Abstract
Autologous fat grafting (AFG) is widely regarded as an important method for breast reconstruction after mastectomy among breast cancer (BC) patients. FTY720 has been proved to affect macrophage polarization and improve the sensitivity of postoperative BC treatment. This study aimed to explore FTY720 function and underlying mechanism in fat transplantation. The C57BL/6 J mice that received AFG were randomly divided into two groups treated with saline and FTY720, respectively. The fat graft samples were obtained at week 1, 2, 4, and 12 post-transplantation. Graft volumes, graft structures, M2 macrophages, and STAT3 protein expression were estimated by histological examination, immunofluorescence, flow cytometry, and western blot, respectively. In vitro, mouse preadipocytes were stimulated with FTY720 treated-M2 macrophages conditioned medium (FTY720-M2-CM) to evaluate the adipogenesis effect. The level of adipogenic mRNA expression in preadipocytes was detected by RT-PCR. The in vivo results showed that FTY720 treatment significantly enhanced the fat graft retention, structure integrity, and neovascularization, indicating the potential of FTY720 in improving graft survival. The histology results showed more polarized M2 macrophage presented in the FTY720 group. In the in vitro assay, after FTY720-M2-CM treatment, the 3T3-L1 preadipocytes showed the increased triglyceride content and adipogenic mRNA expression, including FABP4, C/EBP-α, Adipoq, and PPARγ. Furthermore, FTY720 treatment up-regulated the expression level of M2 biomarker CD206, Arg-1, Fizz-1, which could be weakened by the STAT3 inhibitor. Together, this study confirmed the potential efficacy of FTY720 in improving graft survival in the AFG model, possibly mediated by polarizing macrophages to M2 type through activating the STAT3 pathway.
Keywords: fat graft, FTY720, M2 macrophages, STAT3
Introduction
Autologous fat transplantation (AFG) is an increasingly popular method for repairing soft tissue defects, and is also one of the commonly used techniques to optimize the cosmetic effect of mastectomy 1 –3 . Although AFG is of excellent biocompatibility, simple applicability and low donor site morbidity, the unpredictability of fat graft retention leads to repeated surgery 4 . Various attempts have been made to improve the survival rate of grafts, including platelet-rich plasma 5 , stromal vascular fraction 6 , and adipose-derived stem cells (ADSCs) 7 assisted fat transplantation. However, the outcome of fat transplantation is not very satisfactory, mainly attributing to the complexity of the transplantation microenvironment. The grafts are in an ischemic and hypoxic environment, and the stimulation of various cytokines and the interaction between cells increase the uncertainty of graft survival. Intriguingly, adipocytes are able to secrete various cytokines to regulate the function of immune cells in the fat grafts, like neutrophils and macrophages 8 . These immune cells play vital roles in promoting angiogenesis, affecting fat survival, removing dead cells and grease 9 .
FTY720 is a small molecule agonist of four S1P receptors (S1PRs) and has been clinically approved for multiple sclerosis 10 . In addition, studies have verified that FTY720 possesses strong anti-cancer effects on BC both in vitro and in vivo. FTY720 could significantly potentiate the anti-proliferative and pro-apoptotic effects of radiation on MDA-MB-361 cells 11 . Additionally, FTY720 treatment enhanced the ERα expression and increased the therapeutic sensitivity of ERα-negative syngeneic breast tumors to tamoxifen in vivo 12 . In the obese BC-bearing mice, FTY720 reduced obesity-related inflammation, S1P signaling, and pulmonary metastasis of BC, thereby prolonging the survival of mice 13 . Therefore, FTY720 is potentially suitable for BC patients who choose IBR to achieve the anti-tumor therapeutic effect while improving the survival rate of fat transplantation.
Macrophages are crucial coordinators in the immune response that can be defined as pro-inflammatory macrophages (M1 macrophages) and anti-inflammatory macrophages (M2 macrophages) phenotypes. It is worth noting that M2 macrophages have the potential to benefit fat graft survival attributing to the potential angiogenesis ability. The local depletion of macrophages hindered the extracellular matrix reconstruction process in transferred fat, whereas excessive up-regulation of inflammation inhibited adipogenesis and impaired fat graft retention 14 . Besides, macrophages could recruit hematopoietic stem cells and secrete angiogenic-related factors to improve fat graft survival 15 . The phenotype of macrophage polarization toward M2 macrophage type in the fat graft may be beneficial to the survival of the fat grafts. On the other hand, FTY720 played an anti-inflammatory role by up-regulating the expression of M2-type macrophages in a regenerative model of traumatic skeletal muscle injury 16 . FTY720 could shift microglia from M1 to M2 macrophage polarization via activating the STAT3 signaling pathway to attenuate neuroinflammation and promote oligodendrogenesis after white matter ischemic injury 17 . Therefore, it could be inferred that FTY720 may play a role in fat graft survival through the regulation of macrophages.
Combining the above evidence, we hypothesized that FTY720 can improve graft retention by regulating the polarization of M2 macrophages to improve graft retention. In this study, the in vivo AFG mouse model was constructed with or without the administration of FTY720 to investigate the fat graft retention and histopathological features of graft. Furthermore, the in vitro experiments were performed to explore the underlying effect of FTY720 on adipocytes by regulating M2 macrophages.
