Abstract
Purpose
To determine the ideal location for anterior cruciate ligament (ACL) suspensory cortical button placement on the lateral femur with the highest failure load and to establish the relationship of tunnel diameter and cortical thickness on load to failure.
Methods
Computed tomography (CT) data were obtained from 45 cadaveric distal femurs. A Cartesian coordinate system was established along the lateral femur with the lateral epicondyle (LE) as a reference point. Locations 0, 20 and 30 mm from the LE along lines 0°, 25°, 50°, and 75° posterioproximal from the axial plane were created. Tunnels connecting from each location to the center of the ACL footprint were simulated. Cortical thickness and long axis diameter of the oval cortical holes were determined for each location. Based on the CT data, custom drill guides were created and used to drill 4.5 mm tunnels at each lateral femur location to the ACL footprint on the cadaver femurs. Cortical buttons were placed at each location and pulled using a servohydraulic testing system. The correlation of tunnel diameter and cortical thickness to button failure load were analyzed using a regression analysis.
Results
Significant differences were found for failure load (P<.0001) and cortical thickness between the locations tested (P<.0001). The location 30 mm proximal from the LE and 75⁰ from the axial plane had the highest failure load of 573 N. A regression analysis (R2 = .15) indicated that the cortical thickness was significantly correlated with load to failure (P <.0001), whereas the long-axis diameter was not (P = .33).
Conclusion
The ideal cortical button location on the lateral femur for ACL suspensory fixation was located 30 mm proximal from the lateral epicondyle, based on this area’s high failure load. Oblique tunnel drilling of this proximal location may cause a larger long-axis diameter cortical hole, but the cortex is also thicker, which is more closely correlated with failure load.
Clinical Relevance
Different ACL suspensory cortical button locations on the lateral femur have different failure loads based on the cortical thickness of the bone supporting the button. It is important for surgeons to understand which drilling techniques place the button in a proximal and posterior location, especially if the bone quality of the patient is of concern.
There are several different ways to drill a lateral femur tunnel for suspensory fixation of ACL grafts, including transtibial, accessory anteromedial portal, and outside-in techniques.1, 2, 3 Overall, graft survival rates for ACL reconstruction are excellent, with a report of up to 91% survival at 25 years.4 When ACL suspensory fixation fails due to cortical button fixation, a reported mechanism for failure is cortical penetration at the button site on the lateral femur.5,6 An alternate mechanism of failure for ACL suspensory fixation is due to cyclic loading, as described by Petre et al; however, this mechanism is outside of the scope of this study.7 While most authors agree that the tunnel aperture in the notch should be located at the native ACL footprint in order to provide increased stability and decrease the rate of late degenerative changes in the knee, there is still no consensus regarding the location on the lateral femur for cortical button placement.8
This lack of consensus is evidenced through several studies examining tunnel placement on the lateral femur.5,9 A recent Computed Tomography (CT) study showed evidence that starting 2 cm or more from the lateral epicondyle may make a larger hole due to the obliquity of the drill bit.5 Research has shown that increased tunnel obliquity, along with increased diameter of the oval tunnel aperture, would not provide ample surface area for the cortical button, and the repair would be more likely to fail.5 In contrast, Lubowitz et al. showed that oblique drilling best recreated the ACL footprint, and provides the optimal femoral tunnel.9
The purpose of this study was to determine the ideal location for anterior cruciate ligament (ACL) suspensory cortical button placement on the lateral femur with the highest failure load and establish the relationship of tunnel diameter and cortical thickness on load to failure. Our hypothesis was that more proximal locations would have higher load to failure and that lateral femoral cortex thickness would have a high correlation with load to failure.
Methods
Materials
Forty-five fresh frozen knee specimens were harvested from cadavers with an average age of 71 ± 19 years. The specimens were scanned using a GE scanner (GE Healthcare) with a pixel spacing of 0.496 mm and a slice thickness of 0.625 mm. The obtained images were then converted into 3-dimensional (3D) models using the freeware 3D Slicer10 (www.slicer.org) and exported to Rhinoceros 3D (Robert McNeal, Seattle, WA) in stereolithography format.
