Abstract
A relatively recent addition to the arsenal of antidiabetic drugs used for the treatment of type 2 diabetes mellitus (T2DM) has been the “incretin mimetics,” a group of drugs that work on the glucagon-like peptide-1 (GLP-1) receptor and enhance insulin secretion from the pancreatic β-cells in a glucose-dependent manner, more potently in hyperglycemic conditions, while suppressing glucagon secretion at the same time. Therefore, it was assumed that this class of drugs would have a lower risk of hypoglycemia than insulin secretagogues like sulphonylureas. However, GLP-1 receptor agonists have been proposed to cause hypoglycemia in healthy normoglycemic subjects implying that their action is not as glucose-dependent as once thought. Other studies concluded that they might not induce hypoglycemia and the risk is dependent on other individual factors. However, the FDA announced that the 12 GLP-1 receptor agonists currently available on the market had potential safety signs and evaluated the need for regulatory action. This review provides an overview of the studies that investigated the possible hypoglycemic effect of GLP-1 receptor agonists. In addition, the current review describes other adverse effects of GLP-1 receptor agonist treatment.
Keywords: GLP-1, GLP-1 RA, T2DM, hypoglycemia
Introduction
The word “incretin” was coined by the Belgian physiologist La Barre 1 to describe gut hormones that stimulated pancreatic hormone secretion and proposed it in diabetes treatment. In 1993, Nauck et al 2 demonstrated that an infusion of exogenous GLP-1 agonists normalized fasting plasma glucose in poorly-controlled type 2 diabetic patients. Thereafter it was confirmed that GLP-1 is also able to inhibit the secretion of pancreatic glucagon, providing yet another mechanism to abolish the hyperglycemia in diabetic patients. 3 Additional effects involving satiety slowed gastric emptying, and weight loss has made it a good option used by many doctors. 4 Recently, the American Diabetes Association (ADA) treatment algorithm recommended GLP-1 receptor agonists for the diabetic patients who develop atherosclerotic cardiovascular disease (ASCVD) or chronic kidney disease (CKD) after uncontrolled metformin line of treatment.5-9 However, GLP-1 receptor agonists have been proposed to be associated with many adverse effects, including cancer, systematic complications, and hypoglycemia under combined treatments. 10 In 1998, GLP-1 receptor agonists were suggested to be associated with hypoglycemic symptoms in certain individuals, which proposes the non-glucose-dependent action. 11 In addition, other factors have been assumed to be associated with the action of GLP-1 receptor agonists (Figure 1). 12
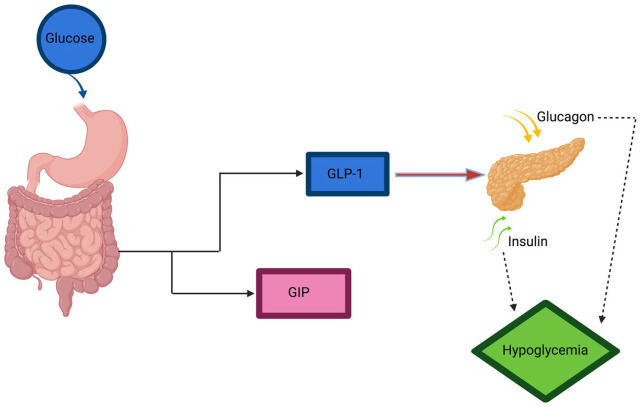
Figure 1.
Schematic of action of GLP-1 on insulin secretion.
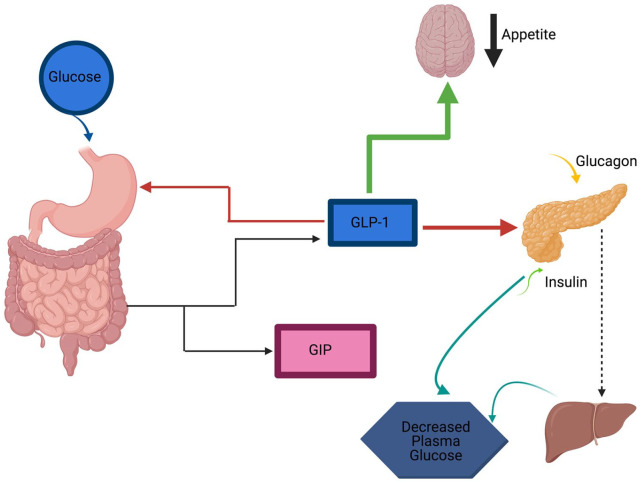
The current review aims to describe and discuss up-to-date literature data related to GLP-1 receptor agonists’ effect on the development of hypoglycemia and some other adverse effects in type 2 diabetic patients (Figure 2).
Figure 2.
Schematic of proposed mechanism of hypoglycemia in GLP-1 receptor agonists.
Definitions of hypoglycemia in diabetes
Hypoglycemia or low blood sugar is defined by the ADA as all episodes of an abnormally low plasma glucose concentration that expose the individual to potential harm, they did not assign a single threshold that defines as glycemic thresholds for symptoms differ between individuals, However, they did identify an alert value which when present should alert the patient to the possibility of the potential clinically evident harm associated with hypoglycemia. This alert value can be used to prompt patients to self-treat (though not always) with oral carbohydrate ingestion, repeat the test or avoid driving and elective exercise until the blood sugar returns to normal. 13
In this review we cite many studies each reporting hypoglycemia in different ways, below is the summary of those ways/values:
| Study | How hypoglycemia was defined in the study |
|---|---|
| Edwards et al 11 | Fall in fasting blood glucose below the normal range (4.22–6.11 mmol) |
| Lerche et al 12 | An abnormally low 3-h oral glucose tolerance test (OGTT) result |
| Nauck et al 14 | Symptoms and plasma glucose <3.1 mmol/l |
| Marre et al 15 | Plasma glucose levels <3.1 mmol/l |
| Nauck et al 16 | Symptoms of hypoglycemia were rated on a visual analog scale ranging from 0 to 10 |
| Gough et al 17 | Hypoglycemia was defined as the occurrence of episodes requiring assistance (severe), or episodes in which plasma glucose concentration (determined from self-monitored glucose) was less than 3.1 mmol/l (56 mg/dl), irrespective of symptoms. |
| Wysham et al 18 | Symptomatic hypoglycaemia with plasma glucose ⩽3.9 mmol/l (⩽70 mg/dl) |
| Vilsbøll et al 19 | Plasma glucose at or below 2.5 mmol/l |
| Buse et al 20 | Major hypoglycaemic episodes were defined as requiring third-party assistance with food only, glucagon, or intravenous glucose. Minor episodes were defined as those that the participant could self-treat and for which the plasma glucose concentration was less than 3.1 mmol/l. At glucose concentrations of 3.1 mmol/l or more, or in the absence of glucose measurements, episodes were regarded as symptoms only |
| Knop et al 21 | Symptoms of hypoglycemia, and biochemical hypoglycemia (with PG concentrations ⩽2.5 mmol/l) |
| Miholic et al 22 | Hypoglycemia (ie, serum glucose <3.8 mmol/l) after the test meal |
| Blevins et al 23 | Hypoglycemic episodes were classified as major or minor. Major hypoglycemia was defined as events that resulted in loss of consciousness, seizure, coma, or other change in mental status consistent with neuroglycopenia, in which symptoms resolved after administration of intramuscular glucagon or IV glucose. Hypoglycemia requiring assistance because of severe impairment in consciousness or behavior accompanied by a blood glucose concentration less than 54 mg/dl (3.0 mmol/l) before treatment was also classified as major. Minor hypoglycemia was defined as events with symptoms consistent with hypoglycemia accompanied by a blood glucose concentration less than 54 mg/dl (3.0 mmol/l) before treatment |
| Ratner et al 24 | Symptomatic hypoglycaemia was defined as symptoms consistent with hypoglycemia, with an accompanying blood glucose <3.3 mmol/l or prompt recovery with carbohydrate. |
Methods
The search was limited to the English language, and we conducted an internet search in Google Scholar, PubMed, and Scopus databases using the following keywords: Glucagon-like protein, glucagon-like peptide receptor agonist, type 2 diabetes mellitus, hypoglycemia.
