Abstract
In this study, we want to investigate the clinical value of each index of thromboelastography (TEG) on the prognosis of infected patients.
The clinical baseline data and TEG test results of 431 infected patients in our hospital’s emergency department between January 2018 and December 2018 were selected. And the patients were divided into death and survival groups to analyze the predictive value of each index of TEG and the joint model on the death of infected patients.
In the correlation study of C-reactive protein (CRP) and procalcitonin (PCT) with each TEG parameter, CRP was positively correlated with maximum amplitude (MA, r = 0.145, P = .003) and elasticity constants (E, r = 0.098, P = .043), respectively. PCT was positively correlated with coagulation reaction time (R, r = 0.124, P = .010) and time to MA (TMA) (r = 0.165, P = .001), respectively; PCT was negatively correlated with α-Angle (r = 0.124, P = .010) and coagulation index (CI, r = −0.108, P = .026), respectively. Multifactorial regression analysis showed that granulocytes, thrombocytes, platelet distribution width (PDW), and infection site were independent influences on infected patients’ death. Diagnostic data showed that all eight TEG indicators had good specificity for predicting death, but all had poor sensitivity; thrombodynamic potential index (TPI) had the best diagnostic value (area under the curve, AUC = 0.609, P = .002). The eight-indicator modeling of TEG showed that the TEG model combined with PCT and CRP, respectively, had lower diagnostic efficacy than PCT (AUC = 0.756, P < .001); however, TEG had better specificity (82.73%) when diagnosed independently.
The granulocytes, thrombocytes, PDW, and infection site are independent influencing factors of death in infected patients. Each index of TEG has better specificity in the diagnosis of death in infected patients.
Keywords: infection, thromboelastography, coagulation function, death, diagnosis
1. Introduction
Infections are local tissue and systemic inflammatory reactions caused by the invasion of bacteria, viruses, fungi, and parasites into the human body, which clinically predisposes patients to pathological responses and damage.1–3 In particular, systemic inflammatory reactions may occur in infected patients when lung tissue infection occurs. 4 Infection activates the inflammatory process by activating inflammatory cells such as neutrophils, macrophages, vascular endothelial cells, and platelets (PLT) that release various inflammatory mediators.5,6
There is an increasing number of studies that show a disturbance of coagulation homeostasis in patients with infections. Recently, a significant retrospective analysis based on machine learning methods classified the phenotypes of sepsis patients into four clinical types (α, β, γ, and δ) that correlate with host response patterns. 7 Among the sepsis types, patients with the δ phenotype, characterized by liver dysfunction and shock, were more inclined to develop coagulation dysfunction and had a higher mortality rate than patients with other phenotypes. 8 When there is an abnormal inflammatory response and hypoxia in patients with pulmonary disease, it can cause damage to the pulmonary vascular endothelium and further activate the coagulation system, causing coagulation abnormalities.9,10 When platelets are activated in response to pathogen stimulation or tissue injury, activated platelets can synthesize and release various substances. 11 These substances are involved in the inflammatory response and the hemostatic and coagulation response processes. However, excessive release of inflammatory factors can lead to coagulation disorders in the body, which can cause blood clots in the microcirculation and eventually trigger disseminated intravascular coagulation (DIC), endangering the life of the patient. 12 Studies have shown that platelet distribution width (PDW) is altered before the platelet count decreases in infected patients. 13 Early observation of changes in platelets and their parameter PDW can be a guide to determine the severity of disease and prognosis of infected patients.
Thromboelastography (TEG) is an effective method for the comprehensive in vitro assessment of coagulation in patients.14,15 Today, TEG is often used for the dynamic monitoring of liver transplantation, 16 severe coagulation disorders, 17 including gastrointestinal hemorrhage, 18 massive postpartum bleeding, 19 severe emergency trauma, 20 bleeding from ruptured ectopic pregnancy, 21 and final coagulation in extracorporeal procedures. The method can be performed on whole blood specimens and provides an accurate and comprehensive picture of the patient’s coagulation status. 22 The design is based on the measurement of clot formation’s physical characteristics at the end of coagulation. When the coagulation process begins, TEG can comprehensively record the entire coagulation process observed in whole blood samples, reflecting the complete picture of coagulation and recording nearly 20 parameter values in the form of curve images. The main parameters of TEG are reaction time (R), K, α-Angle, maximum amplitude (MA), TMA (Time to MA), coagulation index (CI), elasticity constants (E), and platelet kinetic index (TPI) values. There is no exhaustive report on the correlation and consistency between patients’ mortality with studied infections and the various TEG testing indicators.
Procalcitonin (PCT) and C-reactive protein (CRP) are better indicators for the early diagnosis of sepsis, and both have high sensitivity and specificity. PCT is a serum biomarker, which rises rapidly in a short period of time. 23 When patients respond well to treatment, PCT returns to the normal range of levels more quickly than CRP, making it a better guide for the clinical diagnosis of sepsis. 24
In this study, we retrospectively analyzed 431 patients with severe infections in our emergency hospital observation ward. We performed TEG and coagulation function, routine platelet count, CRP, PCT, etc, on the patient. This study sought to investigate the correlation between various parameters of TEG and indicators of standard coagulation tests and the diagnostic and predictive significance of death in infected patients in the hope of providing additional guidance for clinical work.
2. Materials and Methods
2.1. Collection of Patients
The 431 infected patients admitted to Beijing Chaoyang Hospital’s emergency department, Beijing West Campus, were studied from January 1, 2018, to December 31, 2018.. All patients or their families agreed to the TEG test and signed an informed consent form.
Inclusion criteria: precise diagnosis of infection-associated diseases. It mainly included pneumonia, sepsis, septic shock, slow-onset lung, encephalitis, infective endocarditis, etc. Exclusion criteria: (1) those with hematologic disorders; (2) those on long-term anticoagulant drugs; (3) those aged <18 years; (4) those with abnormal mental status and unable to cooperate to complete the study; (5) those with acute cardiovascular and cerebrovascular diseases; and (6) those with a history of surgical procedures in the past 3 months, or severe trauma.
