Abstract
Aims
Aortic valve calcification (AVC) has been shown to be associated with increased cardiovascular disease (CVD) risk; however, whether this is independent of traditional risk factors and coronary artery calcification (CAC) remains unclear.
Methods and results
From the multicentre CAC Consortium database, 10 007 patients (mean 55.8±11.7 years, 64% male) with concomitant CAC and AVC scoring were included in the current analysis. AVC score was quantified using the Agatston score method and categorized as 0, 1–99, and ≥100. The endpoints were all-cause, CVD, and coronary heart disease (CHD) deaths. AVC (AVC>0) was observed in 1397 (14%) patients. During a median 7.8 (interquartile range: 4.7–10.6) years of study follow-up, 511 (5.1%) deaths occurred; 179 (35%) were CVD deaths, and 101 (19.8%) were CHD deaths. A significant interaction between CAC and AVC for mortality was observed (P<0.001). The incidence of mortality events increased with higher AVC; however, AVC ≥100 was not independently associated with all-cause, CVD, and CHD deaths after adjusting for CVD risk factors and CAC (P=0.192, 0.063, and 0.206, respectively). When further stratified by CAC<100 or ≥100, AVC ≥100 was an independent predictor of all-cause and CVD deaths only in patients with CAC <100, after adjusting for CVD risk factors and CAC [hazard ratio (HR): 1.93, 95% confidence interval (CI): 1.14–3.27; P=0.013 and HR: 2.71, 95% CI: 1.15–6.34; P=0.022, respectively].
Conclusion
Although the overall prognostic significance of AVC was attenuated after accounting for CAC, high AVC was independently associated with all-cause and CVD deaths in patients with low coronary atherosclerosis burden.
Keywords: aortic valve sclerosis, cardiovascular mortality, coronary artery calcium, computed tomography, prognosis
Introduction
Aortic valve calcification (AVC) is a marker of aortic valve sclerosis, seen in up to 25% of patients above age 65 years.1 AVC is highly associated with other vascular atherosclerosis, including coronary artery disease. The development and progression of AVC are an indolent process. Cumulative shear stress, endothelial disruption, and lipid infiltration lead to activation of myofibroblasts and leaflet thickening and calcification (sclerosis).2 Based on a definition of aortic sclerosis as focal aortic valve thickening and calcification, ∼10–15% of patients with aortic sclerosis will progress to obstructive valvular disease (stenosis) in their lifetime.3
AVC can be objectively quantified by non-contrast cardiac computed tomography (CT), with low intra- and interobserver variability.4 AVC is an important risk factor for cardiovascular disease (CVD) mortality, even in the absence of aortic stenosis.5,6 AVC and coronary artery calcification (CAC) are closely related to each other as they share similar pathophysiology in development and progression.1,7,8 Prior studies have reported that while AVC is associated with cardiovascular events, the association is attenuated after CAC is taken into account.9,10 The goal of this study was to assess these associations in a larger patient population with long-term follow-up cause-specific mortality data and to examine whether there are subgroups of CAC in which the AVC provides independent prediction of events.
Methods
Study population
The CAC Consortium is an investigator-initiated, large cohort of patients who underwent non-contrast cardiac-gated CAC testing in four US clinical sites, with systematic and prospective long-term follow-up for cause-specific mortality.11 Patients underwent a clinically indicated CAC scan and were at least 18 years old, without previous coronary heart disease (CHD) and were free of clinically significant cardiovascular symptoms (e.g. angina and claudication). CHD was defined as a known history of myocardial infarction, obstructive coronary artery disease, or coronary revascularization.
A total of 66 636 patients were enrolled in the CAC Consortium with baseline CAC testing from 1991 to 2010. In this study, we included 10 007 patients from the one of the four CAC Consortium sites (Cedars-Sinai Medical Center) in whom AVC quantification was performed. All study participants provided informed consent at the time of enrolment and CAC scanning. The study protocol was approved by the Cedars-Sinai Institutional Review Board. The data that support the findings of this study are available on reasonable request to the corresponding author.
