Abstract
Background
Substance use disorders (SUDs) are highly prevalent and associated with a substantial public health burden. Although evidence‐based interventions exist for treating SUDs, many individuals remain symptomatic despite treatment, and relapse is common.Mindfulness‐based interventions (MBIs) have been examined for the treatment of SUDs, but available evidence is mixed.
Objectives
To determine the effects of MBIs for SUDs in terms of substance use outcomes, craving and adverse events compared to standard care, further psychotherapeutic, psychosocial or pharmacological interventions, or instructions, waiting list and no treatment.
Search methods
We searched the following databases up to April 2021: Cochrane Drugs and Alcohol Specialised Register, CENTRAL, PubMed, Embase, Web of Science, CINAHL and PsycINFO. We searched two trial registries and checked the reference lists of included studies for relevant randomized controlled trials (RCTs).
Selection criteria
RCTs testing a MBI versus no treatment or another treatment in individuals with SUDs. SUDs included alcohol and/or drug use disorders but excluded tobacco use disorders. MBIs were defined as interventions including training in mindfulness meditation with repeated meditation practice. Studies in which SUDs were formally diagnosed as well as those merely demonstrating elevated SUD risk were eligible.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
Forty RCTs met our inclusion criteria, with 35 RCTs involving 2825 participants eligible for meta‐analysis. All studies were at high risk of performance bias and most were at high risk of detection bias.
Mindfulness‐based interventions (MBIs) versus no treatment
Twenty‐four RCTs included a comparison between MBI and no treatment. The evidence was uncertain about the effects of MBIs relative to no treatment on all primary outcomes: continuous abstinence rate (post: risk ratio (RR) = 0.96, 95% CI 0.44 to 2.14, 1 RCT, 112 participants; follow‐up: RR = 1.04, 95% CI 0.54 to 2.01, 1 RCT, 112 participants); percentage of days with substance use (post‐treatment: standardized mean difference (SMD) = 0.05, 95% CI ‐0.37 to 0.47, 4 RCTs, 248 participants; follow‐up: SMD = 0.21, 95% CI ‐0.12 to 0.54, 3 RCTs, 167 participants); and consumed amount (post‐treatment: SMD = 0.10, 95% CI ‐0.31 to 0.52, 3 RCTs, 221 participants; follow‐up: SMD = 0.33, 95% CI 0.00 to 0.66, 2 RCTs, 142 participants). Evidence was uncertain for craving intensity and serious adverse events. Analysis of treatment acceptability indicated MBIs result in little to no increase in study attrition relative to no treatment (RR = 1.04, 95% CI 0.77 to 1.40, 21 RCTs, 1087 participants). Certainty of evidence for all other outcomes was very low due to imprecision, risk of bias, and/or inconsistency. Data were unavailable to evaluate adverse events.
Mindfulness‐based interventions (MBIs) versus other treatments (standard of care, cognitive behavioral therapy, psychoeducation, support group, physical exercise, medication)
Nineteen RCTs included a comparison between MBI and another treatment. The evidence was very uncertain about the effects of MBIs relative to other treatments on continuous abstinence rate at post‐treatment (RR = 0.80, 95% CI 0.45 to 1.44, 1 RCT, 286 participants) and follow‐up (RR = 0.57, 95% CI 0.28 to 1.16, 1 RCT, 286 participants), and on consumed amount at post‐treatment (SMD = ‐0.42, 95% CI ‐1.23 to 0.39, 1 RCT, 25 participants) due to imprecision and risk of bias. The evidence suggests that MBIs reduce percentage of days with substance use slightly relative to other treatments at post‐treatment (SMD = ‐0.21, 95% CI ‐0.45 to 0.03, 5 RCTs, 523 participants) and follow‐up (SMD = ‐0.39, 95% CI ‐0.96 to 0.17, 3 RCTs, 409 participants). The evidence was very uncertain about the effects of MBIs relative to other treatments on craving intensity due to imprecision and inconsistency. Analysis of treatment acceptability indicated MBIs result in little to no increase in attrition relative to other treatments (RR = 1.06, 95% CI 0.89 to 1.26, 14 RCTs, 1531 participants). Data were unavailable to evaluate adverse events.
Authors' conclusions
In comparison with no treatment, the evidence is uncertain regarding the impact of MBIs on SUD‐related outcomes. MBIs result in little to no higher attrition than no treatment. In comparison with other treatments, MBIs may slightly reduce days with substance use at post‐treatment and follow‐up (4 to 10 months). The evidence is uncertain regarding the impact of MBIs relative to other treatments on abstinence, consumed substance amount, or craving. MBIs result in little to no higher attrition than other treatments. Few studies reported adverse events.
Plain language summary
Mindfulness‐based interventions for substance use disorders
What is the aim of this review?
The aim of this Cochrane Review was to determine whether mindfulness‐based interventions (MBIs) i.e. interventions involving training in mindfulness meditation improve symptoms of substance use disorders (SUDs) (i.e. alcohol and/or drug use, but excluding tobacco use disorders). Cochrane researchers searched, selected and analyzed all relevant studies to answer this question. We found 40 randomized controlled trials,that assessed MBI as a treatment for SUDs.
Key messages
SUD outcomes were monitored at different time points: directly following completion of the MBIs, and at follow‐up time points, which ranged from 3 months to 10 months after the MBI ended. Relative to other interventions (standard of care, cognitive behavioral therapy (CBT), psychoeducation, support group, physical exercise, medication), MBIs may slightly reduce days with substance use, but it is very uncertain whether they reduce other SUD‐related outcomes. The effects of MBIs relative to no treatment was very uncertain across all SUD‐assessed outcomes, as was the risk for adverse events.
What was studied in this review?
SUDs are very common and associated with negative physical and psychological health outcomes. Although evidence‐based interventions exist for treating SUDs, the standard treatments may not be sufficient and many individuals relapse to substance use. In the past several decades, MBIs have been examined for the treatment of SUDs. MBIs involve training in mindfulness meditation practice, which emphasizes the cultivation of present‐moment, non‐judgmental awareness. MBIs may improve many of the psychological variables involved in substance use and relapse (i.e. depression, anxiety, stress, attention). We studied whether MBIs benefit individuals with SUDs.
We searched for studies that compared an MBI to no treatment or to another treatment (e.g. cognitive behavior therapy, psychoeducation). We studied the results at the end of the intervention and at follow‐up assessments, which occurred 3 to 10 months following the end of the intervention.
What are the main results of this review?
The review authors found 40 relevant studies, of which 45% were focused on individuals with various SUDs with the remaining studies including participants using a specific substance (e.g. alcohol, opioids). Of these 40 studies, 23 were conducted in the USA, 11 were conducted in Iran, two were conducted in Thailand, one was conducted in Brazil, one was conducted in China, one was conducted in Taiwan, and one was conducted in both Spain and the USA. We were able to analyze results of 35 studies composed of 2825 participants; the other five did not report usable results, and requests to the authors for more information were unsuccessful.
When MBIs were compared with other treatments, our review and analysis showed that MBIs may slightly reduce days with substance use at post‐treatment and follow‐up, and show similar study retention. The evidence is uncertain for other SUD‐related outcomes we assessed (continuous abstinence, consumed amount, craving intensity). When MBIs were compared with no treatment, the evidence was uncertain for all SUD‐related outcomes, although MBIs showed similar treatment retention. Adverse effects were only reported on in four studies. However, the available evidence did not suggest MBIs result in adverse events or serious adverse events.
How up‐to‐date is this review?
The review authors searched for studies published up to April 2021.
Study funding sources
Sixteen studies reported no funding. The remaining studies reported one or more sources of funding and support. Nineteen acknowledged federal sources, seven acknowledged internal grants, four acknowledged non‐profit entities, and two acknowledged clinics.
Summary of findings
Summary of findings 1. Summary of findings: mindfulness‐based interventions (MBIs) compared with no treatment for substance use disorders (SUDs).
| Outcomes | Anticipated absolute effects (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Continuous abstinence rate at post‐treatment RR < 1.00 favors MBI |
RR = 0.96 [0.44, 2.14] | 112 (1 RCT) | Very lowa, b, c | The evidence is very uncertain about the effect of MBIs relative to no treatment on continuous abstinence rate at post‐treatment. |
| Continuous abstinence rate at follow‐up (4 months) RR < 1.00 favors MBI |
RR = 1.04 [0.54, 2.01] | 112 (1 RCT) | Very lowa, b, c | The evidence is very uncertain about the effect of MBIs relative to no treatment on continuous abstinence rate at follow‐up. |
| Percentage days with substance use at post‐treatment Lower SMD favors MBI |
SMD = 0.05 [‐0.37, 0.47] | 248 (4 RCTs) | Very lowa, b, c | The evidence is very uncertain about the effect of MBIs relative to no treatment on percentage days with substance use at post‐treatment. |
| Percentage days with substance use at follow‐up (3 to 4 months) Lower SMD favours MBI |
SMD = 0.21 [‐0.12, 0.54] | 167 (3 RCTs) | Very lowb, c, d | The evidence is very uncertain about the effect of MBIs relative to no treatment on percentage days with substance use at follow‐up. |
| Consumed amount at post‐treatment Lower SMD favors MBI |
SMD = 0.10 [‐0.31, 0.52] | 221 (3 RCTs) | Very lowa, b, c | The evidence is very uncertain about the effect of MBIs relative to no treatment on consumed amount at post‐treatment. |
| Consumed amount at follow‐up (3 to 4 months) Lower SMD favors MBI |
SMD = 0.33 [0.00, 0.66] | 142 (2 RCTs) | Very lowb, c, d | The evidence is very uncertain about the effect of MBIs relative to no treatment on consumed amount at follow‐up. |
| Craving intensity at post‐treatment Lower SMD favors MBI |
Could not be pooled because of heterogeneity. Range = ‐4.84 to ‐0.32. | 128 (2 RCTs) | Very Lowa, b, c, e | The evidence is very uncertain about the effect of MBIs relative to no treatment on craving intensity at post‐treatment. |
| Treatment acceptability (attrition) RR < 1.00 favors MBI |
RR = 1.04 [0.77, 1.40] | 1087 (21 RCTs) | High | MBIs result in little to no increase in attrition relative to no treatment. |
| CI: confidence interval; MBI: mindfulness‐based interventions; RCT: randomized controlled trial; RR: risk ratio; SMD: standardized mean difference. | ||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||
a95% CI includes both an appreciable benefit and an appreciable harm. Downgraded one point downgraded for imprecision.
bSample size <400 (less then minimum optimal information size [OIS] recommended for continuous outcomes). Downgraded one point downgraded for imprecision.
cOutcome assessment was unblinded. Downgraded one point for risk of bias.
d95% CI includes both an effect not relevant to participants and an appreciable harm. Downgraded one point downgraded for imprecision.
eEffect sizes were highly heterogeneous (e.g., I2 ≥ 90%). Downgraded one point for inconsistency.
Summary of findings 2. Summary of findings: mindfulness‐based interventions (MBIs) compared with other treatments for substance use disorders (SUDs).
| Outcomes | Anticipated absolute effects (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Continuous abstinence rate at post‐treatment RR < 1.00 favors MBI |
RR = 0.80 [0.45, 1.44] | 286 (1 RCT) | Very Lowa, b, c | The evidence is very uncertain about the effect of MBIs relative to other treatments on continuous abstinence rate at post‐treatment. |
| Continuous abstinence rate at follow‐up (10 months) RR < 1.00 favors MBI |
RR = 0.57 [0.28, 1.16] | 286 (1 RCT) | Very Lowa, b, c | The evidence is very uncertain about the effect of MBIs relative to other treatments on continuous abstinence rate at follow‐up. |
| Percentage days with substance use at post‐treatment Lower SMD favors MBI |
SMD = ‐0.21 [‐0.45, 0.03] | 523 (5 RCTs) | Lowc, d | The evidence suggests that MBIs reduce percentage of days with substance use slightly relative to other treatments at post‐treatment. |
| Percentage days with substance use at follow‐up (4 to 10 months) Lower SMD favors MBI |
SMD = ‐0.39 [‐0.96, 0.17] | 409 (3 RCTs) | Lowc, d | The evidence suggests that MBIs reduce percentage of days with substance use slightly relative to other treatments at follow‐up. |
| Consumed amount at post‐treatment Lower SMD favors MBI |
SMD = ‐0.42 [‐1.23, 0.39] | 25 (1 RCT) | Very Lowa, b, c | The evidence is very uncertain about the effect of MBIs relative to other treatments on consumed amount at post‐treatment. |
| Craving intensity at post‐treatment Lower SMD favors MBI |
Could not be pooled because of heterogeneity. Range from SMD = ‐1.43 to 1.00 | 971 (9 RCTs) | Very lowc, d, e | The evidence is very uncertain about the effect of MBIs relative to other treatments on craving intensity at post‐treatment. |
| Craving intensity at follow‐up (3 to 6 months) Lower SMD favors MBI |
Could not be pooled because of heterogeneity. Range from SMD = ‐2.07 to ‐0.14 | 415 (4 RCTs) | Very lowc, d, e | The evidence is very uncertain about the effect of MBIs relative to other treatments on craving intensity at follow‐up. |
| Treatment acceptability (attrition) RR < 1.00 favors MBI |
RR = 1.06 [0.89, 1.26] | 1531 (14 RCTs) | High | MBIs result in little to no increase in attrition relative to other treatments. |
| CI: confidence interval; MBI: mindfulness‐based interventions; RCT: randomized controlled trial; RR: risk ratio; SMD: standardized mean difference. | ||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||
a95% CI includes both an appreciable benefit and an appreciable harm. Downgraded one point downgraded for imprecision.
bSample size < 400 (less then minimum optimal information size [OIS] recommended for continuous outcomes). Downgraded one point downgraded for imprecision.
cOutcome assessment was unblinded. Downgraded one point for risk of bias.
d95% CI includes both an effect not relevant to participants and an appreciable benefit.
eEffect sizes were highly heterogeneous (e.g., I2 ≥ 90%). Downgraded one point for inconsistency.
Background
Description of the condition
Substance use disorders (SUDs, see Table 3 for a list of all acronyms) are a disease category with a chronic and relapsing nature, characterized by dysfunctional patterns of tobacco, alcohol, prescription or illicit drug use, leading to specific psychophysical, affective and cognitive symptoms and consequences for psychosocial well‐being and health. While the major classification systems Diagnostic and Statistical Manual of Mental Disorders (DSM) (APA 2000) and International Classification of Diseases (ICD) (WHO 2010) have subdivided SUDs into dependence and a secondary category, called "abuse" in Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) and "harmful use" in International Classification of Diseases, Tenth Revision (ICD‐10) (Hasin 2006), the latest version of the DSM system, the DSM‐V (APA 2013) integrates both categories into a single substance use disorder concept that ranges along a continuum from mild to severe (Hasin 2013; Rehm 2013).
1. Acronyms used.
| Acronym | Term |
| SUD | substance use disorder |
| MBI | mindfulness‐based intervention |
| RCT | randomized controlled trial |
| SMD | standardized mean difference |
| CI | confidence interval |
| USA | United States of America |
| MBSR | Mindfulness‐Based Stres Reduction |
| MBCT | Mindfulness‐Based Cognitive Therapy |
| MORE | Mindfulness‐Oriented Recovery Enhancement |
| DBT | Dialectical Behavior Therapy |
| ACT | Acceptance and Commitment Therapy |
| MBRP | Mindfulness‐Based Relapse Prevention |
| SD | standard deviation |
| SE | standard error |
| AE | adverse effects |
| SAE | serious adverse effects |
SUDs are highly prevalent and have a profound public health and economic impact (Degenhardt 2018; Vega 2002). It is estimated that about 4.2% of the global burden of disease as measured in disability adjusted life years (DALYs) is attributable to alcohol and 1.3% to illicit drugs (Degenhardt 2018). Together with mental disorders, SUDs constitute the fifth leading cause of death and disability worldwide (Whiteford 2013). Statistics vary between regions, with higher prevalence of some psychoactive substance use in more highly‐developed, compared to less‐developed, countries (Degenhardt 2018; WHO 2002a). Nevertheless, with the improved industrialization and centralization of alcohol production, alcohol consumption is increasingly becoming a problem in many developing regions (WHO 2002b). Regional shifts also seem to have reshaped the patterns of illicit drug use in the world (Uchtenhagen 2004; UNODC 2013). While some improvements for heroin use are registered in Western Europe, there is a rapid growth of the heroin market in the Afghanistan region and, further, in Central Asia, the Russian Federation and Eastern Europe. With the USA remaining the world's largest market for cocaine, there has been an increase in cocaine trafficking towards Western Europe (UNODC 2013).
The contribution of SUDs to the worldwide burden of disease and the costs to individuals, families and to society associated with substance use are rising (Whiteford 2013). As a large part of the substance‐attributable burden is assumed to be potentially avoidable through the implementation of preventive and therapeutic strategies (Rehm 2009), further effective strategies need to be developed that help individuals with SUDs to discontinue or reduce substance use in a way that increases health and well‐being.
Description of the intervention
Mindfulness is the English translation of Sati in Pali, an ancient language from northern India (Pali Text Society 1992). Rooted in 2500‐year‐old Buddhist philosophy and practice, mindfulness meditation practices such as Vipassana and Zen meditation are mind‐body practices promoting mindfulness as a state of consciousness attending to one’s moment‐to‐moment experience (Brown 2003); and “paying attention in a particular way, on purpose, in the present moment, and nonjudgmentally” (Kabat‐Zinn 1994). Practices like “focused attention meditation”, entailing voluntary and sustained attention on a selected object and “open monitoring meditation”, involving a meta‐cognitive monitoring of automatic cognitive and emotional processes contribute to a mindfulness content of experience with acceptance, patience, and compassion (Lutz 2008; Travis 2010; Vago 2012). By purposefully and nonjudgementally paying attention to the present moment, mindfulness meditation is often distinguished from concentrative meditation, which entails voluntary and sustained attention on a chosen object (Goleman 1988). Nevertheless, current neuroscientific evidence indicates that different types of attentional processes are activated in both meditation types (Lutz 2008). Furthermore, emphasis has been given to further classification criteria such as the role of self‐referential processes in different meditation types (Chiesa 2011).
There are various contemporary definitions of mindfulness in the psychological literature. Bishop 2004 has proposed a two‐component definition, with the first component focusing on self‐regulation of attention towards the immediate present moment and the second as an orientation marked by curiosity, openness and acceptance as fundamental features of mindfulness. Brown 2003 suggests a one‐dimensional definition of mindfulness, focusing on the “receptive attention to and awareness of the present moment”, while Shapiro 2006 developed a three‐component model by adding a motivational factor to Bishop’s components (for an overview see Chiesa 2011). Even though, to date, no consensus has been reached on how to define mindfulness, the two‐factor conceptualization (Bishop 2004) is often applied as an operational definition in research.
Mindfulness meditation was adapted for use in Western cultures in a variety of ways and has been incorporated into psychological treatment, constituting the “third wave” of behavior therapy (Hayes 2004). Combining mindfulness practice with components from mostly behavior and cognitive therapy, mindfulness‐based interventions (MBIs) have been explored to treat a variety of physical and psychological problems and disorders. Mindfulness‐based stress reduction (MBSR), developed by Jon Kabat‐Zinn in 1979 (Kabat‐Zinn 1985; Kabat‐Zinn 1990; Kabat‐Zinn 1992; Kabat‐Zinn 1994; Kabat‐Zinn 2003), integrates mindfulness meditation techniques into a structured clinical program designed to help facilitate adaptation to the stressors of medical illness and assist people in managing pain and stress (Whitebird 2009). By combining Kabat‐Zinn's MBSR with elements of cognitive therapy for depression (Beck 1979), Segal, Williams, and Teasdale developed mindfulness‐based cognitive therapy (MBCT), a program that particularly targets “modes of mind” characteristic for mood disorders (Teasdale 2002). Mindfulness‐oriented recovery enhancement (MORE) ‐ a program integrating mindfulness with reappraisal and savoring skills ‐ has been developed to enhance recovery in people struggling with addiction and the underlying conditions (Garland 2012b). Mindfulness‐based relapse prevention (MBPR) is another MBI designed to target addiction. It integrates mindfulness practices with relapse‐prevention strategies (Witkiewitz 2014b). Dialectical Behavior Therapy (DBT) and Acceptance and Commitment Therapy (ACT) are both innovative behavioral treatments that incorporate mindfulness practices and acceptance‐based interventions into their treatment programs (Chapman 2006). Major influences on DBT derive from behavioral science, dialectical philosophy and Zen practice, while a non‐judgemental observation and experience of thoughts constitutes one of the main elements of ACT. However, ACT and DBT do not emphasize formal training in mindfulness meditation. Following Crane 2017, we considered MBIs to be those that involved "sustained intensive training in mindfulness meditation practice" (p. 993).
Critical issues have been raised about mixing Buddhist elements with current psychological theories in modern MBIs and the resulting consequences for practitioners` aims and attitudes and the underlying psychological mechanism (Chiesa 2011). Some authors considered that influences of ancient Buddhist philosophy are only marginally acknowledged in modern MBIs and even identified misunderstandings of the concept of mindfulness in some modern ways of practicing mindfulness (Rapgay 2009; Chiesa 2011). In turn, it has been called into question whether mindfulness by itself can influence psychopathology without matching the type of mindfulness to a specific form of psycyhopathology (Teasdale 2003).
How the intervention might work
Teaching an attitude of non‐judgement and acceptance with an emphasis on substance use and craving, MBIs are increasingly recognized for their ability to enhance recovery from substance use disorders (Khanna 2013). The idea of experiencing urges without fighting against them has given rise to the term “urge surfing” (Marlatt 1985), in which cravings are conceptualized like waves in the ocean and individuals are encouraged to “surf on”, allowing it to pass instead of being wiped out by giving in to it (Murphy 2014). Through both cognitive behaviorally‐based exercises and mindfulness practices, MBPR practices share the common intention of bringing greater awareness to one’s experiences, with specific emphasis on the sequence of reactions that follow substance‐related cues (Witkiewitz 2014b). For explaining how MBIs may affect substance use, several plausible mechanisms of action emerge from both the addiction as well as the mindfulness perspective. By fostering an increasing ability to “stay in touch” with experiences rather than attempting to escape or distance oneself from unpleasant feelings and sensations, mindfulness practices might help individuals with substance use problems to increase the awareness of habit‐linked, minimally conscious and affective states linked to craving and relapse (Chiesa 2014). By strengthening the ability to “step back” from overwhelming emotions and sensations, while slowing down the chain of automatic processes of substance seeking, mindfulness practices increase the chance to interrupt the cycle of cognitive, affective, and psychophysiological mechanisms underlying craving, relapse and excessive drinking (Witkiewitz 2014b).
Referring to neuropsychological models of craving and self‐control, mindfulness practices have also been put into a neurocognitive perspective (Garland 2014c; Hölzel 2011; Witkiewitz 2014b). Neurocognitive models of self‐regulation hypothesize that the resolving of motivational conflicts to the benefit of intentions requires efficient top‐down control from the prefrontal cortex over subcortical regions, while self‐regulatory failure occurs whenever the balance is tipped in favor of subcortical areas, either due to prefrontal function impairments or particularly strong impulses (Heatherton 2011). Considering that substance‐related cues have acquired exaggerated salience in the course of substance use disorders – a process mainly attributable to neuroadaptive sensitization in the mesolimbic reward system (Robinson 2008) ‐ individuals with SUDs are faced with high demands on top‐down inhibitory control. As a strategy to control strong upwelling motivational drives, individuals with the intention to cut down their drinking often try to inhibit craving through the suppression of substance‐related thoughts (Bowen 2007; Garland 2012b). Thought suppression, in turn, has been shown to have the inverse effect, resulting in an increase, rather than decrease, of unwanted thoughts (Wegner 1994), causing a “behavioral rebound” with greater enactment of consummatory behaviors (Erskine 2010; Garland 2012a). Instead of trying to control strong upwelling motivational drives and to inhibit craving through the suppression of substance‐related thoughts, MBIs prevent swinging “the pendulum of prefrontal regulation from a context of under‐control to one of over‐control” (Garland 2014c), which might “snowball” minor lapses in self‐control into self‐regulatory collapse (Erskine 2010; Garland 2012a; Heatherton 2011).
Why it is important to do this review
MBIs currently receive a lot of attention worldwide and are increasingly suggested as therapeutic approaches for substance use and misuse (Chiesa 2014). In fact, from a theoretic perspective, MBIs appear to bring about meaningful advantages compared to first and second wave therapies. Even though MBIs show promise in the treatment of substance use disorders, findings are rather inconsistent (Murphy 2014). While several studies found positive treatment effects of mindfulness interventions, including reduced quantity and frequency of substance use, a number of studies did not report positive findings. This Cochrane Review on MBIs for SUDs aims to provide a systematic integration of the available evidence to health‐decision makers, therapists and patients; and to offer illustrative measures for estimating the relative benefits of MBIs compared to alternative types of psychotherapy, while indicating gaps of knowledge and methodological demands for future clinical research.
Objectives
To determine the effects of mindfulness‐based interventions (MBIs) for substance use disorder (SUD) (including alcohol and/or drug use disorders but excluding tobacco use disorders) in terms of substance use outcomes, craving and adverse events compared to standard care, further psychotherapeutic, psychosocial, or pharmacological interventions or instructions, waiting lists, and no treatment.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) comparing MBIs for SUDs with other treatments or no intervention were eligible for inclusion in the review. Trials employing a cross‐over design were also eligible, using data from the first active treatment stage only to encounter the risk of carry‐over effects.
Types of participants
RCTs with patients suffering from SUDs including individuals with alcohol, prescription‐, illicit‐ and poly‐substance use disorders were considered as eligible for the review. There was no limitations on age or other participant characteristics. Besides accepted SUD diagnostic criteria including DSM‐III (APA 1980), DSM‐ III‐R (APA 1987), DSM‐IV‐TR (APA 2000), DSM‐V (APA 2013) and ICD‐10 (WHO 1992; WHO 2010), we also included studies in which SUD was not formally diagnosed, acknowledging that diagnostic systems are not consequently used in primary research.
Types of interventions
In accordance with the definition by Bishop and colleagues (Bishop 2004) of mindfulness, we consider as mindfulness‐based all approaches which promote a) an individual's attention towards the immediate present moment experience and b) an open and accepting orientation irrespective of the applied technique. In order to isolate the effects of mindfulness meditation practice specifically, we excluded interventions that involve solely the concept of mindfulness and do not include formal instruction in mindfulness meditation practice e.g. Acceptance and Commitment Therapy (ACT), Dialectical Behaviour Therapy (DBT). This definition of MBIs is in keeping with that proposed by Crane 2017 and widely implemented in the meta‐analytic literature (see Goldberg 2021).
Accordingly, the following experimental interventions were included in the review:
ancient Buddhist meditations such as Vipassana meditation and Zen meditation;
other mindfulness meditation;
modern standardized group‐based meditation practices including mindfulness‐based stress reduction (MBSR), mindfulness‐based cognitive therapy (MBCT) and mindfulness‐based relapse prevention (MBRP) and mindfulness training.
Any type of manually‐based and face‐to‐face treatment delivery including individual therapy and group session format were considered. Media‐ and CD‐supported interventions were accepted as complementary formats of treatment support (e.g. home‐practice formats), but not as exclusive modes of treatment delivery.
All comparators were eligible, with the exception of comparisons with other MBIs or similar mind‐body approaches. Comparisons could include standard care, further psychotherapeutic, psychosocial or pharmacological interventions or instructions, waiting list or no treatment. Comparisons were categorized into no treatment (which included standard care when both the mindfulness and non‐mindfulness arms received this) and other treatment (which included standard care when only the comparison condition received this).
Types of outcome measures
The study endpoints of the primary outcomes were considered essential to determine the effectiveness of MBIs, while secondary outcomes have only complementary value in the interpretation of results. If a study met the inclusion criteria, but did not provide necessary information for estimating effect sizes, while such data were also not available from the authors, the study was excluded from the meta‐analysis, but included in the qualitative analyses.
Primary and secondary outcomes were selected with regard to clinical relevance and conceptual considerations. With the "rate of continuous abstinence", the "per cent days with substance use" limited to non‐abstinent individuals and "consumed amount" limited to days with substance use, primary efficacy outcomes of the review assess conceptually‐distinct achievements in substance use control (Keller 1972); including an individual's ability to a) achieve and maintain continuous abstinence; and b) their ability to refrain from substance use on individual days; and c) to stop substance use once started. Evaluation of adverse effects, serious adverse effects, and treatment acceptability was included to allow evaluation of the safety and acceptability of MBIs relative to controls.
Primary outcomes
Continuous abstinence rate
Percentage of days with substance use
Consumed amount
Adverse event rate
Rate of continuous abstinence is a binary variable comprising the information whether a participant remained fully abstinent until the end of treatment or returned to substance use after detoxification. Accordingly, any substance use irrespective of the consumed amount or frequency of use was considered as treatment failure in the determination of the outcome. Percentage of days with substance use is a continuous measure calculated as the ratio of the total sum of substance use days related to possible exposure days during the treatment phase multiplied by the factor 100. If 'exposure days', representing days at which participants had a chance to use the substance (e.g. not incarcerated or hospitalized), are not available, substance use days were related to the study duration. Consumed amount is also a continuous measure and calculated by dividing the total consumed amount to the number of possible exposure days (or alternatively the entire study duration). Both outcomes, percentage of days with substance use and consumed amount, are measures representing substance use in the entire sample including all participants irrespective of their status of abstinence. Besides efficacy outcomes, harms were assessed with adverse events (AEs), which are binary variables comprising the information if an unfavorable event or symptom occurred during the course of the study or not.
To allow conclusions on the sustainability of treatment effects, post‐treatment efficacy outcomes (follow‐up after treatment termination) were evaluated. Indicators of substance use were considered irrespective of measurement including self‐reports, self‐report questionnaires, documentation templates (substance use diary, monitoring sheets), standardized interviews, observer‐reported measures, laboratory testing and breathalyzer tests. The validation of patient‐reported substance use by objective measures was entered in the risk of bias tables (susceptibility to bias).
Secondary outcomes
Craving intensity
Treatment acceptability (i.e. attrition)
Serious adverse events
Craving intensity occurring in natural settings and in laboratory paradigm was considered as assessed with a standardized tool (visual analog scale (VAS), questionnaire) or by an objective parameter of cue‐reactivity. Treatment acceptability was considered by either a) the number of participants dropping out from treatment for any reason; or b) subjective ratings of acceptance or satisfaction with care; or c) both measures. Serious adverse events (SAEs) were considered to be binary variables comprising the information if a serious unfavorable event such as, for example, suicide, suicide attempts or relapse requiring hospitalization.
Search methods for identification of studies
Electronic searches
The Cochrane Drugs and Alcohol Group Information Specialist conducted systematic searches in the following databases for randomized controlled trials and controlled clinical trials without language, publication year or publication status restrictions:
Cochrane Drugs and Alcohol Group's Specialised Register of Trials (searched on 26 April 2021);
The Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 3);
PubMed (January 1966 to 26 April 2021);
Embase (OVID) (January 1974 to 26 April 2021);
CINAHL (Cumulative Index to Nursing and Allied Health Literature) (EbscoHOST) (1982 to 26 April 2021);
Web of Knowledge, Web of Science (1990 to 26 April 2021);
PsycINFO (OVID) (1806 to 26 April 2021).
The Information Specialist modeled subject strategies for databases on the search strategy designed for PubMed. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying randomized controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6, Lefebvre 2011). Search strategies for major databases are provided in Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6.
We searched the following trials registries on 26 April 2021:
the ISRCTN registry (www.isrctn.com);
ClinicalTrials.gov (clinicaltrials.gov).
Searching other resources
Key informants, primary authors and review authors were contacted with the request to indicate further studies of potential relevance. For this purpose, reference lists with identified studies and criteria of inclusion and exclusion of the review were provided. Finally, handsearching of reference lists of included studies and current reviews was conducted to complete the searches.
Data collection and analysis
Selection of studies
Two review authors independently assessed the eligibility and relevance of trials on the basis of their abstracts retrieved from the electronic searches. For studies that met the inclusion criteria according to the abstract information, we obtained full‐text versions for closer inspection. Full‐text versions were also obtained if review authors differed in their judgement. Again, the relevance and eligibility of studies on the basis of full‐text versions was independently assessed by two review authors. In case of disagreements, the eligibility will be discussed with an additional consultant. The process of study identification and its results are outlined as flow diagrams according to the PRISMA statement (Moher 2009).
Data extraction and management
The review authors had full access to details about authors, institutions, and journals at all times. Information related to the study design and setting, the study participants, the interventions and comparators as well as the outcomes and methods for their assessment was abstracted from the original reports and entered into the study tables. The following information was extracted in detail:.
Study design and setting: design, principle of analysis, setting, study sites, country
Study sample: sample size, diagnosis, specific characteristics, age, gender
Interventions: description of the type of experimental and control intervention, treatment duration, treatment adherence
Outcomes: outcomes, methods of measurement, time points for assessment, compliance
Two review authors independently extracted all relevant outcome data onto pre‐specified data extraction forms and compared data value by value. In case of disagreements, the following sequential procedures were undertaken in descending order.
Comparison of published and extracted information to identify transcription and comprehension errors
Explanation of the coding decisions by each review author, followed by consensus discussion and arbitration
Any disagreement was discussed with an additional expert, and, when necessary, the authors of the studies were contacted for further information. Finally, after comparisons and corrections are concluded, we entered data into the Review Manager software (RevMan 2008).
Assessment of risk of bias in included studies
The risk of bias assessment for RCTs and CCTs in this review was performed using the criteria recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The recommended approach for assessing risk of bias in studies included in Cochrane Reviews is a two‐part tool, addressing seven specific domains, namely sequence generation and allocation concealment (selection bias), blinding of participants and providers (performance bias), blinding of outcome assessor (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other sources of bias. The first part of the tool involves describing what was reported to have happened in the study. The second part of the tool involves assigning a judgement relating to the risk of bias for that entry, in terms of low, high or unclear risk. To make these judgements we used the criteria indicated by the Cochrane Handbook for Systematic Reviews of Interventions adapted to the addiction field. The criteria considered as constitutive for the rating of bias risks are outlined in Appendix 7.
The domains of sequence generation and allocation concealment (avoidance of selection bias) were addressed in the tool by a single entry for each study. We planned to consider blinding of participants, personnel and outcome assessor (avoidance of performance bias and detection bias) separately for objective outcomes (e.g. dropout, substance use measured by urine‐analysis, participants who relapsed at the end of follow‐up,participants engaged in further treatments), and subjective outcomes (e.g. duration and severity of signs and symptoms of withdrawal, patient self‐reported use of substance, side effects, social functioning as integration at school or at work, family relationship). Incomplete outcome data (avoidance of attrition bias) were considered for all outcomes except for the dropout from the treatment, which is very often the primary outcome measure in trials on addiction. We considered the equivalence of baseline characteristics an additional indication of selection bias.
Measures of treatment effect
We measured treatment effects for dichotomous effectiveness outcomes (abstinence rate, retention rate) with a risk ratio (RR). For continuous outcomes (days with substance use, consumed amount per day, craving intensity), we planned to asses treatment effects using the mean differences (MD) for outcomes measured on the same scale.We used the standardized mean difference (SMD) for outcomes measured on different scales. We calculated all treatment effects within 95% confidence intervals (CIs). When effects on binary outcomes reached statistical significance, we calculated the number needed to treat for an additional beneficial outcome (NNTB). A P value of 0.05 and below was chosen to indicate statistical significance of effects.
Unit of analysis issues
Only individually‐randomized trials with the individual participants constituting the unit of analysis were included in the review. To control unit of analysis errors in studies with multiple treatment groups of the same type (e.g. multiple alternative treatment comparisons; Bowen 2014), we combined interventions to create single‐pair comparisons.
Dealing with missing data
Outcome statistics were included as intent‐to‐treat (ITT) analyses. Sample sizes for continuous outcomes which were not explicitly provided in the trial publication were imputed by the size of treatment‐received samples or ‐ if not available ‐ by the size of the randomized sample. An exception was if samples were from analyses explicitly specified as completer analyses, which exclusively reported on patients who completed the trial. For differences in means, missing serious adverse events (SEs) were obtained from standard deviations (SDs), CIs or t values and P values. If only the medians were provided in the trial publications, the outcome statistics were not be included in the meta‐analyses, but we considered the information on the significance of effects in the qualitative discussion of results.
Assessment of heterogeneity
We quantified inconsistency across studies with the I² statistic (Higgins 2003), using threshold values for substantial heterogeneity as outlined by Deeks 2001. The Tau² statistic was additionally considered to provide an estimate of between‐study variance (Rücker 2008) independent of the sample size. A value of P < .10 was considered as significant statistical heterogeneity.
Assessment of reporting biases
When there were more than 10 included studies, we graphically illustrated the risk of publication bias with the funnel plot method (Light 1984; Egger 1997).
Data synthesis
For synthesizing outcome measures, we used a random‐effects model (DerSimonian 1986), with study effects being weighted using the Mantel‐Haenszel approach (Mantel 1959). For outcomes with low effect heterogeneity (I² < 30%), we applied a fixed‐effect model within the scope of sensitivity analyses (see Sensitivity analysis).
Subgroup analysis and investigation of heterogeneity
Inconsistency across studies was quantified as described above (see Assessment of heterogeneity).
Sensitivity analysis
When the number of included studies was sufficient (> 10), we conducted sensitivity analyses to determine the influence of the following variables on the primary outcomes:
the underlying statistical model, by comparing effect sizes for low heterogeneity outcomes (I² < 30%) based on random‐effects models versus fixed‐effect models;
the method of measurement, by comparing effect sizes measured by breathalyzer or laboratory tests versus self‐reported data on alcohol use.
Summary of findings and assessment of the certainty of the evidence
Findings were presented as summarized narrative and by summary of findings tables (GRADE); and the certainty of evidence was assessed with the GRADE approach for each outcome individually.
We created two summary of findings tables using the following outcomes: continuous abstinence, percentage days with substance use, consumed amount, craving intensity, treatment acceptability (attrition). We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to the studies which contribute data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We used methods and recommendations described in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019) using GRADEproGDT software (GRADEpro GDT 2015). We justified all decisions to down‐grade or up‐grade the certainty of studies using footnotes, and we made comments to aid the reader's understanding of the review where necessary.
The GRADE system uses the following criteria for assigning grades of evidence.
High: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
The literature search and included studies are described below.
Results of the search
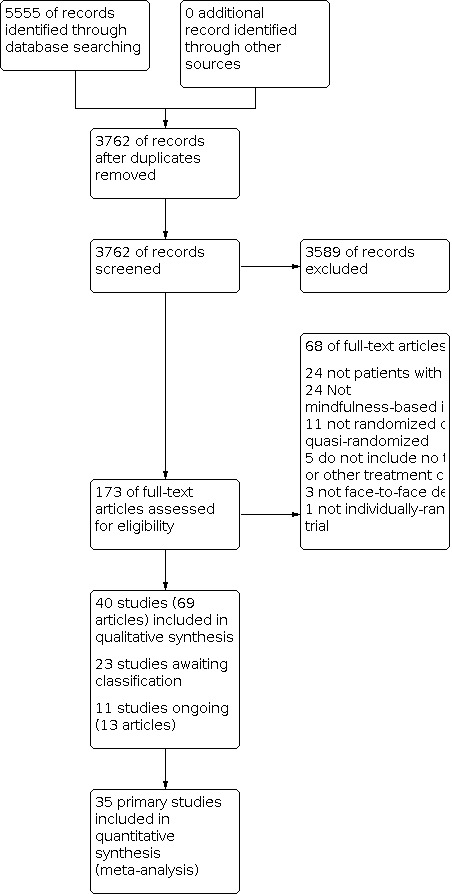
The searches of the seven databases (see Electronic searches) retrieved 5555 records. Our searches of other resources (Grant 2017, Li 2017, Goldberg 2018) identified no additional studies that appeared to meet the inclusion criteria. Our screening of the reference lists of the included publications did not reveal additional randomized controlled trials (RCTs). We therefore had a total of 5555 records.
Once duplicates had been removed, we had a total of 3762 records. We excluded 3598 records based on titles and abstracts. We obtained the full text of 173 records. Of these, 68 records were not eligible to be included (see Characteristics of excluded studies table). We identified 23 studies awaiting classification and 11 ongoing studies reported in 13 references.
We included 40 studies reported in 69 references (as some studies were reported across multiple references). For a further description of our screening process, see the study flow diagram (Figure 1).
1.
Study flow diagram.
Included studies
Forty trials published in 69 publications met our inclusion criteria. Data eligible for meta‐analysis were available from 35 studies (2825) participants (Abed 2019; Alegria 2019; Alizadehgoradel 2019; Alterman 2004; Asl 2014a; Asl 2014b; Bein 2015; Bevan 2012; Black 2019; Bowen 2009; Bowen 2014; Brewer 2009; Brown 2017; Davis 2013; Davis 2018; de Dios 2012; Foroushani 2019; Garland 2010; Garland 2016; Garland 2019; Glasner 2017; Jenaabadi 2017; Imani 2015; Lee 2011; Machado 2020; Marfurt 2007; Margolin 2006; Mermelstein 2015; Shorey 2017; Vowles 2020; Witkiewitz 2014; Wongtongkam 2019; Yaghubi 2017; Zemestani 2016; Zgierska 2017). However, 18 trials only reported data usable for assessing acceptability (i.e. attrition) and did not provide eligible data for assessment of other primary or secondary outcomes. Five trials did not report any outcomes eligible for meta‐analysis (Esmaeili 2017; Himelstein 2015; Ramezani 2019; Wongtongkam 2018; Zhang 2019).
All studies used a randomized controlled trial design. Sixteen studies used an intention‐to‐treat analysis. Principle of analysis was unclear for three studies. In 17 studies, interventions and/or recruitment occurred in a residential treatment setting, while 19 studies did not involve a residential treatment setting. Setting was unclear in four studies. Of the 40 eligible studies, 23 were conducted in the USA, 11 were conducted in Iran, two were conducted in Thailand, one was conducted in China, one was conducted in Taiwan, one was conducted in Brazil, and one was conducted in Spain and the USA. Sample sizes ranged from eight (Bein 2015) to 341 (Alegria 2019), with an average of 76.08 (SD = 71.38).
Details of all 40 studies are available under Characteristics of included studies.
Participants
Eighteen studies included individuals with various substance use disorders (SUDs), 12 were focused on opioids, five on alcohol, three on stimulants, one on marijuana, and one on alcohol and/or cocaine use. Eighteen studies involved a formal diagnosis while 22 did not. Participants were on average 35.38 years old (SD = 8.27, range = 16.45 to 50.50). Samples were on average 31% female (SD = 34%, range = 0% to 100%). Samples were on average 34% non‐Hispanic White (SD = 36%, range = 0% to 100%).
Interventions
Studies implemented a variety of mindfulness‐based interventions (MBIs). Sixteen studies involved mindfulness‐based relapse prevention (MBPR), or an adaptation of this intervention (e.g. mindfulness‐based relapse prevention for alcohol dependence; Zgierska 2017). Four studies involved mindfulness‐oriented recovery enhancement (MORE) or an adaptation of this intervention (e.g., mindfulness‐oriented recovery enhancement for child welfare; Brown 2017). Two studies involved mindfulness‐based cognitive therapy (MBCT), and one study involved mindfulness‐based stress reduction (MBSR). The remaining studies involved other interventions that included mindfulness meditation training.
Three studies included multiple comparison conditions, with two including a second other treatment control conditions (Bowen 2014; Garland 2016) and one including a no treatment control (Jenaabadi 2017). Comparisons were made between MBIs and 19 other treatment conditions and 24 no treatment control conditions. Among the other treatment conditions, seven were standard of care (i.e. treatment as usual), six6 were based on cognitive behavioral therapy (CBT), three were psychoeducation, one was a support group, one was physical exercise, and one was medication. Treatment as usual (TAU) was considered another treatment condition when the control group received treatment that was not also provided to the experimental group (e.g. control group received support group sessions while the experimental group received MBI).
MBIs lasted between one and 12 weeks, with an average duration of 7.17 weeks (SD = 2.49). Other treatment controls lasted between one and 19 weeks, with an average duration of 7.14 weeks (SD = 3.35). The majority (n = 32) of the MBIs used a group delivery format with five using an individual delivery format, and one using a combination of individual or individual and group (Margolin 2006). Delivery format was unclear in two studies. Thirteen of the other treatment control conditions used a group delivery format with two using an individual delivery format. Delivery format was unclear for four other treatment control conditions. Adherence to the MBI was reported in eight studies. All eight studies used a version of outside practice (e.g. minutes of practice, number of practice sessions). Fourteen studies included a mechanism to support provider adherence to the MBI protocol (e.g. recording of sessions, clinical supervision, fidelity checklist). Three studies included a mechanism to support provider adherence to the other treatment control condition protocols (e.g. recording of sessions, clinical supervision).
Outcomes
Primary outcome measures
Two studies reported eligible data for assessing continuous abstinence rate. The specific outcomes assessed included any drug use (Bowen 2014) and any heavy drinking (Zgierska 2017). Nine studies reported eligible data for assessing percentage days with substance use. The specific outcomes assessed included alcohol and other drug use days (Bowen 2009), drug use days (Bowen 2014; Witkiewitz 2014), percentage of days with alcohol use (Brewer 2009), Substance Frequency Scale (Davis 2018), marijuana use days (de Dios 2012), drinking episodes (Mermelstein 2015), and percentage heavy drinking days (Machado 2020; Zgierska 2017). Four studies reported eligible data for assessing consumed amount. The specific outcomes assessed included drinks per week (Davis 2013; Mermelstein 2015), drinks per day (Zgierska 2017), and alcohol consumption (Machado 2020). Four studies reported occurrence of adverse events (Bowen 2014; Brewer 2009; Zemestani 2016; Zgierska 2017). None of the primary outcomes were assessed objectively.
Secondary outcome measures
Eleven studies reported eligible data for assessing craving intensity. The specific outcomes assessed included desire to use from the Heroin Craving Questionnaire (Abed 2019), Alcohol Craving Questionnaire Revised (Bevan 2012), Penn Alcohol Craving Scale (Black 2019; Bowen 2009; Bowen 2014; Garland 2010; Garland 2016; Shorey 2017; Zemestani 2016), subjective craving during stress provocation (Brewer 2009), and the Craving Scale from the GAIN assessment (Davis 2018). Thirty‐four studies reported eligible data for evaluating treatment acceptability in the form of study attrition. Four studies reported occurrence of serious adverse events (Bowen 2014; Brewer 2009; Zemestani 2016; Zgierska 2017). Treatment acceptability in the form of study attrition was the only outcome that was assessed objectively.
Studies awaiting classification and ongoing studies
Twenty‐three studies were identified as awaiting classification. Eleven studies (13 articles) were identified as ongoing studies.
Excluded studies
The full‐text screening resulted in 68 references being excluded due to ineligible criteria. Reasons for exclusion included:
not patients with SUD (24 references);
not MBI (24 references);
not randomized or quasi‐randomized (11 references);
did not include no treatment or other treatment comparison (5 studies);
not face‐to‐face delivery (3 studies);
not individually randomized (1 study);.
Risk of bias in included studies
Results of risk of bias assessment is displayed in Figure 2 and Figure 3.
2.

