Abstract
目的
探讨入院时低CHA2DS2-VASc评分(男性≤1分,女性≤2分)非瓣膜性心房颤动(NVAF)患者心房血栓形成的危险因素。
方法
采用回顾性研究方法收集2009年6月~2019年2月在上海交通大学附属胸科医院行经食管超声心动图检查的NVAF患者临床资料(10 382例)。纳入48例伴有血栓的NVAF患者作为实验组,240例无血栓NVAF患者作为对照组。对两组的基线特征、临床生化指标和超声心动图进行单因素分析及多因素Logistic回归分析,对指标进行Pearson相关分析。
结果
两组研究对象基线资料差异无统计学意义(P > 0.05)。与对照组相比,伴有心房血栓患者的左心房内径增大(P < 0.001),肥厚型心肌病比例更多(P < 0.001),C反应蛋白(P < 0.05)和尿酸(P < 0.001)及红细胞分布宽度标准差(P < 0.001)水平明显增高,差异均有统计学意义。其中左心房内径(P < 0.001)、肥厚型心肌病(P < 0.05)和C反应蛋白(P < 0.05)是影响低评分患者心房血栓形成的独立影响因素。
结论
左心房内径增大、肥厚性心肌病和C反应蛋白升高是低CHA2DS2-VASc评分的NVAF患者心房血栓形成的独立危险因素。临床中对合并上述危险因素的低评分NVAF患者应予积极抗凝,预防卒中发生。
Keywords: 心房颤动, 低CHA2DS2-VASc评分, 心房血栓, 危险因素
Abstract
Objective
To explore the risk factors of atrial thrombosis in patients with nonvalvular atrial fibrillation(NVAF)with low CHA2DS2-VASc scores at admission (≤1 for male and ≤2 for female patients).
Methods
We retrospectively analyzed the clinical data of 10 382 patients with NVAF undergoing transesophageal echocardiography in our hospital from 2009 to 2019, and enrolled 48 NVAF patients with thrombosis as the observation group and another 240 NVAF patients without thrombosis as the control group.The baseline characteristics, biochemical indicators, and echocardiographic findings of the patients were analyzed using univariate analysis, multivariate logistic regression analysis and Pearson correlation analysis.
Results
The baseline data did not differ significantly between the two groups (P > 0.05).Compared with those in the control group, the patients with atrial thrombosis had an increased left atrial diameter (LAD; P < 0.001), a greater likelihood of hypertrophic cardiomyopathy (HCM; P < 0.001), significantly higher levels of C-reactive protein (CRP; P < 0.05) and uric acid (P < 0.001), and greater standard deviation of red blood cell distribution width(RDW-SD; P < 0.001).LAD(P < 0.001), HCM(P < 0.05)and CRP(P < 0.05) were identified as the independent factors affecting the occurrence of atrial thrombosis in patients with low CHA2DS2-VASc scores.
Conclusions
LAD enlargement, HCM, and an elevated CRP level are independent risk factors for atrial thrombosis in NVAF patients with low CHA2DS2-VASc scores.Active anticoagulation therapy should be administered for these patients once these risk factors are detected to prevent the occurrence of stroke.
Keywords: atrial fibrillation, low CHA2DS2-VASc score, atrial thrombosis, risk factors
心房颤动(AF)是世界范围内最常见的一种心律失常, 它是缺血性卒中最重要的危险因素之一, 其主要是由于心房内血栓的形成[1-3]。高达15%的缺血性卒中是由房颤所引起, 而房颤相关的卒中在生存及残障方面的预后最差[4]。尽管现在已经有许多新的抗凝药物及新的治疗理念, 但房颤仍然与患者的发病率与死亡率增加相关[5]。在非瓣膜性房颤(NVAF)患者中, CHA2DS2-VASc评分是对患者进行卒中风险评估的一种有效工具[6]。欧洲心脏病学学会发表的实践指南提出, CHA2DS2-VASc评分≤1的NVAF患者, 为低血栓风险, 不建议进行抗凝治疗。美国心脏协会、美国心脏病学会和美国心律学会发布的心房颤动患者管理指南指出若男性CHA2DS2-VASc评分≥2分, 或女性CHA2DS2-VASc评分≥3分, 建议口服药物进行抗凝治疗[7]。尽管指南中建议低评分患者不进行抗凝治疗, 但是在临床实践中, CHA2DS2-VASc评分较低的NVAF患者如不及时处理, 将会对患者产生严重危害[8]。因此, 仅依靠CHA2DS2-VASc评分系统并不能全面的反映心房颤动患者血栓风险。但目前尚无针对NVAF患者这一亚组中心房血栓风险的专门研究。其他危险因素是否会影响这些NVAF患者中心房血栓的形成仍需进一步探讨。纤维蛋白原水平升高也在左心房和左心耳血栓形成中起着重要作用[9-12]。此外, D-二聚体[13, 14]、左房直径[15-17]、脂蛋白a[9, 18]等因素都与心房颤动患者心房血栓形成相关。但上述研究是针对所有NVAF患者人群, 对低CHA2DS2-VASc评分的房颤患者未独立分析。因此, 我们开展了本项研究, 旨在低CHA2DS2-VASc评分的NVAF患者中, 通过比较形成心房血栓与未形成血栓患者的临床特征、实验室指标以及超声心动图结果, 寻找对血栓形成具有预测价值的指标, 以指导对低CHA2DS2-VASc评分NVAF患者的抗凝治疗。
