Abstract
Purpose
To compare the efficacy of a single, intra-articular, nonconcentrated bone marrow aspirate (BMA) injection in comparison to cortisone for the treatment of glenohumeral joint osteoarthritis (GHJ OA).
Methods
Inclusion criteria were patients between the ages of 18 and 75 with a diagnosis of GHJ OA on radiograph. Patients were randomized to receive an ultrasound-guided, intra-articular cortisone injection or BMA injection (without concentration). The primary outcome measure was the Western Ontario Osteoarthritis of the Shoulder (WOOS) index at 12 months. Secondary outcome measures were the QuickDASH, EuroQOL 5-dimensions 5-level questionnaire (EQ-5D-5L) and visual analogue scale.
Results
The study included 25 shoulders of 22 patients who completed baseline and 12 months’ patient-reported outcome measures (12 shoulders received cortisone, 13 shoulders received BMA) after the study was terminated early by changes in Health Canada regulations. Baseline characteristics demonstrated a significant difference in the ages of the 2 groups, with the BMA group being older (61.6 vs 53.8 mean years, P = 0.021). For the BMA group, a significant improvement was seen in the WOOS index (P = 0.002), the QuickDASH (P < 0.001), and the EQ-5D-5L pain dimension (P = 0.004) between baseline and 12 months. No significant difference was seen for any outcome in the cortisone group between baseline and 12 months. No significant difference was demonstrated between changes in the WOOS scores from baseline to 12 months when compared between groups (P = 0.07). However, a significant difference in changes in scores was seen in the QuickDASH (P = 0.006) and the EQ-5D-5L pain scores (P = 0.003) and the EQ-5D-5L health scores (P = 0.032) in favor of BMA.
Conclusions
The results of this study demonstrate that patients with GHJ OA treated with BMA have superior changes in the QuickDASH and EQ-5D-5L pain and health scores but not in the WOOS outcomes measures at 12 months post injection when compared to patients treated with cortisone. However, because of the limited number of patients as a result of the early termination of the study, larger randomized studies are required to confirm these findings.
Level of Evidence
Level II, randomized controlled trial.
Glenohumeral joint (GHJ) osteoarthritis (OA) is a painful, progressive and debilitating condition that impairs quality of life. Currently, the most common surgical approach is total shoulder arthroplasty, a major operation that carries with it significant risks, including infection,1, 2, 3 and in young or active people, a significant rate of early revision surgery.2,4,5 Therefore, the goal of early shoulder OA management should be symptom control in order to postpone or prevent the need for joint replacement.
Initial nonoperative management options include physical therapy, pain-control medications and intra-articular injections. Corticosteroid injections are the most commonly used injectables for GHJ OA, although the limited evidence available suggests that pain relief occurs for only short periods post treatment.6 Intra-articular hyaluronic acid injections have been proposed as an alternative to corticosteroid injections, and they have demonstrated a clinically significant improvement in pain and function for as long as 6 months after injection,7,8 although with limited effect size.9 There is limited information regarding the use of platelet-rich plasma for GHJ OA.10
Bone marrow aspirate (BMA) and bone marrow aspirate concentrate (BMAC) injections are relatively recent options for the treatment of OA. Typically taken from the patient’s pelvis, BMA has been shown to contain high levels of white blood cells and platelets as well as anti-inflammatory growth factors and cytokines.11 BMA also contains mononuclear cells, of which about 0.001% are mesenchymal stem cells (MSCs), with chondrogenic potential and a paracrine effect in increasing growth factor and cytokine levels.12,13 Intra-articular injections of BMA and BMAC in the setting of knee OA have reported statistically significant benefits in improved pain scores and function,14 although recent level 1 studies failed to demonstrate its superiority to saline15 or to platelet-rich plasma (PRP).16 At this time, there is limited evidence for the use of BMA or BMAC for the treatment of GHJ OA.17,18
The purpose of this study was to compare the efficacy of a single, intra-articular, nonconcentrated BMA injection with cortisone for the treatment of GHJ OA. We hypothesized that BMA would improve patient-reported outcome measures (PROMs) in comparison to cortisone in the setting of GHJ OA at 12 months post injection.
Methods
This study was approved by the institutional research ethics board and registered with ClinicalTrials.gov. Inclusion criteria were men or women between the ages of 18 and 75 with a diagnosis of primary GHJ OA on standardized radiographs (anteroposterior, scapula lateral and axillary lateral) who consented to the study between June 2016 and May 2019. Exclusion criteria were patients with a prior condition resulting in secondary OA (such as trauma, dislocation, avascular necrosis, fracture, inflammatory conditions, rotator cuff arthropathy), previous injection of cortisone or other substances, previous surgical interventions, and inability to comply with rehabilitation or form completion, as well as workplace compensation or lawsuit involvement. All BMA kits were supplied by Marrow Cellutions (Ranfac, Avon, MA). No other funding was received from Marrow Cellutions, nor was the company involved in study design, and at no time were they permitted to access the study’s data.
Patients presenting with a primary diagnosis of Samilson and Prieto stage 1 (osteophytes < 3mm), stage 2 (osteophytes between 3 and 7 mm with slight joint irregularity), or stage 3 (osteophytes > 7 mm with narrowing and sclerosis of the joint) GHJ OA19 were eligible for the study. Those who consented to the study were randomized by a study coordinator to receive an ultrasound-guided, intra-articular cortisone injection (Group 1) or a BMA injection (Group 2) by means of a computerized randomization system (sealedenvelope.com) with a 1:1 allocation between the 2 treatment arms. No sham bone marrow aspirations were performed; thus, patients were not blinded to their treatment group.
