Implications.
Increasing wildlife–livestock interactions enhance opportunities for pathogen transmission and biodiversity erosion.
This increases the risk of emerging diseases in wildlife, livestock, and humans.
Biosecurity measures are needed, but rethinking of livestock production in high biodiversity regions is also required.
A cross-sectoral transdisciplinary approach is required for the effective management of risks at the wildlife–livestock interface.
It is urgent to find models and approaches that allow a better balance between protein production and biodiversity conservation.
Introduction
The ongoing COVID-19 crisis has emphasized more than ever the relevance of wildlife as a potential source of pathogens for other species, including humans, and the potential importance that interactions with wildlife can have on global health. Nevertheless, in the veterinary world, the concept of wildlife as a potential reservoir and source of pathogens detrimental to livestock production and health has been known for centuries.
Well-known examples of livestock diseases in which the interface with wildlife plays, or has played, an important role include rinderpest, avian influenza, foot and mouth disease (FMD), and African swine fever (ASF). Rinderpest, caused by a morbillivirus of the family Paramyxoviridae, is one of only two diseases that have been globally eradicated (the other being smallpox in humans), after having caused major disease outbreaks in domestic and wild artiodactyl species for centuries. After a globally coordinated eradication campaign, the World Organisation for Animal Health (OIE) and the Food and Agriculture Organization (FAO) of the United Nations announced in 2011 that rinderpest virus had been eliminated from livestock, thus declaring global freedom from this disease (Hamilton et al., 2017). Circulation of rinderpest virus in endemic regions in wild susceptible species was an important consideration in the eradication campaign, and lack of recognition of wildlife reservoirs was one of the factors to which failure of initial campaigns in the 1960s and 70s was attributed (Morens et al., 2011).
Other diseases, such as ASF and FMD, are still endemic and expanding across different regions of the world. FMD is estimated to be endemic in 77% of the global livestock population, in Africa, Asia, and some parts of South America (OIE, 2021a) and ASF is becoming endemic in Africa, Europe, Asia, and some parts of Oceania (OIE, 2021b). Efforts to control or eradicate these diseases are challenging, particularly in those areas where wild reservoir hosts contribute to their maintenance and spread. African swine fever virus (ASFV) has been known for more than a century to be maintained in the soft tick-warthog sylvatic cycle in natural savannah environments in East and Southern Africa. Occasional interactions between ASFV-infected ticks and domestic pigs have facilitated the dissemination of several ASFV genotypes into the domestic pig value-chain in Africa and subsequently into other parts of the world (Dixon et al., 2020). During the currently ongoing pandemic of ASF, the wild boar population in Europe has played a central role in the propagation of the virus into new areas. While most ASF spread appears to occur within domestic pig populations due to anthropogenic factors, incursions of ASFV into low biosecurity domestic pig farming systems from wild boar are also important (Brookes et al., 2021). Likewise, transboundary spread of FMD in susceptible domestic livestock such as cattle and pigs is commonly mediated by anthropogenic factors, such as movement of infected livestock, or the feeding of infected products to susceptible species (Di Nardo et al., 2011). However, in East and Southern Africa, the African buffalo interface plays an important role in maintaining FMD virus (FMDV) strains and disseminating them to adjacent susceptible livestock populations (Jori and Etter, 2016).
These examples provide only a snapshot illustration of the potential role of wildlife on livestock disease and demonstrate the importance of the wildlife–livestock interface. At a planetary scale, several factors act as major drivers of increased wildlife–livestock interactions at these interfaces (Magouras et al., 2020). Critical drivers include the need to feed an ever-increasing world human population, which has altered the way in which livestock are farmed, the way in which we interact with the ecosystem, and climate change. These drivers not only increase the intensity and frequency of interactions between wildlife and potential spillover populations (e.g., humans and domesticated animals such as livestock) but also facilitate new transmission pathways for potential emerging pathogens. Some of the impacts of these interactions have been well-described in the literature, particularly those affecting livestock production and health. However, these interactions can also have very significant and devastating effects on wildlife populations and the environment. Importantly, circulation of undetected pathogens in the domestic and wild animal compartments also provides opportunity for the development of potentially dangerous emerging infectious diseases.
In this review, we provide an overview of the drivers of wildlife–livestock interactions and their potential impacts on terrestrial livestock production. We define wildlife as any domesticated or non-domesticated species that is free-ranging and does not depend on mankind for food or reproduction. In addition, we present and discuss the major tools and methods to reduce wildlife–livestock contact and to mitigate its health implications, including biosecurity measures and the approaches and potential solutions for improved cohabitation between livestock and wildlife to encourage biodiversity and reduce negative impacts such as disease spillover.
The Drivers of Wildlife Interactions with Livestock
Global drivers of wildlife–livestock interactions
The world human population is expected to increase by 2 billion people over the next 30 yr, from 7.7 billion today to 9.7 billion in 2050. It could reach a number close to 11 billion people around the year 2100 (Abel et al., 2016). To keep up with this galloping population increase, humanity is faced with the enormous challenge of producing enough protein to meet demand; consequently, protein production is expected to double by 2050. Animal products contribute approximately 67% of total global protein production (Baldi and Gottardo, 2017), and livestock enterprises represent the world’s largest land user, either directly through grazing or indirectly through the production of fodder and grains. This need for space inevitably leads to deforestation, and agriculture is considered responsible for the disappearance of 130 million hectares of tropical forests (equivalent to the size of Brazil) between 1990 and 2015. This process of habitat degradation to increase land for agriculture facilitates increasing areas of interaction between livestock present in these agricultural areas and wildlife inhabiting primary or secondary forest (Jones et al., 2013). In addition, although some mitigating activities are being implemented, livestock production especially cattle farming is a major contributor of carbon release to the atmosphere and subsequent global warming and climate change (Koneswaran and Nierenberg, 2008).
Many infectious diseases are climate sensitive, especially vector-borne diseases because arthropod vectors alter their distribution ranges, introducing vector-borne diseases into new areas. In this manner, tropical vector-borne diseases are progressively expanding into temperate areas of the planet. For example, blue tongue virus, once only found in the tropics, has been expanding into Europe where it has affected European livestock for several decades (Jacquot et al., 2017) and other tropical viruses such as Rift Valley fever virus could follow (Chevalier et al., 2010)
Localized drivers of wildlife–livestock interactions
As well as facilitating large-scale changes in wildlife–livestock interactions by altering the distributions of livestock, wildlife, and the disease vectors, land-use change (and the livestock management practices that go with this) and climate change also facilitate localized interactions. For example, in arid and semi-arid landscapes, the scarcity of water generates increasingly important hotspots of interaction between wild and domestic species around water sources (Jori et al., 2021a). Pastoral and rangeland ecosystems are common areas for ruminant production where they are reared in large extensions. In these ecosystems, interactions between wild and domestic herbivores are particularly common around water or grazing resources (Figure 1). These situations are well known to facilitate the transmission and maintain the circulation of shared diseases between domestic and wild species in all regions of the world, including tropical, temperate, and pre-polar areas (Du Toit et al., 2017). In many resource-poor countries, the boundaries of protected areas are not clearly defined or rely on permeable physical barriers such as poorly maintained fences or seasonal rivers (Figure 2).
