Abstract
Background:
Successful maintenance of a heart transplant (HTx) graft requires adherence to a triple-drug regimen of immunosuppression. However, achieving adequate adherence can be difficult secondary to complicated dosing regimens, side effects, and mental/emotional barriers. A detailed review of current patterns of adherence to immunosuppression in adult HTx recipients is lacking.
Objective:
This systematic review aims to detail the current landscape of adherence to immunosuppression in adult heart transplant patients, including the measurement of adherence, correlates to adherence, health outcomes associated with nonadherence, as well as strategies to improve adherence in HTx patients.
Methods:
We conducted searches in PubMed MEDLINE, Embase, CENTRAL register of Controlled Trials (Wiley), and Scopus, from inception to March 2020. Studies were eligible if they outlined an aspect of adherence (as noted above in the objective) to immunosuppression in adult HTx patients. The HTx cohort had to contain at least 10 patients and measurement of adherence had to be done with an objective or otherwise validated measure of adherence (e.g. drug levels, automated pill bottles or adherence questionnaires). Two authors independently screened the articles for inclusion, then subsequently reviewed the full texts of the included articles. Data was extracted into standardized forms and bias evaluations were done using the Newcastle-Ottawa or modified Newcastle-Ottawa tools, depending on the study type. The authors followed all guidelines for the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
Results:
The titles/abstracts of 880 articles were reviewed. Ultimately, 23 articles were included in the final review. The median number of participants was 101, with a range of 19 to 1397. Studies provided information on baseline levels of adherence (17 studies), correlates to adherence (14 studies), health outcomes related to nonadherence (3 studies) and interventions to improve adherence (3 studies). Baseline adherence estimates varied greatly depending on the adherence measure. Multiple significant correlates to nonadherence exist and appear to affect patients with certain sociodemographic backgrounds, those with psychological/psychiatric comorbidities and those with poor support structures. Nonadherence is associated with transplant coronary artery disease and acute late rejection; it may also be associated with long-term mortality. Finally, a simplified dosing regimen with once-a-day tacrolimus as well as use of a mobile phone-based intervention were associated with improved adherence. Bias scores were most deficient due to self-reported outcomes in 18 studies, and lack of controls/adjustments for confounders, in 7 studies.
Conclusions:
Adherence to immunosuppression in transplant patients varies, but is associated with observable and modifiable factors which are worth addressing. Further high-quality studies regarding strategies to improve adherence are needed in the literature.
Keywords: Heart transplant, solid organ transplant, adherence, compliance, rejection, behaviors
INTRODUCTION
Heart transplant (HTx) is the definitive therapy for those with advanced heart failure. In 2018, 3440 heart transplants were performed in the USA, an increase from 3273 in 2017.1 Recent innovations, including advanced immunosuppressive regimens, thorough preoperative psychosocial evaluations, and increased surveillance have mirrored improved survival.2–5 Despite these advances, HTx recipients face numerous challenges to maintain a healthy graft.
One of these challenges is adherence to immunosuppression (IS). Early in the life of the transplant, HTx patients generally take 3 classes of immunosuppression. These include glucocorticoids, calcineurin inhibitors (ex. cyclosporine, tacrolimus) and antiproliferative agents (ex. azathioprine, mycophenolate mofetil).6, 7 Patients can be maintained on a calcineurin inhibitor with or without an antiproliferative agent long term. Before the widespread use of cyclosporine, patients received only steroids and azathioprine; an early study published in 1984 with patients on this regimen noted 56 nonfatal and 6 cases of fatal rejection in a cohort of only 32 patients over a 3 year follow up.8 A study done soon thereafter showed that compared with dual therapy, triple therapy was associated with less renal failure, infections, lymphoma, and importantly, transplant rejection.9
Despite the clear value of adherence to the multidrug regimen, all three medications have certain risks and side effects to patients.7 These difficulties are mirrored in the HTx precursor population, those with advanced heart failure. Studies note that advanced heart failure patients may have as low as 10% adherence to a medical regimen over 1 year,10 and that <80% adherence to guideline-directed medical therapy for heart failure with reduced ejection fraction was significantly associated with combined outcomes of all-cause mortality and cardiovascular hospitalization.11 By extension, the modern heart transplant population may also experience critical challenges in the sphere of adherence, though this has not been recently reviewed in the literature.
Objectives
The formal exploration of adherence to immunosuppression in HTx has not been performed in a recent review. This manuscript aims to systematically review the measurement of adherence, correlates to adherence, health outcomes associated with nonadherence, as well as interventions to improve adherence as they relate to immunosuppression in HTx recipients. We aim to provide a landscape of current knowledge on adherence as well as underline implemented approaches for the improvement of adherence in HTx patients.
METHODS
Study Design
The authors followed all guidelines for the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
Search Strategy
A systematic review of studies on adherence to immunosuppression in HTx patients was conducted. A research librarian (LO) was responsible for a full literature search. We conducted searches in PubMed MEDLINE, Embase, CENTRAL register of Controlled Trials (Wiley), and Scopus, from inception to March 2020, using search strategies that were collaboratively developed by all authors. The search was employed in PubMed using a combination of MeSH terms for heart transplantation, compliance, and adherence, then applied to the other databases. No language or date limits were used (other than a publication date before March 2020, when the search was done). Search strategies can be found in Appendix 1. TH also hand-searched the bibliographies of relevant review articles and the included articles for additional references. Articles were reviewed in Rayyan by two independent reviewers with conflicts decided by discussion to reach consensus.
Eligibility Criteria
Studies were eligible if they outlined an aspect of adherence to immunosuppression in HTx patients, including but not limited to measurement of adherence, correlates to adherence, health outcomes associated with nonadherence, as well as strategies to improve adherence in HTx. Patients could be of any age, with a plan to divide studies into those primarily in adults (as outlined here) and those in pediatric and young adult patients (published in a separate manuscript). We included studies involving multiple types of solid-organ transplants, but the cohort of HTx had to be at least 10 participants and the data pertaining only to the HTx group needed to be separately reported for inclusion here. Included studies also had to utilize an objective or otherwise validated measure of adherence (eg. drug levels, automated pill bottles or adherence questionnaires). We excluded studies that measured adherence based on unvalidated/subjective metrics including physician report or chart review unless the data was otherwise described and validated. Studies could be in a prospective, observational, cross-sectional survey, or randomized trial format.
Study Selection and Data Extraction
Two authors (TH and SB) independently screened the articles for inclusion, then subsequently reviewed the full-text of the included articles. Discordant assessments were resolved by discussion between the reviewers to reach consensus. Data extraction was standardized to include population, type of article, study design, immunosuppression used, intervention (if any), measure of adherence, duration, number of HTx participants, participant age, study attrition rate, and main outcomes.
Assessment of Risk of Bias
Bias was evaluated by two independent reviewers (TH and KN). We utilized the Newcastle-Ottawa for cohort studies12 and a modified version of the Newcastle-Ottawa by Modesti et al.13 for cross-sectional studies. Disagreements were resolved by discussion between reviewers to reach consensus. Cohort studies could receive up to a total of 10 points via the Newcastle-Ottawa scale and prospective cohort studies could receive up to a total of 9 via the modified Newcastle-Ottawa scale.
Data Synthesis
Data was expected to be heterogenous and primarily presented descriptively. If sufficient homogeneity were found in outcomes, we considered a meta-analysis or effect size analysis.
RESULTS
Literature Search
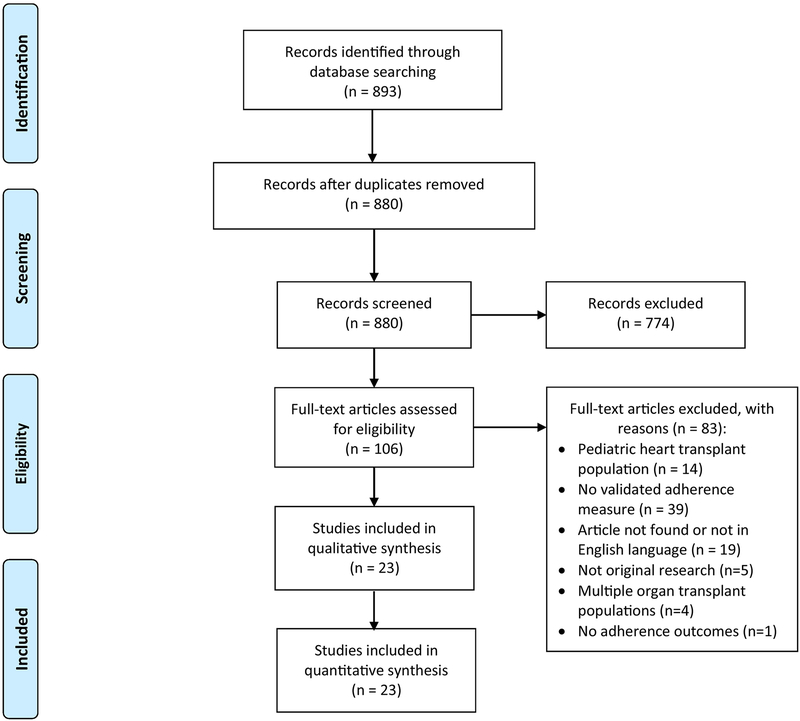
The titles/abstracts of 880 articles published before March 2020 were reviewed. 774 articles were eliminated in the initial screen, leaving 106 articles for full text review. Ultimately, we excluded 83 articles, leaving 23 articles for final inclusion in the review. The study flowchart and further reasoning for article inclusion/exclusion are outlined in the PRISMA flow diagram (Figure 1).
Figure 1:
PRISMA Flow Diagram
Study Characteristics
Study designs are outlined in Table 1. Studies were published between 1998 and 2020. Twelve studies were done in Europe14–25 and 6 in the USA26–31; the remainder were done at multiple international sites (2 studies32, 33), New Zealand (1 study34), China (1 study35) and Israel (1 study36). The median number of participants was 101, with a range of 19 to 1397. Studies were divided into those that reviewed baseline levels of adherence (17 studies), correlates to adherence (14 studies), health outcomes related to nonadherence (3 studies) and interventions to improve adherence (3 studies). Some studies were included in two categories, as applicable. Studies included cross-sectional studies (14 studies) and prospective cohort trials (9 studies). Four studies included immunosuppressant levels or pill bottle monitoring to measure adherence; two of those also included a validated self-report of adherence. A majority of those remaining (18 studies) utilized validated adherence measures based on self-report only.