2.2. Routine Blood Monitoring Method
For routine blood monitoring, 3 ml of venous blood is drawn from the patient and injected into a closed vacuum blood collection tube containing EDTA dipotassium salt (EDTA-K2) EDTA-K2 is an anticoagulant that inhibits peptidases. When testing the blood, attention was paid to uniform shaking to promote its fully mixing. The platelet, neutrophil, and lymphocyte counts were measured by the SYSMEX KX-21 fully automated blood cell analyzer and a cyanide-free haemoglobin (HGB) measurement method within 30 min of blood specimen collection. The values of PLT and PDW parameters were statistically analyzed.
2.3. C-reactive protein and procalcitonin
CRP was measured using turbidimetric inhibition immunoassay (TINIA) with a SIEMENS ADVIA2400 automatic biochemical analyzer. CRP was considered normal when the average level of CRP was <8 mg/L. PCT was measured by quantitative solid-phase immunoassay with a Roche Elecsys 2010 analyzer and was deemed to be expected when PCT was <0.5 ng/ml.
2.4. Thromboelastography Assay
The TEG5000 coagulation testing system, analyzer, supporting reagents, and software system from Haemoscope, were used to perform TEG testing on the patients’ blood. The specific operation was performed strictly according to the instruction manual. Select 1.0 mL of sodium citrate anticoagulated whole blood and add it to the reagent, let it flow down the wall, screw the cap and gently invert the mixture five times without shaking the blood sample. Taken 20 μL of 0. 2 mL/L calcium chloride, added it to the preheated cup of TEG analyzer, and then the assay was started after drawing 340 μL of whole blood and pouring it into the cup until the MA value was determined. Rotated the measurement at 4° and 45° at 37°C, and used the computer to collect TEG parameters. (1) Coagulation reaction time (R, reference range 5–10 min) is the time necessary for initial fibrin formation; (2) clotting time (K, reference range l −3 min): K is the time required from the end of the R time to describe the amplitude of the recording graph up to 20 mm; (3) α-Angle (α-angle, reference range 53°-72°) : represents the rate of thrombin formation; (4) maximum clot strength (MA, reference value 50–70 mm) is used to trace the maximum amplitude on the graph, which is the most comprehensive distance on both sides of the chart; (5) TMA (Times of MA) records the time used from the beginning of clotting to the determination of MA; (6) coagulation index (CI): standard value between (−3)–( + 3), < −3 is the hypocoagulable state, > + 3 is the hypercoagulable state; (7) E constant is the parameter of hardness of blood clot; (8) TPI is the platelet kinetic index, which is used to describe the hemagglutination of citrated whole blood of the patient, with an average interval of 6 to 7.5, when TPI <6 is a hypocoagulable state, >7.5 is a hypercoagulable state.
2.5. Statistical Methods
Statistical data were processed and analyzed using the software SPSS 25.0. Histograms and interfactor correlation studies were plotted using the software GraphPad (9.0); receiver operating characteristic curve (ROC) curves were plotted using the software MedCalc (19.0.4). Count data were tested using the χ2 test. The t-test was used for data conforming to a positive terrestrial distribution; the Mann–Whitney test was not used for a positive-terrestrial distribution. The ROC curves for the parameter diagnoses were tested by using the Z-test. Logistic regression analysis was performed on multivariate regression analysis of patients’ mortality. Differences in results were considered statistically significant when P < .05.
3. Results
3.1. Baseline Data Between nonDeath Group and Death Group
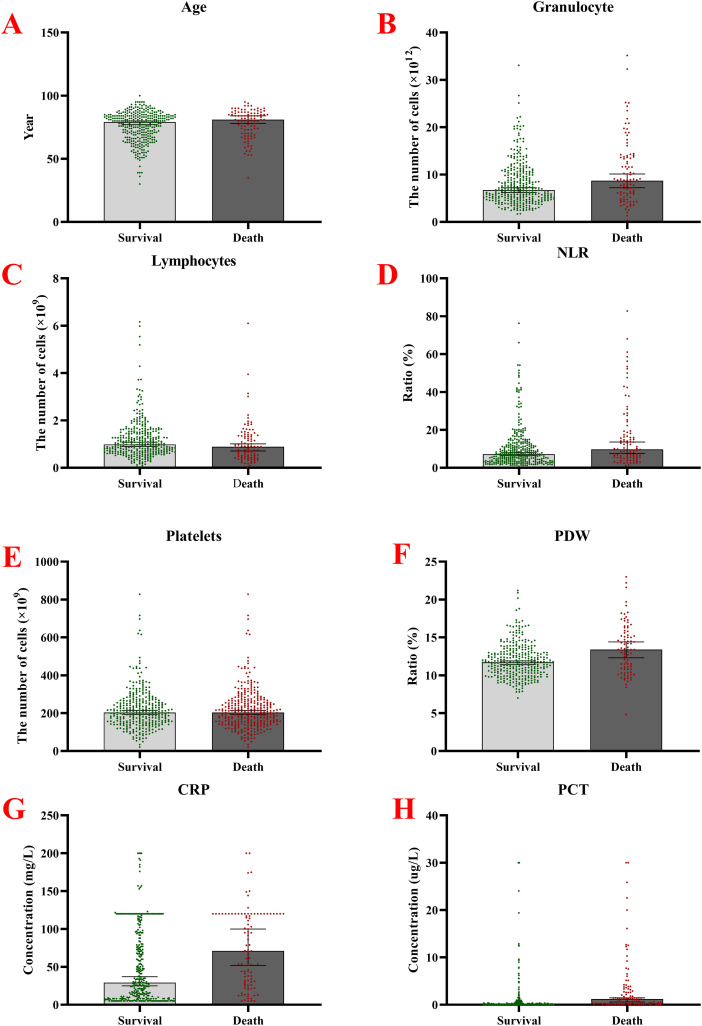
A total of 431 infected patients were included in this study, of which 332 patients (77.03%) were in the nondeath group, and 99 patients (22.97%) were in the death group. Analysis of the patients’ baseline data showed that the patients’ age in the death group was higher than that in the nondeath group, and the difference was statistically significant (P = .047) (Figure 1). The statistics of the patients’ infection sites showed that there were 383 cases of chest infection and 78 deaths (mortality rate is 20.37%); 40 cases of abdominal disease and 16 deaths (mortality rate is 40.00%); the statistical difference between the three groups was statistically significant (P = .001). The results of testing whether the patients were septic or not showed that the mortality rate was higher in patients with sepsis, and the statistical difference was significant (11/21 vs 88/410, P = .003). Patients in the death group had higher sepsis-related organ failure score (SOFA) scores than those in the nondeath group (P < .001). In gender and pneumonia, there was no statistically significant difference between the two groups (P > .05, respectively). More details are shown in Table 1.
