Abstract
This longitudinal study spans two generations of rhesus monkeys, first, investigating the effects of early rearing on the maternal behavior of first generation mothers (rates of premature infant rejection) and, second, investigating the effects of maternal rejection on the behavior of second generation infants. Rhesus macaque mother-infant dyads (Macaca mulatta—N=176) were observed twice weekly, with each session lasting 300 seconds. First-generation mothers were raised in one of three conditions: as mother-reared controls (MR; (n=95)), in peer groups (PR; raised without adults but with constant access to three same-aged peers (n=49)), or with an inanimate surrogate (SPR; raised with an inanimate fleece-covered, surrogate mother and limited daily peer-group interactions (n=32)). Second-generation infants were all reared by their differentially-reared mothers and statistically-grouped into one of two groups: those that were rejected by their mothers beginning at a more-typical weaning age (controls), starting in the third month of life (n=108), and those that were prematurely-rejected, with mothers showing rejections before the third month of life (n=68). Overall, PR mothers exhibited the highest rates of premature infant rejection, except for month-one of infant life, when SPR mothers exhibited the highest rates of rejection. Intriguingly, after month-one, SPR mothers showed high rates of infant cradling and seldom rejected their infants. Independent of their mothers’ early rearing environment, prematurely-rejected infants displayed more aggression and passive vigilance, and were cradled and groomed less by their mothers and there was evidence that the overall rates of rejection after the first two months of life had a cumulative negative effect on the developing infant. Post hoc analyses of plasma cortisol levels showed that the prematurely-rejected infants had higher cortisol concentrations, suggesting a high level of stress in the prematurely-rejected infants. These results suggest that maternal presence during infancy has long-term, intergenerational effects on a female’s future maternal skills which, in turn, have consequences for the socioemotional development of second-generation infants.
Keywords: Early experience, Intergenerational effects, Sensitive parenting, Mother-infant relations, Rhesus monkeys
Introduction
In their seminal article, Greenough and colleagues (1987) proposed the concept of the experience-expectant brain, suggesting a link between the evolutionary process of brain growth and plasticity with the necessity of the evolutionarily expected environmental input at the appropriate developmental time. In most primate species, evolution has selected for mothers to provide such input. As such, the timing and quality of maternal parenting behavior can have lasting consequences on infant developmental outcomes. Mothers that provide a secure base of maternal warmth and sensitivity effectively regulate infant arousal, leading to long-term positive infant outcomes (Lickenbrock & Braungart-Rieker, 2015; Rispoli, McGoey, Koziol, & Schreiber, 2013), producing effects that endure into adulthood (Moran, Turiano, & Gentzler, 2018). Conversely, inappropriate maternal care and insensitivity to infant needs leads to long-term, often intergenerational, negative infant outcomes (for a review, see Lomanowska, Boivin, Hertzman, & Fleming, 2017). Most research investigating the impact of sensitive parenting focuses on the influence of parenting sensitivity on the long-term development of psychopathology (for examples, see Haapasalo & Pokela, 1999; Jaffee, 2017; Perez, Jennings, Piquero, & Baglivio, 2016; Raposo, Mackenzie, Henriksen, & Afifi, 2014). One such extreme example of the impact of inadequate maternal care in infancy on long-term development is the lamentable case of the Romanian orphans (Chugani et al., 2001; Kaler & Freeman, 1994). Experiencing extreme environmental and social deprivation during a sensitive time in neurodevelopment, these orphans exhibit significant reductions in neuronal and synaptic formation, largely explaining their deficits in cognitive and social functioning, when compared to expected norms for their respective ages. Harlow’s now-classic work on the effects of social deprivation demonstrated that early social experience is critical to the development of adequate mothering. He showed that females reared in social isolation mistreated, abused, or at best, neglected their infants (Harlow & Seay, 1966; Seay, Alexander, & Harlow, 1964; Seay, Hansen, & Harlow, 1962). In this extreme case of social isolation, the females were not only deprived of early mothering, but of all other social interactions. Since then, other studies examining the early rearing environment have utilized methodologies that focus only on the effects of maternal absence, while allowing the infant to have more typical social experiences. These later studies show that the psychopathology seen in Harlow’s socially isolated females is largely ameliorated by allowing them experience with other social companions, which has been interpreted as showing that it is not the absence of the mother, but the social isolation and lack of social experience that lead to maternal deficits (Champoux, Byrne, DeLizio, & Suomi, 1992; Novak & Sackett, 2006; Ruppenthal, Arling, Harlow, Sackett, & Suomi, 1976). As evidence of this, the maternal deficits that were exhibited by the motherless mothers were somewhat attenuated when they were reared with constant access to same-aged conspecifics (Novak & Sackett, 2006). Other studies of infants reared in less extreme situations illustrate that insensitive maternal responsiveness or callous infant punishment, particularly when the infant is young, can lead to the development of non-secure attachment bonds, increased anxiety and aggression, and other behavioral pathology (Andrews & Rosenblum, 1991, 1993; Rosenblum & Paully, 1984; Tarantino, Sullivan, & Meltzer-Brody, 2011). In the only study of which the authors are aware comparing the maternal behavior of females reared with or without mothers (surrogate-peer-reared and peer-only reared), few differences were detected in the maternal behaviors of the differentially-reared, now-adult females (Roma, Ruggiero, Schwandt, Higley, & Suomi, 2006). Roma et al. (2006) reported that both the PR and SPR mothers showed species-typical maternal behaviors. This study, however, utilized a much smaller number of mothers that had been reared without mothers in PR and SPR groups than that seen in the present study. They did report, however, that the PR monkey mothers were more likely to show aggression and to reject their infants in month four of life, but that is after normally-reared mothers begin the weaning process, with rejections typically seen at some level in most mothers by month five (Roma et al., 2006). The present study proposes investigating the specific effect of early maternal absence on the long-term, adult female maternal behavior in a large sample of rhesus monkeys.
While it is clear that early parenting can impact infant development, it is less clear whether experiencing inadequate parenting early in life (i.e., during infancy) has a lasting impact on the development of future parenting behaviors. Studies in humans seeking to understand the intergenerational effects of early experience on later parenting behaviors tend to be limited by retrospective methodology (Belsky, 1993; Van IJzendoorn, 1992). Nevertheless, some prospective work suggests that the parenting quality experienced by first-generation females impacts the socioemotional development and later parenting behaviors of second-generation infants (Bailey, Hill, Oesterle, & Hawkins, 2009; Belsky, Jaffee, Sligo, Woodward, & Silva, 2005; Conger, Neppl, Kim, & Scaramella, 2003). While work in human populations assessing intergenerational influences of early experience is valuable, work in human populations suffers from difficulties in controlling environmental influences. Further, longitudinal studies in human populations require a lengthy time period to measure outcomes, and their expense and high rates of attrition makes such research difficult at best.
Nonhuman primate models are well-suited to investigate the intergenerational impact of early life experiences on later parenting behaviors and on the developmental outcomes of offspring. Importantly, there is a long history of utilizing nonhuman primate models to study mother-infant behaviors and developmental outcomes, largely because the environment, including early rearing experience, can be carefully controlled and monitored in a manner that is not possible and is likely unethical in humans. The present study focuses on the impact of aberrant early experiences on later parenting behaviors and on the outcomes of subsequent offspring using a translational rhesus macaque (Macaca mulatta) model. Rhesus macaques possess many genetic (Gibbs et al., 2007) and social similarities (Capitanio, 1985) to humans, exhibiting paralleling developmental pathways and behavioral traits (Barr, Becker, Suomi, & Higley, 2003). Furthermore, as rhesus monkeys mature at a rate that is three-to-four times more rapid than humans (Roth et al., 2004), prospective longitudinal studies investigating the intergenerational impact of first-generation early life experiences on second-generation developmental outcomes can be completed in considerably shorter time than can be done in human studies.
