Abstract
A massive vaccination campaign against the global COVID-19 pandemic caused by SARS-CoV-2 virus began worldwide in January 2021. However, studies continue to investigate the most effective and safe drug therapies to manage the various stages of viral infection. It is critical in the therapeutic management of the patient, with ongoing COVID-19 infection, to reduce viral load and replication, and to regulate the generalized hyperinflammatory state caused by the cytokine storm that occurs in the most severe phases. Probably the right drug therapy is represented by the use of different drugs acting in different modalities and on different targets, to avoid also viral drug resistance. In this article, we describe an interesting scientific pharmacological hypothesis arising from the evidence in the literature; we believe that the association of baricitinib/remdesivir/rhACE2, administered at the right time and dose, represents an important pharmacological synergism that can be therapeutically more effective for the treatment of COVID-19 infection than the single administration of drugs and avoid the phenomenon of drug resistance caused by the virus.
Keywords: COVID-19, SARS-CoV-2, Baricitinib, Remdesivir, rhACE2
Highlights
A new perspective on SARS-CoV-2 management
Baricitinib/remdesivir/rhACE2 may have an effective synergism of action
Avoiding viral drug resistance is of paramount importance
Introduction
COVID-19 infection
The current global pandemic caused by the SARS-coV-2 coronavirus is ongoing. Data recorded as of August 30, 2021, indicate approximately 216 Mln infected and 4.5 Mln dead (World health organization (WHO) 2021; Wang et al. 2020; Huang et al. 2020). In January 2021, a massive vaccination campaign began worldwide (Vitiello et al. 2021a). SARS-CoV-2 infection is responsible for coronavirus disease (COVID-19), and is transmitted from human to human. Viral infection can have an asymptomatic or mildly symptomatic course in most cases. Symptomatic forms include clinical manifestations such as loss of sense of smell, fatigue, fever, cough, and dyspnea (Li et al. 2020; Zhu et al. 2019; Gorbalenya et al. 2020). In severe cases, there may be pulmonary and cardiac injury caused by an abnormal and dysregulated response of the inflammatory/immune system induced by a massive and sudden release of proinflammatory mediators such as cytokines and chemokines (Guarner 2020; Vitiello and Ferrara 2021a; Vitiello et al. 2021b). SARS-CoV-2 coronavirus uses ACE2 glycoprotein as a cellular entry receptor, which is expressed in various tissues (Vitiello and Ferrara 2020a; Vitiello et al. 2021c). ACE2 is a key regulator of the RAS renin-angiotensin system (Vitiello and Ferrara 2021b). Several pharmacological treatments for COVID-19 infection have been used and tested at this time, including anti-inflammatory/immunomodulatory agents, anticoagulants, convalescent plasma, and antivirals indicated for other diseases (Ferrara et al. 2020; Vitiello and Ferrara 2020b; Ferrera and Vitiello 2021). Clinical evidence associates good efficacy in the management of hyperinflammation occurring in the more severe stages of the disease with baricitinib and antiviral activity with remdesivir (Jorgensen et al. 2020; Al-Tawfiq et al. 2020; Vitiello et al. 2020; Favalli et al. 2020). In addition, an interesting line of research proposes the administration of soluble recombinant rhACE2 as a “trap effect” for the circulating virus (Muslim et al. 2020). Probably, the combined use of these three drugs may represent an important pharmacological synergism in the management of COVID-19 infection and avoid the phenomenon of viral drug resistance.