Materials and Methods
Animals and Model Establishment
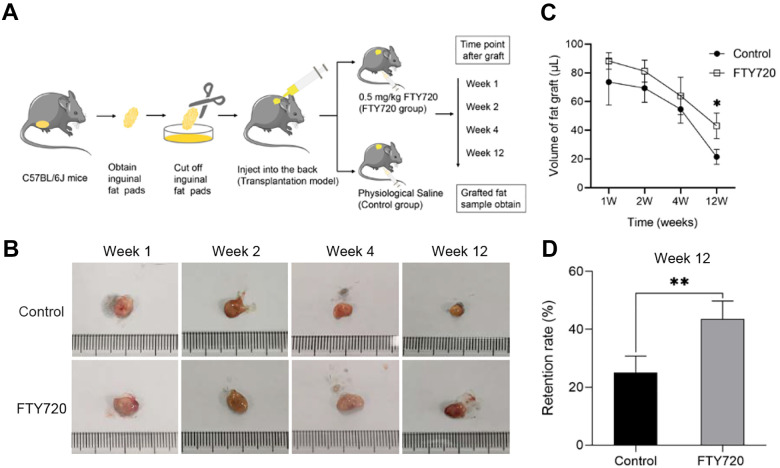
A total of 40 mice (C57BL/6 J, female, 6w) were obtained from the Animal Experimental Center of Tongji Hospital, Huazhong University of Science and Technology (Wuhan, China) and were maintained and operated following the guidelines of the institutional guidelines. The animal experiments were approved by the ethical committee of the Tongji Hospital. All mice were excised to obtain the inguinal fat pads. Then the fat pads were cut into pieces and injected subcutaneously into the back to establish an autologous fat transplantation model. The modeling schematic diagram was shown in Fig. 1A. Then the transplanted mice were randomly divided into two groups, which were intraperitoneally injected with normal saline (control group) and 0.5 mg/kg FTY720 (FTY720 group). Fat grafts were harvested in each group at week 1, 2, 4, and 12 post-transplantation, and their volumes were measured. The liquid overflow method was used to measure the volume of harvested fat grafts 18 . A total of 0.8 mL normal saline was placed in a 1 mL syringe. Then the graft was placed into the syringe and the volume of the graft was determined by the subsequent increase in the volume of saline 19 . Especially, the volume retention of the graft was assessed at week 12 by calculating the final volume/initial volume.
Figure 1.
FTY720 enhanced graft retention in vivo. (A) Flow diagram of in vivo experiment. C57BL/6 J mice were randomly divided into two groups after inguinal fat pad AFG: PBS control group and FTY720 group. (B) Macroscopic morphology of the harvested graft indicated the graft volume changes over time in the control group and FTY720 group. (C) The graft volume in both groups was recorded at each time point. (D) Retention rates of graft in the control group and FTY720 group at week 12 post-transplantation.
Cell Culture
The mouse macrophage RAW264.7 cells were cultured under appropriate criteria 20 . Briefly, the RAW 264.7 cells were cultured with 20 ng/mL IL-4 (BioLegend, San Diego, CA, USA) for 24 h to induce M2 macrophages. The induced M2 macrophages were treated with phosphate-buffered saline (PBS) (Gibco BRL, Waltham, MA, USA) or isopyknic 100 nM FTY720 (Selleck, Houston, USA), named as control and FTY720 group, respectively. The corresponding conditioned medium (CM) in the above groups were obtained and was control and FTY720-M2-CM. Besides, the mouse 3T3-L1 preadipocytes were cultured by 90% Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, CA, USA) and 10% fetal bovine serum (FBS) (Gibco, CA, USA). To induce lipid differentiation, the 3T3-L1 cells were cultured with an inducible mixture of DMEM with 10% FBS, 1 µM dexamethasone, 10 µg/mL insulin, 0.5 mM isobutylmethylxanthine (IBMX) for 8 d, as previously reported 21 .
Histological Examination
For hematoxylin-eosin (HE) staining, the harvested fat grafts in both groups were fixed and embedded in paraffin, and then sectioned and stained with hematoxylin and eosin. Next, the immunohistochemistry (IHC) staining was performed to observe the vascularization of the grafts by staining CD31 (1:200, Abcam, ON, Canada) 22 . The images of HE staining and CD31 expression were obtained by a light microscope (Sdptop, Shanghai, China) and analyzed with Image J software (National Institutes of Health, USA). In the immunofluorescence (IF) assay, the Perilipin (1:200, Signalway Antibody, College Park, Maryland, USA) was stained to detect adipocyte survival. The marker F4/80 was co-stained with CD86 to detect M1 macrophage distribution and F4/80 was co-stained with CD206to detect M2 macrophage distribution. The antibodies of F4/80, CD86, and CD206 were purchased from Servicebio (1:500, Wuhan, China). After staining, the fluorescent pictures were acquired by a fluorescent microscope (Olympus BX60, Tokyo, Japan), and the expression of adipocytes and M1/M2 macrophages was observed.
Flow Cytometry (FCM)
Briefly, the fat grafts were cut and digested with collagenase type I (Sigma, St. Louis, MO, USA) for 60 min at 37°C. The filtered cells were centrifuged for 5 min and labeled with the following antibodies for 30 min: 780-APC-cy, CD45-FITC, F4/80-BV421, CD86-APC, CD206-PE (BioLegend, San Diego, CA, USA). Gates were set to classify the cellular populations: CD45+ F4/80+ CD86+ (M1 macrophages), CD45+ F4/80+ CD206+ (M2 macrophages) 20 . The detection was performed using an LSR II flow cytometer (Becton Dickinson, San Jose, CA, USA) and the results were analyzed by Flowjo software (LLC, Ashland, OR, USA).