During the first month of life, rhesus monkey infants spend almost all of their time clinging to their mother’s ventrum (Fairbanks, 1996; Hinde & Simpson, 1967; Suomi, 2005). During month-two and, to a lesser degree, month-three of infant life, they remain highly dependent on their mothers for nourishment and protection. During this time, mothers typically restrain their infants, restricting their attempts at exploration and play, cradling them to their ventrum, shielding them from harm.
After month-three of life, rhesus monkey infants’ motor skills become more advanced, making time away from mother safer. At this age, their mother’s principle psychological function is to provide a secure base to which infants can return when they are challenged or fearful (Harlow & Harlow, 1965; Harlow & Zimmerman, 1959). Sensitive rhesus monkey mothers effectively monitor their infants, and, when their infants are over-aroused, they provide a secure base, reducing infant arousal. Consequently, infants begin exploring and playing with peers. Typical rhesus monkey mothers begin to show some attenuation of their restrictiveness when infants reach month-three of life, tentatively initiating the process of weaning by allowing the infant to leave their proximity, allowing them more autonomy. While weaning is not completely underway until infants are about six months old (Fairbanks, 1996; Harvey, 1987; Lindburg, 1971), between month-three and -six of infant life, mothers may occasionally reject infant attempts to nurse or initiate physical contact. Once weaning is completely underway, sensitive mothers typically reject infants by turning their back to their infant, covering their ventrum with their arm, or by gently pushing the infant away. Less sensitive mothers may reject their infant by punitive means, such as pushing, hitting or biting them when they attempt to nurse (Hinde & Spencer-Booth, 1967). Fairbanks’ now classic review (1996) of maternal styles across a number of species discusses rejecting and protective maternal styles, illustrating that these styles are trait-like, with some mothers showing consistently high rates of rejection before and after weaning. Fairbanks reviews studies showing that premature rejections are consequential for the developing infant (1996). For example, mothers that fail to restrain their infants and mothers that show rejections that are inappropriate for the infants’ age are more likely to have infants that suffer from premature mortality and attacks from other group members. These infants also tend to exhibit anxiety-like behaviors, showing distress cries, separation anxiety, and tantrums, an indication that the rejections may not be occurring at the appropriate age and that maternal sensitivity may be lacking.
Rhesus monkey mothers that exhibit less skillful or pathological parenting are also less likely to consistently provide a secure base and cradle their infants, showing reduced sensitivity to their infants’ needs and level of maturity, engaging in premature (before month-three) rejections, a pattern of behavior that Greenough would describe as not meeting the experience-expectant needs of the infant (1987). Premature weaning is atypical, and associated with punitive maternal treatment, with studies suggesting that high levels of punitive maternal rejection may lead to elevated infant anxiety and aggression later in life (Maestripieri, McCormack, Lindell, Higley, & Sanchez, 2006). Given the abundance of research confirming the long-lasting impact of early life experiences on later developmental outcomes (for a review, see Britto et al., 2017), it is surprising that so few studies have investigated how early experiences affect later parenting behaviors in nonhuman primates. Specifically, using a large sample size, the present study assesses both the mothers’ and the infants’ behaviors to evaluate the long-term outcome of early maternal absence on infant females that later become mothers, as well as the effect of the parenting of these mothers on their infants’ socioemotional development.
The developmental impact of two early environments where no adult is present—a peer-only rearing condition, where social contact and other experiences with same-aged peers are frequent, and a surrogate-peer rearing condition, where social contact is infrequent, with limited daily social interactions, is investigated.. While first-generation females reared without adults in one of these conditions experience a disproportionate deviation from the experience-expectant life pattern, they tend to carry and cradle their second-generation infants in a manner that is most often within rhesus monkey parenting norms, but outside the norms of high-quality maternal care (Roma, Ruggiero, Schwandt, Higley, & Suomi, 2006). These mothers tend to show weaning-like rejections too early, when security and solicitude are the experience-expectant evolutionary norms.
In accordance with studies showing that harsh maternal treatment of infant rhesus monkeys leads to increased aggression later in infant life (Maestripieri, McCormack, et al., 2006), the present study investigates the effects of premature maternal rejection of infants on infant development. Specifically, the following are assessed: 1) the impact of rhesus monkey females’ early life experiences on their later parenting behaviors, including their rates of infant rejections and 2) the impact of premature rejection on offspring developmental outcomes, including rates of aggression toward conspecifics. It is hypothesized that first-generation rhesus monkey mothers reared in aberrant early conditions (i.e., without adequate maternal care) will exhibit less sensitive maternal care of their own infants, rejecting them more, and cradling them less. It is further hypothesized that, at a time when mothers should provide a reliable, secure base but instead show premature rejections, infants will be cradled less, exhibit more time alone, and exhibit greater than expected rates of aggression.
Methods
Subjects and Data Collection
First-Generation Subjects.
The adult females (n = 176) in the first generation were raised in one of three conditions. Rearing procedures are described in detail elsewhere (see Shannon, Champoux, & Suomi, 1998). Briefly, mother-reared subjects (MR; n = 95) were raised by their biological mothers, in social groups with two-to-three other age-mates, peer-reared subjects (PR; n = 49) were raised without a mother but with constant access to two-to-three other age-mates, and surrogate-peer-reared subjects (SPR; n = 32) were raised with an inanimate surrogate mother and limited peer interactions (i.e., placed in a playroom with their surrogate mother and two-to-three other age-mates for two hours a day, five days a week).
Second Generation Subjects.
The infants (n = 176) born to the first-generation females were all reared in indoor-outdoor enclosures by their mothers in mixed-sex social groups containing two adult males and six-to-eight adult females with their infant offspring, in conditions approximating the natural social setting. In total, from the first and second generations, there were 176 mother-infant dyads. All subjects were housed at the National Institutes of Health Animal Center in Poolesville, MD. All protocols and procedures employed in this study were ethically reviewed and approved by the NIH Animal Care and Use Committee before beginning this study. This study was conducted in compliance with the National Research Council’s Guide for the Care and Use of Laboratory Animals, the US Public Health Service’s Policy on Humane Care and Use of Laboratory Animals, and the Guide for the Care and Use of Laboratory Animals.
Behavioral Observations
Behavioral scoring of mother-infant interactions was conducted by trained observers who achieved inter-observer reliabilities of at least 85% before beginning the study. Monthly interrater reliability checks were performed throughout the course of the study. Behaviors were scored with the infant as the focal subject, except for cases where the mother showed a behavior directed toward her infant (see Table 1 for the behaviors and their definitions). Observations were performed in 300 second sessions, two times a week for the first 24 weeks of infant life. Weekly scores were averaged to create a mean monthly score for each of the behaviors for the first six months of life. All behaviors were scored as duration (seconds), with the exception of rejections and aggression, which were scored as frequencies (see Table 1). In rare instances, data were not obtained due to equipment failure or because an animal was removed from the group for veterinary care. In those cases, the data for the missing day was not included in the calculated means.
Table 1.
Behavior Definitions
| Behavior | Definition |
|---|---|
|
| |
| Infant Aggression* | Infant chasing and threats, as well as bites, slaps, etc. |
| Infant Locomotion | Any self-induced movement across the substrate, by means of walking, running, etc. |
| Infant Passive Vigilance | Absence of directed movement, social behavior, and environmental exploration while awake and vigilant. |
| Maternal Cradling of Infant | Infant is ventrum-to-ventrum with the mother or is on the nipple. |
| Maternal Grooming of Infant | Maternal cleaning and/or grooming of infant by means of scratching, licking, biting, etc. |
| Maternal Rejection of Infant* | Mother rejects approaches made by infant for contact by turning her back, pushing or restraining, hitting, or, in extreme cases, biting the infant. |
Note. The infant was the focal subject in all of the observations. Behaviors recorded as frequencies are marked with an asterisk. Except where noted, all behaviors were recorded as durations (seconds). The ethogram is mutually exclusive and exhaustive.