3.

Allocation
Seventeen studies were at low risk for allocation bias based on reporting adequate methods of sequence generation. The remaining 23 studies were at unclear risk for allocation bias due to insufficient reporting of sequence generation methods. Seven studies were at low risk for allocation bias based on reporting adequate methods of allocation concealment (Alegria 2019; Bevan 2012; Black 2019; Garland 2016; Glasner 2017; Zemestani 2016; Zgierska 2017). The remaining 33 studies were at unclear risk for allocation bias due to insufficient reporting of allocation concealment.
We examined equivalence of baseline characteristics as an additional source of selection bias. Twenty‐nine studies were at low risk for bias due to non‐equivalence of baseline characteristics, with mindfulness‐based intervention (MBI) and control conditions matched at baseline. Five studies were at high risk for bias due to non‐equivalence of baseline characteristics. This source of bias was unclear in six studies.
Blinding
All studies were at high risk for performance bias due to a lack of participant blinding, which is unsurprising given the behavioral nature of the MBIs being evaluated. With the exception of treatment acceptability in the form of attrition, all outcomes were assessed subjectively via self‐report and were therefore at high risk for detection bias (17 studies). Attrition was assessed objectively in all studies were it was assessed (34 studies). Five studies did not include an eligible outcome.
Incomplete outcome data
Fourteen studies were at low risk for attrition bias due to a lack of missing outcome data, adequate treatment of missing outcome data (e.g. through multiple imputation), balanced missingness across groups, and/or similar reasons for missingness across groups. Risk for attrition bias was unclear in 20 studies and high in six studies due to the reasons noted (e.g. missing outcome data that differed in reason and/or amount across conditions).
Selective reporting
Twelves studies were at low risk for reporting bias due to the availability of a study protocol or preregistration with all of the outcomes reported or through the identification of plausible primary outcomes within the published report itself. Risk for reporting bias was unclear in 24 studies. Risk of reporting bias was high in four studies due to the availability of a study protocol but a lack of reporting of pre‐specified outcomes.
Other potential sources of bias
No other potential sources of bias were considered.
Effects of interventions
Mindfulness‐based interventions (MBIs) versus no treatment
Twenty‐four studies included a comparison between MBI and no treatment. As noted, these comparisons may have included standard of care interventions which were received by both the MBI and no treatment conditions (i.e. no additional treatment was provided to the control group).
Primary outcome measures
Continuous abstinence rate
One study with 112 participants (Zgierska 2017) provided results for comparisons with no treatment controls at post‐treatment and follow‐up (four months post‐treatment). The evidence is very uncertain about the effect of MBIs relative to no treatment on continuous abstinence rate at post‐treatment (Analysis 1.1;risk ratio ( RR) = 0.96, 95% confidence interval (CI) 0.44, 2.14) and follow‐up (Analysis 1.2; RR = 1.04, 95% CI 0.54, 2.01).
1.1. Analysis.

Comparison 1: Mindfulness versus no treatment, Outcome 1: Continuous abstinence at post‐treatment
1.2. Analysis.

Comparison 1: Mindfulness versus no treatment, Outcome 2: Continuous abstinence at follow‐up
Percentageof days with substance use
Four studies with 248 participants (de Dios 2012; Machado 2020; Mermelstein 2015; Zgierska 2017) provided results for comparisons with no treatment controls at post‐treatment and three studies with 167 participants (de Dios 2012; Zgierska 2017) provided results for comparisons with no treatment controls at follow‐up (three to four months post‐treatment). The evidence is very uncertain about the effect of MBIs relative to no treatment on percentage days with substance use at post‐treatment (Analysis 1.3; standardized mean difference (SMD) = 0.05, 95% CI ‐0.37, 0.47) and follow‐up (Analysis 1.4; SMD = 0.21, 95% CI ‐0.12, 0.54).
1.3. Analysis.

Comparison 1: Mindfulness versus no treatment, Outcome 3: Percentage days with substance use at post‐treatment
1.4. Analysis.

Comparison 1: Mindfulness versus no treatment, Outcome 4: Percentage days with substance use at follow‐up
Consumed amount
Three studies with 221 participants (Machado 2020; Mermelstein 2015; Zgierska 2017) provided results for comparisons with no treatment controls at post‐treatment and two studies with 142 participants (Machado 2020; Zgierska 2017) provided results for comparisons with no treatment controls at follow‐up (three to four months post‐treatment). The evidence is very uncertain about the effect of MBIs relative to no treatment on consumed amount at post‐treatment (Analysis 1.5; SMD = 0.10, 95% CI ‐0.31, 0.52) and follow‐up (Analysis 1.6; SMD = 0.33, 95% CI 0.00, 0.66).
1.5. Analysis.

Comparison 1: Mindfulness versus no treatment, Outcome 5: Consumed amount at post‐treatment
1.6. Analysis.

Comparison 1: Mindfulness versus no treatment, Outcome 6: Consumed amount at follow‐up
Adverse event rate
One study with 112 participants (Zgierska 2017) provided results for comparisons with no treatment controls. No adverse events were reported in either condition.
Secondary outcome measures
Craving intensity
Two studies with 128 participants (Abed 2019; Bevan 2012) provided results for comparisons with no treatment controls at post‐treatment. The evidence is very uncertain about the effect of MBIs relative to no treatment on craving intensity at post‐treatment (Analysis 1.7; SMD range = ‐4.84 to ‐0.32). Results could not be pooled due to high heterogeneity (I2 ≥ 90%).
1.7. Analysis.

Comparison 1: Mindfulness versus no treatment, Outcome 7: Craving intensity at post‐treatment
Treatment acceptability (attrition)
Twenty‐one studies with 1087 participants (Abed 2019; Alizadehgoradel 2019; Alterman 2004; Asl 2014a; Asl 2014b; Bein 2015; Bevan 2012; Brown 2017; de Dios 2012; Foroushani 2019; Imani 2015; Jenaabadi 2017; Machado 2020; Marfurt 2007; Margolin 2006; Mermelstein 2015; Shorey 2017; Vowles 2020; Wongtongkam 2019; Yaghubi 2017; Zgierska 2017) provided results for comparisons with no treatment controls (Figure 4). MBIs result in little to no increase in study attrition relative to no treatment (Analysis 1.8; RR = 1.04 95% CI 0.77 to 1.40); high‐certainty evidence.
4.

1.8. Analysis.

Comparison 1: Mindfulness versus no treatment, Outcome 8: Treatment acceptability (attrition)
Serious adverse event rate
One study with 112 participants (Zgierska 2017) provided results for comparisons with no treatment controls. No serious adverse events were reported in either condition.
Sensitivity analyses
Sufficient studies to conduct a fixed‐effect model sensitivity analysis (>10 studies) were available only for treatment acceptability. Results indicated that MBIs result in little to no increase in study attrition relative to no treatment (Analysis 1.9; RR = 1.13 95% CI 0.84, 1.50).
1.9. Analysis.

Comparison 1: Mindfulness versus no treatment, Outcome 9: Treatment acceptability (attrition): sensitivity analysis (fixed‐effects model)
Mindfulness‐based interventions (MBIs) versus other treatments
Nineteen studies included a comparison between MBI and another treatment.
Primary outcome measures
Continuous abstinence rate
One study with 286 participants (Bowen 2014) provided results for comparisons with other treatment controls at post‐treatment and follow‐up (10 months post‐treatment). The evidence is very uncertain about the effect of MBIs relative to other treatment controls on continuous abstinence rate at post‐treatment (Analysis 2.1; RR = 0.80, 95% CI 0.45, 1.44) and follow‐up (Analysis 2.2; RR = 0.57, 95% CI 0.28, 1.16).
2.1. Analysis.