CT Data Analysis
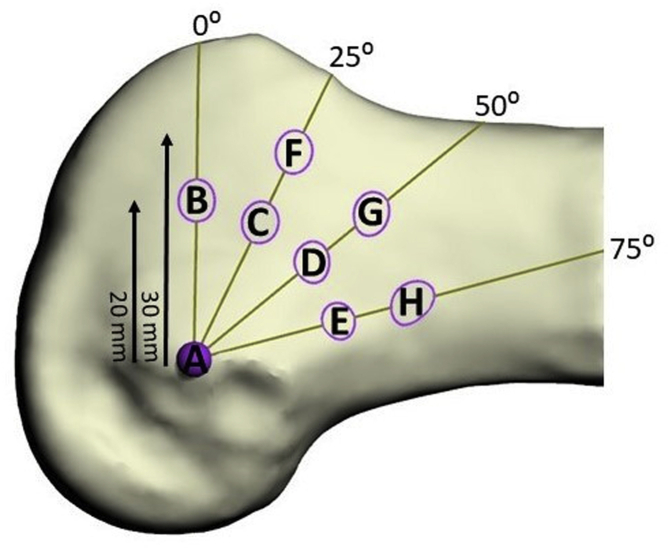
A custom-made script was created to analyze the 3D models. Each reconstructed femur was aligned in the coronal plane so that the 2 condyles would sit on the transverse plane and on the sagittal plane with the anatomic axis parallel to the coronal. An experienced surgeon identified the anterior cruciate ligament (ACL) tunnel entry point at the lateral bifurcate ridge, posterior to the lateral intercondylar ridge on the lateral epicondyle. Similar to that already proposed in a previous study,5 a Cartesian coordinate system was produced on each lateral femur using the lateral epicondyle (LE) as a reference. The algorithm sectioned each knee by intersecting it with the transverse or axial planes drawn at the LE perpendicular to the long axis of the femur.11 We then rotated in the sagittal plane at 0, 25°, 50°, and 75° from the transverse plane (clockwise for a left knee, counterclockwise for a right knee). Starting from the LE on each plane, points were identified at 20 and 30 mm away (Fig 1).
Fig 1.
Cartesian coordinate system and tunnel entry points created by algorithm along the lateral femur with the lateral epicondyle as a reference point. Entry points identified at 0, 20 and 30 mm from the lateral epicondyle 0⁰, 25⁰, 50⁰, and 75° from the transverse plane.
Vectors were drawn from the ACL entry point. Considering a tunnel of a diameter of 4.5 mm, these vectors were used to compute the effective transverse and sagittal inclinations and lengths and the long axis at the outlet point for each tunnel. A set of spheres corresponding to each considered point was transposed in the CT reference frame and exported back to the 3D slicer for the measurement of densities. Cortical density was evaluated as average Hounsfield units measured within the portion of the sphere intersecting the segmented bone; cortical thickness was measured as the mediolateral dimension taken on the transverse slice in correspondence of each point, from the most medial to the most lateral point of the segmentation performed at the threshold values of the cortical bone. Because the cortical button is loaded from the cortical side, the cortical thickness did not include cancellous bone medial to the cortex.
Application of Drill-Guide Template
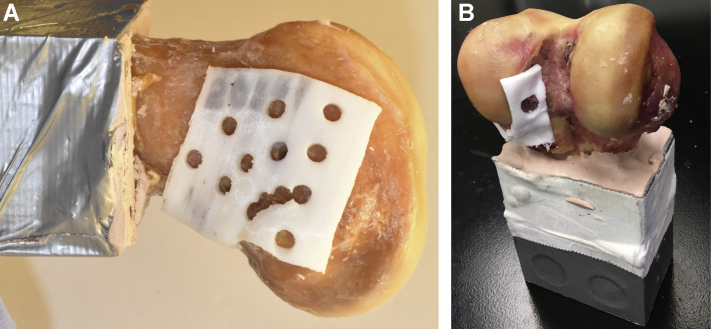
Performing an offset of the femoral geometry in correspondence with the identified holes, a drilling template was then generated for each knee and was 3D-printed to ensure consistency between the analyzed CT data and the subsequently drilled tunnels (Fig 2).
Fig 2.
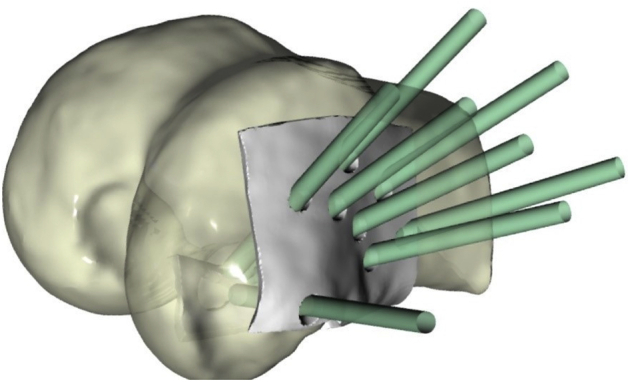
Illustration of 3D-printed drill guide template for accurate tunnel placement. Green cylinders demonstrate trajectories of 8 different tunnels for anterior cruciate ligament suspensory fixation with cortical button on the lateral femur.
