-like expression | Positive | Positive | n.a. | n.a. | Negative | Negative | Negative | n.a. |
| XIV | G1 | <2% | Positive | Positive | Positive | n.a. | n.a. | n.a. | Positive | n.a. | n.a. |
| XV | G2 | 10% | Weak positive | Positive | n.a. | n.a. | n.a. | n.a. | n.a. | Negative | n.a. |
| XVI | G2 | 10% | Dot-like expression | Positive | n.a. | n.a. | n.a. | Negative | n.a. | n.a. | n.a. |
| XVII | G2 | 5% | n.a. | Positive | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
n.a., not assessed.
Thyroid transcription factor 1 (TTF-1), a marker for metastases in NETs of pulmonary origin, and CDX2, a marker for metastases of gastrointestinal origin, were mostly negative in presacral neuroendocrine tumors. Only the presacral NEC was immunohistochemically positive for TTF-1, and one case of presacral NET was positive for CDX-2. Exclusion of a gastrointestinal primary tumor was performed by abdominal CT, gastroscopy, and colonoscopy in this case.
Vimentin—a marker for soft tissue tumors, but also expressed in various epithelial cancers—was examined immunohistochemically in only five presacral NETs, but it was detected in four of five cases.
Four presacral NETs were examined for PSAP by immunohistochemistry, and all resulted in positive detection of PSAP.
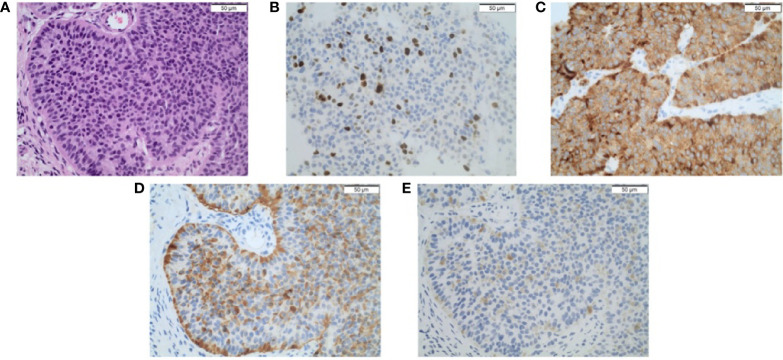
A representative example of microscopic tumor morphology and immunohistochemical stainings is shown in Figures 2A–E . Table 2 summarizes the results of the histopathological reports.
Figure 2.
(A) Primary presacral neuroendocrine neoplasm stained using H&E (100×). Immunohistochemical staining (100×) shows a well-differentiated neuroendocrine neoplasm with a (B) Ki67 index of 7% and positivity for (C) synaptophysin, (D) chromogranin a, and (E) PSAP. Scale bars represent 50 µm.
Cytokeratin 7 (CK7) was not detected by immunohistochemistry (n = 9). Cytokeratin 18 (CK18) was positive (n = 3). CD56, a non-specific marker for neuroendocrine tumors, was positive in four tumors and negative in one.
Molecular Characterization
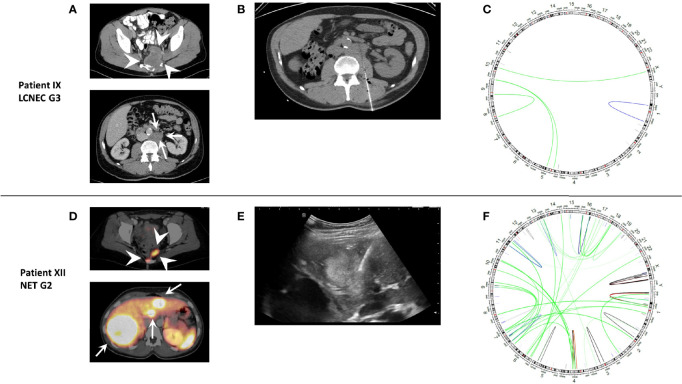
Molecular diagnostics was performed in three patients to identify molecular targets: in one NET G3 (case XIII), a colorectal panel was applied, whereas one NEC G3 (case IX) and one NET G2 (case XII) were enrolled in the MASTER Trial and underwent whole-exome and whole-genome sequencing, respectively ( Figure 3 ). All patients were microsatellite stable. In case XIII, no targetable alterations were detected, and the absence of a pathogenic TP53 mutation confirmed the diagnosis of NET G3. In the NEC G3 case, the tumor mutational burden (TMB) was intermediate with 4.32 non-coding mutations per megabase; besides a TP53 mutation, several cyclin pathway alterations (CDKN2A mutation, CCND1 mutation, CDK6 amplification) were detected. Case XII case showed a SETD2 frameshift insertion with presumably consecutive homologous DNA repair deficiency (HRD). TMB was low with 1.30 mutations per megabase.
Figure 3.
Representative imaging and genomic rearrangements of molecularly characterized patients IX (LCNEC G3) and XII (NET G2). (A) CT and (D) DOTATOC-PET/CT of presacral primary (white arrowheads) and metastases (white arrows). (B) CT-guided biopsy of retroperitoneal lymph node metastasis and (E) ultrasound-guided biopsy of liver metastasis for fresh tissue for genomic analysis. (C, F) Circle plots of genomic rearrangements. Despite slightly lower tumor mutational burden, case XII shows a much higher number of rearrangements as a sign of homologous DNA repair deficiency possibly due to a pathogenic frameshift SETD2 mutation.
Circulating Biomarkers
The general circulating neuroendocrine biomarkers chromogranin A (CgA) were determined in 15 of 17 patients. CgA was only slightly elevated (n = 7) or normal (n = 8) at initial diagnosis. Tumor progression during follow-up was not accompanied by increasing CgA levels.
Serotonin—the marker hormone of the carcinoid syndrome—was determined in seven of our patients with presacral NETs; there was no elevation of serotonin in serum.
Neuron-specific enolase (NSE) is a marker for neuronal tissue, neuroendocrine cells, and in particular a circulating marker for poorly differentiated NEN. NSE serum levels were elevated in 6 of 10 patients, with only two patients showing a pronounced elevation >100 µg/L.
Imaging
Imaging often reveals a solid tumor with sometimes cystic portions in the presacral space (see Figure 1 ). The morphological features of presacral NEN in CT and MR scans were unspecific.
SSTR imaging with specific PET-CTs like 68Ga-DOTATOC-PET/CT or SSTR scintigraphy was performed in 15/17 patients to exclude other potential primaries and for disease staging and treatment planning. Only one tumor showed no detectable SSTR expression. Most presacral NENs showed homogeneous SSTR expression (see for example Figure 3D ), and only two tumors showed heterogeneous expression.
FDG-PET/CT was used in two patients with presacral NET and was not suitable for the detection of the primary tumor.
Treatment in Patients With Presacral NEN
Curative treatment of presacral NENs is only possible in a locally limited stage, when surgical resection of the primary tumor represents the only chance of cure. In our series, most patients with presacral NENs were already in an advanced metastatic stage of disease at the time of diagnosis. Therefore, a palliative systemic therapy was initiated in most cases.
However, even in an already metastatic stage, surgical resection of the primary tumor may be considered for the treatment of symptoms due to the mass effect of the primary tumor. In our cohort, the primary tumor was resected in nine cases, in four patients with localized disease, in two patients with metastatic disease in curative intent (combined with resection of metastases), and in three metastatic patients in palliative intention.
In total, 14 patients with presacral NENs received therapy with SSA. Half of the patients showed stable disease at least until the first follow-up. The other patients underwent therapy escalation due to progression (n = 6) at first follow-up or intolerance (n = 1). During the course of disease, patients frequently showed progression of presacral NEN under SSA; therefore, treatment with SSA was usually not sufficient for growth control in the long term.
In our study population, three patients received treatment with everolimus and did not benefit from this therapy due to progression in the first follow-up. One patient received the tyrosine kinase inhibitor cabozantinib as the seventh line of therapy and showed stable disease [progression-free survival (PFS) >7 months]. The dose of cabozantinib was reduced due to side effects, but treatment was continued at last follow-up.
With good SSTR expression in almost all presacral NEN, PRRT was initiated 15 times in a total of 12 patients. Two patients developed a complete remission, four patients a partial remission, and five patients a stable disease. Two patients developed a mixed response and one a progressive disease. The result of one performed PRRT could not be assessed due to pending staging. Overall, the 14 PRRT responses assessed led to a clinical benefit in 11 cases, giving a clinical benefit rate of 79%.
Liver-directed therapies also proved to be a useful therapeutic approach. One patient developed partial remission after SIRT. Two patients received a transcatheter arterial chemoembolization (TACE) and showed partial remission or stable disease.