Figure 1.
Comparison of baseline information between patients in the infection death group and the non-death group. A. Age; B. The number of granulocytes; C. The number of lymphocytes; D. NLR; E. The number of platelets; F. The PDW; G. Measured CPR; H. Measured values of PCT.
Abbreviations: NLR, Neutrophil-to-Lymphocyte ratio; PDW, platelet distribution width; CPR, C-reactive protein values; PCT, procalcitonin.
Table 1.
The Baseline Data Between the Survival Group and Death Group.
| Factor | Survival group (332) | Death group (99) | Z/X2 | P |
|---|---|---|---|---|
| Age (year) | 79 (67, 84) | 81 (70, 86) | −1.990 | .047 |
| Gender (male, %) | 195 (58.91%) | 53 (53.54%) | 0.903 | .342 |
| Site of infection | ||||
| Chest | 305 (91.87%) | 78 (78.79%) | 13.538 | .001 |
| Abdomen | 24 (7.23%) | 16 (16.16%) | ||
| Others | 3 (0.90%) | 5 (5.05%) | ||
| Severe pneumonia | ||||
| Yes | 10 (3.01%) | 6 (6.06%) | 1.222 | .269 |
| No | 322 (96.99%) | 93 (93.94%) | ||
| Sepsis | ||||
| Yes | 10 (3.01%) | 11 (6.06%) | 9.116 | .003 |
| No | 322 (96.99%) | 88 (93.94%) | ||
| Granulocyte ( × 1012) | 6.73 (4.85, 10.34) | 8.64 (5.84, 13.64) | −2.951 | .003 |
| Lymphocytes ( × 109) | 1.01 (0.68, 1.49) | 0.90 (0.49, 1.34) | −2.358 | .018 |
| NLR (%) | 7.12 (3.60, 11.93) | 9.78 (5.60, 18.80) | −3.558 | <.001 |
| Platelets ( × 109) | 205.00 (153.00, 264.00) | 177.19 ± 99.36 | −4.629 | <.001 |
| PDW (%) | 11.70 (10.30, 13.00) | 13.51 ± 3.34 | −4.338 | <.001 |
| CRP (mg/L) | 29.60 (9.00, 91.93) | 70.50 (27.65, 120.00) | −4.693 | <.001 |
| PCT (ug/L) | 0.050 (0.050, 0.370) | 1.195 (0.188, 3.283) | −8.176 | <.001 |
| R (mm) | 6.10 (5.00, 7.30) | 6.15 (4.83, 8.50) | −0.785 | .433 |
| K (mm) | 1.50 (1.30, 2.00) | 1.80 (1.30, 2.78) | −2.987 | .003 |
| Angle ( 。 ) | 68.85 (62.38, 72.70) | 66.30 (54.98, 71.80) | −2.409 | .016 |
| MA (mm) | 62.90 (58.40, 67.30) | 60.20 (50.15, 67.58) | −2.940 | .003 |
| CI | 0.50 (−1.10, 1.70) | −0.30 (−3.30, 1.68) | −2.428 | .015 |
| TMA (mm) | 24.70 (22.00, 27.30) | 24.30 (22.70, 28.23) | −.770 | .441 |
| E | 169.50 (140.20, 205.70) | 151.30 (100.63, 208.75) | −2.934 | .003 |
| TPI | 57.95 (35.15, 85.63) | 42.30 (15.20, 71.00) | −3.289 | .001 |
| SOFA | 2.00 (1.00, 3.00) | 3.00 (2.00, 5.00) | −.162 | <.001 |
Abbreviations: CRP: C-reactive protein; PCT: procalcitonin; NLR: neutrophil to lymphocyte ratio; PDW: platelet distribution width; TPI, platelet kinetic index; TMA, time to MA; CI, coagulation index; SOFA: sepsis-related organ failure score. Bold: means the difference was statistically significant.
The results of blood cells and related inflammatory factors showed that the expression of granulocytes, neutrophil-to-lymphocyte ratio (NLR), PDW, CRP, and PCT was higher in the death group than in the nondeath group (all P values < .05, respectively). In comparison, lymphocytes and platelets’ expression was lower in the death group than in the nondeath group, and the differences were statistically significant (all P values were <.05, respectively) (Figure 1).
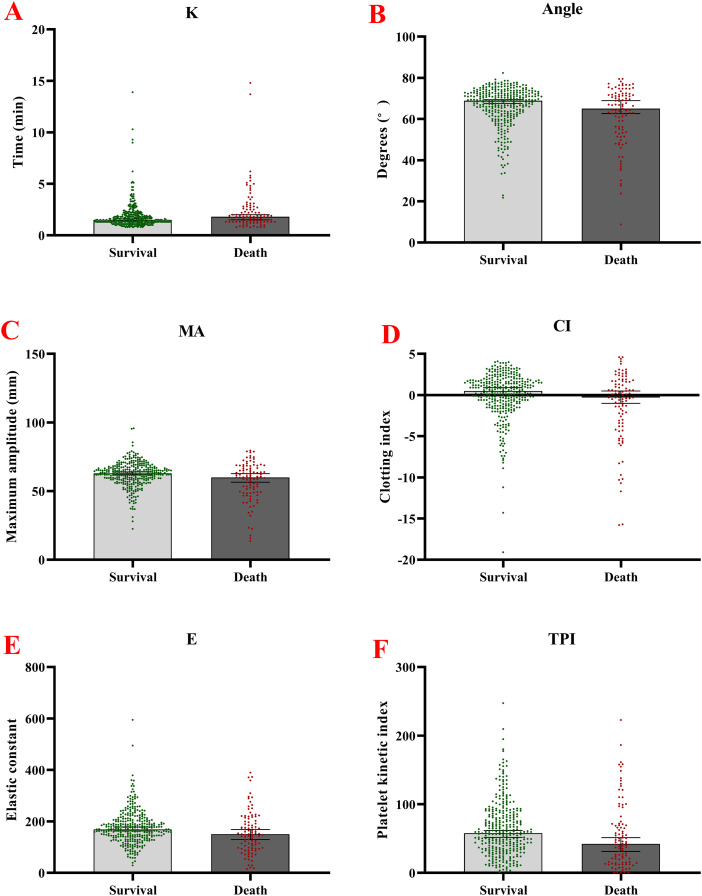
TEG parameters showed the expression of K, α-Angle, MA, CI, and TPI in the death group was statistically significantly lower than in nondeath group (all P values <.05). In contrast, there was no statistical difference between the two groups for R and TMA (all P values > .05) (Figure 2).