Table 1:
Design of all included studies
| Author/Year | Population | Study design | Measure of adherence | Duration | Number of HTx participants | Participant Age (SD) | Attrition rate |
|---|---|---|---|---|---|---|---|
| Blanca Martinez Perez (2013)14 | Pts who received transplant between 2001 and 2011, seen at a large hospital complex in Spain | Cross-sectional study | Morisky-Green test and immunosuppressant drug levels | N/A | 99 | 50 (12) years old | N/A |
| Brocks (2017)15 | Pts at least 18 years old, associated with a German heart transplant clinic who could speak German (survey conducted by mail) | Cross-sectional study | ITAS measure | 4 months | 505 | Range: 18 to 90 years. | N/A |
| De Geest (1998)25 | Adult patients taking cyclosporine as part of their regimen from academic centers in Leuven, Belgium. | Prospective cohort trial | Electronic pill bottle monitor | 3 months | 101 | Median 56.0 (range 20.0–69.0) | 1/101 |
| De Geest (2014)16 | Questionnaires completed by pts who are part of the Swiss Transplant Cohort Study; pts were evaluated and waitlisted for transplantation in Switzerland | Prospective cohort study | Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS) | 3 years | 126 | Age of all solid-organ transplant patients was 52.5 (13.1) | 32/126 |
| Denhaerynck (2018)32 | Pts recruited through the Building Research Initiative Group: Chronic Illness Management and Adherence in Transplantation (BRIGHT) study, which used multistaged sampling to examine 36 HTx centers in Europe, North America, South America, and Australia. | Cross-sectional study | Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS) | Not stated | 1397 | 53.6 (13.2) | N/A |
| Dobbels (2004)17 | Patients recruited at outpatient clinic visits at the Leuven Heart Transplant Program, Belgium | Prospective cohort study | Medication Event Monitoring System (MEMS) | 5 years | 101 | 55 (10) | 0/101 |
| Doesch (2013)18 | Patients were recruited from the Department of Cardiology, Heidelberg University Hospital, Germany. Patients had to be at least 6 months post-HTx and free from acute infection or rejection for 4 months prior to study inclusion. Patients also had to be on stable doses of conventional TAC or CsA for 4 months preceding study entry (as part of a dual immunosuppressive regimen). | Prospective cohort study | Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS) | 8 months | 76 | 46.0 (14.4) | 4/76 |
| Doesch (2010)19 | Patients were recruited from the Department of Cardiology, Heidelberg University Hospital, Germany. Patients had to be at least 6 months post-HTx and free from acute infection or rejection for 4 months prior to study inclusion. Patients also had to be on stable doses of conventional TAC or CsA for 4 months preceding study entry (as part of a dual immunosuppressive regimen). | Prospective cohort study | Visual Analogue Scale (VAS) and Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS) | 4 months | 54 | 46.2 (14.1) | 4/54 |
| Epstein (2014)26 | Data collected from Kaiser Permanente’s Heart Transplant Service in Santa Clara, CA. | Cross-sectional study | Immunosuppressant Therapy Adherence Scale (ITAS) | N/A | 52 | 54.46 (no SD) | N/A |
| Farmer (2013)27 | 4 US medical centers | Prospective cohort study | Assessment of Problems with the Transplant Regimen Scale | 10 years | 555 | 58.58 (8.93) in dead group, 59.50 (9.94)in alive group | 30% |
| Goetzmann (2008)20 | German-speaking patients who had received a heart at University Hospital Zurich, Switzerland | Cross-sectional study | Transplant Effects Questionnaire translated into German (TxEQ-D) | N/A | 41 | At follow up: mean 62.0, range (24–75) | N/A |
| Gomis-Pastor (2020)21 | Adults less than 1.5 years from heart transplant recruited from cardiology outpatient clinic in Barcelona, Spain | Prospective cohort study | Simplified Medication Adherence Questionnaire (SMAQ) | mean 2.3 mos (SD 0.9) | 31 | 54 (12) | 0/31 |
| Grady (2016)28 | Adults transplanted 5–10 years prior at 4 US medical centers | Cross-sectional study | Assessment of Problems with the Heart Transplant Regimen | N/A | 210 | Age of recipient at transplant: women: 48.8 (12.0), men: 57.1 (8.5) | N/A |
| Grady (1998)29 | Adults transplanted 1–2 years ago from two US academic medical centers | Prospective cohort study | Assessment of Problems with Heart Transplant Regimen | 2 years | 120 | None given | 44/120 |
| Grady (1999)30 | Adults transplanted 1 year ago at two US academic medical centers | Cross-sectional study | Assessment of Compliance with Transplant Regimen | N/A | 232 | 53.9 (9.3) | N/A |
| Helmy (2019)33 | Pts recruited through the Building Research Initiative Group: Chronic Illness Management and Adherence in Transplantation (BRIGHT) study, which used multistaged sampling to examine 36 HTx centers in Europe, North America, South America, and Australia. Pts transplanted 1–5 years ago. | Cross-sectional study | Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS) | N/A | 1397 | 53.7 (13.2) | N/A |
| Hugon (2014)22 | French-speaking adult patients who had received a solid organ transplant at least 3 months ago. Questionnaire sent by mail. | Cross-sectional study | Scale based on Morisky-Green; IS trough levels also collected. nonadherent if the self-reported adherence score was ≤ 4 (3) and/or had a ratio of inadequate trough concentrations >0.2. | N/A | 43 | Age of all solid-organ transplant patients was 55.7 (13.0) | N/A |
| Kung (2012)34 | English speaking patients who received transplants at least 3 months prior at one of two solid organ transplant centers in New Zealand. | Cross-sectional study | Immunosuppressant Therapy Adherence Scale (ITAS) based on scoring 12 or less, where a score of 12 indicates perfect adherence | N/A | 87 | 54.4 (11.8) | N/A |
| Milaniak (2014)23 | Adults transplanted at least 3 months ago at a transplant and surgery department in Poland | Cross-sectional study | Transplant Effects Questionnaire (TxEQ) - Subscale scores are expressed as a mean obtained by dividing the total score by the number of items ranging from 1 to 5. Tertiles were used to estimate the emotional response, so that a score of 1–2.3 represents a low emotional response, 2.4–3.6 a moderate response and 3.7–5 a high level of emotional response. | N/A | 46 | 52.36 (13.55) | N/A |
| Scholz (2012)53 | Adults transplanted with a solid organ (heart, liver, kidney, lung) at least 6 months ago at University Hospital Zurich, Switzerland | Cross-sectional study | Five-item adherence subscale of the German version of the Transplant Effects Questionnaire (TxEQ-D) | N/A | 19 | Age of all solid-organ transplant patients was 54.32 (13.32) | N/A |
| Shamaskin (2012)31 | Pts at least 21 years old and 5 years post heart transplant recruited from one of 4 US medical centers | Cross-sectional study | Assessment of Problems with Heart Transplant Regimen | N/A | 555 | Age in three groups: younger: 42.2 (7.1), middle aged: 59.1 (4.2), older: 69.4 (2.8) | N/A |
| Shemesh (2017)36 | Hebrew-speaking patients cared for at an tertiary medical center outpatient clinic in Israel. | Cross-sectional study | Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS) - overall nonadherence defined as that is, missing one or skipping two or more doses, not maintaining a timing of medication intake, altering the prescribed amount, or completely stopping intake of IS medications | N/A | 102 | 56.66 (15.38) | N/A |
| Vitinius (2019)24 | German-speaking patients who were inpatients or outpatients followed at the University of Cologne, Germany; recruited before transplant. | Prospective cohort study | Medication experience scale for immunosuppressants (MESI) | Pre-transplant to 6 months post transplant | 20 | Average age of participants of both studies included in this manuscript: 53.5 (11.75) | 9/20 |
| Zhang (2019)35 | Adult patients at least 3 months from admission for heart transplant from a medical college and university in China. | Cross-sectional study | Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS) | N/A | 186 | 51.5 (interquartile range 41.0–58.0) | 18/186 did not complete instrument |
HTx: Heart transplant; Pt: Patient; N/A: not applicable
Self-reported adherence measures
Table 2 outlines the validated self-reported adherence measures utilized to measure immunosuppressant adherence in heart transplant recipients. Three of the measures (Transplant Effects Questionnaire, Assessment of Problems with the Heart Transplant Regimen and the Medication Experience Scale for Immunosuppression) were developed for use in transplant patients. Three others (Immunosuppressant Therapy Adherence Scale [ITAS], Basel Assessment of Adherence to Immunosuppressive Medications Scale, and Simplified Medication Adherence Questionnaire) were developed in other populations, but later validated in transplant cohorts.
Table 2:
Validated adherence measures based on self-report
| Author | Instrument name | Structure and development/validation references |
|---|---|---|
| Morisky et al. | Morisky-Green test | 4-item scale with yes/no answers54 |
| Chisholm et al. | Immunosuppressant Therapy Adherence Scale (ITAS) | Originally validated 5-item scale with categorical answers55 4-item scale also utilized56 Later validated in solid-organ transplant recipients receiving immunosuppression57 |
| University of Basel | Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS) | Most updated version contains 6 YES/NO items, with additional questioning with 5 response categories if implementation problems are detected.58 Initially validated in patients with HIV59 Later validated in Brazilian kidney-transplant recipients60 |
| Hayes et al. (per Ahearn61) | Visual Analogue Scale (VAS) | Single question: “In a time period, how much medicine did you take as prescribed?” with answers on an analogue scale (0–100%) Validated for HCV patients taking antivirals62 Some data in HIV patients taking ART63 |
| Ziegelmann et al. | Transplant Effects Questionnaire (TxEQ) | 24-item questionnaire around 5 conceptual factors with 5-point Likert scale for answers64 Developed for use in organ transplant patients |
| Knobel et al. | Simplified Medication Adherence Questionnaire (SMAQ) | 6-item scale with different answer types.65 Validated in renal transplant patients66 |
| Grady et al. | Assessment of Problems with the Heart Transplant Regimen | 26-item scale with 4-point Likert scale for answers29 Developed for use in heart transplant patients |
| Goetzmann et al. | Medication Experience Scale for Immunosuppressants (MESI) | 7-item scale with scored answers, total between 4 and 3367 Developed for use in solid organ transplant patients |
HIV: Human immunodeficiency virus; ART: Anti-retroviral therapy;
Baseline adherence
Table 3 outlines the 17 studies that included baseline adherence measurements. Baseline adherence, both in classification as well as apparent value, varied widely. While some studies based their judgments on immunosuppression blood levels, most utilized validated self-report. These self-reports could also further classify nonadherent patients into nonadherence patterns, including taking nonadherence (missing doses), timing nonadherence (straying from the daily dosing schedule), or dose alterations.