Presacral NENs showed limited sensitivity to cytotoxic agents. Overall, presacral NENs responded poorly to chemotherapy. Seven patients received palliative chemotherapy (4× platinum-based chemotherapy with etoposide, 2× temozolomide/capecitabine, and 1× paclitaxel +/− carboplatin). Only the patient with LCNEC showed partial remission under chemotherapy (cisplatin/etoposide). All other patients did not benefit from chemotherapy.
Radiotherapy of the primary tumor was performed in four patients by external beam radiation therapy and in one patient as particle therapy. Three patients developed stable disease and two partial remission after radiotherapy.
Prognosis
Nine patients are currently still alive and in follow-up. Two patients were lost to follow-up. During a median follow-up time of 44.5 months (mean, 56.0 months; range, 0.73–212.4 months), six patients died 0.7–56.2 months after diagnosis of presacral NEN. Three of these patients died of their presacral NEN. The other three had an unknown cause of death.
The Kaplan–Meier plot of duration of observation and overall survival is shown in Figure 4 .
Figure 4.
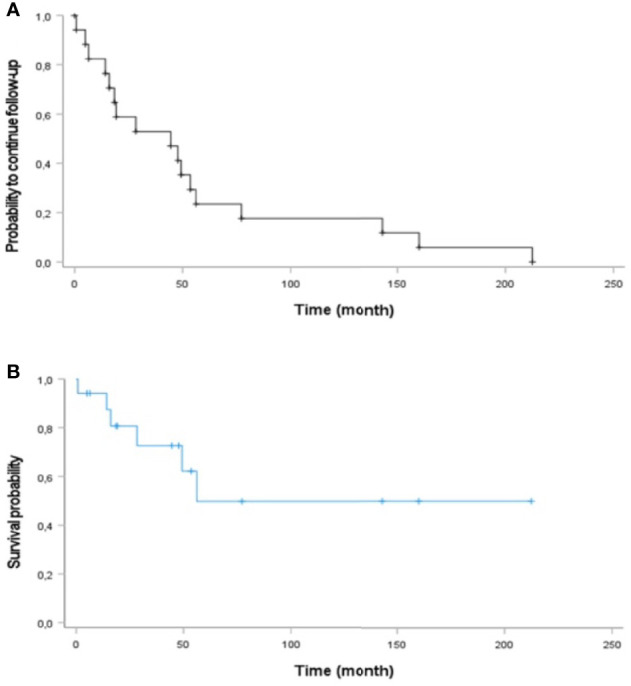
Kaplan–Meier curve analysis of (A) duration of observation and (B) overall survival of patients with primary presacral NEN (n = 17).
Discussion
To the best of our knowledge, this is the largest study of presacral NEN so far. Besides clinical and pathological characteristics, we extensively analyzed the efficacy of different systemic therapeutics.
The pathological characteristics are in line with previous reports on presacral NENs. Expression of the neuroendocrine markers chromogranin A, synaptophysin, and CD56 is common, with CgA staining often being only weakly positive or even negative in some cases. When stained, Ck7, CDX2 (a marker of gastrointestinal origin), and TTF1 (a marker of pulmonary origin in NETs) were mostly negative, whereas PSAP, Ck18, and vimentin were mostly positive. This immunohistochemical profile resembles the profile of rectal NEN (11), which seems quite reasonable considering the hypothesis of a common ontogenetic origin from the embryonal hindgut (34). The similarity to rectal NEN is also supported by the only reported case of molecular profiling in presacral NEN we are aware of (19): here, an intestinal L cell was suggested as a putative cell of origin, with L-cell phenotype being reported in about 80% of rectal NETs (77). Most patients in our study showed well differentiated morphology with the vast majority being classified as NET G2. High-grade histology was detected in four patients, well-differentiated NET G3 in three, and poorly differentiated neuroendocrine carcinoma in one. This NEC was also the only TTF1-positive case in our cohort, demonstrating that TTF1 positivity is commonly observed in NEC of different origins and not a marker of pulmonary primary for NEC (in contrast to NET) (78).
Clinically, many patients showed local symptoms like pain and impairment of defecation as local symptoms of the tumor. However, in a remarkable proportion of patients, presacral NEN was identified as primary tumor of diagnosed NEN metastasis, and patients were referred to the centers as CUP NET cases.
Up to date, most cases of presacral NENs are published as single case reports or small series (14–72) (summarized in Table 3 ). In most of those cases, presacral NENs are treated locally with resection, and there is limited information on follow-up, metastasis, and systemic treatment. The largest case series that we could identify included 10 patients and reported on outcomes of different systemic therapeutic strategies for advanced disease (72). In our cohort, local resection was performed in 8 of 17 patients, half of them in palliative intention to treat local complaints. Several cases were treated with percutaneous radiotherapy, with encouraging results regarding local control and symptomatic improvement. In the literature, an association of presacral NENs with tailgut cysts and teratomas has been described. Additionally, an association with Currarino syndrome can be observed, an autosomal-dominant disorder caused by mutations in the motor neuron and pancreas homeobox 1 (MNX1) gene and characterized by presacral mass, sacral dysgenesis, anorectal anomalies (21, 49, 53). Most remarkably, whereas tailgut cysts or at least partially cystic primary tumors were observed in some of our patients, only one association with teratoma and none of other abnormalities like anorectal malformations or Currarino syndrome were present. Those abnormalities are quite often described in the case reports cited above; in the other larger case series of n = 10, only one patient presented with a teratoma. The observed difference between case reports and case series (including our analysis) could be attributed to a publication bias of more spectacular histological constellation for case reports and referral bias to centers where patients with advanced metastatic are more likely to be referred to. In our cohort, 12 of 17 patients presented with distant metastasis at first diagnosis, whereas more than 80% of the cases reported in the literature were localized or locally advanced.
Table 3.
Previously reported cases of presacral NEN.
| Reference | Age | Sex | Histology | Anomalies | IHC +: positive (+): weak or focal positive −: negative | Metastases synchronous | Metastases metachronous | Treatment (best response, PFS in months) | Follow-up (months) †deceased |
|---|---|---|---|---|---|---|---|---|---|