Baricitinib/remdesivir/rhACE2
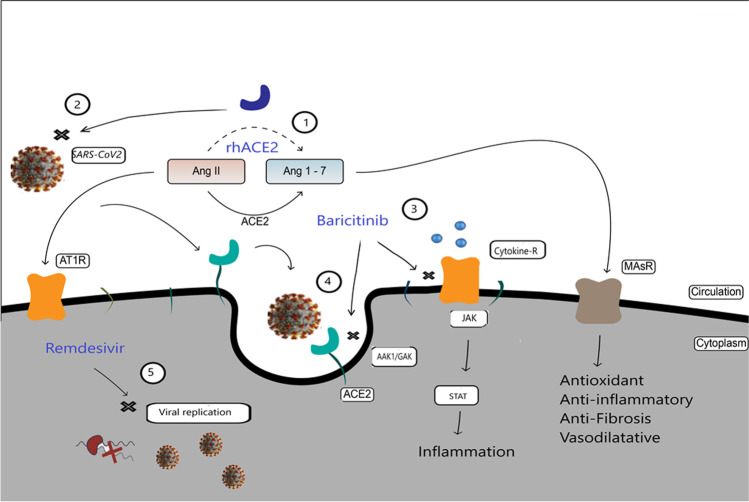
COVID-19 infection is complex. Some individuals are more susceptible to serious complications than others, and the course of the disease is unpredictable in a large percentage of cases. Evidence shows that SARS-CoV-2 infection affects differently men and women, young and old. It seems that elderly subjects and with comorbidities are more at risk of severe COVID-19 infection; despite this, there are cases of young subjects without pre-existing pathologies who have suffered serious consequences. In the scientific world, the idea that genetic factors of the host can influence individual susceptibility to the disease and its progression is increasingly strengthened, suggesting that COVID-19 disease is a true “multifactorial pathology” from this point of view. In view of these aspects, the type of drugs administered, the timing, and the dose appear to be key components to avoid serious complications from COVID-19. Several pharmacological treatments have been tested for the management of COVID-19 viral infection, immunomodulators, anticoagulants, plasma derivatives, and antivirals (Jorgensen et al. 2020; Ferrara and Vitiello 2020). Among the various agents used, we believe that the combined administration of baricitinib, remdesivir, and rhACE2 represents a potential and important pharmacological synergism that can counteract SARS-CoV-2 infection (Vitiello et al. 2021e; Titanji et al. 2020). Specifically, baricitinib is an immunomodulator used in the treatment of rheumatoid arthritis; recently, some evidence shows efficacy against COVID-19 (Favalli et al. 2020; Jorgensen et al. 2020). Baricitinib is a potent and selective JAK1/Jak2; it thereby indirectly inhibits STAT signaling. The inhibition of JAK-STAT is useful to counteract the generalized hyperinflammatory state caused by cytokine storm in severe COVID-19 patients. In addition, baricitinib binds AAK1/GAK whose inhibition has been shown to reduce viral infection in vitro (Hoang et al. 2021). AAK1/GAK is a key regulator of clathrin-mediated endocytosis. Baricitinib therefore has a dual mechanism of anti COVID-19 action, immunomodulatory and antiviral. Remdesivir is among the antivirals used, the one that has found the most positive results against COVID-19. Remdesivir is an antiviral pharmacological agent of the nucleotide analogue family; in vitro studies have shown SARS-CoV-2 inhibition activity (Ferrara et al. 2020). Remdesivir is a pro-drug, which within cells is converted to the active metabolite nucleoside triphosphate and inhibits SARS-CoV-2 viral RNA replication. Recent studies associate remdesivir with continuous and clinically significant improvements in COVID-19 (Vitiello and Ferrara 2020c)-positive patients, resulting in reduced mortality and recovery time hospitalization (McCreary and Angus 2020). Treatment with a soluble recombinant human form of ACE2 (rhACE2) could prove useful as a trap effect for circulating SARS-CoV2 and decrease viral load and hinder infection (Monteil et al. 2020). Administration of recombinant soluble human ACE2 has shown good efficacy in subjects with acute respiratory distress syndrome (ARDS) (Muslim et al. 2020). From a molecular pharmacological point of view, administration of rhACE2 activates the Ang 1–7 and Ang 1–9 synthesis pathway of the RAS system (nonclassical pathway) by decreasing Ang II levels with a tendency to lower cytokine proinflammatory concentration (Gaddam et al. 2014). Administration of the baricitinib/remdesivir/rhACE2 combination acts in different modes of action and on different targets, with anti-inflammatory/immunomodulatory effect, antiviral activity with viral load reduction, inhibition of virus cell penetration, and endocellular viral replication (Fig. 1).
Fig. 1.