Comparison 2: Mindfulness versus other treatments, Outcome 1: Continuous abstinence at post‐treatment
2.2. Analysis.

Comparison 2: Mindfulness versus other treatments, Outcome 2: Continuous abstinence at follow‐up
Percentage days with substance use
Five studies with 523 participants (Bowen 2009; Bowen 2014; Brewer 2009; Davis 2018; Witkiewitz 2014) provided results for comparisons with other treatment controls at post‐treatment and three studies with 409 participants (Bowen 2009; Bowen 2014; Davis 2018) provided results for comparisons with other treatment controls at follow‐up (4 to 10 months post‐treatment). The evidence suggests that MBIs reduce percentage of days with substance use slightly relative to other treatments at post‐treatment (Analysis 2.3; SMD = ‐0.21, 95% CI ‐0.45, 0.03) and follow‐up (Analysis 2.4; SMD = ‐0.39, 95% CI ‐0.96, 0.17); both results low‐certainty evidence.
2.3. Analysis.

Comparison 2: Mindfulness versus other treatments, Outcome 3: Percentage days with substance use at post‐treatment
2.4. Analysis.

Comparison 2: Mindfulness versus other treatments, Outcome 4: Percentage days with substance use at follow‐up
Consumed amount
One study with 25 participants (Davis 2013) provided results for comparisons with other treatment controls at post‐treatment. The evidence is very uncertain about the effect of MBIs relative to other treatments on consumed amount at post‐treatment (Analysis 2.5; SMD = ‐0.42, 95% CI ‐1.23 to 0.39).
2.5. Analysis.

Comparison 2: Mindfulness versus other treatments, Outcome 5: Consumed amount at post‐treatment
Adverse event rate
Two studies with 322 participants (Bowen 2014; Brewer 2009) provided results for comparisons with other treatment controls. No adverse events were reported in either condition. One study with 74 participants (Zemestani 2016) included an other treatment control but results were only available for the MBI condition. No adverse events were reported.
Secondary outcome measures
Craving intensity
Nine studies with 971 participants (Black 2019; Bowen 2009; Bowen 2014; Brewer 2009; Davis 2018; Garland 2010; Garland 2016; Shorey 2017; Zemestani 2016) provided results for comparisons with other treatment controls at post‐treatment. Results could not be pooled due to high heterogeneity (I2 ≥ 90%) (Analysis 2.6; SMD range = ‐1.43 to 1.00). Four studies with 415 participants (Bowen 2009; Bowen 2014; Davis 2018; Zemestani 2016) provided results for comparisons with other treatment controls at follow‐up (three to six months post‐treatment) (Analysis 2.7; SMD range = ‐2.07 to ‐0.14). Results could not be pooled due to high heterogeneity (I2 ≥ 90%).
2.6. Analysis.

Comparison 2: Mindfulness versus other treatments, Outcome 6: Craving intensity at post‐treatment
2.7. Analysis.

Comparison 2: Mindfulness versus other treatments, Outcome 7: Craving intensity at follow‐up
Treatment acceptability (attrition)
Fourteen studies with 1531 participants (Alegria 2019; Black 2019; Bowen 2009; Bowen 2014; Brewer 2009; Davis 2013; Garland 2010; Garland 2016; Garland 2019; Glasner 2017; Jenaabadi 2017; Lee 2011; Witkiewitz 2014; Zemestani 2016) provided results for comparisons with other treatment controls (Figure 5). MBIs result in little to no increase in study attrition relative to other treatment controls (Analysis 2.8; RR = 1.06 95% CI 0.89 to 1.26); high‐certainty evidence.
5.

2.8. Analysis.

Comparison 2: Mindfulness versus other treatments, Outcome 8: Treatment acceptability (attrition)
Serious adverse event rate
Two studies with 322 participants (Bowen 2014; Brewer 2009) provided results for comparisons with other treatment controls. No serious adverse events were reported in either condition. One study with 74 participants (Zemestani 2016) included an other treatment control but results were only available for the MBI condition. No serious adverse events were reported.
Sensitivity analyses
Sufficient studies to conduct a fixed‐effect model sensitivity analysis (>10 studies) were available only for treatment acceptability. Results indicated that MBIs result in little to no increase in study attrition relative to no treatment (Analysis 2.9; RR = 1.07 95% CI 0.91, 1.25).
2.9. Analysis.