Data Analysis
Preliminary analyses showed an effect of infant cohort year on some of the outcome variables of interest. Therefore, infant cohort year was included as a covariate in all analyses. Preliminary analyses showed no effect of infant sex on the outcome variables of interest; therefore, infant sex was not included in the analyses. All analyses were performed in SPSS, version 25.
Effects of Early Maternal Rearing Experience on Infant Outcomes.
To investigate the relationship between the mothers’ early rearing conditions and infant behavioral outcomes, mixed design, repeated-measures ANOVAs were performed with maternal rearing condition (MR, PR, SPR) as the independent variable. Dependent measures included maternal behaviors (rates that mothers rejected their infants and time mothers spent cradling and grooming their infants) and infant behaviors (rates of aggression, time spent in passive vigilance, and locomotion). Months of infant life (1–6) was the repeated measure. When significant effects were found, planned comparisons were calculated using Tukey’s LSD test.
Effects of Premature Maternal Rejection on Infant Outcomes.
In order to examine the effects of premature maternal rejection on the infant behavioral outcomes, mixed design, repeated-measures ANOVAs were performed, with infant rejections (normal or premature) as the independent variable. Dependent measures included rates of infant aggression, infant time spent in passive vigilance, locomotion, being cradled or groomed by mother. Months of infant life (1–6) was the repeated measure. Normal rejections were defined as rejections occurring after infants reached three months of age. Maternal rearing condition for the infants that were rejected at the age-appropriate time was distributed as follows: MR: n = 60; PR: n = 30; SPR: n = 18. Infants were classified as prematurely rejected if they were rejected one or more times in the first two months of life (M = 5 rejections, Range: 1–39 rejections, Mode = 2 rejections). Maternal rearing condition for prematurely rejected infants was distributed as follows: MR: n = 35; PR: n = 19; SPR: n = 14. When significant effects were detected, planned comparisons were calculated using Tukey’s LSD test.
Results
Effects of Maternal Rearing Experience on Infant Outcomes
Infant Rejection.
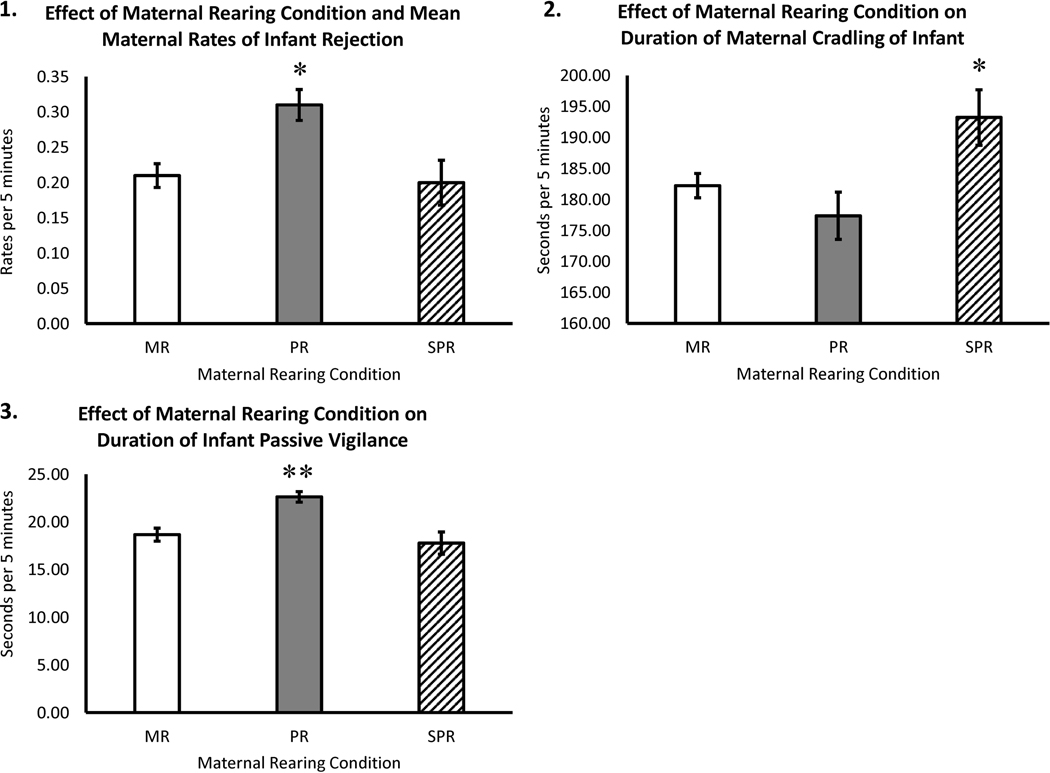
Results showed a statistically significant main effect of mother’s rearing condition on overall rates of infant rejection F(2,169) = 3.49, p = .03). Pairwise comparisons indicated that PR mothers exhibited higher mean rates of rejection across the first six months of infant life, when compared to MR and SPR mothers (p < .05; PR: M = 0.33±0.03; MR: M = 0.23±0.02; SPR: M = 0.22±0.04—see Figure 1, Panel 1).
Figure 1.
Effects of Maternal Rearing Condition on Rates of Rejection, Cradling, and Passive Vigilance
Analyses also showed a two-way mother’s rearing condition by months of infant life interaction for rates of infant rejection (F(2,169) = 3.94, p = .02). Pairwise comparisons indicated that SPR mothers exhibited the highest mean rates of rejection during the first month of infant’s life, when compared to MR and PR mothers (p < .005; SPR: M = 0.28±0.09; MR: M = 0. 12±0.05; PR: M = 0.09±0.074).
There was a two-way rejection condition by months of life interaction (F(5,850) = 3.30, p = .006). Further analyses showed that mothers that prematurely rejected their infants exhibited stable inter-individual rates of rejection across the first six months of infant life (p < .05). Normally-rejected infants did not experience any rejections until after month-three. Thereafter, and through month-five, the two groups were comparable in rates of rejection. In month-six, however, the rates prematurely-rejected infants’ rejections increased to a rate that was higher than that experienced by the normally-rejected infants (p < .05).
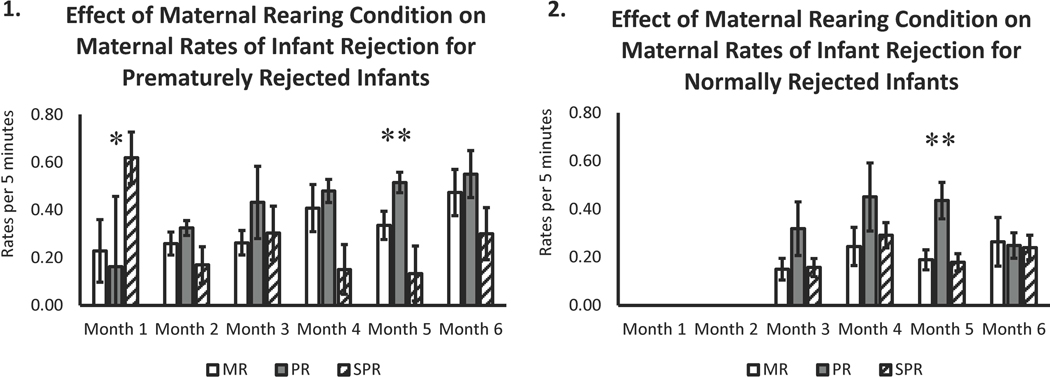
There was also a three-way months of life by mothers’ rearing condition and by rejection condition interaction ((F(2,170) = 3.19, p = .04) see Figure 2, Panels 1–2). Further analyses showed that in month-one, prematurely-rejected infants of SPR mothers were rejected more often than prematurely-rejected infants of mothers from the other rearing conditions (p < .05). Thereafter, their rates fell precipitously, remaining low over the remaining months (p < .05). Within the normally-rejected infant group, PR mothers showed higher rates of rejections during months three-through-five (p < .006), when compared to infants that were normally-rejected by MR and SPR mothers, and their rates of rejections fell to the level of the other mothers in month-six (p < .05).