The resulting drill-guide templates for each femur were 3D-printed using a Monoprice MP Select Mini 3D printer V2 (Monoprice, Brea, CA) with a poly-lactic acid filament. The templates factored into the prechosen drill locations as well as the native ACL footprint location for each femur so as to facilitate drilling. This was done to ensure that the vectors created using the models in the 3D slicer could be prepared accurately on the cadaveric femurs for mechanical testing.
Using the custom 3D-printed drill templates (Fig 3), 4.5 mm tunnels for each lateral femur location were prepared using the outside-in (OI) technique. This was done by placing a 2.4 mm guide pin through an ACL drill guide that was set at each lateral femur location, then reaming with a 4.5 cannulated drill. The 4.5 mm cortical tunnel was used because tunnels this size have been analyzed in previous studies and allow for easy passage of many different cortical buttons.12, 13, 14 Additionally, tunnels of this diameter simulate the outer diameter of the step guide, which some surgeons impact into the lateral cortex when drilling the femur inside-out. The larger femoral tunnel, where the ACL graft sits inside a drilled socket, was not created in this study. For each tunnel location tested, an ACL cortical button (TightRope RT button; Arthrex, Naples, FL) was placed on the lateral cortex with a threaded #5 nonabsorbable suture loaded through the tunnel (FiberWire; Arthrex).
Fig 3.
Right distal femur with 3D-printed custom template guide. 3A, Lateral femur viewed from lateral to medial. The anterior cruciate ligament (ACL) drill guide was placed in 1 of the holes to start. 3B. Distal femur viewed from posterior to anterior. The custom template guide has an exit hole over the ACL footprint. The ACL aiming guide was placed so the drill would exit in the ACL footprint hole.
Mechanical Testing
All 45 tested specimens were potted using a polyester resin (Bondo; 3M, Maplewood, MN) in steel boxes and tested using an Instron 8874 (Instron) mechanical testing system. In each of the 45 femurs, 2 or 3 locations were tested; the locations chosen were those farthest away from each other. Each specimen was loaded with the suture-button complex by manually guiding the suture through the femoral tunnel from lateral to medial along the axis tunnel to ensure absence of contact between the suture and the edges of the tunnel in order to exclude suture failure due to bone cutting. The fixture was free to slide in both directions of the transverse plane to ensure absence of forces with directions different from those of the tunnel (Fig 4). The orientation of the button in respect to the tunnel aperture was not chosen; the button was simply allowed to rest where it settled. This was done because manipulating the button’s orientation is very difficult in vivo. Slippage of the suture-button complex was minimized by means of backup knots, identical to the ones used in the operating room, that were placed on top of the button.
Fig 4.
Failure load testing of a right distal femur.
Specimens were preconditioned with a load of 5 Newtons (N) for tension of the suture and tested until the cortical button penetrated into the femur or the suture failed at a displacement rate of 5 mm/min. Failure was defined as a drop in force greater than 80% from the peak load. The latter was used as reference load to indicate the strength of each tested configuration. The specimens were examined after failure to determine whether the button had penetrated the cortex or the suture had ruptured.
Statistical Analysis
Numeral data from each button location was analyzed using ANOVA. Post hoc analysis to compare data for each button location was performed using the Tukey HSD honestly significant difference. A regression analysis was performed to determine the correlation between cortical thickness and tunnel diameter to failure load.
An a priori power analysis was performed on a small sample of 5 buttons in location A and 5 in location F. Based on a formula for calculating a sample size to determine power or a 1 – B of 0.8, it was determined that the sample size needed in each group was 9.
