Abstract
Background:
Despite a temporal increase in respiratory failure in patients hospitalized with acute heart failure (HF), clinical trials have largely not reported the incidence or associated clinical outcomes for patients requiring mechanical ventilation (MV).
Methods:
After pooling 5 acute HF clinical trials, we utilized multivariable logistic regression adjusted for demographics, comorbidities, exam, and laboratory findings to assess associations between MV and clinical outcomes.
Results:
Among the 8,296 patients, 210 (2.5%) required MV. Age, gender, smoking history, baseline ejection fraction, heart failure etiology, and the proportion of patients randomized to treatment or placebo in the original clinical trial were similar between groups (all, P>0.05). Baseline diabetes mellitus was more common in the MV group (P=0.02), but other comorbidities, including chronic lung disease, were otherwise similar (all, P>0.05). HF rehospitalization at 30-days (12.7% vs 6.6%, P<0.001) and all-cause 60-day mortality (33.3% vs 6.1%, P<0.001) was higher among patients requiring MV. After multivariable adjustment, MV use was associated with an increased 30-day HF rehospitalization (odds ratio [OR] 2.03; 95% confidence interval [CI], 1.29–3.3.21, P=0.002), 30-day mortality (OR 10.40; 95% CI, 7.22–14.98, P<0.001), and 60-day mortality (OR 7.68; 95% CI, 5.50–10.74, P<0.001). The influence of MV did not differ by HF etiology or baseline ejection fraction (both, interaction P>0.20).
Conclusions:
Respiratory failure during an index hospitalization for acute HF was associated with increased rehospitalization and all-cause mortality. The development of respiratory failure during an acute HF admission identifies a particularly vulnerable population, which should be identified for closer monitoring.
Introduction:
Acute heart failure (AHF) remains a complex and challenging syndrome with limited evidenced based treatments.1 One of the more deleterious complications of worsening HF is respiratory failure,2 the treatment of which is largely driven by expert opinion.3 Research investigating the incidence and associated outcomes of respiratory failure complicating HF have largely utilized administrative data,4–6 which has limited granularity (e.g. baseline vital signs, laboratory values, etc.) and lacks post-discharge outcomes. Selected registry reports have included the incidence of respiratory support, but rarely report the associated outcomes.7,8 In addition, little is known about differences in physiologically unique subgroups such as HF etiology (ischemic vs. non-ischemic) or systolic vs. diastolic dysfunction.9 Given these gaps in the literature, especially the lack of post-discharge outcomes, we conducted a pooled analysis of AHF randomized controlled trials to assess the association between mechanical ventilation utilization and short-term clinical outcomes.
Methods:
Data Source and Study Population
We a priori selected all of the available AHF clinical trials at our institution and the University of Alberta. The Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS; ClinicalTrials.gov Identifier: NCT00608491),10 Renal Optimization Strategies Evaluation (ROSE; NCT01132846),11 Diuretic Optimization Strategies Evaluation (DOSE; NCT00577135),12 and Evaluation Study of Congestive heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE; NCT00000619)13 were obtained from the National heart, Lung, and Blood Institute’s Biologic Specimen and Data Repository Information Coordinating Center. The Acute Study of Nesiritide in Decompensated Heart Failure (ASCEND-HF; NCT00475852)14 data was obtained from the Canadian Vigour Centre at the University of Alberta, Canada. All outcomes were previously adjudicated by prespecified criteria in each clinical trial. Specifics of each trial and definition of respiratory support are shown in Supplemental Table 1.
Statistical Analysis
Baseline characteristics were stratified by utilization of mechanical ventilation. Continuous variables were reported with the median and interquartile range (IQR) compared using the Wilcoxon rank-sum test and categorical variables were described as frequencies and percentage using the chi-squared test. The primary outcome was 60-day all-cause mortality corresponding to the longest follow-up for the shortest trials (DOSE and CARRESS). Secondary outcomes included in-hospital mortality, 30-day HF rehospitalization, and 30-day mortality. Our multivariable logistic regression model was constructed by assessing candidate variables, including demographics, comorbidities, and laboratory values. In addition to study of enrollment, covariates were included in the model if their univariate association with the primary outcome was P<0.20. The final model included trial enrollment, age, race, ischemic heart disease, smoking history (yes, no), cerebrovascular disease, hypertension, atrial fibrillation/flutter, peripheral vascular disease, chronic obstructive lung disease, diabetes mellitus, depression as well as baseline heart rate, systolic blood pressure, peripheral edema, jugular venous distension, rales, sodium, and blood urea nitrogen. We used postestimation methods to test final regression model fit with our primary outcome (C-statistic=0.75).
