Abstract
Several lines of evidence point towards the central role of IL-23 as a crucial inflammatory mediator in the pathogenesis of SpA—a group of inflammatory arthritic diseases whose symptoms span the skin, gastrointestinal tract and joints. While therapeutic blockade of IL-23 proved successful in the treatment of IBD, psoriatic skin disease and peripheral SpA, it failed in patients suffering from SpA with predominantly axial involvement. Here we review state-of-the-art discoveries on IL-23 signalling pathways across target tissues involved in SpA. We discuss the discrepancies in resident IL-23–responding cells and their downstream activities across skin, gut and joint that shape the unique immunological landscape of SpA.
Keywords: interleukin 23, spondyloarthritis, psoriasis, inflammatory bowel disease
Rheumatology key messages
The unique composition of tissue-resident and infiltrating immune cells responding to IL-23 across different tissues can account for the divergent physiological effects induced by IL-23 signalling.
Animal studies underline the importance of innate and innate-like immune cells in the early events of IL-23–driven pathology, while findings from patients suggest complex, multidirectional interplay of innate and adaptive pathways in spondyloarthritis.
The kinetics of IL-23 signalling should be addressed to unravel the roles of specific cell populations at different stages of the disease.
The multifaceted nature of SpA
SpA is a group of inflammatory rheumatic arthritides of remarkable clinical heterogeneity. Distinct disease phenotypes are reflected by not only the predominant involvement of axial or peripheral joints, but also the spectrum of other affected organs, spanning from entheses, through skin, nails and gastrointestinal tract to the eye [1]. The search for a common culprit, linking the diverse SpA manifestations, first led to the identification of HLA-B27 as a shared genetic risk factor, followed by the discovery of the IL 23/17 (IL-23/IL-17) axis in immune-mediated inflammatory diseases. Novel therapeutic strategies targeting the key cytokines of the IL-23/IL-17 pathway were developed and employed across the disease spectrum [2]. The clinical trial programs, however, yielded some unexpected surprises that shed new light on the immunobiology of IL-23 signalling in distinct tissues and the pathogenesis of SpA, and urged in-depth research focusing on cellular sources of type 17 cytokines (also known as type 3 immunity of the Th17 response) [3] and upstream pathways leading to IL-17 induction, including their dependency on IL-23 and link with the host–microbiota interaction. While the IL-23/IL-17 blockade was anticipated to effectively tackle all domains of the disease, clinical trials revealed substantial differences in efficacy between disease subsets. These differences are illustrated by the striking lack of response to therapeutic blockade of IL-23 in axial vs peripheral disease and the fact that IL-17 inhibition proved to be efficacious in skin, joint, entheses and spine, while failed or even exacerbated overt IBD.
Regardless of the disease phenotype, we found a high frequency of microscopic gut inflammation in all subsets of SpA, suggesting that the loss of barrier integrity is a common denominator across the entire disease spectrum [4]. Microscopic gut inflammation represents an important risk factor for evolution into full-blown IBD [5]. It has also been linked to SpA disease activity, including the degree of bone marrow oedema in SI joints and structural outcomes [6]. Breach of natural barriers is also exemplified by PsA, a prototypic form of peripheral SpA that usually develops subsequently to skin manifestations [7].
The aforementioned therapeutic discrepancies suggest that distinct mechanisms of immune surveillance could mediate the homeostasis of the varied tissues affected by SpA. This assumption is plausible in view of their divergent functions: while skin and mucosal barriers represent an interface between the external world and the internal immune environment, the primary function of joint tissues is to enable movement. Therefore, it is critically important to demarcate the IL-23/IL-17 pathway in the skin, gut, synovium and entheses of both axial and peripheral joints, including overlapping concepts and differences, and this is the subject of the current review.
IL-23, an IL-12 cytokine family member and its receptor
IL-23 was identified as a member of the IL-12 cytokine family [8]. The IL-12 family cytokines are characteristically composed of an α and a β chain, these chains being uniquely paired across the family members [9]. IL-23 is comprised of the p40 and p19 subunits (IL-23 subunit α), which are shared with IL-12, and a theoretical family member, IL-39, respectively [10]. IL-23 secretion has been reported by all antigen-presenting cells, as well as neutrophils and respiratory and gastrointestinal epithelial and secretory cells [11–14]. The mechanisms of IL-23 secretion regulation, point towards its tight link with host defence and physiological role in barrier tissues homeostasis. Fig. 1 is a schematic representation of IL-23 and IL-12 receptors.
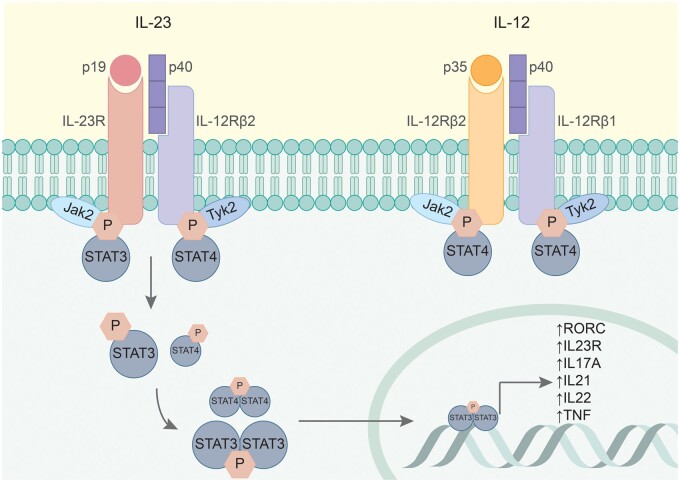
Fig. 1.
IL-23 and IL-12 share the molecular subunit p40 and its receptor IL-12β1, but their signalling cascades proved to exert distinct effects in health and disease
The IL-23 receptor complex is made of the IL-12Rβ1 and the IL-23R transmembrane proteins, with high-affinity binding capacities for the p40 and the p19 cytokine subunits, respectively [21]. Recent studies have shown that cytokine binding occurs in a highly coordinated manner, with the IL-23: IL-23R binary complex as an obligate mechanistic step for the recruitment of the IL-12Rβ1 subunit [22]. The complex has no intrinsic enzymatic activity and is coupled to two Janus-associated kinase (JAK) family members, namely Jak2 and Tyk2. Upon IL-23 binding, receptor complex oligomerization occurs, followed by the phosphorylation and activation cascade of downstream signalling molecules: Jak2, Tyk2 and signal transducer and activator of transcription (STAT) proteins. STAT proteins further undergo dimerization and nuclear translocation, where they bind to the promotor regions of certain immune mediators and transcription factors. IL-23 binding to its receptor preferentially activates STAT3, whereas IL-12 results in predominant STAT4 activation, the STAT proteins engaging different target genes [23]. STAT3 activity upregulates the expression of type 17 immunity signature genes, such as RORC, IL23R, IL17A and IL22.
IL-23 signalling induces the expression of a unique set of inflammatory genes, engaging type 17 immune responses [15]. This includes the retinoic acid receptor-related orphan receptor-γt (RORγt, encoded by the Rorc gene) – a master-regulator of type 17 helper T (Th17) cells [16]. The interaction between IL-23 and RORγt appeared to be bidirectional, as RORγt transcriptional activity is required for the expression of the IL-23 receptor. The IL-23 receptor complex is found on subsets of memory T cells, NKT cells, γδ T cells and innate lymphoid cells (ILCs), but not naïve T cells [16–20]. This finding was initially quite puzzling, given the ascribed role of IL-23 in inducing the distinct type 17 immune phenotype in helper T cells [21]. Consequent studies demonstrated that simultaneous priming with TGF-β, IL-6 and IL-1 activates RORγt, and induces IL-23 receptor expression, enabling further IL-23 signalling [22, 23]. The subsequent IL-23 signal is critical for maturation and stabilization of the proinflammatory Th17 phenotype [21]. Because Th17 cells are crucial for host defence against certain infections such as extracellular microbes and fungi [19, 24], the significance of IL-23 signalling in shaping Th17 effector functions has also been examined in models of both infectious and inflammatory disease. Intriguingly, IL-23 is indispensable for the development of some full-blown immune-mediated inflammation models, but IL-23 blockade did not abolish antimicrobial responses [20, 25]. These observations suggest that Th17 cells activated through different pathways exert distinct functions. These IL-23–independent and IL-23–dependent pathways promote mucosal defence and tissue integrity or reinforce chronic inflammation and even promote autoimmunity, respectively [26].