Figure 2.
Maternal Rearing Condition x Month x Infant Rejection Status on Rates of Infant Rejections
Maternal Cradling.
Results showed a statistically significant main effect of mother’s rearing condition on the overall time that the mothers spent cradling their infants (F (2,170) = 3.01 p = .05), with SPR mothers spending more time, on average, cradling their infants when compared to MR and PR mothers (p < .05; SPR: M = 195.61±5.68; MR: M = 183.29±2.84; PR: M = 178.14±4.25—see Figure 1, Panel 2). A post hoc comparison of the mothers in month-one showed that SPR mothers exhibited more cradling, when compared to the PR and MR mothers (p < .05).
Infant Passive Vigilance.
Results showed a statistically significant main effect of mother’s rearing on overall time infants spent in passive vigilance (sometimes called freezing by others—Kalin et al., 1998), a common measure of infant anxiety in nonhuman primate research, (F(2,169) = 4.48, p = .01) with infants of PR mothers spending, on average, more time in passive vigilance, when compared to infants of MR and SPR mothers (p < .02; PR: M = 23.14±1.26; MR: M = 19.00±0.90; SPR: M = 17.95±1.59—see Figure 1, Panel 3).
Maternal Grooming, Infant Aggression, and Infant Locomotion.
There were no statistically significant effects of mother’s rearing condition on mean time mothers spent grooming their infants (p > .05), mean rates of infant aggression (p > .05), or time that the infants spent in locomotion (p > .05).
Effects of Premature Maternal Rejection on Infant Outcomes
Infant Aggression.
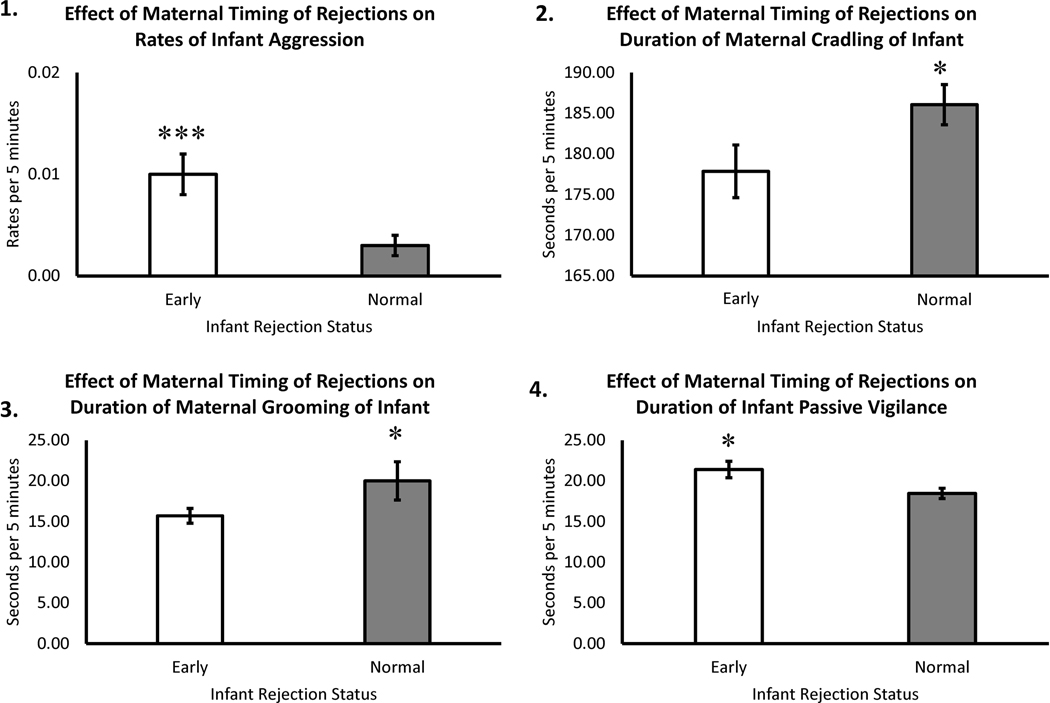
Results showed a statistically significant main effect of maternal timing of rejections on overall rates of infant aggression directed towards others (F(1,165) = 10.97, p = .001), with prematurely-rejected infants exhibiting higher mean rates of aggression (M = 0.01±0.002), when compared to infants that were rejected at the appropriate age (M = 0.003±0.001)—see Figure 3, Panel 1. Results from a post hoc regression analysis showed a statistically significant positive relationship between rates of maternal rejection and rates of infant aggression (β = .58, p = .0001; overall model: F(1, 31) = 15.69, p = .0001, R = .58).
Figure 3.
Effects of Maternal Timing of Rejections on Infant Outcomes
Maternal Cradling.
Analyses showed a statistically significant main effect of maternal rejection status on the overall time that mothers spent cradling their infants (F(1,173) = 4.12, p = .04), with mothers that exhibited premature rejection of their infants cradling their infants less (M = 177.4±3.45), when compared to mothers that rejected their infants at the appropriate age (M = 186.35±2.74)—see Figure 3, Panel 2. Results from a post hoc regression analysis showed a statistically significant negative relationship between rates of maternal rejection and rates of maternal cradling (β = −.18, p = .02; overall model: F(1,167) = 5.83, p = .02, R = .18).
Maternal Grooming of Infant.
Analyses showed a statistically significant main effect of maternal timing of rejections on the overall time mothers spent grooming their infants (F(1,139) = 6.65, p = .01), with mothers that exhibited premature rejection of their infants also showing less grooming (M = 14.25±1.64), when compared to mothers that rejected their infants at the appropriate age (M = 19.96±1.47)—see Figure 3, Panel 3. Results from a post hoc regression analysis did not show a statistically significant relationship between maternal rejections and maternal grooming of infant (p > .05).
Infant Passive Vigilance.
Analyses showed statistically significant main effect of maternal timing of rejections on the overall time infants spent in passive vigilance (F(1,169) = 3.92, p = .049), with prematurely-rejected infants spending more time in passive vigilance (M = 21.45±1.10), when compared to infants that were rejected at the appropriate age (M = 18.61±0.92)—see Figure 3, Panel 4. Results from a post hoc regression analysis showed a statistically significant positive relationship between rates of maternal rejection and rates of infant passive vigilance (β = .26, p = .001; overall model: F(1, 172) = 12.59, p = .001, R = .26).
Infant Locomotion.
There were no statistically significant effects of maternal rejection timing on mean time infants time spent locomoting (p > .05). Post hoc regression analysis did not show a statistically significant relationship between maternal rejections and time infants spent locomoting (p > .05).
Discussion
The findings from this study confirm the hypothesis that first-generation rhesus monkey females reared in aberrant early conditions (i.e., without adequate maternal care) would exhibit less sensitive maternal care when they later became mothers, and, as a consequence, their second-generation infants would exhibit poorer behavioral outcomes. Specifically, mothers that were reared in mother-absent conditions (PR and SPR) were more likely to show insensitive parenting behaviors when compared to control females that were that were raised by their mothers in mixed social-group conditions that approximate the natural environment (MR). Infants were classified as prematurely rejected and normally rejected, with rates of infant rejection substantially higher in the maternally-deprived mothers. At an age when mothers almost never punitively reject their infants, these maternally-deprived females not only rejected their infants at much higher rates (Figure 1, Panel 1, and Figure 2, Panels 1–2), but were also less likely to provide a secure base, as evidenced by the limited time they spent cradling their infants (see Figure 1, Panel 2), with the results showing a significant negative relationship between the infant rejections and infant cradling. Perhaps as a consequence, the infants of maternally-deprived mothers showed more aggression, as well as passive vigilance (see Figure 1, Panel 3), although the differences in the time spent in passive vigilance were not uniform for the infants reared by the PR and SPR mothers, with the PR subjects showing higher rates of passive vigilance.