Figure 2.
Comparison of the TEG parameter between patients in the infection death group and the nondeath group. A. Measured K values; B. Measured α-Angle values; C. Measured MA values; D. Measured CI values. E. Measured E values. F. Measured TPI values.
Abbreviations: TEG, thromboelastography; TPI, platelet kinetic index; CI, index of coagulation.
3.2. The Correlation Between CRP, PCT, SOFA Score, and TEG Parameters
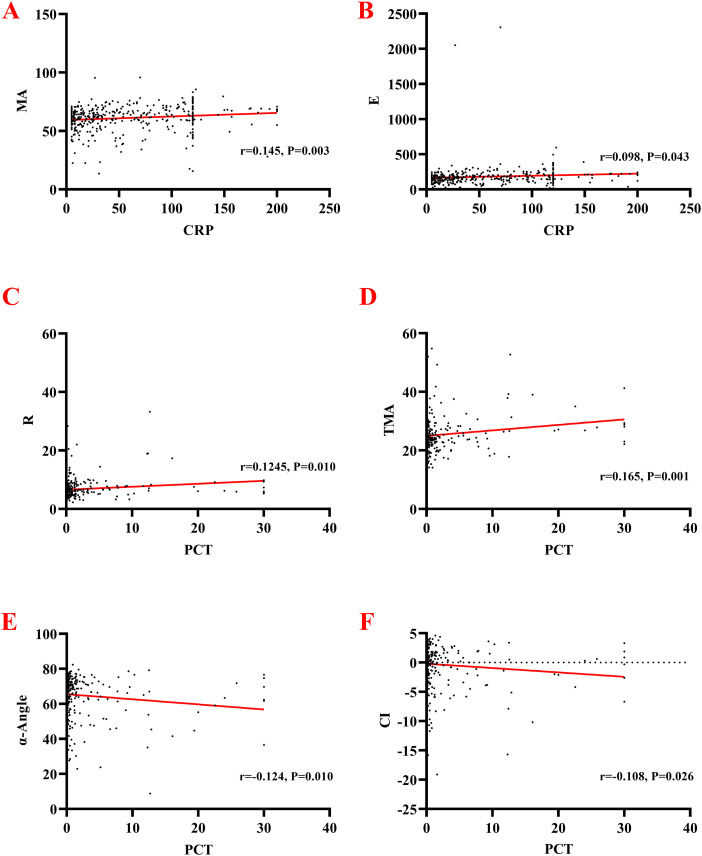
In the correlation study between CRP, PCT, and SOFA with each TEG parameter, CRP showed a low-level positive correlation with MA (r = 0.145, P = .003) and E (r = 0.098, P = .043), respectively, and the differences were all statistically significant. Simultaneously, there was no correlation between CRP and R, K, α-Angle, CI, TMA, TPI (all P values > .05, respectively).
The PCT detection result showed a low-level positive correlation with R (r = 0.124, P = .010) and TMA (r = 0.165, P = .001), respectively. PCT testing showed a low-level negative correlation with α-Angle (r = 0.124, P = .010) and CI, respectively (r = −0.108, P = .026). At the same time, the differences between PCT and K, MA, E, TPI were not statistically significant (all P values > .05, respectively). More details are shown in Table 2 and Fig. 3. SOFA scores were positively correlated with R, K, and TMA; and negatively associated with Angle, MA, CI, E, and TPI (all P values > .05, respectively). More details are shown in Table 2.
Table 2.
The Correlation Between CRP, PCT, and TEG Parameters.
| TEG Parameters | CRP | PCT | SOFA | |||
|---|---|---|---|---|---|---|
| Cor. | P | Cor. | P | Cor. | P | |
| R | 0.056 | .249 | 0.124 | .010 | 0.236 | <.001 |
| K | −0.085 | .081 | 0.048 | .325 | 0.262 | <.001 |
| Angle | 0.046 | .341 | −0.124 | .010 | −0.343 | <.001 |
| MA | 0.145 | .003 | −0.031 | .522 | −0.313 | <.001 |
| CI | 0.048 | .324 | −0.108 | .026 | −0.358 | <.001 |
| TMA | −0.020 | .672 | 0.165 | .001 | 0.342 | <.001 |
| E | 0.098 | .043 | −0.010 | .835 | −0.122 | .011 |
| TPI | 0.093 | .055 | −0.020 | .682 | −0.144 | .003 |
Abbreviations: CRP: C-reactive protein; PCT: procalcitonin; TEG, thromboelastography; TMA, time to MA; TPI, platelet kinetic index; CI, coagulation index. Bold: means the difference was statistically significant.
Figure 3.
Correlation analysis between TEG parameter and CRP, and PCT. A. CRP and MA; B. E and MA; C. PCT and R; D. PCT and TMA; E. PCT and α-Angle; F. PCT and CI.
Abbreviations: TEG, thromboelastography; CRP, C-reactive protein; PCT, procalcitonin; TMA, Time to MA; CI, index of coagulation.
Table 3.
