Abstract
Background:
There is a long history of segregation in the U.S.A with enduring impacts on cancer outcomes today. We evaluated the impact of segregation on racial disparities in Hepatocellular Carcinoma (HCC) treatment and outcomes.
Methods:
We obtained data on black and white patients with HCC from the SEER program (2005–2015) within the 100 most populous participating counties. Our exposure was the index of dissimilarity (IoD), a validated measure of segregation. Outcomes were overall survival, advanced stage at diagnosis (Stage III/IV) and surgery for localized disease (Stage I/II). Cancer-specific survival was assessed using Kaplan-Meier estimates.
Results:
Black patients had a 1.18 times increased risk (95%CI 1.14,1.22) of presenting at advanced stage as compared to white patients and these disparities disappeared at low levels of segregation. In the highest quartile of IoD, black patients had a significantly lower survival than white (17 months vs 27 months, p<0.001), and this difference disappeared at the lowest quartile of IoD.
Conclusions:
Our data illustrate that structural racism in the form racial segregation has a significant impact on racial disparities in the treatment of HCC. Urban and health policy changes can potentially reduce disparities in HCC outcomes.
Introduction:
Over the last two decades the incidence and number of deaths due to liver-related cancers has continued to rise(1), with disproportionate incidence among black individuals (2). The etiology of hepatocellular carcinoma (HCC) is diverse and can present differently in different populations. Worldwide, hepatitis B (HBV) remains the most common etiologic factor associated with progression to cirrhosis and HCC, but alcohol, hepatitis C (HCV), and nonalcoholic fatty liver disease (NAFLD) remain important predisposing factors as well (3). In the United States HCV is the most common cause of HCC, but with newer treatments for HCV, NAFLD and alcoholic liver disease are likely to contribute much more in the coming years (4). Obesity and alcoholism disparately affect marginalized communities and will likely have long-lasting implications in HCC etiology and treatment (5).
There are well-documented disparities in HCC treatment and outcomes between black and white Americans. Black patients often present at more advanced stage than their white counterparts(6–8) and are less likely to receive curative treatment (7,9–11). To add to this, there is a growing body of literature showing that black Americans are much less likely to undergo transplantation for HCC (6,12–15). Furthermore, there is a well-established overall survival disadvantage for black Americans with HCC (6,7,9–11,14,16). The literature is abundant with proposed root causes of these with differences in underlying tumor biology (7) and disease etiology (6)(14) being the most common overall, while HCV and alcoholism are more prominent for marginalized populations.
Structural racism in the form of residential racial segregation has important implications on the health of black Americans. Studies in lung, breast, and colorectal cancer have shown worse treatment and outcomes for black Americans in more segregated areas(17–20). Racial segregation has roots during the Reconstruction period following the Civil War and countless policies of the 20th Century. Black Americans fled violence of the South to urban areas where they were partitioned into certain areas of cities through explicit and veiled discriminatory practices(21). This was solidified during the New Deal Era of the 1930s with racist government-backed housing policies, Redlining, and white flight(22). These racist practices have led to concentration of black Americans into neighborhoods that, through lack of equity, foster significant intergenerational poverty, healthcare and food deserts, and poor access to proper insurance(23,24). It is structural racism and the downstream deleterious socioeconomic effects therein that drive many health disparities between black and white Americans. In the current study, we sought to understand the impacts of structural racism in the form of residential segregation on HCC diagnosis, surgical treatment and survival.
Methods:
Data Sources:
We obtained case data on individuals from the Surveillance, Epidemiology, and End Results (SEER) Program between the years of 2005 and 2015 (25). Cases were selected based on ICDO-3 coding C22.0 and were further limited to only black and white individuals living in the 100 most populous participating counties. We further restricted only to those with HCC as their incident cancer in order to exclude recurrence or those with previous cancers. Sociodemographic data were obtained from the 2010 Decennial Census and the 2013 5-year estimates of the American Community Survey (ACS).
Exposure and Outcomes of Interest:
Our primary exposure of interest was the index of dissimilarity (IoD), a validated measure of segregation(26). Values of IoD range from 0 (no segregation) to 1 (complete integration) and represent the proportion of people within the studied area that would need to move to achieve complete integration. Values were calculated at the county level using race demographic data of Census blocks within each county using the R seg package(27). Primary outcomes of interest included advanced stage at diagnosis (Stage III, IV), surgery for localized disease (Stage I, II), and cancer-specific mortality. Secondarily, we studied the effects of segregation on receipt of ablation, resection, and transplant. Stages were defined based on the American Joint Commission on Cancer (AJCC) Staging Manual, 7th edition (28).
Statistical Analysis
We performed a multivariable modified Poisson regression with robust error variance to assess the impact of IoD on the prevalence ratios of advanced stage at diagnosis and surgical resection for localized disease. We examined the disparity between black and white patients for these outcomes in a stepwise fashion. First, we assessed the crude association of black race on the outcome of interest, then adjusted for sex, age at diagnosis, and IoD in progressive models. The predictive values for the final adjusted model were then plotted to assess disparities between black and white patients across all levels of IoD. To better understand the role of segregation on different types of operations, we performed sensitivity analyses for ablation, resection, and transplant for localized disease. Sub-analyses were performed for ablation and resection for Stage III alone in order to understand the association of race on these surgical outcomes when transplant is not often indicated. Stratified analyses by race were also performed to assess the association of IoD on the outcomes of interest in black and white patients alone. Cancer-specific and overall 5-year survival were assessed by stage using the Kaplan-Meier method. We further used competing risk regression models, controlling for age at diagnosis, sex, and stage, of cancer-specific survival against other causes of death. Unknown values for the outcomes of interest were excluded from analyses.