CAC and AVC testing
Non-contrast cardiac-gated CT scans were performed for CAC scoring at each site according to the common standard protocol. Scanners included the Imatron C-100, C-150, C-300, e-Speed, GE LightSpeed VCT 64-slice platform (GE Medical Systems, Milwaukee, WI, USA), and the 4-slice Somatom Volume Zoom MDCT (Siemens, Munich, Germany). Full details about the equipment, technique, and quality control of CT scanning in the Consortium have been previously published.11
CAC and AVC were computed using the sum of individual area–density products according to the Agatston method.12 Calcium deposits in the aortic valve leaflets were computed as AVC. Calcification in the aortic annulus, root, or nearby coronary arteries was excluded from AVC. In the absence of any AVC, the AVC score was 0. CAC score was categorized as 0, 1–99, 100–399, and ≥400. AVC score was categorized as 0, 1–99, and ≥100.
Definitions of cardiovascular risk factors and outcome adjudication
Hypertension, diabetes, and dyslipidaemia were defined as either a prior diagnosis or treatment with medical therapy for these conditions. Dyslipidaemia was also considered if a lipid panel showed LDL-C >160 mg/dL, HDL-C <40 mg/dL in men or <50 mg/dL in women, or fasting triglycerides >150 mg/dL. A 10-year risk predictor score for atherosclerotic cardiovascular disease (ASCVD) was calculated using baseline data according to the pooled cohort equations.13 A more detailed description of risk factor definitions in the CAC Consortium has been previously published.11
Adjudication of mortality was performed by interrogation of the Social Security Death Index (SSDI) Death Master File, using unique patient identifiers, such as name, date of birth, and social security number (SSN). Cause of death was determined based on International Classification of Diseases (ICD)-9 and ICD-10 codes on the death certificate. CVD death was defined as mortality from CHD, stroke, heart failure, or other cardiovascular conditions. In a separate exploratory analysis, we also considered aortic stenosis as an underlying cause of death identified via ICD codes. Follow-up of the cohort occurred through June 2014 for this report.
Data analysis and statistical methods
Baseline characteristics were reported as total number and percentage of the population for categorical variables and mean±standard deviation or median (interquartile range [IQR]) for continuous variables. Comparisons between AVC categories were performed by use of a one-way analysis of variance or Kruskal–Wallis test for continuous variables and by Pearson’s χ2 test for categorical measures. Survival rates were displayed using the Kaplan–Meier method and compared using log-rank test. The crude incidence of cause-specific mortality events (events per 1000 person-years at risk) was calculated across AVC categories.
Cox proportional hazard regression was performed to estimate risks associated with AVC in two consecutive steps. Model 1 adjusted for age, sex, and conventional CVD risk factors including hypertension, diabetes, hyperlipidaemia, smoking status, and family history of CHD. Model 2 accounted for Model 1 factors and CAC (ln [CAC + 1]). AVC was calculated as ln (AVC + 1) for continuous form. The adjusted hazard ratio (HR) was reported with 95% confidence intervals (CIs). Linear regression analysis was used to assess the relationship between ln (AVC + 1) and ln (CAC + 1). Interaction between AVC and CAC on mortality outcomes was assessed in a Cox model containing AVC and CAC. The Wald P-value for the interaction term was reported. Based on the interaction results, further subgroup analysis with Cox regression model according to CAC [low CAC (CAC < 100) and high CAC (CAC ≥ 100)] was performed. The incremental discriminative value of AVC over CAC was assessed using global χ2 and net classification index (NRI) analyses.14 In the NRI analysis, AVC score ≥100 was considered an upward risk.
In exploratory analysis, we assessed the relationship of AVC to aortic stenosis death. We conducted a 4:1 propensity-matched case–control analysis of patients with and without aortic stenosis death and used a logistic regression model to assess the relationship between aortic stenosis death and AVC score (ln AVC+1) (details in Supplementary data online, Table S1).
Statistical analyses were performed with Stata 11.0 (Stata Corp, College Station, TX, USA). We defined statistical significance as two-side P < 0.05.