Patients randomized to Group 1 received an intra-articular injection of 80 mg Depo Medrol with 4 mL of 1% lidocaine in the office setting, in line with standard treatment practice at our institution. Patients randomized to Group 2 had BMA taken from the posterior superior iliac spine using the Marrow Cellutions bone marrow aspiration system (Ranfac) in an outpatient procedure room. The posterior superior iliac spine was chosen on the basis of evidence that it contains consistently high concentrations of colony-forming units.20 The BMA system was chosen because of familiarity by the surgeons and white-paper evidence of high levels of colony-forming units without the need for a concentration process. Patients were placed in a prone position, and 10 mL of 0.25% lidocaine were used for local anesthesia at the donor site. All instrumentation was rinsed with 2,000 units/mL of heparin prior to use, with .5 mL of the heparin solution retained in the aspirate syringe. After palpation of landmarks, the introducer needle was inserted past the cortex into the medullary space of the posterosuperior iliac spine, the sharp stylet was removed, and 1 mL of marrow was aspirated to ensure proper positioning. A blunt stylet was then inserted, and the access needle was advanced 2 cm farther. Following this, the blunt stylet was removed, and the aspiration cannula and syringe were attached. After each 1 mL of aspirate, the handle was rotated counterclockwise before repeating the 1 mL aspirate, as per manufacturer’s instructions. This was repeated until 10 mL of aspirate were obtained. Instrumentation was removed and dressings applied. The BMA was not centrifuged or manipulated in any way.
The injection procedure was identical in both groups. Patients sat upright, with the appropriate shoulder marked and prepped with 2% chlorhexidine gluconate and 70% isopropyl alcohol. Under ultrasound guidance, local anesthetic (5 mL of 0.5 mL lignocaine) was injected down to capsule of the GHJ using a posterior approach. Following this, using an 18-gauge needle under ultrasound guidance, 10 mL of unmanipulated BMA or 5 mL of the local anesthetic/cortisone mix was injected into the glenohumeral joint. Two orthopedic surgeons performed all bone marrow aspirations and ultrasound-guided injections of both cortisone and BMA.
Subjects in both treatment groups followed the same protocol post procedure. Patients were instructed to rest for 5 days before resuming normal activities and to avoid the use of nonsteroidal anti-inflammatory medication for 2 weeks (although they were allowed to take anti-inflammatories until the day of injection). Physiotherapy was not recommended for either group post injection because not all patients were able to access physiotherapy equally. Patients were followed-up at 3, 6 and 12 months by a research coordinator blinded to the patients’ interventions.
The primary outcome measure was a change in the Western Ontario Osteoarthritis of the Shoulder (WOOS) index from baseline to 12 months postintervention.21 The WOOS index is a disease-specific, patient-reported survey composed of 19 questions and providing scores in 4 domains: (1) physical symptoms, (2) sport, recreation and work, (3) lifestyle, and 94) emotions. Each question is answered on a visual analogue scale, with possible scores ranging from 0 to 100 and with total scores ranging from 0 to 1900; a score of 1900 indicates an extreme decrease in shoulder-related quality of life.22
The secondary outcome measures used were the QuickDash, EQ-5D-5L and visual analogue scale for pain. The QuickDash23,24 is an abbreviated version of the original Disabilities of the Arm, Shoulder and Hand (DASH) outcome measure,25 a region-specific PROM composed of 11 items rather than the original 30-item outcome measure. The QuickDASH score has 2 components: the disability/symptom section (11 items, scored 1-5) and the optional sport and work modules (4 items, scored 1-5). Patients use a 5-point Likert scale form, with the final disability/symptom score ranging from 0 (no disability) to 100% (most severe disability). The minimal clinically important difference for QuickDash is 8% in individuals with shoulder pain.26
The EQ-5D-5L is a general health-status outcome score with 2 parts. The EQ-5D-5L descriptive system comprises 5 dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression.27 Each dimension has 5 levels. The EQ visual analogue scale records the patient’s self-reported health between 0 (the worst health you can imagine) and 100 (the best health you can imagine).
Statistical Analysis
The planned sample size was 44 patients, or 22 patients per group. The sample size calculation was conducted with the Cohen d effect size of 0.98, 80% power of 80%, 2-sided significance level of 0.05, and a 15% attrition rate using R 3.2.0 with the package pwr. The effect size was estimated based on statistics (means and standard deviations) reported in a similar study by Morella et al.6 However, due to Health Canada regulations, the study was stopped before the planned sample size had been reached.
Demographic data and baseline outcome scores were calculated and presented using means and standard deviations, medians and interquartile ranges, or counts and proportions where appropriate. In our primary analysis, we used an unpaired 2-sample t test to compare differences between our primary outcome (change in WOOS scores from baseline to 12 months) between treatment groups. For secondary outcome measures, unpaired 2-sample t tests or Wilcoxon rank sum tests were employed to investigate differences between groups. All tests were 2-sided. The significance level was set at P < 0.05 for all tests. All statistical analysis was performed using SAS s oftware, version 9.4 (SAS Institute, Cary, NC, USA).
Results
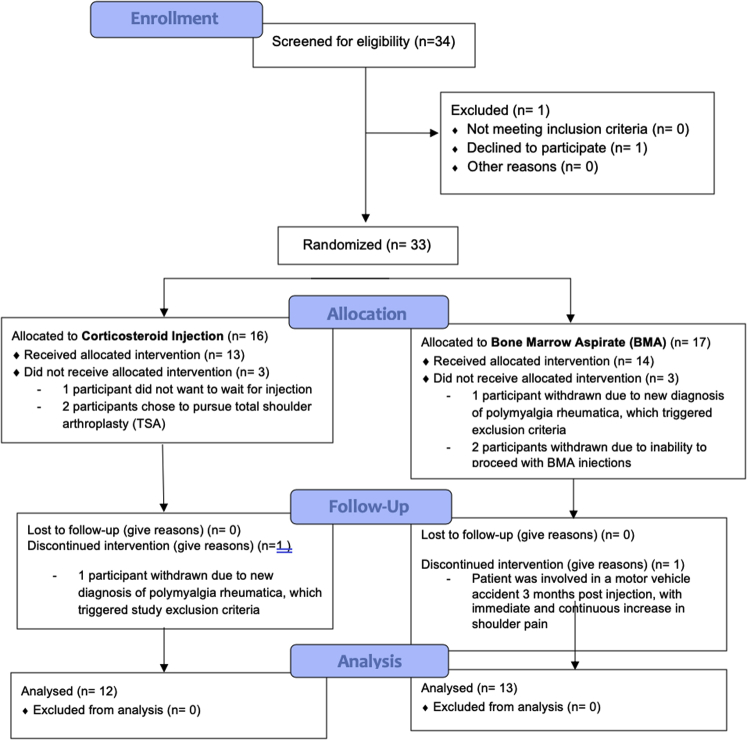
Between June 2016 and May 2019, a total of 33 patients (including 37 shoulders, 4 with bilateral OA) consented to participate in the trial and were randomized. After enrollment, 8 patients were excluded, leaving a total of 25 patients (29 shoulders) in the study. Fig 1 presents the Consolidated Standards of Reporting Trials (CONSORT) flow diagram. On May 15, 2019, Health Canada advised that all cell therapies were to be considered drugs under the Food and Drugs act, requiring authorization by Health Canada to ensure safety and efficacy. For this reason, on May 15, 2019, the study ceased enrolling patients, leaving 2 patients who were randomized to BMA unable to receive their injections. After receiving an injection, 2 patients were excluded from each group, and 1 patient was excluded after a cortisone injection due to a new diagnosis of polymyalgia rheumatica (this patient had also been randomized to receive BMA in her other shoulder, but was excluded prior to this injection). As a result of the new regulations, 25 shoulders (22 patients) were included in the study.