1. 资料和方法
1.1. 研究对象
收集2009年6月~2019年2月期间在上海交通大学附属胸科医院就诊, 射频消融或电复律前进行过经食管超声心动图检查的10 382例房颤患者的临床资料。实验组纳入标准: 房颤患者; 超声提示心房血栓; 入院初评时CHA2DS2-VASc评分男性≤1分, 女性≤2分(血栓形成时间点是院前); 未进行抗凝治疗或未进行有效抗凝治疗。排除标准: 瓣膜病患者, 包括风湿性心脏病、瓣膜置换术或瓣膜修复后的瓣膜病; 左心耳封堵后或左心耳手术结扎/切除术后患者; 既往经TEE或CT诊断左心房血栓; 伴有心脏介入或手术后相关血栓, 包括房间隔封堵器血栓、心内膜垫缺损后心房血栓等; 伴有脑室/主动脉血栓; 心脏占位性病变(如心房黏液瘤)。从中筛选出了48例伴有心房血栓且入院初评时CHA2DS2-VASc低评分的NVAF作为实验组。同时, 将伴有心房血栓的研究对象按1∶5的比例匹配对照组, 对照组研究对象由通过年龄及性别匹配的同一时间段内低CHA2DS2-VASc评分但无血栓形成的患者组成, 排除标准与上述一致, 共纳入240例。研究中所使用的心房颤动诊断标准及分类基于美国心脏病学会-美国心脏协会和欧洲心脏病学会发表的指南。
1.2. 测量仪器和实验室参数
所有患者在进行射频消融或电复律前均接受了经胸超声心动图(TTE)及TEE检查。采用二维TTE进行心房血栓的检测, 同时对左心耳(LAA)进行了二次谐波模式(发射频率1.7~2.0 MHz, 接收频率3.4~4.0 MHz)的详细评估。使用尖端2腔视图可以更好地观察LAA, 有时可以稍微顺时针旋转探针。当LAA显示不理想或需要检测最佳LAA心内膜边界时, 使用改良的心尖5腔视图和/或主动脉瓣水平胸骨旁短轴视图。我们进一步使用经食管超声心动图来确定房颤血栓的存在, 使用5~7 MHz多平面换能器在食管中段位置对LAA进行二维TEE检测, 并在监视器中央获取LAA图像。扫描平面由0°进行性旋转至180°, 以寻找心房内血栓。血栓超声表现为明确的肿块, 在心房体或LAA内表现回声反射, 与底层心内膜不同, 可在多个显像平面显影, 不累及梳状肌, 方可诊断为血栓。研究所得参数由两名独立调查人员以盲法进行分析, 任何测量差异均通过协商解决。
研究对象所有血液样本, 包括血常规、凝血常规、脑利钠肽(BNP)、肝肾功能等实验室指标, 均由操作熟练的护士在病房中使用无菌注射器由肘前静脉采集, 在确保取样位置不出现淤血的同时顺畅引流至EDTA和柠檬酸三钠管中, 同时确保实验人员对临床取样过程不知情。
CHA2DS2-VASc评分包括充血性心力衰竭(1分)、高血压(1分)、年龄75岁以上(2分)、糖尿病(1分)及既往脑卒中(2分)、年龄65~74岁(1分)、女性(1分)及血管疾病(1分)。所有患者均严格按标准进行评估, 并计算分数。
1.3. 统计学分析
正态分布连续变量以均数±标准差表示, 采用独立样本t检验进行检验。非正态分布连续变量用中位数和四分位数表示, 采用Mann-Whitney-U检验进行比较检验。分类变量以数字和百分比表示, 采用χ2进行检验。使用多因素logistic回归分析来确定心房和左心耳血栓形成的独立预测因素, 将单变量分析中P < 0.05的变量纳入模型。同时建立受试者工作特征(ROC)曲线, 评估预测心房和左心耳血栓形成的各项指标最佳截断值。双尾P < 0.05为差异有统计学意义。
2. 结果
2.1. 研究对象基线特征
研究对象均未进行抗凝治疗或未进行有效抗凝治疗。所有研究对象中, 其中高血压患者72例(25.0%)、糖尿病8例(2.8%)、冠心病3例(1.0%)、肥厚性心肌病25例(8.7%)、既往进行过消融术患者21例(7.3%)、左房/LAA血栓形成47例、右房血栓形成1例。与对照组相比, 伴有血栓形成患者的NYHA功能等级较差, 既往持续性房颤病史者较多, 更高HCM患病率(P < 0.001)。而其他特征如年龄、性别、CHA2DS2-VASc评分及既往病史(糖尿病、卒中、冠状动脉疾病)差异无统计学意义(P > 0.05, 表 1)。
1.
实验组及对照组患者临床基线特征对比
Comparison of baseline clinical characteristics between the experimental group and the control group
| Variable | Total group n=288 |
Low score group n=48 |
Control group n=240 |
P |
| Data are expressed as Mean ± SD or as number; AF: Atrial fibrillation; NYHA: New York heart association; HCM: Hypertrophic cardiomyopathy. | ||||
| Age (year) | 56.96±8.446 | 57.77±8.159 | 56.80±8.510 | 0.468 |
| Gender male (n) | 197 | 34 | 163 | 0.692 |
| AF duration (month) | 40.636±56.608 | 44.849±42.120 | 39.794±59.118 | 0.573 |
| AF type | 0.012 | |||
| Paroxysmal | 86 | 7 | 79 | |
| Persistent | 202 | 41 | 161 | |
| NYHA class | < 0.001 | |||
| Ⅰ-Ⅱ | 271 | 36 | 235 | |
| Ⅲ-Ⅳ | 17 | 12 | 5 | |
| Hypertension | 72 | 6 | 66 | 0.028 |
| Diabetes | 8 | 1 | 7 | 748 |
| HCM | 25 | 17 | 8 | < 0.001 |
| Stroke | 0 | 0 | 0 | |
| Thrombosis location | ||||
| Left atrium | 47 | 47 | 0 | |
| Right atrium | 1 | 1 | 0 | |
| CHA2DS2-VASc score | 0.09 | |||
| 0 point | 85 | 8 | 77 | |
| 1 point | 141 | 29 | 112 | |
| 2 point | 62 | 11 | 51 | |
| Coronary heart disease | 3 | 1 | 2 | 0.436 |
| Previous ablation | 21 | 8 | 17 | 0.057 |
| Previous surgery | 9 | 7 | 2 | 0.818 |
2.2. 临床特征
研究人群的临床特征见表 2。从凝血指标来看, 发生血栓患者的血浆纤维蛋白原和D-二聚体水平升高, 差异具有统计学意义(P < 0.05)。与对照组相比, 血栓形成组房颤患者血尿素、肌酐、尿酸水平更高, BNP、GHb、CRP、中性粒细胞、RDE-SD水平在两组间也有显著性差异, 其中尿酸、CRP及RDE-SD显著升高, 差异具有统计学意义(P均 < 0.05)。血栓组超声心动图表现较对照组差, 多数超声指标如左心房直径(LAD)、左心室舒张末期尺寸(LVDd)、左心室射血分数(LVEF)与对照组比较有显著性差异(其中LAD及LEVFP < 0.001)。
2.