Th17 cells, however, are not the only cells responding to IL-23 signalling by a potent production of type 17 cytokines. IL-23 receptor–bearing populations of innate-like T cells: gamma delta (γδ), NKT and mucosal-associated invariant T (MAIT) cells, as well as innate-lymphoid cells (ILCs). These cells are typically enriched in the mucosal sites and have been identified in the skin and joint tissues [27–29]. This is an important discovery as innate production of proinflammatory cytokines is rapid and precedes the adaptive IL-17 response. Indeed, innate-like T cells are often referred to as the first line of defence against pathogens, bridging the innate and adaptive arms of immunity. These innate-like cells have been found to be enriched in inflamed tissues of SpA patients, with distinct subpopulations and phenotypes described across affected organs. Moreover, we and others have demonstrated that major production of IL-17 in SpA can be ascribed to innate-like cells [30]. Studies on animal disease models, including pathologies from the SpA spectrum, underpin the plausible crucial role of innate-like T cells in the development of immune-mediated inflammatory diseases [31], while not disregarding the importance of adaptive, proinflammatory cell populations [32].
The IL-23/IL-17 signalling pathway in innate-like T cells bears similarities to what has been found in conventional T cells. Accordingly, IL-17 production occurs both dependent on and independently from IL-23 signalling (via direct activation of innate immunity receptors by microbial stimuli and endogenous mediators as well as T cell receptor activation) [33–35]. In addition to stimulating IL-17 production, IL-23 triggers further differentiation of γδ-T cells into a γδ17 proinflammatory phenotype [36], promotes the expansion of γδ-T and MAIT cells and induces their migratory properties [37, 38]. As in case of Th17 cells, the effector functions of IL-23-responding NKT and γδ-T cells are dependent on RORγt expression [34, 39–41]. The plasticity of unconventional type 17 T cells has been suggested to even exceed this of Th17 cells, raising hope for possible therapeutic application [42]. Other cells representing the innate immune system, namely ILCs type 3 (ILC3s) and epithelial cells, also respond to IL-23 [43]. These cells have mostly been studied in the context of intestinal tissues and will be discussed below.
An interesting feature of the IL-23 signalling cascade is the positive-feedback loop induced in many cell types. The transcription factors activated by the IL-23 receptor complex binding (STAT3, RORγt, Blimp1) enhance the receptor expression, therefore amplifying its own signalling [9, 44, 45]. Moreover, IL-23 binding has been shown to promote IL-23 receptor endocytosis and recycling in macrophages, which also promotes the signalling cascade [46]. Macrophages themselves are a major source of IL-23 in tissues, and interestingly IL-23 levels correlate with enhanced phagocytosis capacities and a proinflammatory phenotype of macrophages [47, 48]. These findings are particularly interesting in the disease context, since genome-wide association studies identified several polymorphisms in genes encoding IL-23 subunits, their receptors and downstream signalling molecules as associated with increased susceptibility to, or reversely, as variants protective from SpA spectrum pathologies: psoriasis, IBD and arthritis [49]. Mechanistically, it has been shown that the SpA pathology-protective IL23R R381Q variant interferes with IL-23 receptor recycling mechanisms [2, 50]. IL-23 overexpression represents a disease model mimicking many features of SpA pathology, pointing to its crucial role in SpA pathology [42]. At the same time, differential effects of IL-23 signalling across cell types suggests that its ultimate effect depends on the local tissue context. Fig. 2 illustrates IL-23 signalling pathways in the tissues affected by SpA.
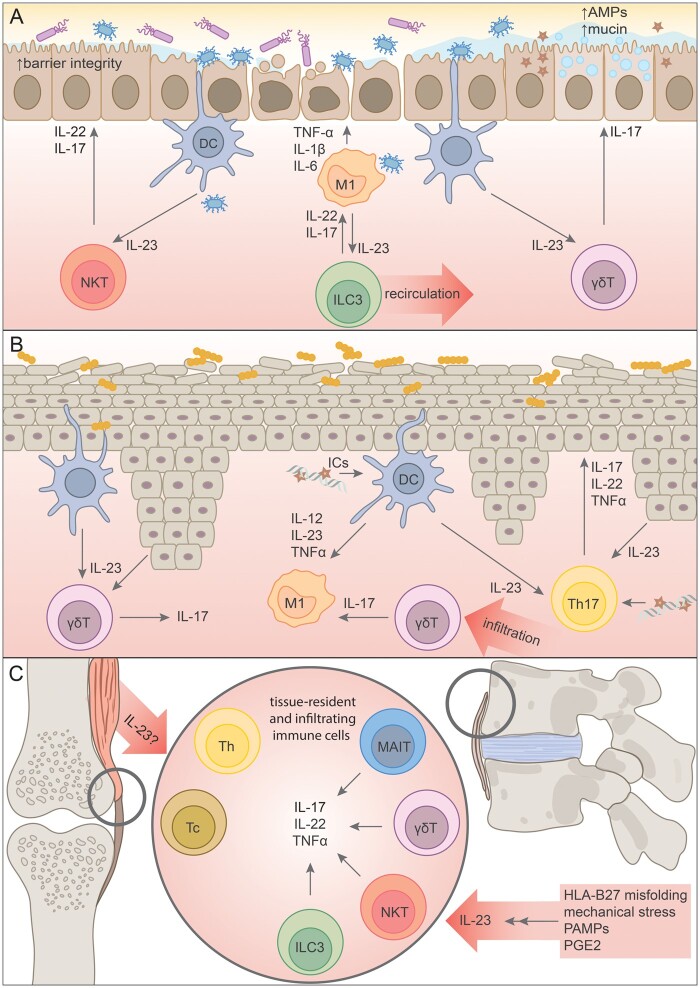
Fig. 2.
Schematic representation of IL-23 signalling across tissues affected by SpA, with a focus on aberrant innate immunity signalling
A—IL-23 signalling in the intestinal mucosa: intestinal dysbiosis is associated with chronic activation of DCs, which abundantly produce IL-23 and activate resident and infiltrating immune cells, including ILC3s, γδT and NKT cells. IL-17 and IL-22 produced by these cells seem to have a favourable effect on intestinal barrier integrity, but are countered by vast amounts of proinflammatory TNFα, IL-1 and IL-6.
B—Psoriatic skin is characterized by elevated levels of IL-23 produced by a plethora of cells, including keratinocytes, an overgrowth of pathogenic bacteria and expansion of proinflammatory macrophages and γδT cells, both resident and tissue-infiltrating. The significance of defective innate immune mechanisms in psoriasis are underlined by the production of antibodies against AMPs and formation of ICs stimulating local antigen-presenting cells. Th17 cells are also highly present in psoriatic skin.
C—Entheses are the soft tissue where ligament, tendon and joint capsules attach to bone. Enthesitis is a pathognomonic symptom of SpA, thought to be dependent on an aberrant IL-23/IL-17 immune axis. Resident populations of innate-like immune cells responding to IL-23 have been identified in healthy entheses, and it has been suggested that they play a critical role in the development of the disease. Adaptive Th and Tc cells producing IL-17 have been identified in SF of PsA patients, but mechanisms for the development of these cell populations are currently unknown.