Given prior literature demonstrating sociodemographic factors including insurance status and poverty(29) as important mediators in the causal pathway between segregation and the outcomes of interest, we did not control for these factors in our models. Similarly, there are regional differences in IoD given historical migration of black Americans during the reconstruction era(21). As such, we did not control for region in our models.
Stata 16 was used for all statistical analyses.(30)
Results:
Baseline Characteristics
In total 37,853 individuals were analyzed, of which 18% were identified as Black (Table 1). There were similar proportions of females in each group (22% in both the Black and White cohorts, p=0.70). Black patients were younger at the time of diagnosis than white patients (60.7 years vs 63.2, p<0.001), and were more heavily concentrated in the South and Midwest (30.7% and 15.6% vs 8.6% and 5.8% for black and white respectively). Overall, black patients were more likely than white to present with Stage IV disease (16.4% vs 13.7%, p<0.001) and less likely to receive any surgical intervention (19.8% vs 22.9%, p<0.001) overall. Black patients tended to live in more segregated areas as compared to white (IoD 0.67 vs 0.60, p<0.001)
Table 1:
Baseline characteristics of black and white patients from the SEER (Surveillance, Epidemiology, and End Results) database with hepatocellular cancer (2005–2015) as identified from the 100 most populous counties from the United States Census (2010)
| All (N= 37,853) |
Black (N=6,740) |
White (N=31,113) |
P-Value | |
|---|---|---|---|---|
| Individual Characteristics | ||||
| Age at diagnosis, mean ± SD | 62.7 ±12.0 | 60.7 ±9.6 | 63.2 ± 12.4 | <0.001 |
| Female, N (%) | 8,285 (21.9) | 1,487 (22.1) | 6,798 (21.9) | 0.70 |
| Region, N (%) | <0.001 | |||
| Northeast | 6,169 (16.3) | 1,069 (15.9) | 5,100 (16.4) | |
| South | 4,751 (12.6) | 2,069 (30.7) | 2,682 (8.6) | |
| Midwest | 2,864 (7.6) | 1,051 (15.6) | 1,813 (5.8) | |
| West | 24,069 (63.6) | 2,551 (37.9) | 21,518 (69.2) | |
| Stage at Diagnosis, N (%) | <0.001 | |||
| Stage I | 11,932 (31.5) | 1,973 (29.3) | 9,959 (32.0) | |
| Stage II | 6,430 (17.0) | 1,026 (15.2) | 5,404 (17.4) | |
| Stage III | 7,360 (19.4) | 1,537 (22.8) | 5,823 (18.7) | |
| Stage IV | 5,379 (14.2) | 1,106 (16.4) | 4,273 (13.7) | |
| Unknown | 6,752 (17.8) | 1098 (16.3) | 5,654 (18.2) | |
| Surgery, N (%) | 8,458 (22.3) | 1,331 (19.8) | 7,127 (22.9) | <0.001 |
| Index of Dissimilarity, mean ± SD | 0.61 ± 0.08 | 0.67 ± 0.10 | 0.60 ± 0.07 | <0.001 |
Advanced Stage at Diagnosis
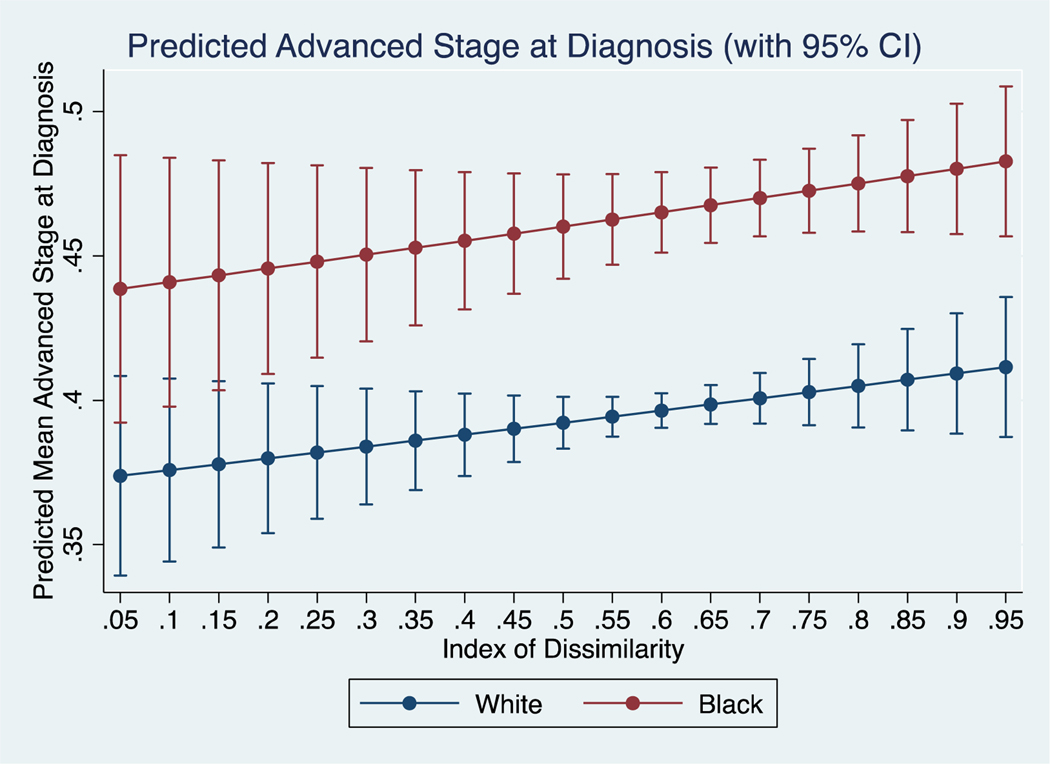
In the unadjusted model (Supplemental Table 1A), black patients had an 18% increased risk (RR 1.18, 95% CI 1.14, 1.22) of presenting at advanced stage as compared to white. After adjusting for age at diagnosis, sex, and IoD this risk persisted (RR 1.18, 95% CI 1.14, 1.22). In plotting predicted values from the final adjusted model (Figure 1), there were no observed differences in presentation at advanced stage between black and white patients at low levels of IoD. In stratified models, neither black (RR 1.26, 95% CI 0.96, 1.64) or white (RR 1.05, 95% CI 0.85, 1.29) patients saw a statistically significant change in risk with increasing segregation.