Quantitative Reverse Transcription-Polymerase (qRT-PCR) Chain Reaction
The graft samples were placed at −80°C for temporary storage. Total RNA was extracted from graft and cell samples by RNAiso Plus (Takara Biomedical Technology, Dalian, China). Then the stable cDNA was obtained by using the Hieff First Strand cDNA Synthesis Super Mix (Yeasen, Shanghai, China). To accurately assess the mRNA expression, the qRT-PCR with SYBR Green real-time PCR Master Mix (Yeasen, Shanghai, China) was used. The β-actin was used as the internal reference. Primer sequences were shown below:
CD206: Forward Primer 5′- CTCTGTTCAGCTATTGGACGC-3′;
Reverse Primer 5′-TGGCACTCCCAAACATAATTTGA-3′;
Arg-1: Forward Primer 5′-CTCCAAGCCAAAGTCCTTAGAG-3′;
Reverse Primer 5′- GGAGCTGTCATTAGGGACATCA-3′;
Fizz-1: Forward Primer 5′- CCAATCCAGCTAACTATCCCTCC-3′;
Reverse Primer 5′-ACCCAGTAGCAGTCATCCCA;
PPARγ: Forward Primer 5′-GGAAGACCACTCGCATTCCTT-3′;
Reverse Primer 5′- GTAATCAGCAACCATTGGGTCA-3′;
Fabp4: Forward Primer 5′-AAGGTGAAGAGCATCATAACCCT-3′;
Reverse Primer 5′- TCACGCCTTTCATAACACATTCC-3′;
C/EBP-α: Forward Primer 5′-CAAGAACAGCAACGAGTACCG-3′;
Reverse Primer 5′-GTCACTGGTCAACTCCAGCAC-3′;
Adipoq: Forward Primer 5′-TGTTCCTCTTAATCCTGCCCA-3′
Reverse Primer 5′-CCAACCTGCACAAGTTCCCTT-3′;
β-actin: Forward Primer 5′-GGCTGTATTCCCCTCCATCG-3′
Reverse Primer 5′-CCAGTTGGTAACAATGCCATGT-3′
Oil red O Staining and Triglyceride Detection
The 3T3-L1 adipocytes were fixed with 4% formaldehyde at day 8 after adipogenic differentiation and then stained with a mixture of oil red O and isopropanol (ratio 3:2). The cell morphology images were observed by a microscope (Sdptop, Shanghai, China). To quantitatively evaluate the lipid accumulation in 3T3-L1 adipocytes, triglyceride levels in the supernatant at day 8 after lipogenesis were measured using the Triglyceride GPO-PAP kit (Jiancheng Biotechnologies, Nanjing, China).
Western Blotting
The cells were washed with cold PBS and lysed in cell lysis buffer (Cell Signaling Technology, Beverly, MA, USA). Total cell extracts were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad, Hercules, CA, USA). Then the PVDF membranes were blocked in 5% non-fat milk in tris-buffered saline with Tween 20 (TBST) for 1 h and blotted with anti-β-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-STAT3 antibody (Abcam, Cambridge, MA, USA), and anti-p-STAT3 antibody ((Bio-Rad, Hercules, CA, USA) overnight at 4°C, separately. Finally, the membranes were washed thrice using TBST and were incubated with the secondary HRP conjugated secondary antibody (1:5000, Proteintech Group, Rosemont, IL, USA). Protein bands were detected and obtained by a ChemiDoc XRS+ imaging system (Bio-Rad, Hercules, CA, USA), and quantitated by ImageLab software. The targeted protein expressions were calculated in relation to β-actin expression.
Statistical Analysis
Two groups analysis was performed one-way analysis of variance with t-test by using Graphpad Prism 8.0 (GraphPad, San Diego, CA, USA). Statistically significant was identified as P < 0.05.
Results
FTY720 Enhanced Graft Retention in Vivo
To evaluate the effects of FTY720 on long-term fat graft survival, autologous fat transplanted mice were treated with saline or FTY720 (Fig. 1A). The volume of fat grafts in both groups decreased gradually with time. As seen in the macroscopic images of harvest grafts, the fat grafts were all covered with a gauzy fibrous capsule, and their soft texture was similar to normal adipose tissue. The surface vascularization was poorer in the control group compared with the FTY720 group (Fig. 1B). More importantly, the fat volume of the FTY720 group was significantly larger than that of the control group (Fig. 1C). At week 12, there was a higher retention rate in the FTY720 group than in the control group (43.55% ± 5.61% vs 25.03% ± 5.15%, p < 0.05) (Fig. 1D). Therefore, it could be inferred that FTY720 significantly enhanced the retention of fat graft in vivo.