AMPs: antimicrobial proteins; M1: proinflammatory macrophages; DC: dendritic cells; NKT: NK T cells; ILC3: innate lymphoid cells type 3; γδT: γδ T cells; Th17: type 17 helper T cells; Tc: cytotoxic T cells; MAIT: mucosal-associated invariant T cells; PAMPs: pathogen-associated molecular patterns; PGE2: prostaglandin E2.
Microbial contribution to type 17 immunity in SpA
In view of the IL-23/IL-17 pathway involvement in the host–microbiome crosstalk, defining the role of microbes in the development of SpA became a crucial goal of many studies [51]. Profound intestinal and skin dysbiosis has been reported in patients with SpA, regardless of clinical manifestations, yet it is currently unclear whether this is the cause or consequence of inflammation [52–55]. Indirect microbial signalling has long been associated with different forms of joint pathology: from migratory arthralgia to overt arthritis. Under certain circumstances, particular microbial triggers may induce reactive arthritis, part of the SpA disease spectrum [56]. Studies in animal models further support the role of host microbiota in the development of arthritis, the HLA-B27 transgenic rats being the prototypical example [57]. The Western-type diet, strongly linked to a proinflammatory status and known to induce intestinal dysbiosis, has recently been demonstrated to facilitate and aggravate joint inflammation in a mouse model of SpA [58]. Furthermore, some bacterial strains, such as segmented filamentous bacteria, are notoriously known to boost type 17 responses in mice [59]. It is not yet known if specific bacteria associated with SpA, such as the Dialister genus [53], also preferentially trigger a type 17 immune response like segmented filamentous bacteria.
Moreover, accumulating evidence is challenging the assumption that joints are isolated from direct microbial influence. Microbial DNA and RNA have been detected in the SF of ReA [60, 61], PsA and RA patients [62], and even in the synovial tissue and cartilage of OA individuals [63]. How pathogens and microbial products translocate to the joints, and how this contributes to non-purulent arthritis remains unknown.
IL-23 as gatekeeper of barrier integrity in the gut
The physiology of IL-23 signalling advocates for its orchestrating role in the maintenance of intestinal barrier homeostasis. Elevated levels of IL-23 have been found in the tissues of patients suffering from overt IBD, as well as in patients with subclinical gut inflammation, both pathologies falling within the SpA spectrum [14]. Intestinal inflammation is associated with enhanced gut barrier permeability and increased evasion of pathogen-associated molecular patterns into the host [64]. This leaky gut results in chronic activation of host defence mechanisms, leading to overproduction of IL-23, thought to play a key role in SpA pathogenesis. The chicken-and-egg conundrum of the IL-23 oversignalling and dysbiosis in SpA remains unresolved. While recently it has been proven that intestinal dysbiosis and consequent inflammation in an animal model of SpA is driven by IL-23 signalling [65], other groups point towards dysbiosis as the causative factor. Environmental factors such as diet were shown to have a predominant effect on the gut microbial community composition, and aggravate susceptibility to arthritis and psoriasis in an IL-23 overexpression-based model [58]. Elucidating the intricacies of the host–microbiome interplay in patients is the focus of multiple research groups [51].
Gut epithelial cells possess the IL-23 receptor complex but, unlike the immune cells localized in the intestine, they do not produce IL-17 nor IL-22. Selective blockade of IL-23 signalling in gut epithelial cells did not evoke spontaneous inflammation but led to increased susceptibility to inflammation induced by epithelial integrity loss [66]. This state was characterized by a decrease in IL-22–producing immune cells, impaired wound-healing mechanisms, and overgrowth of flagellated bacteria, pathogens implicated in IBD development [67, 68]. Exogenous administration of IL-23 restored IL-22 production and gut recovery [69]. IL-22 is known to promote intestinal barrier mechanisms by promoting epithelial cell proliferation and production of mucin and antimicrobial peptides [70], yet may also aggravate intestinal inflammation in some models [71]. IL-22 signalling in IBD is distorted, with elevated levels of the cytokine found in patients’ blood and increase in IL-22–producing ILC3s, countered by a concurrent upregulation of the neutralizing IL-22–binding protein [72–74]. Interestingly, increased numbers of IL-22– and IL-17–producing ILC3s were found not only in the gut, but also in the peripheral blood and SF of SpA patients. These cells expressed homing integrin α4β7, suggesting their recirculation from the gut to the inflamed tissues [75]. The increase in proinflammatory ILC3s in the gut was linked to the ability of IL-23–producing macrophages to promote intestinal inflammation [76, 77].
In contrast to successful therapeutic inhibition of IL-23 in animal models of IBD and in the clinic, the blockade of IL-17A (the most studied member of the IL-17 cytokine family, for simplicity referred to throughout the text as IL-17) demonstrates lack of efficacy and even exacerbation of IBD, despite its efficacy in several preclinical models [78–80]. Intriguingly, intestinal microbiota analyses pinpointed substantial differences in anti-IL-17A vs anti-TNF–treated patients, suggesting a putative link in host–microbial interaction [81]. In this context, IL-17 deficiency exacerbated chemical gut inflammation and promoted intestinal dysbiosis followed by Th17 cell expansion [82, 83]. While the development of intestinal Th17 cells has been linked to the presence of pathogenic microbes, expansion of IL-22–producing, IL-23–responding ILC3s was ascribed to endogenous signalling associated with bacterial colonization [84]. In acute intestinal injury, the production of protective IL-17 was attributed to resident γδ-T cells and found to be IL-23–independent [85].
Latest efforts in therapeutics development included the IL-17F family member [the cytokine family member with the highest homology (50%) to IL-17A]. Although an overlap in IL-17A and IL-17F signalling has been observed, with shared use of receptors resulting in the activation of the TNFα pathway, differences in physiology between the two cytokines are apparent [86]. IL17F is constitutively produced by intestinal cells (including activated monocytes, basophils and mast cells, which do not secrete IL-17A), and, unlike IL-17A, bind with greater affinity to the IL-17RC receptor preferentially expressed on non-hematopoietic cells. A recent study showed that this constitutive production is increased in intestinal inflammation, and that IL-17F or IL-17A/IL-17F deficiency is protective against colitis symptoms [87]. However, in this experimental setting, neither the major source of IL-17F, nor the role of IL-23 signalling were defined. Nevertheless, this finding is of particular importance in the light of the advent of dual IL-17A/IL-17F inhibitors, which proved efficacious and safe in PsA and axial SpA patients [88–90]. The phase II study of the dual neutralization in ulcerative colitis, however, was terminated early due to an imbalance of adverse events with no clear evidence of benefits [91]. Initial molecular studies confirm that synchronized blockade of IL-17A and IL-17F holds the promise of increased efficacy in neutralizing inflammation and establishing new therapeutic targets [88, 92].
IL-23 inducers and responders in the skin: gateway to psoriasis
IL-23 signalling also plays a pivotal role in maintaining barrier homeostasis in the skin, the second major interface between host and environment. IL-23 is upregulated in various skin pathologies, most notably psoriasis, exemplified by the remarkable clinical efficacy data. Interestingly, IL-23 serum levels have been shown to negatively correlate with the disease duration, suggesting the cytokine’s role in early events leading to lesion formation [93]. The sources of IL-23 in the skin include keratinocytes, epidermal Langerhans cells, dermal dendritic cells, and macrophages, cells equipped to respond to microbial and endogenous inflammatory stimuli [94]. Dysbiosis of psoriatic skin microbiota has been suggested as central to chronic stimulation of innate immunity mechanisms and provoking increased IL-23 production [95, 96]. Defective host defence mechanisms in psoriasis are illustrated by the observation that an antimicrobial peptide, cathelicidin LL37, serves as an autoantigen in this disease, as well as PsA [97, 98]. These autoantigens have been shown to bind to DNA and RNA and form immunostimulatory complexes, which are consequently delivered to dendritic cell endosomal TLRs [99]. Activation of TLR8 in dendritic cells induces production of IL-12, TNFα and IL-23 [100].