Figure 1:
Predicted mean proportion of black and white patients presenting at advanced stage across different levels of IoD.
Surgery for Localized Disease
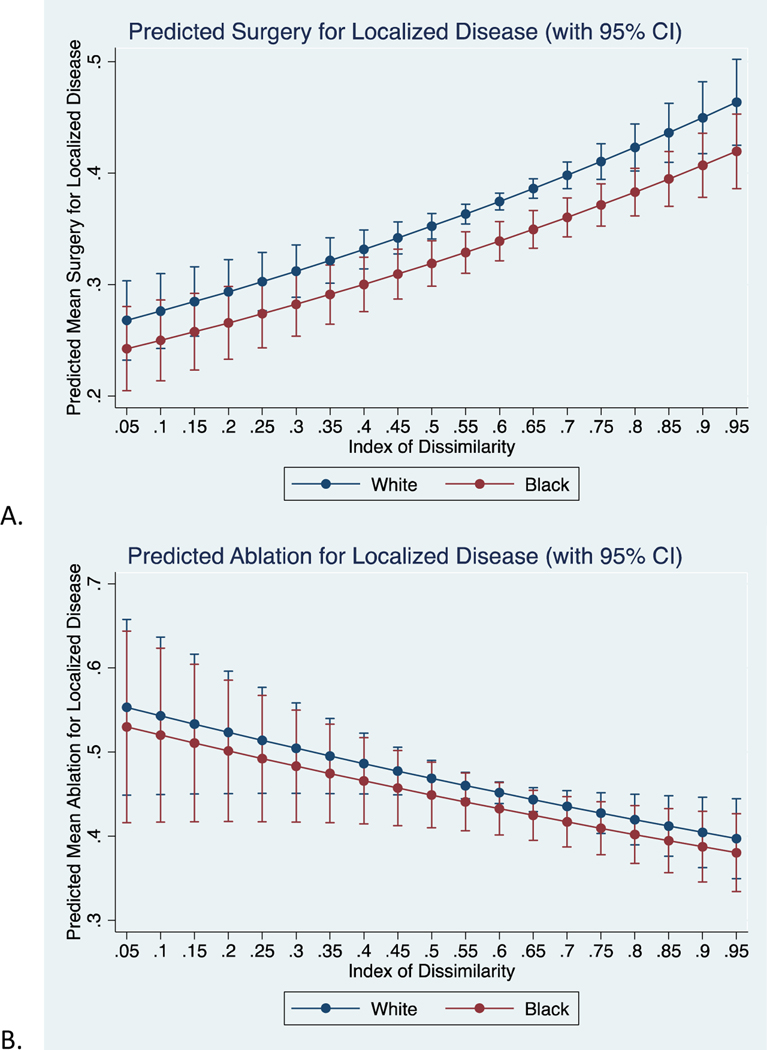
Overall, in unadjusted models, (Supplemental Table 1B) black patients were less likely than white to undergo surgical resection for localized disease (RR 0.94, 95% CI 0.89, 0.99). In the adjusted model, black patients were even less likely to undergo surgical resection (RR 0.89, 95% CI 0.84, 0.94). In plotting the predicted values of the final model (Figure 2A), there were no significant differences at both low and high levels of segregation. Stratified analyses showed no significant difference in likelihood of surgical resection for black patients (RR 0.62, 95% CI 0.39, 1.00); however, white patients saw a significantly increased likelihood (RR 2.65, 95% CI 2.03, 3.46) of surgical resection with increasing IoD.
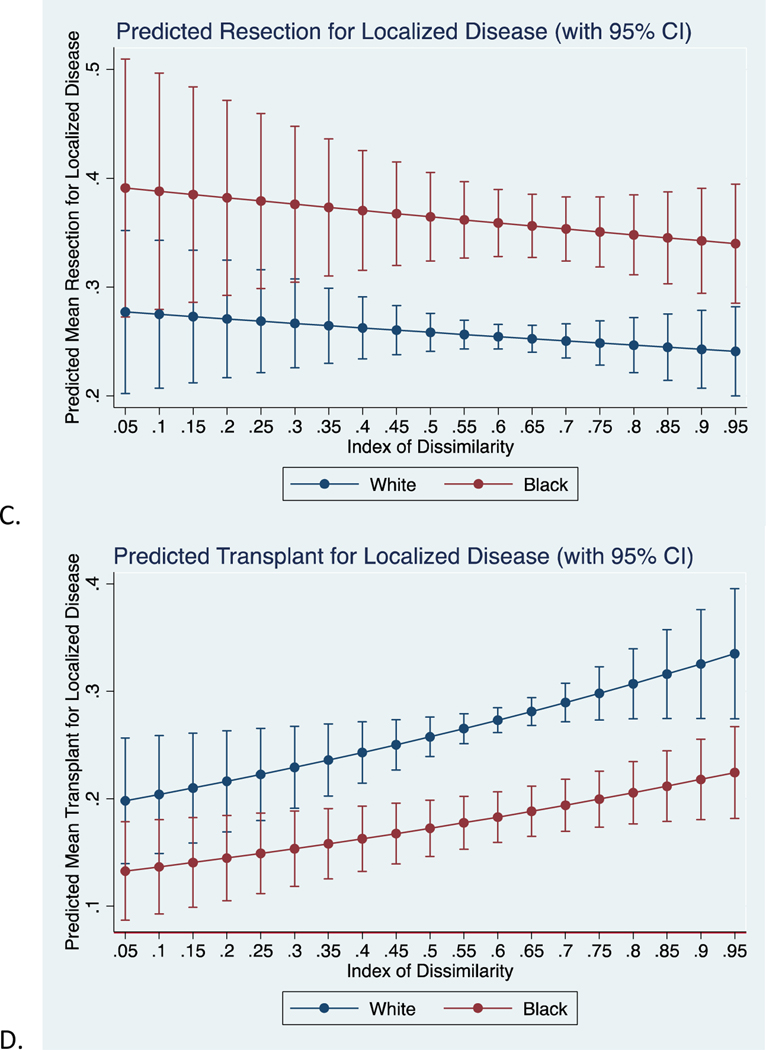
Figure 2:
Predicted mean proportion of black and white patients undergoing A) any surgery, B) ablation, C) resection, or D) transplant for localized disease (Stage I/II) across varying levels of IoD.