Comparison 2: Mindfulness versus other treatments, Outcome 9: Treatment acceptability (attrition): sensitivity analysis (fixed effects model)
Discussion
Summary of main results
Mindfulness‐based interventions (MBIs) were compared with no treatment or other treatments on four primary outcomes (continuous abstinence rate, percentage of days with substance use, consumed amount, adverse event rate) and three secondary outcomes (craving intensity, treatment acceptability, serious adverse events).
Twenty‐four studies included a comparison between MBIs and no treatment. Relative to no treatment, the evidence was very uncertain about the effects of MBIs on all primary and secondary outcomes with the exception of treatment acceptability (differential attrition). MBIs resulted in little to no increase in study attrition relative to no treatment.
Nineteen studies included a comparison between MBI and another treatment. Relative to other treatments, MBIs may reduce percentage of days with substance use slightly at post‐treatment and follow‐up (4 to 10 months). However, the confidence intervals are compatible with both an improvement and little to no difference. MBIs result in little to no increase in study attrition relative to other treatments. The evidence is very uncertain regarding other outcomes including continuous abstinence rate, consumed amount, and craving intensity.
Four studies reported data on adverse events, with all reporting the absence of adverse events.
Overall completeness and applicability of evidence
Of the eligible 40 studies, 35 provided data usable for at least one meta‐analysis. However, no study included data on all outcome measures and many studies included data on only one outcome measure (typically acceptability in the form of differential attrition). Only one study (Foroushani 2019) reported eligible data in a form that did not allow estimation of an effect size. It is possible that other studies measured outcomes that would have been eligible, but data were not available. The limited number of published protocols or preregistrations makes it difficult to determine precisely how much unpublished data may exist.
The majority of studies were conducted in the USA with several also occurring in Iran as well as Thailand, China, Taiwan, and Spain. Studies were conducted between 2004 and 2020. Almost half of the studies included individuals with various substance use disorders (SUDs) with a large proportion focusing on opioids and several focusing on alcohol. Almost half of the studies required a formal SUD diagnosis of some kind for inclusion. Slightly less than half of the studies investigated mindfulness‐based relapse prevention (MBRP) or an adaptation of MBRP with several investigating mindfulness‐oriented recovery enhancement (MORE) or an adaptation of MORE. Most interventions were delivered in a group and were similar in duration to mindfulness‐based stress reduction (MBSR) (i.e. eight weeks). The samples were predominantly male. Given the diversity in study characteristics in terms of the samples and interventions, results of this review can theoretically be applied to MBIs for SUDs generally.
Limited information was available regarding the safety of MBIs for SUDs. However, no adverse events were reported in the four studies including this information.
Quality of the evidence
All studies were at high risk for performance bias due to an inability to blind participants engaging in a behavioral intervention. No studies assessed a substance use outcome objectively, so these outcomes were coded as at high risk for detection bias. Study attrition is by definition an objective (i.e. non‐self‐report) outcome, so treatment acceptability in the form of attrition was assessed as low risk for detection bias. Risk for selection bias due to randomization or allocation procedures was often unclear due to a lack of reporting. Risk of bias due to non‐equivalence at baseline was considered as another source of selection bias and was assessed as high in five studies. Risk of attrition bias was high in six studies. Risk of reporting bias was high in four studies and unclear in 24 studies.
Based on GRADE, the certainty of evidence was high for treatment acceptability (i.e. attrition). Certainty was low for percentage of days with substance use at post‐treatment and follow‐up for comparisons with other treatments due to inconsistency (sample size <400) and risk of bias (unblinded outcome assessment). For all other outcomes, certainty was very low due to inconsistency (sample size <400, 95% CI including both an appreciable benefit and an appreciable harm, 95% CI including both an effect not relevant to participants and an appreciable harm), risk of bias (unblinded outcome assessment), and/or inconsistency (I2 ≥ 90%).
Potential biases in the review process
Publication bias was not evaluated as 10 studies were not available for any of the primary outcomes. We sought to minimize publication bias through an extensive search process of both peer‐reviewed studies and dissertations, reviewing other recent meta‐analyses in this area, as well as contacting authors of ongoing randomized controlled trials (RCTs) of MBIs for SUDs. Nonetheless, publication bias remains a plausible source of bias, particularly given the frequency at which studies were found to be of unclear risk for reporting bias (i.e. selective reporting).
Due to the limited number of available studies for estimating substance use outcomes, we used the last available follow‐up for each study. While this was viewed as providing the most robust estimate of sustained effects by maximizing the amount of data used and follow‐up periods were typically within two to three months of each other, separating follow‐up data into other groupings (e.g. short‐term follow‐up, medium‐term follow‐up, long‐term follow‐up) may have resulted in different results.
Our review protocol prespecified our primary and secondary outcomes. The outcomes assessed did not include some potentially meaningful outcomes (e.g. negative effects of substance use). A future review that includes additional outcome measures may arrive at differing conclusions.
Agreements and disagreements with other studies or reviews
Three meta‐analyses have evaluated the effects of MBIs on SUD outcomes. Li 2017 conducted a meta‐analysis of 34 RCTs, 15 of which were included in the current review. However, Li 2017 included studies focused on smoking cessation as well as interventions that did not emphasize formal mindfulness meditation practice (e.g. Murphy 1986). Li 2017 also did not require a formal or informal SUD diagnosis for inclusion (e.g. Garland 2014a) and analyses collapsed across‐control condition types. Li 2017 reported greater reductions in substance use (standardized mean difference (SMD) = ‐0.33) and craving (SMD = ‐0.68) at post‐treatment for MBIs relative to control conditions.
Grant 2017 conducted a meta‐analysis of nine RCTs testing MBRP for substance abuse. Eight of the included studies were also included in the current review (Uhlig 2009 was not individually randomized). Grant 2017 also collapsed across‐control condition types. Grant 2017 reported that MBRP did not differ from controls on relapse to substance use (odds ratio([OR) = 0.72), frequency of use (SMD = 0.02), quantity of use (SMD = 0.26), or treatment dropout (OR = 0.81). Grant 2017 reported that MBRP was associated with larger reductions in withdrawal and craving symptoms (SMD = ‐0.13) and negative consequences (SMD = ‐0.23).
Goldberg 2018 conducted a meta‐analysis of 142 RCTs testing MBIs for various psychiatric conditions. Effects were estimates for SUDs at post‐treatment and follow‐up. Twelve of the included studies were also included in the current review. Although Goldberg 2018 reported results separated by control condition type, results were collapsed across SUD outcome measure types. Goldberg 2018 included outcomes that were not eligible for inclusion in the current review (e.g. Addiction Severity Index). Goldberg 2018 reported that MBIs did not differ from no treatment on SUD outcomes at post‐treatment (SMD = 0.35), showed larger improvement relative to other treatment controls at post‐treatment (SMD = 0.27), but not at longest follow‐up (SMD = 0.38).
Authors' conclusions
Implications for practice.
Results of this review provide low‐certainty evidence that mindfulness‐based interventions (MBIs) reduce percentage of days with substance use slightly relative to other treatments and high‐certainty evidence that MBIs result in little to no increase in attrition relative to no treatment or other treatments. The evidence for all other outcomes is very uncertain. Data on harm were minimal, although the available data showed no evidence of adverse effects. Indication of slight superiority to other treatments on one substance use outcome (percentage of days with substance use) may support inclusion of MBIs within the available treatment options for substance use disorders SUDs.
Implications for research.
With the exception of estimates related to treatment acceptability (i.e. differential attrition), evidence related to substance use outcomes were of low or very low certainty due to imprecision and inconsistency. It is possible that an updated review with additional studies could result in more reliable estimates of treatment effects.
One of the most notable limitations of the current review is that few studies provided data necessary for estimating substance use outcomes. While it is certainly worthwhile to examine other outcomes within this population (e.g. depression, quality of life), assessing and reporting substance use (e.g. continuous abstinence, percentage of days used, consumed amount) will allow more rigorous evaluation of the effects of MBIs on these key dimensions. It would also be worth examining effects on other dimensions of substance use (e.g. negative effects of substance use). Future studies could more consistently report study design features (e.g. randomization and allocation procedure) and employ procedures to minimize risk of bias (e.g. blind outcome assessment, preregistration). It could be useful in a future and more highly‐powered review to examine moderators such as MBI type (e.g. mindfulness‐based relapse prevention (MBRP), mindfulness‐oriented recovery enhancement (MORE)), substance (e.g. various SUDs, alcohol), and country (e.g. USA, Iran) as well as patient‐level demographic characteristics (e.g. gender, race/ethnicity). Such analyses could determine whether effects vary along these dimensions and could guide decisions regarding when MBIs may or may not be indicated. Efforts to understand the efficacy of MBIs specifically in vulnerable populations (e.g. racial/ethnic minorities) is warranted. Larger RCTs and consistent reporting of adverse effects will also strengthen the certainty of evidence related to the use of MBIs for SUDs.
History
Protocol first published: Issue 6, 2015
Acknowledgements
We thank Zuzana Mitrova and the team of the Cochrane Drugs and Alcohol Group (CDAG, Rome, Italy) for their support in the preparation and conduction of this review. We would also like to thank the staff at Covidence and Cochrane support for their assistance. We thank Marica Ferri for helpful comments on a draft of this study. We thank Sin U Lam for her assistance translating.
Appendices
Appendix 1. CDAG Specialised Register search
April 26, 2021 (215hits)
(acceptance or meditation or mindful* or Vipassana or zen or yoga or yogic or relaxation):ti,ab,kw,xin
Appendix 2. CENTRAL search strategy
CENTRAL (via onlinelibrary.wiley.com)
Issue 3, 2021 (877 hits)
#1 MeSH descriptor: [Substance‐Related Disorders] explode all trees
#2 MeSH descriptor: [Alcohol Drinking] explode all trees
#3 MeSH descriptor: [Amphetamines] explode all trees
#4 MeSH descriptor: [Cannabis] explode all trees
#5 MeSH descriptor: [Cocaine] explode all trees
#6 MeSH descriptor: [Designer Drugs] explode all trees
#7 MeSH descriptor: [Heroin] explode all trees
#8 MeSH descriptor: [Methamphetamine] explode all trees
#9 MeSH descriptor: [Narcotics] explode all trees
#10 MeSH descriptor: [Street Drugs] explode all trees
#11 (alcohol or amphetamine* or drug* or polydrug or substance or cannabis or cocaine or "hash oil*" or hashish or heroin or lsd or marihuana or marijuana or methadone or mdma or morphine or ecstasy or methamphetamine* or narcotics or opioid* or opiate* or opium):ti,ab
#12 (abstin*OR abstain* or abuse* or addict* or dependen* or misuse or overdose or withdrawal* or disorder*):ti,ab,kw
#13 #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11
#14 #12 and #13
#15 #1 or #2 or #14
#16 MeSH descriptor: [Mindfulness] explode all trees
#17 MeSH descriptor: [Meditation] explode all trees
#18 (acceptance or meditation or mindful* or Vipassana or zen or yoga or yogic or relaxation):ti,ab,kw
#19 (breathing near/3 technique):ti,ab,kw
#20 (breathing near/3 exercise):ti,ab,kw
#21 dialectical next behavior next therapy
#22 DBT:ti,ab
#23 (acceptance near/3 therapy):ti,ab
#24 #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23
#25 #14 and #24
Appendix 3. PubMed search strategy
PubMed
April 26, 2021 (1178 hits)
Substance‐Related Disorders[MeSH]
Alcohol Drinking[MeSH]
Amphetamines[MeSH]
Cannabis[MeSH]
Cocaine[MeSH]
Designer Drugs[MeSH]
Heroin[MeSH]
Methamphetamine[MeSH]
Narcotics[MeSH]
Street Drugs[MeSH]
(alcohol[tiab] OR amphetamine*[tiab] OR drug*[tiab] OR polydrug[tiab] OR substance[tiab] OR cannabis[tiab] OR cocaine[tiab] OR "hash oil*"[tiab] OR hashish[tiab] OR heroin[tiab] OR lsd[tiab] OR marihuana[tiab] OR marijuana[tiab] OR methadone[tiab] OR mdma[tiab] OR morphine[tiab] OR ecstasy[tiab] OR methamphetamine*[tiab] OR narcotics[tiab] OR opioid*[tiab] OR opiate*[tiab] OR opium[tiab])
abstin*[tiab] OR abstain*[tiab] OR abuse*[tiab] OR addict*[tiab] OR dependen*[tiab] OR misuse[tiab] OR overdose[tiab] OR withdrawal*[tiab] OR disorder*[tiab]
#3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11
#12 AND #13
#1 OR #2 OR #14
Mindfulness[MeSH]
Meditation[MeSH]
acceptance[tiab] OR meditation[tiab] OR mindfulmindful*[tiab] OR Vipassana[tiab] OR zen[tiab] OR yoga[tiab] OR yogic[tiab] OR relaxation[tiab] OR "breathing technique"[tiab] OR "breathing exercise"[tiab]
"dialectical behavior therapy" OR DBT[tiab]
"acceptance and commitment therapy" OR ACT[tiab]
#16 OR #17 OR #18 OR #19 OR #20
randomized controlled trial [pt]
controlled clinical trial [pt]
randomized [tiab]
placebo [tiab]
drug therapy [sh]
randomly [tiab]
trial [tiab]
groups [tiab]
groups [tiab]
#22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30
(animals [mh] NOT humans [mh])
#31 NOT #32
#15 AND #21 AND #33
Appendix 4. EMBASE search strategy
Embase (OVID)
April 26, 2021 (1204 hits)
1 exp addiction/
2 exp drug abuse/
3 exp alcohol abuse/
4 ((alcohol or amphetamine* or drug* or polydrug or substance or cannabis or cocaine or hashish or heroin or lsd or marihuana or marijuana or methadone or mdma or morphine or ecstasy or methamphetamine* or narcotics or opioid* or opiate* or opium) adj5 (abstin* or abstain* or abuse* or addict* or dependen* or misuse or overdose or withdrawal* or disorder*)).ti,ab.
5 1 or 2 or 3 or 4
6 exp mindfulness/
7 exp meditation/
8 acceptance.ab,ti.
9 meditation.ab,ti.
10 "mindful*".ab,ti.
11 vipassana.ab,ti.
12 zen.ab,ti.
13 (yoga or yogic).ab,ti.
14 relaxation.ab,ti.
15 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14
16 5 and 15
17 exp clinical trial/
18 (clin$ adj3 trial$).tw.
19 exp double blind procedure/
20 exp controlled clinical trial/
21 (placebo or assign* or allocat* or volunteer* or random* or factorial* or crossover).ti,ab.
22 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw.
23 17 or 18 or 19 or 20 or 21 or 22
24 16 and 23
Appendix 5. WOS search strategy
Web of Science (via Web of Knowledge)
April 26, 2021 (699 hits)
Indexes=SCI‐EXPANDED, SSCI, A&HCI, ESCI Timespan=All years
TS=((alcohol OR amphetamine* OR drug* OR polydrug OR substance OR cannabis OR cocaine OR "hash oil*" OR hashish OR heroin OR lsd OR marihuana OR marijuana OR methadone OR mdma OR morphine OR ecstasy OR methamphetamine* OR narcotics OR opioid* OR opiate* OR opium) NEAR/6 (abstin* OR abstain* OR abuse* OR addict* OR dependen* OR misuse OR overdose OR withdrawal* OR disorder*))
TS=(acceptance OR meditation OR mindful* OR Vipassana OR zen OR yoga OR yogic OR relaxation OR "breathing technique" OR "breathing exercise")
TS=((randomi* OR randomly OR trial*))
#1 AND #2 AND #3
Appendix 6. CINAHL search strategy
CINAHL (via EBSCO)
April 26, 2021(637 hits)
S38 S30 AND S36 AND S37
S37 S8 OR S18
S36 S31 OR S32 OR S33 OR S34 OR S35
S35 TX "acceptance and commitment therapy"
S34 TX "dialectical behavior therapy"
S33 TI (acceptance or meditation or mindfulmindful* or Vipassana or zen or yoga or yogic or relaxation or "breathing technique" or "breathing exercise") or AB(acceptance or meditation or mindfulmindful* or Vipassana or zen or yoga or yogic or relaxation or "breathing technique" or "breathing exercise")
S32 (MH "Meditation")
S31 (MH "Mindfulness")
S30 S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29
S29 MH "Quantitative Studies"
S28 TI placebo* or AB placebo*
S27 MH "Placebos"
S26 TI random* allocat* or AB random* allocat*
S25 MH "Random Assignment"
S24 TI randomi?ed control* trial* or AB randomi?ed control* trial*
S23 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* )
S22 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* )
S21 TI clinic* N1 trial* or AB clinic* N1 trial*
S20 PT Clinical trial
S19 MH "Clinical Trials+"
S18 S9 AND S17
S17 S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16
S16 (MH "Ketamine")
S15 (MH "Amphetamines+")
S14 (MH "Methadone")
S13 (MH "Hallucinogens+")
S12 MH "Designer Drugs"
S11 MH "Narcotics"
S10 TX(polydrug or alcohol or opioid or opiate or opium or hallucinogen or cocaine or benzodiazepine* or amphetamine*or “anti‐anxiety‐agents” or barbiturate* or “lysergic acid” or ketamine or cannabis or marihuana or marijuana or hashish or inhalant* or solvent or steroid* or methadone or morphine)
S9 S5 or S6 or S7
S8 S1 or S2 or S3 or S4
S7 TX(use* N2 drug) or TX(use* N2 disorder) or TX(use* N2 illicit)
S6 TX(use* N2 drug) or TX(use* N2 disorder) or TX(use* N2 illicit)
S5 TX(addict* OR overdos* OR intoxicat* OR abstin* OR abstain OR withdraw* OR abus* OR misus* OR disorder* OR dependen*)
S4 TX(substance N3 addict*) or TX(substance N3 dependen*) or TX(substance N3 abuse*) or TX(substance N3 misus*)
S3 TX(drug N3 addict*) or TX(drug N3 dependen*) or TX(drug N3 abuse*) or TX(drug N3 misus*)
S2 (MH "Psychoses, Substance‐Induced+")
S1 (MH "Substance Use Disorders+")
Appendix 7. Critieria for risk of bias assessment
| Item | Judgment | Description |
| 1. Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process such as: random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization |
| High risk | The investigators describe a non‐random component in the sequence generation process such as: odd or even date of birth; date (or day) of admission; hospital or clinic record number; alternation; judgement of the clinician; results of a laboratory test or a series of tests; availability of the intervention | |
| Unclear risk | Insufficient information about the sequence generation process to permit judgement of low or high risk | |
| 2. Allocation concealment (selection bias) | Low risk | Investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled, randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes. |
| High risk | Investigators enrolling participants could possibly foresee assignments because one of the following method was used: open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear risk | Insufficient information to permit judgement of low or high risk This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement | |
| 3. Blinding of participants and providers (performance bias) Objective outcomes |
Low risk | No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk | No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk; | |
| 4. Blinding of participants and providers (performance bias) Subjective outcomes |
Low risk | Blinding of participants and providers ensured and unlikely that the blinding could have been broken; |
| High risk | No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk; | |
| 5. Blinding of outcome assessor (detection bias) Objective outcomes |
Low risk | No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken |
| High risk | No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; Blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk; | |
| 6.blinding of outcome assessor (detection bias) Subjective outcomes |
Low risk | Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken |
| High risk | No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; Blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk; | |
| 7. Incomplete outcome data (attrition bias) For all outcomes except retention in treatment or drop out |
Low risk | No missing outcome data; Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; Missing data have been imputed using appropriate methods; All randomised patients are reported/analysed in the group they were allocated to by randomisation irrespective of non‐compliance and co‐interventions (intention to treat) |
| High risk | Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk (e.g. number randomised not stated, no reasons for missing data provided; number of drop out not reported for each group); | |
| 8 Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk | Not all of the study’s pre‐specified primary outcomes have been reported; One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; The study report fails to include results for a key outcome that would be expected to have been reported for such a study. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk | |
| 9. Other bias (1): equivalence of baseline characteristics | Low risk | The testing of baseline age, gender and baseline drinking (drinking amount, frequency, years of problematic drinking) fulfils at least one of the following conditions: ‐ baseline equivalence between groups was shown for age, gender AND at least one indicator of baseline drinking (e.g. sleep induction, sleep maintenance, insomnia duration) ‐ baseline differences between groups were demonstrated, but adequately controlled in the statistical analyses |
| High risk | Differences between groups in one or more relevant baseline characteristics became evident, but were not controlled in the statistical analyses | |
| Unclear risk | Insufficient reporting of baseline equivalence or its testing to permit judgement of ‘Yes’ or ‘No’ | |
| 9. Other bias (2): equivalence of treatment utilization | Low risk | The equivalence of treatment utilization in the intervention and control group was tested and confirmed |
| High risk | Differences in treatment utilization between the intervention and control group became evident and were not controlled in the statistical analyses | |
| Unclear risk | Insufficient reporting of treatment attendance to permit judgement of ‘Yes’ or ‘No’ |
Appendix 8. PsycINFO search strategy
April 26, 2021 (745)
1 exp exp "substance use disorder"/
2 exp Drug Addiction/
3 exp alcoholism/
4 ((alcohol or amphetamine* or drug* or polydrug or substance or cannabis or cocaine or hashish or heroin or lsd or marihuana or marijuana or methadone or mdma or morphine or ecstasy or methamphetamine* or narcotics or opioid* or opiate* or opium) adj5 (abstin* or abstain* or abuse* or addict* or dependen* or misuse or overdose or withdrawal* or disorder*)).ti,ab.
5 1 or 2 or 3 or 4
6 exp MINDFULNESS/
7 exp MEDITATION/
8 acceptance.ab,ti.
9 meditation.ab,ti.
10 mindful*.ab,ti.
11 vipassana.ab,ti.
12 zen.ab,ti.
13 (yoga or yogic).ab,ti.
14 relaxation.ab,ti.
15 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14
16 5 and 15
17 ("double‐blind" or random* or control).tw.
18 16 and 17
Data and analyses
Comparison 1. Mindfulness versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Continuous abstinence at post‐treatment | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.44, 2.14] |
| 1.2 Continuous abstinence at follow‐up | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.54, 2.01] |
| 1.3 Percentage days with substance use at post‐treatment | 4 | 248 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.37, 0.47] |
| 1.4 Percentage days with substance use at follow‐up | 3 | 167 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.12, 0.54] |
| 1.5 Consumed amount at post‐treatment | 3 | 221 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.31, 0.52] |
| 1.6 Consumed amount at follow‐up | 2 | 142 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [0.00, 0.66] |
| 1.7 Craving intensity at post‐treatment | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.8 Treatment acceptability (attrition) | 21 | 1087 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.77, 1.40] |
| 1.9 Treatment acceptability (attrition): sensitivity analysis (fixed‐effects model) | 21 | 1087 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.84, 1.50] |
Comparison 2. Mindfulness versus other treatments.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Continuous abstinence at post‐treatment | 1 | 286 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.45, 1.44] |
| 2.2 Continuous abstinence at follow‐up | 1 | 286 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.28, 1.16] |
| 2.3 Percentage days with substance use at post‐treatment | 5 | 523 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.45, 0.03] |
| 2.4 Percentage days with substance use at follow‐up | 3 | 409 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.96, 0.17] |
| 2.5 Consumed amount at post‐treatment | 1 | 25 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐1.23, 0.39] |
| 2.6 Craving intensity at post‐treatment | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.7 Craving intensity at follow‐up | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.8 Treatment acceptability (attrition) | 14 | 1531 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.89, 1.26] |
| 2.9 Treatment acceptability (attrition): sensitivity analysis (fixed effects model) | 14 | 1531 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.91, 1.25] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abed 2019.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: opioids Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: undergoing MMT, having at least 2 lapses during MMT Excluded criteria: none Number missing: 5 Reason missing: left the study Baseline differences: no differences Age: 36.6 Percent female: 0% Race/Ethnicity: 100% Iranian |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1: no intervention
|
|
| Outcomes |
Desire to use
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: n/a Country: Iran Setting: not residential Authors name: Abed Institution: Islamic Azad University Email: mohammadrezaabed777@gmail.com Address: Department of Psychology, Najafabad Branch, Islamnic Azad University, Najafabad, Iran COI: none Diagnosis tool: received methadone maintenance treatment Diagnosis type: informal Funding: none reported Journal: Journal of Substance Abuse Publication type: published report Secondary publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Blinding of outcome assessment (detection bias) All non‐attrition outcomes | High risk | Self‐report outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition and higher attrition in MBI, used completer analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, no statement of primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | quote: "no differences in pre‐test scores" p. 640 |
Alegria 2019.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: various substances Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: elevated mental health concerns and substance misuse, 18 to 70 years old, self‐identified as Latino, not receiving or about to receive specialty behavioral health services in the previous 3 month or upcoming month Excluded criteria: lacked capacity to consent, reported imminent suicidal ideation Number missing: 83 Reason missing: not reported Baseline differences: no differences Age: 33.9 Percent female: 51% Race/Ethnicity: 100% Latin |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1
|
|
| Outcomes |
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: n/a Country: USA and Spain Setting: not residential Authors name: Alegria Institution: Harvard Medical School Email: malegria@mgh.harvard.edu Address: Department of Medicine and Psychiatry, Harvard Medical School, Boston, Massachusetts COI: none Diagnosis tool: elevated symptoms on AC‐OK screener Diagnosis type: informal Funding: this study was funded in part by grant R01DA034952 from NIDA of the National Institutes of Health; grant R01MH100155‐01S1 from NIMH; and grants ISCII PI13/02200 and PI16/01852 from Instituto de Salud Carlos III, grant 20151073 from Delegación del Gobierno para el Plan Nacional de Drogas, and grant LSRG‐1‐ 005‐16 from the American Foundation for Suicide Prevention (Dr Baca‐García). Journal: JAMA Network Open Publication type: published report Secondary publications: yes |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified block‐randomization |
| Allocation concealment (selection bias) | Low risk | Blinded project coordinator randomized after baseline |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Research assistant blinded but not participants |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar attrition rates, reasons not given but ITT analyses used |
| Selective reporting (reporting bias) | Low risk | Protocol available and reported primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | No baseline differences |
Alizadehgoradel 2019.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: methamphetamines Baseline characteristics Mindfulness‐based intervention
Control 1
Control 2
Overall
Included criteria: (1) age range of 18 to 21 years, (2) diagnosis a methamphetamine use disorder based on DSM‐V criteria including at least 12‐month history of methamphetamine use before beginning of the experiment, (3) lack of other substance‐related use disorders except for tobacco smoking, as verified by a urine drug screen, (4) lack of other psychiatric disorders except for substance use disorder assessed via a Structured Clinical Interview for DSM‐5 Disorders by an experienced psychiatrist of rehabilitation center for addiction, (5) and not to be on psychotropic medications during the study. Excluded criteria: none reported Number missing: 5 Reason missing: MBSAT: 2 Discontinued MBSAT. Control: 3 Discontinued MBSAT. Baseline differences: no demographic differences, not reported for outcomes Age: 19.5 Percent female: 0% Race/Ethnicity: Iranian |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1: No treatment control
|
|
| Outcomes |
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: none Country: Iran Setting: unclear Comments: Authors name: Jaber Alizadehgoradel Institution: Shahid Beheshti University Email: j_alizadehgoradel@sbu.ac.ir Address: Department of Clinical and Health Psychology, Faculty of Psychology and Educational Sciences, Shahid Beheshti University, P.O. Box: 1983963113, 193954716, Tehran, Iran COI: The authors declare that they have no conflict of interest. Diagnosis tool: DSM‐5 Diagnosis type: formal Funding: none Journal: Neurology Psychiatry and Brain Research Publication type: published report Secondary Publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for dropout not reported, although amount was similar between groups |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, no statement of primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | No demographic differences (p. 16) and figure suggests no differences on outcomes at baseline |
Alterman 2004.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: various substances Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: residents at recovery house Excluded criteria: patients with a psychiatric diagnosis of schizophrenia or borderline personality disorder were excluded Number missing: 6 Reason missing: not reported Baseline differences: mindfulness group higher addiction severity, more recent days of use and years of use, higher ASI psychiatric composite score, medical composite score Age: 36.5 Percent female: 55% Race/Ethnicity: 58.1% were African American; other 41.9% were Caucasian |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1: no treatment control
|
|
| Outcomes |
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: none reported Country: USA Setting: residential Authors name: Arthur I. Alterman Institution: University of Pennsylvania Email: alterman@mail.trc.upenn.edu Address: Treatment and Evaluation Center, 3440 Market Street, Suite 370, Philadelphia, PA 19104, USA COI: not reported Diagnosis tool: receiving substance use treatment at recovery house Diagnosis type: informal Funding: none reported Journal: Journal of Substance Use Publication type: published report Secondary publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number sequence, apportioning subjects to the experimental and control conditions in a 3:2 ratio, was employed |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for dropout not reported, although amount was similar between groups |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, no statement of primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | High risk | Baseline differences not controlled in analyses |
Asl 2014a.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: opioids Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: receiving treatment, BDI‐II score ≥ 14 Excluded criteria: none Number missing: 2 Reason missing: failed to attend 2 sessions Baseline differences: no differences at baseline Age: 29.5 Percent female: 0% Race/Ethnicity: 100% Iranian |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1: No treatment control
|
|
| Outcomes |
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: none reported Country: Turkey, Iran Setting: unclear Authors name: Navidreza Hosseinzadeh Asl Institution: Hacettepe University Email: navidrha@yahoo.com Address: Navidreza Hosseinzadeh Asl, PhD student, Hacettepe University, Ankara, Turkey. COI: not reported Diagnosis tool: receiving treatment through addiction treatment center Diagnosis type: informal Funding: none reported Journal: Archives of Psychiatric Nursing Publication type: published report Secondary publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: attrition in mindfulness intervention only |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol available, no statement of primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | Judgement comment: no differences on BDI (p. 316) or SF‐36 (p. 2) at baseline |
Asl 2014b.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: opioids Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: receiving treatment, BDI‐II score ≥ 14 Excluded criteria: none Number missing: 4 Reason missing: did not continue Baseline differences: no differences on SF‐36 Age: 36.8 Percent female: 0% Race/Ethnicity: 100% Iranian |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1: No treatment control
|
|
| Outcomes |
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: Addiction Treatment Clinic of Milad Country: Iran Setting: unclear Authors name: Navid Reza Hosseinzadeh Asl Institution: Hacettepe University Email: navidrha@yahoo.com Address: Institute of Social Sciences, Hacettepe University, Ankara, Turkey COI: not reported Diagnosis tool: receiving treatment through addiction treatment center Diagnosis type: informal Funding: Addiction Treatment Clinic of Milad Journal: Iranian Red Crescent Medical Journal Publication type: published report Secondary publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: it appears there was no dropout, although not explicitly stated |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol available, no statement of primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | Judgement comment: no baseline differences on SF‐36 (p. 2) |