IL-23 promotes Th17 cell differentiation via STAT3-dependent activation of RORγt [101]. Th17 cells are expanded in the lesional psoriatic skin and secrete classical type 17 cytokines—IL-17, IL-22 and TNF α—acting on keratinocytes and local immune cells in a vicious, positive-feedback loop [100]. Studies on animal models of psoriasis confirm the significance of the IL-23–dependent production of type 17 cytokines [102, 103]. Local administration of IL-23 to the skin (intradermal injection) has been validated as a psoriasis model, which mimics many aspects of human disease [104]. This model has been shown to be TLR dependent, and a comparison of skin transcriptomes between mice subjected to intradermal IL-23 injection and human psoriasis patients showed a significant overlap in differentially expressed genes [105]. Moreover, IL-23 signalling has been shown to result in an infiltration with proinflammatory macrophages and CCR6-expressing γδ-T cells [106]. IL-23 produced by Langerhans cells in the Imiquimod-induced skin inflammation activates skin γδ-T cells to produce IL-17 [107], and induction of psoriasis in γδ-T cell–deficient mice was found to be significantly impaired [108]. It is important to note that the mouse γδ-T cell landscape is unique, including a resident epidermal population (dendritic epidermal T cells), disallowing for direct translation of these mechanisms into humans. At the same time, dermal γδ-T cells were expanded in human psoriatic skin and shown to readily response to IL-23 by increased IL-17 production [108]. Infiltration of circulating γδ-T to psoriatic skin has also been indicated [109]. These findings suggest that γδ-T cells could be the major responders to IL-23 in the skin, although the contribution of other cell types, such as the macrophages and tissue resident memory T cells, has also been reported [110]. This includes identification of oligoclonal populations of IL-17–producing αβ T cells in active and resolved psoriatic lesions, outnumbering γδ-T cells [111]. Autoantigen recognition resulting in robust IL-17 production has also been reported [112], proving that the complex interplay of innate and adaptive, local and infiltrating cell populations remains to be unravelled.
Innate-like T cells as sensors of IL-23 signalling in joints
In contrast to the gastrointestinal membrane and skin (both interfaces of the vast host–microbiome interplay), joint tissues were long considered to be non-immune organs free from microbial stimuli. The emerging roles of tissue-resident immune cells in the joints is challenging this presumption, suggesting that the synovium could in fact constitute an important barrier [113]. Moreover, microbial metabolic products and nucleic acids were found in the circulation and in the joints of non-purulent arthritis patients [54, 62]. Potential translocation of intracellular pathogens targeting immune cells has been suggested, as exemplified by Whipple’s disease, a multisystemic pathology with arthritis and features of spondylodiscitis [114]. Trafficking of intracellular microbes into the joints within lymphoid cells or monocytes has also been shown in ReA [115, 116]. Furthermore, in a mouse model of ReA, dissemination of intracellular bacteria (Chlamydia) was linked to IL-23–mediated joint pathology [116]. Upregulated levels of chemokines have been found on immune cells circulating in the peripheral blood of SpA patients, but whether this was due to intracellular pathogen invasion remains to be determined. Local triggers of IL-23 production, whose levels are upregulated in inflamed joints of SpA and RA patients, remain less evident than in other SpA-affected tissues. Immune cells circulating between the gut and the joints were suggested as one possible source of IL-23 in SpA [75, 117]. Alternatively, the propensity of HLA-B27 heavy chain to misfold, followed by activation of innate immune responses and IL-23 production by myeloid cells was also suggested [118]. PGE2, which represents an endogenous inflammatory stimulus and a classical target of NSAIDs, also upregulates myeloid cell IL-23 production [119]. IL-23 and IL-17 have been shown to exert a profound effect on bone cells, resulting in systemic bone loss and entheseal bone formation. Mechanisms in which these cytokines induce SpA skeletal features have been reviewed in detail by Gravallese et al. [120].
The role of IL-23 in SpA was boosted by the discovery of resident immune cells responsive to IL-23 in the murine enthesis—the area of soft tissue where ligament, tendon and joint capsules attach to bone [27, 121]. Overexpression of IL-23 in susceptible mouse strains results in enthesitis, a hallmark of SpA [27]. Initiation of SpA inflammation in the joints, however, was shown to be independent of Th17 adaptive responses, but rather relying on unconventional innate-like T cells, later identified as entheseal γδ-T cells [27, 121]. They express high levels of the IL-23 receptor and can produce large quantities of IL-17 and IL-22 in an RORγt-dependent manner [122]. An analogous γδ-T cell population has recently been isolated from non-inflamed human entheses [123]. IL-17–producing subpopulations of innate-like T cells, including γδ-T, NKT, MAIT cells and ILC3s were furthermore found to be expanded in the joints and circulation of SpA patients [28, 75, 122]. Recently, a population of resident spinal entheseal γδT-cells capable of IL-23–independent IL-17 production has also been described in healthy, non-inflamed spinal entheses, suggesting these cells may have important homeostatic roles [124].
The observation that innate-like T cells such as γδ-T cells are potent producers of IL-17 in human joints affected by the SpA pathology, coupled with their ability to do this in the presence or absence of IL-23, is of major interest in view of the differential response of axial vs peripheral disease to IL-23 blockade, whereas both respond equally well to anti-IL-17 [125]. MAIT cells in the blood of healthy subjects were shown to produce IL-17F, rather than IL-17A, upon IL-12 and IL-18 signalling [35]. In the blood and peripheral joints of SpA patients, MAIT cells have been shown to acquire an exaggerated type 17 phenotype in response to activation with IL-7 [28], a pathway also described in IL-17–producing γδ-T cells and in the development of ILCs [126, 127]. The upstream signals in the spine leading to IL-17 induction at present are still unclear and have been suggested to include other cytokines such as IL-39, although direct evidence for such pathways is currently lacking [128].
The role of the adaptive arm of the immune system in the production of proinflammatory mediators within the joint, IL-17 in particular, cannot be overlooked. Expansion of IL-17–producing Th and cytotoxic T (Tc) cells has been shown in the joints of patients suffering from RA and PsA [129–131]. These cells were described to have a highly proinflammatory phenotype, associated with IL-23–driven maturation [132]. Innate immunity triggers ultimately affect adaptive immune responses. Although HLA-B27 has mostly been associated with autoinflammation, arthritis-developing, HLA-B27 transgenic rats display expansion of Th17 cells and dysbiosis [133]. A cutting-edge study investigating T cell receptor sequences of the Th and Tc populations in PsA blood and SF determined that the increase in cell numbers was the consequence of clonal expansion; moreover, it established that T cell receptor recognition was shared between patients [134]. This discovery is a significant counter-argument against the hypothesis that PsA pathogenesis is dependent on aberrant innate signalling. Innate and innate-like cells, however, also possess features of adaptive immunity players [134], and adaptive type 17 responses across cell subsets in SpA could account for perpetuation of inflammation.
Concluding remarks
Even though therapeutic inhibition of the IL-23 signalling cascade is successfully exploited in the treatment of various inflammatory, immune-mediated diseases, its role in health and disease remains incompletely understood. Evidence in mouse and man demonstrates a range of IL-23–producing and –responding cells across tissues, enabling a fine-tuned, tissue-specific response. These findings provide a plausible explanation of the seemingly paradoxical effect of blocking of IL-23 and its downstream cytokine IL-17 in various pathologies from the SpA disease spectrum. They also provide a theoretical background to hypothesize that targeting the IL-23 signalling pathway in distinct cell populations could help control pathological processes in certain subsets of SpA patients without interfering with physiological, homeostatic mechanisms. However, it should be noted that discrepant findings from IL-23 overexpression–driven animal models of SpA have been reported, including differential organ involvement and pathology severity across genetic strains, suggesting multifactorial disease mechanisms [27, 102, 135].