Fig 1.
CONSORT (Consolidated Standards of Reporting Trials) flow diagram.
All 22 patients (25 shoulders) completed baseline and 12-months PROMs. Twelve patients received a cortisone injection, and 13 patients received BMA. Baseline characteristics are presented in Table 1. A significant difference was seen in the ages of the 2 groups the BMA group was older (P < 0.05). No complications were seen in either group.
Table 1.
Baseline Characteristic Comparison Between BMA and Cortisone Injection Groups
| BMA N = 13 |
Cortisone N = 12 |
P Value | |
|---|---|---|---|
| Patient demographics | |||
| Age, mean (SD) | 61.6 (8.1) | 53.8 (8.3) | 0.021∗ |
| Sex, n males (%) | 8 (57.1%) | 9 (75.0%) | 0.43† |
| Shoulder characteristics | |||
| Affected side, n right (%) | 5 (35.7%) | 6 (50%) | 0.69† |
| Radiograph arthritis stage | Stage 1: 1 | Stage 1: 0 | 0.23† |
| Stage 2: 11 | Stage 2: 8 | ||
| Stage 3: 1 | Stage 3: 4 | ||
| Baseline outcome measure scores | |||
| WOOS, mean (SD) | 1105.7 (205.3) | 1088.9 (262.8) | 0.86∗ |
| QuickDASH, mean (SD) | 44.5 (12.2) | 39.4 (11.1) | 0.28∗ |
| Work, mean (SD) | 44.3 (22.8) | 32.0 (22.8) | 0.26∗ |
| Sport, mean (SD) | 62.5 (22.0) | 60.9 (11.9) | 0.86∗ |
| Pain VAS, mean (SD) | 4.5 (2.3) | 4.8 (1.8) | 0.72∗ |
| EQ5D-5L Health Score, median (IQR) | 74 (65-85) | 70 (70-75) | 0.57‡ |
Bold values indicate statistical significance (P < 0.05).
IQR, interquartile range; n, number of participants; SD, standard deviation; VAS, visual analogue scale; WOOS, Western Ontario Osteoarthritis of the Shoulder index.
P value produced using 2-sided unpaired t test.
P value produced using Fisher exact test.
P value produced using 2-sided Mann-Whitney U test.
Table 2 presents the difference between the baseline and 12-month outcome scores for the BMA group and for the cortisone group. For the BMA group, a significant difference was seen for the WOOS, the QuickDASH, the QuickDASH work and sports modules, and the EQ-5D-5L pain dimension. No difference was seen for any outcome in the cortisone group.
Table 2.
Differences Between Baseline and 12-month Outcome Scores Within BMA and Cortisone Injection Groups
| Baseline Score | 12-Month Score | P Value | |
|---|---|---|---|
| BMA (N = 13) | |||
| WOOS, mean (SD) | 1082.0 (192.8) | 684.0 (385.3) | 0.002∗ |
| QuickDASH, mean (SD) | 42.5 (10.1) | 25.5 (8.4) | <0.00∗ |
| QuickDASH Work, mean (SD) | 43.2 (23.6) | 18.1 (13.1) | 0.005∗ |
| QuickDASH Sport, mean (SD) | 61.4 (22.7) | 39.2 (25.3) | 0.003∗ |
| Pain VAS, mean (SD) | 4.3 (2.1) | 3.0 (2.8) | 0.13∗ |
| EQ5D-5L Mobility, median (IQR) | 1 (1-1) | 1 (1-1) | 1.00† |
| EQ5D-5L Selfcare, median (IQR) | 2 (1-3) | 2 (1-2) | 0.36† |
| EQ5D-5L Activities, median (IQR) | 2 (2-3) | 2 (2-2) | 0.16† |
| EQ5D-5L Pain, median (IQR) | 3 (3-4) | 2 (2-3) | 0.004† |
| EQ5D-5L Anxiety, median (IQR) | 1 (1-2) | 1 (1-2) | 0.25† |
| EQ5D-5L Health Score, median (IQR) | 75 (70-85) | 81 (75-85) | 0.27† |
| Cortisone (N = 12) | |||
| WOOS, mean (SD) | 1088.9 (262.8) | 1002.7 (429.2) | 0.54∗ |
| QuickDASH, mean (SD) | 39.4 (11.1) | 37.1 (18.7) | 0.59∗ |
| QuickDASH Work, mean (SD) | 32.0 (22.8) | 25.0 (16.5) | 0.47∗ |
| QuickDASH Sport, mean (SD) | 60.9 (11.9) | 49.2 (23.7) | 0.44∗ |
| Pain VAS, mean (SD) | 4.8 (1.8) | 5.1 (3.2) | 0.61∗ |
| EQ5D-5L Mobility, median (IQR) | 1 (1-1) | 1 (1-1) | 0.50† |
| EQ5D-5L Selfcare, median (IQR) | 2 (1.5-2) | 2 (1.5-3) | 1.00† |
| EQ5D-5L Activities, median (IQR) | 3 (2-3) | 3 (2-3) | 1.00† |
| EQ5D-5L Pain, median (IQR) | 3 (2-3.5) | 3 (2.5-3.5) | 0.75† |
| EQ5D-5L Anxiety, median (IQR) | 1 (1-2) | 1 (1-2.5) | 0.25† |
| EQ5D-5L Health Score, median (IQR) | 70 (70-75) | 67.5 (55-72.5) | 0.13† |
Bold values indicate statistical significance (P < 0.05).