Bein 2015.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: various substances Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: participants were English‐speaking patients who experienced at least one cluster of criteria for PTSD. Participants also met full criteria for substance dependence to at least one substance in the past year. Excluded criteria: current psychotic disorder Number missing: 0 Reason missing: n/a Baseline differences: not reported Age: 50.1 Percent female: 0% Race/Ethnicity: 62.5% White, 25% African American, 12.5% mixed ethnicity |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1: no treatment control
|
|
| Outcomes |
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: none reported Country: USA Setting: residential Authors name: Zachary Bein Institution: Alliant International University Los Angeles Email: not reported Address: not reported COI: not reported Diagnosis tool: met full criteria for substance dependence Diagnosis type: formal Funding: none reported Journal: dissertation Publication type: dissertation Secondary publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: no attrition |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol available, no statement of primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | Unclear risk | Judgement comment: not reported |
Bevan 2012.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: various substances Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: receiving treatment at inpatient facility Excluded criteria: psychotic symptoms Number missing: 13 Reason missing: not reported Baseline differences: waitlist more likely to be employed Age: 42 Percent female: 42.86% Race/Ethnicity: 97% White, 1% Black, 1% Asian, 1% unspecified |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1: no treatment control
|
|
| Outcomes |
Alcohol Craving Questionnaire Revised
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: none reported Country: USA Setting: residential Authors name: Edward Bevan Institution: Marywood University Email: not reported Address: not reported COI: not reported Diagnosis tool: receiving treatment at inpatient facility Diagnosis type: informal Funding: none reported Journal: dissertation Publication type: dissertation Secondary publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "table of random numbers" (p. 31) |
| Allocation concealment (selection bias) | Low risk | Blind randomizer |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Blinding of outcome assessment (detection bias) All non‐attrition outcomes | High risk | Self‐report measure |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Group assignment for dropout not reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, no statement of primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | Treatment‐as‐usual control |
Black 2019.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: various substances Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: client at site, female, age 18 to 65, diagnosed with SUD in clinical record, fluent in English Excluded criteria: inability to comprehend or sign consent, cognitive impairment, untreated psychotic disorder or severe chronic mental health conditions, suicidality during the prior 30 days, current prisoner, more than 6 months pregnant, not willing to sign a HIPAA form or be audio‐recorded Number missing: 41 Reason missing: missed first class, not found, passive decline, prison Baseline differences: none Age: 32.5 Percent female: 100% Race/Ethnicity: 58% Latina, 19.5% non‐Hispanic Black, 21% non‐Hispanic White, 1.5% other |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1
|
|
| Outcomes |
Penn Alcohol Craving Scale
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: NIDA, NIAAA Country: USA Setting: residential Authors name: David S. Black Institution: University of Southern California Email: davidbla@usc.edu Address: Keck School of Medicine of the University of Southern California, Los Angeles, CA, 90032. COI: none Diagnosis tool: DSM‐5 Diagnosis type: formal Funding: NIDA, NIAAA Journal: Behaviour Research and Therapy Publication type: published report Secondary publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: urn randomization (p. 4) |
| Allocation concealment (selection bias) | Low risk | Judgement comment: concealed until first group meeting |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding of participants, but blinding of staff |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Blinding of outcome assessment (detection bias) All non‐attrition outcomes | High risk | Self‐report measure |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: similar attrition across groups |
| Selective reporting (reporting bias) | High risk | Judgement comment: not all pre‐specified outcomes reported |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | Judgement comment: no statistically significant differences at baseline |
Bowen 2009.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: various substances Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: 18 to 70 years old, fluent in English, completed intensive outpatient or inpatient treatment in previous 2 weeks, medically cleared for participation Excluded criteria: psychosis, dementia, imminent suicide risk, withdrawal risk, need for more intensive treatment Number missing: 65.52 Reason missing: not reported Baseline differences: MBRP higher proportion White, no other demographic or outcome differences Age: 40.5 Percent female: 32.3% Race/Ethnicity: 51.8% White, 28.6% African American, 15.3% multiracial, 7.7% Native American |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1
|
|
| Outcomes |
AOD days
Penn Alcohol Craving Scale
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: none reported Country: USA Setting: not residential Authors name: Sarah Bowen Institution: University of Washington Email: swbowen@u.washington.edu Address: Department of Psychology, University of Washington, Box 351629, Seattle, WA 98195‐1525, USA COI: not reported Diagnosis tool: received substance abuse treatment Diagnosis type: informal Funding: none reported Journal: Substance Abuse Publication type: published report Secondary publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: quote: "computerized random number generator" p. 298 |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Blinding of outcome assessment (detection bias) All non‐attrition outcomes | High risk | Self‐report |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: no reasons given although attrition did not differ between groups |
| Selective reporting (reporting bias) | Low risk | Judgement comment: clear definition of primary outcomes |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | Judgement comment: differences in race/ethnicity, but controlled for in analyses (p.300) |
Bowen 2014.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: various substances Baseline characteristics Mindfulness‐based intervention
Control 1
Control 2
Overall
Included criteria: 18+, English fluency, medical clearance, ability to attend sessions, agreement to random assignment and follow‐up assessment, completion of initial intensive outpatient or inpatient care Excluded criteria: current psychotic disorder, dementia, suicidality, imminent danger to others, or participation in previous MBRP trials. Number missing: 53 Reason missing: withdrew from study, enrolled as inpatient, incarcerated, refused, unable to contact, died Baseline differences: TAU reported lower severity on SDS Age: 38.4 Percent female: 29.7 Race/Ethnicity: 64% white, 24% black, 12% Hispanic |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1
Control 2
|
|
| Outcomes |
Any drug use
Any heavy drinking
Drug use days
Heavy drinking days
Penn Alcohol Craving Scale
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: NIDA, NIAAA, Recovery Centers of King County Country: USA Setting: residential Comments: Authors name: Sarah Bowen Institution: University of Washington Email: swbowen @uw.edu Address: Center for the Study of Health and Risk Behaviors, University of Washington, 1100 NE 45th St, Ste 300, Seattle, WA 98105 COI: Drs Bowen, Grow, and Chawla conduct MBRP training for which they receive monetary incentives, although the findings presented in this article have not yet been presented as part of these trainings. No other disclosures were reported. Diagnosis tool: received inpatient alcohol use disorder treatment Diagnosis type: informat Funding: NIDA, NIAAA, Recovery Centers of King County Journal: JAMA Psychiatry Publication type: published report Secondary Publications: Carroll et al. (2017); Roos et al. (2019); Roos et al. (2017); Greenfield et al. (2018); Hsiao et al. (2018) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Blinding of outcome assessment (detection bias) All non‐attrition outcomes | High risk | Self‐report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: missing outcome data roughly balanced with similar reasons for attrition, used maximum likelihood estimation |
| Selective reporting (reporting bias) | Low risk | Judgement comment: protocol available and reported primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | Judgement comment: differences on some measures at baseline but controlled in analyses |
Brewer 2009.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: alcohol and/or cocaine use disorder Baseline characteristics Mindfulness‐based intervention
Control 1
Control 2
Overall
Included criteria: English speaking, DSM‐IV criteria for alcohol or cocaine dependence Excluded criteria: younger than 18 years old, suicidal, homicidal, current psychotic disorder, cognitive disorder precluding completion of treatment study, on beta‐blocker Number missing: 22 Reason missing: not reported Baseline differences: fewer married in mindfulness group, no other baseline differences Age: 38.2 Percent female: 28% Race/Ethnicity: 64% White, 24% Black, 12% Hispanic |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1
|
|
| Outcomes |
Percentage days alcohol use
Percentage days cocaine use
Craving during stress provocation
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: NIDA, US VA New England MIRECC, Varela Grant from Mind and Life Institute Country: USA Authors name: Judson Brewer Institution: Yale University Email: judson.brewer@yale.edu Address: Judson A. Brewer, MD, PhD, VA Connecticut Healthcare System, 950 Campbell Avenue, Building 36, Room 142, West Haven, CT, 06516, USA COI: none Diagnosis tool: DSM‐IV criteria for alcohol and/or cocaine abuse or dependence in the past year Diagnosis type: formal Funding: NIDA, US VA New England MIRECC, Varela Grant from Mind and Life Institute Journal: Substance Abuse Publication type: published report Secondary publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: random numbers used |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Blinding of outcome assessment (detection bias) All non‐attrition outcomes | High risk | Self‐report |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: attrition higher in mindfulness; completer analysis used |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | Judgement comment: no significant differences except marital status |
Brown 2017.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: various substances Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: recent report to or open case with child protective services, case low‐to‐moderate risk and involved parental substance use as presenting problem, children remained in the home or parents had weekly visitation, parent English speaking Excluded criteria: case involved sexual abuse, family in extreme crisis Number missing: 7 Reason missing: "too much on plate", moved, personal life changes, unreachable Baseline differences: none Age: 31 Percent female: 81% Race/Ethnicity: 71.4% White, 14.3% Latinx, 9.5% Black, 4.5% other |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1: No treatment control
|
|
| Outcomes |
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: none reported Country: USA Setting: not residential Authors name: Samantha Marie Brown Institution: University of Denver Email: not available Address: not available COI: not reported Diagnosis tool: Mini International Neuropsychiatry Interview; Simple Screening Instrument for Substance Abuse Diagnosis type: formal Funding: none reported Journal: dissertation Publication type: dissertation Secondary Publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: drop out rates and reasons similar across groups (p. 41) |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol available, no statement of primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | Judgement comment: no baseline differences |
Davis 2013.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: alcohol Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: 18 to 29, smoke 10+ cigarettes per day, 5+ alcohol binges per month Excluded criteria: alcohol dependence; diagnosis of schizophrenia, bipolar or delusional disorder; CO breath testing showed CO level of 10 ppm or less Number missing: 30 Reason missing: technical college exams and vacations Baseline differences: none Age: 21.9 Percent female: 29.1% Race/Ethnicity: 90.9% White, 5.5% Latino/Hispanic, 1.8% African American, 1.8% American Indian |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1
|
|
| Outcomes |
Drinks per week
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: NIDA Country: USA Setting: not residential Comments: Authors name: James M. Davis Institution: University of Wisconsin ‐ Madison Email: jjamesdavis@hotmail.com Address: Center for Tobacco Research and Intervention, University of Wisconsin School of Medicine and Public Health, 1930 Monroe Street, Suite 200, 53711 Madison, WI, USA COI: none Diagnosis tool: 5+ alcohol binges per month (5+ drinks per day for males, 4+ for females) Diagnosis type: informal Funding: NIDA Journal: BMC Complementary and Alternative Medicine Publicationptype: published report Secondary Publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: used quote: "random draws" (p. 3) |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: descriptions provided to decrease control group awareness they were assigned to control condition, but no personnel blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Blinding of outcome assessment (detection bias) All non‐attrition outcomes | High risk | Self‐report |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: drop out rates similar but no reasons given |
| Selective reporting (reporting bias) | Low risk | Judgement comment: protocol available and all outcomes included |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | Judgement comment: no baseline differences (p. 6) |
Davis 2018.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: various substances Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: 18 to 29, English proficiency, clear cognitive ability to understand and provide consent Excluded criteria: none Number missing: 14 Reason missing: removed from facility, left facility on own volition, incarcerated, unable to contact Baseline differences: none Age: 25.3 Percent female: 35% Race/Ethnicity: 91.3% White, 7.5% African American, 1.25% Native American |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1
|
|
| Outcomes |
Substance Frequency Scale
Craving Scale
|
|
| Identification |
Sponsorship source: National Institute on Drug Abuse (Grant num.: 1R36DA041538; PI: Davis), Fahs‐Beck Fund for Research and Experimentation (PI: Davis) (082876), Campus Research Board (Grant num.: RB15434; PI: Roberts) Country: USA Setting: residential Authors name: Jordan Davis Institution: University of Southern California Email: jordanpd@usc.edu Address: 669 W 34th Street, Los Angeles, CA 90089, United States. COI: Diagnosis tool: resident at treatment center Diagnosis type: informal Funding: National Institute on Drug Abuse, Fahs‐Beck Fund for Research and Experimentation, Campus Research Board Journal: Journal of Substance Abuse Treatment Publication type: published report Secondary publications: Davis et al. (2019) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: quote: "allocation was performed randomly by an online clinical trial randomizer" p. 39 |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: participants not blinded |
| Blinding of outcome assessment (detection bias) All non‐attrition outcomes | High risk | Self‐report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: drop out rates and reasons similar across groups (p. 41) |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol available; no statement of primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | Judgement comment: no baseline differences (p. 39) |
de Dios 2012.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: marijuana Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: 18 to 29 years old; live within 20 miles of Providence, RI; plan to stay in area for next 3 months; speak English; endorse desire to quit or reduce marijuana use; use marijuana to relax, relieve anxiety, calm down Excluded criteria: severe psychiatric disorders; high use of alcohol or other drugs; past month use of cocaine, heroin, methamphetamines, or other drugs Number missing: 7 Reason missing: not reported Baseline differences: none Age: 23.03 Percent female: 100% Race/Ethnicity: 50% White, 32.4% African American, 5.9% Hispanic, 11.8% other |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1: No treatment control
|
|
| Outcomes |
Marijuana use days
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: NIDA Country: USA Setting: not residential Authors name: Marcel A. de Dios Institution: Brown University Email: mdedios@butler.org Address: Butler Hospital, Providence, RI 02906, USA COI: not reported Diagnosis tool: Used marijuana three or more times in the past month Diagnosis type: informal Funding: NIDA Journal: Journal of Substance Abuse Treatment Publication type: published report SecondarypPublications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Blinding of outcome assessment (detection bias) All non‐attrition outcomes | High risk | Self‐report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: used ITT analysis with all who attended first session (how they defined "enrollled" in the study, p. 58) |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, no statement of primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | Judgement comment: no baseline differences |
Esmaeili 2017.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: 18 to 50 years old, male, 2 to 12 months of methadone maintenance therapy, not participating in other therapy groups Excluded criteria: Use of antipsychotic drugs, inability to answer questions due to physical and psychological problems, lapse, positive results from random urine test (for opium, amphetamines, cannabis, buprenorphin), absence from 2+ training sessions Number missing: unclear Reason missing: not reported Baseline differences: none Age: 32.5 Percent female: 0% Race/Ethnicity: Iranian |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1: No treatment control
|
|
| Outcomes | No eligible outcomes reported | |
| Identification |
Sponsorship source: none reported Country: Iran Setting: not residential Authors name: Mohammad Reza Miri Institution: Birjand University of Medical Sciences Email: miri_moh2516@yahoo.com Address: Social Determinants of Health Research Center, Birjand University of Medical Sciences, Birjand, Iran. COI: none Diagnosis tool: patients referred to outpatient drug addiction treatment and receiving methadone maintenance therapy Diagnosis type: informal Funding: none reported Journal: Journal of Substance Use Publication type: published report Secondary publications: none Substance: opioids |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment:nNo blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: dropout not clearly reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol available, no statement of primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | Judgement comment: no baseline differences |
Foroushani 2019.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: opioids Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: male, receiving methadone maintenance therapy, three lapses during MMT Excluded criteria: absence from 2+ sessions for experimental group Number missing: 5 Reason missing: not reported Baseline differences: unclear age: 35.5 Race/Ethnicity: Iranian |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1: No treatment control
|
|
| Outcomes |
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: internal funds Country: Iran Setting: not residential Authors name: Nahid Sarami Foroushani Institution: Islamic Azad University Email: nahid.sarami97@gmail.com Address: Department of Psychology, Khomeinishar Branch, Islamic Azad University, 581796781, Isfahan, Iran COI: none Diagnosis tool: receiving methadone maintenance therapy at addiction treatment center Diagnosis type: informal Funding: internal funds Journal: Heroin Addiction & Related Clinical Problems Publication type: published report Secondary publications: |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: dropout only in the treatment group, did not use ITT |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol available, no statement of primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | Unclear risk | Judgement comment: not reported |
Garland 2010.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: 18+, alcohol dependence, resided in therapeutic community for 18 months or more Excluded criteria: participants were excluded if they scored less than 16 on the AUDIT, or if they endorsed screening questions indicating active psychosis (Degenhardt et al. 2005) or suicidality Number missing: 16 Reason missing: not reported Baseline differences: none Age: 40.3 Percent female: 20.8% Race/Ethnicity: 60.4% African American, 34.0% White, 5.6% other |
|
| Interventions |
Substance: alcohol Intervention characteristics Mindfulness‐based intervention
Control 1
|
|
| Outcomes |
Penn Alcohol Craving Scale
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: NCCAM, Mind and Life Institute, UNC Chapel Hill School of Social Work Country: USA Setting: residential Authors name: Eric L. Garland Institution: Florida State University Email: elgarlan@gmail.com Address: College of Social Work, Florida State University, University Center, Building C, Tallahassee, FL 32306‐2570 COI: not reported Diagnosis tool: DSM‐IV alcohol dependence Diagnosis type: formal Funding: NCCAM, Mind and Life Institute, UNC Chapel Hill School of Social Work Journal: Journal of Psychoactive Drugs Publication type: published report Secondary publications: Garland (2010) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Blinding of outcome assessment (detection bias) All non‐attrition outcomes | High risk | Self‐report |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: dropout rates similar but no reasons given |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol available, no statement of primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | Judgement comment: no baseline differences |
Garland 2016.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: various substances Baseline characteristics Mindfulness‐based intervention
Control 1
Control 2
Overall
Included criteria: 18 years or older, current substance use disorder diagnosis, current psychiatric disorder diagnosis, homelessness prior to entering the therapeutic community Excluded criteria: active psychosis, substance withdrawal Number missing: 52 Reason missing: dropped out of therapeutic community, relapsed while in treatment Baseline differences: no differences on average number of substance use disorder diagnoses or trauma exposure Age: 37.6 Percent female: 0% Race/Ethnicity: 40 to 44% White, 44 to 45% Black, 12 to 14% Other |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1
Control 2
|
|
| Outcomes |
Penn Alcohol Craving Scale
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: SAMHSA, NIDA Country: USA Setting: residential Authors name: Eric L. Garland Institution: University of Utah Email: eric.garland@socwk.utah.edu Address: 395 South, 1500 East, University of Utah, Salt Lake City, UT, 84112, USA. COI: The first author (ELG) developed the Mindfulness‐Oriented Recovery Enhancement (MORE) intervention, and has received income from the MORE treatment manual (Garland, 2013) and therapist training. Diagnosis tool: MINI Diagnosis type: formal Funding: SAMHSA, NIDA Journal: Behaviour Therapy and Research Publication type: published report Secondary publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: used quote: "randomizer software" |
| Allocation concealment (selection bias) | Low risk | Judgement comment: randomization table created by first author and given to study coordinator |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding of participants, but research assistants blinded |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Blinding of outcome assessment (detection bias) All non‐attrition outcomes | High risk | Self‐report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: dropout rate and reasons similar, used ITT |
| Selective reporting (reporting bias) | Low risk | Judgement comment: no protocol but clear statement of plausible primary outcomes |
| Other bias: equivalence of baseline characteristics (selection bias) | Unclear risk | Judgement comment: no differences on some measures, but not reported for all outcomes |
Garland 2019.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: opioids Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: 18 years or older, admitted to MMT in the past year, chronic non‐cancer pain, English speaking Excluded criteria: none Number missing: 0 Reason missing: n/a Baseline differences: not reported Age: 50.4 Percent female: 50% Race/Ethnicity: 53% African American, 36.7% White, 20% Hispanic |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1
|
|
| Outcomes |
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: NCCAM, NIDA Country: USA Setting: not residential Authors name: Eric L. Garland Institution: University of Utah Email: eric.garland@socwk.utah.edu Address: College of Social Work and Center on Mindfulness and Integrative Health Intervention Development, University of Utah, 395 South, 1500 East, Salt Lake City, UT 84112 USA COI: Eric Garland, PhD, LCSW is the Director of the Center on Mindfulness and Integrative Health Intervention Development. The Center provides Mindfulness‐Oriented Recovery Enhancement (MORE), mindfulness‐based therapy, and cognitive behavioral therapy in the context of research trials for no cost to research participants; however, Dr. Garland has received honoraria and payment for delivering seminars, lectures, and teaching engagements (related to training clinicians in MORE and mindfulness) sponsored by institutions of higher education, government agencies, academic teaching hospitals, and medical centers. Dr. Garland also receives royalties from the sale of books related to MORE. Diagnosis tool: receiving methadone maintenance therapy Diagnosis type: informal Funding: NCCAM, NIDA Journal: Drug and Alcohol Dependence Publication type: published report Secondary publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: similar attrition across groups and not lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Judgement comment: protocol available and not all outcomes reported |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | Judgement comment: no baseline differences |
Glasner 2017.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: stimulants Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: 18+, DSM‐IV diagnosis of stimulant dependence, able to read English, physically able to sit for 30+ minutes Excluded criteria: medical impairment that compromised safety, required medical detoxification, exhibited psychiatric symptoms that warranted hospitalization, were homeless Number missing: 37 Reason missing: absence from protocol participation, not enough compensation, no longer interested, tampering with urine sample Baseline differences: no differences in prevalence of psychiatric disorders, similar in demographics Age: 45.3 Percent female: 28.6% Race/Ethnicity: 44.4% African American, 30.2% White, 20.6% Hispanic, 4.7% other |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1
|
|
| Outcomes |
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: NIDA Country: USA Setting: not residential Authors name: Suzette Glasner Institution: UCLA Email: sglasner@ucla.edu Address: Integrated Substance Abuse Programs, David Geffen School of Medicine at UCLA, Semel Institute for Neuroscience and Human Behavior, 1640 S. Sepulveda Blvd, Suite 120, LosAngeles, CA 90024, USA COI: none Diagnosis tool: MINI Diagnosis type: formal Funding: NIDA Journal: Mindfulness Publication type: published report Secondary publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: quote: "random number table" |
| Allocation concealment (selection bias) | Low risk | Judgement comment: quote: "table was locked in the desk of the study director and after completion of baseline data collection" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: similar attrition but reasons not given |
| Selective reporting (reporting bias) | Low risk | Judgement comment: no protocol but clear statement of plausible primary outcomes |
| Other bias: equivalence of baseline characteristics (selection bias) | Unclear risk | Judgement comment: not reported |
Himelstein 2015.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: various substances Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: not reported Excluded criteria: not reported Number missing: 17 Reason missing: released from detention facility, incomplete self‐report assessment Baseline differences: none Age: 16.4 Percent female: 0% Race/Ethnicity: 70% Latino, 14% African American, 6% Caucasian, 5% Pacific Islander, 5% mixed‐ethnic descent |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1
|
|
| Outcomes | No eligible outcomes reported | |
| Identification |
Sponsorship source: NIDA Country: USA Setting: residential Authors name: Sam Himelstein Institution: Center for Adolescent Studies Email: info@samhimelstein.com Address: Center for Adolescent Studies, Oakland, CA, USA COI: not reported Diagnosis tool: unclear Diagnosis type: formal Funding: NIDA Journal: Mindfulness Publication type: published report Secondary publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: no description |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: high attrition although unclear in what groups; used intention‐to‐treat |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol available, no statement of primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | Judgement comment: no baseline differences |
Imani 2015.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: opioids Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: 18 to 40 years old, 8+ years education, two weeks of medical treatment with opioid agonist medication Excluded criteria: psychosis, dementia, suicide risk organic brain disorder, other drug dependence diagnosis (except nicotine) Number missing: 2 Reason missing: not reported Baseline differences: no significant differences Age: 37.4 percent female: 3.4% Rcae/Ethnicity: Iranian |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1: No treatment control
|
|
| Outcomes |
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: none reported Country: Iran Setting: not residential Authors name: Saeed Imani Institution: Shahid Beheshti University Email: s_imani@sbu.ac.ir Address: Clinical psychology, Department of Clinical Psychology, Shahid Beheshti University, Tehran, IR Iran COI: none Diagnosis tool: DSM‐IV‐TR Diagnosis type: formal Funding: none reported Journal: Iranian J Psychiatry Publication type: published report Secondary publications: |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: dropout only in the treatment group, did not use intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | Judgement comment: protocol available and reported primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | Unclear risk | Judgement comment: no differences on some measures, but not reported for all outcomes |
Jenaabadi 2017.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: opioids Baseline characteristics Mindfulness‐based intervention
Control 1
Control 2
Overall
Included criteria: 20 to 45 years old, elementary school education+, abusing opioids but not dependent on stimulant drugs Excluded criteria: mental retardation, psychotic disorders, structural brain abnormalities, suicidal thoughts Number missing: 18 Reason missing: not reported Baseline differences: differences in quote: "duration of recent treatment" and "number of unsuccessful quits" Age: 32.2 Percent female: 0% Race/Ethnicity: Iranian |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1
Control 2: No treatment control
|
|
| Outcomes |
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: none reported Country: Iran Setting: not residential Comments: Authors name: Amir Hossein Jahangir Institution: Shahid Beheshti University of Medical Sciences Email: jahangir@yahoo.com Address: Department of Clinical Psychology, Taleghani Educational Hospital, School of Medical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, IR Iran COI: not reported Diagnosis tool: DSM‐5 Diagnosis type: formal Funding: none reported Journal: Shiraz E‐Med J Publication type: published report Secondary publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: similar dropout across groups, but no reasons given |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol available, no statement of primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | High risk | Judgement comment: groups differed at baseline on recent treatment and unsuccessful quit attempts |
Lee 2011.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: various substances Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: receiving treatment at drug abuse treatment center, had used illicit drugs in the past, abstinent from illicit drugs for 6 months or more Excluded criteria: psychotic features, delirium, illiteracy Number missing: unclear Reason missing: not reported Baseline differences: differences on DUDIT‐E Age: 40.6 Percent female: 0% Race/Ethnicity: Taiwanese |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1
|
|
| Outcomes |
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: none reported Country: Taiwan Setting: Residential Authors name: Kun‐Hua Lee Institution: Kaohsiung Medical University Email: kunhualee627@gmail.com Address: Kun‐Hua Lee, Department of Psychology, Kaohsiung Medical University (100, Shih‐Chuan 1st Road, Kaohsiung, 80708, Taiwan. Tel: +886‐7‐3215422txt14. E‐mail: kunhualee627@gmail.com COI: none Diagnosis tool: In drug abuse treatment Diagnosis type: Informal Funding: none reported Journal: Journal of Substance Use Publication type: published reported Secondary publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: not clearly reported but likely no dropout (incarcerated) |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol available, no statement of primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | Judgement comment: controlled for baseline differences in analyses |
Machado 2020.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: various substances Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: diagnosis of substance use disorder, having been in substance use disorder treatment for at least a month, literacy, being over 18 Excluded criteria: psychotic disorders, severe cognitive impairment, suicidal ideation Number missing: 13 Reason missing: changed residence, could not be found, relapsed, did not fill out questionnaires Baseline differences: no differences Age: 44 Percent female: 50% Race/Ethnicity: Brazilian |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1: No treatment control
|
|
| Outcomes | Percentage days with heavy alcohol use
Alcohol consumption in standard doses