| (42) Fiandaca 1988 | 35 | F | NET | Teratoma | NR | LYM, HEP, OTH (ovary) | Surgery, PEB (NR) | NR (12 presurgery) | |
| (70) Noshiro 1990 | 48 | F | NET | – | NR | LYM | Surgery (CR, 24 og) | 24 | |
| (60) Addis 1991 | 57 | F | NET | – | CAM5.2+, S100(+), NSE(+), Vimentin-, GFAP- | Surgery (NR, 12 og) | 12 | ||
| (64) Lin 1992 | 18 | F | NET | Tailgut cyst | NR | NR | NR | ||
| (55) Edelstein 1996 | 51 | F | NET | – | NR | Surgery (CR, 18 og) | 18 | ||
| (71) Horenstein 1998 | 19 | F | NET (Ki67 NR) | Tailgut cyst | CgA+, Syn+, NSE+, Cam5.2+, S100−, GFAP− | Surgery (CR, 48 og) | 48 | ||
| (71) Horenstein 1998 | 19 | F | NET (Ki67 NR) | – | CgA+, Syn+, NSE+, Cam5.2+, GFAP−, NeuF−, serotonin−, Somatostatin−, VIP−, Gastrin−, Calcitonin− | 2x Cis/Eto/Ifo, 3x Doxo/DTIC/Cyclo, embolization (SD, 12 og) Surgery (CR, 36 og) |
48 | ||
| (71) Horenstein 1998 | 21 | F | NET (Ki67 NR) | – | CgA+, Syn+, NSE+, Cam5.2+, Ck7−, Ck20−, GFAP−, serotonin−, somatostatin−, VIP−, Gastrin−, Calcitonin− | LR, OTH (Breast) | Surgery (CR, 12) Surgery Breast (CR, 1) |
13 | |
| (68) Gorski 1999 | 42 | F | NR | NR | |||||
| (56) Oyama 2000 | 52 | M | NET G1 (Ki67 NR) | Tailgut cyst | NR | Surgery (CR, 6 og) | 6 | ||
| (43) Prasad 2000 | 69 | F | NEC (Ki67 NR) | Tailgut cyst | Ck+, CgA+ | Surgery(CR, 24og) | 24 | ||
| (58) Theunissen 2001 | 51 | F | NET G2 (“LCNEC”, Ki67 NR) | – | MNF116+, Vimentin(+), CEA−, CA125−, S100−, CgA+, Syn+ | Cis/Eto (SD, 3) | 3 † | ||
| (31) Mourra 2003 | 68 | M | NET/NEC (Ki67 NR) | Tailgut cyst | NSE+, CgA+, Syn+, Ck+, EMA+, PSA−, CD45− | Surgery (CR, 12 og) | 12 | ||
| (26) Jacob 2004 | 42 | F | NR | Tailgut cyst | NR | Surgery (NR) | NR | ||
| (16) Song 2004 | 41 | F | NET (Ki67 NR) | Tailgut cyst | AE1/3+, Syn+, CgA+ | HEP, BRA | Surgery (CR, 12) 5-FU (NR, 3) RT BRA (NR) |
15 | |
| (48) Urioste 2004 | 22 | M | NR | Teratoma, Currarino | NR | NR | |||
| (18) Luong 2005 | 37 | M | NET G1 (Ki67 2.9%) | Teratoma | Ck+, Syn+, NSE+, CgA−, | HEP, LYM, OSS | Lan (PD, 10) Lan (NR, 3) |
18 | |
| (28) Mathieu 2005 | 49 | F | NET (Ki67 NR) | Tailgut cyst | NR | Surgery (CR, 24 og) | 24 | ||
| (34) Kim 2007 | 58 | F | NET (Ki67 NR | Imperforate anus | Syn+, CgA+, NSE+, Ck+, S100− | Surgery (CR, 10 og) | 10 | ||
| (24) Liang 2008 | 51 | F | NET G2? (Ki67 > 1%) | Tailgut cyst | ER+, PR(+), Syn+, CgA+, PanCk+, | Surgery (NR) | NR (3 presurgery) | ||
| (20) La Rosa 2010 | 73 | F | NET G1 (Ki67 < 2%) | Tailgut cyst | Syn+, CgB+, VMAT2+, SSTR2A+, PAP+, Ghrelin+, CgA+, Serotonin+, Somatostatin+, Ck20+, CDX2−, VMAT1−, PP−, YY−, GRP−, Gastrin−, glicentin−, encephalin−, GFAP−, ER−, PR−, AR−, Ck7−, TTF1− | Surgery (CR, 5) | 5 (36 presurgery) | ||
| (65) Pendlimari 2010 (32) Liu 2020 |
22 | F | NET G2 (Ki67 5%) | Currarino, teratoma | CgA+, Syn+, CD56(+) | LYM | Surgery (CR, 24 og) | 24 | |
| (59) Ciotti 2011 | 44 | F | NET G1/G2 (Ki67 < 10%) | Currrarino, teratoma | CgA+, Syn+ | LYM | LR, LYM, HEP | Surgery (CR, 16) Surgery LR, LYM (CR, 24) Octreotide, CT (PD) |
40 † |
| (69) Harbeck 2011 | 39 | F | NET G2 (Ki67 5%)/NEC G3 (Ki67 30%) | – | CgA+, Syn+, Ck+, SSTR2+, serotonin−, glucagon−, somatostatin− | LYM, OSS | Surgery (CR, 30) PRRT (PR, 6 og) |
36 | |
| (30) Spada 2011 | 63 | F | NET G1 (Ki67 < 2%) | Tailgut cyst | AE1/3+, Syn+, PP+, AP+, CgA(+) | HEP | Surgery (CR, 25 og) | 25 | |
| (30) Spada 2011 | 41 | F | NET G2 (Ki67 18%) | Tailgut cyst | CgA+, Syn+, AE1/3+, SSTR2+ | HEP | PLE, OSS, OTH (ovary) | Surgery (PR, NR) Carbo/Eto for HEP (NR, 7) PRRT (PR, NR) Surgery for PLE+Ovary (NR, NR) SSA (SD, NR) PRRT (SD, NR) |
79 |
| (62) Wöhlke 2011 | 55 | F | NET G2 (Ki67 20%) | Tailgut cyst | AE1/3+, Syn+, CgA(+), somatostatin+, glucagon−, insulin−, gastrin−, CDX2− | LYM, HEP | OSS | Surgery, PRRT (CR, 22) PRRT (PR, 8) |
30 |
| (66) Zhong 2012 | 48 | F | NET G1 (Ki67 1%) | – | OTH (muscle) | Surgery, cis/eto/doxo/cyclo RT (SD, 36 og) | 36 | ||
| (63) Zoccali 2012 | 64 | M | NEN (Ki67 NR) | Tailgut cyst | AE1/3+, Syn+, CgA−, p63− | Surgery (NR) | NR | ||
| (57) Damato 2013 | 24 | F | NET (Ki67 NR 5%)? | Tailgut cyst | Vimentin+, Ck+, S100−, Syn+, PSAP+ | Surgery (CR, 3 og) | 3 | ||
| (41) Misawa 2013 | 53 | F | NET/NEC (Ki67 20–60/70%) | AE1/3+, CAM5.1+/−, KL1+/−, S100 +/−, NSE+, Ubiquitin+, CD56+, CgA−, Syn+, LCA−, SMA−, Desmin−, CD10−/+, CD34−, HMB45−, GCDFP15− | LR, PER, PUL | Surgery (NR, 4) RTX for LR (NR, 4) |
11 † | ||
| (67) Simpson 2014 | 64 | F | NEN (Ki67 NR) | Teratoma | NR | NR | Surgery (NR) | NR | |
| (39) Abukar 2014 | 61 | M | NET G2 (Ki67 low) | Tailgut cyst | PAP+, CD56(+), Syn+, CgA+, MNF116+, AE1/3+ | Surgery (NR) | NR | ||
| (17) Charalampakis 2014 | 35 | M | NET G1 (Ki67 < 1%)? | Tailgut cyst | PanCk+, CgA+, Syn+ | Surgery (CR, 36 og) | 36 | ||
| (37) Kim 2014 | 49 | M | NET G2 (Ki67 5%) | Tailgut cyst | CgA+, Syn+, CD56+ | Surgery, w&w for residual tumor (SD, 14 og) | 24 | ||
| (61) Menter 2014 | 69 | M | NET G2 (Ki67 10%), PLE: Ki67 15% | – | PSAP+, TTF1−, PSA−, Ck20(+), CD56+, CgA+, Syn+, SSTR2+, Ck22+, Ck7−, EMA−, ERG−, S100−, | OSS, HEP, PUL, LYM, OTH, heart, duodenum, mesenterium | Surgery, RT (PR, 36) | 72† | |
| (22) Mitsuyama 2015 | 53 | M | NET G2 (Ki67 12.5%) | Tailgut cyst | Vimentin+, panCk(+), EMA−, S100−, CD99−, CgA+, Syn+, SSTR2+ | Surgery (CR, 10) Octreotide (NR) Irradiation (NR, 10) Everolimus (NR, 8 og) |
28 | ||
| (52) Sable 2014 | 35 | F | NET G1 (Ki67 2%) | Teratoma | Ck+, Syn+ | Surgery, CT | NR | ||