Molecular physiology of GLP-1
Stimulation of the pancreatic β-cells to secrete insulin as an incretin hormone is the primary physiological feature of GLP-1. GLP-1 also has additional non-incretin functions, including glucagon secretion repression, gastric motility inhibition, and satiety enhancement. Through sequencing the peptides isolated from gut extraction, naturally occurring GLP-1 was identified in the late 1980s, and subsequent studies showed that GLP1(7-37) and GLP-1 (7-36) amide are the natural bioactive forms of GLP-1. GLP-1, like GIP and GLP-2, has a closely preserved alanine structure at position 9, which makes these peptides suitable substrates for dipeptidyl peptidase 4.25,26
Glucagon is processed by prohormone convertase 2 (PC2) and released from alpha cells, while GLP-1 and GLP-2 are released by PC1/3 from the intestinal L cells as bioactive hormones. As a result, after the absorption of nutrients, GLP-1 and GLP-2 are co-released in a 1:1 ratio, mainly after meals rich in carbohydrates and lipids. In vivo, owing to DPP-4 intervention, GLP-1’s biological half-life is just 1 to 2 minutes. There are several GLP-1 secretagogues, secreted by nutrients such as lipids and carbohydrates. Also, some hormones also control GLP-1 secretion, such as cholecystokinin (CCK), GIP, somatostatin, and various neuromediators.27,28
The human receptor for GLP-1 contains a broad hydrophilic extracellular and 7 hydrophobic transmembrane domains. There are 3 possible N-linked glycosylation sites for the GLP-1 receptor protein where glycosylation can modulate receptor activity. 29
The GLP-1 receptor is functionally linked via the Gs protein signaling cascade to adenyl cyclase (AC). The cAMP is formed and stimulates protein kinase A (PKA) by activation of adenylyl cyclase. Ligand receptor activation also raises intra-cytoplasmic Ca2+ concentration, which is believed to be accomplished both by Na-dependent extracellular Ca2+ uptake and by Ca2+ release from intracellular Ca2+ stores. Increased cytosolic Ca2+ in combination with activated PKA promotes insulin translocation and exocytosis containing secretory granules. 30
GLP1 receptor agonists physiology and hypoglycemia
The response to oral glucose stimulates a higher and more sustained release of insulin than intravenous (IV) glucose, and that this is attributed to the effect of the 2 incretin hormones, glucose-dependent insulinotropic polypeptide (GIP), and glucagon-like peptide-1 (GLP-1). 31 Several GLP-1 receptor agonists are currently available for the treatment of type 2 diabetes mellitus and are classified as short-acting with a duration of action of only a few hours after subcutaneous (s.c.) injections, and long-acting GLP-1 receptor agonists able to maintain plasma levels through the day (Table 1).32,33 For instance, due to the dipeptidyl peptidase-4 (DPP-4) degradation of incretins, a group of GLP-1 agonists resistant to degradation by DPP-4 has been developed such as sitagliptin, vildagliptin, saxagliptin, and linagliptin. 34 In patients with type 2 diabetes and CVD, multiple drugs from this group have shown a lower rate of cardiovascular disease outcomes such as cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke when compared to placebo. For instance, liraglutide, 35 dulaglutide, 36 and semaglutide 35 reduced the risk of cardiovascular events. However, different side effects have been reported in treated patients, including gastrointestinal effects such as nausea, diarrhea, vomiting, constipation, abdominal pain, and dyspepsia. 37 In addition, pancreatitis and progression to pancreatic cancer have been suggested to be associated with GLP-1 agonist treatments. A recent study reported that incretin therapy is not associated with an increased risk of pancreatic cancer in animals. 38 As well, metaanalyses studies supports the lack of association of GLP-1 with pancreatic cancer. 39 However, another study showed GLP-1 receptor agonists’ effect on the β-cell mass in animal models where GLP-1 acted as a β-cell growth factor leading to increased insulin synthesis and β-cell mass 40 ; those results however have not been replicated in humans. Additionally, medullary thyroid cancer was reported in rodents when exposed to GLP-1 receptor agonists but not in primates.37,38
Table 1.
Commercially available GLP-1 agonists.
| Short-acting | Long-acting |
|---|---|
| Exenatide (twice daily within 60 min prior to morning and evening meals) | Exenatide-LAR (once weekly) |
| Lixisenatide (once daily) | Liraglutide (once daily) |
| Oral semaglutide (taken by mouth once daily) | Albiglutide (once weekly) |
| Dulaglutide (once weekly) | |
| Semaglutide (once weekly) |
Table 2 summarizes different types of available GLP-1 receptor agonists used alone or combined with other drugs. Edwards et al concluded that hypoglycemia can be observed following infusion of subcutaneous GLP-1, however, the study was limited to 10 healthy subjects.
Table 2.