Independent of maternal rearing, infants that were prematurely-rejected showed poorer outcomes, including high rates of aggression toward conspecifics (Figure 3, Panel 1) and spent more time in a passively vigilant state (Figure 3, Panel 4). The original hypothesis was that, at a time when mothers should provide a reliable, secure base but instead exhibit weaning-like rejections, infants would be cradled less, exhibit more time alone in a vigilant state, and exhibit greater than expected rates of aggression. One cannot rule out, however, that the cumulative effect of repeated rejections, accompanied by low rates of mothers providing a secure-base, as measured by infant cradling, could play a role in the infant outcomes. When the relationship between the mean rates of early rejections through the first two months of infant life and the mean rates of rejections across months 3–6 was assessed, the correlation failed to achieve traditional statistical significance (β = .14, p = .06; overall model: F(1, 174) = 3.70, p = .06, R = .14), suggesting that rates of early premature rejections may not be driven by the same underlying hypothetical construct as later rejections.
These results suggest that the impact of premature rejections is at least somewhat independent of the impact of cumulative rejections on the infant. Nevertheless, there did appear to be an independent contribution of the cumulative rates of rejections, as shown by the average rates of rejection after month-two of life leading to similar effects (i.e., low rates of maternal cradling, increased time in passive vigilance, and increased rates of infant aggression). These findings suggest that it is likely that both the timing and the cumulative effects of punitive maternal rejections are important, but are somewhat independent in their effects on the young infant. One possible reason for these infant behavioral differences is their limited time in close intimate contact with their mothers, as evidenced by the relatively low time that prematurely-rejected infants spent being cradled or groomed by their mothers (Figure 3, Panels 2–3). Taken together, these findings indicate that there is an intergenerational effect of early life experience on the first-generation females that impacted the second-generation infants’ developmental outcomes. The behavioral differences seen in the second-generation prematurely-rejected infants likely stem from their mothers’ early negative life experiences.
As noted above, the behavioral differences were not uniform across infants from PR and SPR mothers. Overall, on average, the PR mothers exhibited the highest rates of infant rejections across the first six months of infant life, when compared to MR and SPR mothers (Figure 2, Panels 1–2). This finding parallels the findings by Roma and colleagues (2006), who found higher rates of maternal rejections by PR mothers after the PR monkeys’ third month of life. Some level of rejections would be expected by the third month of life, although the rates of rejections by the PR mothers were higher than those seen in the MR and SPR mothers, suggesting an overly punitive maternal style. When discussing the effects of peer-rearing, Harlow remarked that PR infants appear as a “single, two-headed monkey”, referring to their high rates clinging to their peers well beyond the normal age of weaning (Harlow & Harlow, 1965). He found that, in a playroom setting, the tight clinginess of PR monkeys attenuated their time playing and exploring, as they could not escape the grasp of their partners, and noted that the ventral contact seen between the peer-reared subjects was difficult to break. In one author’s experience studying the PR subjects, he reports that often the only way that a PR monkey can escape the grasp of their peers to explore or play is by using physical means such as pushing or biting their partners (JDH, personal observations), with PR subjects showing higher mean rates of aggression than MR controls (Higley, Linnoila, & Suomi, 1994). In a study researching attachment bonds between peers, PR subjects were shown to form anxious-attachment-like bonds with their same-aged peers, showing a strong preference and more time in close proximity to one specific subject within their group (interpreted as their “best buddy”); yet, when compared to MR subjects, PR subjects showed high rates of clinging and seldom explored their environment, an indication that they have difficulty obtaining security from the peer to which they are attached (Higley et al 1992). Similarly, Clarke & Snipes (1998) found that PR monkeys were highly attached to their cagemates in the first six months of life, with the attachment peaking around five months, a time when they observed MR monkeys to be noticeably independent of their mothers. Other studies show that as adolescents and young adults, PR monkeys continue to use aggression at higher than expected rates, oftentimes requiring veterinary intervention or removal from their social group for bulling or repeated wounding (Higley, Linnoila, & Suomi, 1994). Although somewhat speculative, the high rate of rejections by the PR mothers may represent continuity from their own early experiences, aggressively rejecting their own infants as they learned to act aggressively to break contact with their PR age-mates early in life.
Surprisingly, when assessing mean rates of rejection across the first six months of infant life, SPR mothers exhibited the lowest rates of infant rejection (Figure 1, Panel 1). However, a closer assessment of the data showed that SPR mothers engaged in the highest rates of infant rejection during the first month of their infant’s life (Figure 2, Panel 1). This is a developmental period when most MR rhesus monkey infants spend more than 90% of their time being cradled by their mothers, virtually never being rejected or experiencing punitive treatment from their mothers (Harlow & Harlow, 1965). This reversed after the first month of infant life, with SPR mothers thereafter engaging in the lowest rates of infant rejection (Figure 2, Panel 1), and over the next five months, SPR mothers showed more infant cradling (Figure 1, Panel 2). That SPR mothers showed high rates of rejections and high rates of cradling in the first month of life, as well as overall higher rates is seemingly paradoxical. One possible explanation for this finding is related to the early experiences of the SPR mothers. Having never experienced close ventral cradling by their own (inanimate) mothers, they respond to infant attempts to cling with punitive rejections and then engage in high levels of cradling of their own infants after experiencing ventral contact during the first month. Because SPR mothers were deprived of the early experience of being cradled by their own mothers, it may be that their early rejections of their infants may reflect their novel experience of having an infant cling and nurse, while their subsequent clinginess may be an overcompensation from their early life experiences. Regardless of the explanation, the SPR rearing experience appears to have left a lasting signature, resulting in long-term maternal deficits.
Infants of PR mothers also tended to display the highest passive vigilance over the first six months of life (Figure 1, Panel 3), often accompanied by vigilance and immobility, a behavior that is widely interpreted as a sign of anxiety in rhesus monkeys (Coleman & Pierre, 2014; Kalin & Shelton, 2003; Kalin, Shelton, Rickman, & Davidson, 1998; Suomi, Harlow, & Domek, 1970). As PR mothers exhibited the lowest rate of cradling their infants (Figure 1, Panel 2), and the highest rate of rejecting their infants (Figure 1, Panel 1), this vigilant behavior may reflect the PR mother’s reluctance to provide a secure base for their infants, resulting in increased arousal and anxiety in their infants. Because PR mothers are themselves known to exhibit high rates of anxiety-like behavior (Corcoran et al., 2012), this finding suggests that the first-generation mothers’ early rearing experiences leave a more-or-less permanent signature on behavior, extending to the treatment of their second-generation offspring, which may result in increased anxiety-like behaviors in the infants. Whether this is an epigenetic effect that is passed on remains to be determined.
In line with the hypothesis that mothers that exhibit low maternal sensitivity (i.e., engaged in premature rejection of infants) would have infants with poorer behavioral outcomes, rejected infants exhibited more aggression (Figure 3, Panel 1). Aggression is atypical for infants, and while the aggression exhibited across the prematurely-rejected and normally-rejected groups was statistically different, it is worth noting that it was exhibited at low levels. Nevertheless, finding higher rates of aggression in prematurely-rejected infants is consistent with other research showing that a punitive mother-infant relationship can lead to higher probabilities of infants engaging in aggressive behavior that may show continuity into adulthood (Kalin, 1999; Maestripieri, Lindell, & Higley, 2007; Seay & Harlow, 1965). Given that the premature maternal rejections tend to be punitive in nature, the authors cautiously speculate that early aggression by first-generation mothers may increase the probability of second-generation offspring engaging in aggression, something termed the “cycle of violence” in the human literature when referring to later life outcomes of abused human children (Widom, 2000). An alternative interpretation may be that the prematurely-rejected infants become independent earlier, but, because they have less maternal monitoring, they tend to exhibit immature social skills, increasing their aggressive interactions with other group members.