Schematic representation of the pharmacological synergism baricitinib/remdesivir/rhACE2: The different mechanisms of action of the three drugs act on multiple targets, rhACE2 mediates the conversion of Ang 2 to Ang (1–7) with antioxidant anti-inflammatory, anti-fibrosis, and vasodilator effects, mediated by activation of MASr (1), and reduce circulating viral load (2); baricitinib by inhibiting JAKs reduces cytokine hyperinflammatory state (3) and binding AAK1/GAK inhibits clathrin-mediated endocytosis (4); remdesivir inhibits endocellular viral replication (5)
Discussions
Baricitinib/remdesivir/rhACE2 administration may represent an important pharmacological synergism to arrest COVID-19 infection. The enhanced synergism allows for the lowering of the dose of each individual drug, increasing its safety profile. In addition, the pharmacological attack on multiple targets can have a greater effectiveness than the single administration of drugs, and avoid phenomena of drug resistance. Finally, there are no particular risks of drug interactions; baricitinib has a minimal interaction with the relevant CYP enzymes of drug metabolization. During COVID-19 infection, the benefit/risk ratio of drugs administered, and any drug-drug interactions or disease-drug interactions, should be carefully monitored (Ferrara 2020; Ferrara et al. 2020; Vitiello et al. 2021d). In summary, the described combination of drugs can reduce viral infectivity, viral replication, and aberrant inflammatory response of the host. Some clinical evidence shows excellent results when administered in combination baricitinib and remdesivir, and rhACE2 and remdesivir (Kalil et al. 2021; Monteil et al. 2021; Domenico et al. 2021).
Clinical trials
The ACTT-1 study (NCT04280705) demonstrated the efficacy of remdesivir against SARS-CoV-2. ACTT-1 is a double-blind, randomized, placebo-controlled trial that studied intravenous remdesivir in adults who had been hospitalized with COVID-19 and had evidence of lower respiratory tract infection. Patients were randomly assigned to receive remdesivir (200 mg loading dose on day 1, followed by 100 mg daily for up to 9 additional days) or placebo for up to 10 days. The primary outcome was time to recovery. A total of 1062 patients underwent randomization (with 541 assigned to remdesivir and 521 to placebo). Those who received remdesivir had a median recovery time of 10 days compared with 15 days among placebo subjects. Mortality was 6.7% with remdesivir and 11.9% with placebo at day 15 and 11.4% with remdesivir and 15.2% with placebo at day 29. Serious adverse events were reported in 131 of 532 patients receiving remdesivir (24.6%) and in 163 of 516 patients receiving placebo (31.6%). The ACTT-1 study demonstrated that remdesivir was superior to placebo in shortening recovery time in adults who were hospitalized with COVID-19 and had evidence of lower respiratory tract infection (Beigel et al. 2020). On November 19, 2020, the Food and Drug Administration (FDA) approved baricitinib for emergency use (EUA) in combination with remdesivir for the treatment of suspected or laboratory-confirmed COVID-19 disease in certain hospitalized patients requiring supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO). Baricitinib is approved for the treatment of adult patients with moderately to severely active rheumatoid arthritis. Authorization for use in COVID-19 patients was based on review of data from the ACTT-2 clinical trial (NCT04401579), and data from COV-BARRIER (NCT04421027). ACCT-2 is a randomized, double-blind, placebo-controlled trial conducted by the National Institute of Allergy and Infectious Diseases (NIAID) comparing baricitinib in combination with remdesivir and remdesivir as monotherapy. A total of 1033 patients underwent randomization (with 515 assigned to combination treatment and 518 to control). All patients received remdesivir (≤ 10 days) and baricitinib (≤ 14 days) or placebo (control). The primary outcome was time to recovery. Patients who received baricitinib had a median recovery time of 7 days compared with 8 days for control, and a 30% higher probability of improvement in clinical status at day 15. The ACTT-2 study demonstrated that baricitinib plus remdesivir was superior to remdesivir alone in reducing recovery time and accelerating improvement in clinical status among patients with COVID-19, and combination therapy was associated with fewer serious adverse events (Kalil et al. 2021). The COV-BARRIER is a randomized, double-blind, placebo-controlled clinical trial conducted by NIAID comparing treatment with baricitinib with placebo in hospitalized adults with confirmed SARS-CoV-2 infection. A total of 1525 hospitalized adults with COVID-19 receiving standard of care (SOC) were randomly assigned (1:1) to baricitinib 4 mg once daily (N = 764) or placebo (N = 761) for up to 14 days. SOC included systemic corticosteroids in ∼ 79% of participants (dexamethasone ∼ 90%). The primary endpoint was the proportion progressing to high-flow oxygen, noninvasive ventilation, invasive mechanical ventilation, or death by day 28. A key secondary endpoint was all-cause mortality by day 28. Overall, 27.8% of participants who received baricitinib versus 30.5% who received placebo progressed (primary endpoint). The 28-day mortality was 8.1% for baricitinib and 13.1% for placebo, corresponding to a 38.2% reduction in mortality. The frequency of adverse events, serious adverse events, serious infections, and venous thromboembolic events was similar between the groups. The study demonstrated that treatment with baricitinib in addition to SOC (predominantly dexamethasone) significantly reduced mortality with a similar safety profile among the groups of hospitalized COVID-19 participants (Marconi et al. 2021).