Table 3:
Studies exploring baseline adherence levels
| Author (year) | Adherence measure | Definition of adherence | Outcomes |
|---|---|---|---|
| Blanca Martinez Perez (2013)14 | Morisky-Green test and immunosuppressant drug levels | Therapeutic plasma drug levels (specific levels not noted): Tacrolimus levels Cyclosporine levels Everolimus levels Rifampin levels |
Tacolimus: 85 +/− 13% were therapeutic Cyclosporine: 67 +/− 28% were therapeutic Everolimus: 31 +/− 33% were therapeutic Rifampin: 100% were therapeutic |
| Morisky Green test: Said no to all: “Do you ever forget to take your medications?”, “When you feel well, do you stop taking them?”, and “If you feel unwell, do you stop taking the medicines?” and yes to: “Do you take medications at the right times?” |
67/99 were compliant by the Morisky-Green. 30% stated that they sometimes forgot to take their medication and 14% did not take it at the time established. | ||
| Brocks (2017)15 | Immunosuppressant Therapy Adherence Scale (ITAS) | Perfect adherence: 12/12 score on ITAS | 72.4% noted perfect adherence |
| De Geest (1998)25 | Electronic pill bottle monitor | Cluster analysis was done on the basis of specific variables, including medication taking compliance, dosing compliance, variability of dosing intervals, drug holidays, cyclosporine-free days, and interview rating. | The three clusters included 84% as excellent compliers, 7% as minor subclinical noncompliers and 9% as moderate subclinical noncompliers. The groups varied among the above variables p = 0.0001 via MANOVA. |
| De Geest (2014)16 | Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS) | Taking nonadherence: asking “How often did you miss a dose of medication (pretransplant)/immunosuppressive medication (post-transplant) in the past 4 weeks?” | 4 (5.1%) taking nonadherence at 6 months 5 (6.5%) taking nonadherence at 12 months 5 (10.6%) taking nonadherence at 24 months 6 (18.8%) taking nonadherence at 36 months |
| Drug holidays: asking “Did you miss more than one consecutive dose of your (immunosuppressive) medication in the past 4 weeks?” | 0 (0%) drug holidays at 6 months 1 (1.9%) drug holidays at 12 months 1 (3.1%) drug holidays at 24 months 1 (7.1%) drug holidays at 36 months |
||
| Denhaerynck (2018)32 | Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS) | Nonadherence defined as: Overall nonadherence: “any deviation in taking, timing, or dosing” | 34.1% had implementation phase overall nonadherence |
| Taking nonadherence (ie. Missing doses) | 14.7% noted taking nonadherence | ||
| Timing nonadherence (>2 hours deviation from dosing schedule) | 26.5% of patients noted timing nonadherence | ||
| Dobbels (2004)17 | Medication Event Monitoring System (MEMS) | Iterative partitioning methods of cluster analysis identified 1 cluster of compliers, and 2 clusters of non-compliers. | Pts were considered medication non-compliers (n = 17) or compliers (n = 84) |
| Doesch (2013)18 | Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS) | Overall nonadherence defined as missing one or skipping two or more doses, not maintaining a timing of medication intake, altering the prescribed amount, or completely stopping intake of IS medications | Overall nonadherence at baseline for any of the four BAASIS items was 75.0% |
| Doesch (2010)19 | Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS) and Visual Analogue Scale (VAS) | Overall nonadherence defined as missing one or skipping two or more doses, not maintaining a timing of medication intake, altering the prescribed amount, or completely stopping intake of IS medications | Overall nonadherence at baseline for any of the 4 BAASIS items was 74% |
| VAS continuous score on a scale 0–100%. | The VAS score at baseline was 82.3% ± 2.6% | ||
| Goetzmann (2008)20 | Transplant Effects Questionnaire translated into German (TxEQ-D) | Continuous score; average of scores on 5-point Likert scale from “strongly disagree” = 1 to “strongly agree” = 5: (1) Sometimes, I do not take my antirejection medicines; (2) Sometimes, I forget to take my antirejection medicines; (3) When I am too busy, I may forget my antirejection medicines; (4) Sometimes, I think I do not need my antirejection medicines; (5) I find it difficult to adjust to taking my prescribed antirejection drug regime. | Heart transplant patients noted 4.27 (.79) out of 5 score on medication adherence |
| Gomis-Pastor (2020)21 | Simplified Medication Adherence Questionnaire (SMAQ): | Nonadherence defined as any suboptimal answer to: - Do you always take your medications at the appropriate times? (Y/N) - When you feel bad, have you ever discontinued taking your medications? (Y/N) - Have you ever forgotten to take your medications? (Y/N) - Have you ever forgotten to take your medications during the weekend? (Y/N) - In the last week, how many times did you fail to take your prescribed dose? (Never/1–2 times/3–5 times/6–10 times/more than 10 times) - Since your last visit, how many whole days have gone by in which you did not take your medications? |
According to the SMAQ, 39% (12/31) of HTxR were nonadherent to immunosuppressive treatment. |
| Grady (1998)29 | Assessment of Problems with Heart Transplant Regimen | 26-items on a 4-point Likert scale with different choices per question. Only presented these adherence measures: “No difficulty” taking all immunosuppressants | 98% of individuals noted no difficulty taking all immunosuppressants |
| Taking their IS “all of the time” | 99% noted they took their IS “all of the time” | ||
| Taking their prednisone “all of the time” | 100% were adherent to prednisone | ||
| Taking their cyclosporine “all of the time” | 100% were adherent to cyclosporine | ||
| Taking their azathioprine “all of the time” | 100% were adherent to azathioprine | ||
| Helmy (2019)33 | Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS) | Overall nonadherence included any of the below (taking nonadherence, drug holiday, timing nonadherence, and/or dose alteration) | Overall nonadherence rate of 37.4% |
| Taking nonadherence (missing >1 dose) | 15.1%; 95% CI: (13.2, 17.0) experienced taking nonadherence | ||
| Drug holiday (skipping >2 consecutive doses) | 1.4%; (95% CI (0.8, 2.0) endorsed a drug holiday | ||
| Timing nonadherence (taking medication >2 h before or after the prescribed time) | 26.2%; 95% CI (23.9, 28.5) endorsed timing nonadherence | ||
| Dose alteration (taking more or fewer pills than prescribed or changing dosages without a physician’s order) | 1.5%; 95% CI (0.8, 2.1) endorsed dose alteration | ||
| Hugon (2014)22 | Scale based on Morisky-Green; IS trough levels also collected. | Scale was 6 questions with yes/no answers (exact questions unspecified). Considered nonadherent if the self-reported adherence score was ≤ 4 and/or had a ratio of inadequate trough concentrations >0.2. | For heart transplant, adherence rate was 34.9%, significantly lower than other organs. Mean score for self-reported adherence was 4.4 (0.9) |
| Kung (2012)34 | Immunosuppressant Therapy Adherence Scale (ITAS) | Perfect adherence: 12/12 score on ITAS | 37% of HTx patients had perfect adherence. |
| Milaniak (2014)23 | Transplant Effects Questionnaire (TxEQ) | Subscale scores were expressed as a mean obtained by dividing the total score by the number of items ranging from 1 to 5. Tertiles were used to estimate the emotional response; a score of 1–2.3 represents a low emotional response, 2.4–3.6 a moderate response and 3.7–5 a high level of emotional response. | They reported good adherence to medication (avg. 4.02 – third tertile) |
| Shemesh (2017)36 | Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS) | Overall nonadherence defined as missing one or skipping two or more doses, not maintaining a timing of medication intake, altering the prescribed amount, or completely stopping intake of IS medications | 64 patients (64%) were overall nonadherent |
| Timing nonadherence (>2 hours deviation from dosing schedule) | 58 participants (56.9%) noted timing nonadherence | ||
| Zhang (2019)35 | Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS) | Nonadherence defined as: “any deviation in taking, timing, or dosing” | 69 (41.1%) recipients were revealed to be nonadherent in any way |
| Taking nonadherence (ie. Missing doses) | 14.0% of the participants endorsed “taking nonadherence” | ||
| Timing nonadherence (>2 hours deviation from dosing schedule) | 35.1% of the participants endorsed “timing nonadherence” |
IS: immunosuppression
Adherence was between 25–40% in 5 studies.14, 18, 22, 34, 36 The lowest apparent adherence rate of 25% was seen in a study by Doesch et al. defined as self-reported nonadherence to short-acting tacrolimus on any of the 4 BAASIS items.18 The majority of remaining articles noted an adherence rate between 50 and 80%.14, 15, 18–21, 32, 33, 35 Several studies included an adherence rate greater than 80%.16, 17, 19, 23, 25, 29, 32 Interestingly, the same study which observed very low (31 ± 33%) everolimus adherence by blood levels also noted much higher adherence to other IS, including 67% ± 28% for cyclosporine and 85 ± 13% for tacrolimus.14
One study by De Geest et al. monitored “taking” adherence rates over time, defined as missing immunosuppression doses over the past 4 weeks. It found that the nonadherence to immunosuppression steadily rose from 5.1% at 6 months posttransplant to 18.8% taking nonadherence at 36 months.16
Correlates to adherence
Table 4 outlines correlates to adherence and nonadherence. Correlates to adherence were stratified into 5 categories, including sociodemographic, behavioral, support, mental/emotional wellbeing, and health/transplant-related factors.