The Results of Multivariate Regression Analysis.
| Factor | B | SE | Wald | DOF | Sig. | Exp (B) | 95% CI of EXP(B) | |
|---|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||||
| Age (years) | 0.016 | 0.013 | 1.646 | 1 | 0.199 | 1.016 | 0.991 | 1.042 |
| Gender | 0.308 | 0.281 | 1.203 | 1 | 0.273 | 1.361 | 0.784 | 2.362 |
| Granulocyte | 0.050 | 0.021 | 5.473 | 1 | 0.019 | 1.051 | 1.008 | 1.095 |
| Lymphocyte | 0.001 | 0.182 | <0.001 | 1 | 0.996 | 1.001 | 0.701 | 1.429 |
| Thrombocyte | −0.002 | 0.002 | 1.016 | 1 | 0.313 | 0.998 | 0.995 | 1.002 |
| PDW | 0.145 | 0.054 | 7.260 | 1 | 0.007 | 1.156 | 1.040 | 1.285 |
| Infection site | 8.312 | 2 | 0.016 | |||||
| Abdomen | 0.562 | 0.433 | 1.683 | 1 | 0.194 | 1.754 | 0.751 | 4.097 |
| Others | 2.817 | 1.066 | 6.980 | 1 | 0.008 | 16.727 | 2.069 | 135.209 |
| Severe pneumonia | 0.881 | 0.595 | 2.198 | 1 | 0.138 | 2.415 | 0.753 | 7.744 |
| Sepsis | 1.142 | 0.708 | 2.597 | 1 | 0.107 | 3.132 | 0.781 | 12.555 |
| CRP | 0.006 | 0.003 | 3.730 | 1 | 0.053 | 1.006 | 1.000 | 1.011 |
| PCT | 0.032 | 0.031 | 1.066 | 1 | 0.302 | 1.032 | 0.972 | 1.096 |
| SOFA | 0.347 | 0.097 | 12.709 | 1 | <0.001 | 1.415 | 1.169 | 1.713 |
| Constant | −7.340 | 1.676 | 19.177 | 1 | <0.001 | 0.001 | ||
Abbreviations: SE: Standard error; DOF: Degree of freedom; PDW: Platelet distribution width; CRP: C-reactive protein; PCT: Procalcitonin. Bold: means the difference was statistically significant.
3.3. The Results of Multivariate Regression Analysis
The multivariate analysis showed the granulocytes, PDW, SOFA score, and infection site are independent factors that impact the infected patients’ mortality. Among the parameters, the increased number of granulocytes, high PDW and SOFA score, and infection site (abdomen) contributed to infected patients’ death (P values of .019, .007, <.001, and .008, respectively).
3.4. The Diagnosis Results of TEG, CRP, PCT, and SOFA
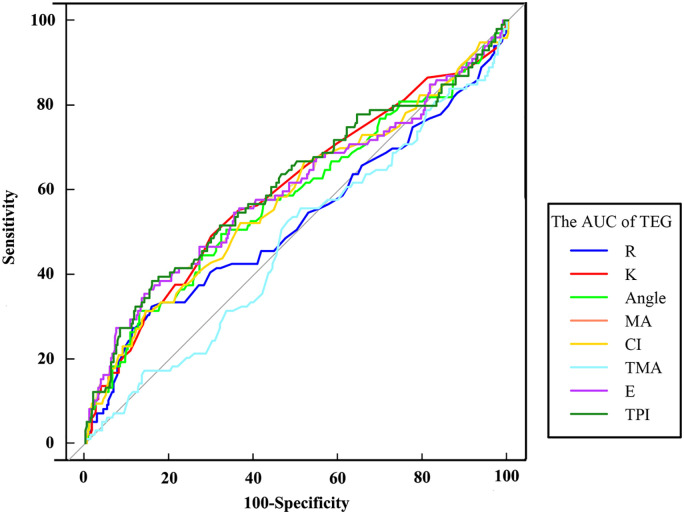
We used eight indices of TEG for the prediction of death in patients. The results showed that TPI had the best diagnostic value with an area under the curve, AUC, being 0.609, sensitivity 38.38%, and specificity 84.24% (Z = 3.079, P = .002). Among all the indicators, the highest diagnostic of sensitivity (48.96%) was the K indicator (Z = 2.848, P = .004), and the best specificity (94.55%) of the TEG parameter was for TMA, but the AUC curve results were not statistically significant (Z = 0.750, P = .453). (Table 4 and Figure 4)
Table 4.
The Diagnostic results of the Thromboelastography Factor.
| Factor | AUC | SE | 95% CI | Sensitivity | Specificity | Youden | AC | Z | P |
|---|---|---|---|---|---|---|---|---|---|
| R | 0.526 | 0.037 | 0.478–0.574 | 32.32 | 84.34 | 0.167 | 7.8 | 0.707 | .480 |
| K | 0.600 | 0.035 | 0.552–0.647 | 48.96 | 70.30 | 0.193 | 1.8 | 2.848 | .004 |
| Angle | 0.580 | 0.035 | 0.531–0.627 | 31.31 | 87.27 | 0.186 | 56.3 | 2.255 | .024 |
| MA | 0.597 | 0.036 | 0.549–0.644 | 35.35 | 86.06 | 0.214 | 54.2 | 2.709 | .007 |
| CI | 0.581 | 0.036 | 0.533–0.629 | 31.25 | 85.76 | 0.170 | −2.1 | 2.283 | .022 |
| TMA | 0.525 | 0.034 | 0.477–0.574 | 14.14 | 94.55 | 0.087 | 31.6 | 0.750 | .453 |
| E | 0.597 | 0.036 | 0.549–0.644 | 35.35 | 86.06 | 0.214 | 118.3 | 2.704 | .007 |
| TPI | 0.609 | 0.035 | 0.561–0.655 | 38.38 | 84.24 | 0.226 | 27.5 | 3.079 | .002 |
Abbreviations: AUC: Area Under Curve; SE: Standard Error; AC: Associated criterion; CI, coagulation index; TMA, time to MA; TPI, platelet kinetic index.
Figure 4.
The AUC diagnostic results of the TEG parameter.
Abbreviations: AUC, area under the curve; TEG, thromboelastography.