Antiviral SARS-CoV-2 drug resistance
Similar to cancer cells that can develop resistance against chemotherapeutic drugs, microorganisms such as viruses can develop drug resistance. The development of viral resistance to SARS-CoV-2 could be an increasing problem with long-term treatment, and specifically could be one of the causes of clinical treatment failure. Surveillance of viral resistance is necessary to choose appropriate empiric therapy and to monitor the spread of resistant virus in the population. Most viruses adapt and mutate to become resistant to antiviral therapy, and this can affect patient and disease management. The prevalence of resistance can be limited with infection control measures and appropriate antiviral treatment, especially used in combinations of multiple effective drugs directed at different targets and proteins within the virus (Duwe 2017a; Ghany and Doo 2009). In this context, surveillance of the potential emergence of antiviral resistance is critical for public health during the COVID-19 pandemic. Antiviral treatments have been shown to lead to the emergence of resistance in hepatitis B virus, human immunodeficiency virus, hepatitis C virus (HCV), and influenza virus (Ghany and Doo 2009; Duwe 2017b) and the ability to develop resistance during single-drug therapies. Similarly, in vitro experiments on SARS-CoV, the causative agent of severe acute respiratory syndrome (SARS), and a relative of SARS-CoV-2, shows that specific SNPs within nsp12, the major RdRp subunit, can alter the efficacy of remdesivir. The Nsp12:Phe480Leu substitution destabilizes the interface between different subdomains of the protein and affects remdesivir binding. The EC50 of remdesivir increased from 0.01 to 0.06 μM in SARS-CoV cultures carrying the Nsp12:Phe480Leu or Nsp12:Val557Leu mutations (Agostini et al. 2018). In the absence of remdesivir, these viral mutants were found to replicate less efficiently and showed substantially reduced viability. Identifying and monitoring transmission of potential antiviral-resistant strains is essential for disease surveillance. A new preprint research paper discusses the emergence of mutations in the SARS-CoV-2 virus that allow it to escape the effects of the drug remdesivir. The researchers found that 12 viral lineages passaged in media containing remdesivir at 1 μM or 2.5 μM showed cytopathic effects (CPE), indicating active replication of the virus in the cell. In two of the passaged viral lineages, replication rate and amplitude showed a change, as did the concentration of remdesivir required to achieve 50% inhibition. These lineages replicated actively in the presence of 7.5 μM remdesivir. However, titers were lower than when cultured without remdesivir. In Vero cells, these actively replicating strains showed a twofold increase in IC50 and a nearly fourfold increase compared to the parental strain. When the same viruses were passaged in media not containing remdesivir, the IC50 remained comparable to the parental strain. This partial resistance to remdesivir was not replicated with other nucleoside analogs such as EIDD2801, indicating the emergence of specific resistance mutations. Overall, the study showed the emergence of a genomic signature conferring partial resistance to remdesivir in the presence of the drug in vitro (Szemiel et al. 2021).