Table 4:
Studies exploring correlates to adherence
| Author/Year | Measure of adherence | Variables explored | Main outcomes |
|---|---|---|---|
| Blanca Martinez Perez (2013)14 | Morisky-Green test and immunosuppressant drug levels | Higher age (years) | Not significant: (OR 1.5; 95% CI: 0.7 – 3.3; p = 1) |
| Sex (binary) | Not significant: (OR 1; 95% CI: 0.4 – 2.3; p = 0.998) | ||
| Urgent vs elective transplant (binary) | Not significant: (OR 0.8; 95% CI: 0.4 – 1.5; p = 1) | ||
| Dyslipidemia (binary) | Not significant: (OR 1; 95% CI: 0.99 – 1.01; p = 0.794) | ||
| Hypertension (binary) | Not significant: (OR 0.9; 95% CI: 0.5–1.4; p =0.442) | ||
| Diabetes mellitus (binary) | Not significant: (OR 1.6; 95% CI: 0.9–2.9; p =0.145) | ||
| Renal failure (binary) | Not significant: (OR 1.9; 95% CI: 0.6–3; p = 0.175) | ||
| Brocks (2017)15 | ITAS measure | Consumption of soft boiled or unpasteurized egg products, scored as (4 = daily; 3 = several times a week; 2 = occasionally; 1 = never) | Significantly associated with nonadherence: (r = −0.130; P < 0.01) |
| Consumption of unpasteurized milk products, scored as (4 = daily; 3 = several times a week; 2 = occasionally; 1 = never) | Significantly associated with nonadherence: (r = −0.128; P < 0.01) | ||
| Higher SF-12 Mental Health Score (units) ranging 0–100 where 0 represents low and 100 represents high level of health. | Significantly associated with adherence: (r = 0.016; 95% CI: 0.003–0.028; p = 0.015) | ||
| Higher age (years) | Significantly associated with adherence: (r = 0.017; 95% CI: 0.08–0.026; p < 0.001) | ||
| Perceptions/miscellaneous | 74.8% noted being at least slightly handicapped by adverse drug effects; fewer than 15% of the group believed any of the following: “I need to take medication too often,” “I need to take too many pills at 1 time,” “I do not know if immunosuppression helps,” “My immunosuppression sometimes runs out,” “Remembering to take immunosuppression is hard,” and “I neglect taking immunosuppression.” | ||
| De Geest (1998)25 | Electronic pill bottle monitor | Appointment noncompliance rates | Appointment noncompliance rates were higher in minor (28.6%) and moderate (11.1%) subclinical noncompliers compared to excellent compliers (3.6%) (p = 0.03). |
| Self efficacy with medication taking as rated on questionnaire on a 5-point scale | Self efficacy was worst in the minor subclinical noncompliers (mean 4.41; Q1: 4.30, Q3: 4.81) and best in the excellent compliers (mean 4.85; Q1: 4.70, Q3: 5.00); Moderate compliers: (mean 4.81; Q1: 4.70, Q3: 4.89) (p = 0.04). | ||
| Miscellaneous | Clusters did not differ significantly on demographic data, BMI, perceived social support, symptom frequency, symptom distress, depressive symptomatology, knowledge of the therapeutic regimen, cardiac functional status, and perceived health status. | ||
| De Geest (2014)16 | Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS) | Greater time since transplant | In the adjusted model: Significantly associated with nonadherence: (aOR: 1.05; 95% CI: 1.01–1.08) |
| Denhaerynck (2018)32 | Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS) | Greater barriers to taking immunosuppression as prescribed, scored 1 (never) to 5 (always) | In the multiple sequential regression model, Significantly associated with nonadherence: (OR = 11.48; 95% CI: 6.66–21.05; p < 0.0001) |
| Currently or recently smoking or having stopped less than a year ago | Significantly associated with nonadherence: (OR: 2.19; 95% CI: 1.35–3.56; p = 0.002) | ||
| Medication pick-up at physician’s office | Significantly associated with nonadherence: (OR = 2.31; 95% CI: 1.24–4.31; p = 0.008) | ||
| Higher monthly immunosuppression cost, scored as 1 ($0–$20), 2 ($20.01–$60), 3 ($60.01–%110) and 4 (>$110) | Significantly associated with nonadherence: (OR = 1.16; 95% CI: 1.02–1.33; p = 0.03) | ||
| Higher frequency of having someone to help read health-related materials, scored 1 (none of the time) to 5 (all of the time) | Significantly associated with adherence: (OR = 0.85; 95% CI: 0.76–0.95; p = 0.004) | ||
| Clinicians reporting nonadherent patients are targeted with adherence interventions, scored 1 (never) to 4 (always) | Significantly associated with adherence: (OR = 0.66; 95% CI: 0.48–0.91; p = 0.01) | ||
| Epstein (2014)26 | Immunosuppressant Therapy Adherence Scale (ITAS) | Chance LOC, defined as feelings of control over health outcomes, measured via the Multidimensional Health Locus of Control tool | Significantly inversely related to occassional missed doses of immunosuppressant medications: (OR 1.1527; 95% CI: 1.0394 – 1.2792); p = 0.0071) |
| African American race | Significant: (p=0.0026). No other data provided. | ||
| Grady (2016)28 | Assessment of Problems with the Heart Transplant Regimen | Female gender | Significantly associated with adherence: (0.05 point difference; 95% CI: 0.02–0.08; p = 0.005) |
| Higher age | Significantly associated with nonadherence: (−0.003 point difference; 95% CI: 0.01 – 0.09; p < 0.0001) | ||
| Idiopathic etiology of heart failure | Significantly associated with adherence: (0.05 point difference; 95% CI: 0.01–0.09; p =0.028) | ||
| Ischemic etiology of heart failure | Significantly associated with adherence: (0.06 point difference; 95% CI: 0.02–0.11; p = 0.003) | ||
| Miscellaneous | “No psychiatric condition”, “No orthopedic illness”, and “No diabetes” were not significantly associated with adherence | ||
| Grady (1998)29 | Assessment of Problems with Heart Transplant Regimen | Higher self-care disability as noted on the Sickness Impact Profile | Significantly associated with nonadherence: (r = 0.32; p < 0.0001) |
| Higher number of complications between 9 and 12 months after transplant as noted in the medical record | Significantly associated with nonadherence: (r = 0.25; p = 0.005) | ||
| Grady (1999)30 | Assessment of Compliance with Transplant Regimen | Life satisfaction as collected from the Quality of Life Index | Significantly associated with adherence: (r = −0.42; p ≤0.0001) |
| Milaniak (2014)23 | Transplant Effects Questionnaire (TxEQ) | Comprehensibility (the idea that confronted stimuli are are structured and predictable) collected from the Sense of Coherence instrument, Polish version | Trend toward adherence but not significant (r = 0.253; p =0.089) |
| Shamaskin (2012)31 | Assessment of Problems with Heart Transplant Regimen | Higher age, divided into older (>60), middle aged between 45–59 and younger (<45). | Significantly associated with adherence (F(2,234.75) = 9.98, p < 0.001) Values were 0.14 (SD 0.12) for younger patients, 0.12 (SD 0.10) for middle aged, and 0.08 (SD 0.07) for older patients |
| Miscellaneous | Higher age was significantly associated with perceived difficulty with adherence (F(2,552) = 11.97; p < 0.001). Values were 0.26 (SD 0.20) for younger patients, 0.24 (SD 0.19) for middle aged and 0.16 (SD 0.17) for older patients | ||
| Shemesh (2017)36 | Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS) | Higher age (years) | Significantly associated with adherence (b=0.036, p=0.04) |
| Greater guilt feelings regarding the heart donation, defined on a scale from 0 (not at all), 1 (slightly), 2 (moderately), 3 (very), and 4 (greatly) | Significantly associated with nonadherence (b=−1.497, p=0.037) | ||
| Time since transplantation as obtained from the medical record | Significantly associated with nonadherence (b=−0.008; p = 0.027) | ||
| Miscellaneous | There was no difference in adherence between men/women (χ2(1)=2.02; p=0.16), employment/student status (χ2(1)=3.32; p = 0.07), transplant center (χ2(1)=0.14, p=0.71) or initial support with LVAD/BiVAD/neither (χ2(1)=.25, P=0.62). | ||
| Vitinius (2019)24 | Medication experience scale for immunosuppressants (MESI) | Anxiety and depression as measured by the HADS-D tool: Seven items form a depression subscale and the other seven items an anxiety subscale. The items are scored from 0 to 3. | Higher scores 6 months after transplant not significantly associated with adherence: (r = 0.44; P = 0.199 and r = 0.54; P = 0.106, respectively) |
| Higher depression scores via the Patients Health Questionnaire (PHQ-D) score: assesses symptoms according to classifications in the DSM-IV | Significantly associated with nonadherence: (r = 0.68, p = 0.031) | ||
| Higher Transplant Evaluation Rating Scale (TERS) score, that represents psychosocial functioning. Scores range from 26.5 to 79.5 and higher represents worse functioning. | Not significantly associated with adherence immediately after transplantation (r = −0.11, P = 0.764) | ||
| However, was significantly associated with nonadherence 6 months after transplantation (r = 0.84; p = 0.001) | |||
| Zhang (2019)35 | Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS) | Lower monthly income (<3000 Chinese Yuan) as obtained via questionnaire. | Significantly associated with nonadherence (OR: 3.11; 95% CI: 1.58–6.12; p=0.001) |
| Number of prescribed concomitant drugs gathered via medical record and patient interview | Significantly associated with nonadherence (OR: 1.23; 95% CI, 1.12–1.50; p=0.003) | ||
| Higher concerns about immunosuppressants as gathered through the Belief Medication Questionnaire (BMQ) Each item is scored on a 5-point scale (1=strongly disagree, 5=strongly agree) and is summed resulting in a score for each subscale. High scores indicate a strong belief in the necessity and low concern for the use of immunosuppressants. | Significantly associated with nonadherence (OR: 1.09; 95% CI, 1.01–1.18; p=0.031) | ||