临床特征及心脏超声测量结果
Comparison of clinical characteristics and echocardiographic measurements between the two groups(Mean±SD)
| Variable | Total group n=288 |
Low score group n=48 |
Control group n=240 |
P |
| Data are expressed as Mean±SD; PT: Prothrombin time; INR: International normalized ratio; APTT: Partially activated thromboplastin time; TT: Thrombin time; GHb: Glycated hemoglobin; CRP: C-reaction protein. | ||||
| PT (s) | 12.85±3.89 | 13.77±4.77 | 12.67±3.68 | 0.143 |
| INR | 1.07±0.32 | 1.15±0.39 | 1.06±0.31 | 0.135 |
| APTT (s) | 29.68±21.37 | 29.45±6.33 | 29.73±23.17 | 0.937 |
| TT (s) | 20.60±7.86 | 20.07±7.68 | 20.70±7.91 | 0.622 |
| Fibrinogen (g/L) | 2.44±0.57 | 2.65±0.69 | 2.40±0.53 | 0.005 |
| D-dimer (mg/L) | 0.42±0.68 | 0.78±0.84 | 0.36±0.62 | 0.003 |
| Urea (mmol/L) | 5.98±1.53 | 6.54±2.04 | 5.87±1.38 | 0.033 |
| Creatinine (μmol/L) | 72.84±18.18 | 84.52±26.17 | 70.50±15.14 | 0.001 |
| Uric acid (μmol/L) | 384.91±109.37 | 453.13±127.42 | 371.27±100.25 | < 0.001 |
| ALT (U/L) | 29.46±27.01 | 36.52±44.89 | 28.05±21.64 | 0.207 |
| AST (U/L) | 26.63±22.54 | 33.29±33.77 | 25.30±19.36 | 0.118 |
| BNP (pg/mL) | 165.04±223.27 | 493.31±420.88 | 118.76±123.28 | < 0.001 |
| GHb (g/L) | 5.91±0.98 | 6.65±1.85 | 5.81±0.75 | 0.025 |
| CRP (mg/L) | 2.31±6.00 | 6.55±13.21 | 1.72±3.80 | 0.035 |
| Neutrophil (×109/L) | 4.19±1.58 | 4.81±2.34 | 4.07±1.37 | 0.048 |
| Lymphocyte (×109/L) | 1.88±1.72 | 2.36±4.00 | 1.79±0.66 | 0.344 |
| Neutrophil percentage (%) | 64.11±9.24 | 64.67±10.60 | 64.00±8.97 | 0.647 |
| RBC (×1012/L) | 4.81±0.51 | 4.74±0.53 | 4.82±0.51 | 0.300 |
| Hemoglobin (g/L) | 147.68±15.49 | 145.98±15.87 | 148.03±15.42 | 0.404 |
| RDW (%) | 15.36±26.60 | 20.53±46.13 | 14.39±21.05 | 0.386 |
| RDW-SD (fL) | 42.80±3.80 | 44.79±3.56 | 42.42±3.73 | < 0.001 |
| PLT (×109/L) | 195.10±54.66 | 168.92±67.05 | 200.33±50.39 | 0.003 |
| PDW (%) | 14.77±2.01 | 15.31±2.13 | 14.67±1.97 | 0.041 |
| MPV (fL) | 11.14±4.75 | 11.14±1.01 | 11.13±5.19 | 1.000 |
| Aorta (mm) | 32.50±3.27 | 32.92±3.65 | 32.41±3.19 | 0.330 |
| LVDs (mm) | 30.68±7.07 | 38.33±11.2 | 29.15±4.61 | < 0.001 |
| LVDd (mm) | 48.19±6.37 | 53.60±8.58 | 47.10±5.21 | < 0.001 |
| LA (mm) | 42.01±6.48 | 49.42±5.82 | 40.53±5.52 | < 0.001 |
| IVS (mm) | 9.83±2.05 | 10.79±2.65 | 9.64±1.86 | 0.006 |
| LVPD (mm) | 9.07±0.98 | 9.52±1.11 | 8.98±0.93 | 0.003 |
| PA (mm) | 23.54±1.89 | 25.40±2.66 | 23.18±1.44 | < 0.001 |
| LVEF (%) | 60.14±8.22 | 50.92±13.39 | 61.98±5.04 | < 0.001 |
| LVEDV (mL) | 99.50±34.06 | 126.54±53.34 | 94.21±25.86 | < 0.001 |
| SV (mL) | 58.91±13.75 | 59.46±14.25 | 58.81±13.68 | 0.766 |
2.3. 诱发因素
2.3.1. ROC曲线
ROC曲线分析显示高血压及左室射血分数与心房血栓的形成无关, 而LAD、HCM、尿酸、RDE-SD、CRP水平与心房血栓显著相关(与血栓事件没有显著相关的参数和与所列类型相同的参数不包括在内)。ROC曲线分析显示, LAD(曲线下面积为0.878, 95%置信区间: 0.827~0.928, P < 0.0001)、CRP(已剔除部分差异过大的病例, 曲线下面积为0.729, 95%置信区间: 0.627~0.832, P < 0.0001)和RDE-SD(曲线下面积为0.704, 95%置信区间: 0.617~0.790, P < 0.0001)的相关性最好。左房内径 > 43.5 mm与心房血栓形成相关, 敏感性为84.4%, 特异性为74.7%。同样, CRP > 1.94 mg/L与心房血栓密切相关, 敏感性为61.3%, 特异性为83.9%。RDE-SD > 为44.85 mg/L与心房血栓相关, 敏感性为53.3%, 特异性为81.0%(图 1)。
1.