IQR, interquartile range; n, number of participants; SD, standard deviation; VAS, Visual Analogue Scale; WOOS, Western Ontario Osteoarthritis of the Shoulder index.
P value produced using 2-sided paired t test.
P value produced using 2-sided Wilcoxon signed rank test.
Table 3 presents a comparison of the 12-month outcome scores for both groups. No significant difference between the BMA group and the cortisone group was seen for the WOOS, the primary outcome measure. A significant difference was seen for the QuickDASH and the EQ-5D-5L pain and health scores.
Table 3.
Comparison of 12-Month Postinjection Outcomes Between BMA and Cortisone groups
| BMA N = 13 | Cortisone N = 12 | P Value | |
|---|---|---|---|
| WOOS, mean (SD) | 684.0 (385.3) | 1002.7 (429.2) | 0.06∗ |
| QuickDASH, mean (SD) | 25.5 (8.4) | 37.1 (18.7) | 0.01∗ |
| QuickDASH, Work, mean (SD) | 18.1 (13.1) | 25.0 (16.5) | 0.36∗ |
| QuickDASH, Sport, mean (SD) | 39.2 (25.3) | 49.2 (23.7) | 0.39∗ |
| Pain VAS, mean (SD) | 3.0 (2.8) | 5.1 (3.2) | 0.09∗ |
| EQ5D-5L | |||
| EQ5D-5L Mobility, median (IQR) | 1 (1-1) | 1 (1-1) | 0.90† |
| EQ5D-5L Selfcare, median (IQR) | 2 (1-2) | 2 (1.5-3) | 0.22† |
| EQ5D-5L Activities, median (IQR) | 2 (2-2) | 3 (2-3) | 0.05† |
| EQ5D-5L Pain, median (IQR) | 2 (2-3) | 3 (2.5-3.5) | 0.028† |
| EQ5D-5L Anxiety, median (IQR) | 1 (1-2) | 1 (1-2.5) | 0.47† |
| EQ5D-5L Health Score, median (IQR) | 81 (75-85) | 67.5 (55-72.5) | 0.015† |
| Patient Satisfaction | |||
| Satisfaction VAS, median (IQR) | 1.3 (0.1-3.5) | 6.8 (4.1-8.6) | 0.06† |
Bold values indicate statistical significance (P < 0.05).
IQR, interquartile range; n, number of participants; SD, standard deviation; VAS, visual analogue scale; WOOS, Western Ontario Osteoarthritis of the Shoulder index.
P value produced using two-sided unpaired t-test
P value produced using 2-sided Mann-Whitney-U test
Table 4 presents a comparison of the changes in outcome scores from baseline to 12 months for the BMA and cortisone injection groups. Again, no significant difference was seen for the WOOS primary outcome measure. A significant difference was seen for the QuickDASH, the QuickDASH sport module, and the EQ-5D-5L pain and health scores in favor of the BMA group.
Table 4.
Comparison of the Change in Outcome Scores From Baseline to 12 Months Postinjection Between BMA and Cortisone Groups
| BMA N = 13 |
Cortisone N = 12 |
P Value | |
|---|---|---|---|
| WOOS, mean (SD) | -398.0 (364.1) | -86.2 (467.8) | 0.07 |
| QuickDASH, mean (SD) | -17.0 (9.9) | -2.3 (14.2) | 0.006 |
| QuickDASH Work, mean (SD) | -18.8 (12.9) | -4.5 (15.2) | 0.07 |
| QuickDASH Sport, mean (SD) | -27.5 (21.5) | -5.4 (17.1) | 0.039 |
| Pain VAS, mean (SD) | -1.3 (2.9) | 0.6 (3.6) | 0.18 |
| EQ5D-5L | |||
| EQ5D-5L Mobility, mean (SD) | 0.1 (0.5) | 0.3 (0.6) | 0.45 |
| EQ5D-5L Selfcare, mean (SD) | -0.3 (0.9) | 0.1 (1.0) | 0.30 |
| EQ5D-5L Activities, mean (SD) | -0.6 (1.3) | 0.1 (0.8) | 0.13 |
| EQ5D-5L Pain, mean (SD) | -0.9 (0.8) | 0.1 (0.7) | 0.003 |
| EQ5D-5L Anxiety, mean (SD) | -0.3 (1.0) | 0.3 (0.5) | 0.10 |
| EQ5D-5L Health Score, mean (SD) | 2.5 (7.5) | -7.3 (13.3) | 0.032 |
Change scores = 12 month scores–baseline scores.
Bold values indicate statistical significance (P < 0.05).
IQR, interquartile range; n, number of participants; SD, standard deviation; VAS, visual analogue scale; WOOS, Western Ontario Osteoarthritis of the Shoulder index.
P values produced using 2-sided unpaired t test.
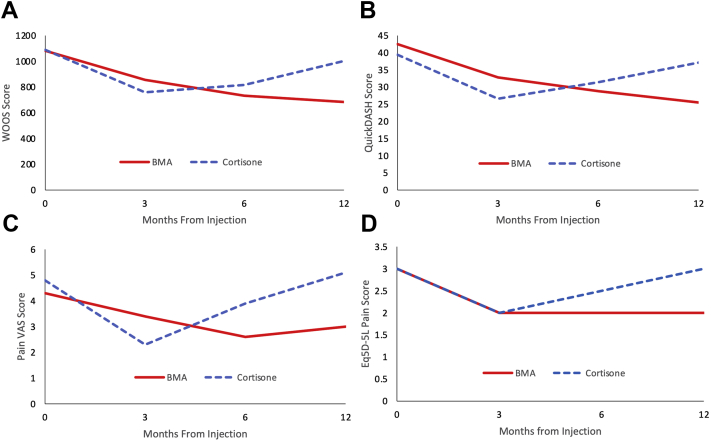
Table 5 and Fig 2 present PROMs at 3,6 and 12 months; some patients did not provide scores at 3 and 6 months. There was no significant difference in PROMs between the 2 groups at the 3- and 6-month time periods (P > 0.05).