| Miscellaneous | Noncompliance was significantly associated with less than a high school education, (53.6% vs. 37.4%; p=0.037), greater likelihood of living in a rural area (36.2% vs. 20.2%; p=0.021); lower median anxiety scores (10.0 vs. 12.0, p=0.041), a poorer Physical Component Summary (PCS) score (44.6 vs. 48.0, p=0.002) and lower Mental Component Summary (MCS) score: (44.4 vs. 49.1, p=0.001) though none were statistically significant in the final logistic model. |
OR: Odds ratio; aOR: Adjusted odds ratio; LVAD: Left-ventricular assist device; BiVAD: bi-ventricular assist device; DSM-IV: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition
In the sociodemographic category, factors positively associated with adherence included higher age15, 31, 36 and female gender.28 Interestingly, one article found higher age to be negatively associated with adherence.28 Sociodemographic factors associated with higher risk of nonadherence included time since transplant,16, 36 African ancestry,26 and lower monthly income.35
In the behavioral category, nonadherence was significantly associated with the consumption of soft-boiled unpasteurized eggs and unpasteurized milk,15 as well as current/recent smoking.32 Appointment noncompliance also correlated with poorer adherence to cyclosporine.25
The support category primarily reviewed the difficulties patients experienced while obtaining their medicines. Denhaerynck et al. noted significantly higher adherence associated with the use of interventions to target adherence after clinician identification, and when patients had someone help them read health-related materials more frequently. The same article noted that nonadherence was significantly associated with medications that were picked up at a physician’s office and when immunosuppression was more costly.32
The mental/emotional status category revolved around perceived barriers as well as psychological and psychiatric comorbidities that could affect adherence. Factors significantly associated with adherence included SF-12 mental health score15, chance Locus of Control (defined as feelings of control over health outcomes),26 self-efficacy with medication-taking25 and life satisfaction.30 Nonadherence-associated factors included greater perceived barriers,32 higher self-care disability,29 greater feelings of guilt,36 higher depression scores and worse psychosocial functioning,24 and high concern regarding the use of immunosuppressants.35
Finally, in terms of health/transplant-related factors, Grady et al. found that patients with both an ischemic as well as idiopathic etiology of their heart failure had significantly higher adherence.28 Zhang et al. also noted that greater numbers of concomitant drugs were associated with nonadherence.35
Health outcomes related to nonadherence
Three studies prospectively reviewed health outcomes related to adherence and are outlined in Table 5. In the first, De Geest et al. clustered patients into different groups by several compliance-related variables. The “excellent compliers” had a 1.2% rate of late acute rejection, while the “minor subclinical noncompliers” had a rate of 14.3% and “moderate subacute noncompliers” had a rate of 22.2%, p = 0.01.25 Dobbels et al. found that nonadherence was significantly associated with any degree of transplant coronary artery disease. Furthermore, adherence was significantly associated with longer event-free time, though it was not significantly associated with late acute rejection, retransplant, or 5-year mortality.17 In contrast, Farmer et al. did note a significant association between both moderate and high adherence and improved mortality rates at 5–10 years.27
Table 5:
Studies exploring health outcomes associated with adherence and nonadherence
| Author (year) | Measure of adherence | Outcome variable | Association with preceding adherence/nonadherence |
|---|---|---|---|
| Dobbels (2004)17 | Medication Event Monitoring System (MEMS) | Transplant coronary artery disease (CAD), defined as any degree of angiographically detectable CAD on routine annual coronary angiogram | Significantly associated with nonadherence: (OR 1.779; 95% CI: 1.20 – 2.64; p = 0.025) |
| Late acute rejection defined as biopsy-proven Grade >1B rejection | Trend toward nonadherence but not significant: (OR 4.941; 95% CI: 0.75 – 32.69; p = 0.131) | ||
| Retransplant as noted in the medical record | Trend toward nonadherence but not significant: (OR 5.4; 95% CI: 0.82 – 35.42; p = 0.114) | ||
| 5-year mortality as noted in the medical record | Not significant (OR 1.098; 95% CI: 0.26 – 4.64; p = 0.999) | ||
| Clinical-event-free time without the above 4 events | Significantly associated with adherence: (mean 1612 days vs. 1318 days; p = 0.043) | ||
| Adjusted relative risk of clinical event per Cox regression analysis | After controlling for other known transplant-related risk factors for poor clinical outcome, trend toward nonadherence but not significant: ARR = 2.03; p = 0.0582 | ||
| Farmer (2013)27 | Assessment of Problems with the Transplant Regimen Scale | Death at 5–10 years as observed in the medical record | High compliance significantly inversely associated with death: (HR 0.26; 95% CI: 0.08–0.81; p=0.02) Moderate compliance significantly inversely associated with death: (HR 0.34; 95% CI: 0.14–0.79; p=0.01) |
| De Geest (1998)25 | Electronic pill bottle monitor | Acute late rejection during the course of the study | Significantly associated with noncompliance: rate 1.2% in excellent compliers, 14.3% in minor subclinical noncompliers and 22.2% in moderate subclinical noncompliers; p = 0.01 |
OR: odds ratio; CI: Confidence interval; HR: Hazard ratio
Interventions to improve adherence
Finally, two interventions were explored to potentially improve adherence, outlined in Table 6. The first strategy employed by Doesch et al. in 201019 and 201318 reviewed the effect of transitioning traditional twice-daily dosed tacrolimus and cyclosporine to once-daily dosed tacrolimus. This intervention in both the preliminary and completed study showed significantly improved nonadherence rates (from 75.0% to 40.3% in the longer 8 month study, p = 0.0001). The second intervention by Gomis-Pastor was a multifaceted mobile app to provide information to transplant patients and also collect data for the physician and research care team. Its use was associated with an improvement in adherence from 61% to 87% (p = 0.005) over 1 month of use.21
Table 6:
Studies evaluating adherence interventions
| Author/Year | Measure of adherence | Description of Intervention | Main outcomes |
|---|---|---|---|
| Doesch (2013)18 | Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS) | Patients were switched from conventional twice-daily tacrolimus or cyclosporine A to once daily modified release tacrolimus regimen. | • Overall nonadherence at baseline for any of the four BAASIS items was 75.0% versus 40.3% after 8 months (P=0.0001). • After 8 months, adherence was improved in 41 patients (56.9%), unchanged in 27 (37.5%), and reduced in four patients (5.6%). • No significant changes were observed for hematological, renal, or liver function parameters after 8 months (all P = NS). |
| Doesch (2010)19 | Visual Analogue Scale (VAS) and Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS) | Patients were switched from conventional twice-daily tacrolimus or cyclosporine A to once daily modified release tacrolimus regimen. | • Overall nonadherence at baseline for any of the 4 BAASIS items was 74% versus 38% after 4 months (P = .0001). • Thereafter, adherence improved in 28 patients (56.0%), was unchanged in 18 (36.0%), and decreased in 4 subjects (8.0%). The VAS score improved from 82.3% +/− 2.6% to 97.5% +/− 4.8% (P = .0001). • No significant changes were observed after 4 months regarding hematologic, renal, or liver function parameters (all P = NS). |
| Gomis-Pastor (2020)21 | Simplified Medication Adherence Questionnaire (SMAQ) | mHeart: mobile app bidirectionally integrated with hospital information system; had functionality to answer patients’ questions about their treatment and health conditions, empower patients in terms of self-care, and facilitate professionals’ interventions regarding symptoms and adverse effects. | • The theory-based multifaceted intervention improved the adherence rate from 61% to 87% (p =0.005) over 1 month. |
NS = Not significant
Studies’ Methodological Quality
Table 7 outlines bias ratings for prospective cohort studies as scored by the Newcastle-Ottawa scale. Table 8 outlines bias ratings for cross-sectional studies as scored by the modified Newcastle-Ottawa scale. Overall, the 9 prospective cohort studies scored between 6 and 9 out of 9 possible points. The majority of points were “lost” due to lack of controls/adjustments for possible confounders during statistical analysis (8 points lost among 4 studies) and only self-reported outcomes (6 points lost among 6 studies). For the 14 cross-sectional studies, scores ranged from 5 to 9 out of a possible 10 points. The most points were lost for only self-reported outcomes (12 points lost among 12 studies) and lack of comparison between respondents and non-respondents (10 points lost among 10 studies). In all studies combined, self-reported outcomes were responsible for the most points lost (18 points lost among 18 studies) and lack of controls/adjustments for confounders (14 points lost among 7 studies).