Supplemental Table 2 presents analyses for ablation, resection, and transplant of localized disease (Stage I/II). There was no significant difference in risk of undergoing ablation between black and white patients (RR 0.98, 95% CI 0.91, 1.06) after adjusting for sex, age at diagnosis, and IoD. On stratified analyses, segregation did not play a role in the likelihood of ablation for black patients (RR 0.99, 95% CI 0.50, 1.96), yet white patients were 38% less likely (RR 0.62, 95% CI 0.42, 0.92) to undergo ablation at higher levels of segregation. After adjusting for previously listed covariates, black patients were 31% less likely to undergo transplant (RR 0.69, 95% CI 0.61, 0.79) and consequently, 42% more likely (RR 1.42, 95% CI 1.29, 1.57) to undergo resection when compared to white patients. For both resection and transplant, the disparities disappear at low levels of segregation (Figure 2C and 2D). On stratified analysis, neither black (RR 0.85, 95% CI 0.38, 1.93) or white (RR 0.88, 95% CI 0.49, 1.59) patients saw a difference in likelihood of resection with increasing segregation. Similarly, black patients saw no change in likelihood of transplant with increasing segregation (RR 1.03, 95% CI 0.28, 3.72), but white patients were much more likely (RR 2.00, 95% CI 1.14, 3.53) to undergo transplant per unit increase in segregation.
Given that transplant is uncommon for Stage III disease, we assessed only the likelihood of ablation and resection in Stage III disease (Supplemental Table 2). In the combined models, we did not observe a statistically significant difference between black and white patients in the unadjusted or adjusted models. Similarly, there were no differences in likelihood of ablation or resection within black or white strata with increasing segregation for Stage III disease.
Cancer-Specific Survival
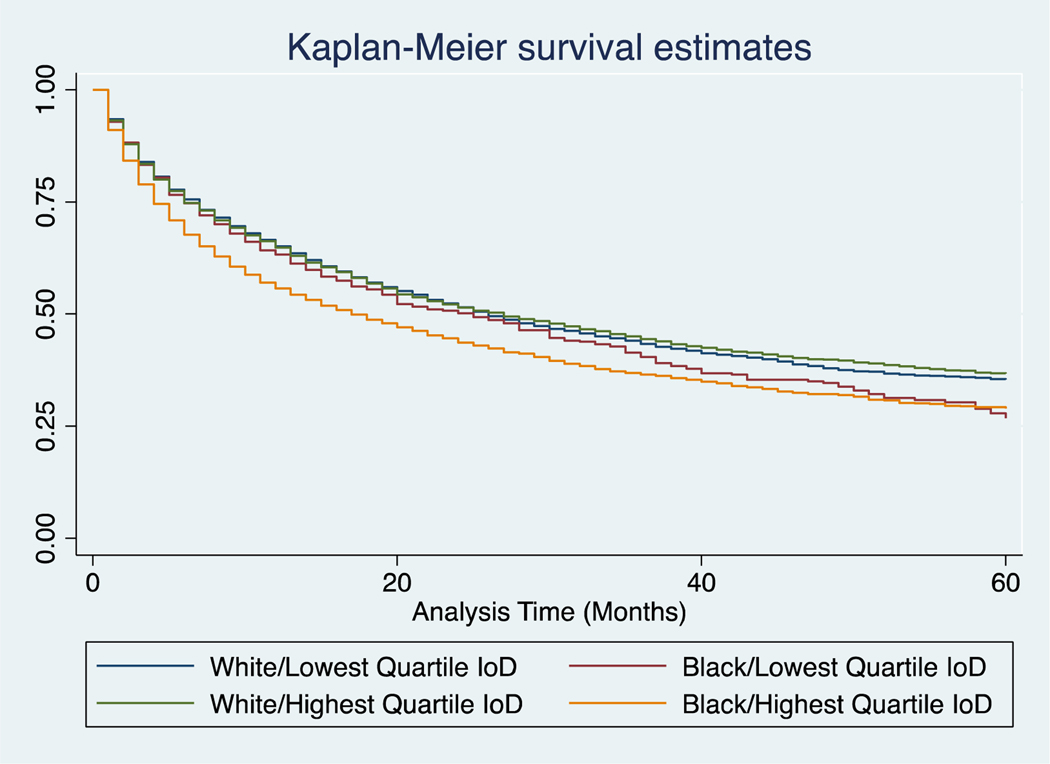
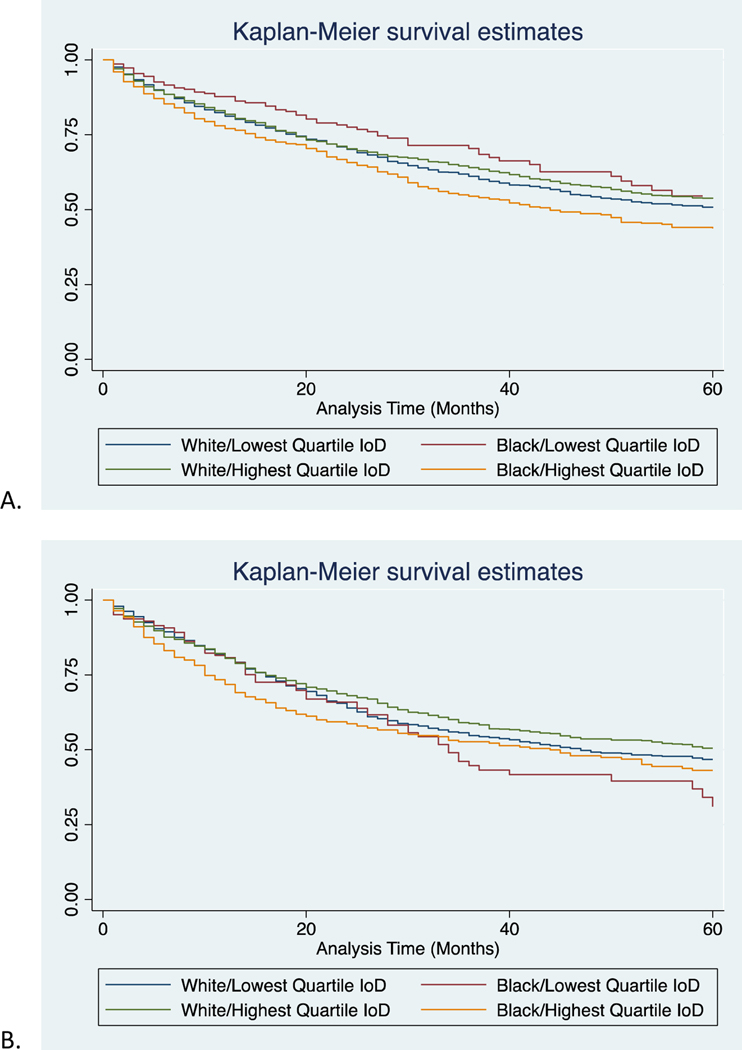
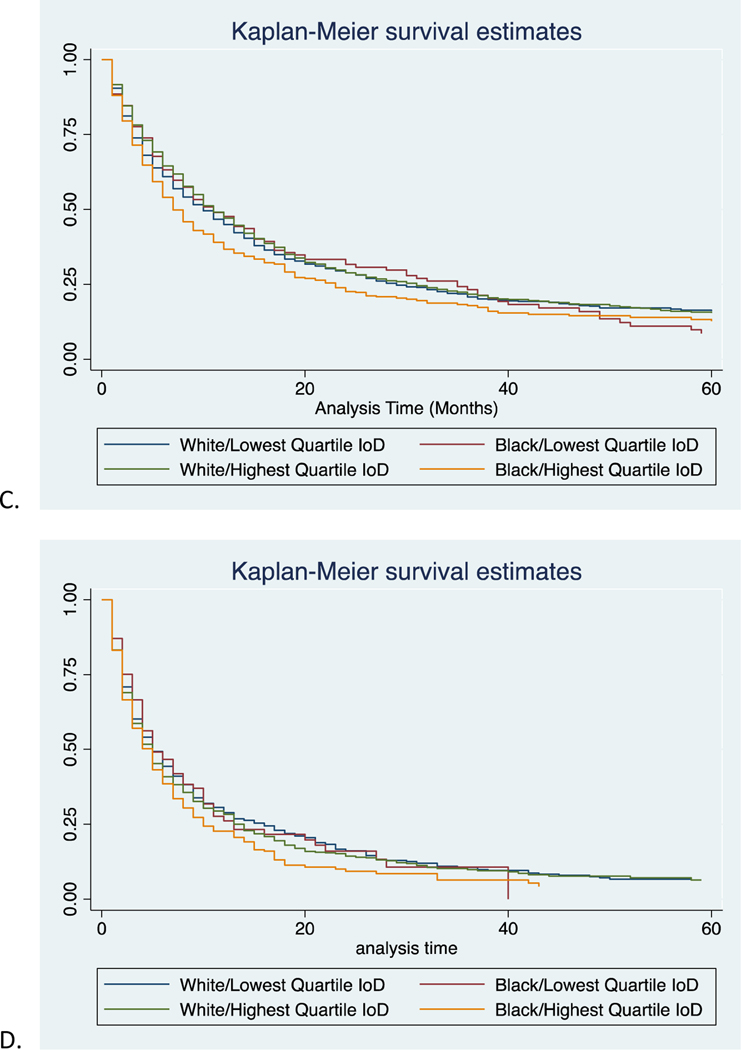
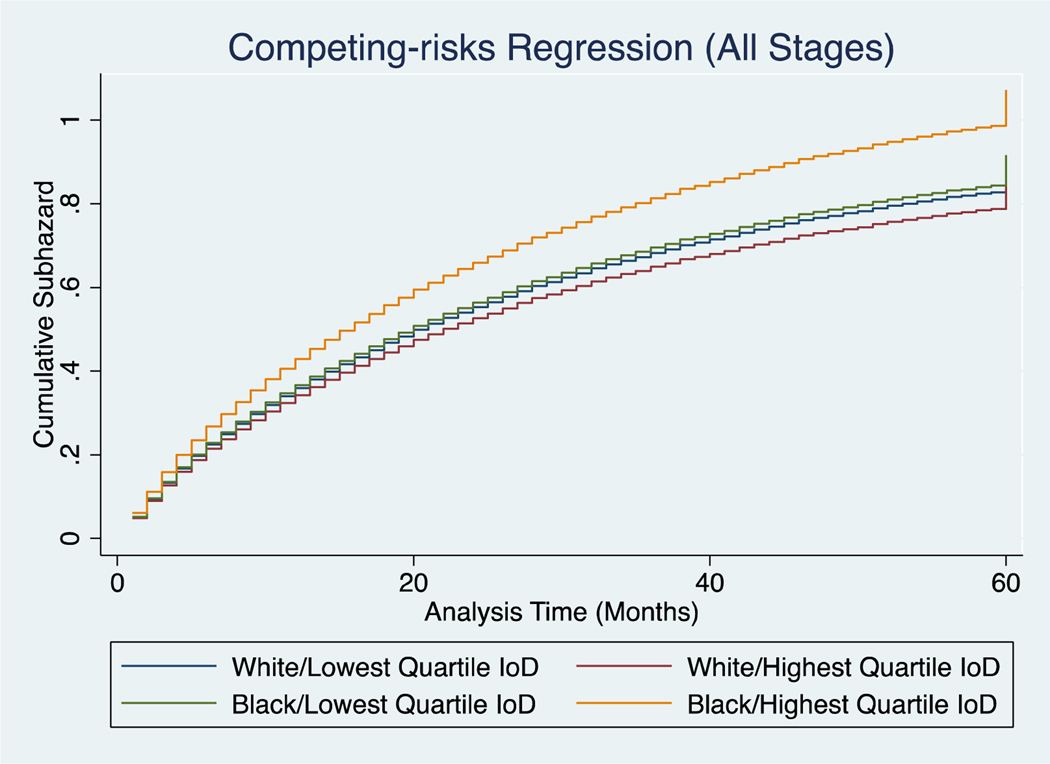