Results
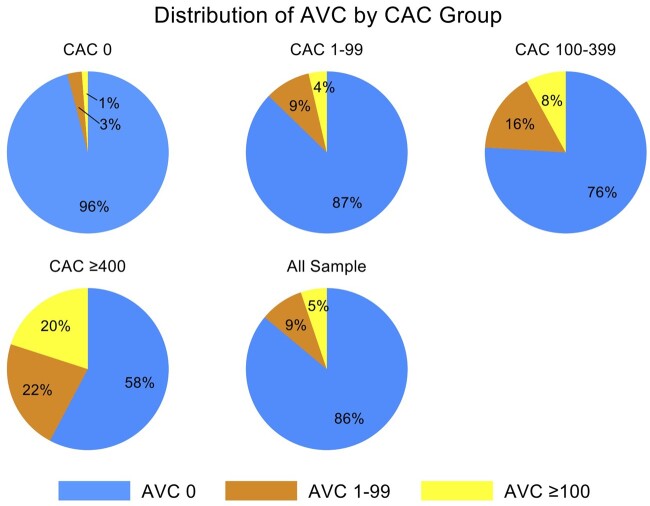
The baseline characteristics of the study population according to AVC category are shown in Table 1. The mean age at enrolment was 55.8 ± 11.7 years old and 64% were men. A total of 8610 (86%) patients had no measurable AVC, 876 (8.8%) patients had AVC 1–99, and 521 (5.2%) patients had AVC ≥100. Patients with high AVC were older with a greater proportion being male (Table 1; both P < 0.001). The prevalence of hypertension, diabetes, and dyslipidaemia was greater in patients with higher AVC (all P < 0.001, Table 1). The proportion of current smokers and patients with family history of CAD did not differ between patients with or without AVC (P = 0.235 and 0.975, respectively). The presence and severity of AVC were strongly associated with higher CAC score (Table 1 and Figure 1;P < 0.001). In patients with CAC of 0, only 4% patients had AVC >0 while 44% patients with CAC >400 had AVC >0 and 89% patients with AVC ≥ 100 had CAC > 0. In the linear regression analysis, a significant correlation existed between CAC and AVC (r = 0.36, P < 0.001; Supplementary data online Figure S1).
Table 1.
Baseline characteristics according to AVC category
| All (N = 10 007) | No AVC (N = 8610) | AVC 1–99 (n = 876) | AVC ≥ 100 (n = 521) | P-value | |
|---|---|---|---|---|---|
| Age | 55.8 ± 11.7 | 53.8 ± 10.7 | 66.1 ± 10.2 | 70.4 ± 10.6 | <0.001 |
| Women | 3603 (36.0) | 3158 (36.7) | 298 (34.0) | 147 (28.2) | <0.001 |
| Hypertension | 4595 (45.9) | 3624 (42.1) | 581 (66.3) | 391 (75.1) | <0.001 |
| Hyperlipidaemia | 5836 (58.3) | 4809 (55.9) | 705 (80.5) | 429 (82.3) | <0.001 |
| Smoker | 874 (8.7) | 764 (8.9) | 75 (8.6) | 35 (6.7) | 0.235 |
| Diabetes | 920 (9.2) | 680 (7.9) | 125 (14.3) | 115 (22.1) | <0.001 |
| Family history | 3742 (37.4) | 3220 (37.4) | 326 (37.2) | 197 (37.8) | 0.975 |
| ASCVD score | 9.7 ± 12.4 | 7.6 ± 9.9 | 19.5 ± 16.3 | 27.9 ± 19.2 | <0.001 |
| CAC score | 2 (0–114) | 0 (0–59) | 164.5 (20–598) | 417 (59–1194) | <0.001 |
| CAC groups | <0.001 | ||||
| 0 | 4842 (48.4) | 4643 (53.9) | 140 (15.9) | 59 (11.3) | |
| 1–99 | 2515 (25.1) | 2195 (25.5) | 229 (26.1) | 91 (17.5) | |
| 100–399 | 1327 (13.3) | 1008 (11.7) | 213 (24.3) | 106 (20.4) | |
| ≥400 | 1323 (13.2) | 764 (8.9) | 294 (33.6) | 265 (50.9) |
Values are expressed as mean ± standard deviation, median (IQR) or n (%).
AVC, aortic valve calcification; ASCVD, atherosclerotic cardiovascular disease; CAC, coronary artery calcification; IQR, interquartile range.
Figure 1.
Distribution of AVC according to strata of CAC.