Table 5.
Outcome Scores Within BMA and Cortisone Injection Groups
| Baseline Score | 3-Month Score | 6-Month Score | 12-Month Score | |
|---|---|---|---|---|
| BMA (N = 13) | N = 13 | N = 8 | N = 10 | N = 13 |
| WOOS, mean (SD) | 1082.0 (192.8) | 856.9 (559.4) | 732.7 (376.3) | 684.0 (385.3) |
| QuickDASH, mean (SD) | 42.5 (10.1) | 32.8 (15.2) | 28.8 (10.0) | 25.5 (8.4) |
| QuickDASH Work, mean (SD) | 43.2 (23.6) | 26.6 (24.5) | 14.1 (17.9) | 18.1 (13.1) |
| QuickDASH Sport, mean (SD) | 61.4 (22.7) | 41.0 (23.4) | 40.6 (23.1) | 39.2 (25.3) |
| Pain VAS, mean (SD) | 4.3 (2.1) | 3.4 (2.6) | 2.6 (2.4) | 3.0 (2.8) |
| EQ5D-5L Mobility, median (IQR) | 1 (1-1) | 1 (1-1) | 1 (1-1) | 1 (1-1) |
| EQ5D-5L Selfcare, median (IQR) | 2 (1-3) | 1.5 (1-2.5) | 1.5 (1-3) | 2 (1-2) |
| EQ5D-5L Activities, median (IQR) | 2 (2-3) | 2 (1.5-2.5) | 2 (2-3) | 2 (2-2) |
| EQ5D-5L Pain, median (IQR) | 3 (3-4) | 2 (2-3) | 2 (1.5-3) | 2 (2-3) |
| EQ5D-5L Anxiety, median (IQR) | 1 (1-2) | 1 (1-1) | 1 (1-1) | 1 (1-2) |
| EQ5D-5L Health Score, median (IQR) | 75 (70-85) | 75 (70-85) | 77.5 (75-85) | 81 (75-85) |
| Cortisone (N = 12) | N = 12 | N = 7 | N = 6 | N = 12 |
| WOOS, mean (SD) | 1088.9 (262.8) | 759.1 (411.0) | 816.6 (316.3) | 1002.7 (429.2) |
| QuickDASH, mean (SD) | 39.4 (11.1) | 26.6 (18.4) | 31.4 (15.7) | 37.1 (18.7) |
| QuickDASH Work, mean (SD) | 32.0 (22.8) | 16.7 (12.9) | 31.3 (18.2) | 25.0 (16.5) |
| QuickDASH Sport, mean (SD) | 60.9 (11.9) | 47.9 (18.8) | 40.6 (22.1) | 49.2 (23.7) |
| Pain VAS, mean (SD) | 4.8 (1.8) | 2.3 (1.4) | 3.9 (3.9) | 5.1 (3.2) |
| EQ5D-5L Mobility, median (IQR) | 1 (1-1) | 1 (1-3) | 1 (1-1) | 1 (1-1) |
| EQ5D-5L Selfcare, median (IQR) | 2 (1.5-2) | 2 (1-1) | 2 (1-2) | 2 (1.5-3) |
| EQ5D-5L Activities, median (IQR) | 3 (2-3) | 2 (1-2) | 2.5 (2-3) | 3 (2-3) |
| EQ5D-5L Pain, median (IQR) | 3 (2-3.5) | 2 (2-3) | 2.5 (2-3) | 3 (2.5-3.5) |
| EQ5D-5L Anxiety, median (IQR) | 1 (1-2) | 1 (1-1) | 1 (1-1) | 1 (1-2.5) |
| EQ5D-5L Health Score, median (IQR) | 70 (70-75) | 75 (60-80) | 75 (60-75) | 67.5 (55-72.5) |
IQR, interquartile range; n, number of participants; SD, standard deviation; VAS, visual analogue scale; WOOS, Western Ontario Osteoarthritis of the Shoulder index.
Fig 2.
Changes in scores over time for both groups. (A) Western Ontario Osteoarthritis of the Shoulder (WOOS) index. No significant difference was seen between the mean scores for the 2 groups at 12 months post injection (P = 0.06). (B) QuickDASH. A significant difference was seen between the mean scores for the 2 groups at 12 months post injection (P = 0.006). (C) Pain Visual Analogue Score. No significant difference was seen between the mean scores for the 2 groups at 12 months post injection (P = 0.09). (D) Eq5D-5L pain score. A significant difference was seen between the mean scores for the 2 groups at 12 months post injection (P = 0.028). A significant difference was also seen for the health score domain (P = 0.015), but not for the mobility, selfcare or activities domain. (BMA, bone marrow aspirate.)
A post hoc power calculation based on the WOOS score, which found that the study was powered to a beta of 0.28 (i.e., 72% of the time, if there was a real difference in WOOS, it would not have been identified). Based on this pilot data, a follow-up study would require 51 patients per group in order to have a beta of 0.8.
Discussion
The findings of this study demonstrate that in comparison to cortisone injections, patients receiving BMA injections showed no significant change between baseline and 12 months on the WOOS primary outcome measure but showed significant improvements on the QuickDASH and on the EQ-5D-5L pain and health scores dimensions. Although there was a nonsignificant difference for superior change in WOOS scores in the BMA group compared to the cortisone group, because of early termination, this study was significantly underpowered, and further study is required to confirm this effect.