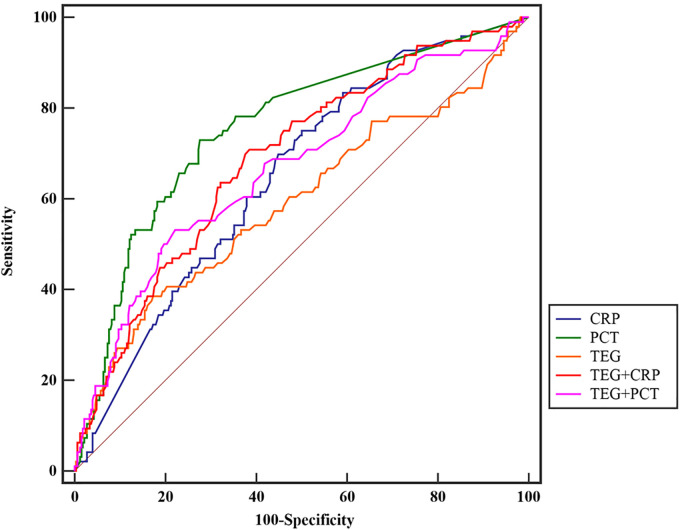
Subsequently, we combined the eight indicators of TEG into a model used to make an overall prediction of death in infected patients. Afterwards, we performed diagnostic tests for CRP, PCT, SOFA score, TEG model, TEG model + CRP, and TEG model + PCT for diagnostic comparison. The results showed that PCT predicted death in infected patients better patients with an AUC of 0.756, the sensitivity of 72.73%, and specificity of 72.29% (Z = 9.187, P < 0.001), and the predictive ability of this factor was better than the TEG model (AUC = 0.593) and CRP (AUC = 0.655). The highest diagnostic sensitivity element was CRP (75.76%), and the highest diagnostic specificity was TEG (82.73%). On the contrary, when TEG was combined with PCT and CRP, the diagnostic effect was lower than each index’s results alone. Detailed results are shown in Table 5 and Figure 5.
Table 5.
Diagnosis Results Between Different Combination Factors.
| Factor | AUC | SE | 95% CI | Sensitivity | Specificity | Youden | AC | Z | P |
|---|---|---|---|---|---|---|---|---|---|
| CRP | 0.655 | 0.030 | 0.608–0.700 | 75.76 | 50.30 | 0.261 | 29.3 | 5.225 | <.001 |
| PCT | 0.756 | 0.028 | 0.712–0.795 | 72.73 | 72.29 | 0.450 | 0.27 | 9.187 | <.001 |
| TEG | 0.593 | 0.036 | 0.545– 0.640 | 38.54 | 82.73 | 0.213 | −2.15 | 2.578 | .010 |
| TEG + CRP | 0.689 | 0.031 | 0.643–0.733 | 70.83 | 61.52 | 0.324 | −1.41 | 6.194 | <.001 |
| TEG + PCT | 0.669 | 0.033 | 0.622–0.713 | 53.13 | 77.88 | 0.310 | −1.27 | 5.119 | <.001 |
| SOFA | 0.699 | 0.030 | 0.654–0.742 | 69.70 | 59.04 | 0.287 | 2.00 | 6.634 | <.001 |
Abbreviations: AUC, Area under the Curve; SE, Standard error; AC, Associated criterion; CRP, C-reactive protein; PCT, Procalcitonin; TEG, Thromboelastogram.
Figure 5.
The result of the AUC between the TEG model, CPR, CRP, and SOFA scores.
Abbreviations: AUC, area under the curve; TEG, thromboelastography; CRP, C-reactive protein, CPR, C-reactive protein values.
4. Discussion
Infected patients are prone to coagulation dysfunction and abnormalities caused by an insufficient synthesis of anticoagulants and procoagulants in the patient’s body. 25 Since conventional coagulation tests can only detect procoagulant features, when patients are given unreasonable plasma infusion during the treatment process, it can disrupt the coagulation homeostasis and aggravate the condition. TEG can characterize the dynamic coagulation profile of patients within a short time, detect high and low coagulation status of blood and fibrinolysis, and simulate the whole process of human coagulation. 26 Therefore, TEG has a high clinical guidance value.
In this study, we used TEG in combination with CRP and PCT to diagnose and evaluate death in infected patients, focusing on the correlation between TEG parameters and the occurrence of death in infected patients and providing implications for the prognosis of infected patients in clinical settings, was mainly correlated with activated partial thromboplastin time, prothrombin time, and fibrinogen (Fbg), but not with prothrombin time. This study showed no statistical difference between the R values of the survival and death groups (P = .433). Both groups’ median values were within the normal range, indicating that the infected patients’ coagulation function was not abnormal and the risk of thrombosis was not elevated. The correlation study between R and PCT showed a synergistic effect (r = 0.124, P = .010). K-value and α-Angle are mainly used to respond to Fbg and platelet function, and a decrease in the levels of both suggests the increase in K value reflects the increase in the rate of clot formation. The K value length is mostly influenced by the Fbg’s level, while the platelet function has less influence. Factors affecting the prolongation of K-values include (1) anticoagulant use; (2) platelet abnormalities; and (3) coagulation factor deficiency disorders. In this study, the k value was higher in the death group than the nondeath group, and the difference between the two groups was statistically significant (P = .003). α-Angle is the result of the combined action of fibrin and platelets at the onset of clot formation. α-Angle and K-value are closely related, reflecting the clot aggregation rate, and the same factors affect α-Angle and K-value. 27 When the blood clot is highly hypocoagulated, the clot amplitude does not reach 20 mm, and the K value cannot be determined at this time.28–30 Therefore, the α-Angle is more valuable than the K value. In this study, α-Angle decreased in the death group compared to the patients’ non-death group, and the difference between the two groups was statistically significant (P = .016). The correlation study between α-Angle and PCT showed a weak negative correlation (r = −0.124, P = .010). The number of platelets was significantly lower in the death group than in the nondeath group (P < .001). The above study shows that it is clear that both α-Angle and platelet counts were lower in the death group than in the nondeath group, while K values were higher than in the nondeath group. Although all three indicators were statistically different between the two groups, all values were within each indicator’s reference values. The MA reflects the fibrin/platelet clot’s maximum strength and is influenced by platelet and Fbg factors. 