During a median 7.8 [IQR: 4.7–10.6] years of study follow-up, a total of 511 (5.1%) deaths, including 179 (1.8%) CVD deaths and 101 (1.0%) CHD deaths, occurred. The incidence of all-cause, CVD, and CHD deaths increased with increasing AVC (Table 2 and Figure 2). The incidence per 1000-person year of all-cause, CVD, and CHD deaths was 5.2 (IQR: 4.7–5.8), 1.6 (1.4–2.0), and 0.8 (0.6–1.1) for the patients without AVC, 16.8 (13.5–20.9), 6.9 (4.9–9.6), and 4.8 (3.2–7.2) for the patients with AVC 1–99, and increasing further to 32.3 (26.1–40.1), 14.3 (10.3–19.7), and 8.5 (5.6–12.9) among patients with AVC ≥ 100. When stratified by age (<65 vs. ≥65) or sex, AVC remained associated with significantly lower survival rates in both groups (Supplementary data online, Figure S2).
Table 2.
Mortality events according to AVC category
| Number of patients | Number of events |
Incidence per 1000-person year |
|||||
|---|---|---|---|---|---|---|---|
| All-cause death | CVD death | CHD death | All-cause death | CVD death | CHD death | ||
| Overall | 10 007 | 511 (5.1) | 179 (1.8) | 101 (1.0) | 6.9 (6.3–7.5) | 2.4 (2.1–2.8) | 1.4 (1.1–1.7) |
| AVC category | |||||||
| AVC = 0 | 8610 | 346 (4.0) | 109 (1.3) | 56 (0.7) | 5.2 (4.7–5.8) | 1.6 (1.4–2.0) | 0.8 (0.6–1.1) |
| AVC 1–99 | 876 | 81 (9.3) | 33 (3.8) | 23 (2.6) | 16.8 (13.5–20.9) | 6.9 (4.9–9.6) | 4.8 (3.2–7.2) |
| AVC ≥ 100 | 521 | 84 (16.1) | 37 (7.1) | 22 (4.2) | 32.3 (26.1–40.1) | 14.3 (10.3–19.7) | 8.5 (5.6–12.9) |
AVC, aortic valve calcification; CHD, coronary heart disease; CVD, cardiovascular disease.
Figure 2.
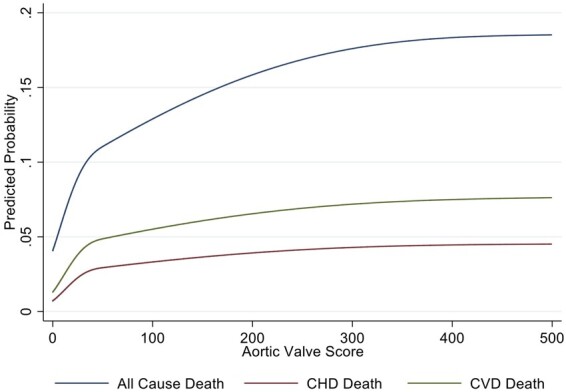
Mortality rates according to AVC score. Higher aortic valve calcification was predictive of all-cause mortality, CHD death, and CVD death in unadjusted analyses. The steeper slope initially represents the increase in risk from AVC of 0 to AVC >0.
The incidence of all-cause, CVD, and CHD deaths according to AVC and CAC categories were shown in Supplementary data online, Table S2. In the Cox regression analysis, the risk of all-cause, CVD, and CHD deaths increased at higher AVC (Table 3). After adjustment for age, sex, and conventional CVD risk factors (Model 1), high AVC scores (≥100 AU) remained significantly associated with ACM (HR: 1.33, 95% CI: 1.02–1.74; P = 0.032), CVD (HR: 1.72, 95% CI: 1.13–2.61; P = 0.011), and borderline significant with CHD deaths (HR: 1.65, 95% CI: 0.95–2.88; P = 0.074), compared to patients without AVC. However, after further adjusting for CAC (Model 2), AVC was not independently associated with mortality events (Table 3, all P > 0.05).
Table 3.