Conclusions
In parallel with the vaccination campaign, research studies should continue to identify effective and safe pharmacological agents for the treatment of COVID-19. Several evidences associate good clinical efficacy treatments with baricitinib and remdesivir. We suggest that patients treated for COVID-19 should be closely monitored for the potential occurrence of adverse reactions and antiviral resistance. Mutations are a well-known viral mechanism to escape drug inhibition, especially during monotherapy. In support of this, we believe that the combination remdesivir/baricitinib/rhACE2 represents an important pharmacological synergism to fight SARS-CoV-2 infection, with a decreased risk of adverse reactions, increased therapeutic efficacy, and decreased risk of antiviral resistance. Well-structured clinical studies could confirm this interesting scientific hypothesis.
Author contribution
AV: conceptualization, writing—original draft, methodology, writing—original draft.
FF: writing—review and editing, supervision, validation.
All authors read and approved the manuscript, and all data were generated in-house and that no paper mill was used.
Data availability
Full availability of data and materials.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
The authors consent to the publication of the manuscript.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Antonio Vitiello, Email: antonio.vitiello2@uslumbria1.it.
Francesco Ferrara, Email: f.ferrara@aslnapoli3sud.it.
References
- Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, Smith EC, Case JB, Feng JY, Jordan R, et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9:e00221-18. doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tawfiq JA, Al-Homoud AH, Memish ZA. Remdesivir as a possible therapeutic option for the COVID-19. Travel Med Infect Dis. 2020;34:101615. doi: 10.1016/j.tmaid.2020.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez-de-Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC, ACTT-1 Study Group Members Remdesivir for the treatment of Covid-19 - Final Report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Domenico M, De Rosa A, Boccellino M. Detection of SARS-COV-2 proteins using an ELISA test. Diagnostics (basel) 2021;11(4):698. doi: 10.3390/diagnostics11040698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duwe S. Influenza viruses - antiviral therapy and resistance. GMS Infect Dis. 2017;5(Doc04):20. doi: 10.3205/id000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duwe S (2017b) Influenza viruses - antiviral therapy and resistance. GMS Infect Dis 5, Doc04 [DOI] [PMC free article] [PubMed]
- Favalli EG, Biggioggero M, Maioli G, Caporali R. Baricitinib for COVID-19: a suitable treatment? Lancet Infect Dis. 2020;20(9):1012–1013. doi: 10.1016/S1473-3099(20)30262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara F. Antirheumatic in SARS-CoV-2: benefit or risk? Ital J Med. 2020;14(2):114–115. doi: 10.4081/itjm.2020.1290. [DOI] [Google Scholar]
- Ferrara F, Vitiello A (2020) Potential pharmacological approach in the regulation of ACE-2 and DPP-IV in diabetic COVID-19 patient. Ital J Med (AOP) 10.4081/itjm.2020.1435
- Ferrara F, Porta R, Santilli P, D’Aiuto V, Vitiello A. Are multiple sclerosis therapies safe in severe acute respiratory syndrome coronavirus 2 times? Indian J Pharmacol. 2020;52(5):441–442. doi: 10.4103/ijp.IJP_417_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara F, Granata G, Pelliccia C, La Porta R, Vitiello A. The added value of pirfenidone to fight inflammation and fibrotic state induced by SARS-CoV-2: anti-inflammatory and anti-fibrotic therapy could solve the lung complications of the infection? Eur J Clin Pharmacol. 2020;76(11):1615–1618. doi: 10.1007/s00228-020-02947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara F, Porta R, D’Aiuto V, Vitiello A. Remdesivir and COVID-19. Ir J Med Sci. 2020;17:1–2. doi: 10.1007/s11845-020-02401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara F, Vitiello A Efficacy of synthetic glucocorticoids in COVID-19 endothelites. Naunyn-Schmiedeberg’s Arch Pharmacol Accepted: 6 January 2021. 10.1007/s00210-021-02049-7 [DOI] [PMC free article] [PubMed]