Results
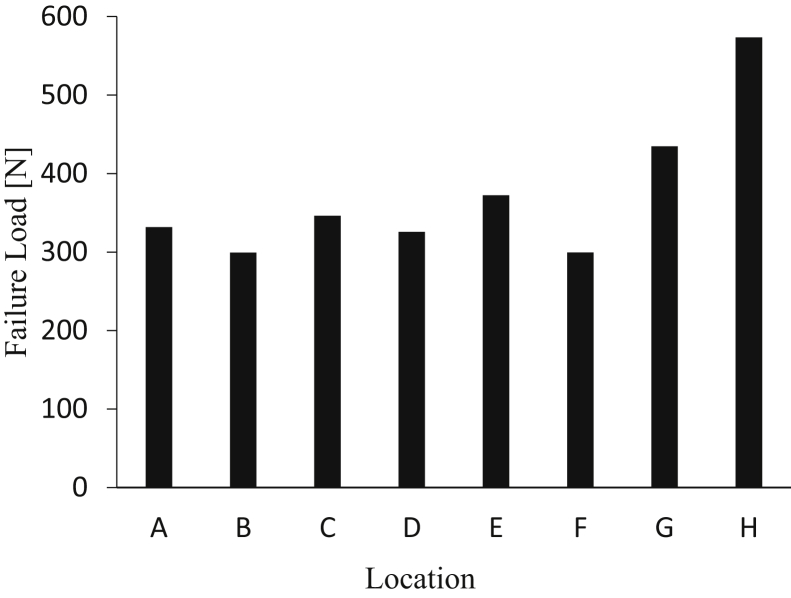
We performed 112 tests on a total of 45 femurs. Each location was tested on 14 different specimens. In each of the 45 femurs, 2 or 3 locations were tested, with locations chosen that were farthest away from each other. There was a difference in failure load (Fig 5) among the 8 locations tested (P<.0001). Location H (the location 30 mm proximal from the lateral epicondyle and 75⁰ from the transverse plane or axial plane) had the highest failure load of 573 N. Post hoc analyses demonstrated that location H had a higher load to failure than locations A through F (P = .003, P = .0005, P = .007, P = .002, P = .03, P = .004, respectively), but no significant difference existed compared to location G (P = .31).
Fig 5.
Failure load (N) of lateral femur suspensory button fixation based on location.
There was a significant difference in the cortical thickness among the various locations tested (P<.0001). Location H had an average cortical thickness of 3.8±1.4 mm and was significantly thicker than locations A, B, C, D, and F (P < .01, P <.0001, P = .001, P = .003, P = .009), but with no difference from locations E (P = .73) and G (P = .21). Location E was significantly thicker than only location B (P = .0045). Locations F, G and H averaged the greatest long-axis tunnel diameter with 6.1±0.5, 6.0±0.5 and 6.0±0.8 mm, respectively. Averages for failure load, long-axis tunnel diameter and cortical thickness are displayed in Table 1. Post hoc analysis showed that locations F, G and H had larger diameters than B, C and D (P<.001).
Table 1.
Average Load to Failure, Largest Tunnel Diameter and Cortical Thickness at Each Button Location on the Lateral Femur For ACL Suspensory Fixation
| Button Location | Failure Load (N) | SD | Largest Tunnel Diameter (mm) | SD | Cortical Thickness (mm) | SD |
|---|---|---|---|---|---|---|
| A | 331.8 | 92.4 | 5.6 | 0.6 | 2.2 | 0.6 |
| B | 299.3 | 150.1 | 5.3 | 0.4 | 1.3 | 0.5 |
| C | 346.3 | 108.3 | 5.3 | 0.4 | 1.7 | 0.7 |
| D | 325.8 | 173.5 | 5.2 | 0.3 | 2.0 | 0.8 |
| E | 372.3 | 188.7 | 5.1 | 0.4 | 3.1 | 1.9 |
| F | 299.5 | 146.1 | 6.1 | 0.5 | 2.1 | 1.4 |
| G | 434.7 | 173.0 | 6.0 | 0.5 | 2.7 | 1.6 |
| H | 573.4 | 211.0 | 6.0 | 0.8 | 3.8 | 1.4 |
A regression analysis provided the relationship between max load (Fmax), tunnel diameter (D), and cortical thickness (tc) displayed in Equation 1. The adjusted R2 was 0.15. Equation 1
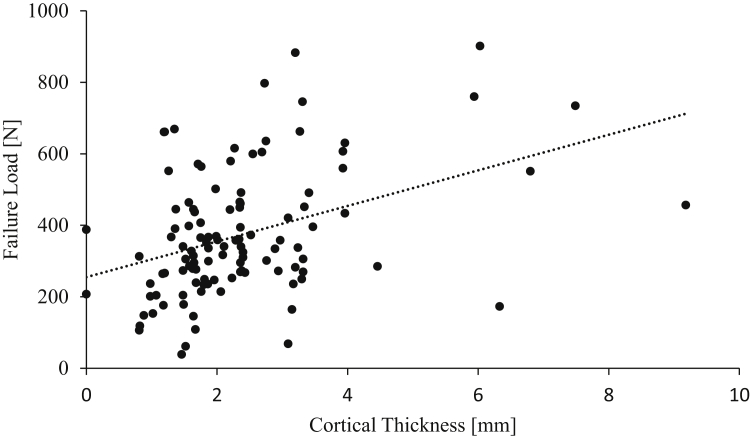
The regression analysis also revealed that cortical thickness was the main factor associated with failure load (P<.0001), whereas diameter was not significantly associated with failure load (P = .33). Additionally, cortical thickness showed a moderate correlation R=.40) (Fig 6), whereas diameter showed a weak correlation (R=.14.