The theory behind the use of BMA and BMAC in the treatment of OA has focused on the presence of MSCs, although the exact mechanism of action is still unclear.12 Both BMA and BMAC have been shown to contain colony-forming units (CFUs) having surface markings consistent with MSCs,11 although MSCs have been shown to represent only 0.001% to 0.02% of mononuclear cells, even after centrifugation.12 Bone marrow is also a rich source of growth factors and cytokines that decrease cell apoptosis and inflammation several times that of PRP,11 and it is thought that nucleated cells in BMA have a paracrine effect by delivering various growth factors and cytokines.13 BMAC has also been shown to have significantly higher levels of platelets compared to PRP.11
The use of both BMA and BMAC has been increasing in orthopedics, whether it be treating cartilage defects in the knee28 or foot and ankle,29 the treatment of bone defects and nonunion,30 or the treatment of degenerative disc disease.31 With regard to the treatment of OA, the most common use has been in the setting of knee OA, and some evidence of efficacy has been shown in nonrandomized trials.14 However, in 2017, Shapiro compared a BMAC injection with a contralateral saline injection in 25 patients with bilateral knee OA and saw no significant difference at either 6 months15 or 12 months post injection.32 Publications regarding the use of BMA or BMAC in the treatment of GHJ OA are extremely limited at this time. In 2015, Centeno published work concerning a series of patients undergoing BMAC injections for both rotator cuff tears and GHJ OA; 34 of 115 (29.6%) patients in this study had GHJ OA, and they showed improvement in the outcome scores.17 Darrow et al. used both whole bone marrow and BMAC to treat 32 cases of shoulder OA, demonstrating improvements in pain and function at a mean of 6 months of follow-up.18 The results of this pilot study demonstrate that patients with GHJ OA who are treated with injections of BMA have improved outcomes at 12 months in the WOOS and QuickDASH outcome measures, as well as in the EQ-5D-5L pain dimension when compared to baseline. This is encouraging and suggests that further, larger scale research regarding the use of BMA and BMAC in this group of difficult-to-treat patients may be rewarding.
There are a variety of products on the market for BMA and BMAC; 2 recent systematic reviews noted that there was inadequate reporting of both preparation protocols and composition in the literature.33,34 In this study, 10 mL of BMA were extracted from the posterior superior iliac spine using the Marrow Cellutions system and injected in the GHJ without a concentration process.35 Alternative systems often use 60 mL or more of extracted bone marrow, followed by a concentration process to increase the number of MSCs relative to baseline.13 However, in both children and adults, it has been shown that after the first few mL of bone marrow aspiration, any increase in volume results primarily in hemodilution.36,37 Further, a study of 30 adults demonstrated that concentrations of MSCs were higher in a 10 mL sample than in a 50 mL sample.38 The Marrow Cellutions system is closed distally to limit peripheral blood infiltration end of the aspiration cannula and obtains multiple small-volume draws (1 mL) from side holes during a single puncture. It also uses technology that allows the aspiration needle to be repositioned after each draw. In 1 study, this technique was shown to produce concentrations of CFUs that were comparable to or greater than samples obtained with larger aspiration volumes and concentrations.35 However, it is important to note that evidence that improving the numbers of CFUs improves outcomes is limited,39 and that further study is required to evaluate the efficacy of different BMA and BMAC systems in the treatment of OA.
There are other, less invasive interventions for the treatment of OA available on the market such as PRP. There is significant evidence for the use of PRP in the setting of knee OA; a systematic review in 2017 found that 9 of 11 randomized controlled trials demonstrated significantly better results with PRP than with hyaluronic acid.40 In theory, BMA and BMAC should be more efficacious anti-inflammatory agents than PRP; they have the same or greater levels of platelets as PRP,11 provide a source of MSCs not found in peripheral blood,41 and are known to contain greater levels of interleukin-1 receptor antagonist,11,42 which work to inhibits interleukin-1 catabolism effects. In 2020, Anz et al. published a randomized trial comparing BMAC to PRP in 90 patients with knee OA and demonstrated no significant improvement in pain relief between the 2 groups at 1 year.16 At this time there are limited studies looking at the use of PRP in GHJ OA, although studies are currently underway. Because of the superiority of PRP over HA in the setting of knee OA and the potential increased efficacy of BMA and BMAC over PRP, the use of BMA was selected for our study in patients with GHJ OA.
Limitations
Despite some of the encouraging findings of this study, it is important to acknowledge a risk of underpowered studies such as ours, including type I errors. Furthermore, any findings are not robust because of the small number of cross-over patients required to change a significant finding into a nonsignificant one.43,44 For example, a recent study looking at significant findings in sports medicine and arthroscopic surgery determined that the median value of the Fragility Index was 2, indicating that changing 2 patients between study groups would reverse the significance.44 There were also limited enrollment numbers over the 3 years because some patients preferred a more permanent solution such as arthroplasty.
There are other limitations in this study. The 2 groups were different in age at baseline, although there was no significant difference in baseline outcomes measures between the 2 groups. There were also more patients with severe GHJ OA in the cortisone group. Although randomization usually serves to limit this occurrence, early termination of the study is likely to be the cause. However, this finding may have affected the outcome of our study. Patients were not blinded to the intervention because no sham BMA procedures were performed. For this reason, some of the improvements in the BMA group may be a result of the placebo effect. However, BMA is not without an element of pain and risk to the patient, and the authors of this study did not believe it was ethical to perform sham procedures. Additionally, we did not perform any product sampling, so we cannot comment on the cellular composition of the BMA injections. Furthermore, no follow-up imaging was used because it was believed the primary function of BMA injections was to reduce pain and inflammation as opposed to cartilage regeneration. The follow-up is also relatively short, at only 12 months, and we will endeavor to follow this group for another year. Only patient-reported outcome measures were used in this study because the primary goal of the intervention was to improve pain, not patient strength or range of motion. Likewise, follow-up imaging was not performed because it was not believed that either injection would result in measurable cartilage regeneration. Finally, BMA was used without any concentration process; it may be that BMAC might have resulted an increased therapeutic effect in this group of patients.
Conclusions
The results of this study demonstrate that patients with GHJ OA that is treated with BMA have superior changes in QuickDASH and EQ-5D-5L pain and health scores but not in WOOS outcomes measures at 12 months post injection, compared to patients treated with cortisone. However, because of the limited number of patients resulting from early termination, larger randomized studies are required to confirm these findings.