- Gaddam RR, Chambers S, Bhatia M. ACE and ACE2 in inflammation: a tale of two enzymes. Inflamm Allergy Drug Targets. 2014;13(4):224–234. doi: 10.2174/1871528113666140713164506. [DOI] [PubMed] [Google Scholar]
- Ghany MG, Doo EC. Antiviral resistance and hepatitis B therapy. Hepatol Baltim Md. 2009;49:S174–184. doi: 10.1002/hep.22900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya AE, et al. Severe acute respiratory syndrome-related coronavirus: the species and its viruses – a statement of the Coronavirus Study Group. Microbiology. 2020 doi: 10.1101/2020.02.07.937862. [DOI] [Google Scholar]
- Guarner J. Three emerging coronaviruses in two decades. Am J Clin Pathol. 2020;153:420–421. doi: 10.1093/ajcp/aqaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang TN, Pino M, Boddapati AK, Viox EG, Starke CE, Upadhyay AA, Gumber S, Nekorchuk M, Busman-Sahay K, Strongin Z, Harper JL, Tharp GK, Pellegrini KL, Kirejczyk S, Zandi K, Tao S, Horton TR, Beagle EN, Mahar EA, Lee MYH, Cohen J, Jean SM, Wood JS, Connor-Stroud F, Stammen RL, Delmas OM, Wang S, Cooney KA, Sayegh MN, Wang L, Filev PD, Weiskopf D, Silvestri G, Waggoner J, Piantadosi A, Kasturi SP, Al-Shakhshir H, Ribeiro SP, Sekaly RP, Levit RD, Estes JD, Vanderford TH, Schinazi RF, Bosinger SE, Paiardini M. Baricitinib treatment resolves lower-airway macrophage inflammation and neutrophil recruitment in SARS-CoV-2-infected rhesus macaques. Cell. 2021;184(2):460–475.e21. doi: 10.1016/j.cell.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(2020):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen SCJ, Tse CLY, Burry L, Dresser LD. Baricitinib: a review of pharmacology, safety, and emerging clinical experience in COVID-19. Pharmacotherapy. 2020;40(8):843–856. doi: 10.1002/phar.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen SCJ, Kebriaei R, Dresser LD. Remdesivir: review of pharmacology, pre-clinical data, and emerging clinical experience for COVID-19. Pharmacotherapy. 2020;40(7):659–671. doi: 10.1002/phar.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, Marconi VC, Ruiz-Palacios GM, Hsieh L, Kline S, Tapson V, Iovine NM, Jain MK, Sweeney DA, El Sahly HM, Branche AR, Regalado Pineda J, Lye DC, Sandkovsky U, Luetkemeyer AF, Cohen SH, Finberg RW, Jackson PEH, Taiwo B, Paules CI, Arguinchona H, Erdmann N, Ahuja N, Frank M, Oh MD, Kim ES, Tan SY, Mularski RA, Nielsen H, Ponce PO, Taylor BS, Larson L, Rouphael NG, Saklawi Y, Cantos VD, Ko ER, Engemann JJ, Amin AN, Watanabe M, Billings J, Elie MC, Davey RT, Burgess TH, Ferreira J, Green M, Makowski M, Cardoso A, de Bono S, Bonnett T, Proschan M, Deye GA, Dempsey W, Nayak SU, Dodd LE, Beigel JH, ACTT-2 Study Group Members Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384(9):795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi VC et al (2021) Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Published: September 01, 2021. 10.1016/S2213-2600(21)00331-3 [DOI] [PMC free article] [PubMed]
- McCreary EK, Angus DC. Efficacy of remdesivir in COVID-19. JAMA. 2020;324(11):1041–1042. doi: 10.1001/jama.2020.16337. [DOI] [PubMed] [Google Scholar]
- Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, Leopoldi A, Garreta E, Hurtado Del Pozo C, Prosper F, Romero JP, Wirnsberger G, Zhang H, Slutsky AS, Conder R, Montserrat N, Mirazimi A, Penninger JM. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181(4):905–913.e7. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteil V, Dyczynski M, Lauschke VM, Kwon H, Wirnsberger G, Youhanna S, Zhang H, Slutsky AS, Hurtado Del Pozo C, Horn M, Montserrat N, Penninger JM, Mirazimi A. Human soluble ACE2 improves the effect of remdesivir in SARS-CoV-2 infection. EMBO Mol Med. 2021;13(1):e13426. doi: 10.15252/emmm.202013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muslim S, Nasrin N, Alotaibi FO, Prasad G, Singh SK, Alam I, Mustafa G. Treatment options available for COVID-19 and an analysis on possible role of combination of rhACE2, angiotensin (1–7) and angiotensin (1–9) as effective therapeutic measure. SN Compr Clin Med. 2020;22:1–6. doi: 10.1007/s42399-020-00407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muslim S, Nasrin N, Alotaibi FO, Prasad G, Singh SK, Alam I, Mustafa G (2020) Treatment options available for COVID-19 and an analysis on possible role of combination of rhACE2, angiotensin (1–7) and angiotensin (1–9) as effective therapeutic measure. SN Compr Clin Med 1–6. 10.1007/s42399-020-00407-9 [DOI] [PMC free article] [PubMed]