Fig 6.
Correlation of cortical thickness (mm) and failure load (N) on the lateral femur for ACL suspensory fixation.
There were 101 failures due to button penetration and 11 failures due to suture rupture (Table 2). There was no difference in the mode of failure among the various button locations (P = .336). When comparing samples that failed due to button penetration and samples that failed due to suture rupture, the load to failure was 615N±129 vs 347±161 respectively (P<.0001).
Table 2.
Mode of Failure for ACL Suspensory Fixation by Location of Button on Lateral Femur Cortex
| Button Location | Suture Rupture | Button Penetration of Cortex |
|---|---|---|
| A | 0 | 14 |
| B | 0 | 14 |
| C | 1 | 13 |
| D | 1 | 13 |
| E | 3 | 11 |
| F | 1 | 14 |
| G | 3 | 11 |
| H | 2 | 13 |
There was no difference in the mode of failure among the various button locations (P=.336).
Discussion
This study found that the ideal cortical button location on the lateral femur was positioned at 30 mm proximal from the lateral epicondyle and 75˚ from the anterior-posterior axis (position H, Fig 1). Button placement in this position can be achieved intraoperatively by the surgeon’s aiming slightly more proximally and posteriorly on the lateral femoral cortex when choosing a starting point for OI drilling. These findings were reached by using cadaveric femurs to evaluate the difference in failure load of cortical buttons in 8 separate locations, using the OI technique. Specifically, this study examined the cortical thickness, tunnel dimensions and pull-out strength of each location. Our study determined that location H (proximal, posterior and lateral) had the highest failure load of all locations because of the greater cortical thickness, despite its larger diameter hole.
The OI technique was used to drill all tunnels in this study in order to drill tunnels precisely at each of the lateral cortex locations. One advantage of the OI technique is that it allows surgeons more freedom in their placement of their femoral guide pin in relation to the lateral aspect of the femur. This contrasts with the transtibial technique, which may result in vertical tunnel placements, and the anteromedial (AM) transportal technique, which may result in shorter tunnels or posterior wall blowout.15, 16, 17, 18, 19 Because of the freedom that exists with tunnel placement in the OI technique, recent literature has attempted to identify the ideal femoral tunnel location. The various lateral cortex locations are also relevant to ACL repair techniques, OI techniques with retrograde reaming, transtibial, and AM drilling techniques. Our study adds to the literature from previous studies because it analyzed failure load of suspensory cortical button fixation at each location in cadavers and also evaluated their relation to cortical thickness and tunnel diameter.
The values obtained in this study for load to failure at the various button locations (between 299.3 N and 573.4 N) are consistent with previous studies that evaluated cadaveric ACL graft suspensory fixation load to failure.6,20 Herbort et al. examined the effect of accidental perforation of the lateral femoral cortex on ACL suspensory button fixation failure.6 They found a maximum failure load of 595.98 N and yield load of 387.9 N. Additionally, they found the predominate mode of failure for most perforation specimens was button pull-through into the tunnel, which was similar to our study, in which 90% of the devices failed due to cortex penetration. Another study, by Weimann et al., demonstrated a maximum load of 618 N and yield load of 360.5 N for an ACL suspensory cortical button.20 These values of failure load are comparable to those in our study. Additionally, Conner et al. also compared their load to failure at both the anterior and lateral femoral cortices.21 The authors found failure loads of 876 N and 987 N for 2 different types of ACL suspensory buttons on the lateral femur.21 These failure loads are higher than those in our study, but they used porcine femurs, which makes a direct comparison difficult.
Lubowitz et al. studied various pin insertion angles in the femur and their effect on the ACL footprint.9 Their study concluded that during OI drilling of the ACL femoral socket, a guide pin entrance angle of 60° to a line perpendicular to the femoral anatomic axis, combined with a guide pin entrance angle of 20° to the transepicondylar axis, resulted in optimal reconstruction of the normal human anatomic ACL femoral footprint length, width, area, and angular orientation. This location described by Lubowitz would likely place the cortical button in a proximal posterolateral location, which is also the region with the highest failure load in our study. Additionally, Gadikota et al. determined that tibial tunnel-independent techniques can produce more anatomic tunnels than the transtibial technique.15 Smith et al. and Forsythe et al. show that the anatomic footprint of the ACL is the most desirable location for the intra-articular aspect of the femoral graft because it allows for a point that is the most isometric during knee flexion.22,23 These studies showed the ideal position in which to reproduce the most anatomic ACL footprint, but they did not evaluate the failure load at each suspensory button location.