ROC curves of C-reactive protein and left atrium diameter for predicting atrial thrombosis.
左房直径C-反应蛋白ROC曲线
2.3.2. 多因素分析
分析结果显示LAD(优势比: 1.2;95%置信区间: 1.1~1.3), HCM(优势比: 3.0;95%置信区间: 1.4~6.5)和CRP(优势比: 1.1;95%置信区间: 1.0~1.1)与心房血栓显著相关(表 3)。
3.
心房血栓的独立预测因素
Independent predictors of atrium thrombosis
| Baseline Characteristic | Univariate | Multivariate | |||
| HR (95% CI) | P | HR (95% CI) | P | ||
| LAD | 7.16-10.61 | < 0.001 | 1.15-1.34 | < 0.001 | |
| Cardiac disease | 0.21-0.61 | < 0.001 | 1.40-6.45 | 0.005 | |
| uric acid | 42.84-120.16 | < 0.001 | 0.99-1.01 | 0.554 | |
| RDE-SD | 1.10-3.39 | < 0.001 | 0.99-1.29 | 0.081 | |
| C-reactive protein | 0.25-6.75 | 0.035 | 1.00-1.12 | 0.049 | |
2.3.3. Pearson相关分析
CRP与左房直径弱相关(r=0.166, P < 0.01, 图 2)。
2.
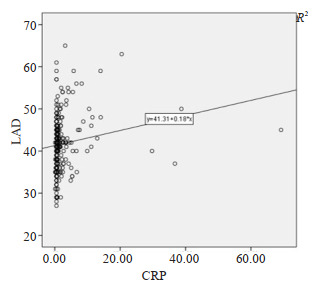
Scatter plot of LAD and C-reactive protein.
LAD与C反应蛋白散点图
3. 讨论
房颤与缺血性卒中风险增加相关, 但血栓形成背后的具体机制仍未完全了解[6]。既往指南提出不建议CHA2DS2-VASc评分低的NVAF进行抗凝治疗。然而, 临床实践中我们发现入院时低评分患者也出现了心房血栓[19], 提示目前CHA2DS2-VASc评分系统的存在假阴性率。因此, 有必要确定低评分患者除此之外的危险因素, 对患者血栓风险进行更全面的评估及预测, 以对患者进行更好的用药指导及管理。既往的临床研究提示, 部分临床危险因素, 如高血压、糖尿病、年龄增加等, 可以预测房颤患者的血栓形成[4]。在本研究中, 实验组患者也存在相关危险因素, 但通过分析研究, 排除了上述因素在此类患者中的相关性。本研究中, 两组研究对象在年龄、糖尿病和冠状动脉疾病方面无差异, 同时高血压与心房血栓的形成无关。值得注意的是, 血栓组的NYHA功能等级较差, 左心房直径较大, 有心肌病病史的患者较多。肥厚性心肌病是心房颤动患者血栓栓塞的危险因素之一[20, 21]。这可能与HCM患者心房直径增大、心力衰竭有关。无CHA2DS2-VASc卒中危险因素的非瓣瓣性房颤HCM患者的卒中风险与无HCM且CHA2DS2-VASc评分为3的患者相似[20]。房颤在HCM中很常见, 并伴有高的血栓栓塞风险[21]。合并HCM的房颤患者左心房血栓发生率高于未合并HCM的房颤患者, 评分为0~1的患者仍可发生左房血栓形成[22]。这些实验结论均与本研究中结果一致。因此, 正如指南所推荐的, 无论CHA2DS2-VASc评分如何, 对于伴有HCM的心房颤动患者, 抗凝治疗都是必要的。
左心房直径增大与房颤的发生、复发甚至死亡相关[23-26]。然而, LAD是否会增加房颤患者的血栓栓塞风险仍有争议。有研究认为左心房直径增大不是心房颤动患者血栓形成的危险因素, 与卒中无关[27, 28]。但最近研究发现在LAD > 45 mm的房颤患者中, 卒中的发生率更高, 而左房增大是NVAF患者卒中和全身栓塞的独立预测因素[29]。然而, 在该项研究中, 左房增大组研究对象年龄较大, CHA2DS2/CHA2DS2-VASc评分较高, 且口服抗凝药物的使用也更为普遍, 这些缺陷使其结论具有局限性。因此, 左房增大是否为卒中的危险因素仍需进一步证实。在本研究中, 我们确认左心房扩张是低CHA2DS2-VASc的NVAF患者血栓形成的独立危险因素, 即使校正了其他混杂因素, 结果提示左房内径 > 43.5 mm与心房血栓相关, 敏感性为84.4%, 特异性为74.7%。因此, 左心房增大可增加NVAF患者血栓形成的风险。这可能与血流动力学障碍、左心耳血流速度降低、左心室顺应性下降、血管内皮功能障碍、凝血系统过度激活有关[30, 31]。总的来说, 左心房大小在评估低评分心房颤动患者血栓形成风险方面可能提供重要的信息。
房颤患者中的CRP较窦性心律患者高[32, 33]。心房颤动持续时间较长与较高的CRP水平和较大的左心房尺寸有关, 支持了心房颤动负担、炎症和结构重构之间存在的联系[34, 35]。尽管炎症和房颤之间存在联系, 但关键问题在于房颤中观察到的炎症反应是否像动脉粥样硬化过程中炎症反应那样会增加血栓栓塞的风险[35]。血浆IL-6和CRP的升高与心房颤动患者血栓前状态指标相关[32]。后续研究也表明, IL-6可以独立预测房颤的血管事件和卒中的发生, CRP与左心房或LAA的自发回声增强相关, 而后者是心房颤动卒中和血栓形成的独立预测因子[33, 36]。这些结论进一步得到了Aulin等人研究的支持和补充, 他们发现CRP和IL-6与房颤患者心肌梗死、血管死亡和血栓栓塞事件的复合风险独立相关[37]。值得注意的是, 炎症和CRP可能通过单核细胞产生的组织因子影响血栓前状态而回声增强[34, 38]。在本研究中, 即使校正了其他参数指标, CRP仍是低评分心房颤动患者血栓形成的独立危险因素。因此, 炎症、心房颤动和血栓形成之间似乎存在一定的联系。但是它们之间的因果关系尚不完全清楚, 仍需要进一步的研究与验证。但无论如何, 对于CHA2DS2-VASc评分较低的心房颤动患者, 仍有评估炎症指标的必要性, 从而更好地评估血栓形成的风险。
本研究创新之处在于: 临床实践中CHA2DS2-VASc评分较低的NVAF患者仍然存在心房血栓风险, 仅依靠CHA2DS2-VASc评分系统并不能全面的反映心房颤动患者血栓风险。本研究首次针对低评分NVAF患者这一亚组中心房血栓的风险进行了研究, 提出左心房内径、肥厚性心肌病和CRP白与血栓形成风险独立相关, 可用于低评分心房颤动患者血栓形成风险的补充评估指标。本研究的不足之处: 这项回顾性研究所使用的的横断面设计不允许我们检查炎症的时间历程并将其与随后的临床结果联系起来。此外, 我们通过评估血浆CRP水平来评估炎症而没有定量其他炎症标记物, 如白细胞介素-6、p-选择素或组织因子等。同时我们研究所使用的样本量相对较小, 因此该结果和结论需要在更大的随机对照试验中得到进一步证实。
综上所述, 本研究回顾性分析了低CHA2DS2-VASc评分患者临床资料, 聚焦于低评分患者形成心房血栓的危险因素。结果显示, 在CHA2DS2-VASc评分低的NVAF中, 左心房内径、肥厚性心肌病和CRP与血栓形成风险独立相关。这些发现表明, 虽然既往指南等认为低评分的患者血栓形成的风险较小, 肥厚性心肌病、左心房功能和炎症状态并非严格相关, 但它们在这些个体中构成了3个主要的、独立的血栓形成的危险因素。结合既往研究及本研究结果, 器质性心脏病, 炎症, 功能指数应该用以评估CHA2DS2-VASc分值较低的房颤患者, 从而准确地量化血栓形成的风险, 正确指导患者使用抗凝剂, 同时预防栓塞并发症。
Biography
周萌萌,在读硕士研究生,E-mail: zhoumm0229@163.com
Funding Statement
国家自然科学基金(81970276);上海申康医院发展中心医院临床科技创新项目(SHDC12018X02)
Supported by National Natural Science Foundation of China (81970276)
Contributor Information
周 萌萌 (Mengmeng ZHOU), Email: zhoumm0229@163.com.
赵 亮 (Liang ZHAO), Email: zhaoliang80112@126.com.
References
- 1.Sheikh A, Patel NJ, Nalluri N, et al Trends in hospitalization for atrial fibrillation: epidemiology, cost, and implications for the future. Prog Cardiovasc Dis. 2015;58(2):105–16. doi: 10.1016/j.pcad.2015.07.002. [Sheikh A, Patel NJ, Nalluri N, et al. Trends in hospitalization for atrial fibrillation: epidemiology, cost, and implications for the future[J]. Prog Cardiovasc Dis, 2015, 58(2): 105-16.] [DOI] [PubMed] [Google Scholar]