Figure 1.
Two examples of hotspots of interaction, where sympatric wild and domestic species are attracted by the presence of water or food resources: (a) Shared meadows between sheep and Alpine ibex (Capra ibex) in the Swiss Alps (Courtesy of M. P. Ryser-Degiorgis). (b) Shared pastures between sheep and wild camelids in Peruvian highlands (Photo: F.J.).
Figure 2.
Patchy or diffuse interface: the boundary between a wildlife and livestock area can be spatially diffuse; for example, when a physical barrier (river) separating wildlife and livestock areas is affected by poor maintenance (fence) or drought (river), such as in this case (Photo: I.M.).
The type of livestock husbandry also influences wildlife–livestock interactions, especially if livestock are kept outdoors. Outdoor husbandry varies from traditional, free-ranging production systems at the village level (also known as backyard farming especially in resource-poor countries; Figure 3a), to outdoor production in middle- to high-income countries with the goal to produce high quality or organic-labeled, niche-market products (Figure 3b). In low- to middle-income countries, backyard farming has very important economic and sociocultural roles for the well-being of rural and peri-urban households, such as food supply, source of income, asset saving, source of employment, soil fertility, livelihoods, transport, agricultural traction, agricultural diversification, and sustainable agricultural production (Banda and Tanganyika, 2021). Such smallholder farmers make up a large proportion of global rural communities. Particularly, in rural areas, this livestock is often raised under very poor biosecurity conditions allowing frequent and regular contacts with wildlife species (Jori et al., 2021a). In middle- to high-income countries, rising consumer demand for improved animal welfare through animal-friendly practices, as well as perceived better-quality products, has triggered the increase in outdoor production in systems that traditionally were indoors. However, these systems allow greater contact with wild mammals and birds and pose serious challenges when it comes to mitigating negative impacts such as the increased risk of exposure of domestic animals to pathogens carried by wildlife, predation, and environmental contaminants (Åkerfeldt et al., 2020).
Figure 3.
Two examples of free-ranging pigs in different settings. (a) Backyard pig farming in Northern Vietnam (Photo: I.M.). (b) In Corsica and other Mediterranean islands, it is traditional to rear pigs in extensive farming systems. There is an increasing consumer demand for the resulting pork products (Photo: F.J.).
The Impacts of Wildlife on Livestock
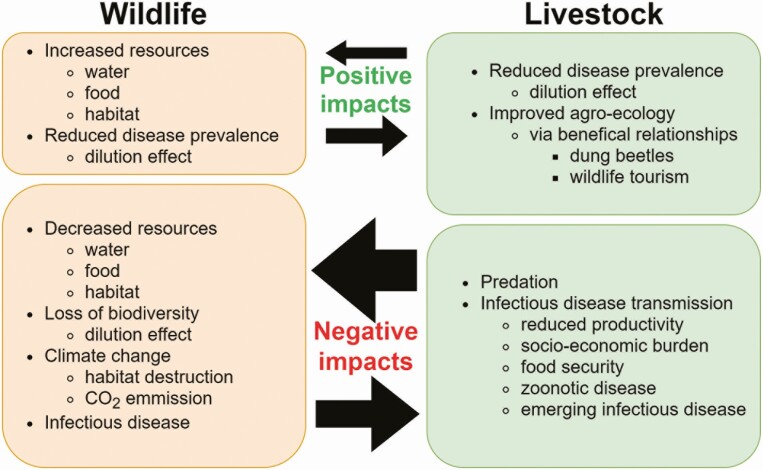
The growing need to expand livestock production suggests that increasing interactions between wildlife and livestock will become more important in the next decades and, as a result, impacts on livestock and wildlife populations will intensify. Figure 4 illustrates some of these negative and positive impacts, which are described in more detail below.
Figure 4.
The impacts of interactions at the wildlife–livestock interface. The size of the arrows and compartments are schematic representations of the relative difference in size of the influence and the overall impacts between wildlife and livestock (Design: V.J.B.).
Negative impacts
Examples of negative impacts of wildlife interactions on livestock production are well known. They include morbidity and mortality due to infectious diseases, impacts on people due to reduced food security, zoonotic disease or economic burden, and death or injury of livestock due to predator–prey interactions.
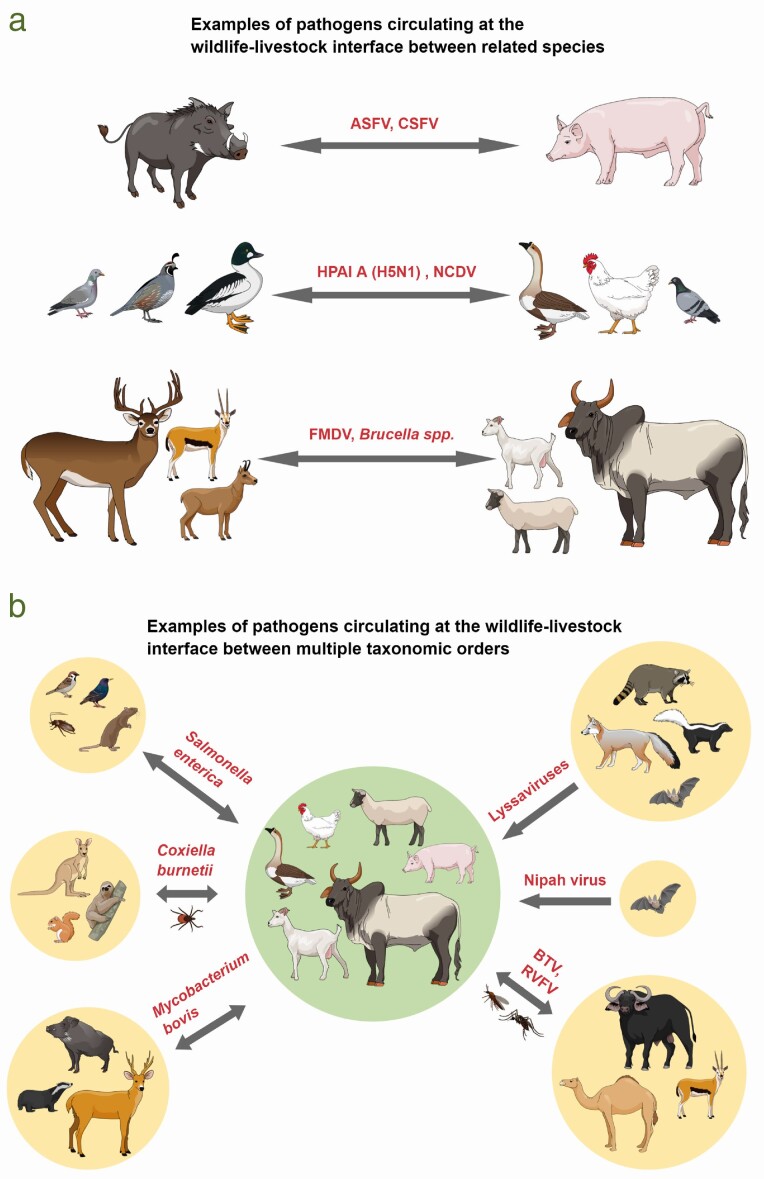
With respect to infectious diseases, there are many examples of wildlife species acting as reservoirs of pathogens that have a serious impact on livestock production (Figure 5). In most of those cases, the disease does not cause apparent symptoms in the wildlife host but can have devastating impacts on livestock populations. Examples include wild waterfowl acting as reservoirs of highly pathogenic avian influenza viruses (Globig et al., 2009), wild birds as reservoirs of Newcastle Disease viruses for domestic chickens (Ayala et al., 2020), warthogs being able to act as reservoirs of ASFV, and the African buffalo that is well known as the major wild reservoir of FMDV (Jori and Etter, 2016; Jori et al., 2021a). Direct or indirect contacts of such reservoirs with susceptible livestock populations can initiate sporadic outbreaks that can develop into epidemics as disease spreads between farms. Such regional spread can be due to ongoing transmission from wildlife or more commonly, anthropogenic factors such as movement of infected livestock or contaminated fomites (Dixon et al., 2020). Impacts are then twofold; besides the direct effects of animal sickness causing loss of animals and decreased productivity, indirect losses also occur due to the instigation of treatment for individuals or control measures. In high-income countries, strict and dramatic control measures can be implemented to control highly infectious diseases such as FMD, and although financial compensation might be provided by governments (e.g., a joint agreement between livestock industries and governments in Australia outlines the shared costs of the control of diseases such as FMD and ASF in the event of an incursion (Anonymous, 2020), the genetic value and relationship to individual animals are non-compensable losses. Finally, infectious disease outbreaks in a country do not only impact affected farms but can also have substantial impacts on the international trade of animals and animal products from that country, not just directly due to lost income during export bans but also subsequently due to loss of trust in the integrity of the market (Dixon et al., 2020). Indeed, outbreaks of some transboundary animal diseases such as FMD or ASF can cause huge losses due to export bans, eradication measures, proof of freedom surveys, and ongoing surveillance (Domenech et al., 2006).