Prespecified subgroup analyses included HF etiology (ischemic vs. non-ischemic) and HF with reduced ejection fraction (HFrEF, EF<40%) vs. preserved EF (HFpEF, EF≥40%). In addition, we performed several sensitivity analyses. First, since some participants could have been coded for mechanical ventilation due to a surgical procedure, we performed an analysis excluding patients undergoing a cardiac surgical procedure during their index admission. Second, given differences between the two cohorts, we repeated our primary analysis with N-terminal pro B-type natriuretic peptide (NT-proBNP) in the model for those with available levels at baseline (n=3,308). Finally, we assessed for differences in hemodynamics for patients with pulmonary artery catheterization data at baseline (n=225). Analyses were performed on STATA 16.0 (Stata Corp, College Station, TX) with statistical significance considered at a two-tailed P<0.05.
Results:
Baseline characteristics stratified by the utilization of mechanical ventilation are shown in Table 1. Among the 8,296 patients, 210 (2.5%) required mechanical ventilation. Demographics, smoking status, baseline EF, HF etiology, and the proportion of patients randomized to treatment or placebo in the original clinical trial were similar between groups (all, P>0.05). Baseline medical comorbidities, including chronic lung disease (all, P>0.05), were statistically similar with the exception of diabetes mellites which had a higher proportion in the mechanical ventilation group (P=0.02). Ventilated patients were more likely to present with New York Heart Association class III or IV heart failure (80.4% vs. 89.5%, P=0.002). Baseline NT-proBNP was approximately two times higher in those requiring mechanical ventilation (4,460 pg/mL vs. 8,298 pg/mL, P<0.001). All other laboratory values were similar.
Table 1.
Patient Characteristics Stratified by Mechanical Ventilation Use
| No Mechanical Ventilation | Mechanical Ventilation | P Value | |
|---|---|---|---|
|
| |||
| Demographics | |||
| Age, years | 67 (56–76) | 66 (56–74) | 0.18 |
| Men | 5,416 (67.0%) | 152 (72.4%) | 0.10 |
| Race | <0.001 | ||
| Black | 1,331 (16.5%) | 22 (10.5%) | |
| White | 4,716 (58.3%) | 91 (43.3%) | |
| Other | 2,038 (25.2%) | 97 (46.2%) | |
| Body mass index, kg/m2 | 28 (24–33) | 25 (22–31) | <0.001 |
| New York Heart Association class | |||
| I-II | 1,337 (19.6%) | 20 (10.5%) | 0.002 |
| III-IV | 5,496 (80.4%) | 171 (89.5%) | 0.002 |
| Ejection fraction | 29 (20–38) | 28 (10–35) | 0.28 |
| Smoking history | 1,581 (20.3%) | 34 (16.3%) | 0.16 |
| Randomized to treatment in primary trial | 4,102 (50.7%) | 98 (46.7%) | 0.24 |
| Physical examination | |||