For all stage cancer-specific survival in the highest quartile of IoD, black patients had significantly lower survival than white (17 months vs 27 months, p<0.001, Supplemental Table 3). However, in the lowest quartile of IoD, black and white patients had more similar mortality rates (25 and 26 months respectively, p=0.06). Survival curves for all stages are provided in Figure 3. These findings were also seen when evaluating stages I-III (Table IV, Figure 4). For these stages, black patients in the highest quartile of IoD had significantly lower survival than their white counterparts, and even more telling, they have lower survival than their black counterparts in the lowest quartile of IoD. In fact, black patients in the lowest quartile of IoD have similar mortality rates to whites (601 vs 624 deaths per 1000 person-years for stage III, p=0.92). Not surprisingly, there was no significant difference in survival for black or white patients in the highest or lowest quartile of IoD for stage IV disease. In assessing competing risks cumulative hazards for all stage mortality, we see similar trends (Figure 5). Black patients in the highest quartile of IoD had significant higher hazards of death compared to black patients in the lowest quartile of IoD (HR 1.17, 95% CI 1.04, 1.31). These black patients in the lowest quartile of IoD had no significantly different hazards of death when compared to white patients in the lowest (HR 1.02, 95% CI 0.91, 1.14) or highest (HR 1.07, 95% CI 0.96, 1.19) quartile of IoD. Similar trends were found when evaluating overall survival (Supplemental Table 4).
Figure 3:
Kaplan-Meier cancer-specific survival estimates of black and white patients in the lowest and highest quartiles of IoD for all stages at presentation.
Figure 4:
Kaplan-Meier cancer-specific survival estimates of black and white patients in the lowest and highest quartiles of IoD for A) Stage I, B) Stage II, C) Stage III, and D) Stage IV disease.
Figure 5:
Competing risks regression of cancer-specific mortality against other-cause mortality in black and white patients in the highest and lowest levels of segregation.
Discussion:
Our findings highlight the impact of structural racism in the form of residential segregation on treatment and outcomes of patients with HCC. We find that black patients are more likely than white to present at advanced stage and have worse survival. While this is in line with other studies on race and survival for HCC, what is most striking in our data is that these differences vanish at low levels of segregation. Similarly, we find that black patients are less likely than white patients to undergo transplant for localized disease – which would be curative of their malignancy and their underlying cirrhosis – and instead are more likely to undergo resection, which does not address their underlying disease. These disparities also disappear at low levels of segregation.
Our results support previous studies in localities such as Florida and Texas, as well as those using SEER, that show more advanced stage at presentation for black patients when compared to white. What we think is remarkable is that stage for stage, in areas where there is the least racial segregation, there is no survival difference between black and white patients. These findings build on previous literature showing worse mortality for black patients overall with the observed differences being partly attributable to differences in treatment (6,10,14). Studies in other cancers have shown lower access to care for black individuals due to geographic isolation and lack of sufficient transportation(31), which may be compounded with worse insurance coverage due to significant poverty associated with segregation(32). Taken together this could lead to late presentation of symptoms or lower rates of screening for high-risk patients. Given that differences occur in stages that are amenable to surgical intervention, it is possible that the survival benefits of living in less segregated areas may be attributable to differences in surgical intervention.