Table 7:
Bias ratings of prospective cohort studies, via Newcastle-Ottawa scores
| File name | Representativeness of the exposed cohort | Selection of nonexposed cohort | Ascertainment of exposure | that outcome of interest was not present at the start of the study | Comparability | Assessment of outcome | Length of follow-up | Adequacy of follow-up | Bias rating | Bias reasoning |
|---|---|---|---|---|---|---|---|---|---|---|
| De Geest (1998)25 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 out of 9 | No controls/adjustment |
| De Geest (2014)16 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 7 out of 9 | Self-reported outcomes High attrition rate |
| Dobbels (2004)17 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 out of 9 | N/A |
| Doesch (2013)18 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 6 out of 9 | No controls/adjustment Self-reported outcomes |
| Doesch (2010)19 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 6 out of 9 | No controls/adjustment Self-reported outcomes |
| Gomis-Pastor (2020)21 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 6 out of 9 | No controls/adjustment Self-reported outcomes |
| Grady (1998)29 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 7 out of 9 | Self-reported outcomes High attrition rate |
| Farmer (2013)27 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 out of 9 | N/A |
| Vitinius (2019)24 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 8 out of 9 | Self-reported outcomes |
N/A: not applicable
Table 8:
Bias rating of cross-sectional studies, via modified Newcastle-Ottawa scores
| File name | Representativeness of the sample | Sample size | Non-respondents | Ascertainment of the exposure (risk factor) | Comparability | Assessment of the outcome | Statistical test | Bias rating | Bias reasoning |
|---|---|---|---|---|---|---|---|---|---|
| Blanca Martinez Perez (2013)14 | 1 | 1 | 1 | 2 | 0 | 2 | 1 | 8 out of 10 | No controls/adjustments done |
| Brocks (2017)15 | 1 | 1 | 0 | 2 | 2 | 1 | 1 | 8 out of 10 | No description of non-respondents Self-reported outcomes only |
| Denhaerynck (2018)32 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 9 out of 10 | Self-reported outcomes only |
| Epstein (2014)26 | 1 | 1 | 0 | 2 | 2 | 1 | 1 | 8 out of 10 | No description of non-respondents Self-reported outcomes only |
| Goetzmann (2008)20 | 1 | 1 | 0 | 2 | 0 | 1 | 1 | 6 out of 10 | No description of non-respondents No controls/adjustments done Self-reported outcomes only |
| Grady (2016)28 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 9 out of 10 | Self-reported outcomes only |
| Grady (1999)30 | 1 | 1 | 0 | 2 | 2 | 1 | 0 | 7 out of 10 | No description of non-respondents Self-reported outcomes only p-values given but no confidence intervals given for adherence scores |
| Helmy (2019)33 | 1 | 1 | 0 | 2 | 2 | 1 | 1 | 8 out of 10 | No description of non-respondents Self-reported outcomes only |
| Hugon (2014)22 | 1 | 1 | 0 | 2 | 2 | 2 | 0 | 8 out of 10 | No description of non-respondents No p-values or confidence intervals given specifically for heart transplant patients |
| Kung (2012)34 | 1 | 1 | 0 | 2 | 2 | 1 | 0 | 7 out of 10 | No description of non-respondents Self-reported outcomes only No p-values or confidence intervals given specifically for heart transplant patients |
| Milaniak (2014)23 | 1 | 1 | 0 | 2 | 0 | 1 | 0 | 5 out of 10 | No description of non-respondents No controls/adjustments done Self-reported outcomes only R given but no confidence interval |
| Shamaskin (2012)31 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 9 out of 10 | Self-reported outcomes only |
| Shemesh (2017)36 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 7 out of 10 | No description of non-respondents Exposure tool non-validated Self-reported outcomes only |
| Zhang (2019)35 | 1 | 1 | 0 | 2 | 2 | 1 | 1 | 8 out of 10 | No description of non-respondents Self-reported outcomes only |
N/A: not applicable
Discussion
This systematic review analyzed multiple aspects of adherence to immunosuppression in adult heart transplant recipients. Adherence in this population has been measured via blood immunosuppression levels, electronic pill bottle monitoring, or most commonly, a variety of validated self-report questionnaires. Baseline adherence estimates vary greatly depending on the definition and measurement of adherence. Multiple significant correlates to nonadherence exist and appear to affect patients with certain sociodemographic backgrounds, those with psychological/psychiatric comorbidities and those with poor support structures. Nonadherence is associated with transplant coronary artery disease and acute late rejection; it may also be associated with long-term mortality. Finally, a simplified dosing regimen with once-a-day tacrolimus as well as use of a mobile phone-based intervention may improve adherence. Bias scores were most deficient due to only self-reported outcomes as noted in 18 studies and lack of controls/adjustments for confounders, as seen in 7 studies.
Our findings suggest a wide range of baseline adherence estimates, as well as several risk factors, some modifiable, that also correlate with adherence. But what rate of adherence is adequate? There is no clear value widely touted in transplant research. In solid-organ transplants, studies range from ≥80%37 up to <98%38, 39 as cutoffs for nonadherence; the stringent higher limit was recommended in our included article by De Geest et al. in 1998 who identified the correlation between even subclinical noncompliance to cyclosporine and increased incidence of late acute rejections.25 In the HIV literature, older manuscripts suggested ≥95% adherence was needed for adequate virologic suppression, though more recent studies with modern regimens note that even ≥80% may not be statistically suboptimal.40
Despite this varying set of definitions, our study suggests that populations exist where the adherence to immunosuppression still remains concerningly low or variable. Inadequate adherence to common agents such as tacrolimus may place patients at risk of acute and chronic rejection, donor-specific anti-HLA antibodies, and progressive fibrotic damage that can lead to graft failure.41 At its extreme, poor adherence has been associated with mortality in the pediatric heart transplant literature,42 while few studies exist regarding outcomes in the adult literature as evidenced in our study. Thus, there remains a clear need to identify and maintain a threshold of adequate adherence.
Challenges remain in the measurement and management of immunosuppressant adherence. A study by Shi et al. done in late 2020 reviewed interventions tested by RCT to improve adherence in solid-organ transplant recipients.43 They found significantly better pooled risk ratios for overall adherence, dosing adherence, and timing adherence between the intervention groups and controls, but no significant improvement in immunosuppressant blood concentrations. This reflects a pattern seen in our review, in which the vast majority (18/23 studies) utilized self-report measures for adherence and only 4 utilized immunosuppressant levels or pill bottle monitors to more accurately observe adherence. A 2020 publication stratified measurement tools in transplant recipients and noted that rich and reliable data could be collected through more direct measures (electronic monitoring, ingestible smart sensors), though many teams continue to use more limited and biased measures including retrospective questionnaires, pharmacy refill data, and patient diaries.44 Further studies and implemented programs may benefit in terms of limiting bias and social desirability by utilizing more objective measures of adherence.
Further studies on effective interventions in the heart transplant literature are needed. There does exist promising data that some interventions are feasible and to patients’ satisfaction45, but other strategies can suffer from high attrition as a barrier46. Some interventions have been associated with improved adherence in areas such as adolescent kidney transplant47 and even solid organ transplant recipients as a whole48. Unfortunately, there remains a lack of data on demonstrated survival outcomes, as well as utilization of taxonomies (for example, division into initiation, implementation, and discontinuation phase-focused tools) to better understand effective interventions.44 As underlined in a 2017 review of 21 interventions to improve adherence in solid-organ transplant recipients, no strategies were associated with improved transplant outcomes49. Those authors suspected a “streetlight effect” is in play – that is, adherence researchers are looking for outcomes where and how they are easy to come across, rather than focusing on the right population, interventions and outcomes. To combat this, the authors recommend further studies that 1) focus on nonadherent patients, rather than a convenience sample, 2) use direct measures of adherence and survival, and 3) utilize effective, low-effort, high-engagement methods to recruit patients who already struggle with complex adherence tasks. The takeaways from our study are similar – that evidence regarding effective interventions and ultimately, the link between them and survival, are yet to be explored fully. To this end, mHealth, or mobile health interventions, are of potential use in this sphere as they have the advantage of being low-cost, easily disseminated, and customizable to patients at the individual level.50
Strengths and Limitations
The primary strength of this manuscript is its novelty and systematic approach to evaluating multiple aspects of IS adherence in adult heart transplant patients. At least 2 authors independently screened all articles and evaluated for bias, which adds credibility to our gathered conclusions. We have thoroughly examined and organized our data with the intention that a multitude of questions can be answered by perusing this manuscript and its included tables. While the data is heterogeneous and sparse in some areas, we hope that this in itself is a call to action for teams performing further research in this field.
Limitations of this paper include multiple sources of bias, especially from the focus on self-reported outcomes, as well as lack of controls/adjustment in data analysis. It remains a challenge for teams to measure medication adherence at least somewhat objectively, either through drug levels or less direct pill-bottle measurement systems. With smaller cohorts, there is also difficulty in recruiting enough participants to effectively analyze or control for multiple variables. Furthermore, some independent variables, such as race, are societal constructs and may have confounders that affect observed correlates, as seen in prior studies.51, 52 Studies should carefully state how statistical analyses take into account controlling/adjusting for baseline factors, including age, socio-demographics, and transplant characteristics, when relevant and possible. A final challenge of ours was that we found very heterogeneous qualitative and quantitative outcomes, precluding a true meta-analysis.
Conclusions
Heart transplant is an extremely focused and resource-intensive specialty. We provide evidence that rates of IS adherence vary but that many correlates to adherence are observable and modifiable factors. Thus, we hope to provide direction for potential further intervention and action. Further high-quality studies regarding strategies to improve IS adherence are needed in the adult heart transplant literature.
Highlights:
Adherence to immunosuppression in heart transplant patients varies considerably
Socio-demographics, mental health, and poor support correlate with nonadherence
Nonadherence may lead to coronary artery disease and acute late rejection
Simplified dosing regimen and a mobile app intervention were associated with improved adherence
Acknowledgments
Conflict of Interest/Funding Information: This project was supported by a grant from the National Heart, Lung, and Blood Institute of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the National Institutes of Health.
Declaration of Interest
This project was also supported by grant (K23HL150232, PI: Badawy) from the National Heart, Lung, and Blood Institute of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the National Institutes of Health.