| (35) Falkmer 2015 | 57 | M | NET G2 (Ki67 5–10%) | – | AE1/3+, Syn+, CgA+, CgB+, Ghrelin+, PYY(+), Motilin(+), VMAT2−, Serotonin−, Gastrin−, GIP−, CGRP−, CART−, Calcitonin−, ACTH−, Secretin−, VIP−, NRK−, Insulin−, IAPP−, glucagon−, GLP1−, GRP−, neurotensin− | LYM, OSS | LR, SKI, BRA, OTH (soft tissue, kidney, heart) | Watch&wait (NR, 27) Surgery (8) RTX (4) STZ/5-FU (PD, NR) PRRT (NR,19) Octreotide (NR, NR) Octreotide-HD (NR, NR) RTX (NR, 13) PRRT (NR, 25) PRRT (PR, 9) |
135 † |
| (38) Jehangir 2016 | 74 | M | NET (Ki67 NR) | Tailgut cyst | Syn+, NSE+, CgA− | Surgery (CR, 60 og) | 60 | ||
| (40) Ferrer-Márquez 2017 | NR | NR | NET (Ki67 NR) | NR | NR | Surgery (CR, 24 og) | 24 | ||
| (40) Ferrer-Márquez 2017 | NR | NR | NET (Ki67 NR) | NR | Surgery (CR, 24 og) | 24 | |||
| (40) Ferrer-Márquez 2017 | NR | NR | NET (Ki67 NR) | NR | Surgery (CR, 6 og) | 6 | |||
| (45) Mora-Guzmán 2017 | 56 | F | NET G1 (Ki67 < 2%) | Tailgut cyst | AE1/3+, CD56+, Syn+, CgA+ | Surgery (CR) | 7 | ||
| (29) Al Khaldi 2018 | 53 | F | NET G2 (Ki67 5-10%) | Tailgut cyst | CgA+, Syn+, Cam5.2+, AE1/3(+), CD56(+) | LYM, PUL, HEP | Surgery (CR, 24) SSA for LYM+PUL (NR, 6) SZT/5-FU (SD, 12) Everolimus (PD, 4) |
46 | |
| (19) Erdrich 2018 | 77 | M | NET G2 (Ki67 8.6%) HEP: NET G2 (Ki67 6.4%) |
Tailgut cyst | CgA(+), Syn+ | HEP | Surgery primary + HEP | NR | |
| (14) Iwata 2019 | 25 | F | NET G2, (Ki67 20%) | Tailgut cyst | Ck+, Syn+, CgA+, ER−, PR− | Surgery (NR) | NR (8 presurgery) | ||
| (51) Soyer 2018 | 14 | M | Tailgut cyst | NR | |||||
| (72) Yang 2018 | 39 | F | NET G2 (Ki67 5–10%) | Tailgut cyst | CgA+, Syn+, Serotonin−, TTF1−, CDX2−, PAX8−, PP− | LR, HEP, PUL, PER, OTH (ovaries) | Surgery (CR, 12) Surgery + SSA for LR (CR,12) Surgery LR + PER, ablation (NR, 41) Surgery + RT for LR + PER + OTH (NR, 36) SIRT + SSA (NR, NR) |
120 | |
| (72) Yang 2018 | 41 | F | NET (Ki67 NR) | Teratoma | Syn+, CgA+, Ck+, AE1/3+, S100+ | OTH (ovaries, retroperitoneal) | Surgery, SSA (CR, 48) Surgery (ovaries, retroperitoneal) (NR, NR) |
48 | |
| (72) Yang 2018 | 45 | F | NET (Ki67 NR), BRA: NET G2, Ki67 18% | Anterior sacral meningocele, tailgut cyst | CAM5.2+, CgA+, Syn+, CDX2+ | BRA, LYM, HEP, OSS | Surgery (CR, 9) Surgery brain (CR, 3) Octreotide, PRRT LR, LYM, HEP (SD, 36) PRRT for HEP, OSS (PR, NR) |
46 (156 presurgery | |
| (72) Yang 2018 | 46 | M | NET G2 (Ki67 15%) BRA: NET G2 (Ki67 12%) |
Tailgut cyst | Syn+, CgA+, AE1/3+, TTF1−, CDX2− | HEP, OSS | FOLFOX + Bev (SD?, 14 og) Everolimus + Oct, Irradiation (NR, 12) Surgery (BRA) |
28 | |
| (72) Yang 2018 | 75 | M | NET G1, Ki67 < 1% | – | LYM, OSS, PUL | HEP | Surgery, RTX, Oct (NR, 47) SIRT, Oct (PR, 27 og) |
78 | |
| (72) Yang 2018 | 42 | F | NET G2 (Ki67 6%) | – | Syn+, CD56+, NSE+, WT1+, | HEP | Surgery (CR, 12) Oct (NR, 25) Oct + CC-223 (NR, 9) SIRT (PR, 11) Surgery pancreas (NR, NR) Tem (PD, NR) Sunitinib (NR, NR) PRRT (SD, NR) |
68 | |
| (72) Yang 2018 | 41 | F | NET G2 (Ki67 13%) | – | PUL, HEP, OSS | BRA | Lan + IFN (NR, 9) Pazopanib (NR, 16) RT OSS BRA (SD, 6 og) |
36 | |
| (72) Yang 2018 | 44 | F | NEC G3 (Ki67 80–90%) | – | Ck+, Syn+, CD56+ | LYM | Cis/Eto, RTX (PR, 5 og) | 5 | |
| (72) Yang 2018 | 77 | F | Large-cell NET/NEC (Ki67 50%) | – | Syn+, CD56+, Villin+ | Carbo/Eto (SD, 3 og) Oct (PD, 4) |
7 | ||
| (72) Yang 2018 | 50 | F | NET (Ki67 NR) | – | LYM, HEP, OTH (pancreas) | Tem/Cap (NR), Oct (NR) | NR | ||
| (21) Chatani 2019 | 59 | F | NET G2 (Ki67 NR), Adenocarcinoma | Teratoma, Currarino | Syn+, CgA+ | Resection, RT (CR, 8 og) |
8 | ||
| (49) Coetzee 2019 | 60 | F | NET G2 (Ki67 14%) | Teratoma, Currarino | CgA+, Syn+ | Surgery (CR, 18 og) | 18 | ||
| (53) Colombo 2019 | 46 | M | NET (Ki67 NR) | Currarino, dermoid cyst | NR | NR | |||
| (36) Kim 2019 | 78 | M | NET G2 (Ki67 6.6%) | – | CgA+, Syn+, CD56+, Ck7-, TTF1-, CDX2- | HEP | PER | Surgery HEP (CR, 48) Octreotide + everolimus (SD, 14) Pazopanib (NR, 14) PRRT (SD, 2 og) |
96 |
| (15) Lee 2019 | 33 | F | NET G1 (Ki67 1-2%) | Tailgut cyst | AE1/3+, Syn+, CgA+, CDX2(+), ER(+), Ck7(+), Ck20- | Surgery (NR, NR) | NR (96 presurgery) | ||
| (46) Olczak 2019 | 49 | M | |||||||
| (50) Rod 2019 | 51 | M | NET (Ki67 NR) | Currarino | NR | Surgery (NR, NR) | 60 | ||
| (27) Sakr 2019 | M | NET (Ki67 NR) | Tailgut cyst | NR | Surgery (NR, NR), RT (NR, NR) | NR | |||
| (33) Singh 2019 | 63 | M | NET G1 (Ki67 < 1%) | Tailgut cyst | Syn+, Ck+, CgA−, GFAP−, SMHC−, p63−, CD56− | Surgery (NR, NR) | NR | ||
| (25) Zhang 2019 | 36 | F | NET G3 (Ki67 30%) | – | Syn+, CD56+, Ck+ | Surgery (CR, 1 og) | 1 | ||
| (23) Kodera 2020 | 68 | F | NET G1 (Ki67 < 2%) | Tailgut cyst | CD56+, SSTR2A+, PP+, PR(+), CgA−, p53−, ER−, gastrin−, serotonin−, somatostatin−, CDX2−, TTF1− | Surgery (CR, 12 og) | 12 | ||
| (32) Liu 2020 | 75 | F | NET G2 (Ki67 3%), HEP: NET G2 (Ki67 6.8%) | Tailgut cyst, Currarino | NR | HEP | Octreotide (PD, 11) PRRT (NR, NR) |
11 | |
| (32) Liu 2020 (54) Scott 2021 |
35 | F | NET G2 (Ki67 4%) | Currarino, tailgut cyst | Syn+, SSTR2A+, Islet-1+, CgA−, Ck20−, TTF1−, CDX2−, PAX8−, GATA3−, Inhibin−Desmin−, S100− | LYM, OSS | Surgery, Octreotide (SD, 22 og) PRRT (NR, NR) Oct high dose (SD, NR) |
36 | |
| (47) Rebelo 2020 | 48 | M | NET G2 (Ki67 6%) | Tailgut cyst, Currarino | CD56+, Syn+, CgA− | HEP, OSS, OTH (spleen) | Surgery (CR, 18) CT |
18 | |
| (54) Scott 2021 | 38 | M | NET G2 (Ki67 7.5%), LYM: NET G2 (Ki67 9%) | Currarino, tailgut cyst | Syn+, CgA+, SSTR2A+, Islet−1+, PAX6+, CDX2−, TTF1− | LYM | Surgery (CR, 24) Surgery LYM (CR, NR) |
24 | |
| (54) Scott 2021 | 62 | F | NET G1 (Ki67 < 1%) | Currarino, teratoma | AE1/3+, CAM 5.2+, Syn+, CD56(+), Ck5/6−, p63−, S100−, desmin−, CD34−, CD45−, CgA− | Surgery (CR, 12 og) | 12 |
Histopathology was adapted to the most current WHO 2019 classification according to the description in the report.