Figure 5.
Diagram showing different infectious pathogens transmitted between wild and domestic species in different contexts: (a) examples of pathogens transmitted between closely related species (ruminants, birds, and suids) and (b) examples of opportunistic pathogens shared by a diversity of species (Design: I.M.).
Another, more unusual, example of transmission from a wildlife reservoir to livestock is found in tropical America, where the common vampire bat (Desmodus rotundus) not only causes bite damage when it feeds on the blood of livestock (causing hide damage), poultry, wildlife, and humans, but can also transmit rabies virus (Mayen, 2003a, 2003b). Losses of tens of thousands of cattle due to paralytic rabies are estimated to occur annually across affected countries. Control strategies include vaccination of susceptible livestock, such as cattle, and bat population control using poisons to reduce the extent of the bat–cattle interface (Johnson et al., 2014).
As well as the socioeconomics of disease due to loss of productivity and increased costs, some diseases that have wildlife reservoirs are also zoonoses (Magouras et al., 2020). Examples of classical zoonoses include Japanese encephalitis, brucellosis, tuberculosis, and rabies, and disease is seen in both livestock and humans. In contrast, some zoonotic pathogens do not cause clinical signs in animals (e.g., salmonellosis and campylobacteriosis) and are, therefore, more difficult to monitor and control (Chlebicz and Śliżewska, 2018). As pathogens circulate in livestock species, this also provides opportunity for pathogen evolution and amplification, thus increasing the probability of emerging zoonotic diseases. An example is Nipah virus emergence in pigs in Malaysia in 1998 following repeated spillover events from bats (Daszak et al., 2013). The characteristics of interfaces of livestock with wildlife are critical factors in the emergence of previously unknown diseases, and, in this case, the large population of domestic pigs provided opportunity for circulation and amplification of the virus and its evolution to infect humans.
Positive impacts
An important positive impact of the conservation of biodiversity on livestock production is the ecological process known as the “dilution effect” (Khalil et al., 2016). This suggests that the net effects of biodiversity (including host and nonhost species) reduce the risk of certain pathogens to affect target species of interest such as livestock or people. Therefore, a habitat with higher biodiversity could act as a protective buffer for the emergence of certain diseases affecting livestock and domestic animals (Civitello et al., 2015). While the generalization of this concept of disease ecology is often the source of controversy and speculation, it is more widely accepted for some examples of disease emergence such as Lyme disease.
Other examples of positive impacts of livestock–wildlife cohabitation on animal production systems are described in the context of extensive livestock production systems in which there is acceptance of biodiversity and integration of farming practices with the natural environment (Pywell et al., 2012). For instance, this includes the benefit of insect species to livestock. The availability of dung beetles on grazing pastures degrades ruminant feces and thus increases the ruminant production outputs due to lowered parasite burden, reduces the impact of flies in insect-borne diseases such as infectious keratoconjunctivitis, and increases pasture productivity (Nichols et al., 2008; Lopez-Collado et al., 2017).
Another example of potential benefits of wildlife conservation on animal farms is the development of wildlife tourism, which can also have indirect positive impacts on livestock via effects on rural livelihoods and agro-ecological sustainability (Melita and Mendlinger, 2013). In extensive livestock systems such as the Brazilian Pantanal (Figure 6), the development of extensive livestock ranching has to cope with the presence of large cats such as jaguar and puma that live in the same habitat and generate livestock predation losses. In this context, the tourist attraction generated by the presence of those predators can generate considerable income for cattle ranches and positively impact livestock production systems by encouraging the maintenance of habitat for the wildlife, thus, biodiversity. In this case, wildlife tourism represents an additional source of income that boosts the tolerance of large cats in private ranches. This partnership between ecotourism and cattle ranchers, in which cattle losses induced by predation can be compensated by tourism revenues, provides an interesting model of coexistence between traditional livestock husbandry and biodiversity conservation (Tortato et al., 2017). Due to the requirement of integration with wildlife habitat, this coexistence of wildlife with livestock production systems is only possible in extensive livestock production systems with relatively low outputs. Such systems are usually associated with traditional farming methods and not intensive agriculture (in which livestock are housed in groups either wholly or partially during their production cycle, and inputs such as labor and capital are high relative to the land area being farmed), which is the major driver of land-use change. Although impacts of wildlife tourism are complex and not always positive for the environment, this kind of approach is worth exploring in other areas affected by problems with livestock–carnivore coexistence. In many countries, carnivore populations such as bears, wolves, or native wild dogs cause significant losses to livestock in their distribution areas (Katel et al., 2014; Gastineau et al., 2019).