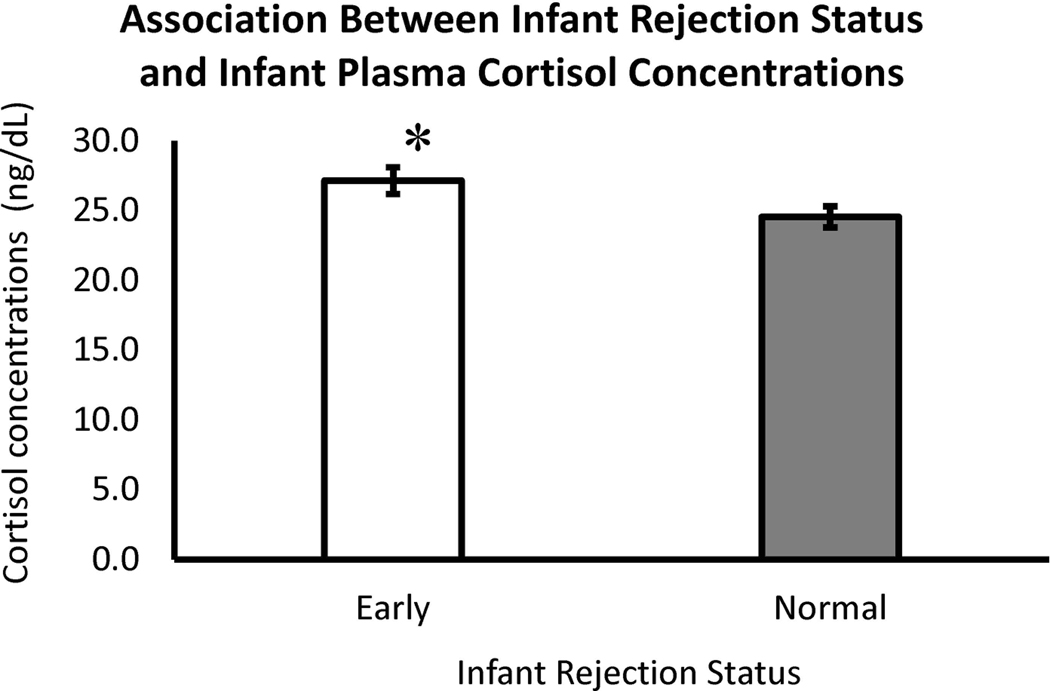
Prematurely-rejected infants also exhibited more passive vigilance or freezing than infants that were rejected at the age-appropriate time (Figure 3, Panel 4). Freezing is considered an indication of anxiety, as noted above (Kalin & Shelton 1998; Harlow & Suomi, 1970). Positive, close physical contact (contact comfort) reduces arousal, leading to exploration, a defining characteristic of secure attachment bonds in nonhuman primates (Harlow & Harlow, 1965; Weaver & de Waal, 2002). Mothers that showed high rates of infant rejection also exhibited low rates of infant cradling, suggesting a non-secure mother-infant attachment bond and, likely, likely resulting from a failure to reliably provide a secure base to reduce infant arousal (Harlow & Harlow, 1965). Moreover, mothers that rejected their infants showed less grooming. Grooming is seen as evidence of social support and bonding in Old World monkeys (Carpenter, 1942). Given these deficits, it is not surprising that infants that experienced early rejections showed more anxiety-like passive vigilance. As cortisol is often used as a marker of anxiety or stress (Capitanio 2018; Higley, Suomi, & Chaffin, 2011; Kagan, Reznick, & Snidman, 1988), plasma cortisol was statistically compared using a subset of the these subjects whose blood was obtained during another study at month-two of life. Subjects were capture and a blood sample was obtained within 5 minutes. A post hoc analysis of this plasma cortisol data showed that the infants that were rejected prematurely had significantly higher levels of plasma cortisol (M=27.15, ± 0.84) than infants for which rejections began at the expected time (M=24.55, ± 0.70), further suggesting that the prematurel -rejected infants were experiencing more stress or trait-like anxiety than their peers that experienced maternal rejection at a more age-appropriate time; t(157) = 2.35, p = .02 (see Figure 4).
Figure 4.
Effects of Maternal Timing of Rejections on Infant Plasma Cortisol Concentrations
Overall, the results from this study suggest that there are poorer outcomes for infants of mothers that had aberrant early rearing experiences. They also suggest that maternal rejections must occur at the optimal developmental time, which is typically when weaning takes place, a time when the infant is sufficiently mature, for it to lead to positive outcomes such as independence, something Greenough (1987) would call experience-expectant timing. It would be interesting to assess long-term developmental outcomes of the prematurely-rejected infants, particularly to compare the infants that were rejected early with those that were not but who later experienced high rates of maternal rejection to assess differences in outcomes are similar or different. These results show that premature maternal rejections may be detrimental to infant socioemotional development, increasing their aggression and in particular, increasing their anxiety-like behaviors. As other studies have found that males receive more punitive treatment and are retrieved less often than are female infants (Mitchell, 1968; Eaton, Johnson, Glick, & Worlein, 1985), it is noteworthy that there were no sex differences in rates of rejection detected in this study. As noted by Fairbanks (1996) sex differences in rejection are not uniform across studies (Brown and Dixon, 2000), and studies that have looked at sex differences in weaning and independence by and large have not assessed rates at such an early age nor have they assessed rejections in the face of variation of early maternal experiences.
Other studies show that PR and SPR rearing conditions lead to dysregulations in the serotonin system in rhesus monkeys (Bennett et al., 2002), a risk factor for aggression and a difference also noted in abused infants that experienced high rates of maternal rejections (Maestripieri, Higley, et al., 2006). Maestripieri and colleagues (2006) suggest that low serotonergic functioning resulting from early rejection plays a role in the transmission of abuse from one generation to the next. Furthermore, females that were abused in infancy and went on to become abusive mothers themselves had lower serotonergic function than the abused females that did not become abusers. This interpretation fits well with the findings of the present study that aberrant early rearing experiences (maternal absence) in first-generation females can lead to negative behavioral and social outcomes for second generation infants. It would be of interest to analyze central serotonin functioning from these prematurely-rejected infants to assess for impaired serotonin functioning.
This study highlights the importance of early experience, including its role in an individual’s own parenting behavior and its consequences for the next generation. As illustrated by the behavior of the PR and SPR mothers in this study, the negative effects of poor early-rearing experiences may be intergenerational. While it was not possible to assess molecular evidence of epigenetic changes in the mothers or the prematurely-rejected infants, other studies show that maternal absence leads to long-term changes at the epigenetic level (Lindell et al., 2012; Massart et al., 2016). While it would not be surprising if the mothers that lacked a mother when they were infants showed rearing induced epigenetic marks, it would be of interest to assess whether the second-generation infants that were reared by PR and SPR mothers also showed epigenetic marks paralleling those found in the mothers reared in mother-absent conditions.
To the extent that these results generalize to humans, the present findings have implications for human parenting as well as for infant developmental outcomes. The effects of inadequate first-generation mothering appear to leave a more-or-less lasting impact, which may be carried into adulthood, leading to impaired, insensitive mothering. This in turn may adversely affect the development of second-generation infants and children, leading to intergenerational consequences for offspring development. The task at hand is to learn how to intervene and prevent the long-term deleterious outcomes of poor early experiences on first-generation individuals and their subsequent second-generation offspring.