Studies on the role of IL-23 have also reinforced the notion of host–microbiome interactions as modulators of both local and systemic immune responses. IL-23 has been shown to restore epithelial barrier integrity and promote defence mechanisms against pathogens. Interestingly, many of the cell types involved in host defence are also potent producers of proinflammatory cytokines in SpA pathology, suggesting contribution of skewed antimicrobial responses to the disease pathogenesis. Unambiguous conclusions, however, are momentarily still lacking. The observation that innate-like cells highly responsive to IL-23 are present at anatomical sites affected by SpA, including non-barrier tissues such as entheses, points towards their shared homeostatic role, but also possible utility as a therapeutic target. Production of immune mediators by cells representing the innate arm of the immune system outpaces the adaptive responses, suggesting the identified innate-like cells could be the early effectors inciting inflammation in the SpA pathogenesis. This hypothesis is further encouraged by studies on animal models of the disease, in which experimental depletion of innate-like cell populations has been shown to be protective against the development of the pathology. Investigation of these steps in humans remains challenging. Elevated levels of IL-23 in patients with active, established disease could be attributed to the positive feedback loop in IL-23 signalling. It seems plausible that early-responding innate-like cells support differentiation and phenotype maturation of adaptive type 17 immune cells identified in the joints of patients with overt inflammatory arthritis. The emergence of autoantigens and oligoclonal Tc and Th populations in psoriasis and PsA underpins the importance of adaptive responses across tissues and acts in favour of an autoimmune rather than autoinflammatory nature of the disease [136].
Importantly, in both innate-like and adaptive immune cells the production of IL-17 can be induced independently of IL-23 signalling. This potential is of particular interest in the light of the confirmed homeostatic role of the IL-23–independent production of IL-17 in the gut, and the lack of therapeutic efficacy of IL-23 inhibition in axial SpA. The recent discovery of resident γδ-T cells capable of IL-23–independent IL-17 production in spinal entheses could be the first step into understanding of the latter. MAIT cells, ILC3s and NKT cells—subsets implicated in the pathogenesis of SpA—have also been shown to strengthen IL-17 proinflammatory signalling and contribute to the development of arthritis independently of IL-23 priming, Furthermore, inflammatory cytokine signalling cascades are interconnected, and parallel pathways converge for perpetuation of inflammation. Elucidating the kinetics of IL-23 signalling in the pathogenesis of SpA is therefore of critical importance.
Clinical trials and everyday practice with biologics have provided crucial real-world data on the intricacies of IL-23/IL-17 signalling in disease. As new therapeutic targets are being investigated, our understanding of common and divergent mechanisms across cell types and tissues is broadening. Therapeutic strategies targeting different steps in the IL-23 signalling cascade may unravel the discrepancies seen between SpA subtypes. These efforts are represented by the development of Jak and Tyk2 tyrosine kinase inhibitors and blockers of transcription factors, such as RORγt [101, 103]. Blockade of RORγt-inhibited IL-17 production while not affecting IL-22 [122], whereas Tyk2 blockade inhibited IL-22 production with a lesser effect on IL-17 [103]. Such highly selective therapeutic approaches could represent a new area for the development of precision medicine. Results of these ongoing studies will complement our knowledge on IL-23 signalling across the tissues affected by SpA.
Funding: This paper was published as part of a supplement sponsored by the Janssen Pharmaceutical Companies of Johnson & Johnson.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.
References
- 1. Dougados M, Baeten D.. Spondyloarthritis. Lancet 2011;377:2127–37. [DOI] [PubMed] [Google Scholar]
- 2. Gaffen SL, Jain R, Garg AV, Cua DJ.. The IL-23–IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol 2014;14:585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Annunziato F, Romagnani C, Romagnani S.. The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol 2015;135:626–35. [DOI] [PubMed] [Google Scholar]
- 4. Van Praet L, Van den Bosch FE, Jacques P. et al. Microscopic gut inflammation in axial spondyloarthritis: a multiparametric predictive model. Ann Rheum Dis 2013;72:414–7. [DOI] [PubMed] [Google Scholar]
- 5. Mielants H, Veys EM, Cuvelier C. et al. The evolution of spondyloarthropathies in relation to gut histology. III. Relation between gut and joint. J Rheumatol 1995;22:2279–84. [PubMed] [Google Scholar]
- 6. Van Praet L, Jans L, Carron P. et al. Degree of bone marrow oedema in sacroiliac joints of patients with axial spondyloarthritis is linked to gut inflammation and male sex: results from the GIANT cohort. Ann Rheum Dis 2014;73:1186–9. [DOI] [PubMed] [Google Scholar]
- 7. Ritchlin CT, Colbert RA, Gladman DD.. Psoriatic arthritis. N Engl J Med 2017;376:957–70. [DOI] [PubMed] [Google Scholar]
- 8. Oppmann B, Lesley R, Blom B. et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 2000;13:715–25. [DOI] [PubMed] [Google Scholar]
- 9. Vignali DA, Kuchroo VK.. IL-12 family cytokines: immunological playmakers. Nat Immunol 2012;13:722–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bridgewood C, Alase A, Watad A. et al. The IL-23p19/EBI3 heterodimeric cytokine termed IL-39 remains a theoretical cytokine in man. Inflamm Res 2019;68:423–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sieper J, Poddubnyy D, Miossec P.. The IL-23–IL-17 pathway as a therapeutic target in axial spondyloarthritis. Nat Rev Rheumatol 2019;15:747–57. [DOI] [PubMed] [Google Scholar]
- 12. Lim KS, Yong ZWE, Wang H. et al. Inflammatory and mitogenic signals drive interleukin 23 subunit alpha (IL23A) secretion independent of IL12B in intestinal epithelial cells. J Biol Chem 2020;295:6387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bosmann M, Grailer JJ, Russkamp NF. et al. CD11c+ alveolar macrophages are a source of IL-23 during lipopolysaccharide-induced acute lung injury. Shock 2013;39:447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ciccia F, Bombardieri M, Principato A. et al. Overexpression of interleukin-23, but not interleukin-17, as an immunologic signature of subclinical intestinal inflammation in ankylosing spondylitis. Arthritis Rheum 2009;60:955–65. [DOI] [PubMed] [Google Scholar]
- 15. Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL.. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem 2003;278:1910–4. [DOI] [PubMed] [Google Scholar]
- 16. Ivanov II, McKenzie BS, Zhou L. et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006;126:1121–33. [DOI] [PubMed] [Google Scholar]
- 17. Bettelli E, Carrier Y, Gao W. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006;441:235–8. [DOI] [PubMed] [Google Scholar]
- 18. Awasthi A, Riol-Blanco L, Jäger A. et al. Cutting edge: IL-23 receptor GFP reporter mice reveal distinct populations of IL-17–producing cells. J Immunol 2009;182:5904–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van de Veerdonk FL, Marijnissen RJ, Kullberg BJ. et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe 2009;5:329–40. [DOI] [PubMed] [Google Scholar]