| Heart rate, beats/min | 81 (70–94) | 88 (76–100) | <0.001 |
| Systolic blood pressure, mm Hg | 120 (110–138) | 120 (106–135) | 0.02 |
| Peripheral edema | 6,142 (76.5%) | 160 (76.9%) | 0.89 |
| Jugular venous distension | 4,988 (62.2%) | 139 (66.2%) | 0.24 |
| Rales | 6,601 (81.9%) | 191 (91.0%) | <0.001 |
| Comorbidities | |||
| Heart failure etiology | 0.76 | ||
| Ischemic etiology | 3,782 (46.8%) | 99 (47.1%) | |
| Non-ischemic etiology | 3,324 (41.1%) | 89 (42.4%) | |
| Dilated/idiopathic | 1,569 (19.4% | 42 (20.0%) | 0.83 |
| Hypertensive | 959 (11.9%) | 19 (9.0%) | 0.21 |
| Valvular | 327 (4.0%) | 13 (6.2%) | 0.12 |
| Other | 552 (6.8%) | 11 (5.2%) | 0.37 |
| Coronary artery disease | 4,132 (51.1%) | 103 (49.0%) | 0.55 |
| Hypertension | 5,821 (72.0%) | 143 (68.1%) | 0.21 |
| Diabetes mellitus | 3,503 (43.4%) | 108 (51.4%) | 0.02 |
| Atrial fibrillation/flutter | 3,004 (38.6%) | 68 (32.7%) | 0.08 |
| Cerebrovascular disease | 992 (12.3%) | 24 (11.4%) | 0.71 |
| Peripheral vascular disease | 893 (11.0%) | 22 (10.5%) | 0.79 |
| Chronic lung disease | 1,420 (17.6%) | 37 (17.6%) | 0.98 |
| Hepatic disease | 255 (3.2%) | 7 (3.3%) | 0.88 |
| Cancer history | 347 (4.3%) | 7 (3.3%) | 0.50 |
| Chronic alcohol use | 683 (8.9%) | 19 (9.5%) | 0.79 |
| Laboratory value | |||
| Sodium, mEq/L | 139 (136–141) | 137 (134–140) | <0.001 |
| Creatinine, mg/dL | 1.29 (1.01–1.64) | 1.24 (1.00–1.68) | 0.42 |
| BUN, mg/dL | 10.5 (6.8–18.2) | 10.3 (6.8–17.3) | 0.38 |
| AST, U/L | 28 (21–39) | 28 (21–45) | 0.33 |
| ALT, U/L | 26 (17–40) | 28 (20–43) | 0.11 |
| Total bilirubin, mg/dL | 0.9 (0.6–1.3) | 1.0 (0.6–1.4) | 0.29 |
| Hemoglobin, g/dL | 12.6 (11.2–13.9) | 12.4 (11.0–14.4) | 0.71 |
| BNP, pg/mL* | 962 (509–1816) | 1,238 (570–1860) | 0.25 |
| NT-proBNP, pg/mL* | 4,460 (2136–9168) | 8,298 (2957–13,790) | <0.001 |
| Hospital outcomes | |||
| Hospital length of stay, days | 6 (4–10) | 13 (5–28) | <0.001 |
| Vasoactive drug use | 4,077 (54.7%) | 165 (78.9%) | <0.001 |
| In-hospital mortality | 112 (1.4%) | 55 (26.2%) | <0.001 |
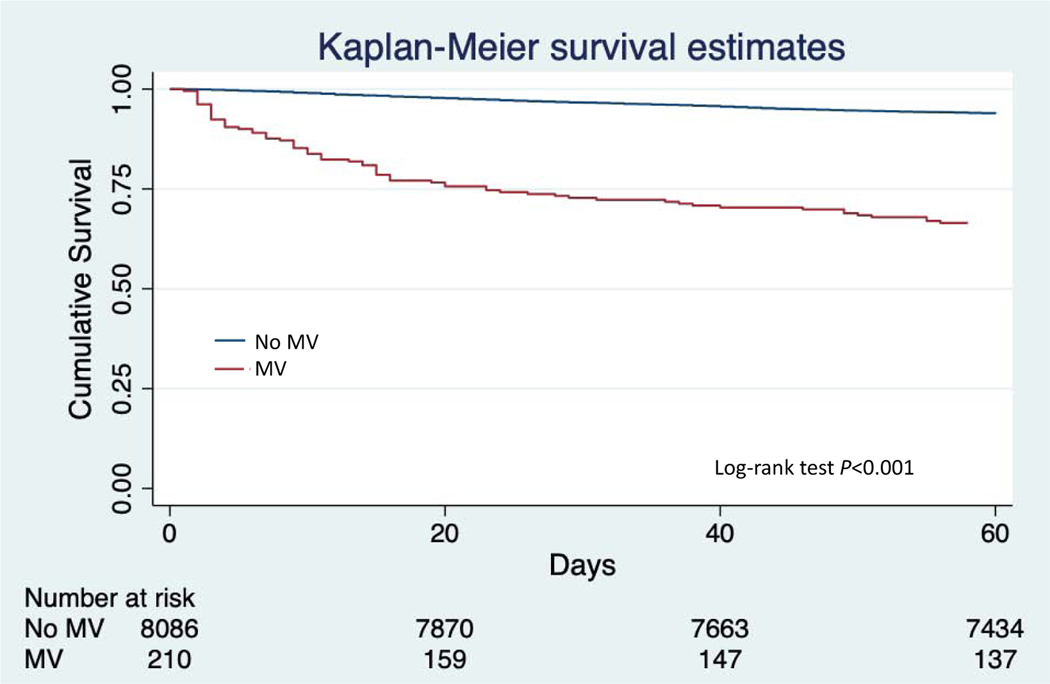
The median length of stay was 7 days longer in ventilated compared to non-ventilated patients (P<0.001). The proportion of patients with a 30-day HF rehospitalization was nearly double in those requiring mechanical ventilation during their index admission (6.6% vs. 12.7%, P<0.001). The 30-day mortality was 3.4% in those without mechanical ventilation and 27.1% in those with mechanical ventilation (P<0.001). At 60-days, the all-cause mortality was 6.1% in those not requiring mechanical ventilation and 33.3% in the mechanical ventilation group (Figure 1, P<0.001).