Acknowledgements
The authors would like to thank the research and animal care staff, as well as the graduate students and the post-docs at the National Institutes of Health Animal Center for their assistance in data collection. This work was supported by the National Institute of Alcohol Abuse and Alcoholism, Intramural Program, Eunice Kennedy Shriver National Institute of Child and Health and Human Development, Intramural Program, and by small grants provided by Brigham Young University.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request and with permissions from the National Institutes of Health.
References
- Andrews MW, & Rosenblum LA (1991). Attachment in monkey infants raised in variable- and low-demand environments. Child Dev, 62(4), 686–693. [PubMed] [Google Scholar]
- Andrews MW, & Rosenblum LA (1993). Assessment of attachment in differentially reared infant monkeys (Macaca radiata): response to separation and a novel environment. J Comp Psychol, 107(1), 84–90. [DOI] [PubMed] [Google Scholar]
- Bailey JA, Hill KG, Oesterle S, & Hawkins JD (2009). Parenting practices and problem behavior across three generations: monitoring, harsh discipline, and drug use in the intergenerational transmission of externalizing behavior. Dev Psychol, 45(5), 1214–1226. doi: 10.1037/a0016129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Becker ML, Suomi SJ, & Higley JD (2003). Relationships among CSF monoamine metabolite levels, alcohol sensitivity, and alcohol-related aggression in rhesus macaques. Aggress Behav, 29, 288–301). [Google Scholar]
- Belsky J. (1993). Etiology of child maltreatment: a developmental-ecological analysis. Psychol Bull, 114(3), 413–434. [DOI] [PubMed] [Google Scholar]
- Belsky J, Jaffee SR, Sligo J, Woodward L, & Silva PA (2005). Intergenerational transmission of warm-sensitive-stimulating parenting: a prospective study of mothers and fathers of 3-year-olds. Child Dev, 76(2), 384–396. doi: 10.1111/j.1467-8624.2005.00852.x [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, . . . Higley JD (2002). Early Experience and Serotonin Transporter Gene Variation Interact to Influence Primate CNS Function. Molecular Psychiatry, 7, 118–122. doi: 10.1038/sj/mp/4000949 [DOI] [PubMed] [Google Scholar]
- Britto PR, Lye SJ, Proulx K, Yousafzai AK, Matthews SG, Vaivada T, . . . Fernald LC (2017). Nurturing care: promoting early childhood development. Lancet, 389(10064), 91–102. [DOI] [PubMed] [Google Scholar]
- Brown GR, & Dixson AF (2000). The development of behavioural sex differences in infant rhesus macaques (Macaca mulatta). Primates, 41(1), 63–77. [DOI] [PubMed] [Google Scholar]
- Capitanio JP (1985). Early experience and social processes in rhesus macaques (Macaca mulatta): II. Complex social interaction. J Comp Psychol, 99(2), 133–144. [PubMed] [Google Scholar]
- Capitanio JP (2018). Behavioral inhibition in nonhuman primates: the elephant in the room. In Perez-Edgar K, and Fox NA, (Eds.), Behavioral Inhibition (pp. 17–33). Springer: Boston. [Google Scholar]
- Carpenter CR (1942). Sexual behavior of free-ranging rhesus monkeys (Macaca mulatta) -- as reprinted in 1964 volume. In Carpenter CR (Ed.), Naturalistic Behavior of Nonhuman Primates (pp. 289–318). University Park: Penn State U.P. [Google Scholar]
- Champoux M, Byrne E, DeLizio R, & Suomi SJ (1992). Motherless mothers revisited: Rhesus maternal behavior and rearing history. Primates, 33(2), 251–255. [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhász C, Nagy F, & Chugani DC (2001). Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. Neuroimage, 14(6), 1290–1301. [DOI] [PubMed] [Google Scholar]
- Clarke AS, & Snipes M. (1998). Early behavioral development and temperamental traits in mother- vs peer-reared rhesus monkeys. Primates, 39(4), 433–448. doi: 10.1007/BF02557567 [DOI] [Google Scholar]
- Coleman K, & Pierre PJ (2014). Assessing anxiety in nonhuman primates. Ilar Journal, 55(2), 333–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger RD, Neppl T, Kim KJ, & Scaramella L. (2003). Angry and aggressive behavior across three generations: a prospective, longitudinal study of parents and children. J Abnorm Child Psychol, 31(2), 143–160. [DOI] [PubMed] [Google Scholar]
- Corcoran CA, Pierre PJ, Haddad T, Bice C, Suomi SJ, Grant KA, . . . Bennett AJ (2012). Long-term effects of differential early rearing in rhesus macaques: Behavioral reactivity in adulthood. Dev Psychobio, 54(5), 546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton GG, Johnson DF, Glick BB, & Worlein JM (1985). Development in Japanese macaques (Macaca fuscata): Sexually dimorphic behavior during the first year of life. Primates, 26(3), 238–247. [Google Scholar]
- Fairbanks LA (1996). Individual differences in maternal style: Causes and consequences for mothers and offspring. Advances in the Study of Behavior, 25, 579–611. [Google Scholar]
- Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, . . . Consortium R. M. G. S. a. A. (2007). Evolutionary and biomedical insights from the rhesus macaque genome. Science, 316(5822), 222–234. doi: 10.1126/science.1139247 [DOI] [PubMed] [Google Scholar]
- Greenough WT (1987). Experience and brain development. Child Development, 58, 539–559. [PubMed] [Google Scholar]
- Greenough WT, Black JE, & Wallace CS (1987). Experience and brain development. Child Dev, 58(3), 539–559. [PubMed] [Google Scholar]
- Haapasalo J, & Pokela E. (1999). Child-rearing and child abuse antecedents of criminality. Aggress Violent Behav, 4(1), 107–127. [Google Scholar]
- Harlow HF, & Harlow MK (1965). The affectional systems. In Schrier AM, Harlow HF, & Stollinitz F. (Eds.), Behavior of Nonhuman Primates (Vol. 2, pp. 287–334). New York: Academic Press. [Google Scholar]
- Harlow H, & Seay B. (1966). Mothering in motherless mother monkeys. Br J Soc Psychi, 1, 63–69. [Google Scholar]
- Harlow HF, & Suomi SJ (1970).The nature of love – Simplified. Am Psychol, 25, 161–168. [DOI] [PubMed] [Google Scholar]
- Harlow HF, & Zimmerman RR (1959). Affectional responses in the infant monkey. Science, 130, 421–432. [DOI] [PubMed] [Google Scholar]
- Harvey P. J. P. s. (1987). Life histories in comparative perspective. In Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, and Struhsaker TT (Eds.), Primate Societies, pp. 179–180. Chicago: University of Chicago Press. [Google Scholar]
- Higley JD, Linnoila M, & Suomi SJ (1994). Ethological contributions: Experiential and genetic contributions to the expression and inhibition of aggression in primates. In Hersen M, Ammerman RT, & Sisson L. (Eds.), Handbook of Aggressive and Destructive Behavior in Psychiatric Patients (Vol. 17–32). New York: Plenum Press. [Google Scholar]
- Higley JD, Hopkins WD, Thompson WW, Byrne EA, Hirsch RM, Suomi SJ (1992) Peers as primary attachment sources in yearling rhesus monkeys (Macaca mulatta). Dev Psycho, 28, 1163–1171. [Google Scholar]
- Higley JD, Suomi SJ, & Chaffin AC (2011) Reactivity and Behavioral Inhibition as Personality Traits in Nonhuman Primates. In Weiss A. and King J, (Eds), Personality and Behavioral Syndromes in Nonhuman Primates: Developments in Primatology, pp. 285–312. Springer Press: New York. [Google Scholar]
- Hinde RA, & Simpson MJA (1967). Qualities of mother-infant relationships in monkeys. Ciba Found Symp, 33, 39–68. [DOI] [PubMed] [Google Scholar]