- 2.Zimetbaum P Atrial fibrillation. Ann Intern Med. 2017;166(5):ITC33–48. doi: 10.7326/AITC201703070. [Zimetbaum P. Atrial fibrillation[J]. Ann Intern Med, 2017, 166(5): ITC33-48.] [DOI] [PubMed] [Google Scholar]
- 3.Chung MK, Eckhardt LL, Chen LY, et al Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American heart association. http://d.wanfangdata.com.cn/periodical/11927f2db649ef0b01d1a8002075d6d2. Circulation. 2020;141(16):e750–72. doi: 10.1161/CIR.0000000000000748. [Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American heart association[J]. Circulation, 2020, 141(16): e750-72.] [DOI] [PubMed] [Google Scholar]
- 4.Wolf PA, Abbott RD, Kannel WB Atrial fibrillation as an independent risk factor for stroke: the framingham study. Stroke. 1991;22(8):983–8. doi: 10.1161/01.STR.22.8.983. [Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the framingham study[J]. Stroke, 1991, 22(8): 983-8.] [DOI] [PubMed] [Google Scholar]
- 5.Lamassa M, di Carlo A, Pracucci G, et al Characteristics, outcome, and care of stroke associated with atrial fibrillation in europe: data from a multicenter multinational hospital-based registry(The European Community Stroke Project) Stroke. 2001;32(2):392–8. doi: 10.1161/01.STR.32.2.392. [Lamassa M, di Carlo A, Pracucci G, et al. Characteristics, outcome, and care of stroke associated with atrial fibrillation in europe: data from a multicenter multinational hospital-based registry(The European Community Stroke Project)[J]. Stroke, 2001, 32(2): 392-8.] [DOI] [PubMed] [Google Scholar]
- 6.Pistoia F, Sacco S, Tiseo C, et al The epidemiology of atrial fibrillation and stroke. Cardiol Clin. 2016;34(2):255–68. doi: 10.1016/j.ccl.2015.12.002. [Pistoia F, Sacco S, Tiseo C, et al. The epidemiology of atrial fibrillation and stroke[J]. Cardiol Clin, 2016, 34(2): 255-68.] [DOI] [PubMed] [Google Scholar]
- 7.Kirchhof P, Benussi S, Kotecha D, et al 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–962. doi: 10.1093/eurheartj/ehw210. [Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS[J]. Eur Heart J, 2016, 37(38): 2893-962.] [DOI] [PubMed] [Google Scholar]
- 8.January CT, Wann LS, Calkins H, et al 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation. 2019;140(2):e125–51. doi: 10.1161/CIR.0000000000000665. [January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons[J]. Circulation, 2019, 140(2): e125-51.] [DOI] [PubMed] [Google Scholar]
- 9.Ding WY, Protty MB, Davies IG, et al Relationship between lipoproteins, thrombosis and atrial fibrillation. Cardiovasc Res. 2021 doi: 10.1093/cvr/cvab017.Onlineaheadofprint. [Ding WY, Protty MB, Davies IG, et al. Relationship between lipoproteins, thrombosis and atrial fibrillation[J]. Cardiovasc Res, 2021: doi:10.1093/cvr/cvab017.Onlineaheadofprint.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murugesan V, Pulimamidi VK, Rajappa M, et al Elevated fibrinogen and lowered homocysteine-vitamin determinants and their association with left atrial Thrombus in patients with rheumatic mitral Stenosis. Br J Biomed Sci. 2015;72(3):102–6. doi: 10.1080/09674845.2015.11666804. [Murugesan V, Pulimamidi VK, Rajappa M, et al. Elevated fibrinogen and lowered homocysteine-vitamin determinants and their association with left atrial Thrombus in patients with rheumatic mitral Stenosis[J]. Br J Biomed Sci, 2015, 72(3): 102-6.] [DOI] [PubMed] [Google Scholar]
- 11.Bartus K, Litwinowicz R, Natorska J, et al Coagulation factors and fibrinolytic activity in the left atrial appendage and other heart Chambers in patients with atrial fibrillation: is there a local intracardiac prothrombotic state?(HEART-CLOT study) http://www.sciencedirect.com/science/article/pii/s0167527319340173. Int J Cardiol. 2020;301(2):103–7. doi: 10.1016/j.ijcard.2019.09.053. [Bartus K, Litwinowicz R, Natorska J, et al. Coagulation factors and fibrinolytic activity in the left atrial appendage and other heart Chambers in patients with atrial fibrillation: is there a local intracardiac prothrombotic state?(HEART-CLOT study)[J]. Int J Cardiol, 2020, 301(2): 103-7.] [DOI] [PubMed] [Google Scholar]