31 (1) When the MA decreases, the patient’s blood is diluted and prone to bleeding; (2) the patient’s body is deficient or depleted in clotting factors; and (3) abnormalities in the quality or quantity of platelets occur.32,33 When MA increases, it means that the patient’s blood is in a hypercoagulable state and is prone to arterial and venous thrombosis. This study showed that MA was lower in patients in the infection death group than in the nondeath group, and the difference was statistically significant (P = .003). Since the median MA was within the normal interval in both groups, this implies that patients in the infected death group had a higher propensity to bleed than those in the nondeath group. And there was a synergistic effect of elevated MA and CRP expression in patients in the death group (r = 0.145, P = .003). However, TMA (Time to MA) assay results for the time taken for blood to clot from the onset of clotting to MA values’ determination showed no statistical difference between the two groups (P = .441). The correlation study between TMA and PCT showed a weak positive correlation (r = 0.165, P = .001). These study results are the opposite of Sharma et al. 27 in a study of thromboelastography for DIC assessment. Their findings showed higher R and K times (within normal values), lower α-Angle, and MA length in the DIC group than in the non-DIC group. Their results using the TEM indicators for diagnosing DIC showed that the sensitivity of the TEM indicators for the diagnosis of DIC was better. The CI is a composite index of coagulation, which can reflect the overall coagulation status of the body. This study showed that the CI values of patients in the death group were lower than those in the nondeath group. The difference between the two groups was statistically significant (P = .015). However, the CI of both groups was within the normal reference range, which reflected the lower ability of patients in the infected death group to clot than in the nondeath group. The correlation study between CI and PCT showed a weak negative correlation (r = −0.108, P = .010). E is the elasticity constant, and MA can calculate the maximum amplitude of E through the formula. The value of E in the death group in this study was lower than that in the nondeath group, and the difference between the two groups was statistically significant (P = .003). This study is consistent with the results of the MA test. TPI is a platelet kinetic index, an index used to describe the clot’s kinetic potential.34,35 There was a statistically significant difference in TPI’s comparison between the two groups in this study (P = .001). The TPI level was significantly higher than the reference range (6–15) in both groups. The other performance of the TEG assay indicators proved that the infected patients had abnormal coagulation function.
We then did a diagnostic analysis of several TEG factors related to death in infected patients. The results showed that all the indicators had a low sensitivity (the highest was K value, with a sensitivity of only 48.96%). Still, all these factors had a high diagnostic specificity (Table 4). We then combined all the indicators into a diagnostic model and combined CRP and PCT for further diagnostic treatment. The results showed that the model combined with both hands had the average diagnostic effect (PCT was the indicator with the largest AUC area, CRP was the indicator with the best sensitivity, and the TEG model was the best specificity). The above study showed that although each TEG indicator’s specificity and the combined model were high in predicting patient death, the sensitivity was low. Besides, the TEG model combined with CRP and PCT was less effective.
Multivariate regression analysis showed that increased granulocyte count, increased PDW values, SOFA score, and abdominal infection were independent risk factors for poor prognosis in infected patients. Thrombocytopenia is associated with increased mortality in patients in the intensive care unit (ICU). A study by Cengizhan Sezgi et al. 36 in 175 patients treated in the ICU showed that levels of leukocytes, mean platelet volume (MPV), and PDW decreased in patients in the nondeath group compared to admission levels; in the death group, admission MPV and PDW levels increased while levels of platelet count decreased. Zhang et al. 37 showed that the platelet index is a new predictor of in-hospital mortality in ICU patients; higher MPV and PDW were associated with an increased risk of patient death, while a decrease in plateletcrit was associated with an increased risk of death. These studies are consistent with our findings. The multivariate regression analysis shows that the infected patients cause an inflammatory response in the body and at the same time trigger the development of disorders of coagulation balance in the patient’s body. Therefore, this study provides a new perspective on infected patients’ clinical prognosis by analyzing baseline data of the detection of each parameter of TEG, CRP, and PCT from 431 patients with acute infections and using TEG to explore the relationship between patients’ coagulation function and death.
Acknowledgements
This study was supported by Shijingshan District Medical Key Support Specialty Construction.
Footnotes
Authors’ Contributions: JCX and BW contributed to the conception and design of the study; JCX and JYW performed the experiments, collected and analyzed data; JCX and BW wrote the manuscript. All authors reviewed and approved the final version of the manuscript.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The study protocol was approved by the Ethics Committee of Beijing Chaoyang Hospital (No. 2016-KE-143).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed Consent: All patients or their families agreed to the thromboelastography test and signed an informed consent form.