Cox regression analysis for all cause, CVD, and CHD death
| All cause death |
CVD death |
CHD death |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Unadjusted | |||||||||
| AVC = 0 | 1 (ref) | 1 (ref) | 1 (ref) | ||||||
| AVC > 0 | 3.98 | 3.30–4.79 | <0.001 | 5.12 | 3.79–6.94 | <0.001 | 6.16 | 4.15–9.15 | <0.001 |
| AVC 1–99 | 3.02 | 2.37–3.85 | <0.001 | 3.75 | 2.53–5.54 | <0.001 | 4.89 | 3.01–7.97 | <0.001 |
| AVC ≥ 100 | 5.74 | 4.52–7.30 | <0.001 | 7.66 | 5.26–11.15 | <0.001 | 8.46 | 5.15–13.89 | <0.001 |
| ln (AVC+1) | 1.35 | 1.30–1.39 | <0.001 | 1.40 | 1.32–1.48 | <0.001 | 1.42 | 1.31–1.53 | <0.001 |
| Model 1 (adjusted for age, sex, hypertension, diabetes, hyperlipidaemia, smoking, and family history) | |||||||||
| AVC = 0 | 1 (ref) | 1 (ref) | 1 (ref) | ||||||
| AVC > 0 | 1.15 | 0.94–1.42 | 0.183 | 1.46 | 1.04–2.04 | 0.030 | 1.59 | 1.02–2.47 | 0.039 |
| AVC 1–99 | 1.02 | 0.79–1.32 | 0.854 | 1.27 | 0.84–1.92 | 0.252 | 1.54 | 0.92–2.58 | 0.097 |
| AVC ≥ 100 | 1.33 | 1.02–1.74 | 0.032 | 1.72 | 1.13–2.61 | 0.011 | 1.65 | 0.95–2.88 | 0.074 |
| ln (AVC+1) | 1.05 | 1.00–1.09 | 0.030 | 1.09 | 1.02–1.17 | 0.011 | 1.08 | 0.98–1.17 | 0.106 |
| Model 2 (adjusted for model 1 + CAC score [ln (CAC+1)]) | |||||||||
| AVC = 0 | 1 (ref) | 1 (ref) | 1 (ref) | ||||||
| AVC > 0 | 1.05 | 0.86–1.30 | 0.624 | 1.30 | 0.93–1.81 | 0.131 | 1.41 | 0.91–2.22 | 0.123 |
| AVC 1–99 | 0.95 | 0.74–1.23 | 0.712 | 1.16 | 0.77–1.74 | 0.487 | 1.40 | 0.84–2.33 | 0.196 |
| AVC ≥ 100 | 1.20 | 0.92–1.55 | 0.192 | 1.48 | 0.98–2.25 | 0.063 | 1.42 | 0.82–2.46 | 0.206 |
| ln (AVC+1) | 1.03 | 0.99–1.07 | 0.205 | 1.06 | 0.99–1.14 | 0.071 | 1.05 | 0.96–1.14 | 0.294 |
AVC, aortic valve calcification; CAC, coronary artery calcification; CHD, coronary heart disease; CVD, cardiovascular disease.
There was a significant interaction between CAC and AVC for mortality events (P < 0.001). When further stratified by CAC <100 or ≥100, high AVC (≥100) was a significant predictor of all-cause and CVD deaths in patients with CAC <100, after adjusting for age, sex, CVD risk factors, and CAC (Table 4, HR: 1.93, 95% CI: 1.14–3.27; P = 0.013 and HR: 2.71, 95% CI: 1.15–6.34; P = 0.022, respectively). Conversely, AVC was not independently associated with any types of mortality events in patients with CAC ≥100.
Table 4.