- 20. Langrish CL, Chen Y, Blumenschein WM. et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 2005;201:233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ghoreschi K, Laurence A, Yang XP. et al. Generation of pathogenic Th17 cells in the absence of TGF-β signalling. Nature 2010;467:967–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B.. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17–producing T cells. Immunity 2006;24:179–89. [DOI] [PubMed] [Google Scholar]
- 23. Mangan PR, Harrington LE, O’Quinn DB. et al. Transforming growth factor-beta induces development of the TH17 lineage. Nature 2006;441:231–4. [DOI] [PubMed] [Google Scholar]
- 24. Happel KI, Zheng M, Young E. et al. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol 2003;170:4432–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chackerian AA, Chen SJ, Brodie SJ. et al. Neutralization or absence of the interleukin-23 pathway does not compromise immunity to mycobacterial infection. Infect Immun 2006;74:6092–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stadhouders R, Lubberts E, Hendriks RW.. A cellular and molecular view of T helper 17 cell plasticity in autoimmunity. J Autoimmun 2018;87:1–15. [DOI] [PubMed] [Google Scholar]
- 27. Sherlock JP, Joyce-Shaikh B, Turner SP. et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4–CD8– entheseal resident T cells. Nat Med 2012;18:1069–76. [DOI] [PubMed] [Google Scholar]
- 28. Gracey E, Qaiyum Z, Almaghlouth I. et al. IL-7 primes IL-17 in mucosal-associated invariant T (MAIT) cells, which contribute to the Th17-axis in ankylosing spondylitis. Ann Rheum Dis 2016;75:2124–32. [DOI] [PubMed] [Google Scholar]
- 29. Middendorp S, Nieuwenhuis EE.. NKT cells in mucosal immunity. Mucosal Immunol 2009;2:393–402. [DOI] [PubMed] [Google Scholar]
- 30. Mortier C, Govindarajan S, Venken K, Elewaut D.. It takes “guts” to cause joint inflammation: role of innate-like T cells. Front Immunol 2018;9:1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sutton CE, Lalor SJ, Sweeney CM. et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 2009;31:331–41. [DOI] [PubMed] [Google Scholar]
- 32. Glatigny S, Fert I, Blaton MA. et al. Proinflammatory Th17 cells are expanded and induced by dendritic cells in spondylarthritis-prone HLA-B27-transgenic rats. Arthritis Rheum 2012;64:110–20. [DOI] [PubMed] [Google Scholar]
- 33. Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M.. Interleukin-17–producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity 2009;31:321–30. [DOI] [PubMed] [Google Scholar]
- 34. Rachitskaya AV, Hansen AM, Horai R. et al. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol 2008;180:5167–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cole S, Murray J, Simpson C. et al. Interleukin (IL)-12 and IL-18 synergize to promote MAIT Cell IL-17A and IL-17F production independently of IL-23 signaling. Front Immunol 2020;11:585134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Papotto PH, Gonçalves-Sousa N, Schmolka N. et al. IL-23 drives differentiation of peripheral γδ17 T cells from adult bone marrow–derived precursors. EMBO Rep 2017;18:1957–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang H, Kjer-Nielsen L, Shi M. et al. IL-23 costimulates antigen-specific MAIT cell activation and enables vaccination against bacterial infection. Sci Immunol 2019;4:eaaw0402. [DOI] [PubMed] [Google Scholar]
- 38. Álvarez-Salamero C, Castillo-González R, Pastor-Fernández G. et al. IL-23 signaling regulation of pro-inflammatory T-cell migration uncovered by phosphoproteomics. PLoS Biol 2020;18:e3000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barros-Martins J, Schmolka N, Fontinha D. et al. Effector γδ T cell differentiation relies on master but not auxiliary Th cell transcription factors. J Immunol 2016;196:3642–52. [DOI] [PubMed] [Google Scholar]
- 40. Moreira-Teixeira L, Resende M, Coffre M. et al. Proinflammatory environment dictates the IL-17–producing capacity of human invariant NKT cells. J Immunol 2011;186:5758–65. [DOI] [PubMed] [Google Scholar]
- 41. Michel ML, Mendes-da-Cruz D, Keller AC. et al. Critical role of ROR-γt in a new thymic pathway leading to IL-17–producing invariant NKT cell differentiation. Proc Natl Acad Sci USA 2008;105:19845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schmolka N, Wencker M, Hayday AC, Silva-Santos B.. Epigenetic and transcriptional regulation of γδ T cell differentiation: programming cells for responses in time and space. Semin Immunol 2015;27:19–25. [DOI] [PubMed] [Google Scholar]
- 43. Takatori H, Kanno Y, Watford WT. et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med 2009;206:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jain R, Chen Y, Kanno Y. et al. Interleukin-23–induced transcription factor blimp-1 promotes pathogenicity of T helper 17 cells. Immunity 2016;44: 131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hirota K, Duarte JH, Veldhoen M. et al. Fate mapping of IL-17–producing T cells in inflammatory responses. Nat Immunol 2011;12:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sun R, Hedl M, Abraham C.. IL23 induces IL23R recycling and amplifies innate receptor-induced signalling and cytokines in human macrophages, and the IBD-protective IL23R R381Q variant modulates these outcomes. Gut 2020;69:264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang Y, Edelmayer R, Wetter J. et al. Monocytes/macrophages play a pathogenic role in IL-23 mediated psoriasis-like skin inflammation. Sci Rep 2019;9:5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hou Y, Zhu L, Tian H. et al. IL-23–induced macrophage polarization and its pathological roles in mice with imiquimod-induced psoriasis. Protein Cell 2018;9:1027–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bianchi E, Rogge L.. The IL-23/IL-17 pathway in human chronic inflammatory diseases—new insight from genetics and targeted therapies. Genes Immun 2019;20:415–25. [DOI] [PubMed] [Google Scholar]
- 50. Sarin R, Wu X, Abraham C.. Inflammatory disease protective R381Q IL23 receptor polymorphism results in decreased primary CD4+ and CD8+ human T-cell functional responses. Proc Natl Acad Sci USA 2011;108:9560–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dumas E, Venken K, Rosenbaum JT, Elewaut D.. Intestinal microbiota, HLA-B27, and spondyloarthritis: dangerous liaisons. Rheum Dis Clin North Am 2020;46:213–24. [DOI] [PubMed] [Google Scholar]
- 52. Breban M, Tap J, Leboime A. et al. Faecal microbiota study reveals specific dysbiosis in spondyloarthritis. Ann Rheum Dis 2017;76:1614–22. [DOI] [PubMed] [Google Scholar]
- 53. Tito RY, Cypers H, Joossens M. et al. Brief report: dialister as a microbial marker of disease activity in spondyloarthritis. Arthritis Rheumatol 2017;69:114–21. [DOI] [PubMed] [Google Scholar]