FTY720 Improved Fat Graft Structure Integrity in Vivo
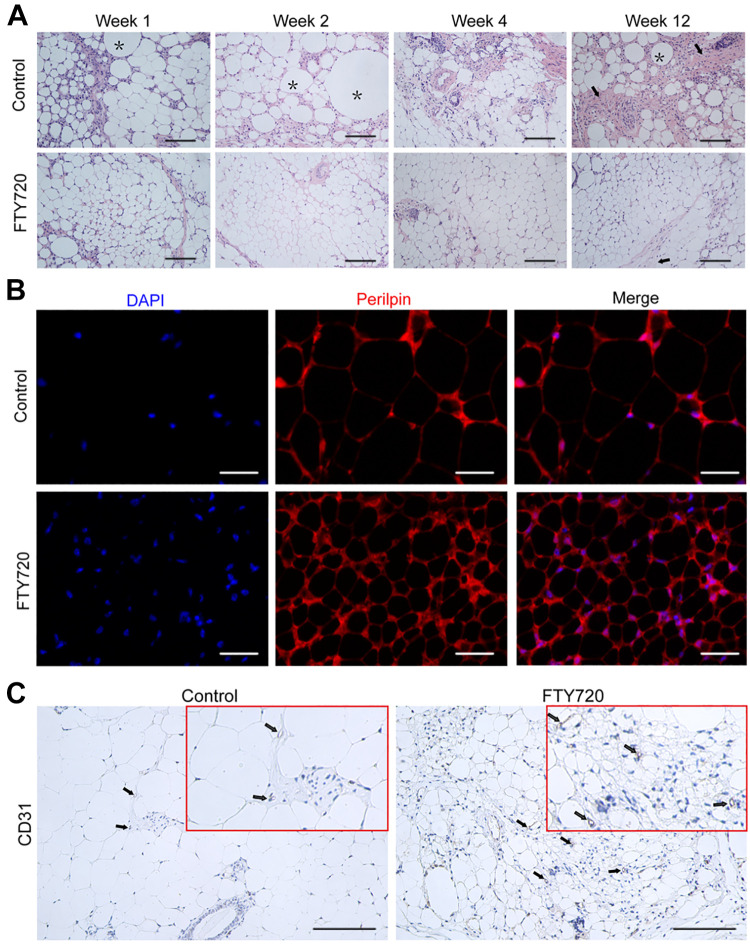
The histologic assessment of fat grafts was performed to investigate the impact of FTY720 on the structural integrity of fat grafts. The HE staining showed that inflammatory cell infiltration in the control group was higher than that in the FTY720 group at week 1, 2, 4, and 12 post-transplantation, especially at week 1 (Fig. 2A). Furthermore, HE staining also showed that a large area of tissue fibrosis and a large number of fat vacuoles appeared in the control group at week 12, while adipocytes in the FTY720 group were of regular size and had better structural integrity (Fig. 2A). In the IF assay, the harvested grafts were stained for perilipin (red) and DAPI (blue) at week 12, and the results showed there were more positive mature adipocytes marked with perilipin in the FTY720 group (Fig. 2B). The IHC assay exhibited the consistent result that the number of CD31-positive cells (the newly generated microvessel) in the FTY720 group was much higher than that in the control group, which might indicate that the FTY720 group had a better survival environment ( Fig. 2C ). These results further revealed that FTY720 could indeed possess the ability to promote adipocyte survival.
Figure 2.
FTY720 improved fat graft structure integrity in vivo. (A) The histological changes of the fat graft at week 12 were detected by HE staining. Oil cysts and fibrosis structures were indicated by black asterisks and black arrows, respectively. Scale bars = 120 µm. (B) Perilipin immunostaining of the fat graft at week 12 was used to stain the mature adipocytes. Scale bars = 60 µm. (C) Representative IHC staining images of CD31 in the FTY720 group and control group at week 12. Scale bars = 120 µm.
FTY720 Promoted the Polarization of M2 Macrophages in Vivo
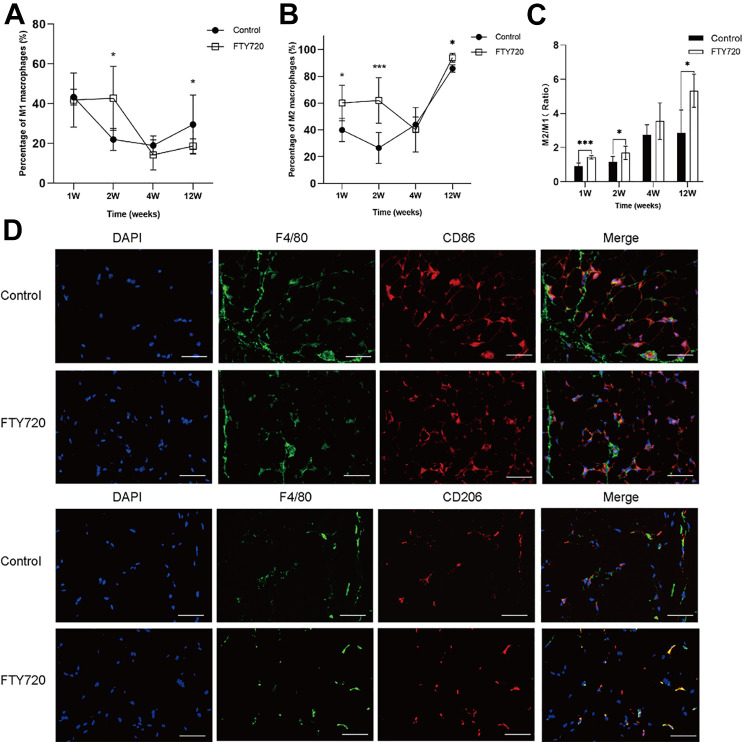
Next, the FCM analysis was used to explore the effects of FTY720 on macrophage polarization in vivo. FCM analysis showed that, compared with the control group, the M1 macrophages (CD45+ F4/80+ CD86+) were decreased and M2 macrophages (CD45+ F4/80+ CD206+) were increased in the fat grafts at week 12 (Fig. 3A, B ). Moreover, the M2/M1 ratio in the FTY720 group was greater than in the control group at week 12 (Fig. 3C). The IF staining results of macrophage distribution showed that there were fewer M1 macrophages (CD45+ F4/80+ CD86+) and more M2 macrophages in the FTY720 group compared with the control group (Fig. 3D). These results consistently suggested that M2 macrophages were the dominant phenotype in the FTY720 group, thereby presuming that FTY720 reduced inflammation in fat grafts by shifting proinflammatory M1 macrophages to anti-inflammatory M2 macrophages.