Summary of the literature that described the hypoglycemic effect of GLP1 receptor agonists.
| Study | GLP-receptor agonist used | Other drugs used | Study population | Percentage of participants who experienced hypoglycaemic events |
|---|---|---|---|---|
| Edwards et al 11 | Subcutaneous GLP-1 | — | 10 healthy subjects | 10% |
| Lerche et al 12 | Continuous infused native GLP-1 | — | 8 healthy men | 37.5% |
| Nauck, M., et al 14 | Once daily liraglutide in combination with metformin | Glimepiride + metformin or placebo + metformin | 1091 subjects previously treated with oral antidiabetic (OAD) therapy. | ∼3% of subjects in the placebo and liraglutide groups and 17% in the glimepiride group |
| Marre et al 15 | Liraglutide + glimepiride | Rosiglitazone + glimepiride Placebo + glimepiride |
1041 adults with type 2 diabetes mellitus | <10% of subjects for any treatment glimepiride monotherapy 2.6%, -liraglutide 0.6 mg 5.2%, rosiglitazone 4.3% |
| Nauck et al 2 | IV GLP-1 | Regular insulin | Nine healthy volunteers with normal oral glucose tolerance | N/A |
| Buse et al 20 | Liraglutide once a day or exenatide twice a day in addition to their previous oral antidiabetic therapy. | Metformin or A sulphonylurea | Adults with inadequately controlled type 2 diabetes on maximally tolerated doses of metformin, sulphonylurea, or both | Minor hypoglycemia: 26% with liraglutide vs 34% with exenatide |
| Blevins et al 23 | Exenatide once weekly and exenatide twice daily | — | 252 intent-to-treat patients with type 2 diabetes | Minor hypoglycemia occurred only among subjects using a concomitant sulphonylurea 6.75% in the exenatide once weekly with a SU (sulphonylurea) group 5.4% in the exenatide twice daily with a SU group |
| Ratner et al 24 | Subcutaneous lixisenatide | Placebo | 542 patients with Type 2 diabetes inadequately controlled on metformin | (0.9%-5.7%) per group |
| Vilsbøll et al 19 | Subcutaneous injection of GLP-1 plus IV glucose bolus | — | Eight Type 2 diabetic patients and 7 non-diabetic subjects | 71% of the healthy subjects but none of the subjects with type 2 diabetes |
| Knop et al 21 | Subcutaneous injection of GLP-1 plus IV glucose bolus | — | Eight lean type 2 diabetic patients and 8 patients with type 2 diabetes secondary to chronic pancreatitis | Neither symptoms of hypoglycemia nor biochemical hypoglycemia were observed in any patient |
| Gough et al 17 | A fixed-ratio combination of insulin degludec and liraglutide (IDegLira) | Insulin degludec alone liraglutide alone | 1663 adults with type 2 diabetes | Number of confirmed hypoglycaemic events per patient year was 1.8 for IDegLira, 0.2 for liraglutide, and 2.6 for insulin degludec |
On the other hand, the other studies reported hypoglycemic phenotype when GLP-1 agonists were given alongside other drugs such as sulphonylureas and the only other instance of hypoglycemia was observed in healthy subjects injected with GLP-1 but not type 2 diabetic patients, potentially due to their impaired insulin sensitivity and insulin resistance.
Since their discovery, GLP-1 receptor agonists have been considered a good treatment option mainly because of their favorable effect on glycemic control, which can sometimes be challenging to achieve. They have also been the right choice for people who cannot tolerate metformin or cannot use insulin because of its weight gaining side effects. 41 Glucagon-like peptide 1 (GLP-1)-based therapies (GLP-1 receptor agonists, dipeptidyl peptidase 4 inhibitors); a class of drugs with none of the adverse effects commonly seen in insulin and oral sulphonylureas seemed like the superior option.
However, the possibility of hypoglycemia was raised in different reports. For instance, in 1998 a double-blind, randomized study of 10 healthy subjects injected with GLP-1 or saline subcutaneously after a 16-hour fast was performed and argued that a GLP-1 injection could cause hypoglycemia in healthy subjects who did not have hyperglycemia. Interestingly, the results showed that GLP-1 receptor agonists acted as insulinotropic in a glucose-independent manner. 11 On the other hand, a randomized double-blind placebo-controlled study was conducted including 8 healthy men to assess the safety, in terms of hypoglycemia, of a continuously infused pharmacological dose of native GLP-1 during long-term fasting. The results showed that plasma glucose levels were similar during GLP-1 versus placebo infusions, and therefore, it was concluded the use of long-acting GLP-1 receptor agonists might not cause hypoglycemia. 12 Many similar reports followed, which all confirmed that GLP-1 receptor agonists could cause only minor and non-significant hypoglycemic events, and even those occurred at a low rate. In 2009, Nauck et al assessed the efficacy and safety of adding liraglutide to metformin compared with placebo or glimepiride to metformin in subjects previously treated with oral antidiabetes (OAD) therapy. One thousand ninety-one subjects with type 2 diabetes were randomly assigned to once-daily liraglutide, placebo, or glimepiride. All treatments were given in combination with metformin. At the end of the 26-week study, it was observed that the incidence of minor hypoglycemia with liraglutide was comparable to that with placebo but less than that with glimepiride. However, no major hypoglycemic events were reported. 14 In addition, some cases of GLP-1 overdoses were reported in accidental occasions, however, the overdose of GLP-1 receptor agonist did not show an impact on hypoglycemia development. 10
Additionally, GLP-1 therapy may be beneficial in elderly but then discourages use in this cohort. There is potential that GLP-1 therapy may benefit comorbidities and preclinical data on memory and dementia may be translatable to humans. Polypharmacy, which is frequently observed in the elderly, increases risk of drug—drug and drug—disease interactions and therefore using 1 medication (the GLP-1 receptor agonist) may help reduce the burden of polypharmacy, medication cost, and medication non-adherence. However, the elderly have a higher risk of adverse drug effects, including hypoglycemia due to polypharmacy, age-related changes affecting drug metabolism, and non-adherence to medication. 42
Is the hypoglycemia related to additional risk factors?