Figure 6.
Livestock reared extensively in rich wildlife habitats such as the Brazilian Pantanal. Sympatric wildlife species have the potential to attract tourists to these agricultural areas (Photo: F.J.).
The Impacts of Livestock on Wildlife
Livestock production generates major impacts on wildlife populations through land-use change and encroachment on wildlife habitats worldwide (Gordon, 2018). This increased competition with livestock results in reduced habitat, food, and water for wildlife populations, and an impact on wildlife numbers and diversity. As described above, in the context of disease, this decline in biodiversity can result in loss of the “dilution effect” on disease prevalence; if susceptible species become dominant in an ecosystem (i.e., due to biodiversity loss), disease prevalence can increase. For example, excessive grazing pressure due to high stocking densities or grazing over long periods can reduce vegetation abundance and diversity, which reduce both forage availability and suitable habitat space for wildlife species (Boone et al., 2005).
Infectious diseases of livestock can also spill over to wildlife populations and cause high mortality and losses. For example, ASFV is able to spread among domestic pigs and some wild suid species. The introduction of genotype II ASFV into the Caucasus in 2007 has resulted in relentless ASF propagation via slow geographical expansion through European wild boar populations. To date, at least 12 European Union countries have reported cases of ASF in wild boar, with more than 30,000 cases reported only in the last 5 yr (Viltrop et al., 2021). In addition, ASFV spread across the Asian continent has started to affect some populations of endemic suids such as the Sumatran bearded pig (Sus barbatus) and is seriously threatening several highly endangered pig species in Asia (Luskin et al., 2020).
Another example of a disease that spread from livestock to wildlife is bovine tuberculosis which was first detected in Kruger National Park in a single African buffalo (Syncerus caffer) in 1990. After molecular analysis, it was inferred that Mycobacterium bovis was probably introduced in 1963 through an interaction with infected cattle. Since then, M. bovis has spread among the abundant buffalo populations and at least 23 mammalian species, including large predators (Hlokwe et al., 2016).
Such spillover episodes are particularly serious when they affect vulnerable or endangered species already threatened by extinction. Examples include the recent cases of bovine tuberculosis found in endangered African wild dog (Lycaon pictus) populations (Higgitt et al., 2019) or the outbreaks of rabies reported among Ethiopian wolf (Canis simensis) populations (Johnson et al., 2014).
Measures and Strategies to Reduce Wildlife–Livestock Interactions or Mitigate Their Impact
Given the significant risk of disease transmission posed by wildlife–livestock interactions and their associated economic, social, and environmental impacts, as well as their contribution to the emergence of zoonotic pathogens of public health significance, regulations, policies, and guidelines have been developed worldwide to minimize these interactions and mitigate their impacts. For effective mitigation of this risk, a long-term, cross-sectoral, collaborative, and transdisciplinary approach across government and industry stakeholders and the community is needed. However, major differences in interests and priorities of different organizations and stakeholders have in some instances hampered or limited the success of the implementation of available mitigation strategies. The tools available for minimizing livestock–wildlife interactions relevant for infectious disease transmission are mainly based on three types of strategies: those aimed at the reduction of the population of wildlife species in livestock production areas, those focusing on monitoring and controlling the occurrence of disease in wildlife populations, and those focusing on implementation of on-farm management practices that reduce the likelihood of direct or indirect contacts. Usually, integrated control strategies based on combinations of some of these tools are used.
Reduction of wildlife in livestock areas
If we focus on the first type of strategy, wildlife culling has been a commonly used control method for both reducing the risk of disease transmission and predation of domestic animals by large predators worldwide. However, in developed economies, the reduction in biodiversity and increasing public concerns regarding the involved ethical issues have progressively challenged the use of this practice, and currently, the resettlement or translocation of problematic animals is a preferred option. Before wildlife culling is undertaken, pathogen transmission pattern, host contact pattern, regulatory processes, seasonality, spatial structure, and environmental sources of infection should be thoroughly analyzed to understand the complexity of host–pathogen associations at ecological level and the benefits and costs of such a disease management approach (Miguel et al., 2020). Another potential method to reduce wildlife population in specific areas is the translocation of problematic individuals to other sites; however, this method is mostly used as a wildlife management tool for reducing the impact of human activities on large predators with high conservation value.
Pest control strategies at a farm level also aim at reducing or eliminating wildlife species considered a nuisance for farm operations, due to their potential risk of disease transmission. Having an appropriate pest management plan in place, including infestation monitoring, recording, and control, is a biosecurity requirement for commercial livestock operations (Figure 7). However, previous studies have identified the need to improve pest control. As an example, some authors identified a risk of transmission of production-limiting pathogens from rats to domestic pigs in Australia (Pearson et al., 2016).
Figure 7.
Fences are used worldwide to separate wildlife and livestock populations. They can be regularly challenged by wild animals and need regular maintenance to remain effective. (a) An electric fence being challenged by an elephant in South Africa (Photo: F.J.). (b) Livestock being separated by a fence at the edge of the Kruger National Park, South Africa (Photo: F.J.). (c) In Southern Africa, fences at the edge of protected areas can be several hundreds of kilometers long (Photo: F.J.). (d) A fence to protect a pig farm from terrestrial wildlife incursions in Australia (Photo: V.J.B.).
Monitoring and control of diseases in wildlife
To monitor diseases at the wildlife–livestock interface, surveillance efforts should ideally target both wildlife and livestock populations. An example of an active surveillance program is the Australian National Avian Influenza wild bird surveillance program, which contributes to understanding of the ecology and epidemiology of avian influenza viruses and supports industry, human, and wildlife risk management strategies (Hansbro et al., 2010). However, in practice, sampling wildlife requires specific expertise and considerable resources. A common alternative to this constraint is to monitor pathogens of interest in domestic species exposed to wildlife contacts. Exposed individuals can act as sentinels for certain pathogens circulating in wild species. For example, during the large epidemic of avian influenza H5N1 in wildfowl in 2006 in Europe, sentinel ducks were sampled on a regular basis as an early warning surveillance system (Globig et al., 2009). Other alternatives to the capture and restraint of wild species for surveillance are the possibility to sample game gathered by hunters or the use of noninvasive methods to collect biological material from wildlife (Bataille et al., 2019). Given the previously described issues associated with wildlife population control, wildlife vaccination has been used occasionally to reduce the risk of disease transmission to domestic livestock, with the vaccine being administered orally using baits. An example is the control of rabies in foxes and racoons in Europe, the United States, and Canada (Freuling et al., 2013). Another example is the control of M. bovis in possums in New Zealand (Nugent et al., 2016); however, the effectiveness of this strategy has only been demonstrated experimentally. Despite being an effective control strategy, issues such as safety for nontarget species and stability and resistance to environmental conditions need to be considered (Gortazar et al., 2014). An alternative method for controlling vector-borne diseases in wildlife is controlling the arthropod vectors, which could be done using insecticides and acaricides (Wilson et al., 2020)
Reduction of interactions between wildlife and livestock
The most common strategies to reduce the risk of disease transmission at the wildlife–livestock interface are the implementation of on-farm practices that minimize the risk of direct and indirect contacts. Most of these practices are commonly known as biosecurity. Physical barriers, such as appropriate fences, and deterrent methods, such as the use of guard dogs, the reduction of suitable and attractive habitats around livestock premises, and the avoidance of sharing of resources, will minimize direct and indirect contact and the probability of disease spillover from wildlife to livestock.