Cox regression analysis for all-cause mortality, CVD death, and CHD death after stratified by CAC < 100 and CAC ≥ 100
| All cause death |
CVD death |
CHD death |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Low CAC (<100) | |||||||||
| AVC = 0 | 1 (ref) | 1 (ref) | 1 (ref) | ||||||
| AVC > 0 | 1.16 | 0.78–1.72 | 0.462 | 1.28 | 0.63–2.60 | 0.491 | 1.10 | 0.42–2.89 | 0.846 |
| AVC 1–99 | 0.85 | 0.51–1.41 | 0.518 | 0.68 | 0.24–1.94 | 0.465 | 0.54 | 0.12–2.37 | 0.411 |
| AVC ≥ 100 | 1.93 | 1.14–3.27 | 0.013 | 2.71 | 1.16–6.34 | 0.022 | 2.39 | 0.77–7.47 | 0.133 |
| ln (AVC+1) | 1.09 | 0.99–1.18 | 0.057 | 1.15 | 0.99–1.34 | 0.051 | 1.13 | 0.93–1.37 | 0.234 |
| High CAC (≥100) | |||||||||
| AVC = 0 | 1 (ref) | 1 (ref) | 1 (ref) | ||||||
| AVC > 0 | 1.03 | 0.81–1.31 | 0.804 | 1.32 | 0.90–1.94 | 0.154 | 1.53 | 0.93–2.53 | 0.095 |
| AVC 1–99 | 0.99 | 0.74–1.32 | 0.932 | 1.32 | 0.84–2.07 | 0.231 | 1.71 | 0.97–3.02 | 0.063 |
| AVC ≥ 100 | 1.08 | 0.80–1.47 | 0.604 | 1.32 | 0.82–2.12 | 0.246 | 1.34 | 0.72–2.49 | 0.361 |
| ln (AVC+1) | 1.01 | 0.96–1.06 | 0.594 | 1.04 | 0.97–1.12 | 0.278 | 1.03 | 0.94–1.14 | 0.539 |
Adjusting by age, sex, hypertension, diabetes, hyperlipidaemia, smoking, family history, and log CAC + 1.
AVC, aortic valve calcification; CAC, coronary artery calcification; CHD, coronary heart disease; CVD, cardiovascular disease.
In patients with CAC <100, global χ2 analysis showed that the addition of AVC to CAC improved the discrimination for all-cause, CVD, and CHD mortality outcomes (Table 5, all P < 0.01). In the NRI analysis, AVC score ≥100 resulted in a net increase of 8.4% and 11.9% in cases, and a net decrease of 1.9% and 2% in controls correctly classified, with overall NRI of 6.5% and 9.9% for all-cause and CVD mortality, respectively (Table 5, both P < 0.05). A similar trend was shown in CHD mortality events (NRI: 10.9%, case: 12.9%, control: −2%); however, the result did not reach statistical significance (P = 0.091).
Table 5.
Global χ2 values and net reclassification index for the addition of AVC to CAC in patients with CAC <100
| χ 2 for CAC | χ 2 for CAC+AVC | χ 2 improvement | P-value | NRI (95% CI) | Net cases (%) | Net control (%) | P-value | |
|---|---|---|---|---|---|---|---|---|
| ACM | 64.1 | 93.5 | 29.5 | <0.001 | 6.5% (2.7–10.3) | 8.4 | −1.9 | 0.001 |
| CVD death | 20.2 | 34.0 | 13.8 | <0.001 | 9.9% (1.6–18.2) | 11.9 | −2.0 | 0.027 |
| CHD death | 23.1 | 30.1 | 7.0 | 0.008 | 10.9% (−0.1–22.7) | 12.9 | −2.0 | 0.091 |
AVC, aortic valve calcification; CAC, coronary artery calcification; NRI, net reclassification index.
In the exploratory analysis regarding AVC and aortic stenosis death, increased AVC score (log transformed AVC) was associated with increased likelihood of aortic stenosis deaths (odds ratio: 1.98, 95% CI: 1.11–3.51; P = 0.020) (Supplementary data online, Table S1).
Discussion
In this large cohort with AVC quantification from non-contrast CT scans, the prevalence of AVC was 14% of the study population and 5% had an AVC score ≥100. Subsequent rates of all-cause, CVD, and CHD deaths were significantly elevated among individuals with higher AVC. In the overall population, AVC was not independently predictive of mortality events after adjustment for clinical CVD risk factors and CAC. However, when further stratified by CAC <100 and CAC ≥100, AVC ≥100 was an independent predictor of total mortality and CVD death in patients with CAC <100. Furthermore, AVC assessment improved the discrimination and reclassification for the prediction of all-cause and CVD mortality events.
This study data confirm previous findings of the association between AVC and increased incidence of adverse cardiovascular outcomes. In the landmark Cardiovascular Health Study, AVC identified by echocardiography was associated with a 50% increase in CVD deaths over a 5-year period in individuals >65 years old, after adjustment for age, sex, and clinical risk factors.5 In the population from the CAC Consortium, we found a similar relative risk increase in CVD mortality (HR: 1.46, 95% CI: 1.04–2.04) in those with AVC by cardiac CT, when adjusting for conventional CVD risk factors.