It can be argued that the possible hypoglycemic effect is due to concomitant use of other therapies that cause hypoglycemia (eg, sulphonylurea) and it was demonstrated in clinical trials studying a combination of a GLP-1 receptor agonist and a sulphonylurea that the risk of hypoglycemia was higher when compared to placebo. 15 The hypoglycemic effect has been attributed to the uncoupling of GLP-1 from its glucose dependence by the Sulphonylurea. 43 A clinical trial in 2002 concluded that the suppression of glucagon by GLP-1 occurs at euglycemia. 16 Additionally, the hypoglycemic effect of GLP-1 receptor agonists was attributed to the suppression of glucagon secretion. In another study, a case report of a 54-year-old Caucasian female with type 2 diabetes treated with a 3 drugs regimen, 1 of which was a GLP-1 agonist (Liraglutide) after which she experienced confusion and an episode of hypoglycemia for which she was hospitalized and found to have a pancreatic mass that was confirmed to be a benign insulinoma. Interestingly, the benign insulinomas overexpressed GLP-1 receptors and the administration of a GLP-1 agonist provoked this hypoglycemia. Therefore the case report concluded that an insulinoma should be considered when a patient develops severe hypoglycemia on a GLP-1 agonist. 44
The combination of insulin and a GLP-receptor agonist
A clinical trial published in The Lancet in 2014 compared the efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone. In the 26-week open-label, randomized trial, adults with type 2 diabetes were randomly assigned to daily injections of IDegLira, insulin degludec, or liraglutide. The number of confirmed hypoglycemic events per patient-year was 1.8 for IDegLira, 0.2 for liraglutide, and 2.6 for insulin degludec, confirming the assumption that a combination of insulin and a GLP-1 receptor agonist would have a higher risk of hypoglycemia. 17 The LixiLan-L trial evaluated 736 patients with type 2 diabetes mellitus inadequately controlled on basal insulin alone or in combination with 1 or 2 glucose-lowering drugs. Patients treated with iGlarLixi (insulin glargine/lixisenatide titratable fixed-ratio combination) showed greater reductions in HbA1c and no major differences in hypoglycemia incidence than patients treated with insulin glargine. 18
Interestingly, it was demonstrated that subcutaneous GLP-1 plus intravenous-glucose induced reactive hypoglycemia in healthy subjects, but not in Type 2 diabetic patients. The study included 8 patients with type 2 diabetes and 7 non-diabetic subjects were given a subcutaneous injection of GLP-1 and an intravenous injection of glucose; hypoglycemia was observed in 5 of the 7 healthy subjects but none the patients with diabetes therefore it was concluded that hypoglycemia shouldn’t be expected in patients with type 2 diabetes treated with GLP-1 based therapies. 19 The possible proposed reason for the absence of hypoglycemia is the impaired insulin response and increased insulin resistance in those with type 2 diabetes. Therefore, it was also essential to investigate diabetic patients with normal or close to normal insulin sensitivity. Another study supported this assumption that included 8 lean type 2 diabetic patients and 8 patients with type 2 diabetes secondary to chronic pancreatitis. All patients were given a subcutaneous injection of GLP-1 and an intravenous glucose bolus. Both groups showed impaired insulin secretory capacity and close to normal insulin sensitivity without hypoglycemic impact. Therefore, it was concluded that GLP-1 receptor agonists based therapy could not cause hypoglycemia even in insulin-sensitive type 2 diabetic patients. 21
Bariatric surgery and hypoglycemia
Dumping syndrome occurs when food moves from the stomach into the duodenum too quickly. The food passage stimulates the release of gut hormones, and water shifts from the bloodstream into the intestines leading to bloating, diarrhea, hypotension, tachycardia, and reactive hypoglycemia due to the exaggerated release of insulin. This phenomenon occurs mostly following different types of bariatric surgery, such as sleeve gastrectomy and Roux-en-Y gastric bypass. It has been proposed that GLP-1 may contribute to the exaggerated insulin surge and resultant hypoglycemia. This hypothesis is supported after reporting exaggerated GLP-1 secretion and hypoglycemia in patients who underwent gastrectomy.22,45 So patients with rapid gastric emptying may have an exaggerated response to GLP-1 receptor agonists.
Do different GLP-1 receptor agonists have different risks of hypoglycemia?
In a randomized control trial that included adults with inadequately controlled type 2 diabetes on maximally tolerated doses of metformin, sulphonylurea, or both, the participants were randomly assigned additional liraglutide once a day or exenatide twice a day and it was demonstrated that minor hypoglycemia was less frequent with liraglutide than with exenatide. 20 A meta-analysis study showed that long and short-acting agonists had different rates of hypoglycemia occurrence. For instance, randomized controlled trials comparing the coadministration of short- or long-acting GLP-1 RAs and basal insulin with basal insulin ± placebo were identified, and data about differences in HbA1c, fasting plasma glucose, body weight, and adverse events were extracted. Hypoglycemia as a side effect occurred more frequently in patients treated with GLP-1 RAs and basal insulin than in those treated with basal insulin ± placebo. Patients reporting symptomatic but not severe hypoglycemia were fewer with long- versus short-acting GLP-1 RAs added to insulin. 46 Those results were supported by another randomized control study that compared the effects of exenatide once weekly and exenatide twice daily and reported no major hypoglycemic events at all. 23
Short-acting agonists were evaluated in several studies as well. For instance, Lixisenatide was evaluated for safety, efficacy, and adverse events in a randomized, double-blind, placebo-controlled, parallel-group, 13-week study of 542 patients with Type 2 diabetes inadequately controlled on metformin (⩾1000 mg/day) treated with subcutaneous lixisenatide doses of 5, 10, 20, or 30 μg once daily or twice daily or placebo. In terms of hypoglycemia, a dose relationship between symptomatic hypoglycaemic episodes and severe hypoglycemia was not observed at all. 24
GLP-1 receptor agonists and other adverse effects
Gastrointestinal adverse effects
Nausea, vomiting, and diarrhea are the most commonly shown side effects of GLP-1 agonists. In addition, this class of drugs increases satiety which may cause temporary and mild nausea if taken on a rather full stomach. Pruritus and erythema at the injection site are also prevalent, particularly with the longer-acting drugs in this class. 47 There was less incidence of nausea for exenatide once weekly than with twice-daily exenatide or liraglutide. In comparison with exenatide twice daily, lixisenatide also showed decreased rates of nausea. Albiglutide had lower rates of nausea than liraglutide among the 2 most recently approved GLP-1RAs, whereas dulaglutide had similar rates compared to liraglutide; however, taspoglutide (long-acting glucagon-like peptide 1 receptor agonist) had by far the highest rates of nausea: 53% and 59% with 10 and 20 mg once weekly, respectively, compared with 35% among participants treated with exenatide twice daily. This was one of the key reasons why taspoglutide clinical research was stopped. 48 . In general, it was found that a less frequent weekly dose is more beneficial in terms of gastrointestinal compliance for the patents in comparison to the daily dose regimens. 49 .