Figure 1.
Survival in patients stratified by utilization of mechanical ventilation
MV = Invasive mechanical ventilation
*Note = Time zero starts at study enrollment
After multivariable adjustment, mechanical ventilation utilization was associated with increased 30-day HF rehospitalization (odds ratio [OR] 2.03; 95% confidence interval [CI], 1.29–3.21, P=0.002), 30-day all-cause mortality (OR 10.40; 95% CI, 7.22–14.98, P<0.001), and 60-day all-cause mortality (OR 7.68; 95% CI, 5.50–10.73, P<0.001) (Table 2). Amongst hospital survivors, 60-day mortality was similarly elevated for those requiring respiratory support (OR 2.70; 95% CI, 1.58–4.62, P<0.001). When stratified by ischemic vs. non-ischemic etiology or HFrEF vs. HFpEF, the odds of 60-day mortality were similar (both, interaction P>0.20). There was no interaction between clinical trials or between patients randomized to treatment compared to placebo (all, interaction P>0.50).
Table 2.
Clinical outcomes stratified by mechanical ventilation utilization
| Endpoint | Adjusted Odds Ratio (95% CI) * | P Value |
|---|---|---|
| 30-day HF rehospitalization | 2.03 (1.29–3.21) | 0.002 |
| 30-day mortality | 10.40 (7.22–14.98) | <0.001 |
| 60-day mortality | 7.68 (5.50–10.74) | <0.001 |
| Ischemic HF | 7.84 (4.81–12.76) | <0.001 |
| Non-ischemic HF | 7.89 (4.87–12.44) | <0.001 |
| EF ≥ 40% | 8.84 (3.64–21.49) | <0.001 |
| EF < 40% | 7.94 (5.26–11.99) | <0.001 |
CI = Confidence interval; HF = Heart failure; EF = Ejection fraction
Adjusted for trial enrollment, age, race, ischemic heart disease, smoking history (yes, no), cerebrovascular disease, hypertension, atrial fibrillation/flutter, peripheral vascular disease, chronic obstructive lung disease, diabetes mellitus, depression, heart rate, systolic blood pressure, edema, jugular venous distension, rales, sodium, and blood urea nitrogen.
In sensitivity analysis, excluding patients who underwent a cardiac surgical procedure, those requiring mechanical ventilation continued to have a substantially higher 60-day mortality (OR 9.93; 95% CI, 6.89–14.31, P<0.001). Second, in the cohort with NT-proBNP available, the inclusion of NT-proBNP in the model did not significantly change the 60-day mortality (OR 8.08; 95% CI, 4.66–14.01, P<0.001). Finally, in the much smaller population of patients (n=225) with hemodynamic data available at baseline, patients requiring mechanical ventilation had a higher median right atrial pressures (12 mmHg vs. 20 mmHg, P=0.003) (Supplemental Table 2). All other measurements were statistically similar (all, P>0.05).
Discussion:
In this pooled analysis of AHF clinical trials, we found that patients requiring mechanical ventilation had substantially worse short-term clinical outcomes. Specifically, patients requiring mechanical ventilation during their index admission were over two times more likely to be hospitalized for HF at 30-days. We also found that 30-day and 60-day all-cause mortality was substantially higher than those not requiring mechanical ventilation. In addition, there were no significant differences in patient outcomes when stratified by HF etiology or HFrEF vs. HFpEF. In aggregate, these data underscore the importance of a poorly studied subgroup of AHF patients and stress the need for therapies and strategies to improve outcomes in these patients.