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: n/a Country: Brazil Setting: not residential Comments: Authors name: Machado Institution: Universidade Federal de São Paulo Email: mayra.pamachado@gmail.com Address: Mayra Pires Alves Machado, Rua Botucatu, 862, 1o andar, Vila Clementino, CEP 04023‐062, São Paulo, SP, Brazil COI: none Diagnosis tool: not reported Diagnosis type: formal Funding: Fundac ¸a ~o de Amparo a` Pesquisa do Estado de São Paulo (FAPESP; grant 2015/19472‐5), the Conselho Nacional de Desenvolvimento Cient ´ıfico e Tecnolo ´ gico (CNPq; process 142267/2015‐5), and the Associac ¸a ~o de Fundo e Incentivo a` Pesquisa (AFIP) Journal: Brazilian Journal of Psychiatry Publication type: published report Secondary publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Noo blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Blinding of outcome assessment (detection bias) All non‐attrition outcomes | High risk | Self‐report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | similar attrition, used intention‐to‐treat analyses |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, no statement of primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | No baseline differences in outcomes |
Marfurt 2007.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: various substances Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: women between the ages 22 to 60 with at least 90 days of sobriety, resident at the facility, history of alcohol or drug abuse, speak and understand English Excluded criteria: none Number missing: 4 Reason missing: withdrew at the baseline measurement for new work assignments, missed 4+ meditation sessions Baseline differences: younger age in waitlist Age: 42.4 Percent female: 100% Race/Ethnicity: 100% White |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1: no treatment control
|
|
| Outcomes |
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: none reported Country: USA Setting: residential Authors name: Stephanie Marfurt Institution: Texas Woman's University Email: not available Address: not available COI: not reported Diagnosis tool: Receiving residential treatment and history of substance abuse Diagnosis type: Informal Funding: not reported Journal: Dissertation Publication type: dissertation Secondary publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: similar attrition but reasons not given |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol available, no statement of primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | High risk | Baseline differences on age |
Margolin 2006.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: heroin Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: methadone‐maintained clients with opioid use disorder Excluded criteria: none Number missing: 11 Reason missing: not reported Baseline differences: none between MBI and control Age: 41.5 Percent female: 65% Race/Ethnicity: 44% to 47% White, 26% to 44% African American, 12% to 26% Hispanic |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1: no treatment control
|
|
| Outcomes |
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: NIDA Country: USA Setting: not residential Authors name: Arthur Margolin Institution: Yale University Email: arthur.margolin@yale.edu Address: Yale University School of Medicine, Welch Center, 495 Congress Ave., New Haven, CT 06519 COI: not reported Diagnosis tool: DSM‐IV Diagnosis type: formal Funding: NIDA Journal: AIDS Education and Prevention Publication type: published report Secondary publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: similar attrition but reasons not given |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol available, no statement of primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | Judgement comment: no baseline differences when collapsed across two active groups |
Mermelstein 2015.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: alcohol Baseline characteristics Mindfulness‐based intervention
No treatment
Overall
Included criteria: 18 to 24, at least one binge drinking episode in past 2 weeks, full‐time non‐commuter student, not currently under the influence of alcohol or illicit substances Excluded criteria: self‐reported diagnosis of schizophrenia or other psychotic disorder Pretreatment: no differences on consequences of alcohol use at baseline, no other baseline between‐group tests reported Number missing: 3 Reason missing: lost to follow‐up, reasons unknown Baseline differences: no differences on consequences of alcohol use at baseline, no other baseline between‐group tests reported Age: 19.1 Percent female: 50% Race/Ethnicity: 91% white, 4% black, 4% multiracial, 1% Latino |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1: no treatment control
|
|
| Outcomes | Drinking episodes
Total drinks per week
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: none reported Country: USA Setting: not residential Authors name: Liza C. Mermelstein Institution: Brown University Email: lizamermelstein@gmail.com Address: Liza C. Mermelstein, Alpert Medical School of Brown University–Psychiatry and Human Behavior, 222 Richmond Street, Providence, RI 02903 COI: not reported Diagnosis tool: at least one binge drinking episode in past 2 weeks Diagnosis type: Informal Funding: none reported Journal: Psychology of Addictive Behaviors Publication type: published report Secondary publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: no description |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Blinding of outcome assessment (detection bias) All non‐attrition outcomes | High risk | Self‐report |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: similar attrition rates but reasons not given |
| Selective reporting (reporting bias) | Low risk | Judgement comment: no protocol but clear statement of plausible primary outcomes |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | Judgement comment: no baseline differences |
Ramezani 2019.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: opioids Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: receiving treatment at substance dependence clinic, not attending other treatment Excluded criteria: absence from 2+ therapy sessions, unwillingness to continue treatment, lack of methadone consumption, being illiterate Number missing: not reported Reason missing: not reported Baseline differences: not reported Age: 33.3 Percent female: 0% Race/Ethnicity: 100% Iranian |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1: no treatment control
|
|
| Outcomes | No eligible outcomes reported | |
| Identification |
Sponsorship source: n/a Country: Iran Setting: unclear Authors name: Ramezani Institution: University of Mohaghegh Ardabili Email: lavinramezani@yahoo.com Address: Department of Psychology, Faculty of Education and Psychology, University of Mohaghegh Ardabili, Ardabil, Iran COI: none Diagnosis tool: receiving treatment at substance dependence clinic Diagnosis type: informal Funding: The Deputy of Research and Technology of Kurdistan University of Medical Sciences Journal: Journal of Practice in Clinical Psychology Publication type: published report Secondary publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout not reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, no statement of primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | Unclear risk | Not reported |
Shorey 2017.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: various substance Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: 18+ years old, cleared from withdrawal by medical staff Excluded criteria: psychotic symptoms, cognitive impairment Baseline differences: more women in mindfulness group Number missing: 8 Reason missing: disobeyed unit rules, left unit voluntarily Age: 41.3 Percent female: 26% Race/Ethnicity: 92.2% white, 3.4% African American, 1.7% Hispanic, 1.7% Asian American, 0.9% Indian |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1: no treatment control
|
|
| Outcomes |
Penn Alcohol Craving Scale ‐ alcohol
Penn Alcohol Craving Scale ‐ drug
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: NIAAA Country: USA Setting: residential Authors name: Ryan C. Shorey Institution: Ohio University Email: shorey@ohio.edu Address: Department of Psychology, Ohio University, 239 Porter Hall, Athens, OH, 45701, USA. COI: Ryan C. Shorey and Gregory L. Stuart received consulting compensation from the Cornerstone of Recovery. Diagnosis tool: receiving treatment at residential substance use program Diagnosis type: informal Funding: NIAAA Journal: Substance Use & Misuse Publication type: published report Secondary publications: |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: random number generator used |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Blinding of outcome assessment (detection bias) All non‐attrition outcomes | High risk | Self‐report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: similar attrition rates with similar reasons |
| Selective reporting (reporting bias) | Low risk | Judgement comment: no protocol but clear statement of plausible primary outcomes |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | Judgement comment: no baseline differences |
Vowles 2020.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: opioids Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: receiving treatment for chronic pain through VA, prescribed at least one opioid medication for chronic pain, show evidence of opioid misuse, speak and read English Excluded criteria: history of suicide attempt in past 12 months, current buprenorphine prescription, uncontrolled psychosis Number missing: 7 Reason missing: taken off opioids, suicide attempt, no response, lost to follow‐up Baseline differences: lower prescribed opioid dose in usual care group Age: 50.5 Percent female: 14% Race/Ethnicity: 51.4% White, 28.6% Latinx, 17.1% Native American, 2.9% other |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1: no treatment control
|
|
| Outcomes |
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: NCCIH Country: USA Setting: not residential Authors name: Kevin E. Vowles Institution: Queen's University Belfast Email: kvowles@unm.edu Address: School of Psychology, Queen’s University Belfast, David Keir Building, 18‐30 Malone Rd, Belfast BT9 5BN, Northern Ireland, United Kingdom COI: not reported Diagnosis tool: Current Opioid Misuse Measure (COMM) established cut point of 9+ and/or meeting criteria for opioid use disorder by DSM 5 Diagnosis type: informal/formal Funding: NCCIH Journal: Journal of Pain Publication type: published report Secondary publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: numerically higher attrition in usual‐care arm |
| Selective reporting (reporting bias) | Low risk | Judgement comment: protocol available and reported primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | No baseline differences |
Witkiewitz 2014.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: various substances Baseline characteristics Mindfulness‐based intervention
Relapse prevention
Overall
Included criteria: residency at the treatment center, proficiency in the English language, willingness to be randomized to treatment condition, and sufficient self‐reported cognitive ability to understand and provide consent Excluded criteria: none Number missing: 34 Reason missing: switched groups before starting treatment, opted out of study, left center, failed to respond Baseline differences: none Age: 34.0 Percent female: 100 % Race/Ethnicity: 34.5% to 51.0% white, 10.2% to 12.7% African American, 7.3% to 10.2% Native American, 2% to 3.6% Asian, 0% to 1.8% Hispanic/Latinx |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1
|
|
| Outcomes |
Drug use days
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: Washington State University Vancouver grant Country: USA Setting: residential Authors name: Katie Witkiewitz Institution: University of New Mexico Email: katiew@unm.edu Address: Dr Katie Witkiewitz, PhD, Department of Psychology, Center on Alcoholism, Substance Abuse, and Addictions, University of New Mexico, 2650 Yale Blvd SE, Albuquerque, NM, USA COI: none Diagnosis tool: receiving residential treatment Diagnosis type: informal Funding: Washington State University Vancouver grant Journal: Substance Use & Misuse Publication type: published report Secondary publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: quote: "random number generator" |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Blinding of outcome assessment (detection bias) All non‐attrition outcomes | High risk | Self‐report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: reasons for dropout and amount of dropout similar across groups |
| Selective reporting (reporting bias) | Low risk | Judgement comment: no protocol but clear statement of plausible primary outcomes |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | Judgement comment: no baseline differences |
Wongtongkam 2018.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: alcohol Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: age 18 years and older, with a diagnosis of alcohol dependence and proficiency in spoken Thai language Excluded criteria: psychotic symptoms, disrupting other participants, unable to control behaviors while meditating Number missing: not reported Reason missing: n/a Baseline differences: mindfulness group higher on personal distress Age: 40.2 Percent female: % Race/Ethnicity: Thai |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1
|
|
| Outcomes | No eligible outcomes reported | |
| Identification |
Sponsorship source: not reported Country: Australia, Thailand Setting: residential Comments: Authors name: Nualnong Wongtongkam Institution: Charles Sturt University Email: nwongtongkam@csu.edu.au Address: School of Biomedical Sciences, Charles Sturt University, Bathurst, New South Wales 2795, Australia. COI: the authors report no conflicts of interest. Diagnosis tool: diagnosis of alcohol dependence Diagnosis type: formal Funding: not reported Journal: Alcoholism Treatment Quarterly Publication type: published report Secondary publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: not reported, although likely no dropout (residential setting) |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol available, no statement of primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | High risk | Judgement comment: differences at baseline |
Wongtongkam 2019.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: various substances Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: 18+, receiving treatment at rehabilitation center, had illegal substance use problems, proficient in spoken and written Thai Excluded criteria: showing severe psychotic symptoms, disrupting others while meditating Number missing: 20 Reason missing: not reported Baseline differences: no differences Age: 29.5 Percent female: 100% Race/Ethnicity: 100% Thai |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1: no treatment control
|
|
| Outcomes |
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: n/a Country: Thailand Setting: residential Comments: Authors name: Wongtongkam Institution: Charles Sturt University Email: nualnongw@gmail.com Address: School of Biomedical Sciences, Charles Sturt University, Albury, Australia COI: none Diagnosis tool: receiving treatment at rehabilitation center Diagnosis type: informal Funding: none reported Journal: Therapeutic Communities Publication type: published report Secondary publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "simple random allocation by a nurse," but not further specified |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | no blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | similar attrition but reasons not given |
| Selective reporting (reporting bias) | Unclear risk | no protocol available, no statement of primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | no baseline differences |
Yaghubi 2017.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: opioids Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: age between 20 to 45 years, psychiatric or medical references regarding the original diagnosis and diagnostic criteria for substance dependence according to the Diagnostic and Statistical Manual of Mental Disorders‐5th edition (DSM‐5), not having severe psychiatric disorders (schizophrenia, depression and bipolar disorder), and having the least degree of junior high school Excluded criteria: not wanting to continue the meetings, the absence at more than two sessions, participating in other health programs simultaneously, and having a long‐term dependence on simultaneous multi‐drug Number missing: 10 Reason missing: irregular presence and non‐completion of questionnaires Baseline differences: none Age: 30. Percent female: 0% Race/Ethnicity: Iranian |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1: no treatment control
|
|
| Outcomes |
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: Isfahan University of Medical Sciences and Health Services Country: Iran Setting: not residential Authors name: Fatemeh Zargar Institution: Isfahan University of Medical Sciences Email: fatemehzargar@gmail.com Address: Behavioral Sciences Research Center and Department of Psychiatry, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran COI: none Diagnosis tool: DSM‐IV Diagnosis type: formal Funding: Isfahan University of Medical Sciences and Health Services Journal: Addiction & Health Publication type: published report Secondary publications: Yaghubi et al. (2018) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: quote: "table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: imbalance in number of dropouts |
| Selective reporting (reporting bias) | High risk | Judgement comment: pProtocol available but not all outcomes reported (distress tolerance not reported) |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | Judgement comment: no baseline differences on demographics, also appears no differences on outcomes either |
Zemestani 2016.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: various substances Baseline characteristics Mindfulness‐based intervention
Treatment as usual
Overall
Included criteria: DSM‐IV‐TR substance dependence, 2+ weeks in inpatient or outpatient treatment and completion of detoxification, BDI‐II score in moderate range, speak and read Persian Excluded criteria: psychotic disorder, suicide risk, withdrawal risk, need for more intensive treatment, did not complete inpatient or outpatient treatment Number missing: 8 Reason missing: dropped out, missed 4+ sessions, lost to 3‐month follow‐up Baseline differences: none Age: 30.1 Percent female: 20.3% Race/Ethnicity: Iranian |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1
|
|
| Outcomes |
Penn Alcohol Craving Scale
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: Italian Ministry of Health Country: Iran, Italy Setting: residential Authors name: Mehdi Zemestani Institution: University of Kurdistan Email: m.zemestan@gmail.com Address: Social Sciences, University of Kurdistan, Sanandaj, Iran COI: none Diagnosis tool: DSM‐IV SCID‐IV Diagnosis type: formal Funding: Italian Ministry of Health Journal: Mindfulness Publication Ttpe: published report Secondary publications: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: quote: "pre‐prepared blocked randomization lists" |
| Allocation concealment (selection bias) | Low risk | Judgement comment: allocated by first author who was not involved in recruitment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding of research staff but not participants |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Blinding of outcome assessment (detection bias) All non‐attrition outcomes | High risk | Self‐report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: similar attrition rates, reasons not given but intention‐to‐treat analyses used |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol available, no statement of primary outcome |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | Judgement comment: no baseline differences |
Zgierska 2017.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Substance: alcohol Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: 18+, English fluency, alcohol dependence diagnosis, early recovery (quit date within the prior 2‐14 weeks), completion of ≥2 weeks outpatient treatment for alcohol dependence, elevated Perceived Stress Scale‐10 (score ≥14) Excluded criteria: inability to reliably participate, current meditation practice, current pregnancy, schizophrenia, delusional, or bipolar disorders; acute drug use disorder based on SCID Number missing: 11 Reason missing: lack of time or changed life circumstances, withdrawn by PI due to disruptiveness Baseline differences: control more likely to be employed Age: 41 Percent female: 44% Race/Ethnicity: 91.0% White, 4.5% African‐American, 4.5% other, 2.7% Hispanic/Latinx |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1: no treatment control
|
|
| Outcomes |
Percentage heavy drinking days
Number of drinks per day
Any heavy drinking
Treatment acceptability (attrition)
|
|
| Identification |
Sponsorship source: NIAAA, NCATS Country: USA Setting: not residential Authors name: Aleksandra E. Zgierska Institution: University of Wisconsin ‐ Madison Email: aleksandra.zgierska@fammed.wisc.edu Address: School of Medicine and Public Health, Department of Family Medicine and Community Health, University of Wisconsin‐Madison, 1100 Delaplaine Ct., Madison, WI 53715, USA COI: none Diagnosis tool: SCID for DSM‐IV‐TR alcohol dependence Diagnosis type: formal Funding: NIAAA, NCATS Publication type: published report Secondary publications: Zgierska et al. (2019) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: study statistician prepared randomization envelopes |
| Allocation concealment (selection bias) | Low risk | Judgement comment: randomization envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding |
| Blinding of outcome assessment (detection bias) Treatment acceptability (attrition) | Low risk | Objective measure |
| Blinding of outcome assessment (detection bias) All non‐attrition outcomes | High risk | Self‐report |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: numerically higher attrition in mindfulness condition, not all participants included in analysis |
| Selective reporting (reporting bias) | Low risk | Judgement comment: no protocol but clear statement of plausible primary outcomes |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | Judgement comment: no baseline differences |
Zhang 2019.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Mindfulness‐based intervention
Control 1
Overall
Included criteria: DSM‐5 stimulant use disorder, receiving treatment at mandated drug rehabilitation center Excluded criteria: cognitive disability, serious physical health condition, previous mindfulness‐related practice experience (e.g., qigong), had participated in previous study, participating in concurrent study Number missing: not reported Reason missing: not reported Baseline differences: intervention group younger age, no differences in drug use history or other demongraphics Age: 34.4 Percent female: 0% Race/Ethnicity: 100% Chinese |
|
| Interventions |
Intervention characteristics Mindfulness‐based intervention
Control 1: no treatment control
|
|
| Outcomes | No eligible outcomes reported | |
| Identification |
Sponsorship source: n/a Country: China Setting: residential Authors name: Zhang Institution: Shanghai Jiao Tong University Email: dujiangdou@163.com Address: Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai 20030, China COI: not reported Diagnosis tool: DSM‐5 Diagnosis type: formal Funding: none reported Journal: National Natural Science Foundation of China, Project of Science and Technology Commission of Shanghai Municipality Publication type: published report Secondary publications: none Substance: stimulants |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized number table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout not reported |
| Selective reporting (reporting bias) | High risk | Protocol available and not all outcomes reported |
| Other bias: equivalence of baseline characteristics (selection bias) | Low risk | Treatment‐as‐usual control |
CBT: cognitive behavioral therapy;COI: conflict of interestDSM‐5: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; ITT: intention‐to‐treat; MBI: mindfulness‐based interventions; MBRP: mindfulness‐based relapse prevention; MBSR; mindfulness‐based stress reduction; PTSD: post‐traumatic stress disorder; SCID: structured interview guide;SD: standard deviation; SUD: substance use disorderTAU: treatment as usual.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alexander 2019 | Not randomized or quasi‐randomized |
| Amaro 2017 | Not patients with SUD |
| Bandawar 2016 | Not face‐to‐face delivery |
| Bowen 2006 | Not randomized or quasi‐randomized |
| Bowen 2012 | Not randomized or quasi‐randomized |
| Bowen 2017 | Not randomized or quasi‐randomized |
| Carpentier 2015 | Does not include no treatment or other treatment comparison |
| Caselli 2016 | Not mindfulness‐based intervention |
| Chen 2019 | Not mindfulness‐based intervention |
| Chouhan 2011 | Not patients with SUD |
| Collins 2009 | Does not include no treatment or other treatment comparison |
| Crescentini 2015 | Not individually randomized trial |
| Crowfoot 2014 | Not mindfulness‐based intervention |
| DRKS00015678 | Not patients with SUD |
| Enkema 2017 | Does not include no treatment or other treatment comparison |
| Fonagy 2010 | Not patients with SUD |
| Garland 2014 | Not patients with SUD |
| Garland 2014a | Not patients with SUD |
| Garland 2014b | Not patients with SUD |
| Garland 2017 | Not patients with SUD |
| Garland 2017a | Not patients with SUD |
| Garland 2017b | Not patients with SUD |
| Garland 2018 | Not patients with SUD |
| Garland 2019a | Not patients with SUD |
| Garland 2019b | Not patients with SUD |
| Garland 2019c | Not patients with SUD |
| Garland 2020 | Not patients with SUD |
| Gayner 2012 | Not patients with SUD |
| Gibson 2019 | Does not include no treatment or other treatment comparison |
| Grow 2015 | Not randomized or quasi‐randomized |
| Hai 2021 | Not mindfulness‐based intervention |
| Hargreaves 1974 | Not patients with SUD |
| Hruschak 2021 | Not mindfulness‐based intervention |
| Iranshahri 2015 | Not randomized or quasi‐randomized |
| IRCT20150413021727N2 | Not randomized or quasi‐randomized |
| IRCT2015042420961N | Not mindfulness‐based intervention |
| Kamboj 2017 | Not face‐to‐face delivery |
| Lee 2017 | Not mindfulness‐based intervention |
| Lyons 2019 | Not patients with SUD |
| Magidson 2011 | Not mindfulness‐based intervention |
| Malouf 2017 | Not patients with SUD |
| Marcus 2001 | Not randomized or quasi‐randomized |
| Marcus 2009 | Not randomized or quasi‐randomized |
| Murphy 2014 | Not mindfulness‐based intervention |
| Nakamura 2015 | Not mindfulness‐based intervention |
| NCT01505101 | Not patients with SUD |
| NCT04082637 | Not mindfulness‐based intervention |
| NCT04160754 | Not patients with SUD |
| NCT04567043 | Not patients with SUD |
| NCT04769986 | Not mindfulness‐based intervention |
| Nice 2008 | Not patients with SUD |
| Ojehagen 1992 | Not mindfulness‐based intervention |
| Parker 1978 | Not mindfulness‐based intervention |
| Parker 1978a | Not mindfulness‐based intervention |
| Price 2012 | Not mindfulness‐based intervention |
| Price 2012a | Not mindfulness‐based intervention |
| Price 2016 | Not mindfulness‐based intervention |
| Price 2017 | Not mindfulness‐based intervention |
| Price 2018 | Not mindfulness‐based intervention |
| Price 2019 | Not mindfulness‐based intervention |
| Price 2019a | Not mindfulness‐based intervention |
| Rentala 2020 | Not mindfulness‐based intervention |
| Russell 2019 | does not include no treatment or other treatment comparison |
| Simpson 2015 | Not mindfulness‐based intervention |
| Tang 2016 | Not patients with SUD |
| Temme 2012 | Not randomized or quasi‐randomized |
| Vinci 2014 | Not face‐to‐face delivery |
| Wupperman 2015 | Not randomized or quasi‐randomized |
SUD: Substance use disorder.
Characteristics of studies awaiting classification [ordered by study ID]
ACTRN12613000193774.
| Methods | |
| Participants | adults with alcohol dependence |
| Interventions | mindfulness‐based cognitive therapy vs. alcohol support group |
| Outcomes | Brief Symptom Inventory, Penn Alcohol Craving Scale, Impaired Alcohol Response Inhibition Scale, Perceived Stress Scale, White Bear Suppression Inventory, psychophysiological cue reactivity, Kentucky Inventory of Mindfulness Skills, Attitudes Towards Treatment Questionnaire |
| Notes | Contact email: chris.lee@murdoch.edu.au, anticipated enrollment date of February 20, 2013 |
Baldus 2018.
| Methods | |
| Participants | 340 13 to 19 year olds receiving inpatient or day treatment targeting substance use |
| Interventions | mindfulness‐based psychotherapy with standard substance use treatment vs. standard substance use treatment |
| Outcomes | substance use, substance use symptoms, comorbid symptoms |
| Notes |
Becker 2017.
| Methods | |
| Participants | 60 adults with comorbid alcohol dependence and depression |
| Interventions | mindfulness‐based training vs. behavioral activation |
| Outcomes | default mode network activity, craving, depression, relapse rates |
| Notes |
c9njc, R. B. R.
| Methods | |
| Participants | not available |
| Interventions | not available |
| Outcomes | not available |
| Notes |
CasasGaviln 2018.
| Methods | |
| Participants | 162 patients diagnosed with alcohol use disorder |
| Interventions | mindfulness‐based relapse prevention vs. control |
| Outcomes | unclear |
| Notes |
Chen 2018.
| Methods | |
| Participants | 180 participants with methamphetamine use disorder |
| Interventions | mindfulness‐based relapse prevention with virtual reality cue exposure vs. treatment‐as‐usual |
| Outcomes | craving, virtual cue reactivity, anxiety, depression, emotion regulation, mindfulness, drug‐related attention bias |
| Notes |
Connors 2011.
| Methods | |
| Participants | 92 participants |
| Interventions | mindfulness‐based stress reduction vs. healthy lifestyle lectures |
| Outcomes | stress, hassles, anxiety, psychiatric symptoms |
| Notes |
CTRI/2018/07/014994.
| Methods | |
| Participants | not available |
| Interventions | not available |
| Outcomes | not available |
| Notes |
Garland 2016a.
| Methods | |
| Participants | not available |
| Interventions | not available |
| Outcomes | not available |
| Notes |
IRCT2013031612826N1.
| Methods | |
| Participants | 30 patients who are dependent on opioids receiving treatment with methadone in the Iranian National Center for Addiction Study |
| Interventions | mindfulness‐based group therapy vs. control |
| Outcomes | craving, alcohol and drug use consequences, mindfulness, acceptance, Difficulties in Emotion Regulation Scale; Addiction Severity Index; Depression, Anxiety, and Stress Scale |
| Notes | Contact email: psychology2008@gmail.com, expected recruitment end date July 21, 2013 |
IRCT2015041321727N1.
| Methods | |
| Participants | 24 male participants with substance dependence recruited from the Yavaran Omid Addiction Treatment Clinic |
| Interventions | 6 one‐hour group therapy sessions based on detached mindfulness techniques vs. "common group therapy program of the clinic" |
| Outcomes | Relapse Prediction Scale, Meta‐Cognitive Questionnaire‐30, Beck Depression and Anxiety Inventory, Clinica Global Improvement, Client Satisfaction Questionnaire |
| Notes | Contact email: zahragholami014@gmail.com, expected recruitment end date December 22, 2013 |
IRCT2015061522749N1.
| Methods | |
| Participants | 36 patients with heroin use being treated with methadone and without acute mental disorder |
| Interventions | mindfulness‐based cognitive therapy vs. control |
| Outcomes | Obsessive Compulsive Drug Use Scale (OCDUS) and Ruminative Response Scale (RRS) |
| Notes | Contact email: ali91haghnazari@gmail.com, expected completion date: March 21, 2014 |
IRCT2015121925603N1.
| Methods | |
| Participants | 90 males with methamphetamine addiction |
| Interventions | 12 mindfulness sessions vs. control |
| Outcomes | affective control, self‐regulation, perceived stress |
| Notes | Contact email: psyk13t@yahoo.com, expected recruitment end date August 21, 2016 |
Irct20170702034844N.
| Methods | |
| Participants | not available |
| Interventions | not available |
| Outcomes | not available |
| Notes |
IRCT2017081325160N7.
| Methods | |
| Participants | 40 males with stimulant abuse/dependence |
| Interventions | mindfulness‐based cognitive therapy vs. control |
| Outcomes | psychological symptoms, craving beliefs, self‐efficacy |
| Notes | Contact email: ahmadij@sums.ac.ir, expected recruitment end date August 22, 2018 |
NCT01211418.
| Methods | |
| Participants | 66 adults meeting DSM‐IV criteria for cocaine dependence or abuse and seeking treatment |
| Interventions | integrative meditation vs. supportive counseling |
| Outcomes | cocaine urine toxicology, use of drugs and alcohol, heart rate variability, Addiction Severity Index, length of time in drug treatment program, cocaine cravings, Beck Depression Inventory II, Spielberger State‐Trait Anxiety Inventory, Self‐Efficacy and Self‐Esteem |
| Notes | Contact information: Mary Bahr‐Robertson, Research Supervisor, University of Maryland, College Park Last updated October, 15, 2018 |
NCT02147483.
| Methods | |
| Participants | 4 participants with DSM‐IV‐TR diagnosis of alcohol dependence |
| Interventions | mindfulness‐based relapse prevention vs. treatment‐as‐usual |
| Outcomes | mindfulness, craving, depression, anxiety, perceived stress, obsessive thoughts of alcohol/compulsive drinking, drinking behavior |
| Notes | Contact information: Jennifer Kim Penberthy, Associate Professor, University of Virginia Last updated: April 17, 2019 |
NCT03366909.
| Methods | |
| Participants | 40 participants with cannabis use |
| Interventions | mindfulness‐based relapse prevention vs. classic therapy |
| Outcomes | cannabis use, treatment retention, withdrawal symptoms, electroretinogram, retinal thickness |
| Notes | Contact information: Vincent LaprevoteCentre Psychothérapique de Nancy, last updated April 19, 2018 |
NCT03748875.
| Methods | |
| Participants | 200 adults with DSM 5 amphetamine use disorder |
| Interventions | mindfulness‐based relapse prevention |
| Outcomes | treatment‐as‐usual |
| Notes |
NCT03894501.
| Methods | |
| Participants | 30 participants in methadone treatment for at least 3 months with non‐malignant pain for 2 months or longer |
| Interventions | mindfulness‐oriented recovery enhancement vs. methadone program treatment‐as‐usual |
| Outcomes | expressing interest in the study, refusing study participation, number screened, number consented, number refusing participation after/during consent, number of sessions completed by study participants, percentage of sessions completed by study participants, number of participants who drop out, number of completed assessments |
| Notes | Contact information: Nina A. Cooperman, Psy. D., Associate Professor, Rutgers, The State University of New Jersey Last updated: September 4, 2019 |
Negrei 2015.
| Methods | |
| Participants | 60 Romanian patients |
| Interventions | mindfulness‐based cognitive therapy vs. medication treatment |
| Outcomes | depression, anxiety |
| Notes |
Park 2005.
| Methods | |
| Participants | not available |
| Interventions | not available |
| Outcomes | not available |
| Notes |
RBR‐4br6q5.
| Methods | |
| Participants | 40 crack users following the 12 steps program in a therapeutic community |
| Interventions | 8 weeks of meditation for stress reduction vs. control |
| Outcomes | perceived stress |
| Notes | Contact email: mseleghim@yahoo.com, anticipated first enrollment date October 5, 2016 |
DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; DSM‐5: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.
Characteristics of ongoing studies [ordered by study ID]
DRKS00014041.
| Study name | Treatment of mindfulness‐based psychotherapy in adolescent inpatients with substance use disorders |
| Methods | |
| Participants | 246 adolescents (13 to 19 years) with substance use disorders |
| Interventions | 12 group therapy sessions of a mindfulness‐based therapy vs. standard substance use disorder treatment |
| Outcomes | days with substance use, craving, well‐being / quality of life, abstinence motivation, severity of dependence, mindfulness skill, impulsivity, perceived stress, disability days, comorbid diagnoses, general psychosocial functioning, changes in meditation, treatment adherence |