External property perimeter fences are used to reduce the ease of access to the property, whereas long perimeter fences are used to restrict access and minimize the spread of a disease into new territories. The latter are often used for the separation of populations based on their health status, allowing compartmentalization or zoning, often used to safeguard international trade from specific production areas. However, long perimeter fences are often criticized on the basis of their environmental impact (Mysterud and Rolandsen, 2019). Despite fences being an effective method for limiting wildlife–livestock contact, their design should be adapted to circulating wildlife species (Figure 7), and it is crucial to establish a regular monitoring and maintenance system to guarantee their efficacy (Jori et al., 2011; Gortazar et al., 2014).
In poultry enterprises, wild bird proofing of sheds, preventing wild bird access to animal feed, and protecting water sources will reduce the likelihood of transmission of diseases and pathogens. However, these measures are more difficult to implement in free-range poultry enterprises, with these enterprises facing a higher risk of disease transmission than indoor enterprises (Scott et al., 2018).
In addition to these biosecurity measures, a very important and common health management practice to protect livestock from disease transmission from wildlife and minimize spread within domestic populations is the use of vaccination in livestock. A clear example is the vaccination of poultry against H5N1 avian influenza in countries with endemic H5N1 viruses, such as China. Vaccination of poultry is the most effective method for reducing infection in poultry, the viral environmental contamination, and subsequently the risk of re-introduction of the virus from wild birds to domestic populations. Vaccination against classical swine fever virus in domestic pigs, in conjunction with wild boar vaccination, has been used for controlling the disease in endemic European regions where domestic and wild pig populations were infected.
While some biosecurity measures can be implemented in most farms, farm-specific biosecurity plans that consider region and farm characteristics and wildlife species posing a risk are needed. Many of the above-described measures are very often used in combination. For example, in several Southern African countries that are beef exporters (South Africa, Namibia, and Botswana), contacts with infected African buffalo can jeopardize beef exports due to potential FMDV transmission. Therefore, preventive measures consist of a combination of zoning or compartmentalization of the territory according to OIE guidelines, the erection of fences to separate cattle from potentially infected buffalo (Figure 7), and the regular vaccination of livestock against FMDV serotypes present in buffalo populations (Jori and Etter 2016). In addition, the possibility of exporting manufactured beef products processed in such a way that freedom of FMD virus is guaranteed (a process known as Commodity Based Trade) could potentially improve market access for beef from communal areas where cattle are exposed to buffalo contacts (Naziri et al., 2015). Similarly, in the European Union, the control of ASF in wild boar populations is addressed with a combination of fencing, zoning or regionalization, wild boar culling, and passive surveillance (Jori et al., 2021b).
Conclusions
Global drivers of a changing world are making wildlife–livestock interactions more frequent, difficult to avoid, and increasingly challenging for animal production systems. It is clear that at those interfaces, wildlife and livestock populations tend to have major negative impacts on each other. In the case of livestock, while the major impacts are loss of productivity and increased socioeconomic costs, these interactions also increase the risk of emerging diseases, including zoonoses. These have the capacity to generate global health impacts, such as the COVID-19 pandemic we are currently experiencing. Biosecurity measures implemented in the livestock sector can reduce some of these interactions and their impact on livestock production. However, this is a challenge that cannot be addressed by biosecurity measures alone. In part, this is because their implementation is not always cost-effective and applicable in all contexts. The costs and benefits of implementing specific biosecurity measures and stakeholder acceptance of these measures must be considered to ensure their implementation, maintenance, and success. However, in the long term, addressing the global drivers increasing wildlife–livestock interactions and their underlying determinants (e.g., human migration due to conflict and climate change due to fossil fuel usage) is becoming critical. Particularly in the case of wildlife, the negative impacts of those interactions, such as biodiversity loss and wildlife population decline, due to habitat degradation and resource reduction, land-use change, or introduction of pathogens or pollutants (pesticides and toxic residues), are often irreversible. With the unprecedented rates of human population growth and needs for additional livestock production, these impacts are not expected to decrease. Therefore, urgent action is needed to reverse or at least slow down this negative trend and subsequent global health risks.
There is a need to search for alternative methods of protein production that are more compatible with wildlife–livestock interactions and more respectful and compatible with conservation of biodiversity. The possibility of compensating for the negative impacts on wildlife by added benefits from tourism or the production and promotion of better-quality products that are ecologically friendly are some initiatives suggesting that finding a balance between animal production and nature conservation is possible. In addition, it is important to find approaches and methods to raise the awareness of consumers, particularly in developed economies, of the impacts of livestock production on the environment and the need to reduce the impacts of protein production to benefit the environment, human society, and global health.
Lastly, any strategy or approach taken to minimize the risks and impacts at the wildlife–livestock interface must be dynamic, adaptable to constantly changing global systems, and consider the specificities of the local context. It is a fundamental requirement for animal health and production professionals to promote the advantages of a cross-sectoral, transdisciplinary perspective that considers health promotion as a globally integrated activity that encompasses not only links between animal, human, and environmental health in a broad sense but also specifically the determinants of wildlife–livestock interactions and their mitigation. As the global human population rapidly increases along with the need for protein production, this will be of paramount importance when considering the design and implementation of education programs for future generations of animal science professionals.
About the Authors

Ferran Jori is a Spanish veterinary epidemiologist working on wildlife use and disease risks for more than 25 yr. After graduating in Veterinary Medicine in Barcelona in 1990, he started his career working on international cooperation and rural development in Central America and soon moved into the field of tropical wildlife management by doing his MSc and subsequent PhD on wildlife farming and production in Central Africa. Employed by CIRAD (French International Research Centre for Tropical Agricultural Development) since 1998, he has had worked on wild animal production and the study of the epidemiology and ecology of infectious diseases in many developing countries in Africa and Latin America. During the last 15 yr, he has concentrated on the study of transboundary infectious diseases circulating at the wildlife–livestock interface such as foot and mouth disease and African swine fever, particularly in Southern Africa. Since 2017, he supervises postgraduate students and coordinates research activities in tropical countries and Europe from CIRAD headquarters in Southern France.