Figure 3.
FTY720 promoted the polarization of M2 macrophages in vivo. The percentage of M1 macrophages (A) and M2 macrophages (B) in CD45+ lymphocytes of fat grafts in the control and FTY720 group at week1, 2, 4, and 12, detected by FCM. (C) The according to M2/M1 ratio of fat grafts in control and FTY720 group. (D) The expression and location of M1 macrophage markers (F4/80 and CD86) and M2 macrophage markers (F4/80 and CD206) in control and FTY720 group at week 12, by IF. Scale bars = 60 µm.
FTY720-M2 CM Promoted Adipogenic Differentiation of 3T3-L1 Preadipocytes in Vitro
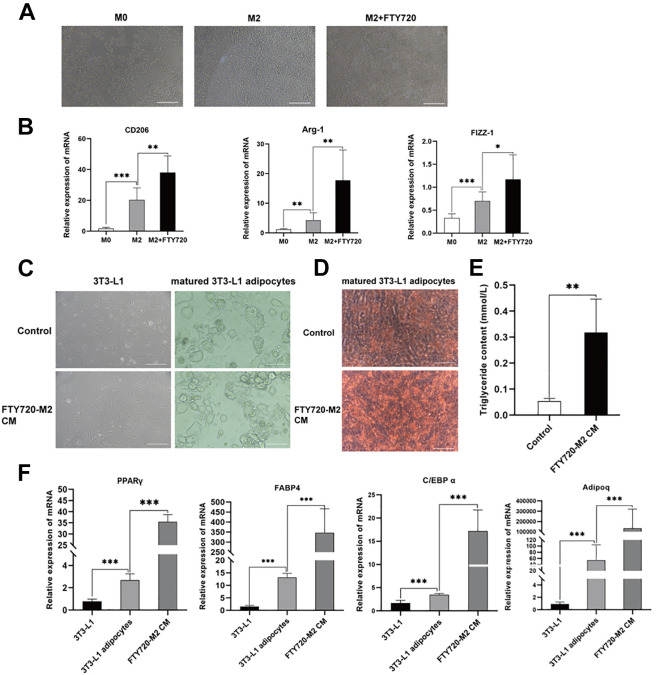
M2 Macrophages were characterized based on morphology and molecular marker. The morphology of M2 macrophages was highly refractive, large, and round under light microscopy. There was no significant change in the morphology of macrophages in each group (Fig. 4A). And RT-qPCR analysis showed that the expression of macrophage marker M2 (CD206, Arg-1, FIZZ-1) increased significantly after FTY720 treatment (Fig. 4B). To further clarify the relationship between M2 macrophage polarization and fat graft survival, the M2 macrophages CM with or without FTY720 treatment were used to co-culture with 3T3-L1 cell lines, respectively. Next, the bright field of the microscope indicated that FTY720-M2 CM could promote the lipid drop formation than normal M2 CM (representative micrograph in Fig. 4C). In the Oil Red-O Staining assay, FTY720-M2 CM promoted more stained lipid drops, indicating more mature adipocytes were induced (Fig. 4D). Besides, the matured 3T3-L1 adipocytes cells treated by FTY720-M2 CM resulted in a significant increase of triglyceride content, inferring more lipid accumulation (Fig. 4E). Meanwhile, the PPARγ mRNA in the FTY720-M2 CM group were increased dramatically than that of the control group (Fig. 4F). Similarly, other adipocyte-related markers, such as FABP4, C/EBP-α, Adipoq, also presented an elevated expression level in the FTY720-M2 CM group than the control. M2 CM could promote adipogenic differentiation of 3T3-L1 preadipocytes in vitro, which could further enhance by FTY720 stimulation. These in vitro results were consistent with the in vivo results, suggesting that FTY720 could induce the M2 polarization and adipogenic differentiation function.
Figure 4.
M2 macrophages promoted adipogenic differentiation of 3T3-L1 preadipocytes in vitro. (A) The general morphology of M0, M2 and FTY720-treated M2 RAW264.7 cells under a light microscope. Scale bars = 120 µm. (B) Relative mRNA expression of M2 macrophage markers (CD206, Arg-1, Fizz-1) in M0, M2 and FTY720-treated M2 macrophages, by using RT-PCR. The general morphology of 3T3-L1 cells treated with or without FTY720-M2 CM in adipogenic medium (C) and Oil Red O staining identification (D). Scale bars = 120 µm. (E) Triglyceride content was measured after adipogenic induction of 3T3-L1 cells treated with or without FTY720-M2 CM. (F) Relative mRNA expression of adipogenic related genes PPARγ, FABP4, C/EBP-α, and Adipoq in 3T3-L1 cells, matured 3T3-L1 adipocytes cells and matured 3T3-L1 adipocytes cells treated with FTY720-M2 CM.