Abbreviations
- HTx
heart transplant
- IS
immunosuppression
Appendix 1: Search Strategy
PubMed
((“Heart Transplantation”[MeSH Terms] OR “heart transplant*”[Title/Abstract]) OR ((“Organ Transplantation”[MeSH Terms:noexp] OR “transplant*”[Title/Abstract]) AND (“heart”[MeSH Terms] OR “heart*”[Title/Abstract]))) AND (((((((((“Medication Adherence”[MeSH Terms] OR “Patient Compliance”[MeSH Terms]) OR “Adherence”[Title/Abstract]) OR “Nonadherence”[Title/Abstract]) OR “non adherence”[Title/Abstract]) OR “non adherence”[Title/Abstract]) OR “Compliance”[Title/Abstract]) OR “Noncompliance”[Title/Abstract]) OR “non compliance”[Title/Abstract]) OR “non compliance”[Title/Abstract])
Remove review[pt] and animals in title
Embase
(‘heart transplantation’/exp OR ‘heart transplant*’:ti,ab OR ((‘organ transplantation’/exp OR transplant*:ti,ab) AND (‘heart’/exp OR heart:ti,ab))) AND (‘medication compliance’/exp OR ‘adherence’:ti,ab OR ‘nonadherence’:ti,ab OR ‘non adherence’:ti,ab OR ‘compliance’:ti,ab OR ‘noncompliance’:ti,ab OR ‘non compliance’:ti,ab)
Remove review and meeting abstracts and animals in title
CENTRAL
#1 MeSH descriptor: [Heart Transplantation] explode all trees
#2 “heart transplant*”:ti,ab
#3 #1 OR #2
#4 MeSH descriptor: [Organ Transplantation] explode all trees
#5 transplant*:ti,ab
#6 MeSH descriptor: [Heart] explode all trees
#7 heart:ti,ab
#8 #4 OR #5
#9 #6 OR #7
#10 #8 AND #9
#11 #3 OR #10
#12 MeSH descriptor: [Medication Adherence] explode all trees
#13 MeSH descriptor: [Patient Compliance] explode all trees
#14 (“adherence” OR “nonadherence” OR “non adherence” OR “compliance” OR “noncompliance” OR “non compliance”).ti,ab
#15 #12 OR #13 OR #14
#16 #11 AND #15
Scopus
TITLE-ABS-KEY (“heart transplant*”) AND TITLE-ABS-KEY (adherence OR nonadherence OR “non adherence” OR compliance OR noncompliance OR “non compliance”) AND (LIMIT-TO (DOCTYPE, “ar”))
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Colvin M, Smith JM, Hadley N, et al. OPTN/SRTR 2018 Annual Data Report: Heart. Am J Transplant. January 2020;20 Suppl s1:340–426. doi: 10.1111/ajt.15676 [DOI] [PubMed] [Google Scholar]
- 2.Goldraich LA, Leitao SAT, Scolari FL, et al. A Comprehensive and Contemporary Review on Immunosuppression Therapy for Heart Transplantation. Curr Pharm Des. 2020;26(28):3351–3384. doi: 10.2174/1381612826666200603130232 [DOI] [PubMed] [Google Scholar]
- 3.Wilhelm MJ. Long-term outcome following heart transplantation: current perspective. J Thorac Dis. March 2015;7(3):549–51. doi: 10.3978/j.issn.2072-1439.2015.01.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colvin M, Smith JM, Skeans MA, et al. Heart. Am J Transplant. January 2016;16 Suppl 2:115–40. doi: 10.1111/ajt.13670 [DOI] [PubMed] [Google Scholar]
- 5.Long EF, Swain GW, Mangi AA. Comparative survival and cost-effectiveness of advanced therapies for end-stage heart failure. Circ Heart Fail. May 2014;7(3):470–8. doi: 10.1161/CIRCHEARTFAILURE.113.000807 [DOI] [PubMed] [Google Scholar]
- 6.Birati EY, Rame JE. Post-heart transplant complications. Crit Care Clin. July 2014;30(3):629–37. doi: 10.1016/j.ccc.2014.03.005 [DOI] [PubMed] [Google Scholar]
- 7.Lindenfeld J, Miller GG, Shakar SF, et al. Drug therapy in the heart transplant recipient: part II: immunosuppressive drugs. Circulation. December 21 2004;110(25):3858–65. doi: 10.1161/01.CIR.0000150332.42276.69 [DOI] [PubMed] [Google Scholar]
- 8.Copeland JG, Mammana RB, Fuller JK, Campbell DW, McAleer MJ, Sailer JA. Heart transplantation. Four years’ experience with conventional immunosuppression. JAMA. March 23–30 1984;251(12):1563–6. doi: 10.1001/jama.251.12.1563 [DOI] [PubMed] [Google Scholar]
- 9.Olivari MT, Kubo SH, Braunlin EA, Bolman RM, Ring WS. Five-year experience with triple-drug immunosuppressive therapy in cardiac transplantation. Circulation. November 1990;82(5 Suppl):IV276–80. [PubMed] [Google Scholar]
- 10.Monane M, Bohn RL, Gurwitz JH, Glynn RJ, Avorn J. Noncompliance with congestive heart failure therapy in the elderly. Arch Intern Med. February 28 1994;154(4):433–7. [PubMed] [Google Scholar]
- 11.Fitzgerald AA, Powers JD, Ho PM, et al. Impact of medication nonadherence on hospitalizations and mortality in heart failure. J Card Fail. August 2011;17(8):664–9. doi: 10.1016/j.cardfail.2011.04.011 [DOI] [PubMed] [Google Scholar]
- 12.Wells GA SB, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2021; [Google Scholar]
- 13.Modesti PA, Reboldi G, Cappuccio FP, et al. Panethnic Differences in Blood Pressure in Europe: A Systematic Review and Meta-Analysis. PLoS One. 2016;11(1):e0147601. doi: 10.1371/journal.pone.0147601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanca Martinez Perez A, Lopez Suarez A, Rodriguez Rodriguez J, Sobrino Marquez JM, Lage Galle E. Medication adherence in patients who undergo cardiac transplantation. Transplant Proc. 2013;45(10):3662–4. doi: 10.1016/j.transproceed.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 15.Brocks Y, Zittermann A, Grisse D, et al. Adherence of Heart Transplant Recipients to Prescribed Medication and Recommended Lifestyle Habits. Prog Transplant. June 2017;27(2):160–166. doi: 10.1177/1526924817699959 [DOI] [PubMed] [Google Scholar]
- 16.De Geest S, Burkhalter H, Bogert L, et al. Describing the evolution of medication nonadherence from pretransplant until 3 years post-transplant and determining pretransplant medication nonadherence as risk factor for post-transplant nonadherence to immunosuppressives: the Swiss Transplant Cohort Study. Transpl Int. July 2014;27(7):657–66. doi: 10.1111/tri.12312 [DOI] [PubMed] [Google Scholar]
- 17.Dobbels F, De Geest S, van Cleemput J, Droogne W, Vanhaecke J. Effect of late medication non-compliance on outcome after heart transplantation: a 5-year follow-up. J Heart Lung Transplant. November 2004;23(11):1245–51. doi: 10.1016/j.healun.2003.09.016 [DOI] [PubMed] [Google Scholar]
- 18.Doesch AO, Mueller S, Akyol C, et al. Increased adherence eight months after switch from twice daily calcineurin inhibitor based treatment to once daily modified released tacrolimus in heart transplantation. Drug Des Devel Ther. 2013;7:1253–8. doi: 10.2147/DDDT.S52820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doesch AO, Mueller S, Konstandin M, et al. Increased adherence after switch from twice daily calcineurin inhibitor based treatment to once daily modified released tacrolimus in heart transplantation: a pre-experimental study. Transplant Proc. December 2010;42(10):4238–42. doi: 10.1016/j.transproceed.2010.09.074 [DOI] [PubMed] [Google Scholar]
- 20.Goetzmann L, Sarac N, Ambuhl P, et al. Psychological response and quality of life after transplantation: a comparison between heart, lung, liver and kidney recipients. Swiss Med Wkly. August 23 2008;138(33–34):477–83. doi:2008/33/smw-12160 [DOI] [PubMed] [Google Scholar]
- 21.Gomis-Pastor M, Roig E, Mirabet S, et al. A Mobile App (mHeart) to Detect Medication Nonadherence in the Heart Transplant Population: Validation Study. JMIR Mhealth Uhealth. February 4 2020;8(2):e15957. doi: 10.2196/15957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hugon A, Roustit M, Lehmann A, et al. Influence of intention to adhere, beliefs and satisfaction about medicines on adherence in solid organ transplant recipients. Transplantation. July 27 2014;98(2):222–8. doi: 10.1097/TP.0000000000000221 [DOI] [PubMed] [Google Scholar]
- 23.Milaniak I, Wilczek-Ruzyczka E, Wierzbicki K, Sadowski J, Przybylowski P. The influence of sense of coherence on emotional response in heart transplant recipients - a preliminary report. Kardiochir Torakochirurgia Pol. June 2014;11(2):220–4. doi: 10.5114/kitp.2014.43856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vitinius F, Reklat A, Hellmich M, et al. Prediction of survival on the waiting list for heart transplantation and of posttransplant nonadherence-Results of a prospective longitudinal study. Clin Transplant. July 2019;33(7):e13616. doi: 10.1111/ctr.13616 [DOI] [PubMed] [Google Scholar]
- 25.De Geest S, Abraham I, Moons P, et al. Late acute rejection and subclinical noncompliance with cyclosporine therapy in heart transplant recipients. J Heart Lung Transplant. September 1998;17(9):854–63. [PubMed] [Google Scholar]
- 26.Epstein FR, Kale PP, Weisshaar DM. Association between the chance locus of control belief with self-reported occasional nonadherence after heart transplantation: a pilot study. Transplantation. July 15 2014;98(1):e4. doi: 10.1097/TP.0000000000000196 [DOI] [PubMed] [Google Scholar]
- 27.Farmer SA, Grady KL, Wang E, McGee EC Jr., Cotts WG, McCarthy PM. Demographic, psychosocial, and behavioral factors associated with survival after heart transplantation. Ann Thorac Surg. March 2013;95(3):876–83. doi: 10.1016/j.athoracsur.2012.11.041 [DOI] [PubMed] [Google Scholar]
- 28.Grady KL, Andrei AC, Li Z, et al. Gender differences in appraisal of stress and coping 5 years after heart transplantation. Heart Lung. Jan-Feb 2016;45(1):41–7. doi: 10.1016/j.hrtlng.2015.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grady KL, Jalowiec A, White-Williams C. Patient compliance at one year and two years after heart transplantation. J Heart Lung Transplant. April 1998;17(4):383–94. [PubMed] [Google Scholar]
- 30.Grady KL, Jalowiec A, White-Williams C. Predictors of quality of life in patients at one year after heart transplantation. J Heart Lung Transplant. March 1999;18(3):202–10. doi: 10.1016/s1053-2498(98)00048-5 [DOI] [PubMed] [Google Scholar]
- 31.Shamaskin AM, Rybarczyk BD, Wang E, et al. Older patients (age 65+) report better quality of life, psychological adjustment, and adherence than younger patients 5 years after heart transplant: A multisite study. J Heart Lung Transplant. May 2012;31(5):478–84. doi: 10.1016/j.healun.2011.11.025 [DOI] [PubMed] [Google Scholar]