- 54. Ciccia F, Guggino G, Rizzo A. et al. Dysbiosis and zonulin upregulation alter gut epithelial and vascular barriers in patients with ankylosing spondylitis. Ann Rheum Dis 2017;76:1123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chang HW, Yan D, Singh R. et al. Alteration of the cutaneous microbiome in psoriasis and potential role in Th17 polarization. Microbiome 2018;6:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fendler C, Laitko S, Sörensen H. et al. Frequency of triggering bacteria in patients with reactive arthritis and undifferentiated oligoarthritis and the relative importance of the tests used for diagnosis. Ann Rheum Dis 2001;60:337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Taurog JD, Richardson JA, Croft JT. et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med 1994;180:2359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shi Z, Wu X, Santos RC. et al. Short-term Western diet intake promotes IL-23‒mediated skin and joint inflammation accompanied by changes to the gut microbiota in mice. J Invest Dermatol 2021; Advance Access published 22 January 2021, doi: 10.1016/j.jid.2020.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ivanov II, Atarashi K, Manel N. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009;139:485–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Taylor-Robinson D, Gilroy CB, Thomas BJ, Keat AC.. Detection of Chlamydia trachomatis DNA in joints of reactive arthritis patients by polymerase chain reaction. Lancet 1992;340:81–2. [DOI] [PubMed] [Google Scholar]
- 61. Granfors K, Jalkanen S, Lindberg AA. et al. Salmonella lipopolysaccharide in synovial cells from patients with reactive arthritis. Lancet 1990;335:685–8. [DOI] [PubMed] [Google Scholar]
- 62. van der Heijden IM, Wilbrink B, Tchetverikov I. et al. Presence of bacterial DNA and bacterial peptidoglycans in joints of patients with rheumatoid arthritis and other arthritides. Arthritis Rheum 2000;43:593–8. [DOI] [PubMed] [Google Scholar]
- 63. Zhao Y, Chen B, Li S. et al. Detection and characterization of bacterial nucleic acids in culture-negative synovial tissue and fluid samples from rheumatoid arthritis or osteoarthritis patients. Sci Rep 2018;8:14305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ciccia F, Ferrante A, Triolo G.. Intestinal dysbiosis and innate immune responses in axial spondyloarthritis. Curr Opin Rheumatol 2016;28:352–8. [DOI] [PubMed] [Google Scholar]
- 65. Rehaume LM, Matigian N, Mehdi AM. et al. IL-23 favours outgrowth of spondyloarthritis-associated pathobionts and suppresses host support for homeostatic microbiota. Ann Rheum Dis 2019;78:494–503. [DOI] [PubMed] [Google Scholar]
- 66. Aden K, Rehman A, Falk-Paulsen M. et al. Epithelial IL-23R signaling licenses protective IL-22 responses in intestinal inflammation. Cell Rep 2016;16:2208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shih VF, Cox J, Kljavin NM. et al. Homeostatic IL-23 receptor signaling limits Th17 response through IL-22–mediated containment of commensal microbiota. Proc Natl Acad Sci USA 2014;111:13942–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tran HQ, Ley RE, Gewirtz AT, Chassaing B.. Flagellin-elicited adaptive immunity suppresses flagellated microbiota and vaccinates against chronic inflammatory diseases. Nat Commun 2019;10:5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ngo VL, Abo H, Maxim E. et al. A cytokine network involving IL-36γ, IL-23, and IL-22 promotes antimicrobial defense and recovery from intestinal barrier damage. Proc Natl Acad Sci USA 2018;115:e5076–e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Keir M, Yi Y, Lu T, Ghilardi N.. The role of IL-22 in intestinal health and disease. J Exp Med 2020;217:e20192195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bernshtein B, Curato C, Ioannou M. et al. IL-23 producing IL-10Rα–deficient gut macrophages elicit an IL-22–driven proinflammatory epithelial cell response. Sci Immunol 2019;4:eaau6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pelczar P, Witkowski M, Perez LG. et al. A pathogenic role for T cell–derived IL-22BP in inflammatory bowel disease. Science 2016;354:358–62. [DOI] [PubMed] [Google Scholar]
- 73. Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity 2009;31:15–23. [DOI] [PubMed] [Google Scholar]
- 74. Geremia A, Arancibia-Cárcamo CV, Fleming MP. et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med 2011;208:1127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ciccia F, Guggino G, Rizzo A. et al. Type 3 innate lymphoid cells producing IL-17 and IL-22 are expanded in the gut, in the peripheral blood, synovial fluid and bone marrow of patients with ankylosing spondylitis. Ann Rheum Dis 2015;74:1739–47. [DOI] [PubMed] [Google Scholar]
- 76. Bauché D, Joyce-Shaikh B, Jain R. et al. LAG3. Immunity 2018;49:342–52.e5. [DOI] [PubMed] [Google Scholar]
- 77. Savage AK, Liang HE, Locksley RM.. The development of steady-state activation hubs between adult LTi ILC3s and primed macrophages in small intestine. J Immunol 2017;199:1912–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Teng MW, Bowman EP, McElwee JJ. et al. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med 2015;21:719–29. [DOI] [PubMed] [Google Scholar]
- 79. Sands BE, Sandborn WJ, Panaccione R. et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2019;381:1201–14. [DOI] [PubMed] [Google Scholar]
- 80. Maxwell JR, Zhang Y, Brown WA. et al. Differential roles for interleukin-23 and interleukin-17 in intestinal immunoregulation. Immunity 2015;43:739–50. [DOI] [PubMed] [Google Scholar]
- 81. Manasson J, Wallach DS, Guggino G. et al. Interleukin-17 inhibition in spondyloarthritis is associated with subclinical gut microbiome perturbations and a distinctive interleukin-25–driven intestinal inflammation. Arthritis Rheumatol 2020;72:645–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kumar P, Monin L, Castillo P. et al. Intestinal interleukin-17 receptor signaling mediates reciprocal control of the gut microbiota and autoimmune inflammation. Immunity 2016;44:659–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yang XO, Chang SH, Park H. et al. Regulation of inflammatory responses by IL-17F. J Exp Med 2008;205:1063–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Atarashi K, Tanoue T, Ando M. et al. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 2015;163:367–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lee JS, Tato CM, Joyce-Shaikh B. et al. Interleukin-23–independent IL-17 production regulates intestinal epithelial permeability. Immunity 2015;43:727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Iwakura Y, Ishigame H, Saijo S, Nakae S.. Functional specialization of interleukin-17 family members. Immunity 2011;34:149–62. [DOI] [PubMed] [Google Scholar]
- 87. Tang C, Kakuta S, Shimizu K. et al. Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing Treg cells through modification of the intestinal microbiota. Nat Immunol 2018;19:755–65. [DOI] [PubMed] [Google Scholar]
- 88. Glatt S, Baeten D, Baker T. et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis 2018;77:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ritchlin CT, Kavanaugh A, Merola JF. et al. Bimekizumab in patients with active psoriatic arthritis: results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial. Lancet 2020;395:427–40. [DOI] [PubMed] [Google Scholar]
- 90. van der Heijde D, Gensler LS, Deodhar A. et al. Dual neutralisation of interleukin-17A and interleukin-17F with bimekizumab in patients with active ankylosing spondylitis: results from a 48-week phase IIb, randomised, double-blind, placebo-controlled, dose-ranging study. Ann Rheum Dis 2020;79:595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Disclosure CTRR. Clinical Trial Results: A multicenter, subject-blind, investigator-blind, randomized, placebo-controlled study evaluating the efficacy, safety, tolerability and pharmacokinetics of an iv loading dose followed by sc administration of bimekizumab (UCB4940) in subjects with moderate to severe active ulcerative colitis 2019. https://www.clinicaltrialsregister.eu/ctr-search/trial/2016-000420-26/results (15 June 2021, date last accessed).