Footnotes
Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
References
- 1.Anthony C.A., Westermann R.W., Gao Y., Pugely A.J., Wolf B.R., Hettrich C.M. What are risk factors for 30-day morbidity and transfusion in total shoulder arthroplasty? A review of 1922 cases. Clin Orthop Relat Res. 2015;473:2099–2105. doi: 10.1007/s11999-014-4107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craig R.S., Lane J.C.E., Carr A.J., Furniss D., Collins G.S., Rees J.L. Serious adverse events and lifetime risk of reoperation after elective shoulder replacement: Population-based cohort study using hospital episode statistics for England. BMJ. 2019;364:l298. doi: 10.1136/bmj.l298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eck C.F., Neumann J.A., Limpisvasti O., Adams C.R. Lack of level I evidence on how to prevent infection after elective shoulder surgery. Knee Surg Sports Traumatol Arthrosc. 2018;26:2465–2480. doi: 10.1007/s00167-018-4832-7. [DOI] [PubMed] [Google Scholar]
- 4.Denard P.J., Raiss P., Sowa B., Walch G. Mid- to long-term follow-up of total shoulder arthroplasty using a keeled glenoid in young adults with primary glenohumeral arthritis. J Shoulder Elbow Surg. 2013;22:894–900. doi: 10.1016/j.jse.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Dillon M.T., Inacio M.C., Burke M.F., Navarro R.A., Yian E.H. Shoulder arthroplasty in patients 59 years of age and younger. J Shoulder Elbow Surg. 2013;22:1338–1344. doi: 10.1016/j.jse.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 6.Merolla G., Sperling J.W., Paladini P., Porcellini G. Efficacy of Hylan G-F 20 versus 6-methylprednisolone acetate in painful shoulder osteoarthritis: A retrospective controlled trial. Musculoskelet Surg. 2011;95:215–224. doi: 10.1007/s12306-011-0138-3. [DOI] [PubMed] [Google Scholar]
- 7.Kwon Y.W., Eisenberg G., Zuckerman J.D. Sodium hyaluronate for the treatment of chronic shoulder pain associated with glenohumeral osteoarthritis: A multicenter, randomized, double-blind, placebo-controlled trial. J Shoulder Elbow Surg. 2013;22:584–594. doi: 10.1016/j.jse.2012.10.040. [DOI] [PubMed] [Google Scholar]
- 8.Zhang B., Thayaparan A., Horner N., Bedi A., Alolabi B., Khan M. Outcomes of hyaluronic acid injections for glenohumeral osteoarthritis: A systematic review and meta-analysis. J Shoulder Elbow Surg. 2019;28:596–606. doi: 10.1016/j.jse.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Colen S., Geervliet P., Haverkamp D., Van Den Bekerom M.P. Intra-articular infiltration therapy for patients with glenohumeral osteoarthritis: A systematic review of the literature. Int J Shoulder Surg. 2014;8:114–121. doi: 10.4103/0973-6042.145252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossi L.A., Piuzzi N.S., Shapiro S.A. Glenohumeral osteoarthritis: The role for orthobiologic therapies: Platelet-rich plasma and cell therapies. JBJS Rev. 2020;8 doi: 10.2106/JBJS.RVW.19.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassano J.M., Kennedy J.G., Ross K.A., Fraser E.J., Goodale M.B., Fortier L.A. Bone marrow concentrate and platelet-rich plasma differ in cell distribution and interleukin 1 receptor antagonist protein concentration. Knee Surg Sports Traumatol Arthrosc. 2018;26:333–342. doi: 10.1007/s00167-016-3981-9. [DOI] [PubMed] [Google Scholar]
- 12.Dragoo J.L., Guzman R.A. Evaluation of the consistency and composition of commercially available bone marrow aspirate concentrate systems. Orthop J Sports Med. 2020;8 doi: 10.1177/2325967119893634. 2325967119893634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim G.B., Seo M.S., Park W.T., Lee G.W. Bone marrow aspirate concentrate: Its uses in osteoarthritis. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21093224. 3224-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chahla J., Dean C.S., Moatshe G., Pascual-Garrido C., Serra Cruz R., LaPrade R.F. Concentrated bone marrow aspirate for the treatment of chondral injuries and osteoarthritis of the knee: A systematic review of outcomes. Orthop J Sports Med. 2016;4 doi: 10.1177/2325967115625481. 2325967115625481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shapiro S.A., Kazmerchak S.E., Heckman M.G., Zubair A.C., O'Connor M.I. A prospective, single-blind, placebo-controlled trial of bone marrow aspirate concentrate for knee osteoarthritis. Am J Sports Med. 2017;45:82–90. doi: 10.1177/0363546516662455. [DOI] [PubMed] [Google Scholar]
- 16.Anz A.W., Hubbard R., Rendos N.K., Everts P.A., Andrews J.R., Hackel J.G. Bone marrow aspirate concentrate is equivalent to platelet-rich plasma for the treatment of knee osteoarthritis at 1 year: A prospective, randomized trial. Orthop J Sports Med. 2020;8 doi: 10.1177/2325967119900958. 2325967119900958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centeno C.J., Al-Sayegh H., Bashir J., Goodyear S., Freeman M.D. A prospective multi-site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. J Pain Res. 2015;8:269–276. doi: 10.2147/JPR.S80872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darrow M., Shaw B., Schmidt N., Boeger G., Budgett S. Treatment of shoulder osteoarthritis and rotator cuff tears with bone marrow concentrate and whole bone marrow injections. Cogent Med. 2019;6:1628883. [Google Scholar]
- 19.Samilson R.L., Prieto V. Dislocation arthropathy of the shoulder. J Bone Joint Surg Am. 1983;65:456–460. [PubMed] [Google Scholar]
- 20.Otto A., Muench L.N., Kia C., et al. Proximal humerus and ilium are reliable sources of bone marrow aspirates for biologic augmentation during arthroscopic surgery. Arthroscopy. 2020;36:2403–2411. doi: 10.1016/j.arthro.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Lo I.K., Griffin S., Kirkley A. The development of a disease-specific quality of life measurement tool for osteoarthritis of the shoulder: The Western Ontario Osteoarthritis of the Shoulder (WOOS) index. Osteoarthrit Cartil. 2001;9:771–778. doi: 10.1053/joca.2001.0474. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen J.V., Jakobsen J., Olsen B.S., Brorson S. Translation and validation of the Western Ontario Osteoarthritis of the Shoulder (WOOS) index: The Danish version. Patient Relat Outcome Meas. 2013;4:49–54. doi: 10.2147/PROM.S50976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gummesson C., Ward M.M., Atroshi I. The shortened Disabilities of the Arm, Shoulder and Hand questionnaire (QuickDASH): Validity and reliability based on responses within the full-length DASH. BMC Musculoskelet Disord. 2006;7:44. doi: 10.1186/1471-2474-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matheson L.N., Melhorn J.M., Mayer T.G., Theodore B.R., Gatchel R.J. Reliability of a visual analog version of the QuickDASH. J Bone Joint Surg Am. 2006;88:1782–1787. doi: 10.2106/JBJS.F.00406. [DOI] [PubMed] [Google Scholar]