We found that black patients were less likely to undergo liver transplant compared to white patients for Stage I/II disease. This is borne out in the literature with multiple studies showing socioeconomic status seems to play an outsized role as insurance status is highly correlative with transplant for HCC(12,13,15). In their study, Sarpel et al found that those with government insurance were less likely to undergo transplant overall, but even when controlling for these factors, blacks continued to be less likely to undergo transplant(13). Franco et al proposed geography as an important driver of these disparities in their comparison of HCC treatment in the North and South(2). Danos, et al studied concentrated disadvantage as a drive of HCC and found that when controlling for race, concentrated disadvantage was associated with increased incidence of HCC(33). Interestingly, we find that black patients seem to be proportionally more likely to undergo resection than transplant, as compared to whites, suggesting that black patients may be more likely receiving resection over more curative transplant.
As the level of segregation increases, white patients are more and more likely to undergo liver transplant, as well as surgery overall, while less likely to undergo the sometimes less curative intervention of ablation. This suggests that segregation may not only be disadvantageous to black patients but may offer advantageous to white patients - via improved access to healthcare, better referral patterns, or other means. While not directly addressed in the medical literature, there is evidence that given a concentrated disadvantage to black Americans in more segregated areas, there may be a concentrated advantage to white Americans in those same areas(34).
Taken together with the previous literature, our results highlight the enduring impact of history and policy on the health of black Americans today. Fleeing white on black violence in the South and searching for steady work, black families largely ended up in urban areas during the Reconstruction era(21). They were often partitioned to certain areas of cities, separated from white Americans through discriminatory practices. This segregation was solidified in the 1930s during the New Deal era with the formation of the Home Owner’s Loan Corporation (HOLC), which was charged with providing government backed loans to Americans to rescue the housing market in the wake of the recession. However, these loans were not offered to black Americans or areas that had a significant black population (now colloquially known as redlined areas)(22,35). Residential segregation has been shown to have important implications on the built environment with increased poverty, lower rates of insurance and greater concentrated disadvantage in these redlined areas (23,24).
We would suggest that this history of government-complicit segregation led to the conditions offered as causes for black / white disparities by so many in the literature. We believe that this structural racism and the resultant intergenerational socioeconomic deprivation, in a sense precludes many black patients from undergoing liver transplant. Of utmost importance, though, is that our data show that these disparities evaporate at low levels of segregation. Policies aimed at reinvestment in black communities in conjunction with improved infrastructure in these neighborhoods will be important to resolve black/white disparities in hepatocellular carcinoma. Hospitals, particularly Accountable Care Organizations, can serve as models for larger policy change by investing in the marginalized communities that they serve through hiring practices and targeted investments in the black community, such as changing purchasing practices to focus on black-owned businesses. These actions can serve as evidence for larger policy change focused on reparations, not only for slavery, but for the perpetuated governmentdriven segregation and disinvestment of black communities.
There are important limitations to our study. Our analyses rely on retrospective information, precluding causative analyses. Additionally, we use Census data that is static in nature, which does not adequately address the changing nature of neighborhoods over time. We are limited to analysis at the county level, which does not allow for more granular analysis of segregation and redlining within cities, thus we are likely underestimating the true effect. Similarly, we are unable to assess if patients are seeking care within their own county of residence, but we would expect this to be the case given that we limit only to urban counties. We are limited only to the county level segregation measure but are unable to assess biases implicit to providers who may be providing care. Additionally, we are unable to assess adjuvant therapy, which may also be impacted by segregation.
Conclusions
Overall, we show that at low levels of segregation there are no observed black-white disparities in stage at diagnosis, survival, or surgical interventions, with clear disadvantages to black patients and advantages for white patients with increasing segregation. These are stark findings that highlight the continued effects of structural racism on the health of patients today, likely through intergenerational poverty, insurance status, and access to appropriate healthcare. Solutions to these disparities will inevitably come from changes to urban and health policy focused on historical structural inequities based in racist governmental policies.
Supplementary Material
Acknowledgments
Funding: Michael Poulson is supported by a T32 training grant (HP10028). Alaina Geary is supported by a T32 training grant (GM86308). Teviah Sachs is the Perlman Scholar for Pancreatic Cancer Research.