Marta Hernandez-Jover is an associate professor in Veterinary Epidemiology and Public Health at the School of Animal and Veterinary Science, and a member of the Graham Centre for Agricultural Innovation at Charles Sturt University (CSU) in Wagga Wagga. She completed her Bachelor of Veterinary Sciences and her PhD in Barcelona and moved to Australia in 2005. She has over 15 yr of experience working as a veterinary epidemiologist in Australia and is a member of the Epidemiology Chapter of the Australian and New Zealand Chapter of Veterinary Scientists. Her main interests and current research focus on biosecurity, disease surveillance, and risk analysis methods applied to infectious animal diseases and public health. She has also contributed to research on biosecurity and disease surveillance among livestock producers in Australia, by investigating the drivers for engagement with biosecurity and health management practices.

Ioannis Magouras is an assistant professor at the Department of Infectious Diseases and Public Health, and a member of the Centre for Applied One Health Research and Policy Advice (OHRP) at City University of Hong Kong. He holds a veterinary degree from the University of Veterinary Medicine in Budapest and completed a PhD in veterinary virology at the University of Bern in Switzerland. He has also worked as a clinical microbiologist at the Institute for Infectious Diseases in Bern, where he gained extensive knowledge of the laboratory diagnosis and the epidemiology of infectious diseases in humans. In his last position at the Veterinary Public Health Institute (University of Bern, Switzerland), he led projects on the epidemiology of Q fever in animals and humans, on-farm biosecurity measures, and antimicrobial usage and resistance in livestock. His research at City University in Hong Kong currently focuses on the epidemiology of antimicrobial resistance and zoonoses at the wildlife–livestock–human interface, through the application of various methodological approaches, such as spatial and molecular epidemiology and social sciences.

Salome Dürr is an assistant professor at the Veterinary Public Health Institute at the University of Bern, Switzerland. She is a veterinarian and epidemiologist (dipl. ECVPH), with over 20 yr of research experience in this field in universities and research institutes in Switzerland, Australia, the United States, and Chad. Her main research area lies in the prevention, surveillance and control of zoonotic diseases, integrating human, domestic animals, wildlife, and ecosystem sectors following the One Health approach. Development and application of epidemiological models to combat infectious diseases are one of her areas of expertise, demanding close interaction and communication with stakeholders such as governmental institutions and the private livestock sector. She has a large international professional network, including collaborations in sub-Saharan Africa and Southeast Asia. She contributes to the scientific community by supervising MSc and PhD students and residents, publishing and reviewing scientific articles, and serving as a journal editor.

Victoria J. Brookes is a veterinary epidemiologist and a senior lecturer in Population Health at the School of Animal and Veterinary Sciences, and a member of the Graham Centre for Agricultural Innovation at Charles Sturt University, Australia. Her main research interests are emerging and neglected infectious diseases, and the application of quantitative methods such as disease modeling and risk analysis. Prior to working in academia, Victoria spent several years in mainly large animal practice in the United Kingdom and Australia. She has on-the-ground emergency animal disease response experience from the 2001 outbreak of FMD in the United Kingdom and the 2007 outbreak of equine influenza in Australia, and field-research experience in investigation and preparedness for emerging and transboundary diseases in Southeast Asia and Papua New Guinea, and remote communities in northern Australia.
Literature Cited
- Abel, G.J., Barakat B., Kc S., and Lutz W.. 2016. Meeting the sustainable development goals leads to lower world population growth. Proc. Natl. Acad. Sci. U. S. A. 113:14294–14299. doi: 10.1073/pnas.1611386113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åkerfeldt, M.P., Gunnarsson S., Bernes G., and Blanco-Penedo I.. 2020. Health and welfare in organic livestock production systems—a systematic mapping of current knowledge. Org. Agric. ( 11):105–132. doi: 10.1007/s13165-020-00334-y [DOI] [Google Scholar]
- Anonymous. 2020. Emergency Animal Disease Response Agreement (EADRA). Available from www.animalhealthaustralia.com.au/download/1048/ [accessed May 25, 2021].
- Ayala, A.J., Yabsley M.J., and Hernandez S.M.. 2020. A review of pathogen transmission at the backyard chicken–wild bird interface. Front. Vet. Sci. 7(662). doi: 10.3389/fvets.2020.539925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi, A., and Gottardo D.. 2017. Livestock production to feed the planet. Animal protein: a forecast of global demand over the next years. Relat. Beyond Anthr.5(1):67–71. doi: 10.7358/rela-2017-001-bald [DOI] [Google Scholar]
- Banda, L.J., and Tanganyika J.. 2021. Livestock provide more than food in smallholder production systems of developing countries. Anim. Front. 11:7–14. doi: 10.1093/af/vfab001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataille, A., Kwiatek O., Belfkhi S., Mounier L., Parida S., Mahapatra M., Caron A., Chubwa C.C., Keyyu J., Kock R., . et al. 2019. Optimization and evaluation of a non-invasive tool for peste des petits ruminants surveillance and control. Sci. Rep. 9:4742. doi: 10.1038/s41598-019-41232-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone, R., BurnSilver S., Thornton P., Worden J., Galvin K., and Are A.. 2005. Quantifying declines in livestock due to land subdivision. Rangeland Ecol. Manag.58(5):523–532. doi: 10.2111/1551-5028(2005)58[523:QDILDT]2.0.CO;2 [DOI] [Google Scholar]
- Brookes, V.J., Barrett T.E., Ward M.P., Roby J.A., Hernandez-Jover M., Cross E.M., Donnelly C.M., Barnes T.S., Wilson C.S., and Khalfan S.. 2021. A scoping review of African swine fever virus spread between domestic and free-living pigs. Transbound Emerg. Dis. 00:1–14. doi: 10.1111/tbed.13993 [DOI] [PubMed] [Google Scholar]
- Chevalier, V., Pépin M., Plée L., and Lancelot R.. 2010. Rift valley fever—a threat for Europe? Euro Surveill.15(10):19506. doi: 10.2807/ese.15.10.19506-en [DOI] [PubMed] [Google Scholar]