- 12.Meus R, Son M, Sobczyk D, et al Prothrombotic state in patients with a left atrial appendage Thrombus of unknown origin and cerebrovascular events. Stroke. 2016;47(7):1872–8. doi: 10.1161/STROKEAHA.116.012856. [Meus R, Son M, Sobczyk D, et al. Prothrombotic state in patients with a left atrial appendage Thrombus of unknown origin and cerebrovascular events[J]. Stroke, 2016, 47(7): 1872-8.] [DOI] [PubMed] [Google Scholar]
- 13.Almorad A, Ohanyan A, Pintea Bentea G, et al D-dimer blood concentrations to exclude left atrial Thrombus in patients with atrial fibrillation. Heart. 2021;107(3):195–200. doi: 10.1136/heartjnl-2020-317612. [Almorad A, Ohanyan A, Pintea Bentea G, et al. D-dimer blood concentrations to exclude left atrial Thrombus in patients with atrial fibrillation[J]. Heart, 2021, 107(3): 195-200.] [DOI] [PubMed] [Google Scholar]
- 14.Tarnowski D, Poitz DM, Plichta L, et al Comparison of diverse platelet activation markers as indicators for left atrial Thrombus in atrial fibrillation. Platelets. 2018;29(1):41–7. doi: 10.1080/09537104.2017.1293805. [Tarnowski D, Poitz DM, Plichta L, et al. Comparison of diverse platelet activation markers as indicators for left atrial Thrombus in atrial fibrillation[J]. Platelets, 2018, 29(1): 41-7.] [DOI] [PubMed] [Google Scholar]
- 15.Song ZL, Xu K, Hu XF, et al A study of cardiogenic stroke risk in non-valvular atrial fibrillation patients. http://www.researchgate.net/publication/346798033_A_Study_of_Cardiogenic_Stroke_Risk_in_Non-valvular_Atrial_Fibrillation_Patients. Front Cardiovasc Med. 2020;25(7):604795. doi: 10.3389/fcvm.2020.604795. [Song ZL, Xu K, Hu XF, et al. A study of cardiogenic stroke risk in non-valvular atrial fibrillation patients[J]. Front Cardiovasc Med, 2020, 25(7): 604795.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Çakır OM Low vitamin D levels predict left atrial thrombus in nonvalvular atrial fibrillation. Nutr Metab Cardiovasc Dis. 2020;30(7):1152–60. doi: 10.1016/j.numecd.2020.03.023. [Çakır OM. Low vitamin D levels predict left atrial thrombus in nonvalvular atrial fibrillation[J]. Nutr Metab Cardiovasc Dis, 2020, 30(7): 1152-60.] [DOI] [PubMed] [Google Scholar]
- 17.Lin WD, Xue YM, Liu FZ, et al Left atrial enlargement and non-paroxysmal atrial fibrillation as risk factors for left atrial Thrombus/spontaneous Echo contrast in patients with atrial fibrillation and low CHA2DS2-VASc score. http://www.cnki.com.cn/Article/CJFDTotal-JOGC202003005.htm. J Geriatr Cardiol. 2020;17(3):155–9. doi: 10.11909/j.issn.1671-5411.2020.03.001. [Lin WD, Xue YM, Liu FZ, et al. Left atrial enlargement and non-paroxysmal atrial fibrillation as risk factors for left atrial Thrombus/spontaneous Echo contrast in patients with atrial fibrillation and low CHA2DS2-VASc score[J]. J Geriatr Cardiol, 2020, 17(3): 155-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Igarashi Y, Yamaura M, Ito M, et al Elevated serum lipoprotein(a)is a risk factor for left atrial Thrombus in patients with chronic atrial fibrillation: a transesophageal echocardiographic study. Am Heart J. 1998;136(6):965–71. doi: 10.1016/S0002-8703(98)70151-6. [Igarashi Y, Yamaura M, Ito M, et al. Elevated serum lipoprotein(a)is a risk factor for left atrial Thrombus in patients with chronic atrial fibrillation: a transesophageal echocardiographic study[J]. Am Heart J, 1998, 136(6): 965-71.] [DOI] [PubMed] [Google Scholar]
- 19.Cheng CD, Gu X, Li HX, et al Can men with atrial fibrillation really rest easy with a CHA2DS2-VASc score of 0. BMC Cardiovasc Disord. 2019;19(1):178. doi: 10.1186/s12872-019-1150-z. [Cheng CD, Gu X, Li HX, et al. Can men with atrial fibrillation really rest easy with a CHA2DS2-VASc score of 0[J]? BMC Cardiovasc Disord, 2019, 19(1): 178.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung H, Yang PS, Sung JH, et al Hypertrophic cardiomyopathy in patients with atrial fibrillation: prevalence and associated stroke risks in a nationwide cohort study. Thromb Haemost. 2019;119(2):285–93. doi: 10.1055/s-0038-1676818. [Jung H, Yang PS, Sung JH, et al. Hypertrophic cardiomyopathy in patients with atrial fibrillation: prevalence and associated stroke risks in a nationwide cohort study[J]. Thromb Haemost, 2019, 119(2): 285-93.] [DOI] [PubMed] [Google Scholar]