It is also worth mentioning that albiglutide was discontinued in 2017 due to limited prescribing of the drug and declining sales. 50
Pancreatitis
Concerns about the potential relationship between GLP-1 receptor agonist therapy and pancreatic inflammation and pancreatitis have been introduced. In this aspect, animal research has demonstrated that these medications have a potentially adverse effect on pancreatic tissue, ranging from chronic pancreatic damage to lab animals, characterized by acinar cell pyknosis, increased cytoplasmic vacuoles, expanded cell distance, till pancreatic tissue inflammatory cell infiltration.51,52 The concern was augmented, causing the US Food and Drug Administration (FDA) to investigate the risk of pancreatitis associated with the use of incretin agonist drugs. In one of them, the usage of incretin-mimetic therapies was assessed for 30 days to 2 years, which is correlated with an elevated risk of acute pancreatitis relative to non-users. A combined review of phase III clinical tests and 2 outcome analyses revealed a marginally greater (but not significant) risk of GLP-1 receptor agonist pancreatitis relative to alternative treatment results. 53
On the other hand, most recent trials and meta-analyses have failed to demonstrate an elevated risk of pancreatitis.54,55 Also, compared to controls, a more recent meta-analysis of 55 randomized clinical trials found no elevated risk of pancreatitis with GLP-1 agonists. 56 Similarly, no critical link between incretin-based therapy and acute pancreatitis was observed in another recent meta-analysis of 9 observational trials. 57 Moreover, more than 250 toxicology tests and more than 200 studies have recently been re-evaluated by the FDA and the European Medicines Agency (EMA) in a collaborative review; both agencies agreed that the concerns raised by many writers and the media concerning a potential causal interaction of incretin-mimetic drugs with acute pancreatitis are inconsistent with the concerns expressed by many authors and the media. 58 No clear cause-and-effect association between GLP-1 receptor agonists and pancreatitis has been shown, but before this issue is eventually settled, it may be advisable not to prescribe GLP-1 receptor agonists to patients with many pancreatitis risk factors, such as extreme hypertriglyceridemia or alcohol consumption. 59
Cardiovascular effect
It has been shown that GLP-1 receptor agonists decrease systolic blood pressure (SBP) and to a lesser degree, diastolic blood pressure (DBP). GLP-1 receptor agonists were associated with substantial decreases in SBP for dulaglutide and albiglutide relative to placebo in a network meta-analysis of 60 clinical trials. A marked decrease in DBP compared to placebo was found for exenatide only. 60 Another favorable outcome during therapy with GLP-1 receptor agonists was that a decrease in lipid levels has also been noted. A 35 clinical trial network meta-analysis found that these compounds were associated with substantial declines in LDL cholesterol and total cholesterol versus control. 61
The safety of incretin-based drugs in heart failure has been addressed in several studies. A study in 2016 examined existing data from multiple cohorts of patients to determine whether the use of incretin-based drugs, as compared with oral antidiabetic-drug combinations, in routine clinical practice is associated with an increased risk of heart failure and they found that it was not associated with an increased risk of hospitalization for heart failure. 62 Another study that evaluated clinical outcomes in patients with diabetes, treated by cardiac resynchronization therapy with a defibrillator (CRT-d), and glucagon-like peptide 1 receptor agonists (GLP-1 RA) in addition to conventional hypoglycemic therapy versus CRTd patients under conventional hypoglycemic drugs found that CRTd patients with diabetes treated by GLP-1 RA therapy versus CRTd patients with diabetes that did not receive GLP-1 RA therapy, experienced a significant reduction of NYHA class, associated to higher values of 6 minutes walking, and higher rate of CRTd responders. CRTd responders were defined as HFrEF patients with diabetes who received cardiac resynchronization therapy with a defibrillator (CRT-d) treatment and displayed improved cardiac performance and functional New York Association Heart (NYHA) class, and a reduction in hospital admissions and mortality. GLP-1 RA patients versus controls at follow up end experienced lower AF events (P value <0.05), lower VT events (P value <0.05), lower rate of hospitalization for heart failure worsening (P value <0.05), and higher rate of CRTd responders (P value <0.05), therefore, GLP-1 RA therapy added to conventional hypoglycemic therapy may play a protective role against arrythmias in HFrEF patients who receive CRTd therapy. 63
SIRT6 is a gene that encodes a member of the sirtuin family of NAD-dependent enzymes that are implicated in cellular stress resistance, genomic stability, aging, and energy homeostasis. The encoded protein is localized to the nucleus, exhibits ADP-ribosyl transferase and histone deacetylase activities, and plays a role in DNA repair, maintenance of telomeric chromatin, inflammation, lipid, and glucose metabolism. 64 A study from 2015 hypothesized that, by acting on SIRT6, diabetes may enhance the inflammatory potential of atherosclerotic plaques, favoring their instability, and evaluated the effect of incretin therapy in diabetic patients on SIRT6 expression in carotid plaques and early outgrown circulating endothelial progenitor cells (EPCs), it found that compared with nondiabetic plaques, diabetic plaques had more inflammation and oxidative stress, along with a lesser SIRT6 expression and collagen content. Compared with non-GLP-1 therapy-treated plaques, GLP-1 therapy-treated plaques presented greater SIRT6 expression and collagen content, and less inflammation and oxidative stress, indicating a more stable plaque phenotype. 65
In addition, incretin-based therapies have shown benefits in patients with NSTEMI-NOCS (non-obstructive coronary artery stenosis [NOCS]—Non-ST-Elevation Myocardial Infarction [NSTEMI]). A recent study from 2017 investigated the 12-months prognosis in the cohort of NSTEMI-NOCS diabetics, previously treated by incretin-based therapy, compared with a matched cohort of NSTEMI-NOCS diabetics never treated with such therapy and results showed that 1-year mortality and adverse cardiovascular outcomes were higher in patients with NOCS-NSTEMI and type 2 diabetes mellitus as opposed to patients with NOCS-NSTEMI alone. Also diabetic never-incretin-users had a worse prognosis as compared to diabetic current-incretin-users (6 months, GLP-1 agonists or DPP-4 inhibitors). 66 In STEMI, type 2 diabetic patients with STEMI-Mv-NOCS (multivessel non obstructive coronary stenosis), showed a higher incidence of 1-year mortality and adverse cardiovascular outcomes, as compared to non-diabetic STEMI-Mv-NOCS patients. And again, in diabetic patients, never-incretin-users had a worse prognosis as compared to current-incretin-users. 67