| Starting date | August 16, 2018 |
| Contact information | Tanja.Legenbauer at rub.de |
| Notes |
Ellingson 2018.
| Study name | |
| Methods | |
| Participants | 36 adults seeking treatment for alcohol use disorder |
| Interventions | mindfulness‐based relapse prevention vs. relapse prevention |
| Outcomes | alcohol dependence, depression, anxiety, mindfulness |
| Starting date | |
| Contact information | |
| Notes |
NCT02755103.
| Study name | Mindfulness meditation for the treatment of women with comorbid PTSD and SUD |
| Methods | |
| Participants | 102 females with DSM 5 alcohol or substance use disorder and PTSD |
| Interventions | mindfulness‐based relapse prevention vs. treatment‐as‐usual |
| Outcomes | PTSD symptoms, days of substance use, amount of substance use, emotion regulation, mindfulness |
| Starting date | June 1, 2016 |
| Contact information | Therese K. Killeen, Research Professor, Medical University of South Carolina |
| Notes |
NCT03734666.
| Study name | Development of a mindfulness‐based treatment for the reduction of alcohol use and smoking cessation |
| Methods | |
| Participants | 80 participants who smoke and with elevated alcohol use |
| Interventions | mindfulness‐based relapse prevention vs. cognitive behavioral therapy |
| Outcomes | participant satisfaction, rate of recruitment, participant retention, questionnaire completion, smoking abstinence, alcohol use |
| Starting date | November 1, 2018 |
| Contact information | Mikaela.Hemenway@moffitt.org |
| Notes |
NCT03883646.
| Study name | Mindfulness for alcohol abusing offenders (MIT) |
| Methods | |
| Participants | 480 females with alcohol use disorder released from incarceration > 3 months |
| Interventions | mindfulness‐based relapse prevention vs. relapse prevention |
| Outcomes | alcohol craving, alcohol consumption, temptation to drink alcohol, criminal behavior |
| Starting date | July 1, 2018 |
| Contact information | Jenna Shold, PhD 505‐400‐5241 jshold@mrn.org |
| Notes |
NCT04112186.
| Study name | Mindfulness‐Oriented Recovery Enhancement (MORE) in heroin addiction |
| Methods | |
| Participants | 300 adults with opioid use disorder with heroin as primary drug of choice, stabilized on methadone or other form of MAT |
| Interventions | 8‐weeks of group therapy using psychological principles including mindfulness training vs. 8‐weeks of group therapy using psychological principles not including mindfulness training |
| Outcomes | fMRI BOLD signal during tasks of reward, control reactivity, cue reactivity, during resting‐state functional connectivity, voxel‐based morphometry, urine drug test |
| Starting date | October 21, 2020 |
| Contact information | Rita Goldstein, PhD Icahn School of Medicine at Mount Sinai, rita.goldstein@mssm.edu |
| Notes |
NCT04278352.
| Study name | Mindfulness‐based relapse prevention for opioid and alcohol use disorders (MBRP) |
| Methods | |
| Participants | 240 adults who completed behavioral health treatment for opioid use disorder (OUD) or alcohol use disorder (AUD) within previous 8 weeks, meeting DSM‐5 criteria for OUD or AUD |
| Interventions | mindfulness‐based relapse prevention vs. waitlist control |
| Outcomes | opioid / alcohol use, opioid alcohol craving, withdrawal symptoms, quality of life, perceived stress, posttraumatic stress symptoms, pain severity, medication adherence, mindfulness skills, emotion regulation skills, executive functioning, savoring, affect |
| Starting date | July 1, 2020 |
| Contact information | Heidi Zinzow, Ph.D. 864‐656‐4376 hzinzow@clemson.edu |
| Notes |
NCT04278586.
| Study name | Effect of mindfulness on opioid use and anxiety during primary care buprenorphine treatment (R33 phase) (Mindful‐OBOT) |
| Methods | |
| Participants | 236 adults with opioid use disorder diagnosis, prescribed buprenorphine, less than 90 days of abstinence |
| Interventions | Live‐online Mindful Recovery OUD Care continuum vs. Live‐Online Control |
| Outcomes | opioid abstinence, cocaine toxicology, benzodiazepine toxicology, anxiety, pain interference, pain catastrophizing, substance craving, mental health, treatment retention, emotion regulation, self‐compassion, internalized stigma, decentering, rumination, experiential avoidance, perceived stress, interoceptive awareness, mindfulness |
| Starting date | January 6, 2021 |
| Contact information | Kayley Okst, BA 857‐270‐0372 kokst@challiance.org |
| Notes |
NCT04491968.
| Study name | Mindfulness oriented recovery enhancement for chronic pain and opioid relapse |
| Methods | |
| Participants | 154 adults currently on methadone, experiencing non‐malignant pain for ≥3 months |
| Interventions | Mindfulness‐oriented recovery enhancement vs. methadone treatment‐as‐usual |
| Outcomes | opioid relapse, opioid abstinence vs. any opioid use, drug abstinence vs. other drug use, number of days of opioid use, number of days of other drug use, craving, pain, emotional distress |
| Starting date | August 13, 2020 |
| Contact information | Nina Cooperman, PsyD732‐235‐8569 cooperna@rwjms.rutgers.edu |
| Notes |
NCT04584502.
| Study name | Mindful Moms in Recovery (MMORE) |
| Methods | |
| Participants | 120 adults receiving comprehensive medication treatment for opioid use disorder at maternity care practice |
| Interventions | Mindful Moms yoga mindfulness intervention vs. treatment‐as‐usual |
| Outcomes | retention in medication treatment for opioid use disorder, opioid abstinence, opioid and other substance use, depression, anxiety, stress, post‐traumatic stress, mindfulness, pain, quality of life |
| Starting date | June 2021 |
| Contact information | Ashley E Maher, BA606‐646‐7039 ashley.e.maher@dartmouth.edu |
| Notes |
NCT04648228.
| Study name | Pain and opioids: integrated treatment In veterans |
| Methods | |
| Participants | 160 adults, 21 years or older, stabilized on buprenorphine dose for 1 to 6 months, enrolled in VA Co‐Occurring Disorders clinic, chronic pain for < 6 months |
| Interventions | Acceptance and commitment therapy and Mindfulness‐Based Relapse Prevention vs. education control |
| Outcomes | pain interference, opioid misuse, pain intensity, depression, pain‐related fear, alcohol and other drug use |
| Starting date | June 11, 2021 |
| Contact information | Zachary Schmidt, PhD 505‐265‐1711 ext 6079 Zachary.Schmidt2@va.gov |
| Notes |
BOLD:blood‐oxygen‐level‐dependent; DSM‐5: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; fMRI: functional magnetic resonance imaging; MRI: magnetic resonance imaging; PTSD: post‐traumatic stress disorder.
Differences between protocol and review
Study selection
Jonathan Livingstone is producing a Cochrane Review with the topic mindfulness and tobacco, therefore we decided to exclude tobacco from our review. In order to isolate the effects of training in mindfulness meditation (Crane 2017), we excluded interventions that did not involve instruction in mindfulness meditation (e.g. Acceptance and Commitment Therapy and Dialectical Behavior Therapy were not eligible). We did not include quasi‐randomized studies.
Analysis
Sensitivity analyses were not conducted as there were insufficient studies (≤ 10) for all outcomes with the exception of treatment acceptability (differential attrition). Therefore, we did not conduct subgroup analyses separated by substance. Analyses were separated by control condition type based on evidence that the strength of the control condition impacts the magnitude of between‐group effects for MBIs (Goldberg 2018; Goldberg 2021). We omitted assessment of risk of bias related to equivalence of treatment utilization as this was not relevant when a no treatment comparison condition or when an other treatment comparison condition with a different intended duration or intensity was used. Blinding of pf participants, personnel, and outcome assessor (performance and detection bias) was not assessed separately for objective and subjective outcomes. This was because, with the exception of treatment acceptability in the form of study attrition, all outcomes were assessed subjectively via self‐report.
Contributions of authors
SIMON B. GOLDBERG
study selection
data extraction
data management
analysis of data
interpretation and discussion of results
writing of the review
securing funding
BRIAN T. PACE
study selection
data extraction
data management
interpretation and discussion of results
writing of the review
securing funding
MATAS GRISKAITIS
study selection
data extraction
data management
interpretation and discussion of results
writing of the review
securing funding
REINHARD WILLUTZKI
protocol elaboration
study selection
participation in consent discussions
interpretation and discussion of results
NICOLE SKOETZ
interpretation and discussion of results
writing of the review
SVEN THOENES
protocol elaboration
study selection
participation in consent discussions
ALEKSANDRA ZGIERSKA
protocol elaboration
study selection
participation in consent discussions
interpretation and discussion of results
SUSANNE RÖSNER
protocol elaboration
study selection
data extraction
data management
participation in consent discussions
analysis of data
interpretation and discussion of results
writing of the review
securing funding
Sven Thoenes died in October 2020 before the publication of this review. The contributions as stated above were provided before the author died.
Sources of support
Internal sources
-
Forel Klinik, Switzerland
Provision of infrastructure, related services and salery
External sources
-
National Institutes of Health, USA
National Center for Complementary & Integrative Health Award Number K23AT010879
Declarations of interest
SIMON B. GOLDBERG: No conflict of interest known
BRIAN T. PACE: No conflict of interest known
MATAS GRISKAITIS: No conflict of interest known
REINHARD WILLUTZKI: No conflict of interest known
NICOLE SKOETZ: No conflict of interest known
SVEN THOENES: (deceased, October 2020). No conflict of interest known. This declaration of interest was provided before the author died.
ALEKSANDRA ZGIERSKA: Dr. Zgierska is a member of the Board of Directors for the American Society of Addiction Medicine.
SUSANNE RÖSNER: No conflict of interest known
Deceased
New
References
References to studies included in this review
Abed 2019 {published data only}
- Abed M, Ansari Shahidi M. Mindfulness-based relapse prevention to reduce lapse and craving. Journal of Substance Use 2019;24(6):638-42. [DOI: 10.1080/14659891.2019.1640305] [DOI] [Google Scholar]
Alegria 2019 {published data only}
- Alegria M, Falgas-Bague I, Collazos F, Camacho RC, Markle SL, Wang Y, et al. Evaluation of the integrated intervention for dual problems and early action among latino immigrants with co-occurring mental health and substance misuse symptoms: a randomized clinical trial. JAMA Network Open 2019;2(1):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuna LR, Falgas-Bague I, Ramos Z, Porche MV, Alegria M. Development of a cognitive behavioral therapy with integrated mindfulness for latinx immigrants with co-occurring disorders: analysis of intermediary outcomes. Psychological Trauma: Theory, Research, Practice, and Policy 2020;12(8):825-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alizadehgoradel 2019 {published data only}
- Alizadehgoradel J, Imani S, Nejati V, Fathabadi J. Mindfulness-based substance abuse treatment (MBSAT) improves executive functions in adolescents with substance use disorders. Neurology Psychiatry and Brain Research 2019;34:13-21. [DOI: ] [Google Scholar]
Alterman 2004 {published data only}
- Alterman AI, Koppenhaver JM, Mulholland E, Ladden LJ, Baime MJ. Pilot trial of effectiveness of mindfulness meditation for substance abuse patients. Journal of Substance Use 2004;9(6):259-68. [DOI: ] [Google Scholar]
Asl 2014a {published data only}
- Asl NH, Barahmand U. Effectiveness of mindfulness-based cognitive therapy for co-morbid depression in drug-dependent males. Archives of Psychiatric Nursing 2014;28(5):314-8. [DOI: 10.1016/j.apnu.2014.05.003] [DOI] [PubMed] [Google Scholar]
Asl 2014b {published and unpublished data}
- Asl NRH, Hosseinalipour F. Effectiveness of mindfulness-based stress reduction intervention for health-related quality of life in drug-dependent males. Iranian Red Crescent Medical Journal 2014;16(9):1-4. [DOI: 10.5812/ircmj.12608] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bein 2015 {published data only}
- Bein Z. A pilot study of an 8-week mindfulness-based intervention for veterans with posttraumatic symptoms and co-occurring substance use disorders. Dissertation Abstracts International: Section B: The Sciences and Engineering 2015;75(10-B(E)).
Bevan 2012 {published and unpublished data}
- Bevan E. The effect of mindfulness training on drug craving is moderated by level of negative affect. Dissertation Abstracts International: Section B: The Sciences and Engineering 2012;72(7-B):4312.
Black 2019 {published data only}
- Black DS, Amaro H. Moment-by-moment in women's recovery (MMWR): mindfulness-based intervention effects on residential substance use disorder treatment retention in a randomized controlled trial. Behaviour Research and Therapy 2019;120:1-11. [DOI: 10.1016/j.brat.2019.103437] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02977988. Women's treatment and early recovery. https://clinicaltrials.gov/show/NCT02977988 2016.
Bowen 2009 {published data only}
- Bowen S, Chawla N, Collins SE, Witkiewitz K, Hsu S, Grow J, et al. Mindfulness-based relapse prevention for substance use disorders: a pilot efficacy trial. Substance Abuse 2009;30(4):295-305. [DOI: 10.1080/08897070903250084] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SH, Collins SE, Marlatt GA. Examining psychometric properties of distress tolerance and its moderation of mindfulness-based relapse prevention effects on alcohol and other drug use outcomes. Addictive Behaviors 2013;38(3):1852-8. [DOI: 10.1016/j.addbeh.2012.11.002] [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Bowen S, Douglas H, Hsu SH. Mindfulness-based relapse prevention for substance craving. Addictive Behaviors 2013;38(2):1563-71. [DOI: 10.1016/j.addbeh.2012.04.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Bowen S. Depression, craving, and substance use following a randomized trial of mindfulness-based relapse prevention. Journal of Consulting and Clinical Psychology 2010;78(3):362-74. [DOI: 10.1037/a0019172] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowen 2014 {published data only (unpublished sought but not used)}
- Bowen S, Witkiewitz K, Clifasefi SL, Grow J, Chawla N, Hsu SH, et al. Relative efficacy of mindfulness-based relapse prevention, standard relapse prevention, and treatment as usual for substance use disorders: a randomized clinical trial. JAMA psychiatry 2014;71(5):547-56. [DOI: 10.1001/jamapsychiatry.2013.4546] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll H, Lustyk MK. Mindfulness-based relapse prevention for substance use disorders: effects on cardiac vagal control and craving under stress. Mindfulness 2017;9(2):488-99. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle T, Bowen S. Evaluating substance use treatment efficacy for younger and older adults. Addictive Behaviors 2021;112(106618):1-4. [DOI] [PubMed] [Google Scholar]
- Greenfield BL, Roos C, Hagler KJ, Stein E, Bowen S, Witkiewitz KA. Race/ethnicity and racial group composition moderate the effectiveness of mindfulness-based relapse prevention for substance use disorder. Addictive Behaviors 2018;81:96-103. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao YY, Tofighi D, Kruger ES, Lee Van Horn M, MacKinnon DP, Witkiewitz K. The (lack of) replication of self-reported mindfulness as a mechanism of change in mindfulness-based relapse prevention for substance use disorders. Mindfulness 2018;10(4):724-36. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos C, Bowen S, Witkiewitz K. Approach coping and substance use outcomes following mindfulness-based relapse prevention among individuals with negative affect symptomatology. Mindfulness 2020;11:2397-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos CR, Bowen S, Witkiewitz K. Baseline patterns of substance use disorder severity and depression and anxiety symptoms moderate the efficacy of mindfulness-based relapse prevention. Journal of Consulting and Clinical Psychology 2017;85(11):1041-51. [DOI: 10.1037/ccp0000249] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos CR, Stein E, Bowen S, Witkiewitz K. Individual gender and group gender composition as predictors of differential benefit from mindfulness-based relapse prevention for substance use disorders. Mindfulness 2019;10(8):1560-67. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brewer 2009 {published data only}
- Brewer JA, Sinha R, Chen JA, Michalsen RN, Babuscio TA, Nich C, et al. Mindfulness training and stress reactivity in substance abuse: results from a randomized, controlled stage I pilot study. Substance Abuse 2009;30(4):306-17. [DOI: 10.1080/08897070903250241] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2017 {published data only}
- Brown SM, Bender KA, Bellamy JL, Garland EL, Dmitrieva J, Jenson JM. A pilot randomized trial of a mindfulness-informed intervention for child welfare-involved families. Mindfulness 2021;12:420-35. [Google Scholar]
- Brown SM. A mindfulness-based intervention to improve family functioning among child welfare-involved families with substance use. Dissertation Abstracts International Section A: Humanities and Social Sciences 2017;77(11-A(E)):1-21. [Google Scholar]
Davis 2013 {published data only}
- Davis JM, Mills DM, Stankevitz KA, Manley AR, Majeskie MR, Smith SS. Pilot randomized trial on mindfulness training for smokers in young adult binge drinkers. BMC Complementary and Alternative Medicine 2013;13(1):215-24. [DOI: 10.1186/1472-6882-13-215] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 2018 {published data only (unpublished sought but not used)}
- Davis JP, Barr N, Dworkin ER, Dumas TM, Berey B, DiGuiseppi G, et al. Effect of mindfulness-based relapse prevention on impulsivity trajectories among young adults in residential substance use disorder treatment. Mindfulness 2019;10(10):1997-2009. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JP, Berry D, Dumas TM, Ritter E, Smith DC, Menard C, et al. Substance use outcomes for mindfulness based relapse prevention are partially mediated by reductions in stress: results from a randomized trial. Journal of Substance Abuse Treatment 2018;91:37-48. [DOI: 10.1016/j.jsat.2018.05.002] [DOI] [PubMed] [Google Scholar]
- Davis JP. Effect of mindfulness based relapse prevention on developmental trends, stress, and substance use among young adults in residential substance use treatment: a randomized controlled trial. Dissertation Abstracts International Section A: Humanities and Social Sciences 2019;80(5-A(E)):1-229. [Google Scholar]
de Dios 2012 {published and unpublished data}
- Dios MA, Herman DS, Britton WB, Hagerty CE, Anderson BJ, Stein MD. Motivational and mindfulness intervention for young adult female marijuana users. Journal of Substance Abuse Treatment 2012;42(1):56-64. [DOI: 10.1016/j.jsat.2011.08.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
Esmaeili 2017 {published data only (unpublished sought but not used)}
- Esmaeili A, Khodadadi M, Norozi E, Miri MR. Effectiveness of mindfulness-based cognitive group therapy on cognitive emotion regulation of patients under treatment with methadone. Journal of Substance Use 2017;23(1):58-62. [DOI: 10.1080/14659891.2017.1348553] [DOI] [Google Scholar]
Foroushani 2019 {published data only (unpublished sought but not used)}
- Foroushani NS. The impact of mindfulness-based relapse prevention on craving, lapse and mindfulness fostering in addicted patients in methadone maintenance treatment. Heroin Addiction and Related Clinical Problems 2019;21(5):33-40. [Google Scholar]
Garland 2010 {published data only}
- Garland EL, Gaylord SA, Boettiger CA, Howard MO. Mindfulness training modifies cognitive, affective, and physiological mechanisms implicated in alcohol dependence: results of a randomized controlled pilot trial. Journal of Psychoactive Drugs 2010;42(2 XOID - PSYCINFO):177-92. [DOI: 10.1080/02791072.2010.10400690] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL. Biopsychosocial assessment of a mindfulness-oriented cognitive intervention for alcohol dependent adults. Dissertation Abstracts International Section A: Humanities and Social Sciences 2010;71(1-A):333. [Google Scholar]
Garland 2016 {published data only (unpublished sought but not used)}
- Garland EL, Roberts-Lewis A, Tronnier CD, Graves R, Kelley K. Corrigendum to "Mindfulness-oriented recovery enhancement versus CBT for co-occurring substance dependence, traumatic stress, and psychiatric disorders: Proximal outcomes from a pragmatic randomized trial" (Behav. Res. Ther. vol 77, pg 7, 2016). Behaviour Research and Therapy 2018;100:78. [DOI: 10.1016/j.brat.2017.09.007] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Roberts-Lewis A, Tronnier CD, Graves R, Kelley K. Mindfulness-Oriented Recovery Enhancement versus CBT for co-occurring substance dependence, traumatic stress, and psychiatric disorders: Proximal outcomes from a pragmatic randomized trial. Behaviour research and therapy 2016;77:7-16. [DOI: 10.1016/j.brat.2015.11.012] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garland 2019 {published data only}
- Garland EL, Hanley AW, Kline A, Cooperman NA. Mindfulness-Oriented Recovery Enhancement reduces opioid craving among individuals with opioid use disorder and chronic pain in medication assisted treatment: ecological momentary assessments from a stage 1 randomized controlled trial. Drug and Alcohol Dependence 2019;203:61‐5. [DOI: 10.1016/j.drugalcdep.2019.07.007] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glasner 2017 {published data only}
- Glasner-Edwards S, Mooney L, Ang A, Garneau HC, Hartwell EE, Brecht ML, et al. Mindfulness based relapse prevention improves stimulant use among adults with major depression and generalized anxiety disorder. Drug and Alcohol Dependence 2015;156:e80. [DOI: ] [Google Scholar]
- Glasner S, Mooney LJ, Ang A, Garneau HC, Hartwell E, Brecht ML, et al. Mindfulness-based relapse prevention for stimulant dependent adults: a pilot randomized clinical trial. Mindfulness 2017;8(1):126-35. [DOI: 10.1007/s12671-016-0586-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Himelstein 2015 {published data only (unpublished sought but not used)}
- Himelstein S, Saul S, Garcia-Romeu A. Does mindfulness meditation increase effectiveness of substance abuse treatment with incarcerated youth? A pilot randomized controlled trial. Mindfulness 2015;6(6):1472-80. [DOI: 10.1007/s12671-015-0431-6] [DOI] [Google Scholar]
Imani 2015 {published data only}
- Imani S, Vahid MK, Gharraee B, Habibi M, Bowen, S, Noroozi A. Comparing mindfulness-based group therapy with treatment as usual for opioid dependents: a pilot randomized clinical trial study protocol. Iranian Journal of Psychiatry and Behavioral Sciences 2015;9(1):1-4. [DOI: 10.5812/ijpbs.216] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imani S, Vahid MK, Gharraee B, Noroozi A, Habibi M, Bowen S. Effectiveness of mindfulness-based group therapy compared to the usual opioid dependence treatment. Iranian Journal of Psychiatry 2015;10(3):175-84. [PMC free article] [PubMed] [Google Scholar]
Jenaabadi 2017 {published data only}
- Jenaabadi H, Jahangir AH. Comparing the effectiveness of mindfulness-based group therapy and methadone maintenance therapy on psychological symptoms (obsession, interpersonal sensitivity, depression, anxiety, and aggression) among opioid-dependent patients. Shiraz E Medical Journal 2017;18(6):e45224. [DOI: ] [Google Scholar]
Lee 2011 {published and unpublished data}
- Lee KH, Bowen S, An-Fu B. Psychosocial outcomes of mindfulness-based relapse prevention in incarcerated substance abusers in Taiwan: a preliminary study. Journal of Substance Use 2011;16(6):476-83. [DOI: 10.3109/14659891.2010.505999] [DOI] [Google Scholar]
Machado 2020 {published data only}
- Machado MP, Fidalgo, TM, Brasiliano S, Hochgraf PB, Noto AR. The contribution of mindfulness to outpatient substance use disorder treatment in Brazil: a preliminary study. Brazilian Journal of Psychiatry 2020;42(5):527-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marfurt 2007 {published data only}
- Marfurt S. Reducing stress in women recovering from substance abuse. Dissertation Abstracts International: Section B: The Sciences and Engineering 2007;68(1-B):209.
Margolin 2006 {published data only}
- Margolin A, Beitel M, Schuman-Olivier Z, Avants SK. A controlled study of a spirituality-focused intervention for increasing motivation for HIV prevention among drug users. AIDS Education and Prevention : official publication of the International Society for AIDS Education 2006;18(4):311-22. [DOI: 10.1521/aeap.2006.18.4.311] [DOI] [PubMed] [Google Scholar]
Mermelstein 2015 {published data only}
- Mermelstein LC, Garske JP. A brief mindfulness intervention for college student binge drinkers: a pilot study. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors 2015;29(2):259-69. [DOI: 10.1037/adb0000040] [DOI] [PubMed] [Google Scholar]
- Mermelstein LC. A brief mindfulness intervention to decrease binge drinking among college students: a controlled study. Dissertation Abstracts International: Section B: The Sciences and Engineering 2015;76(5-B(E)):1-157. [Google Scholar]
Ramezani 2019 {published data only}
- Ramezani S, Afkhamzadeh AR, Qorbani H, Naimi S, Rahmani S. Effect of mindfulness-based cognitive therapy on substance dependence intensity and cognitive emotion regulation in patients under methadone maintenance treatment. Journal of Practice in Clinical Psychology 2019;7(3):225-34. [Google Scholar]
Shorey 2017 {published data only}
- Shorey RC, Elmquist J, Gawrysiak MJ, Strauss, C, Haynes E, Anderson S, et al. A randomized controlled trial of a mindfulness and acceptance group therapy for residential substance use patients. Substance Use & Misuse 2017;52(11):1400-10. [DOI: 10.1080/10826084.2017.1284232] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vowles 2020 {published data only}
- Pilot study of combined treatment for veterans with chronic pain & opiate misuse. https://clinicaltrials.gov/show/NCT02423772.
- Vowles KE, Edwards KA, Witkiewitz K, Cusack KJ, Cardon KE, Gilliam WP, et al. Integrated behavioral treatment for veterans with co-morbid chronic pain and hazardous opioid use: a randomized controlled pilot trial. Journal of Pain 2020;21(7-8):798-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Witkiewitz 2014 {published data only}
- Witkiewitz K, Greenfield BL, Bowen S. Mindfulness-based relapse prevention with racial and ethnic minority women. Addictive Behaviors 2013;38(12):2821-4. [DOI: 10.1016/j.addbeh.2013.08.018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Warner K, Sully B, Barricks A, Stauffer C, Thompson BL, et al. Randomized trial comparing mindfulness-based relapse prevention with relapse prevention for women offenders at a residential addiction treatment center. Substance Use & Misuse 2014;49(5):536-46. [DOI: 10.3109/10826084.2013.856922] [DOI] [PubMed] [Google Scholar]
Wongtongkam 2018 {published data only (unpublished sought but not used)}
- Wongtongkam N, Lampoo S, Choocherd P, Chiangkuntod S. Partial efficacy of vipassana mindfulness approach in alcohol-dependent persons. Alcoholism Treatment Quarterly 2018;36(1):3-14. [DOI: ] [Google Scholar]
Wongtongkam 2019 {published data only}
- Wongtongkam N, Bhavanaveeranusith P. A pilot study of Vipassana meditation with female drug users at a rehabilitation centre, Thailand. Therapeutic Communities 2019;40(3/4):132-41. [Google Scholar]
Yaghubi 2017 {published data only}
- Yaghubi M, Zargar F, Akbari H. Comparing effectiveness of mindfulness-based relapse prevention with treatment as usual on impulsivity and relapse for methadone-treated patients: a randomized clinical trial. Addiction & Health 2017;9(3):156‐65. [PMC free article] [PubMed] [Google Scholar]
- Yaghubi M, Zargar F. Effectiveness of mindfulness-based relapse prevention on quality of life and craving in methadone-treated patients: a randomized clinical trial. Addiction & Health 2018;10(4):250‐9. [DOI: 10.22122/ahj.v10i4.573] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghubi M. Effect of mindfulness based relapse prevention model on impulsivity, distress tolerance and relapse prevention in opioid dependent patient. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2016010525870N1 2016.
Zemestani 2016 {published data only}
- Zemestani M, Ottaviani C. Effectiveness of mindfulness-based relapse prevention for co-occurring substance use and depression disorders. Mindfulness 2016;7(6):1347-55. [DOI: 10.1007/s12671-016-0576-y] [DOI] [Google Scholar]
Zgierska 2017 {published data only}
- NCT01056484. Mindfulness Meditation for Health. https://clinicaltrials.gov/show/NCT01056484 2010.
- Zgierska A, Rabago D, Lerner F, Goodman V, Coe C. Biological effects of mindfulness-based relapse prevention in alcohol dependence: pilot clinical trial. Journal of Investigative Medicine 2010;58(4):651. [DOI: 10.231/JIM.0b013e3181d85541] [DOI] [Google Scholar]
- Zgierska AE, Burzinski CA, Mundt MP, McClintock AS, Cox J, Coe CL, et al. Mindfulness-based relapse prevention for alcohol dependence: findings from a randomized controlled trial. Journal of Substance Abuse Treatment 2019;100:8‐17. [DOI: 10.1016/j.jsat.2019.01.013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgierska AE, Shapiro J, Burzinski CA, Lerner F, Goodman-Strenski V. Maintaining treatment fidelity of mindfulness-based relapse prevention intervention for alcohol dependence: a randomized controlled trial experience. Evidence-Based Complementary and Alternative Medicine 2017;2017:1-12. [DOI: 10.1155/2017/9716586] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2019 {published data only}
- Zhang JT, Zhang JY, Du ZY, Li J, Lu CH, Du J. Effect of mindfulness-based intervention on functional connectivity of resting state electroencephalogram of amphetamine-type stimulants use patients. Journal of Shanghai Jiao Tong University 2019;39(12):1416-21. [Google Scholar]
References to studies excluded from this review
Alexander 2019 {published data only}
- Alexander K, Kronk R, Sekula K, Short V, Abatemarco D. Implementation of a mindfulness intervention for women in treatment for opioid use disorder and its effects on depression symptoms. Issues in Mental Health Nursing 2019;40:690-6. [DOI: ] [DOI] [PubMed] [Google Scholar]
Amaro 2017 {published data only}
- Amaro H, Black DS. Moment-by-Moment in Women's Recovery: Randomized controlled trial protocol to test the efficacy of a mindfulness-based intervention on treatment retention and relapse prevention among women in residential treatment for substance use disorder. Contemporary Clinical Trials 2017;62:146-52. [DOI: 10.1016/j.cct.2017.09.004] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bandawar 2016 {published data only}
- Bandawar MS, Field M. Effects of inhibition training and a brief mindfulness intervention on event-related potential markers of inhibitory control in heavy drinkers. Alcoholism: Cclinical and Experimental Research. Conference: 39th annual scientific meeting of the research society on alcoholism. New orleans, LA united states. Conference start: 20160625. Conference end: 20160629. Conference publication: (var.pagings) 2016;40:212a. [DOI: 10.1111/acer.13084] [DOI] [Google Scholar]
Bowen 2006 {published data only}
- Bowen S, Witkiewitz K, Dillworth TM, Chawla N, Simpson TL, Ostafin BD, et al. Mindfulness meditation and substance use in an incarcerated population. Psychology of Addictive Behaviors 2006;20(3):343-7. [DOI: ] [DOI] [PubMed] [Google Scholar]
Bowen 2012 {published data only}
- Bowen S, Kurz AS. Between- session practice and therapeutic alliance as predictors of mindfulness after mindfulness-based relapse prevention. Journal of Clinical Psychology 2012;68(3):236-45. [DOI: 10.1002/jclp.20855] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowen 2017 {published data only}
- Bowen S, De Boer D, Bergman AL. The role of mindfulness as approach-based coping in the PTSD-substance abuse cycle. Addictive Behaviors 2017;64:212-6. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carpentier 2015 {published data only}
- Carpentier D, Romo L, Bouthillon-Heitzmann P, Limosin F. Mindfulness-based-relapse prevention (MBRP): evaluation of the impact of a group of mindfulness therapy in alcohol relapse prevention for alcohol use disorders. L'Encephale 2015;41(6):521-6. [DOI: 10.1016/j.encep.2015.05.003] [DOI] [PubMed] [Google Scholar]
Caselli 2016 {published data only}
- Caselli G, Gemelli A, Spada MM, Wells A. Experimental modification of perspective on thoughts and metacognitive beliefs in alcohol use disorder. Psychiatry Research 2016;244:57-61. [DOI: 10.1016/j.psychres.2016.07.029] [DOI] [PubMed] [Google Scholar]
Chen 2019 {published data only}
- Chen JY, Yu JC, Cao JP, Xiao Y, Gu H, Zhong RL, et al. Abstinence following a motivation-skill-desensitization-mental energy intervention for heroin dependence: a three-year follow-up result of a randomized controlled trial. Current Medical Science 2019;39(3):472-82. [DOI: 10.1007/s11596-019-2062-y] [DOI] [PubMed] [Google Scholar]
Chouhan 2011 {published data only}
- Chouhan S, Kumar S. Comparative study between effectiveness of dance movement therapy and progressive relaxation therapy with music for stress management in college students. Indian Journal of Physiotherapy & Occupational Therapy 2011;5(2):172-5. [Google Scholar]
Collins 2009 {published data only}
- Collins SE, Chawla N, Hsu SH, Grow J, Otto JM, Marlatt GA. Language-based measures of mindfulness: initial validity and clinical utility. Psychology of Addictive Behaviors : Journal of the Society of Psychologists in Addictive Behaviors 2009;23(4):743-9. [DOI: 10.1037/a0017579] [DOI] [PMC free article] [PubMed] [Google Scholar]
Crescentini 2015 {published data only}
- Crescentini C, Matiz A Fabbro F. Improving personality/character traits in individuals with alcohol dependence: the influence of mindfulness-oriented meditation. Journal of Addictive Diseases 2015;34(1):75-87. [DOI: 10.1080/10550887.2014.991657] [DOI] [PubMed] [Google Scholar]
Crowfoot 2014 {published data only}
- Crowfoot K. The effects of meditation on the perceived stress of participants in an outpatient substance abuse program. Dissertation Abstracts International: Section B: The Sciences and Engineering 2014;74(11-B(E)):Not-specified. [Google Scholar]
DRKS00015678 {published data only}
- DRKS00015678. Substance use prevention in adolescence by the mindfulness-augmented Strengthening Families Program 10-14 („SFP-Mind“). http://www.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00015678 2019.
Enkema 2017 {published data only}
- Enkema MC, Bowen S. Mindfulness practice moderates the relationship between craving and substance use in a clinical sample. Drug and Alcohol Dependence 2017;179:1-7. [DOI: 10.1016/j.drugalcdep.2017.05.036] [DOI] [PubMed] [Google Scholar]
Fonagy 2010 {published data only}
- Fonagy P. The changing shape of clinical practice: driven by science or by pragmatics? Psychoanalytic Psychotherapy 2010;24(1):22-43. [DOI: 10.1080/02668731003590139] [DOI] [Google Scholar]
Garland 2014 {published data only}
- Garland EL, Froeliger B, Howard MO. Effects of mindfulness-oriented recovery enhancement on reward responsiveness and opioid cue-reactivity. Psychopharmacology 2014;231(16):3229-38. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garland 2014a {published data only}
- Garland EL, Manusov EG, Froeliger B, Kelly A, Williams JM, Howard MO. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: results from an early-stage randomized controlled trial. Journal of Consulting and Clinical Psychology: 2014;82(3):448-59. [DOI: 10.1037/a0035798] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garland 2014b {published data only}
- Garland EL, Black DS. Mindfulness for chronic pain and prescription opioid misuse: novel mechanisms and unresolved issues. Substance Use & Misuse 2014;49(5):608-11. [DOI: 10.3109/10826084.2014.852801] [DOI] [PubMed] [Google Scholar]