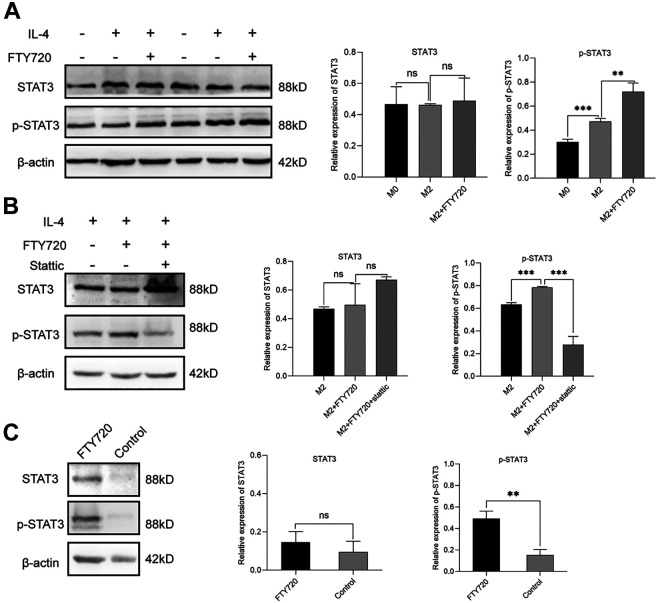
FTY720 Facilitated Macrophages to M2 Phenotype Switch via STAT3 Pathway in Vitro
Finally, to further explore the potential mechanism of FTY720 in M2 macrophage polarization, western blot was performed to detect the protein alteration of STAT3 signaling pathway 17 . In M2 macrophages, FTY720 stimulation obviously enhanced the expression level of p-STAT3 protein (Fig. 5A). Besides, as one of the highly selective blocking inhibitors of the STAT3 pathway 23 , stattic was used to co-incubate RAW264.7 cells with FTY720. The result showed that the enhanced STAT3 activity induced by FTY720 was significantly inhibited in the stattic group (Fig. 5B). In addition, the in vivo results of the western blot assay also showed that the p-STAT3 protein was also increased in the fat graft of the FTY720 group, which was similar to the in vitro analysis (Fig. 5C). Together, it could speculate that the FTY720 facilitated the M2 polarization state through the STAT3 signaling pathway.
Figure 5.
FTY720 facilitated macrophages switch to M2 macrophages via STAT3 pathway in vitro. (A) Western blot analysis and quantification of STAT3 and p-STAT3 expression in M0, M2, and FTY720 treated M2 macrophages. (B) Western blot analysis and quantification of STAT3 and p-STAT3 expression in M2, FTY720 treated M2 and static plus FTY720 treated M2 macrophages. (C) The STAT3 and p-STAT3 protein expression of fat grafts in FTY720 group and control group at week 12.
Discussion
Fat absorption is thought to an inevitable event and strikingly compromises the outcomes of fat grafts. In our study, the results demonstrated that FTY720 significantly intensified the polarization of M2 macrophages in the grafts and protected adipose structures, leading to enhanced fat retention in vivo. Moreover, FTY720 treatment could also promote the M2 macrophage polarization via STAT3 pathway signaling in vitro. To our knowledge, this is the first study to investigate the protective effect and underlying mechanism of FTY720 in fat transplantation. Therefore, FTY720 is expected to improve the retention rate of autologous auntie transplantation.
Nowadays, emerging evidence has proved that macrophages are very important for tissue repair. Some studies indicated that the levels of macrophage polarization affect tip cell migration and assist tip cell fusion in fat grafts, thereby promoting tissue revascularization 24 . In addition, M2 macrophages are beneficial in stimulating angiogenesis and inducing adipogenic C/EBP-α mRNA expression in ADSCs 25 . However, some researchers have suggested that fat grafts might not benefit from high levels of M2 macrophage infiltration, which may promote more serious fat fibrosis 26 . For instance, adipose tissue fibrosis in obese or diabetic patients has also been shown to be related to macrophage infiltration 27 . More interestingly, Cai et al. evaluated the effect of different doses of granulocyte colony-stimulating factor (G-CSF), which increased macrophage infiltration in fat grafts 28 . A slight increase in macrophage infiltration induced by low-dose G-CSF increased the survival rate of fat graft by inducing increased angiogenesis. However, high levels of macrophage infiltration induced by high doses of G-CSF caused severe graft fibrosis. 28 Consequently, the effect of macrophage polarization in fat transplantation is a double-edged sword. The moderate increase of M2 macrophage infiltration may contribute to the improved survival rate of fat graft. In our research, it was observed that M2 macrophages significantly protected transplanted adipose tissue in vivo. To further illustrate how do M2 macrophages promote fat graft reservation, 3T3-L1 cells were treated with CM from activated macrophages. In vitro, M2 macrophage CM could largely enhance lipid accumulation and promote adipogenic differentiation of 3T3-L1 cells. Our results indicated that M2 macrophages potentially increased the graft retention rate by promoting the differentiation of preadipocytes.
There are still some limitations that need to be addressed in this study. Firstly, the receptor S1P is widely and differentially expressed in various cell types, including immune cells 29 and adipocytes 30 . In the in vivo transplantation model, whether FTY720 could also improve adipocyte survival by affecting other related cells was an issue worth considering. Secondly, the in vitro experimental results showed that M2 CM could promote the differentiation of preadipocytes. But it is worth noting that M2 CM was a mixed component derived from cell culture, thus a single component or multi-components synergistically exert the actively biological effect by M2 macrophage is worthy of further discussion. Finally, there are species differences in animal models and clinical applications, that is, animal levels cannot truly reflect or predict clinical effects. Therefore, the application of FTY720 in clinical fat transplantation still has a long way to go and remains to be verified by large-scale clinical researches.