- 21.Guttmann OP, Rahman MS, O'Mahony C, et al Atrial fibrillation and thromboembolism in patients with hypertrophic cardiomyopathy: systematic review. Heart. 2014;100(6):465–72. doi: 10.1136/heartjnl-2013-304276. [Guttmann OP, Rahman MS, O'Mahony C, et al. Atrial fibrillation and thromboembolism in patients with hypertrophic cardiomyopathy: systematic review[J]. Heart, 2014, 100(6): 465-72.] [DOI] [PubMed] [Google Scholar]
- 22.Cui J, Du X, Wu JH, et al Clinical characteristics of left atrial appendage Thrombus in patients with hypertrophic cardiomyopathy and non-valvular atrial fibrillation. Zhonghua Xin Xue Guan Bing Za Zhi. 2019;47(12):956–62. doi: 10.3760/cma.j.issn.0253-3758.2019.12.003. [Cui J, Du X, Wu JH, et al. Clinical characteristics of left atrial appendage Thrombus in patients with hypertrophic cardiomyopathy and non-valvular atrial fibrillation[J]. Zhonghua Xin Xue Guan Bing Za Zhi, 2019, 47(12): 956-62.] [DOI] [PubMed] [Google Scholar]
- 23.Proietti M, Raparelli V, Basili S, et al Relation of female sex to left atrial diameter and cardiovascular death in atrial fibrillation: The AFFIRM Trial. Int J Cardiol. 2016;207(10):258–63. doi: 10.1016/j.ijcard.2016.01.169. [Proietti M, Raparelli V, Basili S, et al. Relation of female sex to left atrial diameter and cardiovascular death in atrial fibrillation: The AFFIRM Trial[J]. Int J Cardiol, 2016, 207(10): 258-63.] [DOI] [PubMed] [Google Scholar]
- 24.Nakamori S, Ngo LH, Tugal D, et al Incremental value of left atrial geometric remodeling in predicting late atrial fibrillation recurrence after pulmonary vein isolation: a cardiovascular magnetic resonance study. http://www.ncbi.nlm.nih.gov/pubmed/30371333. JAm HeartAssoc. 2018;7(19):e009793. doi: 10.1161/JAHA.118.009793. [Nakamori S, Ngo LH, Tugal D, et al. Incremental value of left atrial geometric remodeling in predicting late atrial fibrillation recurrence after pulmonary vein isolation: a cardiovascular magnetic resonance study[J]. JAm HeartAssoc, 2018, 7(19): e009793.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sardana M, Lessard D, Tsao CW, et al Association of left atrial function index with atrial fibrillation and cardiovascular disease: the Framingham offspring study. J Am Heart Assoc. 2018;7(7):e008435. doi: 10.1161/JAHA.117.008435. [Sardana M, Lessard D, Tsao CW, et al. Association of left atrial function index with atrial fibrillation and cardiovascular disease: the Framingham offspring study[J]. J Am Heart Assoc, 2018, 7(7): e008435.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McManus DD, Yin XY, Gladstone R, et al Alcohol consumption, left atrial diameter, and atrial fibrillation. http://pdfs.semanticscholar.org/a04f/2a6fc875401238916323b7651fa8c3a02d50.pdf. J Am Heart Assoc. 2016;5(9):e004060. doi: 10.1161/JAHA.116.004060. [McManus DD, Yin XY, Gladstone R, et al. Alcohol consumption, left atrial diameter, and atrial fibrillation[J]. J Am Heart Assoc, 2016, 5(9): e004060.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olshansky B, Heller EN, Mitchell LB, et al Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke?Results from the Atrial Fibrillation Follow-Up Investigation of Rhythm Management(AFFIRM)study. J Am Coll Cardiol. 2005;45(12):2026–33. doi: 10.1016/j.jacc.2005.03.020. [Olshansky B, Heller EN, Mitchell LB, et al. Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke?Results from the Atrial Fibrillation Follow-Up Investigation of Rhythm Management(AFFIRM)study[J]. J Am Coll Cardiol, 2005, 45(12): 2026-33.] [DOI] [PubMed] [Google Scholar]
- 28.Broughton ST, O'Neal WT, Salahuddin T, et al The influence of left atrial enlargement on the relationship between atrial fibrillation and stroke. J Stroke Cerebrovasc Dis. 2016;25(6):1396–402. doi: 10.1016/j.jstrokecerebrovasdis.2016.02.004. [Broughton ST, O'Neal WT, Salahuddin T, et al. The influence of left atrial enlargement on the relationship between atrial fibrillation and stroke[J]. J Stroke Cerebrovasc Dis, 2016, 25(6): 1396-402.] [DOI] [PubMed] [Google Scholar]