The available data do not indicate any increase in severe cardiovascular adverse events with GLP-1 receptor agonists. 68 However, administration of the GLP-1 receptor agonist has been linked with a minor rise in heart rate. A meta-analysis of 22 experiments found that GLP-1 agonists resulted in a significant increase in heart rate compared with placebo, with a weighted mean difference of 1.86 beats per minute (bpm) and 1.90 bpm compared with active control. Daily and weekly doses, in addition to short or long-acting GLP-1 agonists, slightly affected the overall outcome of tachycardia. 69 Whereas in general, the increase in heart rate with GLP-1 receptor agonists is minimal but remains clinically relevant as heart rate is a marker of cardiovascular adverse effects. 70
In contrast, DPP-4 inhibitors have not shown similar positive effects on cardiovascular outcomes. Based on currently available evidence DPP-4 inhibitors show only a neutral effect on cardiovascular outcomes in patients with high risk or established CVD in diabetes. 71
Allergy and angioedema
Due to the nature of their route of administration, as subcutaneously injected peptides, and the different immunogenic properties between the class members, hypersensitivity, and immunological reactions were anticipated with the concomitant use. The occurrence of immune-related and hypersensitivity reactions was demonstrated following re-exposure to exenatide was evaluated in 58 T2D patients in a multicenter, open-label, 24-week trial. Treatment-emergent adverse effects were observed in 40% and 47% of patients with positive and negative treatment-emergent antibodies, respectively. 72 Further studies on exenatide, found that in the small subset of patients with higher antibody titers (5%), the decrease in HbA1c was not significantly reduced twice-daily with exenatide. In addition, a significant decrease in efficacy was observed in the subset of patients who received exenatide once weekly and who had high antibody titers (12%). These findings represent a clinically relevant finding that exenatide decreases exenatide efficacy in patients who develop high titers of emergent treatment antibodies. 73 Severe anaphylactic reactions have not been reported with GLP-1 receptor agonists. However, post-marketing reports showed that anaphylactic reactions rarely occur with liraglutide, exenatide very rarely, and lixisenatide uncommonly. In addition, rare post-marketing reports of exenatide, liraglutide, and lixisenatide have been described for pruritus, urticaria, and angioneurotic edema.74-77
Injection-site reactions
Because of variations in recording outcomes methods, it is difficult to compare injection site reactions between experiments. Overall, GLP-1RAs once a week tend to be linked with higher incidences of an injection-site reaction than twice-daily exenatide or once-daily liraglutide. In the HARMONY-7 trial, for example, injection site reactions with albiglutide (13%) occurred more often than with liraglutide. 78 In the DURATION trial, a similar finding was made in which patients administered with exenatide once weekly than liraglutide reported higher incidences of nodules at the injection, pruritus at the injection site, and erythema at the injection site. Higher rates of injection site reactions reported in these studies are consistent with outcomes observed with other injectable drug formulations. 79 Exenatide and albiglutide have the highest incidence of skin reaction compared to lixisenatide having the least.76,77,80 Reactions at the injection site are recorded more often with long-acting receptor agonists than with short-acting GLP-1 receptor agonists. Pruritus in the primary injecting site reaction is the most prominent. These reactions are more commonly temporary and do not usually require medication to be stopped. Interestingly, patients receiving GLP-1 receptor analogs and producing anti-drug antibodies appear to have more injection site reactions because patients who do not produce antibodies experience comparable frequency and forms of adverse effects to those experienced. 81
Conclusions
Up to date, there is no clear evidence supporting the probability of sole GLP-1 agonist-induced hypoglycemia. GLP-1 receptor agonists have shown effective outcomes of glycemic control in type 2 diabetic patients especially in the elderly patients who are already taking other drugs for different conditions. GLP-1 receptor agonists have not been linked to weight gain and the possible risk of hypoglycemia has not been demonstrated. Our review suggests that hypoglycemia cannot occur in type 2 diabetic patients because of their insulin resistance and impaired insulin response. However, 1 clinical trial, 11 reported 3 instances of hypoglycemia out of 10 healthy patients.
Acknowledgments
We are very thankful to the Deanship of Scientific Research and Graduate Study for their support to complete and publish this work. Many thanks to Mr. Adel Rababh for the fine art production.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author contribution: All authors contributed towards writing the initial draft, revising the manuscript and reading the final proof.
ORCID iDs: Mazhar Salim Al Zoubi  https://orcid.org/0000-0003-0248-4777
https://orcid.org/0000-0003-0248-4777
Murtaza M Tambuwala  https://orcid.org/0000-0001-8499-9891
https://orcid.org/0000-0001-8499-9891
References
- 1. Labarre J. Sur Les Possibilites d’un Traitement Du Diabete par I’incretine. 1932. [Google Scholar]
- 2. Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741-744. [DOI] [PubMed] [Google Scholar]
- 3. Näslund E, Bogefors J, Skogar S, et al. GLP-1 slows solid gastric emptying and inhibits insulin, glucagon, and PYY release in humans. Am J Physiol. 1999;277:R910-R916. [DOI] [PubMed] [Google Scholar]
- 4. Shah M, Vella A. Effects of GLP-1 on appetite and weight. Rev Endocr Metab Disord. 2014;15:181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davies MJ, D’Alessio DA, Fradkin J, et al. Correction to: management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the american Diabetes association (ADA) and the european association for the study of Diabetes (EASD). Diabetologia. 2019;62:873-2498. [DOI] [PubMed] [Google Scholar]
- 6. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care. 2019;42:S90-S102. [DOI] [PubMed] [Google Scholar]
- 7. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care. 2019;42:S13-S28. [DOI] [PubMed] [Google Scholar]
- 8. American Diabetes Association. 1. Improving care and promoting health in populations: standards of medical care in diabetes-2019. Diabetes Care. 2019;42:S7-S12. [DOI] [PubMed] [Google Scholar]
- 9. American Diabetes Association. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2019. Diabetes Care. 2019;42:S103-S123. [DOI] [PubMed] [Google Scholar]