Although there is no agreed upon definition, in-hospital worsening HF is an increasingly recognized event with a considerably high adverse event rate.15 Perhaps the most severe form of worsening HF is respiratory failure, which unfortunately is rarely reported in clinical trials.9 However, reporting the incidence of and associated clinical outcomes is integral in order to understand the scope of the problem as well as establish a foundation for identifying potential treatment options. More specifically, outstanding questions remain regarding methods to identify early or potentially prevent respiratory failure, positive pressure ventilation best practices, and how it may differentially affect specific HF populations (HFrEF vs. HFpEF), right versus left ventricular failure, and approaches to better define appropriate liberation from mechanical ventilation.
In addition, our results highlight the discrepancy between clinical trial populations and “real-world” patients. While the incidence of respiratory failure we found (2.5%) was similar to rates from previous administrative databases (2.1–3.2%),4,6 HF registries have reported between 5–14% of AHF patients require some form of respiratory support.7,8 Future clinical trials, potentially pragmatistic trials leveraging the electronic health record, may be one strategy to better study this critically ill HF subgroup.
Limitations
There are several limitations that require mention. First, we were unable to account for the timing of mechanical ventilation in relation to a surgical procedure. However, exclusion of these patients in our sensitivity analyses actually increased the point estimates associated with 60-day mortality. Second, the use of non-invasive modalities both before and/or after invasive mechanical ventilation was not captured in all of the clinical trials, and therefore not included in our analysis. Finally, although our study has included several novel aspects compared to previous analyses, we lack data on some important variables such as mechanical ventilation length, ventilator settings, and concomitant sedation practices.
Conclusions
In conclusion, those requiring mechanical ventilation during an episode of AHF represent an especially sick population with substantially worse short-term clinical outcomes. Future studies are needed in order to develop predictive models of acute worsening HF, especially respiratory failure, as well as investigate specific treatment strategies for these critically ill patients.
Supplementary Material
Acknowledgments
Funding: Dr. Miller reports funding through by the Yale National Clinician Scholars Program and by CTSA Grant Number TL1 TR001864 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Allen LA, Hernandez AF, O’Connor CM, Felker GM. End points for clinical trials in acute heart failure syndromes. J Am Coll Cardiol. 2009;53(24):2248–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metkus TS, Miller PE, Alviar CL, et al. Advanced Respiratory Support in the Contemporary Cardiac ICU. Crit Care Explor. 2020;2(9):e0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alviar CL, Miller PE, McAreavey D, et al. Positive Pressure Ventilation in the Cardiac Intensive Care Unit. J Am Coll Cardiol. 2018;72(13):1532–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller PE, Patel S, Saha A, et al. National Trends in Incidence and Outcomes of Patients With Heart Failure Requiring Respiratory Support. Am J Cardiol. 2019. [DOI] [PubMed] [Google Scholar]
- 5.Metkus TS, Stephens RS, Schulman S, Hsu S, Morrow DA, Eid SM. Utilization and outcomes of early respiratory support in 6.5 million acute heart failure hospitalizations. Eur Heart J Qual Care Clin Outcomes. 2020;6(1):72–80. [DOI] [PubMed] [Google Scholar]
- 6.de Miguel-Diez J, Jimenez-Garcia R, Mendez-Bailon M, et al. National trends in mechanical ventilation among patients hospitalized with heart failure: a population-based study in Spain (2001–2017). Eur J Intern Med. 2020;78:76–81. [DOI] [PubMed] [Google Scholar]
- 7.Adams KF Jr., Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149(2):209–216. [DOI] [PubMed] [Google Scholar]
- 8.Nieminen MS, Brutsaert D, Dickstein K, et al. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27(22):2725–2736. [DOI] [PubMed] [Google Scholar]
- 9.Miller PE, van Diepen S, Ahmad T. Acute Decompensated Heart Failure Complicated by Respiratory Failure. Circ Heart Fail. 2019;12(5):e006013. [DOI] [PubMed] [Google Scholar]
- 10.Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367(24):2296–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA. 2013;310(23):2533–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364(9):797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Binanay C, Califf RM, Hasselblad V, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294(13):1625–1633. [DOI] [PubMed] [Google Scholar]
- 14.O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365(1):32–43. [DOI] [PubMed] [Google Scholar]
- 15.Butler J, Gheorghiade M, Kelkar A, et al. In-hospital worsening heart failure. Eur J Heart Fail. 2015;17(11):1104–1113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.