AP, acid phosphatase; AR, androgen receptor; BRA, brain; CAM, cell adhesion molecule; Cap, capecitabine; Cis, cisplatin; Ck, cytokeratin; CT, chemotherapy; CgA, chromogranin A; CgB, chromogranin B; CR, complete remission; cyclo, cyclophosphamide; doxo, doxorubicin; DTIC, dacarbazin; EMA, epithelial membrane antigen, ER, estrogen receptor; eto, etoposide; F, female, GCDFP, gross cystic disease fluid protein; GFAP, glial fibrillary acidic protein; GIP, gastric inhibitory peptide; GRP, gastrin releasing peptide; HEP, liver; Lan, lanreotide, LR, local recurrence; LYM, lymph nodes; M, male; NF, neurofilament; NR, not reported; NSE, neuron-specific enolase; Oct, octreotide; og, ongoing; OSS, bone; OTH, other, PD, progressive disease; PER, peritoneum; PgR, progesterone receptor; PLE, pleura; PP, pancreatic polypeptide; PR, partial remission; PRRT, peptide receptor radionuclide therapy; PSAP, prostatic specific acidic phosphatase; PUL, lung; PYY, peptide YY; RT, radiotherapy; SD, stable disease; SKI, skin; SSA, somatostatin analogue; SSTR, somatostatin receptor; syn, synaptophysin; Tem, temozolomide; VIP, vasoactive intestinal peptide; VMAT, vesicular monoamine transporter; w&w: watch & wait.
When taking the systemic treatments applied for presacral NEN in our analysis into context, beside comparing them to NEN in general, a special focus should be laid on rectal NEN regarding their resemblance to presacral NEN as discussed above.
SSAs are among the first approved drugs for disease stabilizations for well-differentiated NETs, with octreotide for midgut NET in the PROMID trial (79) and lanreotide for enteropancreatic NET in the CLARINET trial (80). However, the use of SSAs in rectal NET is up for debate, since lanreotide failed to show PFS benefit vs. placebo in the CLARINET trial with very few rectal NET patients included. Nevertheless, SSAs are also commonly reported as effective treatment for presacral NET, with octreotide in 13 cases (22, 32, 36, 72), lanreotide in 1 case (72), and an unspecified SSA in 4 cases (29, 72). Fifteen patients in our analysis received SSAs, half of them lanreotide. Disease stabilization was observed in 50% (7/14).
PRRT is an effective treatment for NET of various locations since the mid-90s. With the conclusion of the NETTER trial, PRRT has shown its efficacy in a randomized phase III setting for intestinal NET (81), gaining approval in many countries. In rectal NET, PRRT has shown efficacy in case series (82). PRRT is reported in nine cases with presacral NET, all with encouraging long-term stabilizations and remission (32, 35, 36, 72). In accordance to this, PRRT was also one of the most effective treatments observed in our cohort, resulting in 43% responses (6/14) and 36% disease stabilizations (5/14).
The mTOR inhibitor everolimus has been approved for extrapancreatic NET after the positive phase III RADIANT-4 trial (83). The subgroup of rectal NETs in this trial showed significant PFS prolongation in this trial. Four cases of treatment of presacral NET are reported in the literature (22, 36, 72), with one showing long-term disease control, two short-term stabilizations, and one progressive disease. In our analysis, all three patients receiving everolimus showed disease progression.
The multi-tyrosine kinase inhibitor cabozantinib has shown promising antitumor activity in a preliminary report of a phase II trial for pancreatic and extrapancreatic NETs (72). We report the first patient receiving cabozantinib for metastatic presacral NET with an encouraging long-lasting disease stabilization in a heavily pretreated patient.
Cytotoxic chemotherapy is the main systemic treatment modality for NEN G3 and an effective option for pancreatic NET G1/G2 (73). In presacral NET, applications of the protocols FOLFOX (n = 1) (72), temozolomide + capecitabine (n = 2) (72), cisplatin + etoposide (n = 4) (58, 72), and carboplatin-etoposide (n = 2) (72) were reported, with heterogenous results. In our analysis, chemotherapy was applied seven times to six patients, mostly with unfavorable response. While well-differentiated presacral NET did not sufficiently respond to chemotherapy, treatment of the presacral neuroendocrine carcinoma with cisplatin + etoposide resulted in a partial remission.
Of three patients receiving molecular diagnostics, potential targetable alterations were detected in two patients: alterations in the cyclin pathway as potential target for a CDK inhibitor and HRD as a potential target for PARP inhibition and platinum-based chemotherapy most probable due to a SETD2 frameshift insertion (84–86). This is remarkable, since the only other case of molecularly profiled presacral NET reported earlier (19) showed a BRCA1 mutation, which also commonly leads to HRD.
Our study has several limitations, mainly due to its retrospective nature. On the other hand, considering the rarity of the disease, a prospective or even randomized trial is most likely not feasible. Furthermore, a central pathological or radiological review was not performed. However, all patients were included by experienced high volume NEN centers with well-established multidisciplinary diagnostic and therapeutic pathways.
In conclusion, we report the largest analysis of clinicopathological characteristics and treatment outcomes for presacral NEN so far. Presacral NENs are usually non-functioning and primarily cause locoregional symptoms. Plasma CgA levels are usually not elevated. Presacral NEN should be considered as possible primary in CUP-NET, especially when the immunohistochemical profile resembles a hindgut NET, and a rectal primary is excluded endoscopically. Functional imaging with SSR-based PET-CT is helpful for primary tumor identification and treatment planning. Local control could be achieved via radiotherapy. SSAs demonstrated limited efficacy, whereas PRRT showed promising activity for advanced disease. In our cohort, everolimus and chemotherapy were largely ineffective. Molecular diagnostics showed potential targetable alterations in selected cases. Further prospective evaluation and molecular characterization of this rare tumor entity are needed.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: The dataset supporting the conclusions of this article is available on request by contacting the authors. Tables 1 , 2 and Figure 3 build the dataset. Requests to access these datasets should be directed to sprengea@uni-marburg.
Ethics Statement
The study was conducted in accordance with the Declaration of Helsinki. All patients were included in the local disease databases conducted with approval of the local ethics committees at the respective sites. Written informed patient consent and approval for data collection and analysis were obtained upon admission to our institutions. For the use of the images, an additional consent was obtained in the selected cases.
Author Contributions
ARi and DL contributed to conception and design of the study. SM, LA, JS, HL, SKru, ARi, ZK, and AK carried out the acquisition of data. SM, LA, and ARi analyzed and interpreted the data and drafted the manuscript. SM performed statistical analysis. ARa performed immunohistochemical stainings and pathological review. DL contributed PET-CT images. SKre provided molecular data and participated in data interpretation. TG was involved in data interpretation and critical review of the manuscript draft. All authors contributed to the article and approved the submitted version.