A sub-study of the Losartan Intervention for End-Point Reduction in Hypertension (LIFE) trial reported that aortic sclerosis was associated with a two-fold increase in cardiovascular risk.6 In a study of 8401 asymptomatic subjects, the presence of AVC predicted all-cause mortality independent of traditional risk factors.15 In the Heinz Nixdorf Recall Study (HNRS), among nearly 4000 patients with a mean age of 59 and followed for an average of 9 years, the incidence of major adverse CVD events was two-fold higher in those with AVC >0 vs. AVC=0.9
Although the presence and severity of AVC were associated with all cause, CHD, and CVD deaths, AVC was not an independent predictor of mortality outcomes once regression models accounted for CAC. This finding is consistent with prior observations that prognostic value of AVC was not significant after adjusting for CAC. In data from the HNRS, AVC remained independently associated with CVD and CHD events after adjustment for Framingham risk factors, but not following adjustment for CAC.9 In 6685 patients from the Multi-Ethnic Study of Atherosclerosis (MESA) study, followed for a median of 5.8 years, AVC was no longer predictive of CVD and CHD event risk after adjustment for CAC.10
AVC and CAC are closely related to each other as they share similar pathophysiology in development and progression.1,7,8 Our findings extend prior investigations to explore the potential interaction between AVC and CAC for prognosis. In patients with a high coronary atherosclerotic burden (CAC ≥ 100), CAC remained the single most important predictor and AVC ≥ 100 did not show prognostic significance. However, AVC ≥ 100 was an independent prognostic marker in patients with CAC<100, after adjusting for conventional CVD risk factors and CAC.
A potential explanation of the current finding is that despite the similarities in the clinical risk factors associated with aortic valve sclerosis and coronary atherosclerosis, mechanical stress and subsequent activation of fibroblast are the primary mechanisms of initiation and progression of aortic valve sclerosis.2,16 In addition, calcific changes on the aortic valve occur at earlier stages of atherosclerosis when compared with coronary artery plaques.2,17 Therefore, in patients with a low burden of coronary atherosclerosis, elevated AVC might be an independent prognostic indicator of atherosclerosis associated with an increased risk of adverse CVD outcomes. Further studies are warranted to verify the interaction between AVC and CAC as prognosticators in the asymptomatic population.
Our exploratory analysis showed a relationship between AVC and aortic stenosis death. A very strong relationship between the severity of AVC and the severity of aortic stenosis has previously been documented.18 These findings suggest that including AVC quantification in reporting may be useful for the detection and assessment of aortic stenosis.19 Further study of the relationship between AVC and aortic stenosis in population-based studies with both AVC and echocardiographic data are needed to provide evidence supporting this recommendation.
AVC can be measured by standard software without additional technologist time. Based on the findings of the current and prior studies, we believe that quantitative assessment of AVC should become a part of routine reporting of CAC scans.
This study has several limitations. Given the retrospective and observational nature of the current study, we cannot discount the potential for unmeasured confounding factors, which might have influenced the clinical endpoints of this study. In addition, the cohort was from a single centre of the CAC Consortium, which might affect the generalizability of the results. Information regarding downstream pharmacological and/or interventional management after CAC scan was unavailable. Patients’ management strategies for atherosclerotic disease are likely to have evolved since initial enrolment in 1991 through 2010. Therefore, persons enrolled later into the study might have received different treatment strategies compared to those enrolled earlier, which could affect the prognostic significance of AVC. The findings of exploratory propensity-matched analysis for AVC and aortic stenosis death may be underpowered and requires further assessment.
Conclusion
The incidence of CHD, CVD, and total mortality increased with higher AVC burden; however, the prognostic significance of AVC was attenuated after accounting for CAC. When further stratified by CAC burden, AVC ≥100 was independently associated with CVD and all-cause death after adjustment for conventional CAD risk factors and CAC, in patients with CAC <100. In patients with low coronary atherosclerotic burden, presence and severity of AVC may provide a predictive utility beyond current available CVD risk assessment.
Supplementary data
Supplementary data are available at European Heart Journal - Cardiovascular Imaging online.