- Hinde RA, & Spencer-Booth Y. (1967). The behaviour of socially living rhesus monkeys in their first two and a half years. Anim Behav, 15(1), 169–196. [DOI] [PubMed] [Google Scholar]
- Jaffee SR (2017). Child Maltreatment and Risk for Psychopathology in Childhood and Adulthood. Annu Rev Clin Psychol, 13, 525–551. doi: 10.1146/annurev-clinpsy-032816-045005 [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, & Snidman N. (1988). Biological bases of childhood shyness. Science 240(4849), 167. [DOI] [PubMed] [Google Scholar]
- Kaler SR, & Freeman BJ (1994). Analysis of environmental deprivation: cognitive and social development in Romanian orphans. J Child Psychol Psychiatry, 35(4), 769–781. [DOI] [PubMed] [Google Scholar]
- Kalin NH (1999). Primate models to understand human aggression. J Clin Psychiatry, 60 Suppl 15, 29–32. [PubMed] [Google Scholar]
- Kalin NH, & Shelton SE (2003). Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Ann NY Acad Sci, 1008, 189–200. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Rickman M, & Davidson RJ (1998). Individual differences in freezing and cortisol in infant and mother rhesus monkeys. Behav Neurosci, 112(1), 251–254. [DOI] [PubMed] [Google Scholar]
- Lickenbrock DM, & Braungart-Rieker JM (2015). Examining antecedents of infant attachment security with mothers and fathers: An ecological systems perspective. Infant Behav Dev, 39, 173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindburg DG (1971). The rhesus monkey in North India: an ecological and behavioral study. In Primate behavior: developments in field laboratory research (Vol. 2, pp. 1–106). [Google Scholar]
- Lindell SG, Yuan Q, Zhou Z, Goldman D, Thompson RC, Lopez JF, . . . Barr, C. S. (2012). The serotonin transporter gene is a substrate for age and stress dependent epigenetic regulation in rhesus macaque brain: potential roles in genetic selection and gene x environment interactions. Dev Psychopathol, 24(4), 1391–1400. doi: 10.1017/s0954579412000788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomanowska AM, Boivin M, Hertzman C, & Fleming AS (2017). Parenting begets parenting: A neurobiological perspective on early adversity and the transmission of parenting styles across generations. Neuroscience, 342, 120–139. doi: 10.1016/j.neuroscience.2015.09.029 [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Higley JD, Lindell SG, Newman TK, McCormack KM, & Sanchez MM (2006). Early maternal rejection affects the development of monoaminergic systems and adult abusive parenting in rhesus macaques (Macaca mulatta). Behav Neurosci, 120, 1017–1024. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Lindell SG, & Higley JD (2007). Intergenerational transmission of maternal behavior in rhesus macaques and its underlying mechanisms. Dev Psychobio, 49, 165–171. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, McCormack K, Lindell SG, Higley JD, & Sanchez MM (2006). Influence of parenting style on the offspring’s behaviour and CSF monoamine metabolite levels in crossfostered and noncrossfostered female rhesus macaques. Behav Brain Res, 175, 90–95. [DOI] [PubMed] [Google Scholar]
- Massart R, Nemoda Z, Suderman MJ, Sutti S, Ruggiero AM, Dettmer AM, . . . Szyf M. (2016). Early life adversity alters normal sex-dependent developmental dynamics of DNA methylation. Dev Psychopathol, 28(4pt2), 1259–1272. doi: 10.1017/s0954579416000833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GDJCD (1968). Attachment differences in male and female infant monkeys. 611–620. [PubMed] [Google Scholar]
- Moran KM, Turiano NA, & Gentzler AL (2018). Parental warmth during childhood predicts coping and well-being in adulthood. J Fam Psychol, 32(5), 610–621. doi: 10.1037/fam0000401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak MA, & Sackett GP (2006). The effects of rearing experiences: The early years. In Nursery rearing of nonhuman primates in the 21st century. (Eds) Sackett GP, and Ruppenthal GC, Elias K. (pp. 5–19), Springer: Boston. [Google Scholar]
- Perez NM, Jennings WG, Piquero AR, & Baglivio MT (2016). Adverse Childhood Experiences and Suicide Attempts: The Mediating Influence of Personality Development and Problem Behaviors. J Youth Adolesc, 45(8), 1527–1545. doi: 10.1007/s10964-016-0519-x [DOI] [PubMed] [Google Scholar]
- Raposo SM, Mackenzie CS, Henriksen CA, & Afifi TO (2014). Time does not heal all wounds: older adults who experienced childhood adversities have higher odds of mood, anxiety, and personality disorders. Am J Geriatr Psychiatry, 22(11), 1241–1250. doi: 10.1016/j.jagp.2013.04.009 [DOI] [PubMed] [Google Scholar]
- Rispoli KM, McGoey KE, Koziol NA, & Schreiber JB (2013). The relation of parenting, child temperament, and attachment security in early childhood to social competence at school entry. J Sch Psychol, 51(5), 643–658. [DOI] [PubMed] [Google Scholar]
- Roma PG, Ruggiero AM, Schwandt M, Higley JD, & Suomi SJ (2006). The kids are alright: Maternal behavioral interactions and stress reactivity in infants of differentially reared rhesus monkeys. Journal of Developmental Processes, 1, 103–122. [Google Scholar]
- Rosenblum LA, & Paully GS (1984). The effects of varying environmental demands on maternal and infant behavior. Child Dev, 55(1), 305–314. [PubMed] [Google Scholar]
- Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA, & Ingram DK (2004). Aging in rhesus monkeys: relevance to human health interventions. Science, 305(5689), 1423–1426. doi: 10.1126/science.1102541 [DOI] [PubMed] [Google Scholar]
- Ruppenthal GC, Arling GL, Harlow HF, Sackett GP, & Suomi SJ (1976). A 10-year perspective of motherless-mother monkey behavior. J Abnorm Psychol, 85(4), 341–349. [DOI] [PubMed] [Google Scholar]
- Seay B, & Harlow HF (1965). Maternal separation in the rhesus monkey. J Nerv, 140, 434–444. [DOI] [PubMed] [Google Scholar]
- Seay B, Alexander BK, & Harlow HF (1964). Maternal behavior of socially deprived rhesus monkeys. J Abnorm Psychol, 69(4), 345. [DOI] [PubMed] [Google Scholar]
- Seay B, Hansen E, Harlow HF (1962). Mother-infant separation in monkeys. J Child Psychol Psychiat, 3(3-4), 123–132. [DOI] [PubMed] [Google Scholar]
- Shannon C, Champoux M, & Suomi SJ (1998). Rearing condition and plasma cortisol in rhesus monkey infants. Am J Primatol, 46, 311–321. [DOI] [PubMed] [Google Scholar]
- Suomi. (2005). Mother-infant attachment, peer relationships, and the development of social networks in rhesus monkeys. Hum Dev, 48, 67–79. [Google Scholar]
- Suomi Harlow, & Domek. (1970). Effect of repetitive infant-infant separation of young monkeys. J Abnorm Psychol, 76, 161–172. [DOI] [PubMed] [Google Scholar]
- Tarantino LM, Sullivan PF, & Meltzer-Brody S. (2011). Using animal models to disentangle the role of genetic, epigenetic, and environmental influences on behavioral outcomes associated with maternal anxiety and depression. Front Psychiatry, 2, 44. doi: 10.3389/fpsyt.2011.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van IJzendoorn MH (1992). Intergenerational transmission of parenting: A review of studies in nonclinical populations. Dev Rev, 12, 76–99. [Google Scholar]
- Weaver A, & de Waal FB (2002). An index of relationship quality based on attachment theory. J Comp Psychol, 116(1), 93. [DOI] [PubMed] [Google Scholar]
- Widom CS (2000). Motivation and mechanisms in the “cycle of violence”. Nebraska Symposium on Motivation, 46, 1–37. [PubMed] [Google Scholar]