Conflict of Interest
ARi has received honoraria for presentations and advisory boards from AAA, Advanz Pharma, Falk, IPSEN and Novartis. LA has received honoraria and travel expenses from Ipsen and Novartis. JS has received honoraria for presentations and advisory boards from Advanz Pharma, IPSEN and Novartis, and research grants from Riemser Pharma and Novartis. HL reports personal fees and grants from Novartis, and personal fees from Ipsen and AAA, outside the submitted work. AK has received honoraria for presentations from Ipsen and Novartis. TMG has received funding from IPSEN, Pfizer, and Novartis for joined research projects, participation in advisory boards, and lectures.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Lloyd RV. Practical Markers Used in the Diagnosis of Neuroendocrine Tumors. Endocr Pathol (2003) 14:293–301. doi: 10.1385/EP:14:4:293 [DOI] [PubMed] [Google Scholar]
- 2. Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol (2017) 3:1335–42. doi: 10.1001/jamaoncol.2017.0589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maasberg S, Pape U-F, Fottner C, Goretzki PE, Anlauf M, Hörsch D, et al. [Neuroendocrine Neoplasia Within the German NET Registry]. Z Gastroenterol (2018) 56:1237–46. doi: 10.1055/a-0661-6099 [DOI] [PubMed] [Google Scholar]
- 4. Garcia-Carbonero R, Capdevila J, Crespo-Herrero G, Díaz-Pérez JA, Martínez Del Prado MP, Alonso Orduña V, et al. Incidence, Patterns of Care and Prognostic Factors for Outcome of Gastroenteropancreatic Neuroendocrine Tumors (GEP-Nets): Results From the National Cancer Registry of Spain (RGETNE). Ann Oncol (2010) 21:1794–803. doi: 10.1093/annonc/mdq022 [DOI] [PubMed] [Google Scholar]
- 5. Lombard-Bohas C, Mitry E, O’Toole D, Louvet C, Pillon D, Cadiot G, et al. And FFCD-ANGH-GERCOR, Thirteen-Month Registration of Patients With Gastroenteropancreatic Endocrine Tumours in France. Neuroendocrinology (2009) 89:217–22. doi: 10.1159/000151562 [DOI] [PubMed] [Google Scholar]
- 6. Bozkurt MF, Virgolini I, Balogova S, Beheshti M, Rubello D, Decristoforo C, et al. Guideline for PET/CT Imaging of Neuroendocrine Neoplasms With. Eur J Nucl Med Mol Imaging (2017) 44:1588–601. doi: 10.1007/s00259-017-3728-y [DOI] [PubMed] [Google Scholar]
- 7. Prasad V, Ambrosini V, Hommann M, Hoersch D, Fanti S, Baum RP. Detection of Unknown Primary Neuroendocrine Tumours (CUP-NET) Using (68)Ga-DOTA-NOC Receptor PET/CT. Eur J Nucl Med Mol Imaging (2010) 37:67–77. doi: 10.1007/s00259-009-1205-y [DOI] [PubMed] [Google Scholar]
- 8. Hermann G, Konukiewitz B, Schmitt A, Perren A, Klöppel G. Hormonally Defined Pancreatic and Duodenal Neuroendocrine Tumors Differ in Their Transcription Factor Signatures: Expression of ISL1, PDX1, NGN3, and CDX2. Virchows Arch (2011) 459:147–54. doi: 10.1007/s00428-011-1118-6 [DOI] [PubMed] [Google Scholar]
- 9. Perren A, Couvelard A, Scoazec JY, Costa F, Borbath I, Delle Fave G, et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pathology: Diagnosis and Prognostic Stratification. Neuroendocrinology (2017) 105:196–200. doi: 10.1159/000457956 [DOI] [PubMed] [Google Scholar]
- 10. Kimura N, Sasano N. Prostate-Specific Acid Phosphatase in Carcinoid Tumors. Virchows Arch A Pathol Anat Histopathol (1986) 410:247–51. doi: 10.1007/BF00710831 [DOI] [PubMed] [Google Scholar]
- 11. Koenig A, Krug S, Mueller D, Barth PJ, Koenig U, Scharf M, et al. Clinicopathological Hallmarks and Biomarkers of Colorectal Neuroendocrine Neoplasms. PloS One (2017) 12:e0188876. doi: 10.1371/journal.pone.0188876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bullard Dunn K. Retrorectal Tumors. Surg Clin North Am (2010) 90:163–71. Table of Contents. doi: 10.1016/j.suc.2009.09.009 [DOI] [PubMed] [Google Scholar]
- 13. Lev-Chelouche D, Gutman M, Goldman G, Even-Sapir E, Meller I, Issakov J, et al. Presacral Tumors: A Practical Classification and Treatment of a Unique and Heterogeneous Group of Diseases. Surgery (2003) 133:473–8. doi: 10.1067/msy.2003.118 [DOI] [PubMed] [Google Scholar]
- 14. Iwata E, Orosz Z, Teh J, Reynolds J, Whitwell D, Tanaka Y, et al. Neuroendocrine Tumor Arising in a Tailgut Cyst: A Rare Presacral Tumor. Int J Surg Pathol (2019) 27:336–42. doi: 10.1177/1066896918796291 [DOI] [PubMed] [Google Scholar]
- 15. Lee A, Suhardja TS, Nguyen TC, Teoh WM. Neuroendocrine Tumour Developing Within a Long-Standing Tailgut Cyst: Case Report and Review of the Literature. Clin J Gastroenterol (2019) 12:539–51. doi: 10.1007/s12328-019-00998-4 [DOI] [PubMed] [Google Scholar]
- 16. Song DE, Park JK, Hur B, Ro JY. Carcinoid Tumor Arising in a Tailgut Cyst of the Anorectal Junction With Distant Metastasis: A Case Report and Review of the Literature. Arch Pathol Lab Med (2004) 128:578–80. doi: 10.5858/2004-128-578-CTAIAT [DOI] [PubMed] [Google Scholar]
- 17. Charalampakis V, Stamatiou D, Christodoulakis M, Kafousi M, Chryssou E, de Bree E, et al. Large Presacral Tailgut Cyst With a Carcinoid Tumor in a Male: Report of a Case. Surg Today (2014) 44:961–6. doi: 10.1007/s00595-012-0482-4 [DOI] [PubMed] [Google Scholar]
- 18. Luong TV, Salvagni S, Bordi C. Presacral Carcinoid Tumour. Review of the Literature and Report of a Clinically Malignant Case. Dig Liver Dis (2005) 37:278–81. doi: 10.1016/j.dld.2004.10.014 [DOI] [PubMed] [Google Scholar]
- 19. Erdrich J, Schaberg KB, Khodadoust MS, Zhou L, Shelton AA, Visser BC, et al. Surgical and Molecular Characterization of Primary and Metastatic Disease in a Neuroendocrine Tumor Arising in a Tailgut Cyst. Cold Spring Harb Mol Case Stud (2018) 4:a003004. doi: 10.1101/mcs.a003004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. La Rosa S, Boni L, Finzi G, Vigetti D, Papanikolaou N, Tenconi SM, et al. Ghrelin-Producing Well-Differentiated Neuroendocrine Tumor (Carcinoid) of Tailgut Cyst. Morphological, Immunohistochemical, Ultrastructural, and RT-PCR Study of a Case and Review of the Literature. Endocr Pathol (2010) 21:190–8. doi: 10.1007/s12022-010-9127-6 [DOI] [PubMed] [Google Scholar]
- 21. Chatani S, Onaya H, Kato S, Inaba Y. Adenocarcinoma and Neuroendocrine Tumor Arising Within Presacral Teratoma Associated With Currarino Syndrome: A Case Report. Indian J Radiol Imaging (2019) 29:327–31. doi: 10.4103/ijri.IJRI_148_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mitsuyama T, Kubota M, Nakamura Y, Yuzurihara M, Hoshi K, Okada Y. Neuroendocrine Tumor Arising From Tailgut Cyst With Spinal Cord Tethering: Case Report and Literature Review. Spine J (2015) 15:e1–8. doi: 10.1016/j.spinee.2014.09.027 [DOI] [PubMed] [Google Scholar]
- 23. Kodera K, Eto S, Fukasawa N, Kai W, Matsumoto T, Hirabayashi T, et al. Laparoscopic Resection of a Neuroendocrine Tumor That Almost Fully Replaced Tailgut Cysts: A Case Report. Surg Case Rep (2020) 6:269. doi: 10.1186/s40792-020-01044-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liang JJ, Alrawi S, Fuller GN, Tan D. Carcinoid Tumors Arising in Tailgut Cysts May be Associated With Estrogen Receptor Status: Case Report and Review of the Literature. Int J Clin Exp Pathol (2008) 1:539–43. [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang R, Zhu Y, Huang XB, Deng C, Li M, Shen GS, et al. Primary Neuroendocrine Tumor in the Presacral Region: A Case Report. World J Clin cases (2019) 7:1884–91. doi: 10.12998/wjcc.v7.i14.1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jacob S, Dewan Y, Joseph S. Presacral Carcinoid Tumour Arising in a Tailgut Cyst–a Case Report. Indian J Pathol Microbiol (2004) 47:32–3. [PubMed] [Google Scholar]