Conclusions
In conclusion, the present study proved the potential efficacy of FTY720 in improving graft survival in the autologous fat transplantation model. Mechanically, FTY720 regulated the polarization of macrophages to the M2 macrophages through the STAT3 pathway, thus resulted in improved graft retention by promoting adipocyte regeneration. Therefore, we demonstrate a preliminary mechanism by which FTY720 promotes fat transplantation, which may provide a novel and effective strategy for fat transplantation and even post-BC reconstruction.
List of Abbreviations
- BC
breast cancer
- IBR
immediate breast reconstruction
- DBR
delayed breast reconstruction
- AFG
Autologous fat grafting
- ADSCs
adipose-derived stem cells
- S1PRs
S1P receptors
- CM
conditioned medium
- DMEM
Dulbecco’s Modified Eagle Medium
- FBS
fetal bovine serum
- IBMX
isobutylmethylxanthine
- HE
hematoxylin-eosin
- IHC
immunohistochemistry
- IF
immunofluorescence
- FCM
flow cytometer
- PVDF
polyvinylidene fluoride
- TBST
tris-buffered saline with Tween
- G-CSF
granulocyte colony-stimulating factor
Footnotes
Author contributions: YY and WH performed this experiment and wrote the manuscript. YW, QZ, and MW conceived the project and revised the manuscript. WL, CZ, and MX edited the manuscript. All authors reviewed the manuscript and all approved of the final version.
Both Yi Yi and Weijie Hu contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This animal experiment was approved by the ethical committee of the Tongji Hospital.
Statement of Human and Animal Rights: All procedures in this study were performed in accordance with the ethical policy and guidelines of Animal Experimental Center of Tongji Hospital, Huazhong University of Science and Technology for laboratory animals.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by China Guanghua Science and Technology Foundation (grant number 2019JZXM001) and Wuhan Science and Technology Bureau (grant 2020020601012241).
ORCID iDs: Qi Zhang  https://orcid.org/0000-0003-0564-9215
https://orcid.org/0000-0003-0564-9215
Yiping Wu  https://orcid.org/0000-0002-4815-0732
https://orcid.org/0000-0002-4815-0732
References
- 1. Bennett KG, Qi J, Kim HM, Hamill JB, Wilkins EG, Mehrara BJ, Kozlow JH. Association of fat grafting with patient-reported outcomes in postmastectomy breast reconstruction. JAMA Surg. 2017;152(10):944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davis MJ, Perdanasari AT, Abu-Ghname A, Gonzalez SR, Chamata E, Rammos CK, Winocour SJ. Application of fat grafting in cosmetic breast surgery. Semin Plast Surg. 2020;34(01):024–029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dolen U, Cohen JB, Overschmidt B, Tenenbaum MM, Myckatyn TM. Fat grafting with tissue liquefaction technology as an adjunct to breast reconstruction. Aesthetic Plast Surg. 2016;40(6):854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lv Q, Li X, Qi Y, Gu Y, Liu Z, Ma G. Volume retention after facial fat grafting and relevant factors: a systematic review and meta-analysis. Aesthetic Plast Surg. 2021;45(2):506–520. [DOI] [PubMed] [Google Scholar]
- 5. Mammoto T, Jiang A, Jiang E, Mammoto A. Platelet rich plasma extract promotes angiogenesis through the angiopoietin1-Tie2 pathway. Microvasc Res. 2013;89:15–24. [DOI] [PubMed] [Google Scholar]
- 6. Gontijo-de-Amorim NF, Charles-de-Sá L, Rigotti G. Mechanical supplementation with the stromal vascular fraction yields improved volume retention in facial lipotransfer: a 1-year comparative study. Aesthetic Surg J. 2017;37(9):975–985. [DOI] [PubMed] [Google Scholar]
- 7. Mou S, Zhou M, Li Y, Wang J, Yuan Q, Xiao P, Sun J, Wang Z. Extracellular vesicles from human adipose-derived stem cells for the improvement of angiogenesis and fat-grafting application. Plast Reconstr Surg. 2019;144(4):869–880. [DOI] [PubMed] [Google Scholar]
- 8. Bouglé A, Rocheteau P, Hivelin M, Haroche A, Briand D, Tremolada C, Mantz J, Chrétien F. Micro-fragmented fat injection reduces sepsis-induced acute inflammatory response in a mouse model. Br J Anaesth. 2018;121(6):1249–1259. [DOI] [PubMed] [Google Scholar]
- 9. Liu K, Cai J, Li H, Feng J, Feng C, Lu F. The Disturbed function of neutrophils at the early stage of fat grafting impairs long-term fat graft retention. Plast Reconstr Surg. 2018;142(5):1229–1238. [DOI] [PubMed] [Google Scholar]
- 10. Chen W, Ghobrial RM, Li XC, Kloc M. Inhibition of RhoA and mTORC2/Rictor by Fingolimod (FTY720) induces p21-activated kinase 1, PAK-1 and amplifies podosomes in mouse peritoneal macrophages. Immunobiology. 2018;223(11):634–647. [DOI] [PubMed] [Google Scholar]