- 25.Beaton D.E., Katz J.N., Fossel A.H., Wright J.G., Tarasuk V., Bombardier C. Measuring the whole or the parts? Validity, reliability, and responsiveness of the Disabilities of the Arm, Shoulder and Hand outcome measure in different regions of the upper extremity. J Hand Ther. 2001;14:128–146. [PubMed] [Google Scholar]
- 26.Mintken P.E., Glynn P., Cleland J.A. Psychometric properties of the shortened disabilities of the Arm, Shoulder, and Hand Questionnaire (QuickDASH) and Numeric Pain Rating Scale in patients with shoulder pain. J Shoulder Elbow Surg. 2009;18:920–926. doi: 10.1016/j.jse.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Herdman M., Gudex C., Lloyd A., et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavinatto L., Hinckel B.B., Tomlinson R.E., Gupta S., Farr J., Bartolozzi A.R. The role of bone marrow aspirate concentrate for the treatment of focal chondral lesions of the knee: A systematic review and critical analysis of animal and clinical studies. Arthroscopy. 2019;35:1860–1877. doi: 10.1016/j.arthro.2018.11.073. [DOI] [PubMed] [Google Scholar]
- 29.Murphy E.P., McGoldrick N.P., Curtin M., Kearns S.R. A prospective evaluation of bone marrow aspirate concentrate and microfracture in the treatment of osteochondral lesions of the talus. Foot Ankle Surg. 2019;25:441–448. doi: 10.1016/j.fas.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Imam M.A., Holton J., Ernstbrunner L., et al. A systematic review of the clinical applications and complications of bone marrow aspirate concentrate in management of bone defects and nonunions. Int Orthop. 2017;41:2213–2220. doi: 10.1007/s00264-017-3597-9. [DOI] [PubMed] [Google Scholar]
- 31.Hirase T., Jack R.A., 2nd, Sochacki K.R., Harris J.D., Weiner B.K. Systematic review: Is intradiscal injection of bone marrow concentrate for lumbar disc degeneration effective? Cureus. 2020;12 doi: 10.7759/cureus.9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shapiro S.A., Arthurs J.R., Heckman M.G., et al. Quantitative T2 MRI mapping and 12-month follow-up in a randomized, blinded, placebo controlled trial of bone marrow aspiration and concentration for osteoarthritis of the knees. Cartilage. 2019;10:432–443. doi: 10.1177/1947603518796142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray I.R., Robinson P.G., West C.C., et al. Reporting standards in clinical studies evaluating bone marrow aspirate concentrate: A systematic review. Arthroscopy. 2018;34:1366–1375. doi: 10.1016/j.arthro.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 34.Piuzzi N.S., Hussain Z.B., Chahla J., et al. Variability in the preparation, reporting, and use of bone marrow aspirate concentrate in musculoskeletal disorders: A systematic review of the clinical orthopaedic literature. J Bone Joint Surg Am. 2018;100:517–525. doi: 10.2106/JBJS.17.00451. [DOI] [PubMed] [Google Scholar]
- 35.Scarpone M., Kuebler D., Chambers A., et al. Isolation of clinically relevant concentrations of bone marrow mesenchymal stem cells without centrifugation. J Transl Med. 2019;17:10. doi: 10.1186/s12967-018-1750-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yandow S.M., Van de Velde S.K., Siebert J., Perkins S.L. The influence of aspiration volume on the number of osteoblastic progenitors obtained from bone marrow in children. J Pediatr Orthop. 2019;39:382–386. doi: 10.1097/BPO.0000000000000949. [DOI] [PubMed] [Google Scholar]
- 37.Muschler G.F., Boehm C., Easley K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: The influence of aspiration volume. J Bone Joint Surg Am. 1997;79:1699–1709. doi: 10.2106/00004623-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Hernigou P., Homma Y., Flouzat Lachaniette C.H., et al. Benefits of small volume and small syringe for bone marrow aspirations of mesenchymal stem cells. Int Orthop. 2013;37:2279–2287. doi: 10.1007/s00264-013-2017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pettine K.A., Suzuki R.K., Sand T.T., Murphy M.B. Autologous bone marrow concentrate intradiscal injection for the treatment of degenerative disc disease with three-year follow-up. Int Orthop. 2017;41:2097–2103. doi: 10.1007/s00264-017-3560-9. [DOI] [PubMed] [Google Scholar]
- 40.Chevrier A., Darras V., Picard G., et al. Injectable chitosan-platelet-rich plasma implants to promote tissue regeneration: In vitro properties, in vivo residence, degradation, cell recruitment and vascularization. J Tissue Eng Regen Med. 2018;12:217–228. doi: 10.1002/term.2403. [DOI] [PubMed] [Google Scholar]
- 41.Kuznetsov S.A., Mankani M.H., Leet A.I., Ziran N., Gronthos S., Robey P.G. Circulating connective tissue precursors: extreme rarity in humans and chondrogenic potential in guinea pigs. Stem Cells. 2007;25:1830–1839. doi: 10.1634/stemcells.2007-0140. [DOI] [PubMed] [Google Scholar]
- 42.Ziegler C.G., Van Sloun R., Gonzalez S., et al. Characterization of growth factors, cytokines, and chemokines in bone marrow concentrate and platelet-rich plasma: A prospective analysis. Am J Sports Med. 2019;47:2174–2187. doi: 10.1177/0363546519832003. [DOI] [PubMed] [Google Scholar]
- 43.Walsh M., Srinathan S.K., McAuley D.F., et al. The statistical significance of randomized controlled trial results is frequently fragile: A case for a fragility index. J Clin Epidemiol. 2014;67:622–628. doi: 10.1016/j.jclinepi.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 44.Khan M., Evaniew N., Gichuru M., et al. The fragility of statistically significant findings from randomized trials in sports surgery: A systematic survey. Am J Sports Med. 2017;45:2164–2170. doi: 10.1177/0363546516674469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.