ORCID iD: Bing Wei https://orcid.org/0000-0002-9740-2086
Reference
- 1.Chang RYK, Wallin M, Lin Y, et al. Phage therapy for respiratory infections. Adv Drug Delivery Rev. 2018;133:76‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leber AL, Everhart K, Balada-Llasat JM, et al. Multicenter evaluation of BioFire FilmArray meningitis/encephalitis panel for detection of Bacteria, viruses, and yeast in cerebrospinal fluid specimens. J Clin Microbiol. 2016;54(9):2251‐2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erickson AK, Jesudhasan PR, Mayer MJ, et al. Bacteria facilitate enteric virus Co-infection of mammalian cells and promote genetic recombination. Cell Host Microbe. 2018;23(1):77‐88. e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu D, Taylor P, Fletcher D, et al. Interleukin-17 pathophysiology and therapeutic intervention in cystic fibrosis lung infection and inflammation. Infect Immun. 2016;84(9):2410‐2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris G, Bortolasci CC, Puri BK, et al. Preventing the development of severe COVID-19 by modifying immunothrombosis. Life Sci. 2021;264(118617). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mussbacher M, Salzmann M, Brostjan C, et al. Cell type-specific roles of NF-kappaB linking inflammation and thrombosis. Front Immunol. 2019;10(85). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seymour CW, Kennedy JN, Wang S, et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA. 2019;321(20):2003‐2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasegawa D, Nishida O. Individualized recombinant human thrombomodulin (ART-123) administration in sepsis patients based on predicted phenotypes. Crit Care. 2019;23(1):231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grover SP, Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol. 2018;38(4):709‐725. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Yin M, Zhang H, et al. BMX Represses thrombin-PAR1-mediated endothelial permeability and vascular leakage during early sepsis. Circ Res. 2020;126(4):471‐485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matowicka-Karna J. Markers of inflammation, activation of blood platelets and coagulation disorders in inflammatory bowel diseases. Postepy Hig Med Dosw (Online. 2016;70:305‐312. [DOI] [PubMed] [Google Scholar]
- 12.Chang JC. Sepsis and septic shock: endothelial molecular pathogenesis associated with vascular microthrombotic disease. Thromb J. 2019;17(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad MS, Waheed A. Platelet counts, MPV and PDW in culture proven and probable neonatal sepsis and association of platelet counts with mortality rate. J Coll Physicians Surg Pak. 2014;24(5):340‐344. [PubMed] [Google Scholar]
- 14.Hartert H. Blood clotting studies with thrombus stressography; a new investigation procedure. Klin Wochenschr. 1948;26(37-38):577‐583. [DOI] [PubMed] [Google Scholar]
- 15.Liu Z, Chai E, Chen H, et al. Comparison of thrombelastography (TEG) in patients with acute cerebral hemorrhage and cerebral infarction. Med Sci Monit. 2018;24:6466‐6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mallett SV. Clinical utility of viscoelastic tests of coagulation (TEG/ROTEM) in patients with liver disease and during liver transplantation. Semin Thromb Hemost. 2015;41(5):527‐537. [DOI] [PubMed] [Google Scholar]
- 17.Hunt H, Stanworth S, Curry N, et al. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma induced coagulopathy in adult trauma patients with bleeding. Cochrane Database Syst Rev. 2015;2:CD010438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.da Rocha EC V, D'Amico EA, Caldwell SH, et al. A prospective study of conventional and expanded coagulation indices in predicting ulcer bleeding after variceal band ligation. Clin Gastroenterol Hepatol. 2009;7(9):988‐993. [DOI] [PubMed] [Google Scholar]
- 19.Rigouzzo A, Louvet N, Favier R, et al. Assessment of coagulation by thromboelastography during ongoing postpartum hemorrhage: a retrospective cohort analysis. Anesth Analg. 2020;130(2):416‐425. [DOI] [PubMed] [Google Scholar]
- 20.George MJ, Burchfield J, MacFarlane B, et al. Multiplate and TEG platelet mapping in a population of severely injured trauma patients. Transfus Med. 2018;28(3):224‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rai R, Tuddenham E, Backos M, et al. Thromboelastography, whole-blood haemostasis and recurrent miscarriage. Hum Reprod. 2003;18(12):2540‐2543. [DOI] [PubMed] [Google Scholar]
- 22.Bolliger D, Seeberger MD, Tanaka KA. Principles and practice of thromboelastography in clinical coagulation management and transfusion practice. Transfus Med Rev. 2012;26(1):1‐13. [DOI] [PubMed] [Google Scholar]
- 23.Standage SW, Wong HR. Biomarkers for pediatric sepsis and septic shock. Expert Rev Anti Infect Ther. 2011;9(1):71‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nath SR, Jayapalan S, Nair H, et al. Comparative diagnostic test evaluation of serum procalcitonin and C-reactive protein in suspected bloodstream infections in children with cancer.. J Med Microbiol. 2017;66(5):622‐627. 10.1099/jmm.0.000478.Epub2017May15.PMID:28504925. [DOI] [PubMed] [Google Scholar]
- 25.Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58(7):1116‐1120. [DOI] [PubMed] [Google Scholar]
- 26.Akay OM. The double hazard of bleeding and thrombosis in hemostasis from a clinical point of view: a global assessment by rotational thromboelastometry (ROTEM). Clin Appl Thromb Hemost. 2018;24(6):850‐858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou W, Zhou W, Bai J, et al. TEG In the monitoring of coagulation changes in patients with sepsis and the clinical significance. Exp Ther Med. 2019;17(5):3373‐3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shamseddeen H, Patidar KR, Ghabril M, et al. Features of blood clotting on thromboelastography in hospitalized patients With cirrhosis. Am J Med. 2020;133(12):1479‐1487. e1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie X, Wang M, Lu Y, et al. Thromboelastography (TEG) in normal pregnancy and its diagnostic efficacy in patients with gestational hypertension, gestational diabetes mellitus, or preeclampsia. J Clin Lab Anal. 2021;35(2):e23623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilic Y, Topcu I, Bambal H, et al. Thromboelastography in the evaluation of coagulation disorders in patients with sepsis. Turk J Med Sci. 2014;44(2):267‐272. [DOI] [PubMed] [Google Scholar]
- 31.van Rooyen LJ, Hooijberg EH, Schoeman JP, et al. Thromboelastographic platelet mapping in dogs with complicated Babesia rossi infection. Vet Clin Pathol. 2019;48(1):11‐18. [DOI] [PubMed] [Google Scholar]
- 32.Kreutz RP, Schmeisser G, Schaffter A, et al. Prediction of ischemic events after percutaneous coronary intervention: thrombelastography profiles and factor XIIIa activity. TH Open. 2018;2(2):e173‐e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kornblith LZ, Kutcher ME, Redick BJ, et al. Fibrinogen and platelet contributions to clot formation: implications for trauma resuscitation and thromboprophylaxis. J Trauma Acute Care Surg. 2014;76(2):255‐256. discussion 262-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersen NG, Mowinckel MC, Sunde K, et al. Utility of coagulation analyses to assess thromboprophylaxis with dalteparin in intensive care unit patients. Acta Anaesthesiol Scand. 2020. [DOI] [PubMed] [Google Scholar]
- 35.Lu S, Kang R, Wang Y, et al. Altered TEG parameters identify hypercoagulablilty and are of diagnosis value for papillary thyroid carcinoma patients. Exp Clin Endocrinol Diabetes. 2020;128(5):297‐302. [DOI] [PubMed] [Google Scholar]
- 36.Sezgi C, Taylan M, Kaya H, et al. Alterations in platelet count and mean platelet volume as predictors of patient outcome in the respiratory intensive care unit. Clin Respir J. 2015;9(4):403‐408. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z, Xu X, Ni H, et al. Platelet indices are novel predictors of hospital mortality in intensive care unit patients. J Crit Care. 2014;29(5):885. e881-886. [DOI] [PubMed] [Google Scholar]