- 11. Marvaso G, Barone A, Amodio N, Raimondi L, Agosti V, Altomare E, Scotti V, Lombardi A, Bianco R, Bianco C, Caraglia M, et al. Sphingosine analog fingolimod (FTY720) increases radiation sensitivity of human breast cancer cells in vitro. Cancer Biol Ther. 2014;15(6):797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hait NC, Avni D, Yamada A, Nagahashi M, Aoyagi T, Aoki H, Dumur CI, Zelenko Z, Gallagher EJ, Leroith D, Milstien S, et al. The phosphorylated prodrug FTY720 is a histone deacetylase inhibitor that reactivates ERα expression and enhances hormonal therapy for breast cancer. Oncogenesis. 2015;4(6):e156–e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nagahashi M, Yamada A, Katsuta E, Aoyagi T, Huang W-C, Terracina KP, Hait NC, Allegood JC, Tsuchida J, Yuza K, Nakajima M, et al. Targeting the SphK1/S1P/S1PR1 axis that links obesity, chronic inflammation, and breast cancer metastasis. Cancer Res. 2018;78(7):1713–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cai J, Li B, Liu K, Li G, Lu F. Macrophage infiltration regulates the adipose ECM reconstruction and the fibrosis process after fat grafting. Biochem Biophys Res Commun. 2017;490(2):560–566. [DOI] [PubMed] [Google Scholar]
- 15. Cai J, Feng J, Liu K, Zhou S, Lu F. Early macrophage infiltration improves the fat graft survival by inducing angiogenesis and hematopoietic stem cell recruitment. Plast Reconstr Surg. 2017;79(1):146–158. [DOI] [PubMed] [Google Scholar]
- 16. San Emeterio CL, Olingy CE, Chu Y, Botchwey EA. Selective recruitment of non-classical monocytes promotes skeletal muscle repair. Biomaterials. 2017;117:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qin C, Fan W-H, Liu Q, Shang K, Murugan M, Wu L-J, Wang W, Tian D-S. Fingolimod protects against ischemic white matter damage by modulating microglia toward M2 polarization via STAT3 pathway. Stroke. 2017;48(12):3336–3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cells EP. Enhancement of viability of fat grafts in nude mice by endothelial progenitor Cells. Dermatol Surg. 2006;32(12):1437–1443. [DOI] [PubMed] [Google Scholar]
- 19. Huang H, Feng S, Zhang W, Li W, Xu P, Wang X, Ai A. Bone marrow mesenchymal stem cell-derived extracellular vesicles improve the survival of transplanted fat grafts. Mol Med Rep. 2017;16(3):3069–3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu H, Wu L, Liu L, Ruan Q, Zhang X, Hong W, Wu S, Jin G, Bai Y. Quercetin ameliorates kidney injury and fibrosis by modulating M1/M2 macrophage polarization. Biochem Pharmacol. 2018;154:203–212. [DOI] [PubMed] [Google Scholar]
- 21. Lee J, Hong BS, Ryu HS, Lee H-B, Lee M, Park IA, Kim J, Han W, Noh D-Y, Moon H-G. Transition into inflammatory cancer-associated adipocytes in breast cancer microenvironment requires microRNA regulatory mechanism Ahmad A, editor. PLoS One. 2017;12(3):e0174126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liang Y, Song X, Li Y, Chen B, Zhao W, Wang L, Zhang H, Liu Y, Han D, Zhang N, Ma T, et al. LncRNA BCRT1 promotes breast cancer progression by targeting miR-1303/PTBP3 axis. Mol Cancer. 2020;19(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23. Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol. 2006;13(11):1235–1242. [DOI] [PubMed] [Google Scholar]
- 24. Dong Z, Fu R, Liu L, Lu F. Stromal vascular fraction (SVF) cells enhance long-term survival of autologous fat grafting through the facilitation of M2 macrophages. Cell Biol Int. 2013;37(8):855–859. [DOI] [PubMed] [Google Scholar]
- 25. Phipps KD, Gebremeskel S, Gillis J, Hong P, Johnston B, Bezuhly M. Alternatively activated M2 macrophages improve autologous fat graft survival in a mouse model through induction of angiogenesis. Plast Reconstr Surg. 2015;135(1):140–149. [DOI] [PubMed] [Google Scholar]
- 26. Mineda K, Kuno S, Kato H, Kinoshita K, Doi K, Hashimoto I, Nakanishi H, Yoshimura K. Chronic inflammation and progressive calcification as a result of fat necrosis. Plast Reconstr Surg. 2014;133(5):1064–1072. [DOI] [PubMed] [Google Scholar]
- 27. Keophiphath M, Achard V, Henegar C, Rouault C, Clement K, Lacasa D. Macrophage-secreted factors promote a profibrotic phenotype in human preadipocytes. Mol Endocrinol. 2009;23(1):11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cai J, Li B, Liu K, Feng J, Gao K, Lu F. Low-dose G-CSF improves fat graft retention by mobilizing endogenous stem cells and inducing angiogenesis, whereas high-dose G-CSF inhibits adipogenesis with prolonged inflammation and severe fibrosis. Biochem Biophys Res Commun. 2017;491(3):662–667. [DOI] [PubMed] [Google Scholar]
- 29. Hunter SF, Bowen JD, Reder AT. The direct effects of fingolimod in the central nervous system: implications for relapsing multiple sclerosis. CNS Drugs. 2016;30(2):135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. JEONG J-K, MOON M-H, PARK S-Y. Modulation of the expression of sphingosine 1-phosphate 2 receptors regulates the differentiation of pre-adipocytes. Mol Med Rep. 2015;12(5):7496–7502. [DOI] [PubMed] [Google Scholar]