In addition to evaluating the ACL footprint, some studies have evaluated suspensory cortical button hole diameter in various tunnel locations. Herbort et al. showed that when the lateral cortex is penetrated with drill sizes larger than 6 mm, cortical ACL suspensory fixation has worse properties.6 Okazaki et al. concluded that the risk of fixation failure of a cortical button increases if the entry point for drilling is 2 cm or farther from the lateral epicondyle and the tunnel diameter is more than 5 mm.5 They came to this conclusion by demonstrating that as the tunnel entry point in the OI femoral drilling technique moves away from the lateral epicondyle, the drilling angle becomes more oblique relative to the cortical surface, and the oval-shaped aperture becomes longer. In contrast, our results show that cortical thickness, not diameter, is the most important variable in the location of the button on the lateral femur. As shown above, location H, despite having the largest diameter of the 8 locations tested, had the highest load to failure. Through a regression analysis, our study showed that cortical thickness was the main factor associated with failure load (P<.0001), whereas diameter was not significantly associated with failure load (P=.33). It should be noted that the largest average diameter we tested (location F) was 6.1 mm, and the largest individual sample diameter was 7.1 mm. This is compared to a button size of 20 mm by 5 mm, which was used in this study (ACL TightRope button; Arthrex). Testing much larger diameters would be likely to contribute to failure at the interface of the button and the lateral cortex.
The current study focused on OI drilling techniques, but some surgeons prefer other drilling techniques that may place the suspensory fixation button in locations similar to those in our study. Gadikota et al. studied transtibial, anteromedial and OI techniques, and their study placed cortical buttons near our locations G, E and D, respectively.15 Osaki et al. evaluated 2 bundle techniques in 120 degrees of flexion and 135° of flexion.24 In 120° of flexion, their suspensory fixation was near our location H, and at 135° of flexion, it was near our location E through an anteromedial portal. In summary, most transtibial techniques place the button around location G. Most anteromedial drilling techniques place the button around location E or H, which are regions with the thickest cortex
One of the advantages of OI drilling techniques is that one can place the button in any of the previously mentioned locations. More proximal button locations may have larger diameter holes due to more oblique drilling, but they also have thicker associated cortices. This concept of varying cortical thickness or densities was demonstrated throughout various regions of the distal lateral femur in our study. We determined that the more proximal regions had cortical thicknesses averaging from 2.7 mm to 3.8 mm, whereas the more distal and anterior regions had lower cortical thicknesses of 1.3 mm. The lateral epicondyle, which was also a distal region, however, had an intermediate thickness of 2.2 mm. These different regions can be described as densitome regions of the same bone with similar densities or cortical thickness. Overall, our data demonstrate that the lateral femur cortical thickness is much more important than the hole diameter when it comes to button failure. When placing the button in a more posterior lateral location on the distal femur cortex, the hole may be larger, but the cortex is also thicker.
Limitations
Possible limitations of this study include the use of an OI technique for all button locations, including locations typically seen in transtibial and anteromedial drilling techniques. Possible future studies could attempt to reproduce the data from this study using transtibial and anteromedial techniques. Because of the use of fully dissected femoral specimens, the creation of the tunnels using the OI technique in this study does not fully resemble tunnel creation in vivo. The method we used was employed to ensure consistency and precision in using a 3D template without needing to take into account the surrounding soft tissue. Another possible limitation of this study is that the orientation of the button was not being controlled in relation to the size of each drill hole. The decision not to control the orientation of the button in this study was made purposefully, because button orientation is often difficult to control clinically. Additionally, some surgeons use a 3.5 mm or 4 mm hole for passing cortical buttons, whereas our study used 4.5 mm drills. This was done so as to use the largest size described for passing suspensory buttons in various described techniques.12, 13, 14 Also, suture slippage in the button was not evaluated in this study; however, it was minimized by placing several backup knots over the top of the button. Last, an ACL tendon graft was not included in the testing. This was done to reduce the variability of load-to-failure measurements induced by the graft-suture interface.
Conclusions
The ideal cortical button location on the lateral femur for ACL suspensory fixation was located 30 mm proximal from the lateral epicondyle, based on this area’s high failure load. Oblique tunnel drilling in this proximal location may cause a larger long-axis-diameter cortical hole, but the cortex is also thicker, which is more closely correlated with failure load.