Footnotes
Disclosure: The authors have no financial nor personal disclosures or conflicts of interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, based on November 2018 submission data (1999–2016). U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. 2019. [Google Scholar]
- 2.Franco RA, Fan Y, Jarosek S, Bae S, Galbraith J. Racial and Geographic Disparities in Hepatocellular Carcinoma Outcomes. Am J Prev Med. 2018;55:S40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mittal S, El-Serag HB. Epidemiology of HCC: Consider the Population. J Clin Gastroenterol. 2013;47(0):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Setiawan VW, Lim U, Lipworth L, Lu SC, Shepherd J, Ernst T, et al. Sex and Ethnic Differences in the Association of Obesity With Risk of Hepatocellular Carcinoma. Clin Gastroenterol Hepatol. 2016;14(2):309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones PD, Scheinberg AR, Muenyi V, Gonzalez-Diaz J, Martin PM, Kobetz E. Socioeconomic And Survival Differences Among Minorities With Hepatocellular Carcinoma In Florida. J Hepatocell Carcinoma. 2019;Volume 6:167–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rich NE, Hester C, Odewole M, Murphy CC, Parikh ND, Marrero JA, et al. Racial and Ethnic Differences in Presentation and Outcomes of Hepatocellular Carcinoma. Clin Gastroenterol Hepatol. 2019;17(3):551–559.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu L, Kim Y, Spolverato G, Gani F, Pawlik TM. Racial disparities in treatment and survival of patients with hepatocellular carcinoma in the United States. Hepatobiliary Surg Nutr. 2016;5(1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franco EL, Shinder GA, Tota JE, Isidean SD. An elusive low-hanging fruit for public health: GUN violence prevention. Prev Med (Baltim) [Internet]. 2015;79:1–2. Available from: 10.1016/j.ypmed.2015.06.008 [DOI] [PubMed] [Google Scholar]
- 10.Mathur AK, Osborne NH, Lynch RJ, Ghaferi AA, Dimick JB, Sonnenday CJ. Racial/ethnic disparities in access to care and survival for patients with early-stage hepatocellular carcinoma. Arch Surg. 2010;145(12):1158–63. [DOI] [PubMed] [Google Scholar]
- 11.Wong RJ, Corley DA. Survival differences by race/ethnicity and treatment for localized hepatocellular carcinoma within the united states. Dig Dis Sci. 2009;54(9):2031–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dakhoul L, Gawrieh S, Jones KR, Ghabril M, McShane C, Orman E, et al. Racial Disparities in Liver Transplantation for Hepatocellular Carcinoma Are Not Explained by Differences in Comorbidities, Liver Disease Severity, or Tumor Burden. Hepatol Commun. 2019;3(1):52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarpel U, Suprun M, Sofianou A, Berger Y, Tedjasukmana A, Sekendiz Z, et al. Disentangling the effects of race and socioeconomic factors on liver transplantation rates for hepatocellular carcinoma. Clin Transplant. 2016;30(6):714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Artinyan A, Mailey B, Sanchez-Luege N, Khalili J, Sun CL, Bhatia S, et al. Race, ethnicity, and socioeconomic status influence the survival of patients with hepatocellular carcinoma in the United States. Cancer. 2010;116(5):1367–77. [DOI] [PubMed] [Google Scholar]
- 15.Yu JC, Neugut AI, Wang S, Jacobson JS, Ferrante L, Khungar V, et al. Racial and insurance disparities in the receipt of transplant among patients with hepatocellular carcinoma. Cancer. 2010;116(7):1801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart SL, Kwong SL, Bowlus CL, Nguyen TT, Maxwell AE, Bastani R, et al. Racial/ethnic disparities in hepatocellular carcinoma treatment and survival in California, 1988–2012. World J Gastroenterol. 2016;22(38):8584–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas JS, Earle CC, Orav JE, Brawarsky P, Neville BA, Williams DR. Racial segregation and disparities in cancer stage for seniors. J Gen Intern Med. 2008;23(5):699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayanga AJ, Zeliadt SB, Backhus LM. Lung cancer mortality and residential segregation in the United States. JAMA Surg. 2013;148(1):37–42. [DOI] [PubMed] [Google Scholar]
- 19.Johnson AM, Johnson A, Hines RB, Bayakly R. The effects of residential segregation and neighborhood characteristics on surgery and survival in patients with early-stage non?small cell lung cancer. Cancer Epidemiol Biomarkers Prev. 2016;25(5):750–8. [DOI] [PubMed] [Google Scholar]
- 20.Dai D Black residential segregation, disparities in spatial access to health care facilities, and late-stage breast cancer diagnosis in metropolitan Detroit. Heal Place [Internet]. 2010;16(5):1038–52. Available from: 10.1016/j.healthplace.2010.06.012 [DOI] [PubMed] [Google Scholar]
- 21.Berlin I The Making of African America: The Four Great Migrations. Vol. 9. New York, NY: The Penguin Group; 2010. [Google Scholar]
- 22.Rothstein R The Color of Law: A Forgotten History of How our Government Segregated America. First Edit. New York, London: Liveright Publishing Corporation, a division of W.W. Norton & Company; 2017. [Google Scholar]
- 23.Aaronson D, Hartley D, Mazumder B. The Effects of the 1930s HOLC “Redlining” Maps. Fed Reserv Bank Chicago. 2019;Working pa(February).
- 24.Mitchell B, Franco J. HOLC “Redlining” Maps: The Persistent Structure of Segregation and Economic Inequality. Natl Community Reinvestment Coalit [Internet]. 2018; Available from: ncrc.umich.edu/about-ncrc
- 25.National Cancer Institute, DCCPS SRP. Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Research Data; (1975–2016). [Google Scholar]
- 26.Bureau USC. Appendix B. Measures of residential segregation. Racial Ethn Resid Segreg United States 1980–2000. 2000;(1988):119–23.
- 27.Hong S-Y, O’Sullivan D. Seg Package. R Foundation; 2019. [Google Scholar]
- 28.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Andy Trotti I. AJCC Cancer Staging Manual. American Joint Committee On Cancer. 2017.
- 29.Ko NY, Hong S, Winn RA, Calip GS. Association of Insurance Status and Racial Disparities with the Detection of Early-Stage Breast Cancer. JAMA Oncol. 2020;6(3):385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; [Google Scholar]
- 31.Altaweel M How Redlining Communities Affects Health [Internet]. Geography Realm. [cited 2020 Dec 7]. Available from: https://www.geographyrealm.com/how-redliningcommunities-affects-health/
- 32.Singal A, Li X, Tiro J, Kandunoori P, Adams-Huet B, Nehra M, et al. Racial, Social, and Clinical Determinants of Hepatocellular Carcinoma Surveillance Amit. Am J Med [Internet]. 2015;128(1):1–7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3624763/pdf/nihms412728.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danos D, Leonardi C, Gilliland A, Shankar S, Srivastava RK, Simonsen N, et al. Increased risk of hepatocellular carcinoma associated with neighborhood concentrated disadvantage. Front Oncol. 2018;8(SEP):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beaulieu M, Continelli T. Benefits of Segregation for White Communities: A Review of the Literature and Directions for Future Research. J African Am Stud. 2011;15(4):487–507. [Google Scholar]
- 35.Coates T-N. The Case for Reparations. The Atlantic. 2014. June;
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.