Garland 2017 {published data only}
- Garland EL, Howard MO, Zubieta JK, Froeliger B. Restructuring hedonic dysregulation in chronic pain and prescription opioid misuse: effects of mindfulness-oriented recovery enhancement on responsiveness to drug cues and natural rewards. Psychotherapy and Psychosomatics 2017;86(2):111-2. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garland 2017a {published data only}
- Garland EL, Bryan CJ, Finan PH Thomas EA Priddy SE, Riquino MR, et al. Pain, hedonic regulation, and opioid misuse: modulation of momentary experience by mindfulness-oriented recovery enhancement in opioid-treated chronic pain patients. Drug and Alcohol Dependence 2017;173 Suppl 1:65-72. [DOI: 10.1016/j.drugalcdep.2016.07.033] [DOI] [PubMed] [Google Scholar]
Garland 2017b {published data only}
- Garland EL, Baker AK, Howard MO. Mindfulness- oriented recovery enhancement reduces opioid attentional bias among prescription opioid-treated chronic pain patients. Journal of the Society for Social Work and Research 2017;8(4):493-509. [DOI: 10.1086/694324] [DOI] [Google Scholar]
Garland 2018 {published data only}
- Garland EL, Howard MO. Enhancing natural reward responsiveness among opioid users predicts chronic pain relief: eeg analyses from a trial of mindfulness-oriented recovery enhancement. Journal of the Society for Social Work and Research 2018;9(2):285-303. [DOI: 10.1086/697685] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garland 2019a {published data only}
- Garland EL, Bryan MA, Priddy SE, Riquino MR, Froeliger B, Howard MO. Effects of mindfulness-oriented recovery enhancement versus social support on negative affective interference during inhibitory control among opioid-treated chronic pain patients: a pilot mechanistic study. Annals of behavioral medicine 2019;53(10):865‐76. [DOI: 10.1093/abm/kay096] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garland 2019b {published data only}
- Garland EL, Hanley AW, Riquino MR, Reese SE, Baker AK, Salas K, et al. Mindfulness-oriented recovery enhancement reduces opioid misuse risk via analgesic and positive psychological mechanisms: a randomized controlled trial. Journal of Consulting and Clinical Psychology 2019;87(10):927-40. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garland 2019c {published data only}
- Garland EL, Atchley RM, Hanley AW, Zubieta JK, Froeliger B. Mindfulness- oriented recovery enhancement remediates hedonic dysregulation in opioid users: neural and affective evidence of target engagement. Science Advances 2019;5(10):1-12. [DOI: 10.1126/sciadv.aax1569] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garland 2020 {published data only}
- Garland EL, Hudak J, Hanley AW, Nakamura Y. Mindfulness-oriented recovery enhancement reduces opioid dose in primary care by strengthening autonomic regulation during meditation. American Psychologist 2020;75(6):840-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gayner 2012 {published data only}
- Gayner B, Esplen M, DeRoche P, Wong J, Bishop S, Kavanagh L, et al. A randomized controlled trial of mindfulness-based stress reduction to manage affective symptoms and improve quality of life in gay men living with HIV. Journal of Behavioral Medicine 2012;35(3):272-85. [DOI: 10.1007/s10865-011-9350-8] [DOI] [PubMed] [Google Scholar]
Gibson 2019 {published data only}
- Gibson BC, Votaw VR, Stein ER, Clark VP, Witkiewitz K. Increases in mindfulness following mindfulness-based relapse prevention and transcranial direct current stimulation to reduce heavy drinking. Alcoholism: Clinical and Experimental Research 2019;43(Supplement 1):56A. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grow 2015 {published data only}
- Grow JC, Collins SE, Harrop EN, Marlatt GA. Enactment of home practice following mindfulness-based relapse prevention and its association with substance-use outcomes. Addictive Behaviors 2015;40:16-20. [DOI: 10.1016/j.addbeh.2014.07.030] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hai 2021 {published data only}
- Hai H, Wigmore B, Franklin C, Shorkey C, Sternberg K, Cole AH, et al. Efficacy of two-way prayer meditation in improving the psychospiritual well-being of people with substance use disorders: a pilot randomized controlled trial. Substance Abuse 2021;in press:Epub ahead of print. [DOI] [PubMed] [Google Scholar]
Hargreaves 1974 {published data only}
- Hargreaves WA, Showstack J, Flohr R, Brady C, Harri S. Treatment acceptance following intake assignment to individual therapy, group therapy, or contact group. Archives of General Psychiatry 1974;31(3):343-9. [DOI] [PubMed] [Google Scholar]
Hruschak 2021 {published data only}
- Hruschak V, Rosen D, Tierney M, Eack SM, Wasan AD, Cochran G. Integrated Psychosocial Group Treatment: a randomized pilot trial of a harm reduction and preventive approach for patients with chronic pain at risk of opioid misuse. Pain Medicine 2021;22(9):2007-18. [DOI] [PubMed] [Google Scholar]
Iranshahri 2015 {published data only}
- Iranshahri B, Jenaabadi H. The effectiveness of mindfulness therapy in controlling under treatment addicts’ drug cravings. Open Journal of Medical Psychology 2015;04(03):88-98. [DOI: 10.4236/ojmp.2015.43009] [DOI] [Google Scholar]
IRCT20150413021727N2 {published data only}
- IRCT20150413021727N2. The effect of mindfulness-based group therapy in relapse prevention of methamphetamine-addicted males. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20150413021727N2 2017.
IRCT2015042420961N {published data only}
- IRCT2015042420961N. The effect of wells’ metacognitive therapy on dysfunctional thoughts in addicts with drug abuse. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2015042420961N3 2015.
Kamboj 2017 {published data only}
- Kamboj SK, Irez D, Serfaty S, Thomas E, Das RK, Freeman TP. Ultra- brief mindfulness training reduces alcohol consumption in at-risk drinkers: a randomized double-blind active-controlled experiment. International Journal of Neuropsychopharmacology 2017;20(11):936-47. [DOI: 10.1093/ijnp/pyx064] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2017 {published data only}
- Lee MY, Zaharlick A, Akers D. Impact of meditation on mental health outcomes of female trauma survivors of interpersonal violence with co-occurring disorders: a randomized controlled trial. Journal of interpersonal Violence 2017;32(14):2139-65. [DOI: 10.1177/0886260515591277] [DOI] [PubMed] [Google Scholar]
Lyons 2019 {published data only}
- Lyons T, Womack VY, Cantrell WD, Kenemore T. Mindfulness-based relapse prevention in a jail drug treatment program. Substance Use & Misuse 2019;54(1):57-64. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Magidson 2011 {published data only}
- Magidson JF, Gorka SM, MacPherson L, Hopko DR, Blanco C, Lejuez CW, et al. Examining the effect of the Life Enhancement Treatment for Substance Use (LETS ACT) on residential substance abuse treatment retention. Addictive Behaviors 2011;36(6):615-23. [DOI: 10.1016/j.addbeh.2011.01.016] [DOI] [PMC free article] [PubMed] [Google Scholar]
Malouf 2017 {published data only}
- Malouf ET, Youman K, Stuewig J, Witt EA, Tangney JP. A pilot rct of a values-based mindfulness group intervention with jail inmates: evidence for reduction in post-release risk behavior. Mindfulness 2017;8(3):603-14. [DOI: 10.1007/s12671-016-0636-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marcus 2001 {published data only}
- Marcus MT, Fine M, Kouzekanani K. Mindfulness-based meditation in a therapeutic community. Journal of Substance Use 2001;5(4):305-11. [Google Scholar]
Marcus 2009 {published data only}
- Marcus MT, Schmitz J, Moeller G, Liehr P, Cron SG, Swank P, et al. Mindfulness-based stress reduction in therapeutic community treatment: a stage 1 trial. American Journal of Drug and Alcohol Abuse 2009;35(2):103-8. [DOI: 10.1080/00952990902823079] [DOI] [PubMed] [Google Scholar]
Murphy 2014 {published data only}
- Murphy CM, MacKillop J. Mindfulness as a strategy for coping with cue-elicited cravings for alcohol: an experimental examination. Alcoholism, Clinical and Experimental Research 2014;38(4):1134-42. [DOI: 10.1111/acer.12322] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakamura 2015 {published data only}
- Nakamura Y, Lipschitz DL, Kanarowski E, McCormick T, Sutherland D, Melow-Murchie M. Investigating Impacts of Incorporating an adjuvant mind-body intervention method into treatment as usual at a community-based substance abuse treatment facility: a pilot randomized controlled study. Sage Open 2015;5(1):1-18. [DOI: 10.1177/2158244015572489] [DOI] [Google Scholar]
NCT01505101 {published data only}
- NCT01505101. Mindfulness- oriented recovery enhancement for chronic pain patients receiving opioid therapy. https://clinicaltrials.gov/show/NCT01505101 2012.
NCT04082637 {published data only}
- NCT04082637. Mindful body awareness with buprenorphine for opioid use disorder treatment. https://clinicaltrials.gov/show/NCT04082637 2019.
NCT04160754 {published data only}
- Mindfulness for at risk youth: understanding substance use and important mechanisms of change. https://clinicaltrials.gov/show/NCT04160754.
NCT04567043 {published data only}
- Treating opioid misuse via mindfulness-based just-in-time adaptive intervention. https://clinicaltrials.gov/show/NCT04567043.
NCT04769986 {published data only}
- Mobile mindfulness for alcohol use and PTSD among veterans. https://clinicaltrials.gov/show/NCT04769986.
Nice 2008 {published data only}
- Nice PR. Mindfulness interventions in the treatment of substance and mood disorders. Dissertation Abstracts International: Section B: The Sciences and Engineering 2008;69(3-B):1982. [Google Scholar]
Ojehagen 1992 {published data only}
- Ojehagen A, Berglund M. Acceptance, attrition, and outcome in an outpatient treatment programme for alcoholics. A comparison between a randomized and a non-randomized process-outcome study. European Archives of Psychiatry and Clinical Neuroscience 1992;242(2-3):82-4. [DOI] [PubMed] [Google Scholar]
Parker 1978 {published data only}
- Parker JC, Gilbert GS, Thoreson RW. Anxiety management in alcoholics: a study of generalized effects of relaxation techniques. Addictive Behaviors 1978;3(2):123-7. [DOI] [PubMed] [Google Scholar]
Parker 1978a {published data only}
- Parker JC, Gilbert GS, Thoreson RW. Reduction of autonomic arousal in alcoholics: a comparison of relaxation and meditation techniques. Journal of Consulting and Clinical Psychology 1978;46(5):879-86. [DOI: ] [DOI] [PubMed] [Google Scholar]
Price 2012 {published data only}
- Price CJ, Wells EA, Donovan DM, Rue T. Mindful awareness in body-oriented therapy as an adjunct to women's substance use disorder treatment: A pilot feasibility study. Journal of Substance Abuse Treatment 2012;43(1):94-107. [DOI: 10.1016/j.jsat.2011.09.016] [DOI] [PMC free article] [PubMed] [Google Scholar]
Price 2012a {published data only}
- Price CJ, Wells EA, Donovan DM, Brooks M. Implementation and acceptability of mindful awareness in body-oriented therapy in women's substance use disorder treatment. Journal of Alternative and Complementary Medicine 2012;18(5):454-62. [DOI: 10.1089/acm.2011.0126] [DOI] [PMC free article] [PubMed] [Google Scholar]
Price 2016 {published data only}
- Price C, Crowell S. Interoceptive awareness training for emotion regulation through mindful body awareness for women in treatment for substance use disorder. Journal of Alternative and Complementary Medicine 2016;22(6):A19. [DOI: ] [Google Scholar]
Price 2017 {published data only}
- Price C, Crowell SE, Cheng C, Thompson E. Interoceptive awareness training for women in SUD treatment. Drug and Alcohol Dependence. Conference: 2016 annual meeting of the college on problems of drug dependence, CPDD 2016. United states 2017;171:e169. [DOI: 10.1016/j.drugalcdep.2016.08.464] [DOI] [Google Scholar]
Price 2018 {published data only}
- Price CJ, Thompson EA, Crowell SE, Pike K, Cheng SC, Parent S, et al. Immediate effects of interoceptive awareness training through mindful awareness in body-oriented therapy (mabt) for women in substance use disorder treatment. Substance Abuse 2019;40:102-15. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Price 2019 {published data only}
- Price CJ, Thompson EA, Crowell S, Pike K. Longitudinal effects of interoceptive awareness training through mindful awareness in body-oriented therapy (MABT) as an adjunct to women's substance use disorder treatment: a randomized controlled trial. Drug and Alcohol Dependence 2019;198:140-9. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Price 2019a {published data only}
- Price CJ, Merrill JO, McCarty RL, Pike KC, Tsui JI. A pilot study of mindful body awareness training as an adjunct to office-based medication treatment of opioid use disorder. Journal of Substance Abuse Treatment 2019;108:123-8. [DOI: 10.1016/j.jsat.2019.05.013] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rentala 2020 {published data only}
- Rentala S, Ng S, Chan CLW, Bevoor P, Nayak RB, Desai M. Effect of holistic relapse prevention intervention among individuals with alcohol dependence: a prospective study at a mental health care setting in India. Journal of Ethnicity in Substance Abuse 2020;in press:Epub ahead of print. [DOI] [PubMed] [Google Scholar]
Russell 2019 {published data only}
- Russell BS, Hutchison M, Fusco A. Emotion regulation outcomes and preliminary feasibility evidence from a mindfulness intervention for adolescent substance use. Journal of Child & Adolescent Substance Abuse 2019;28:21-31. [DOI: ] [Google Scholar]
Simpson 2015 {published data only}
- Simpson TL, Stappenbeck CA, Luterek JA, Rosenthal CF, Gurrad B, Kaysen D. Outcomes of an rct comparingtwo copingskills among dually diagnosed individuals with alcohol dependence and PTSD. Alcoholism: Clinical and Experimental Research. 2015;39:299a. [DOI: 10.1111/acer.12742] [DOI] [Google Scholar]
Tang 2016 {published data only}
- Tang YY, Tang R, Posner MI. Mindfulness meditation improves emotion regulation and reduces drug abuse. Drug and Alcohol Dependence 2016;163 Suppl 1:S13-8. [DOI: 10.1016/j.drugalcdep.2015.11.041] [DOI] [PubMed] [Google Scholar]
Temme 2012 {published data only}
- Temme LJ, Fenster J, Ream GL. Evaluation of meditation in the treatment of chemical dependency. Journal of Social Work Practice in the Addictions 2012;12(3):264-81. [DOI: 10.1080/1533256X.2012.702632] [DOI] [Google Scholar]
Vinci 2014 {published data only}
- Vinci C, Peltier MR, Shah S, Kinsaul J, Waldo K, McVay MA, et al. Effects of a brief mindfulness intervention on negative affect and urge to drink among college student drinkers. Behaviour Research and Therapy 2014;59:82-93. [DOI: 10.1016/j.brat.2014.05.012] [DOI] [PubMed] [Google Scholar]
Wupperman 2015 {published data only}
- Wupperman P, Cohen MG, Haller DL Flom P, Litt LC, Rounsaville BJ. Mindfulness and modification therapy for behavioral dysregulation: a comparison trial focused on substance use and aggression. Journal of Clinical Psychology 2015;71(10):964-78. [DOI: 10.1002/jclp.22213] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ACTRN12613000193774 {published data only}
- ACTRN12613000193774. The effects of Mindfulness training people recovering from alcohol dependence. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12613000193774 2013.
Baldus 2018 {published data only}
- Baldus C, Mokros L, Daubmann A, Arnaud N, Holtmann M, Thomasius R, et al. Treatment effectiveness of a mindfulness-based inpatient group psychotherapy in adolescent substance use disorder - study protocol for a randomized controlled trial. Trials 2018;19(1):706. [DOI: 10.1186/s13063-018-3048-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Becker 2017 {published data only}
- Becker A, Ehret AM, Kirsch P. From the neurobiological basis of comorbid alcohol dependence and depression to psychological treatment strategies: study protocol of a randomized controlled trial. BMC Psychiatry 2017;17(1):153. [DOI: 10.1186/s12888-017-1324-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
c9njc, R. B. R. {published data only}
- Effects of mindfulness on impulsivity of peope with alcohol use disorder. http://www.who.int/trialsearch/Trial2.aspx?TrialID=RBR-6c9njc.
CasasGaviln 2018 {published data only}
- Casas Gavilán E, Peña Lorente T. Eficacia del programa de prevención de recaídas basado en mindfulness para la disminución del craving del paciente alcohólico. Nure Investigación 2018;15(93):1-10. [Google Scholar]
Chen 2018 {published data only}
- Chen XJ, Wang DM, Zhou LD, Winkler M, Pauli P, Sui N, et al. Mindfulness-based relapse prevention combined with virtual reality cue exposure for methamphetamine use disorder: study protocol for a randomized controlled trial. Contemporary Clinical Trials 2018;70:99‐105. [DOI: 10.1016/j.cct.2018.04.006] [DOI] [PubMed] [Google Scholar]
Connors 2011 {published data only}
- Connors GJ, Walitzer KS, Smyth NS, Reschke JE. Changes in stress and drinking following MBSR training in alcoholism treatment. Alcohol 2011;45(3):301. [Google Scholar]
CTRI/2018/07/014994 {published data only}
- CTRI/2018/07/014994. Holistic relapse prevention for alcoholic patients. http://www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/07/014994 2018.
Garland 2016a {published data only}
- Garland E. Mindfulness promotes positive emotion-cognition interactions integral to human flourishing: evidence from research on mindfulness-oriented recovery enhancement. Psychosomatic Medicine 2016;78(3):A9. [DOI: 10.1097/PSY.0000000000000343] [DOI] [Google Scholar]
IRCT2013031612826N1 {published data only}
- Irct2013031612826N. Effectiveness of mindfulness based group therapy reduction of risk factors for relapse in opioid addicted. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2013031612826N1 2013.
IRCT2015041321727N1 {published data only}
- IRCT2015041321727N1. The effectiveness of group therapy based on detached mindfulness (DM) techniques in decreasing the tendency and the probability using in drug dependents under methadone maintenance treatment. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2015041321727N1 2015.
IRCT2015061522749N1 {published data only}
- IRCT2015061522749N1. The effectiveness of mindfulness on addiction. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2015061522749N1 2015.
IRCT2015121925603N1 {published data only}
- IRCT2015121925603N. The effectiveness of mindfulness therapy on the methamphetamine addicts. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2015121925603N1 2016.
Irct20170702034844N {published data only}
- Effectiveness of mindfulness-based relapse prevention group therapy among people with opioids use disorder. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20170702034844N5.
IRCT2017081325160N7 {published data only}
- IRCT2017081325160N7. The Efficacy of mindfulness-based cognitive therapy on reduction of psychological symptoms,craving beliefs and increase self-efficacy in opioid abusers. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2017081325160N7 2017.
NCT01211418 {published data only}
- NCT01211418. Integrative meditation (IM) for cocaine addiction. https://clinicaltrials.gov/show/NCT01211418 2010.
NCT02147483 {published data only}
- NCT02147483. Mindfulness- based relapse prevention for alcohol use disorders in remission. https://clinicaltrials.gov/show/NCT02147483 2013.
NCT03366909 {published data only}
- NCT03366909. Mindfulness meditation and cannabis dependence: therapy effectiveness. https://clinicaltrials.gov/show/NCT03366909 2017.
NCT03748875 {published data only}
- NCT03748875. The effect of mindfulness-based relapse prevention on impulsive control circuit among methamphetamine dependents. https://clinicaltrials.gov/show/NCT03748875 2018.
NCT03894501 {published data only}
- NCT03894501. Pilot of mindfulness oriented recovery enhancement in methadonetreatment. https://clinicaltrials.gov/show/NCT03894501 2019.
Negrei 2015 {published data only}
- Negrei C, Stan M, Balalau C, Ginghina O, Baconi DL, Craciun B. The effectiveness of combining pharmacologic strategies with MCBT for diminishing the level of depression and anxiety at patients diagnosed with addiction. Toxicology Letters. 2015;238(2 suppl. 1):S150. [DOI: 10.1016/j.toxlet.2015.08.468] [DOI] [Google Scholar]
Park 2005 {published data only}
- Park HN, Yoo SJ. Effects of meditation training program on self concept, abstinence self-efficacy, and abstinence in alcoholic patients. Journal Korean Academy Psychology Mental Health Nusrsing 2005;14(3):304-12. [Google Scholar]
RBR‐4br6q5 {published data only}
- R B R br6q. The effect of mindfulness meditation on crack cocaine users. http://www.who.int/trialsearch/Trial2.aspx?TrialID=RBR-4br6q5 2016.
References to ongoing studies
DRKS00014041 {published data only}
- DRKS00014041. Treatment of mindfulness-based psychotherapy in adolescent inpatients with substance use disorders. http://www.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00014041 2018.
Ellingson 2018 {published data only}
- Ellingson JM, York Williams S, Hagerty SL, Bryan AD, Hutchison KE. Is mindfulness a mechanism of change in co-occurring Aud and internalizing symptoms: a pilot study of individual MBRP. Alcoholism: Clinical and Experimental Research 2018;42:260. [DOI: 10.1111/acer.13747] [DOI] [Google Scholar]
- York Williams SL, Hagerty SL, Ellingson J, Bryan A, Hutchison KE. Preliminary results on non-abstinence focused treatment for alcohol use disorder in treatment seeking adults. Alcoholism: Clinical and Experimental Research 2018;42:259. [DOI: 10.1111/acer.13747] [DOI] [Google Scholar]
- Zabelski AE, Karoly HC, Hutchison KE, Ross JM. Investigating alcohol and cannabis co-use patterns in a sample of treatment-engaged heavy drinkers. Alcoholism: Clinical and Experimental Research 2020;44:162A. [Google Scholar]
NCT02755103 {published data only}
- NCT02755103. Mindfulness meditation for the treatment of women with comorbid PTSD and SUD. https://clinicaltrials.gov/show/NCT02755103 2016.
NCT03734666 {published data only}
- NCT03734666. Development of a mindfulness-based treatment for the reduction of alcohol use and smoking cessation. https://clinicaltrials.gov/show/NCT03734666 2018.
NCT03883646 {published data only}
- NCT. Mindfulness for alcohol abusing offenders. https://clinicaltrials.gov/show/NCT03883646 2019.
NCT04112186 {published data only}
- NCT. Mindfulness-Oriented Recovery Enhancement (MORE) in heroin addiction. https://clinicaltrials.gov/show/NCT04112186 2019.
NCT04278352 {published data only}
- Mindfulness-based relapse prevention for opioid use disorders. https://clinicaltrials.gov/show/NCT04278352.
NCT04278586 {published data only}
- Effect of mindfulness on opioid use and anxiety during primary care buprenorphine treatment (R33 phase). https://clinicaltrials.gov/show/NCT04278586.
NCT04491968 {published data only}
- Mindfulness oriented recovery enhancement for chronic pain and opioid relapse. https://clinicaltrials.gov/show/NCT04491968.
NCT04584502 {published data only}
- Mindful Moms in Recovery. https://clinicaltrials.gov/show/NCT04584502.
NCT04648228 {published data only}
- Pain and opioids: integrated treatment in veterans. https://clinicaltrials.gov/show/NCT04648228.
Additional references
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (3rd ed). Washington, DC: APA, 1980. [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (3rd ed, rev). Washington, DC: APA, 1987. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: APA, 2000. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. Washington, DC: APA, 2013. [Google Scholar]
Atkins 2004
- Atkins D, Eccles M, Flottorp S, Guyatt GH, Henry D, Hill S, et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Services Research 2004;4(38):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beck 1979
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. New York: The Guilford Press, 1979. [Google Scholar]
Bishop 2004
- Bishop SR, Lau M, Shapiro S, Carlson L, Anderson N, Carmody J, et al. Mindfulness: a proposed operational definition. Clinical Psychology 2004;11:230-41. [Google Scholar]
Bowen 2007
- Bowen S, Witkiewitz K, Dillworth TM, Marlatt GA. The role of thought suppression in the relationship between mindfulness meditation and alcohol use. Adaptive Behavior 2007;32(10):2324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2003
- Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. Journal of Personality and Social Psychology 2003;84:822-48. [DOI] [PubMed] [Google Scholar]
Chapman 2006
- Chapman AL. Acceptance and mindfulness in behavior therapy: a comparison of dialectical behavior therapy and acceptance and commitment therapy. International Journal of Behavioral Consultation and Therapy 2006;2(3):308-13. [Google Scholar]
Chiesa 2011
- Chiesa A, Malinowski, P. Mindfulness based approaches: are they all the same? Journal of Clinical Psychology 2011;67(4):1-21. [DOI] [PubMed] [Google Scholar]
Chiesa 2014
- Chiesa A, Serretti A. Are mindfulness-based interventions effective for substance use disorders? A systematic review of the evidence. Substance Use and Misuse 2014;49:492-512. [DOI] [PubMed] [Google Scholar]
Crane 2017
- Crane RS, Brewer J, Feldman C, Kabat-Zinn J, Santorelli S, Williams JM, et al. What defines mindfulness-based programs? The warp and the weft. Psychological Medicine 2017;47(6):990-9. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors(s). Systematic Reviews in Health Care: Meta-analysis in Context. 2nd edition. London (UK): BMJ Publication Group, 2001. [Google Scholar]
Degenhardt 2018
- Degenhardt L, Charlson F, Ferrari A, Santomauro D, Erskine H, Mantilla-Herrara A, et al. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Psychiatry 2018;5(12):987-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird NM. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7:177-88. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Erskine 2010
- Erskine JA, Georgiou GJ, Kvavilashvili L. I suppress, therefore I smoke: effects of thought suppression on smoking behavior. Psychological Science 2010;21(9):1225-30. [DOI] [PubMed] [Google Scholar]
Garland 2012a
- Garland EL, Boettiger CA, Gaylord S, Chanon VW, Howard MO. Mindfulness is inversely associated with alcohol attentional bias among recovering alcohol-dependent adults. Cognitive Therapy and Research 2012;36:441-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garland 2012b
- Garland EL, Carter K, Ropes K, Howard MO. Thought suppression, impaired regulation of urges, and Addiction-Stroop predict affect-modulated cue-reactivity among alcohol dependent adults. Biological Psychology 2012;89(1):87-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garland 2014c
- Garland EL, Froeliger B, Howard MO. Mindfulness training targets neurocognitive mechanisms of addiction at the Attention-Appraisal-Emotion Interface. Frontiers in Psychiatry 2014;4:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldberg 2018
- Goldberg SB, Tucker RP, Greene PA, Davidson RJ, Wampold BE, Kearney DJ, et al. Mindfulness-based interventions for psychiatric disorders: a systematic review and meta-analysis. Clinical Psychology Review 2018;59:52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldberg 2021
- Goldberg SB, Riordan K, Sun S, Davidson RJ. The empirical status of mindfulness-based interventions: a systematic review of 44 meta-analyses of randomized controlled trials. Perspectives on Psychological Science 2021;in press:1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goleman 1988
- Goleman D. The Meditative Mind: the Varieties of Meditative Experience. New York: Perigee Books, 1988. [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADEpro. GRADEpro GDT. Hamilton, ON, Canada: McMaster University, 2015.
Grant 2017
- Grant S, Colaiaco B, Motala A, Shanman R, Booth M, Sorbero M, et al. Mindfulness-based relapse prevention for substance use disorders: a systematic review and meta-analysis. Journal of Addiction Medicine 2017;11(5):386-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hasin 2006
- Hasin D, Hatzenbuehler ML, Keyes K, Ogburn E. Substance use disorders: Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) and International Classification of Diseases, tenth edition (ICD-10). Addiction 2006;101 Suppl 1:59-75. [DOI] [PubMed] [Google Scholar]
Hasin 2013
- Hasin DS, O'Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, et al. DSM-5 criteria for substance use disorders: recommendations and rationale. The American Journal of Psychiatry 2013;170:834-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayes 2004
- Hayes SC. Acceptance and commitment therapy, relational frame theory, and the third wave of behavioral and cognitive therapies. Behavior Therapy 2004;35(4):639-65. [DOI] [PubMed] [Google Scholar]
Heatherton 2011
- Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends in Cognitive Sciences 2011;15:132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Higgins 2019
- Higgins JP, Thomas J. Cochrane Handbooks for Systematic reviews of Interventions (Second ed.). Oxford: Cochrane Collaboration and John Wiley & Sons, 2019. [Google Scholar]
Hölzel 2011
- Hölzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago DR, Ott U. How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspectives on Psychological Science 2011;6:537-59. [DOI] [PubMed] [Google Scholar]
Kabat‐Zinn 1985
- Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. Journal of Behavioral Medicine 1985;8(2):163-90. [DOI] [PubMed] [Google Scholar]
Kabat‐Zinn 1990
- Kabat-Zinn J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness. New York: Dell Publishing, 1990. [Google Scholar]
Kabat‐Zinn 1992
- Kabat-Zinn J, Massion AO, Kristeller J, Peterson LG, Fletcher KE, Pbert L, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. American Journal of Psychiatry 1992;149:936-43. [DOI] [PubMed] [Google Scholar]
Kabat‐Zinn 1994
- Kabat-Zinn J. Wherever You Go, There You Are: Mindfulness Meditation in Everyday Life. New York: Hyperion, 1994. [Google Scholar]
Kabat‐Zinn 2003
- Kabat-Zinn J. Mindfulness-based interventions in context: past, present, and future. Clinical Psychology: Science and Practice 2003;10(2):144-56. [Google Scholar]
Keller 1972
- Keller M. On the loss-of-control phenomenon in alcoholism. British Journal of Addiction 1972;67:153-66. [DOI] [PubMed] [Google Scholar]
Khanna 2013
- Khanna S, Greeson JM. A narrative review of yoga and mindfulness as complementary therapies for addiction. Complementary Therapies in Medicine 2013;21(3):244-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S (editors). Cochrane Handbook forSystematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The CochraneCollaboration, 2011. Available from www.cochrane-handbook.org.
Li 2017
- Li W, Howard MO, Garland EL, McGovern P, Lazar M. Mindfulness treatment for substance misuse: A systematic review and meta-analysis. Journal of Substance Abuse Treatment 2017;75:62-96. [DOI] [PubMed] [Google Scholar]
Light 1984
- Light RJ, Pillemer BD. Summing Up: The Science of Reviewing Research. Cambridge (MA): Harvard University Press, 1984. [Google Scholar]
Lutz 2008
- Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends in Cognitive Sciences 2008;12(4):163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective disease. Journal of National Cancer Institute 1959;22:719-48. [PubMed] [Google Scholar]
Marlatt 1985
- Marlatt GA, Gordon JR. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. New York: Guilford Press, 1985. [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Medicine 2009;3(3):e123-30. [PMC free article] [PubMed] [Google Scholar]
Murphy 1986
- Murphy TJ, Pagano RR, Marlatt GA. Lifestyle modification with heavy alcohol drinkers: Effects of aerobic exercise and meditation. Addictive Behaviors 1986;11(2):175-86. [DOI] [PubMed] [Google Scholar]
Pali Text Society 1992
- Pali Text Society. The Pali Text Society's Pali-English Dictionary. http://dsal.uchicago.edu/dictionaries/pali/, 1992. [Google Scholar]
Rapgay 2009
- Rapgay L, Bystrisky A. Classical mindfulness: an introduction to its theory and practice for clinical application. Annals of the New York Academy of Sciences 2009;1172:148-62. [DOI] [PubMed] [Google Scholar]
Rehm 2009
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009;373(9682):2223-33. [DOI] [PubMed] [Google Scholar]
Rehm 2013
- Rehm J, Marmet S, Anderson P, Gual A, Kraus L, Nutt D J, et al. Defining substance use disorders: do we really need more than heavy use? Alcohol and Alcoholism (Oxford, Oxfordshire) 2013;48(6):633-40. [DOI] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- Review Manager (RevMan). The Cochrane Collaboration, Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Robinson 2008
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 2008;363(1507):3137-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rücker 2008
- Rücker G, Schwarzer G, Carpenter JR, Schumacher M. Undue reliance on I² in assessing heterogeneity may mislead. BMC Medical research Methology 2008;8:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shapiro 2006
- Shapiro S, Carlson L, Astin J, Freedman B. Mechanisms of mindfulness. Journal of Clinical Psychology 2006;62:373–86. [DOI] [PubMed] [Google Scholar]
Teasdale 2002
- Teasdale JD, Moore RG, Hayhurst H, Pope M, Williams S, Segal ZV. Metacognitive awareness and prevention of relapse in depression: empirical evidence. Journal of Consulting and Clinical Psychology 2002;70(2):275-87. [DOI] [PubMed] [Google Scholar]
Teasdale 2003
- Teasdale JD, Segal Z, Williams J. Mindfulness training and problem formulation. Clinical Psychology Practice 2003;10:157-60. [Google Scholar]
Travis 2010
- Travis F, Shear J. Focused attention, open monitoring and automatic self-transcending: categories to organize meditations from Vedic, Buddhist and Chinese traditions. Consciousness and Cognition 2010;19(4):1110-8. [DOI] [PubMed] [Google Scholar]
Uchtenhagen 2004
- Uchtenhagen A. Substance use problems in developing countries. Bulletin of the World Health Organization 2004;82(9):641. [PMC free article] [PubMed] [Google Scholar]
Uhlig 2009
- Uhlig DJ. Mindfulness based relapse prevention and the matrix model in substance abuse relapse prevention. Dissertation Abstracts 2009.
UNODC 2013
- United Nations Office on Drugs and Crime. World Drug Report 2013. United Nations 2013;Sales No. E.13.XI.6.
Vago 2012
- Vago DR, Silbersweig DA. Self-awareness, self-regulation, and self-transcendence (S-ART): a framework for understanding the neurobiological mechanisms of mindfulness. Frontiers in Human Neuroscience 2012;6:296. [DOI: 10.3389/fnhum.2012.00296.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vega 2002
- Vega WA, Aguilar-Gaxiola S, Andrade L, Bijl R, Borges G, Caraveo-Anduaga JJ, et al. Prevalence and age of onset for drug use in seven international sites: results from the international consortium of psychiatric epidemiology. Drug and Alcohol Dependence 2002;68(3):285-97. [DOI] [PubMed] [Google Scholar]
Wegner 1994
- Wegner DM, Zanakos S. Chronic thought suppression. Journal of Personality 1994;62(4):616-40. [DOI] [PubMed] [Google Scholar]
Whitebird 2009
- Whitebird RR, Kreitzer MJ, O'Connor PJ. Mindfulness-based stress reduction and diabetes. Diabetes spectrum: a publication of the American Diabetes Association 2009;22(4):226-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiteford 2013
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 2013;382(9904):1575-86. [DOI] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems. Geneva: WHO, 2010. [Google Scholar]
WHO 2002a
- World Health Organization. The world health report 2002 – Reducing risks to health, promoting healthy life. Geneva: WHO 2002.
WHO 2002b
- World Health Organization. Alcohol in developing societies: a public health approach. Geneva: WHO 2002.
WHO 2010
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems. Fourth Edition. Geneva: WHO, 2010. [Google Scholar]
Witkiewitz 2014b
- Witkiewitz K, Bowen S, Harrop EN, Douglas H, Enkema M, Sedgwick C. Mindfulness-based treatment to prevent addictive behavior relapse: theoretical models and hypothesized mechanisms of change. Substance Use & Misuse 2014;49:513-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Rösner 2015
- Rösner S, Willutzki R, Zgierska A. Mindfulness‐based interventions for substance use disorders. Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No: CD011723. [DOI: 10.1002/14651858.CD011723] [DOI] [PMC free article] [PubMed] [Google Scholar]