- 10. Filippatos TD, Panagiotopoulou TV, Elisaf MS. Adverse effects of GLP-1 receptor agonists. Rev Diabetic Stud 2014;11:202-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edwards CM, Todd JF, Ghatei MA, Bloom SR. Subcutaneous glucagon-like peptide-1 (7-36) amide is insulinotropic and can cause hypoglycaemia in fasted healthy subjects. Clin Sci. 1998;95:719-724. [DOI] [PubMed] [Google Scholar]
- 12. Lerche S, Soendergaard L, Rungby J, et al. No increased risk of hypoglycaemic episodes during 48 h of subcutaneous glucagon-like-peptide-1 administration in fasting healthy subjects. Clin Endocrinol. 2009;71:500-506. [DOI] [PubMed] [Google Scholar]
- 13. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and Diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36:1384-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marre M, Shaw J, Brändle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabetic Med. 2009;26:268-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nauck MA, Heimesaat MM, Behle K, et al. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002;87:1239-1246. [DOI] [PubMed] [Google Scholar]
- 17. Gough SC, Bode B, Woo V, et al. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2:885-893. [DOI] [PubMed] [Google Scholar]
- 18. Wysham C, Bonadonna RC, Aroda VR, et al. Consistent findings in glycaemic control, body weight and hypoglycaemia with iGlarLixi (insulin glargine/lixisenatide titratable fixed-ratio combination) vs insulin glargine across baseline HbA1c, BMI and diabetes duration categories in the LixiLan-L trial. Diabetes Obes Metab. 2017;19:1408-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vilsbøll T, Krarup T, Madsbad S, Holst JJ. No reactive hypoglycaemia in type 2 diabetic patients after subcutaneous administration of GLP-1 and intravenous glucose. Diabetic Med. 2001;18:144-149. [DOI] [PubMed] [Google Scholar]
- 20. Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374:39-47. [DOI] [PubMed] [Google Scholar]
- 21. Knop FK, Vilsbøll T, Larsen S, Madsbad S, Holst JJ, Krarup T. No hypoglycemia after subcutaneous administration of glucagon-like peptide-1 in lean type 2 diabetic patients and in patients with diabetes secondary to chronic pancreatitis. Diabetes Care. 2003;26:2581-2587. [DOI] [PubMed] [Google Scholar]
- 22. Miholic J, Orskov C, Holst JJ, Kotzerke J, Meyer HJ. Emptying of the gastric substitute, glucagon-like peptide-1 (GLP-1), and reactive hypoglycemia after total gastrectomy. Dig Dis Sci. 1991;36:1361-1370. [DOI] [PubMed] [Google Scholar]
- 23. Blevins T, Pullman J, Malloy J, et al. DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96:1301-1310. [DOI] [PubMed] [Google Scholar]
- 24. Ratner RE, Rosenstock J, Boka G. Dose-dependent effects of the once-daily GLP-1 receptor agonist lixisenatide in patients with type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled trial. Diabetic Med. 2010;27:1024-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park MK. Glucagon-Like peptide-1. Handb Horm. 2016;135:e17C-5. [Google Scholar]
- 26. Fehmann H-C, Göke R, Göke B. Glucagon-like peptide-1(7-37)/(7-36)amide is a new incretin. Mol Cell Endocrinol. 1992;85:C39-C44. [DOI] [PubMed] [Google Scholar]
- 27. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409-1439. [DOI] [PubMed] [Google Scholar]
- 28. Leech CA, Dzhura I, Chepurny OG, et al. Molecular physiology of glucagon-like peptide-1 insulin secretagogue action in pancreatic β cells. Prog Biophys Mol Biol. 2011;107:236-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen Q, Miller LJ, Dong M. Role of N-linked glycosylation in biosynthesis, trafficking, and function of the human glucagon-like peptide 1 receptor. Am J Physiol. 2010;299:E62-E68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci. 1987;84:3434-3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Elrick H, Stimmler L, Hlad CJ, Arai Y. Plasma insulin response to oral and intravenous glucose administration1. J Clin Endocrinol Metab. 1964;24:1076-1082. [DOI] [PubMed] [Google Scholar]
- 32. Holst JJ. Long-acting glucagon-like peptide-1 receptor agonist-status December 2018. Ann Transl Med. 2019;7:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Das A, Geetha KM, Hazarika I. Contemporary updates on the physiology of glucagon like peptide-1 and its agonist to treat type 2 Diabetes mellitus. Int J Pept Res Ther. 2020;26:1211-1221. [Google Scholar]
- 34. Brunton S. GLP-1 receptor agonists vs. DPP-4 inhibitors for type 2 diabetes: is one approach more successful or preferable than the other? Int J Clin Pract. 2014;68:557-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. New Engl J Med. 2016;375:311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121-130. [DOI] [PubMed] [Google Scholar]
- 37. Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141:150-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gotfredsen CF, Mølck A-M, Thorup I, et al. The human GLP-1 analogs liraglutide and semaglutide: absence of histopathological effects on the pancreas in nonhuman primates. Diabetes. 2014;63:2486-2497. [DOI] [PubMed] [Google Scholar]
- 39. Cao C, Yang S, Zhou Z. GLP-1 Receptor Agonists and Pancreatic Safety Concerns in Type 2 Diabetic Patients: Data From Cardiovascular Outcome Trials. Springer; 2020. [DOI] [PubMed] [Google Scholar]
- 40. Fusco J, Xiao X, Prasadan K, et al. GLP-1/Exendin-4 induces β-cell proliferation via the epidermal growth factor receptor. Sci Rep. 2017;7:9100-9106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Irons B, Minze M. Drug treatment of type 2 diabetes mellitus in patients for whom metformin is contraindicated. Diabetes Metab Syndr Obes. 2014;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Onoviran OF, Li D, Toombs Smith S, Raji MA. Effects of glucagon-like peptide 1 receptor agonists on comorbidities in older patients with diabetes mellitus. Ther Adv Chronic Dis. 2019;10:2040622319862691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Heer J, Holst JJ. Sulfonylurea compounds uncouple the glucose dependence of the insulinotropic effect of glucagon-like peptide 1. Diabetes. 2007;56:438-443. [DOI] [PubMed] [Google Scholar]
- 44. Ruby RJ, Armato JP, Pyke C, Peters AL. GLP-1 provoked severe hypoglycemia in an individual with type 2 diabetes and a benign insulinoma. Diabetes Care. 2014;37:e177-e178. [DOI] [PubMed] [Google Scholar]
- 45. Palladino AA, Sayed S, Levitt Katz LE, Gallagher PR, De León DD. Increased glucagon-like peptide-1 secretion and postprandial hypoglycemia in children after Nissen fundoplication. J Clin Endocrinol Metab. 2008;94:39-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huthmacher JA, Meier JJ, Nauck MA. Efficacy and safety of short- and long-acting glucagon-like peptide 1 receptor agonists on a background of basal insulin in type 2 diabetes: a meta-analysis. Diabetes Care. 2020;43:2303-2312. [DOI] [PubMed] [Google Scholar]