Acknowledgments
The authors acknowledge Angelia Lewis and Curtiss Walters for the CT imaging and Mr. Alan Ogden for assistance in mechanical testing.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: P.A.M. received grants from Arthrex and honoraria from Vericel, outside the submitted work. R.S.B. received educational support from Midsouth Orthopedics, consulting fees and hospitality payments from Zimmer Biomet, and hospitality payments from Arthrex, outside the submitted work. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
References
- 1.Giron F., Cuomo P., Edwards A., Bull A.M.J., Amis A.A., Aglietti P. Double-bundle “anatomic” anterior cruciate ligament reconstruction: A cadaveric study of tunnel positioning with a transtibial technique. Arthrosc J Arthrosc Relat Surg. 2007;23:7–13. doi: 10.1016/j.arthro.2006.08.008. https://doi.10.1016/j.arthro.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Kaseta M.K., DeFrate L.E., Charnock B.L., Sullivan R.T., Garrett W.E. Reconstruction technique affects femoral tunnel placement in ACL reconstruction. Clin Orthop Relat Res. 2008;466:1467–1474. doi: 10.1007/s11999-008-0238-z. https://doi.10.1007/s11999-008-0238-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lubowitz J.H. Anteromedial portal technique for the anterior cruciate ligament femoral socket: Pitfalls and solutions. J Arthrosc Relat Surg. 2009;25:95–101. doi: 10.1016/j.arthro.2008.10.012. https://doi.10.1016/j.arthro.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Sanders T.L., Pareek A., Hewett T.E., et al. Long-term rate of graft failure after ACL reconstruction: A geographic population cohort analysis. Knee Surgery, Sport Traumatol Arthrosc. 2017;25:222–228. doi: 10.1007/s00167-016-4275-y. https://doi.10.1007/s00167-016-4275-y. [DOI] [PubMed] [Google Scholar]
- 5.Okazaki K., Matsubara H., Osaki K., et al. Femoral tunnel apertures on the lateral cortex in anterior cruciate ligament reconstruction: An analysis of cortical button fixation. J Arthrosc Relat Surg. 2014;30:841–848. doi: 10.1016/j.arthro.2014.03.004. https://doi.10.1016/j.arthro.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Herbort M., Heletta S., Raschke M.J., et al. Accidental perforation of the lateral femoral cortex in ACL reconstruction: An investigation of mechanical properties of different fixation techniques. J Arthrosc Relat Surg. 2012;28:382–389. doi: 10.1016/j.arthro.2011.10.028. https://doi.10.1016/j.arthro.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 7.Petre B.M., Smith S.D., Jansson K.S., et al. Femoral cortical suspension devices for soft tissue anterior cruciate ligament reconstruction: A comparative biomechanical study. Am J Sports Med. 2013;41:416–422. doi: 10.1177/0363546512469875. https://doi.10.1177/0363546512469875. [DOI] [PubMed] [Google Scholar]
- 8.Hensler D., Working Z.M., Illingworth K.D., Thorhauer E.D., Tashman S., Fu F.H. Medial portal drilling: Effects on the femoral tunnel aperture morphology during anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2011;93:2063–2071. doi: 10.2106/JBJS.J.01705. https://doi.10.2106/JBJS.J.01705. [DOI] [PubMed] [Google Scholar]
- 9.Lubowitz J.H., Akhavan S., Waterman B.R., Aalami-Harandi A., Konicek J. Technique for creating the anterior cruciate ligament femoral socket: Optimizing femoral footprint anatomic restoration using outside-in drilling. J Arthrosc Relat Surg. 2013;29:522–528. doi: 10.1016/j.arthro.2012.10.007. https://doi.10.1016/j.arthro.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Fedorov A., Beichel R., Kalpathy-Cramer J., et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30:1323–1341. doi: 10.1016/j.mri.2012.05.001. https://doi.10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moatshe G., Brady A.W., Slette E.L., et al. Multiple ligament reconstruction femoral tunnels. Am J Sports Med. 2017;45:563–569. doi: 10.1177/0363546516673616. https://doi.10.1177/0363546516673616. [DOI] [PubMed] [Google Scholar]