- Chlebicz, A., and Śliżewska K.. 2018. Campylobacteriosis, salmonellosis, yersiniosis, and listeriosis as zoonotic foodborne diseases: a review. Int. J. Environ. Res. Public Health 15(5):863. doi: 10.3390/ijerph15050863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitello, D.J., Cohen J., Fatima H., Halstead N.T., Liriano J., McMahon T.A., Ortega C.N., Sauer E.L., Sehgal T., Young S., . et al. 2015. Biodiversity inhibits parasites: broad evidence for the dilution effect. Proc. Natl. Acad. Sci. U. S. A. 112:8667–8671. doi: 10.1073/pnas.1506279112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daszak, P., Zambrana-Torrelio C., Bogich T.L., Fernandez M., Epstein J.H., Murray K.A., and Hamilton H.. 2013. Interdisciplinary approaches to understanding disease emergence: the past, present, and future drivers of Nipah virus emergence. Proc. Natl. Acad. Sci. U. S. A. 110 (Suppl 1):3681–3688. doi: 10.1073/pnas.1201243109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nardo, A., Knowles N.J., and Paton D.J.. 2011. Combining livestock trade patterns with phylogenetics to help understand the spread of foot and mouth disease in sub-Saharan Africa, the Middle East and Southeast Asia. Rev. Sci. Tech. 30:63–85. doi: 10.20506/rst.30.1.2022 [DOI] [PubMed] [Google Scholar]
- Dixon, L.K., Stahl K., Jori F., Vial L., and Pfeiffer D.U.. 2020. African swine fever epidemiology and control. Annu. Rev. Anim. Biosci. 8:221–246. doi: 10.1146/annurev-animal-021419-083741 [DOI] [PubMed] [Google Scholar]
- Domenech, J., Lubroth J., Eddi C., Martin V., and Roger F.. 2006. Regional and international approaches on prevention and control of animal transboundary and emerging diseases. Ann. N. Y. Acad. Sci. 1081:90–107. doi: 10.1196/annals.1373.010 [DOI] [PubMed] [Google Scholar]
- Du Toit, J.T., Cross P.C., and Valeix M.. 2017. Managing the livestock–wildlife interface on rangelands. In: Briske, D.D., editor, Rangeland systems: processes, management and challenges. Springer International Publishing, Switzerland; p. 395–425. [Google Scholar]
- Freuling, C.M., Hampson K., Selhorst T., Schröder R., Meslin F.X., Mettenleiter T.C., and Müller T.. 2013. The elimination of fox rabies from Europe: determinants of success and lessons for the future. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 368:20120142. doi: 10.1098/rstb.2012.0142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastineau, A., Robert A., Sarrazin F., Mihoub J.-B., and Quenette P.-Y.. 2019. Spatiotemporal depredation hotspots of brown bears, Ursus arctos, on livestock in the Pyrenees, France. Biol. Cons. 238:108210. doi: 10.1016/j.biocon.2019.108210 [DOI] [Google Scholar]
- Globig, A., Baumer A., Revilla-Fernández S., Beer M., Wodak E., Fink M., Greber N., Harder T. C., Wilking H., Brunhart I., . et al. 2009. Ducks as sentinels for avian influenza in wild birds. Emerg. Infect. Dis. 15:1633–1636. doi: 10.3201/eid1510.090439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, I.J. 2018. Review: livestock production increasingly influences wildlife across the globe. Animal. 12(s2):s372–s82. doi: 10.1017/S1751731118001349 [DOI] [PubMed] [Google Scholar]
- Gortazar, C., Diez-Delgado I., Barasona J.A., Vicente J., De La Fuente J., and Boadella M.. 2014. The wild side of disease control at the wildlife-livestock-human interface: a review. Front. Vet. Sci. 1:27. doi: 10.3389/fvets.2014.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, K., Baron M.D., Matsuo K., and Visser D.. 2017. Rinderpest eradication: challenges for remaining disease free and implications for future eradication efforts. Rev. Sci. Tech. 36:579–588. doi: 10.20506/rst.36.2.2676 [DOI] [PubMed] [Google Scholar]
- Hansbro, P.M., Warner S., Tracey J.P., Arzey K.E., Selleck P., O′Riley K., Beckett E.L., Bunn C., Kirkland P.D., Vijaykrishna D., . et al. 2010. Surveillance and analysis of avian influenza viruses, Australia. Emerg. Infect. Dis. 16:1896–1904. doi: 10.3201/eid1612.100776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgitt, R.L., Van Schalkwyk O.L., de Klerk-Lorist L.M., Buss P.E., Caldwell P., Rossouw L., Manamela T., Hausler G.A., Hewlett J., Mitchell E.P., and van Helden P.D.. 2019. Mycobacterium bovis Infection in African Wild Dogs, Kruger National Park, South Africa. Emerg. Infect. Dis. 25(7):1425–1427. doi: 10.3201/eid2507.181653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlokwe, T.M., De Klerk-Lorist L.M., and Michel A.L.. 2016. Wildlife on the move: a hidden tuberculosis threat to conservation areas and game farms through introduction of untested animals. J. Wildl. Dis. 52:837–843. doi: 10.7589/2015-10-281 [DOI] [PubMed] [Google Scholar]
- Jacquot, M., Nomikou K., Palmarini M., Mertens P., and Biek R.. 2017. Bluetongue virus spread in Europe is a consequence of climatic, landscape and vertebrate host factors as revealed by phylogeographic inference. Proc. R. Soc. B: Biol. Sci. 284(1864):20170919. doi: 10.1098/rspb.2017.0919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, N., Aréchiga-Ceballos N., and Aguilar-Setien A.. 2014. Vampire bat rabies: ecology, epidemiology and control. Viruses 6:1911–1928. doi: 10.3390/v6051911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, B.A., Grace D., Kock R., Alonso S., Rushton J., Said M.Y., McKeever D., Mutua F., Young J., McDermott J., . et al. 2013. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl. Acad. Sci. U. S. A. 110:8399–8404. doi: 10.1073/pnas.1208059110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jori, F., Brahmbhatt D., Fosgate G.T., Thompson P.N., Budke C., Ward M.P., Ferguson K., and Gummow B.. 2011. A questionnaire-based evaluation of the veterinary cordon fence separating wildlife and livestock along the boundary of the Kruger National Park, South Africa. Prev. Vet. Med. 100:210–220. doi: 10.1016/j.prevetmed.2011.03.015 [DOI] [PubMed] [Google Scholar]
- Jori, F., De Nys H., Faye B., and Molia S.. 2021a. Characteristics and perspectives of disease at the wildlife-livestock interface in Africa. In: Vicente, V. K. C. J., and Gortázar C., editors. Diseases at the wildlife—livestock interface. Wildlife Research Monographs, Vol. 3. Springer; p. 181–2015. [Google Scholar]
- Jori, F., and Etter E.. 2016. Transmission of foot and mouth disease at the wildlife/livestock interface of the Kruger National Park, South Africa: can the risk be mitigated? Prev. Vet. Med. 126:19–29. doi: 10.1016/j.prevetmed.2016.01.016 [DOI] [PubMed] [Google Scholar]