- 27. Sakr A, Kim HS, Han YD, Cho MS, Hur H, Min BS, et al. Single-Center Experience of 24 Cases of Tailgut Cyst. Ann Coloproctol (2019) 35:268–74. doi: 10.3393/ac.2018.12.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mathieu A, Chamlou R, Le Moine F, Maris C, Van de Stadt J, Salmon I. Tailgut Cyst Associated With a Carcinoid Tumor: Case Report and Review of the Literature. Histol Histopathol (2005) 20:1065–9. doi: 10.14670/HH-20.1065 [DOI] [PubMed] [Google Scholar]
- 29. Al Khaldi M, Mesbah A, Dubé P, Isler M, Mitchell A, Doyon J, et al. Neuroendocrine Carcinoma Arising in a Tailgut Cyst. Int J Surg Case Rep (2018) 49:91–5. doi: 10.1016/j.ijscr.2018.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spada F, Pelosi G, Squadroni M, Lorizzo K, Farris A, de Braud F, et al. Neuroendocrine Tumour Arising Inside a Retro-Rectal Tailgut Cyst: Report of Two Cases and a Review of the Literature. Ecancermedicalscience (2011) 5:201. doi: 10.3332/ecancer.2011.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mourra N, Caplin S, Parc R, Flejou JF. Presacral Neuroendocrine Carcinoma Developed in a Tailgut Cyst: Report of a Case. Dis Colon Rectum (2003) 46:411–3. doi: 10.1007/s10350-004-6564-7 [DOI] [PubMed] [Google Scholar]
- 32. Liu AJ, Halfdanarson TR, Sonbol MB. Currarino Syndrome: A Rare Condition With Potential Connection to Neuroendocrine Tumors. Pancreas (2020) 49:1104–8. doi: 10.1097/MPA.0000000000001632 [DOI] [PubMed] [Google Scholar]
- 33. Singh A, Karnik S, Khedkar B, Deshmukh S, Deodhar K. A Well-Differentiated Neuroendocrine Tumor (Grade I) Arising in a Tailgut Cyst. J Cancer Res Ther (2019) 15:258–60. doi: 10.4103/0973-1482.189236 [DOI] [PubMed] [Google Scholar]
- 34. Kim T, Grobmyer SR, Liu C, Hochwald SN. Primary Presacral Neuroendocrine Tumor Associated With Imperforate Anus. World J Surg Oncol (2007) 5:115. doi: 10.1186/1477-7819-5-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Falkmer UG, Gustafsson T, Wenzel R, Wierup N, Sundler F, Kulkarni H, et al. Malignant Presacral Ghrelinoma With Long-Standing Hyperghrelinaemia. Ups J Med Sci (2015) 120:299–304. doi: 10.3109/03009734.2015.1054453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim MR, Shim HK. Long-Term Follow-Up of a Patient With Primary Presacral Neuroendocrine Tumor: A Case Report With Literature Review. Am J Case Rep (2019) 20:1969–75. doi: 10.12659/AJCR.921439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim JH, Jin SY, Hong SS, Lee TH. A Carcinoid Tumour Arising Within a Tailgut Cyst: A Diagnostic Challenge. Scott Med J (2014) 59:e14–7. doi: 10.1177/0036933013519029 [DOI] [PubMed] [Google Scholar]
- 38. Jehangir A, Le BH, Carter FM. A Rare Case of Carcinoid Tumor in a Tailgut Cyst. J Community Hosp Intern Med Perspect (2016) 6:31410. doi: 10.3402/jchimp.v6.31410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abukar AA, Parcell BJ, Lim CB, Patil PV, Ramsanahie A, Carey F, et al. Malignancy Within a Tail Gut Cyst: A Case of Retrorectal Carcinoid Tumour. Case Rep Surg (2014) 2014:454502. doi: 10.1155/2014/454502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ferrer-Márquez M, Rubio-Gil F, Ortega-Ruiz S, Blesa-Sierra I, Álvarez-García A, Jorge-Cerrudo J, et al. Transanal Endoscopic Microsurgery for the Treatment of Uncommon Rectal Lesions. Cir Esp (2017) 95:335–41. doi: 10.1016/j.ciresp.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 41. Misawa S, Horie H, Yamaguchi T, Kobayashi S, Kumano H, Lefor AT, et al. A Unique Retrorectal Tumor With Neuroendocrine Differentiation: Case Report and Review of the Literature. Int J Surg Pathol (2013) 21:271–7. doi: 10.1177/1066896913476738 [DOI] [PubMed] [Google Scholar]
- 42. Fiandaca MS, Ross WK, Pearl GS, Bakay RA. Carcinoid Tumor in a Presacral Teratoma Associated With an Anterior Sacral Meningocele: Case Report and Review of the Literature. Neurosurgery (1988) 22:581–8. doi: 10.1227/00006123-198803000-00025 [DOI] [PubMed] [Google Scholar]
- 43. Prasad AR, Amin MB, Randolph TL, Lee CS, Ma CK. Retrorectal Cystic Hamartoma: Report of 5 Cases With Malignancy Arising in 2. Arch Pathol Lab Med (2000) 124:725–9. doi: 10.5858/2000-124-0725-RCH [DOI] [PubMed] [Google Scholar]
- 44. Schnee CL, Hurst RW, Curtis MT, Friedman ED. Carcinoid Tumor of the Sacrum: Case Report. Neurosurgery (1994) 35:1163–7. doi: 10.1227/00006123-199412000-00024 [DOI] [PubMed] [Google Scholar]
- 45. Mora-Guzmán I, Alonso-Casado A, Rodríguez Sánchez A, Bermejo Marcos E. Neuroendocrine Tumour Arising Inside a Tailgut Cyst. Ann R Coll Surg Engl (2017) 99:e91–3. doi: 10.1308/rcsann.2016.0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Olczak B, Zaforemska A, Cialkowska-Rysz A. Long-Term Analgesic Pharmacotherapy in Addiction to Intranasal Fentanyl. BMJ Support Palliat Care (2019). doi: 10.1136/bmjspcare-2019-001990 [DOI] [PubMed] [Google Scholar]
- 47. Rebelo J, Marques A, Vilares T, Silva N, Silva R, Cunha R, et al. Currarino Triad: A Case Report of a 48-Year-Old Patient With a Neuroendocrine Tumor. Radiol Case Rep (2020) 15:1555–61. doi: 10.1016/j.radcr.2020.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Urioste M, Garcia-Andrade M, Valle L, Robledo M, González-Palacios F, Méndez R, et al. Malignant Degeneration of Presacral Teratoma in the Currarino Anomaly. Am J Med Genet A (2004) 128A:299–304. doi: 10.1002/ajmg.a.30028 [DOI] [PubMed] [Google Scholar]
- 49. Coetzee E, Malaka S. Malignant Neuroendocrine Tumour in an Adult Female Diagnosed With Currarino Syndrome. S Afr J Surg (2019) 57:44. doi: 10.17159/2078-5151/2019/v57n4a3145 [DOI] [PubMed] [Google Scholar]
- 50. Rod J, Cretolle C, Faivre L, Jacquot C, Yacoub O, Ravasse P, et al. Malignant Transformation of Presacral Mass in Currarino Syndrome. Pediatr Blood Cancer (2019) 66:e27659. doi: 10.1002/pbc.27659 [DOI] [PubMed] [Google Scholar]
- 51. Soyer T, Aydin B, Orhan D, Tanyel FC. Neuroencorine Tumor Arising Within a Tailgut Cyst in an Adolescent Boy. Fetal Pediatr Pathol (2018) 37:270–5. doi: 10.1080/15513815.2018.1472355 [DOI] [PubMed] [Google Scholar]
- 52. Sable MN, Nath D, Chumbar S, Das CJ, Priyadarshini P, Kaur K, et al. Pelvic Mature Cystic Teratoma With Neuroendocrine Carcinoma: Report of a Rare Association and Review of Literature. Indian J Pathol Microbiol (2014) 57:113–5. doi: 10.4103/0377-4929.130916 [DOI] [PubMed] [Google Scholar]
- 53. Colombo F, Janous P, Buxton N. Carcinoid Transformation of Presacral Dermoid Cyst in Patient With Currarino Syndrome: A Case Report. Br J Neurosurg (2019) 33:285–6. doi: 10.1080/02688697.2017.1339226 [DOI] [PubMed] [Google Scholar]
- 54. Scott AT, Tessmann JB, Braun T, Brown B, Breheny PJ, Darbro BW, et al. Presacral Neuroendocrine Tumors Associated With the Currarino Syndrome. Am J Med Genet A (2021) 185:1582–8. doi: 10.1002/ajmg.a.62145 [DOI] [PubMed] [Google Scholar]
- 55. Edelstein PS, Wong WD, La Valleur J, Rothenberger DA. Carcinoid Tumor: An Extremely Unusual Presacral Lesion. Report of a Case. Dis Colon Rectum (1996) 39:938–42. doi: 10.1007/BF02053995 [DOI] [PubMed] [Google Scholar]
- 56. Oyama K, Embi C, Rader AE. Aspiration Cytology and Core Biopsy of a Carcinoid Tumor Arising in a Retrorectal Cyst: A Case Report. Diagn Cytopathol (2000) 22:376–8. doi: [DOI] [PubMed] [Google Scholar]