- Szemiel AM et al (2021) In vitro evolution of remdesivir resistance reveals genome plasticity of SARS-CoV-2. bioRxiv preprint. 10.1101/2021.02.01.429199v1. https://www.biorxiv.org/content/. Accessed August 2021
- Titanji BK, Farley MM, Mehta A, Connor-Schuler R, Moanna A, Cribbs SK, O’Shea J, DeSilva K, Chan B, Edwards A, Gavegnano C, Schinazi RF, Marconi VC (2020) Use of baricitinib in patients with moderate and severe COVID-19. Clin Infect Dis ciaa879. 10.1093/cid/ciaa879 [DOI] [PMC free article] [PubMed]
- Vitiello A, Ferrara F. Correlation between renin-angiotensin system and severe acute respiratory syndrome coronavirus 2 infection: what do we know? Eur J Pharmacol. 2020;15(883):173373. doi: 10.1016/j.ejphar.2020.173373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello A, Ferrara F. Pharmacological agents to therapeutic treatment of cardiac injury caused by Covid-19. Life Sci. 2020;1(262):118510. doi: 10.1016/j.lfs.2020.118510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello A, Ferrara F. Remdesivir versus ritonavir/lopinavir in COVID-19 patients. Ir J Med Sci. 2020;18:1–2. doi: 10.1007/s11845-020-02440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello A, Ferrara F (2021a) Colchicine and SARS-CoV-2: management of the hyperinflammatory state. Respir Med 178:106322, ISSN 0954-6111, 10.1016/j.rmed.2021.106322 [DOI] [PMC free article] [PubMed]
- Vitiello A, Ferrara F. Therapeutic strategies for SARS-CoV-2 acting on ACE-2. Eur J Pharm Sci. 2021;1(156):105579. doi: 10.1016/j.ejps.2020.105579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello A, Porta R, Pianesi L, Ferrara F. COVID-19 pandemic: vaccine and new monoclonal antibodies, point of view. Ir J Med Sci. 2021 doi: 10.1007/s11845-021-02584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello A, La Porta R, D’Aiuto V, Ferrara F (2021b) Pharmacological approach for the reduction of inflammatory and prothrombotic hyperactive state in COVID-19 positive patients by acting on complement cascade. Hum Immunol ISSN 0198-8859, 10.1016/j.humimm.2021.01.007 [DOI] [PMC free article] [PubMed]
- Vitiello A, Pelliccia C, Ferrara F (2021c) Drugs acting on the renin–angiotensin system and SARS-CoV-2. Drug Discov Today ISSN 1359-6446, 10.1016/j.drudis.2021.01.010 [DOI] [PMC free article] [PubMed]
- Vitiello A, La Porta R, D’Aiuto V, et al. The risks of liver injury in COVID-19 patients and pharmacological management to reduce or prevent the damage induced. Egypt Liver Journal. 2021;11:11. doi: 10.1186/s43066-021-00082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello A, La Porta R, Ferrara F (2021e) Scientific hypothesis and rational pharmacological for the use of sacubitril/valsartan in cardiac damage caused by COVID-19. Med Hypotheses 110486,ISSN 0306-9877 [DOI] [PMC free article] [PubMed]
- Vitiello A, Ferrara F, La Porta R Remdesivir and COVID-19 infection, therapeutic benefits or unnecessary risks? Received: 9 December 2020 /Accepted: 16 December 2020 Irish Journal of Medical Science (1971 -) 10.1007/s11845-020-02482-2 [DOI] [PMC free article] [PubMed]
- Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World health organization (WHO) https://www.who.int/emergencies/diseases/novel coronavirus2019/situation-reports (Situation Reports March 2021)
- Zhu N, et al. A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2019;382(2020):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Full availability of data and materials.