- 92. Shah M, Maroof A, Gikas P. et al. Dual neutralisation of IL-17F and IL-17A with bimekizumab blocks inflammation-driven osteogenic differentiation of human periosteal cells. RMD Open 2020;6:e001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Schön MP, Erpenbeck L.. The interleukin-23/interleukin-17 axis links adaptive and innate immunity in psoriasis. Front Immunol 2018;9:1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Piskin G, Sylva-Steenland RM, Bos JD, Teunissen MB.. In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin. J Immunol 2006;176:1908–15. [DOI] [PubMed] [Google Scholar]
- 95. Alekseyenko AV, Perez-Perez GI, De Souza A. et al. Community differentiation of the cutaneous microbiota in psoriasis. Microbiome 2013;1:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Fahlén A, Engstrand L, Baker BS, Powles A, Fry L.. Comparison of bacterial microbiota in skin biopsies from normal and psoriatic skin. Arch Dermatol Res 2012;304:15–22. [DOI] [PubMed] [Google Scholar]
- 97. Ganguly D, Chamilos G, Lande R. et al. Self-RNA–antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med 2009;206:1983–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Frasca L, Palazzo R, Chimenti MS. et al. Anti-LL37 antibodies are present in psoriatic arthritis (PsA) patients: new biomarkers in PsA. Front Immunol 2018;9:1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Takahashi T, Kulkarni NN, Lee EY. et al. Cathelicidin promotes inflammation by enabling binding of self-RNA to cell surface scavenger receptors. Sci Rep 2018;8:4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lowes MA, Suárez-Fariñas M, Krueger JG.. Immunology of psoriasis. Annu Rev Immunol 2014;32:227–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ghoreschi K, Balato A, Enerbäck C, Sabat R.. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet 2021;397:754–66. [DOI] [PubMed] [Google Scholar]
- 102. Chen L, Deshpande M, Grisotto M. et al. Skin expression of IL-23 drives the development of psoriasis and psoriatic arthritis in mice. Sci Rep 2020;10:8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gracey E, Hromadová D, Lim M. et al. TYK2 inhibition reduces type 3 immunity and modifies disease progression in murine spondyloarthritis. J Clin Invest 2020;130:1863–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gauld SB, Gauvin D, Olson L. et al. Mechanistic and pharmacological assessment of murine IL-23 mediated psoriasiform dermatitis; implications for drug discovery. J Dermatol Sci 2018;92:45–53. [DOI] [PubMed] [Google Scholar]
- 105. Suárez-Fariñas M, Arbeit R, Jiang W. et al. Suppression of molecular inflammatory pathways by Toll-like receptor 7, 8, and 9 antagonists in a model of IL-23–induced skin inflammation. PLoS One 2013;8:e84634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Campbell JJ, Ebsworth K, Ertl LS. et al. IL-17–secreting γδ T cells are completely dependent upon CCR6 for homing to inflamed skin. J Immunol 2017;199:3129–36. [DOI] [PubMed] [Google Scholar]
- 107. Yoshiki R, Kabashima K, Honda T. et al. IL-23 from Langerhans cells is required for the development of imiquimod-induced psoriasis-like dermatitis by induction of IL-17A–producing γδ T cells. J Invest Dermatol 2014;134:1912–21. [DOI] [PubMed] [Google Scholar]
- 108. Cai Y, Shen X, Ding C. et al. Pivotal role of dermal IL-17–producing γδ T cells in skin inflammation. Immunity 2011;35:596–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Laggner U, Di Meglio P, Perera GK. et al. Identification of a novel proinflammatory human skin-homing Vγ9Vδ2 T cell subset with a potential role in psoriasis. J Immunol 2011;187:2783–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lowes MA, Kikuchi T, Fuentes-Duculan J. et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol 2008;128:1207–11. [DOI] [PubMed] [Google Scholar]
- 111. Matos TR, O’Malley JT, Lowry EL. et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17–producing αβ T cell clones. J Clin Invest 2017;127:4031–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Arakawa A, Siewert K, Stöhr J. et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med 2015;212:2203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Culemann S, Grüneboom A, Nicolás-Ávila J. et al. Locally renewing resident synovial macrophages provide a protective barrier for the joint. Nature 2019;572:670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Berthelot JM, Puéchal X.. Impaired intracellular pathogen clearance and inflammatory joint disease: is Whipple’s disease a guiding light? Joint Bone Spine 2018;85:531–6. [DOI] [PubMed] [Google Scholar]
- 115. Sibilia J, Limbach FX.. Reactive arthritis or chronic infectious arthritis? Ann Rheum Dis 2002;61:580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Romand X, Liu X, Rahman MA. et al. Chlamydia-infected macrophages mediate interleukin-23 and tumor necrosis factor–driven reactive arthritis in SKG mice. Arthritis Rheumatol 2021; Advance Access published 16 January 2021, doi: 10.1002/art.41653 [DOI] [PubMed] [Google Scholar]
- 117. Varkas G, Thevissen K, De Brabanter G. et al. An induction or flare of arthritis and/or sacroiliitis by vedolizumab in inflammatory bowel disease: a case series. Ann Rheum Dis 2017;76:878–81. [DOI] [PubMed] [Google Scholar]
- 118. Taurog JD, Chhabra A, Colbert RA.. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med 2016;374:2563–74. [DOI] [PubMed] [Google Scholar]
- 119. Sheibanie AF, Tadmori I, Jing H, Vassiliou E, Ganea D.. Prostaglandin E2 induces IL-23 production in bone marrow–derived dendritic cells. FASEB J 2004;18:1318–20. [DOI] [PubMed] [Google Scholar]
- 120. Gravallese EM, Schett G.. Effects of the IL-23–IL-17 pathway on bone in spondyloarthritis. Nat Rev Rheumatol 2018;14:631–40. [DOI] [PubMed] [Google Scholar]
- 121. Reinhardt A, Yevsa T, Worbs T. et al. Interleukin-23–dependent γ/δ T cells produce interleukin-17 and accumulate in the enthesis, aortic valve, and ciliary body in mice. Arthritis Rheumatol 2016;68:2476–86. [DOI] [PubMed] [Google Scholar]
- 122. Venken K, Jacques P, Mortier C. et al. RORγt inhibition selectively targets IL-17 producing iNKT and γδ-T cells enriched in spondyloarthritis patients. Nat Commun 2019;10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Cuthbert RJ, Fragkakis EM, Dunsmuir R. et al. Brief report: group 3 innate lymphoid cells in human enthesis. Arthritis Rheumatol 2017;69:1816–22. [DOI] [PubMed] [Google Scholar]
- 124. Cuthbert RJ, Watad A, Fragkakis EM. et al. Evidence that tissue resident human enthesis γδT-cells can produce IL-17A independently of IL-23R transcript expression. Ann Rheum Dis 2019;78:1559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Deodhar A, Gensler LS, Sieper J. et al. Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis Rheumatol 2019;71:258–70. [DOI] [PubMed] [Google Scholar]
- 126. Michel ML, Pang DJ, Haque SF. et al. Interleukin 7 (IL-7) selectively promotes mouse and human IL-17-producing γδ cells. Proc Natl Acad Sci USA 2012;109:17549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Mauro D, Macaluso F, Fasano S, Alessandro R, Ciccia F.. ILC3 in axial spondyloarthritis: the gut angle. Curr Rheumatol Rep 2019;21:37. [DOI] [PubMed] [Google Scholar]
- 128. Siebert S, Millar NL, McInnes IB.. Why did IL-23p19 inhibition fail in AS: a tale of tissues, trials or translation? Ann Rheum Dis 2019;78:1015–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Gullick NJ, Evans HG, Church LD. et al. Linking power Doppler ultrasound to the presence of Th17 cells in the rheumatoid arthritis joint. PLoS One 2010;5:e12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Jandus C, Bioley G, Rivals JP. et al. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum 2008;58:2307–17. [DOI] [PubMed] [Google Scholar]
- 131. Menon B, Gullick NJ, Walter GJ. et al. Interleukin-17+CD8+ T cells are enriched in the joints of patients with psoriatic arthritis and correlate with disease activity and joint damage progression. Arthritis Rheumatol 2014;66:1272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Steel KJA, Srenathan U, Ridley M. et al. Polyfunctional, proinflammatory, tissue-resident memory phenotype and function of synovial interleukin-17A+CD8+ T cells in psoriatic arthritis. Arthritis Rheumatol 2020;72:435–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Asquith MJ, Stauffer P, Davin S. et al. Perturbed mucosal immunity and dysbiosis accompany clinical disease in a rat model of spondyloarthritis. Arthritis Rheumatol 2016;68:2151–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Netea MG, Domínguez-Andrés J, Barreiro LB. et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol 2020;20:375–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Souza D II. IL23 overexpression demonstrates gut–joint inflammation link and increased expression of spondyloarthopathy associated genes in vivo. ACR/ARHP Annual Meeting, Boston, MA, November 14–19, 2014.
- 136. Penkava F, Velasco-Herrera MDC, Young MD. et al. Single-cell sequencing reveals clonal expansions of pro-inflammatory synovial CD8 T cells expressing tissue-homing receptors in psoriatic arthritis. Nat Commun 2020;11:4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.