- 47. Rehfeld JF. The origin and understanding of the incretin concept. Front Endocrinol. 2018;9:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Madsbad S. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes Metab. 2016;18:317-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sun F, Yu K, Yang Z, et al. Impact of GLP-1 receptor agonists on major gastrointestinal disorders for type 2 diabetes mellitus: a mixed treatment comparison meta-analysis. Exp Diabetes Res. 2012;2012:230624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hoerman J. Tanzeum (Albiglutide) discontinued after FDA warns of risk of anaphylaxis reaction. [Google Scholar]
- 51. Yu X, Tang H, Huang L, Yang Y, Tian B, Yu C. Exenatide-induced chronic damage of pancreatic tissue in rats. Pancreas. 2012;41:1235-1240. [DOI] [PubMed] [Google Scholar]
- 52. Rouse R, Xu L, Stewart S, Zhang J. High fat diet and GLP-1 drugs induce pancreatic injury in mice. Toxicol Appl Pharmacol. 2014;276:104-114. [DOI] [PubMed] [Google Scholar]
- 53. Singh S, Chang H-Y, Richards TM, Weiner JP, Clark JM, Segal JB. Glucagonlike peptide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus: a population-based matched case-control study. JAMA Intern Med. 2013;173:534-539. [DOI] [PubMed] [Google Scholar]
- 54. Li X, Zhang Z, Duke J. Glucagon-like peptide 1-based therapies and risk of pancreatitis: a self-controlled case series analysis. Pharmacoepidemiol Drug Saf. 2014;23:234-239. [DOI] [PubMed] [Google Scholar]
- 55. Giorda CB, Sacerdote C, Nada E, Marafetti L, Baldi I, Gnavi R. Incretin-Based Therapies and Acute Pancreatitis Risk: A Systematic Review and Meta-Analysis of Observational Studies. Springer; 2015. [DOI] [PubMed] [Google Scholar]
- 56. Li L, Shen J, Bala MM, et al. Incretin treatment and risk of pancreatitis in patients with type 2 diabetes mellitus: systematic review and meta-analysis of randomised and non-randomised studies. BMJ. 2014;348:g2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang T, Wang F, Gou Z, et al. Using real-world data to evaluate the association of incretin-based therapies with risk of acute pancreatitis: a meta-analysis of 1,324,515 patients from observational studies. Diabetes Obes Metab. 2015;17:32-41. [DOI] [PubMed] [Google Scholar]
- 58. Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs — FDA and EMA assessment. New Engl J Med. 2014;370:794-797. [DOI] [PubMed] [Google Scholar]
- 59. Filippatos TD, Elisaf MS. Recommendations for severe hypertriglyceridemia treatment, are there new strategies? Curr Vasc Pharmacol. 2014;12:598-616. [DOI] [PubMed] [Google Scholar]
- 60. Sun F, Wu S, Guo S, et al. Impact of GLP-1 receptor agonists on blood pressure, heart rate and hypertension among patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Res Clin Pract. 2015;110:26-37. [DOI] [PubMed] [Google Scholar]
- 61. Sun F, Wu S, Wang J, et al. Effect of glucagon-like peptide-1 receptor agonists on lipid profiles among type 2 diabetes: a systematic review and network meta-analysis. Clin Ther. 2015;37:225-241.e8. [DOI] [PubMed] [Google Scholar]
- 62. Filion KB, Azoulay L, Platt RW, et al. A multicenter observational study of incretin-based drugs and heart failure. New Engl J Med. 2016;374:1145-1154. [DOI] [PubMed] [Google Scholar]
- 63. Sardu C, Paolisso P, Sacra C, et al. Cardiac resynchronization therapy with a defibrillator (CRTd) in failing heart patients with type 2 diabetes mellitus and treated by glucagon-like peptide 1 receptor agonists (GLP-1 RA) therapy vs. Conventional hypoglycemic drugs: arrhythmic burden, hospitalizations for heart failure, and CRTd responders rate. Cardiovasc Diabetol. 2018;17:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. NCBI. SIRT6 Sirtuin 6 [Homo sapiens (human)]. https://www.ncbi.nlm.nih.gov/gene/51548
- 65. Balestrieri ML, Rizzo MR, Barbieri M, et al. Sirtuin 6 expression and inflammatory activity in diabetic atherosclerotic plaques: effects of incretin treatment. Diabetes. 2015;64:1395-1406. [DOI] [PubMed] [Google Scholar]
- 66. Marfella R, Sardu C, Calabrò P, et al. Non-ST-elevation myocardial infarction outcomes in patients with type 2 diabetes with non-obstructive coronary artery stenosis: effects of incretin treatment. Diabetes Obes Metab. 2018;20:723-729. [DOI] [PubMed] [Google Scholar]
- 67. Marfella R, Sardu C, Balestrieri ML, et al. Effects of incretin treatment on cardiovascular outcomes in diabetic STEMI-patients with culprit obstructive and multivessel non obstructive-coronary-stenosis. Diabetol Metab Syndr. 2018;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schmidt LJ, Habacher W, Augustin T, Krahulec E, Semlitsch T. A systematic review and meta-analysis of the efficacy of lixisenatide in the treatment of patients with type 2 diabetes. Diabetes Obes Metab. 2014;16:769-779. [DOI] [PubMed] [Google Scholar]
- 69. Robinson LE, Holt TA, Rees K, Randeva HS, O’Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open. 2013;3:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Böhm M, Reil J-C, Deedwania P, Kim JB, Borer JS. Resting heart rate: risk indicator and emerging risk factor in cardiovascular disease. Am J Med. 2015;128:219-228. [DOI] [PubMed] [Google Scholar]
- 71. Khalse M, Bhargava A. A review on cardiovascular outcome studies of Dipeptidyl peptidase-4 inhibitors. Indian J Endocrinol Metab. 2018;22:689-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Faludi P, Brodows R, Burger J, Ivanyi T, Braun DK. The effect of exenatide re-exposure on safety and efficacy. Peptides. 2009;30:1771-1774. [DOI] [PubMed] [Google Scholar]
- 73. Fineman MS, Mace KF, Diamant M, et al. Clinical relevance of anti-exenatide antibodies: safety, efficacy and cross-reactivity with long-term treatment. Diabetes Obes Metab. 2012;14:546-554. [DOI] [PubMed] [Google Scholar]
- 74. AstraZeneca U. Limited. Byetta (Exenatide) Summary of Product Characteristics. 2016. 2016. [Google Scholar]
- 75. Lilly E. Company. Trulicity (Dulaglutide 1.5 and 3.0 mg/mL) solution for injection. summary of product characteristics (EU); 2015. 2015. [Google Scholar]
- 76. Sanofi-Aventis. Lyxumia®(lixisenatide) summary of product characteristics. 2014. [Google Scholar]
- 77. Croom KF, McCormack PL. Liraglutide: a review of its use in type 2 diabetes mellitus. Drugs. 2009;69:1985-2004. [DOI] [PubMed] [Google Scholar]
- 78. Pratley RE, Nauck MA, Barnett AH, et al. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet Diabetes Endocrinol. 2014;2:289-297. [DOI] [PubMed] [Google Scholar]
- 79. Buse JB, Nauck M, Forst T, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet. 2013;381:117-124. [DOI] [PubMed] [Google Scholar]
- 80. Otto T, Myland M, Jung H, Lebrec J, Richter H, Norrbacka K. Utilization patterns of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes mellitus in Germany: a retrospective cohort study. Curr Med Res Opin. 2019;35:893-901. [DOI] [PubMed] [Google Scholar]
- 81. Madsbad S, Kielgast U, Asmar M, Deacon CF, Torekov SS, Holst JJ. An overview of once-weekly glucagon-like peptide-1 receptor agonists–available efficacy and safety data and perspectives for the future. Diabetes Obes Metab. 2011;13:394-407. [DOI] [PubMed] [Google Scholar]