- 57. Damato A, Pusceddu S, Milione M, Mazzaferro V, Magli M, Seregni E, et al. Well-Differentiated Neuroendocrine Tumor of Tailgut Cyst. A Rare Entity With Controversial Medical Opportunities. Tumori (2013) 99:e148–51. doi: 10.1700/1361.15113 [DOI] [PubMed] [Google Scholar]
- 58. Theunissen P, Fickers M, Goei R. Primary Large Cell Neuroendocrine Carcinoma of the Presacral Region. J Clin Pathol (2001) 54:880–2. doi: 10.1136/jcp.54.11.880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ciotti P, Mandich P, Bellone E, Ceppa P, Bovio M, Ameri P, et al. Currarino Syndrome With Pelvic Neuroendocrine Tumor Diagnosed by Post-Mortem Genetic Analysis of Tissue Specimens. Am J Med Genet A (2011) 155A:2750–3. doi: 10.1002/ajmg.a.34031 [DOI] [PubMed] [Google Scholar]
- 60. Addis BJ, Rao SG, Finnis D, Carvell JE. Pre-Sacral Carcinoid Tumour. Histopathology (1991) 18:563–5. doi: 10.1111/j.1365-2559.1991.tb01486.x [DOI] [PubMed] [Google Scholar]
- 61. Menter T, Fischmann A, Glatz K. [PSAP Expression in a Primary Presacral Neuroendocrine Tumor. Potential for Confusion With Prostate Cancer]. Pathologe (2014) 35:277–82. doi: 10.1007/s00292-013-1855-1 [DOI] [PubMed] [Google Scholar]
- 62. Wöhlke M, Sauer J, Dommisch K, Görling S, Valdix A, Hinze R. [Primary Metastatic Well-Differentiated Neuroendocrine Tumor Arising in a Tailgut Cyst]. Pathologe (2011) 32:165–7. doi: 10.1007/s00292-010-1390-2 [DOI] [PubMed] [Google Scholar]
- 63. Zoccali M, Hart J, Fichera A. Image of the Month. Low-Grade Neuroendocrine Carcinoma Arising From a Tailgut Cyst. Arch Surg (2012) 147:93–4. doi: 10.1001/archsurg.2011.702a [DOI] [PubMed] [Google Scholar]
- 64. Lin SL, Yang AH, Liu HC. Tailgut Cyst With Carcinoid: A Case Report. Zhonghua Yi Xue Za Zhi (Taipei) (1992) 49:57–60. [PubMed] [Google Scholar]
- 65. Pendlimari R, Leonard D, Dozois EJ. Rare Malignant Neuroendocrine Transformation of a Presacral Teratoma in Patient With Currarino Syndrome. Int J Colorectal Dis (2010) 25:1383–4. doi: 10.1007/s00384-010-0953-2 [DOI] [PubMed] [Google Scholar]
- 66. Zhong W, You C, Chen H, Huang S. Primary Presacral Carcinoid Tumor With Gluteal Muscle Metastasis. Neurol India (2012) 60:544–5. doi: 10.4103/0028-3886.103219 [DOI] [PubMed] [Google Scholar]
- 67. Simpson PJ, Kehl J, Rose PS, Dozois EJ. Heterogenous Malignant Presacral Teratoma With a Locally Destructive Benign Intrasacral Component. Tech Coloproctol (2014) 18:605–6. doi: 10.1007/s10151-013-1087-7 [DOI] [PubMed] [Google Scholar]
- 68. Gorski T, Khubchandani IT, Stasik JJ, Riether R. Retrorectal Carcinoid Tumor. South Med J (1999) 92:417–20. doi: 10.1097/00007611-199904000-00014 [DOI] [PubMed] [Google Scholar]
- 69. Harbeck B, Anlauf M, Klöppel G, Bröring D, Lehnert H, Mönig H. An Unusual Case of a Retrorectal Neuroendocrine Tumor With High- and Low-Grade Differentiation. Int J Colorectal Dis (2012) 27:1241–2. doi: 10.1007/s00384-011-1389-z [DOI] [PubMed] [Google Scholar]
- 70. Noshiro H, Satoh K, Gondoh T, Iwata T, Fujii T, Mezuki M, et al. A Case of Rectal Carcinoid With Extensive Sacral Breakage. Jpn J Surg (1990) 20:443–7. doi: 10.1007/BF02470829 [DOI] [PubMed] [Google Scholar]
- 71. Horenstein MG, Erlandson RA, Gonzalez-Cueto DM, Rosai J. Presacral Carcinoid Tumors: Report of Three Cases and Review of the Literature. Am J Surg Pathol (1998) 22:251–5. doi: 10.1097/00000478-199802000-00015 [DOI] [PubMed] [Google Scholar]
- 72. Yang G, Dhall D, Yu R, Tuli R, Amersi FF, Friedman ML, et al. The Clinicopathological Aspects of Primary Presacral Neuroendocrine Neoplasms: One Center Experience. Pancreas (2018) 47:122–9. doi: 10.1097/MPA.0000000000000954 [DOI] [PubMed] [Google Scholar]
- 73. Pavel M, O’Toole D, Costa F, Capdevila J, Gross D, Kianmanesh R, et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology (2016) 103:172–85. doi: 10.1159/000443167 [DOI] [PubMed] [Google Scholar]
- 74. Horak P, Klink B, Heining C, Gröschel S, Hutter B, Fröhlich M, et al. Precision Oncology Based on Omics Data: The NCT Heidelberg Experience. Int J Cancer (2017) 141:877–86. doi: 10.1002/ijc.30828 [DOI] [PubMed] [Google Scholar]
- 75. Heining C, Horak P, Uhrig S, Codo PL, Klink B, Hutter B, et al. NRG1 Fusions in KRAS Wild-Type Pancreatic Cancer. Cancer Discovery (2018) 8:1087–95. doi: 10.1158/2159-8290.CD-18-0036 [DOI] [PubMed] [Google Scholar]
- 76. Gröschel S, Hübschmann D, Raimondi F, Horak P, Warsow G, Fröhlich M, et al. Defective Homologous Recombination DNA Repair as Therapeutic Target in Advanced Chordoma. Nat Commun (2019) 10:1635. doi: 10.1038/s41467-019-09633-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lee SH, Kim BC, Chang HJ, Sohn DK, Han KS, Hong CW, et al. Rectal Neuroendocrine and L-Cell Tumors: Diagnostic Dilemma and Therapeutic Strategy. Am J Surg Pathol (2013) 37:1044–52. doi: 10.1097/PAS.0b013e3182819f0f [DOI] [PubMed] [Google Scholar]
- 78. Agoff SN, Lamps LW, Philip AT, Amin MB, Schmidt RA, True LD, et al. Thyroid Transcription Factor-1 Is Expressed in Extrapulmonary Small Cell Carcinomas But Not in Other Extrapulmonary Neuroendocrine Tumors. Mod Pathol (2000) 13:238–42. doi: 10.1038/modpathol.3880044 [DOI] [PubMed] [Google Scholar]
- 79. Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, et al. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients With Metastatic Neuroendocrine Midgut Tumors: A Report From the PROMID Study Group. J Clin Oncol (2009) 27:4656–63. doi: 10.1200/JCO.2009.22.8510 [DOI] [PubMed] [Google Scholar]
- 80. Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, et al. Lanreotide in Metastatic Enteropancreatic Neuroendocrine Tumors. N Engl J Med (2014) 371:224–33. doi: 10.1056/NEJMoa1316158 [DOI] [PubMed] [Google Scholar]
- 81. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med (2017) 376:125–35. doi: 10.1056/NEJMoa1607427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kong G, Grozinsky-Glasberg S, Hofman MS, Akhurst T, Meirovitz A, Maimon O, et al. Highly Favourable Outcomes With Peptide Receptor Radionuclide Therapy (PRRT) for Metastatic Rectal Neuroendocrine Neoplasia (NEN). Eur J Nucl Med Mol Imaging (2019) 46:718–27. doi: 10.1007/s00259-018-4196-8 [DOI] [PubMed] [Google Scholar]
- 83. Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, et al. RAD001 in Advanced Neuroendocrine Tumours, Everolimus for the Treatment of Advanced, Non-Functional Neuroendocrine Tumours of the Lung or Gastrointestinal Tract (RADIANT-4): A Randomised, Placebo-Controlled, Phase 3 Study. Lancet (2015) 387:968–77. doi: 10.1016/S0140-6736(15)00817-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Carvalho S, Vítor AC, Sridhara SC, Martins FB, Raposo AC, Desterro JM, et al. SETD2 is Required for DNA Double-Strand Break Repair and Activation of the P53-Mediated Checkpoint. Elife (2014) 3:e02482. doi: 10.7554/eLife.02482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pfister SX, Ahrabi S, Zalmas LP, Sarkar S, Aymard F, Bachrati CZ, et al. SETD2-Dependent Histone H3K36 Trimethylation Is Required for Homologous Recombination Repair and Genome Stability. Cell Rep (2014) 7:2006–18. doi: 10.1016/j.celrep.2014.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Park IY, Powell RT, Tripathi DN, Dere R, Ho TH, Blasius TL, et al. Dual Chromatin and Cytoskeletal Remodeling by SETD2. Cell (2016) 166:950–62. doi: 10.1016/j.cell.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: The dataset supporting the conclusions of this article is available on request by contacting the authors. Tables 1 , 2 and Figure 3 build the dataset. Requests to access these datasets should be directed to sprengea@uni-marburg.