- 32.Denhaerynck K, Berben L, Dobbels F, et al. Multilevel factors are associated with immunosuppressant nonadherence in heart transplant recipients: The international BRIGHT study. Am J Transplant. June 2018;18(6):1447–1460. doi: 10.1111/ajt.14611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helmy R, Scalso de Almeida S, Denhaerynck K, et al. Prevalence of Medication Nonadherence to Co-medication Compared to Immunosuppressants in Heart Transplant Recipients: Findings From the International Cross-sectional BRIGHT Study. Clin Ther. January 2019;41(1):130–136. doi: 10.1016/j.clinthera.2018.11.007 [DOI] [PubMed] [Google Scholar]
- 34.Kung M, Koschwanez HE, Painter L, Honeyman V, Broadbent E. Immunosuppressant nonadherence in heart, liver, and lung transplant patients: associations with medication beliefs and illness perceptions. Transplantation. May 15 2012;93(9):958–63. doi: 10.1097/TP.0b013e31824b822d [DOI] [PubMed] [Google Scholar]
- 35.Zhang M, Zhou H, Nelson RS, et al. Prevalence and Risk Factors of Immunosuppressant Nonadherence in Heart Transplant Recipients: A Single-Center Cross-Sectional Study. Patient Prefer Adherence. 2019;13:2185–2193. doi: 10.2147/PPA.S223837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shemesh Y, Peles-Bortz A, Peled Y, et al. Feelings of indebtedness and guilt toward donor and immunosuppressive medication adherence among heart transplant (HTx) patients, as assessed in a cross-sectional study with the Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS). Clin Transplant. October 2017;31(10)doi: 10.1111/ctr.13053 [DOI] [PubMed] [Google Scholar]
- 37.Su GC, Greanya ED, Partovi N, Yoshida EM, Shapiro RJ, Levy RD. Assessing medication adherence in solid-organ transplant recipients. Exp Clin Transplant. December 2013;11(6):475–81. doi: 10.6002/ect.2013.0060 [DOI] [PubMed] [Google Scholar]
- 38.Ko H, Kim HK, Chung C, et al. Association between medication adherence and intrapatient variability in tacrolimus concentration among stable kidney transplant recipients. Sci Rep. March 8 2021;11(1):5397. doi: 10.1038/s41598-021-84868-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schafer-Keller P, Steiger J, Bock A, Denhaerynck K, De Geest S. Diagnostic accuracy of measurement methods to assess non-adherence to immunosuppressive drugs in kidney transplant recipients. Am J Transplant. March 2008;8(3):616–26. doi: 10.1111/j.1600-6143.2007.02127.x [DOI] [PubMed] [Google Scholar]
- 40.Bezabhe WM, Chalmers L, Bereznicki LR, Peterson GM. Adherence to Antiretroviral Therapy and Virologic Failure: A Meta-Analysis. Medicine (Baltimore). April 2016;95(15):e3361. doi: 10.1097/MD.0000000000003361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuypers DRJ. Intrapatient Variability of Tacrolimus Exposure in Solid Organ Transplantation: A Novel Marker for Clinical Outcome. Clin Pharmacol Ther. February 2020;107(2):347–358. doi: 10.1002/cpt.1618 [DOI] [PubMed] [Google Scholar]
- 42.Oliva M, Singh TP, Gauvreau K, Vanderpluym CJ, Bastardi HJ, Almond CS. Impact of medication non-adherence on survival after pediatric heart transplantation in the U.S.A. J Heart Lung Transplant. September 2013;32(9):881–8. doi: 10.1016/j.healun.2013.03.008 [DOI] [PubMed] [Google Scholar]
- 43.Shi YX, Liu CX, Liu F, et al. Efficacy of Adherence-Enhancing Interventions for Immunosuppressive Therapy in Solid Organ Transplant Recipients: A Systematic Review and Meta-Analysis Based on Randomized Controlled Trials. Front Pharmacol. 2020;11:578887. doi: 10.3389/fphar.2020.578887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Geest SM, Ribaut J, Denhaerynck K, Dobbels F. Adherence measurement in transplantation. In: Cukor D, Cohen S, Kimmel PL, eds. Psychosocial Aspects of Chronic Kidney Disease. 1st ed. Academic Press; 2020:409–448:chap 19. [Google Scholar]
- 45.Jung HY, Jeon Y, Seong SJ, et al. ICT-based adherence monitoring in kidney transplant recipients: a randomized controlled trial. BMC Med Inform Decis Mak. June 10 2020;20(1):105. doi: 10.1186/s12911-020-01146-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han A, Min SI, Ahn S, et al. Mobile medication manager application to improve adherence with immunosuppressive therapy in renal transplant recipients: A randomized controlled trial. PLoS One. 2019;14(11):e0224595. doi: 10.1371/journal.pone.0224595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foster BJ, Pai ALH, Zelikovsky N, et al. A Randomized Trial of a Multicomponent Intervention to Promote Medication Adherence: The Teen Adherence in Kidney Transplant Effectiveness of Intervention Trial (TAKE-IT). Am J Kidney Dis. July 2018;72(1):30–41. doi: 10.1053/j.ajkd.2017.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dobbels F, De Bleser L, Berben L, et al. Efficacy of a medication adherence enhancing intervention in transplantation: The MAESTRO-Tx trial. J Heart Lung Transplant. May 2017;36(5):499–508. doi: 10.1016/j.healun.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 49.Duncan S, Annunziato RA, Dunphy C, LaPointe Rudow D, Shneider BL, Shemesh E. A systematic review of immunosuppressant adherence interventions in transplant recipients: Decoding the streetlight effect. Pediatr Transplant. February 2018;22(1)doi: 10.1111/petr.13086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hussain T, Nassetta K, Badawy SM. Adherence to Immunosuppression Medications among Heart Transplant Recipients: Challenges, Opportunities, and Potential Role of Digital Approaches in the COVID-19 Era. J Cardiovasc Dev Dis. 2021;8(68)doi: 10.3390/jcdd8060068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Breathett K, Yee E, Pool N, et al. Association of Gender and Race With Allocation of Advanced Heart Failure Therapies. JAMA Netw Open. July 1 2020;3(7):e2011044. doi: 10.1001/jamanetworkopen.2020.11044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frech A, Tarrence J, Natale G, Tumin D. Ventricular Assist Device Technology and Black-White Disparities on the Heart Transplant Wait List. Prog Transplant. March 2021;31(1):80–87. doi: 10.1177/1526924820978591 [DOI] [PubMed] [Google Scholar]
- 53.Scholz U, Klaghofer R, Dux R, et al. Predicting intentions and adherence behavior in the context of organ transplantation: gender differences of provided social support. J Psychosom Res. March 2012;72(3):214–9. doi: 10.1016/j.jpsychores.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 54.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. January 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007 [DOI] [PubMed] [Google Scholar]
- 55.Chisholm MA, Lance CE, Williamson GM, Mulloy LL. Development and validation of the immunosuppressant therapy adherence instrument (ITAS). Patient Educ Couns. October 2005;59(1):13–20. doi: 10.1016/j.pec.2004.09.003 [DOI] [PubMed] [Google Scholar]
- 56.Chisholm MA, Lance CE, Mulloy LL. Patient factors associated with adherence to immunosuppressant therapy in renal transplant recipients. Am J Health Syst Pharm. September 1 2005;62(17):1775–81. doi: 10.2146/ajhp040541 [DOI] [PubMed] [Google Scholar]
- 57.Wilks SE, Spivey CA, Chisholm-Burns MA. Psychometric re-evaluation of the immunosuppressant therapy adherence scale among solid-organ transplant recipients. J Eval Clin Pract. February 2010;16(1):64–8. doi: 10.1111/j.1365-2753.2008.01115.x [DOI] [PubMed] [Google Scholar]
- 58.De Geest S The Basel Assessment of Adherence to immunoSuppressive medications Scale 2021;
- 59.Dobbels F, Berben L, De Geest S, et al. The psychometric properties and practicability of self-report instruments to identify medication nonadherence in adult transplant patients: a systematic review. Transplantation. July 27 2010;90(2):205–19. doi: 10.1097/TP.0b013e3181e346cd [DOI] [PubMed] [Google Scholar]
- 60.Marsicano Ede O, Fernandes Nda S, Colugnati F, et al. Transcultural adaptation and initial validation of Brazilian-Portuguese version of the Basel assessment of adherence to immunosuppressive medications scale (BAASIS) in kidney transplants. BMC Nephrol. May 21 2013;14:108. doi: 10.1186/1471-2369-14-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahearn EP. The use of visual analog scales in mood disorders: a critical review. J Psychiatr Res. Sep-Oct 1997;31(5):569–79. doi: 10.1016/s0022-3956(97)00029-0 [DOI] [PubMed] [Google Scholar]
- 62.Burton MJ, Voluse AC, Patel AB, Konkle-Parker D. Measuring Adherence to Hepatitis C Direct-Acting Antiviral Medications: Using the VAS in an HCV Treatment Clinic. South Med J. January 2018;111(1):45–50. doi: 10.14423/SMJ.0000000000000750 [DOI] [PubMed] [Google Scholar]
- 63.Amico KR, Fisher WA, Cornman DH, et al. Visual analog scale of ART adherence: association with 3-day self-report and adherence barriers. J Acquir Immune Defic Syndr. August 1 2006;42(4):455–9. doi: 10.1097/01.qai.0000225020.73760.c2 [DOI] [PubMed] [Google Scholar]
- 64.Ziegelmann JP, Griva K, Hankins M, et al. The Transplant Effects Questionnaire (TxEQ): The development of a questionnaire for assessing the multidimensional outcome of organ transplantation - example of end stage renal disease (ESRD). Br J Health Psychol. November 2002;7(Part 4):393–408. doi: 10.1348/135910702320645381 [DOI] [PubMed] [Google Scholar]
- 65.Knobel H, Alonso J, Casado JL, et al. Validation of a simplified medication adherence questionnaire in a large cohort of HIV-infected patients: the GEEMA Study. AIDS. March 8 2002;16(4):605–13. doi: 10.1097/00002030-200203080-00012 [DOI] [PubMed] [Google Scholar]
- 66.Ortega Suarez FJ, Sanchez Plumed J, Perez Valentin MA, et al. Validation on the simplified medication adherence questionnaire (SMAQ) in renal transplant patients on tacrolimus. Nefrologia. 2011;31(6):690–6. doi: 10.3265/Nefrologia.pre2011.Aug.10973 [DOI] [PubMed] [Google Scholar]
- 67.Goetzmann L, Klaghofer R, Spindler A, Wagner-Huber R, Scheuer E, Buddeberg C. [The “Medication Experience Scale for Immunosuppressants” (MESI): initial results for a new screening instrument in transplant medicine]. Psychother Psychosom Med Psychol. February 2006;56(2):49–55. Die “Medikamenten-Erfahrungs-Skala fur Immunsuppressiva” (MESI) -- erste Ergebnisse zu einem neuen Screeninginstrument in der Transplantationsmedizin. doi: 10.1055/s-2005-867060 [DOI] [PubMed] [Google Scholar]