- 29.Hamatani Y, Ogawa H, Takabayashi K, et al Left atrial enlargement is an independent predictor of stroke and systemic embolism in patients with non-valvular atrial fibrillation. Sci Rep. 2016;6(3):31042. doi: 10.1038/srep31042. [Hamatani Y, Ogawa H, Takabayashi K, et al. Left atrial enlargement is an independent predictor of stroke and systemic embolism in patients with non-valvular atrial fibrillation[J]. Sci Rep, 2016, 6(3): 31042.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim TW, Jung SW, Song IU, et al Left atrial dilatation is associated with severe ischemic stroke in men with non-valvular atrial fibrillation. J Neurol Sci. 2015;354(1-2):97–102. doi: 10.1016/j.jns.2015.05.008. [Kim TW, Jung SW, Song IU, et al. Left atrial dilatation is associated with severe ischemic stroke in men with non-valvular atrial fibrillation[J]. J Neurol Sci, 2015, 354(1-2): 97-102.] [DOI] [PubMed] [Google Scholar]
- 31.Mondillo S, Sabatini L, Agricola E, et al Correlation between left atrial size, prothrombotic state and markers of endothelial dysfunction in patients with lone chronic nonrheumatic atrial fibrillation. Int J Cardiol. 2000;75(2-3):227–32. doi: 10.1016/S0167-5273(00)00336-3. [Mondillo S, Sabatini L, Agricola E, et al. Correlation between left atrial size, prothrombotic state and markers of endothelial dysfunction in patients with lone chronic nonrheumatic atrial fibrillation[J]. Int J Cardiol, 2000, 75(2-3): 227-32.] [DOI] [PubMed] [Google Scholar]
- 32.Conway DS, Buggins P, Hughes E, et al Relationship of interleukin-6 and C-reactive protein to the prothrombotic state in chronic atrial fibrillation. JAm Coll Cardiol. 2004;43(11):2075–82. doi: 10.1016/j.jacc.2003.11.062. [Conway DS, Buggins P, Hughes E, et al. Relationship of interleukin-6 and C-reactive protein to the prothrombotic state in chronic atrial fibrillation[J]. JAm Coll Cardiol, 2004, 43(11): 2075-82.] [DOI] [PubMed] [Google Scholar]
- 33.Conway DS, Buggins P, Hughes E, et al Prognostic significance of raised plasma levels of interleukin-6 and C-reactive protein in atrial fibrillation. Am Heart J. 2004;148(3):462–6. doi: 10.1016/j.ahj.2004.01.026. [Conway DS, Buggins P, Hughes E, et al. Prognostic significance of raised plasma levels of interleukin-6 and C-reactive protein in atrial fibrillation[J]. Am Heart J, 2004, 148(3): 462-6.] [DOI] [PubMed] [Google Scholar]
- 34.Chung MK, Martin DO, Sprecher D, et al C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104(24):2886–91. doi: 10.1161/hc4901.101760. [Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation[J]. Circulation, 2001, 104(24): 2886-91.] [DOI] [PubMed] [Google Scholar]
- 35.Boos CJ, Anderson RA, Lip GY. Is atrial fibrillation an inflammatory disorder[J]?Eur Heart J, 2006, 27(2): 136-49.
- 36.Conway DS, Buggins P, Hughes E, et al Relation of interleukin-6, Creactive protein, and the prothrombotic state to transesophageal echocardiographic findings in atrial fibrillation. Am J Cardiol. 2004;93(11):1368–73. doi: 10.1016/j.amjcard.2004.02.032. [Conway DS, Buggins P, Hughes E, et al. Relation of interleukin-6, Creactive protein, and the prothrombotic state to transesophageal echocardiographic findings in atrial fibrillation[J]. Am J Cardiol, 2004, 93(11): 1368-73.] [DOI] [PubMed] [Google Scholar]
- 37.Aulin J, Siegbahn A, Hijazi Z, et al Interleukin-6 and C-reactive protein and risk for death and cardiovascular events in patients with atrial fibrillation. Am Heart J. 2015;170(6):1151–60. doi: 10.1016/j.ahj.2015.09.018. [Aulin J, Siegbahn A, Hijazi Z, et al. Interleukin-6 and C-reactive protein and risk for death and cardiovascular events in patients with atrial fibrillation[J]. Am Heart J, 2015, 170(6): 1151-60.] [DOI] [PubMed] [Google Scholar]
- 38.Cermak J, Key NS, Bach RR, et al C-reactive protein induces human peripheral blood monocytes to synthesize tissue factor. Blood. 1993;82(2):513–20. doi: 10.1182/blood.V82.2.513.513. [Cermak J, Key NS, Bach RR, et al. C-reactive protein induces human peripheral blood monocytes to synthesize tissue factor[J]. Blood, 1993, 82(2): 513-20.] [DOI] [PubMed] [Google Scholar]