- Jori, F., Massei G., Licoppe A., Ruiz-Fons F., Linden A., Václavek P., Chenais E., and Rosell C.I.E.P.. 2021b. Management of wild boar populations in the European Union before and during the ASF crisis. In: Iacolina, L., Penrith M., Bellini S., Chenais E., Jori F., Montoya M., Ståhl K., and Gavier-Widén D., editors. Understanding and combatting African swine fever. A European perspective. Wageningen: ( The Netherlands: ): Wageningen Academic Publishers; p. 197–228. [Google Scholar]
- Katel, O., Pradhan S., and Schmidt-Vogt D.. 2014. A survey of livestock losses caused by Asiatic wild dogs, leopards and tigers, and of the impact of predation on the livelihood of farmers in Bhutan. CSIRO Wild. Res.41(4):300–310. doi: 10.1071/WR14013 [DOI] [Google Scholar]
- Khalil, H., Ecke F., Evander M., Magnusson M., and Hörnfeldt B.. 2016. Declining ecosystem health and the dilution effect. Sci. Rep. 6:31314. doi: 10.1038/srep31314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koneswaran, G., and Nierenberg D.. 2008. Global farm animal production and global warming: impacting and mitigating climate change. Environ. Health Perspect. 116:578–582. doi: 10.1289/ehp.11034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Collado, J., Cruz-Rosales M., Vilaboa-Arroniz J., Martínez-Morales I., and Gonzalez-Hernandez H.. 2017. Contribution of dung beetles to cattle productivity in the tropics: a stochastic-dynamic modeling approach. Agric. Sys. 155:78–87. doi: 10.1016/j.agsy.2017.05.001 [DOI] [Google Scholar]
- Luskin, M.S., Meijaard E., Surya S., Sheherazade, C. Walzer, and Linkie M.. 2020. African swine fever threatens Southeast Asia’s 11 endemic wild pig species. Cons. Lett. 14(3):e12784. doi: 10.1111/conl.12784 [DOI] [Google Scholar]
- Magouras, I., Brookes V.J., Jori F., Martin A., Pfeiffer D.U., and Dürr S.. 2020. Emerging zoonotic diseases: should we rethink the animal–human interface? Front. Vet. Sci. 7(748): 1– 6. doi: 10.3389/fvets.2020.582743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayen, F. 2003a. Haematophagous bats in Brazil, their role in rabies transmission, impact on public health, livestock industry and alternatives to an indiscriminate reduction of bat population. J. Vet. Med. B. Infect. Dis. Vet. Public Health 50:469–472. doi: 10.1046/j.1439-0450.2003.00713.x [DOI] [PubMed] [Google Scholar]
- Mayen, F. 2003b. Haematophagous bats in Brazil, their role in rabies transmission, impact on public health, livestock industry and alternatives to an indiscriminate reduction of bat population. J. Vet. Med. B. Infect. Dis. Vet. Public Health 50:469–472. doi: 10.1046/j.1439-0450.2003.00713.x [DOI] [PubMed] [Google Scholar]
- Melita, A., and Mendlinger S.. 2013. The impact of tourism revenue on the local communities’ livelihood: a case study of Ngorongoro conservation area, Tanzania. J. Serv. Sci. Manag. 06:117–126. doi: 10.4236/jssm.2013.61012 [DOI] [Google Scholar]
- Miguel, E., Grosbois V., Caron A., Pople D., Roche B., and Donnelly C.A.. 2020. A systemic approach to assess the potential and risks of wildlife culling for infectious disease control. Commun. Biol. 3(1):353. doi: 10.1038/s42003-020-1032-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens, D.M., Holmes E.C., Davis A.S., and Taubenberger J.K.. 2011. Global rinderpest eradication: lessons learned and why humans should celebrate too. J. Infect. Dis. 204:502–505. doi: 10.1093/infdis/jir327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysterud, A., and Rolandsen C. M.. 2019. Fencing for wildlife disease control. J. Appl. Ecol. 56(3):519–525. doi: 10.1111/1365-2664.13301 [DOI] [Google Scholar]
- Naziri, D., Rich K., and Bennett B.. 2015. Would a commodity-based trade approach improve market access for Africa? A case study of the potential of beef exports from communal areas of Namibia. Develop. Pol. Rev. 33(2):195–219. doi: 10.1111/dpr.12098 [DOI] [Google Scholar]
- Nichols, E., Spector S., Louzada J., Larsen T., Amezquita S., and Favila M.E.. 2008. Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol. Conserv. 141(6):1461–1474. doi: 10.1016/j.biocon.2008.04.011 [DOI] [Google Scholar]
- Nugent, G., Yockney I.J., Whitford E.J., Cross M.L., Aldwell F.E., and Buddle B.M.. 2016. Field trial of an aerially-distributed tuberculosis vaccine in a low-density wildlife population of Brushtail Possums (Trichosurus vulpecula). PLoS One 11:e0167144. doi: 10.1371/journal.pone.0167144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson, H.E., Toribio J.L.M.L., Lapidge S.J., and Hernández-Jover M.. 2016. Evaluating the risk of pathogen transmission from wild animals to domestic pigs in Australia. Prev. Vet. Med. 123:39–51. doi: 10.1016/j.prevetmed.2015.11.017 [DOI] [PubMed] [Google Scholar]
- Pywell, R.F., Heard M.S., Bradbury R.B., Hinsley S., Nowakowski M., Walker K.J., and Bullock J.M.. 2012. Wildlife-friendly farming benefits rare birds, bees and plants. Biol. Lett. 8:772–775. doi: 10.1098/rsbl.2012.0367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, A.B., Toribio J.A., Singh M., Groves P., Barnes B., Glass K., Moloney B., Black A., Hernandez-Jover M.. 2018. Low pathogenic avian influenza exposure risk assessment in australian commercial chicken farms. Front. Vet. Sci. 5. doi: 10.3389/fvets.2018.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortato, F.R., Izzo T.J., Hoogesteijn R., and Peres C.A.. 2017. The numbers of the beast: valuation of jaguar (Panthera onca) tourism and cattle depredation in the Brazilian Pantanal. Glob. Ecol. Conserv. 11:106–114. doi: 10.1016/j.gecco.2017.05.003 [DOI] [Google Scholar]
- Viltrop, A., Boinas F., Depner K., Jori F., Kolbasov D., Laddomada A., Ståhl K., and Chenais E.. 2021. African swine fever epidemiology, surveillance and control. In: Iacolina, L., Penrith M., Bellini S., Chenais E., Jori F., Montoya M., Ståhl K. and Gavier-Widén D., editors. Understanding and combatting African Swine Fever. A European perspective. Wageningen Academic Publishers, Wageningen, The Netherlands; pp. 229–261. [Google Scholar]
- Wilson, A.L., Courtenay O., Kelly-Hope L.A., Scott T.W., Takken W., Torr S.J., and Lindsay S.W.. 2020. The importance of vector control for the control and elimination of vector-borne diseases. Plos Negl. Trop. Dis. 14:e0007831. doi: 10.1371/journal.pntd.0007831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Animal Health (OIE). 2021a. African swine fever. Available from https://www.oie.int/en/disease/african-swine-fever/ [accessed May 25, 2021].
- World Animal Health (OIE). 2021b. Food and mouth disease. Available from https://www.oie.int/en/disease/foot-and-mouth-disease/ [accessed May 25, 2021].