Funding
National Institutes of Health Grant L30 HL110027 and Miriam and Sheldon G. Adelson Medical Research Foundation.
Conflict of interest: M.J.B. receives modest grant support from General Electric. All other authors have no conflicts of interest to report.
Supplementary Material
All authors had access to the data and a role in writing the manuscript.
References
- 1. Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE. et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997;29:630–4. [DOI] [PubMed] [Google Scholar]
- 2. Freeman RV, Otto CM.. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation 2005;111:3316–26. [DOI] [PubMed] [Google Scholar]
- 3. Otto CM, Prendergast B.. Aortic-valve stenosis–from patients at risk to severe valve obstruction. N Engl J Med 2014;371:744–56. [DOI] [PubMed] [Google Scholar]
- 4. Budoff MJ, Mao S, Takasu J, Shavelle DM, Zhao XQ, O'Brien KD.. Reproducibility of electron-beam CT measures of aortic valve calcification. Acad Radiol 2002;9:1122–7. [DOI] [PubMed] [Google Scholar]
- 5. Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS.. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med 1999;341:142–7. [DOI] [PubMed] [Google Scholar]
- 6. Olsen MH, Wachtell K, Bella JN, Gerdts E, Palmieri V, Nieminen MS. et al. Aortic valve sclerosis relates to cardiovascular events in patients with hypertension (a LIFE substudy). Am J Cardiol 2005;95:132–6. [DOI] [PubMed] [Google Scholar]
- 7. Messika-Zeitoun D, Bielak LF, Peyser PA, Sheedy PF, Turner ST, Nkomo VT. et al. Aortic valve calcification: determinants and progression in the population. Arterioscler Thromb Vasc Biol 2007;27:642–8. [DOI] [PubMed] [Google Scholar]
- 8. Owens DS, Katz R, Takasu J, Kronmal R, Budoff MJ, O'Brien KD.. Incidence and progression of aortic valve calcium in the Multi-ethnic Study of Atherosclerosis (MESA). Am J Cardiol 2010;105:701–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kalsch H, Lehmann N, Mahabadi AA, Bauer M, Kara K, Huppe P. et al. ; on behalf of the Investigator Group of the Heinz Nixdorf Recall Study. Beyond Framingham risk factors and coronary calcification: does aortic valve calcification improve risk prediction? The Heinz Nixdorf Recall Study. Heart 2014;100:930–7. [DOI] [PubMed] [Google Scholar]
- 10. Owens DS, Budoff MJ, Katz R, Takasu J, Shavelle DM, Carr JJ. et al. Aortic valve calcium independently predicts coronary and cardiovascular events in a primary prevention population. JACC Cardiovasc Imaging 2012;5:619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blaha MJ, Whelton SP, Al Rifai M, Dardari ZA, Shaw LJ, Al-Mallah MH. et al. Rationale and design of the coronary artery calcium consortium: a multicenter cohort study. J Cardiovasc Comput Tomogr 2017;11:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R.. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–32. [DOI] [PubMed] [Google Scholar]
- 13. Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB Sr, Gibbons R. et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pencina MJ, D'Agostino RB Sr, Steyerberg EW.. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Statist Med 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blaha MJ, Budoff MJ, Rivera JJ, Khan AN, Santos RD, Shaw LJ. et al. Relation of aortic valve calcium detected by cardiac computed tomography to all-cause mortality. Am J Cardiol 2010;106:1787–91. [DOI] [PubMed] [Google Scholar]
- 16. Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M. et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation 2003;107:2181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Warren BA, Yong JL.. Calcification of the aortic valve: its progression and grading. Pathology 1997;29:360–8. [DOI] [PubMed] [Google Scholar]
- 18. Pawade T, Clavel MA, Tribouilloy C, Dreyfus J, Mathieu T, Tastet L. et al. Computed tomography aortic valve calcium scoring in patients with aortic stenosis. Circ Cardiovasc Imaging 2018;11:e007146. [DOI] [PubMed] [Google Scholar]
- 19. Hecht HS, Blaha MJ, Kazerooni EA, Cury RC, Budoff M, Leipsic J. et al. CAC-DRS: Coronary Artery Calcium Data and Reporting System. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). J Cardiovasc Comput Tomogr 2018;12:185–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.