- 12.Colombet P., Graveleau N. An anterior cruciate ligament reconstruction technique with 4-strand semitendinosus grafts, using outside-in tibial tunnel drilling and suspensory fixation devices. Arthrosc Tech. 2015;4:e507–e511. doi: 10.1016/j.eats.2015.05.014. https://doi.10.1016/j.eats.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eguchi A., Ochi M., Adachi N., Deie M., Nakamae A., Usman M.A. Mechanical properties of suspensory fixation devices for anterior cruciate ligament reconstruction: Comparison of the fixed-length loop device versus the adjustable-length loop device. Knee. 2014;21:743–748. doi: 10.1016/j.knee.2014.02.009. https://doi.10.1016/j.knee.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Lanzetti R.M., Monaco E., De Carli A., et al. Can an adjustable-loop length suspensory fixation device reduce femoral tunnel enlargement in anterior cruciate ligament reconstruction? A prospective computer tomography study. Knee. 2016;23:837–841. doi: 10.1016/j.knee.2016.01.015. https://doi.10.1016/j.knee.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Gadikota H.R., Sim J.A., Hosseini A., Gill T.J., Li G. The relationship between femoral tunnels created by the transtibial, anteromedial portal, and outside-in techniques and the anterior cruciate ligament footprint. Am J Sports Med. 2012;40:882–888. doi: 10.1177/0363546511434276. https://doi.10.1177/0363546511434276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bedi A., Raphael B., Maderazo A., Pavlov H., Williams R.J. Transtibial versus anteromedial portal drilling for anterior cruciate ligament reconstruction: A cadaveric study of femoral tunnel length and obliquity. J Arthrosc Relat Surg. 2010;26:342–350. doi: 10.1016/j.arthro.2009.12.006. https://doi.10.1016/j.arthro.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Dargel J., Schmidt-Wiethoff R., Fischer S., Mader K., Koebke J., Schneider T. Femoral bone tunnel placement using the transtibial tunnel or the anteromedial portal in ACL reconstruction: A radiographic evaluation. Knee Surg Sport Traumatol Arthrosc. 2009;17:220–227. doi: 10.1007/s00167-008-0639-2. https://doi.10.1007/s00167-008-0639-2. [DOI] [PubMed] [Google Scholar]
- 18.Matsubara H., Okazaki K., Osaki K., et al. Optimal entry position on the lateral femoral surface for outside-in drilling technique to restore the anatomical footprint of anterior cruciate ligament. Knee Surg Sport Traumatol Arthrosc. 2016;24:2758–2766. doi: 10.1007/s00167-014-3460-0. https://doi.10.1007/s00167-014-3460-0. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura M., Deie M., Shibuya H., et al. Potential risks of femoral tunnel drilling through the far anteromedial portal: A cadaveric study. J Arthrosc Relat Surg. 2009;25:481–487. doi: 10.1016/j.arthro.2008.11.010. https://doi.10.1016/j.arthro.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Weimann A., Zantop T., Herbort M., Strobel M., Petersen W. Initial fixation strength of a hybrid technique for femoral ACL graft fixation. Knee Surg Sport Traumatol Arthrosc. 2006;14:1122–1129. doi: 10.1007/s00167-006-0159-x. https://doi.10.1007/s00167-006-0159-x. [DOI] [PubMed] [Google Scholar]
- 21.Conner C.S., Perez B.A., Morris R.P., Buckner J.W., Buford W.L., Ivey F.M. Three femoral fixation devices for anterior cruciate ligament reconstruction: Comparison of fixation on the lateral cortex versus the anterior cortex. J Arthrosc Relat Surg. 2010;26:796–807. doi: 10.1016/j.arthro.2009.10.015. https://doi.10.1016/j.arthro.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Smith J.O., Yasen S., Risebury M.J., Wilson A.J. Femoral and tibial tunnel positioning on graft isometry in anterior cruciate ligament reconstruction: A cadaveric study. J Orthop Surg. 2014;22:318–324. doi: 10.1177/230949901402200310. https://doi.10.1177/230949901402200310. [DOI] [PubMed] [Google Scholar]
- 23.Forsythe B., Lansdown D., Zuke W.A., et al. Dynamic 3-dimensional mapping of isometric anterior cruciate ligament attachment sites on the tibia and femur: Is anatomic also isometric? J Arthrosc Relat Surg. 2018;34:2466–2475. doi: 10.1016/j.arthro.2018.03.033. https://doi.10.1016/j.arthro.2018.03.033. [DOI] [PubMed] [Google Scholar]
- 24.Osaki K., Okazaki K., Tashiro Y., Matsubara H., Iwamoto Y. Influences of knee flexion angle and portal position on the location of femoral tunnel outlet in anterior cruciate ligament reconstruction with anteromedial portal technique. Knee Surgery, Sport Traumatol Arthrosc. 2015;23:777–784. doi: 10.1007/s00167-013-2705-7. https://doi.10.1007/s00167-013-2705-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.