Abstract
Citrus grandis or Citrus maxima, widely recognized as Pomelo is widely cultivated in many countries because of their large amounts of functional, nutraceutical and biological activities. In traditional medicine, various parts of this plant including leaf, pulp and peel are used for generations as they are scientifically proven to have therapeutic potentials and safe for human use. The main objective of this study was to review the different therapeutic applications of Citrus grandis and the phytochemicals associated with its medicinal values. In this article different pharmacological properties like antimicrobial, antitumor, antioxidant, anti-inflammatory, anticancer, antiepileptic, stomach tonic, cardiac stimulant, cytotoxic, hepatoprotective, nephroprotective, and anti-diabetic activities of the plant are highlighted. The enrichment of the fruit with flavonoids, polyphenols, coumarins, limonoids, acridone alkaloids, essential oils and vitamins mainly helps in exhibiting the pharmacological activities within the body. The vitamins enriched fruit is rich in nutritional value and also has minerals like calcium, phosphorous, sodium and potassium, which helps in maintaining the proper health and growth of the bones as well as the electrolyte balance of the body. To conclude, various potential therapeutic effects of Citrus grandis have been demonstrated in recent literature. Further studies on various parts of fruit, including pulp, peel, leaf, seed and it essential oil could unveil additional pharmacological activities which can be beneficial to the mankind.
Keywords: Citrus grandis, Citrus maxima, pomelo, phytomedicine
Background
The connection between human and nature have been prevalent for thousands of years. This connection led to the discovery and utilization of multifarious plants bearing medicinal properties in the treatment and curing of numerous diseases. 1 Medicinal plants usage is as old as the existence of mankind. According to WHO, botanical garden is still a source of reliance of 80% of the global population and many medicines owe their origin to medicinal plants. 2 It has been estimated that for medicinal purposes, up to 74% of pharmacologically active plant derived components were discovered. 3 Despite of having scientific advancement and globalization, medicinal plant based drug discovery is still playing a significant role in the exploration of various pharmacological activities and the discovery of new drugs. 4 In fact, phytomedicines, nutraceuticals, herbal medicines and plant-based different preparations in various dosage forms are extensively investigated by the researchers, herbal and nutraceutical industries aiming to the discovery and development of effective and safe complementary and alternative therapies for the development of medicaments against diabetes, 5 –8 cancer, 9 –11 autoimmune and immunocompromised conditions, 12 –18 inflammation and infectious diseases, 19 –21 hyperlipidemia 22,23 and neurological and adaptogenic problems 24 due to the limitations of conventional drugs, because of the development of drug resistance and adverse effects and chronic toxicities in long term treatment.
This led us to grow further interest to carry out newer studies and analysis on botanical plants for inventing and discovering new methods of treatment of the existing and upcoming diseases.
Plant Introduction
The plant Citrus grandis or Citrus grandis, commonly known as Pomelo, belongs to the family Rutaceae and is considered as the major ancestor of the grapefruit. 25 –27 Citrus grandis, indigenous to China and different areas of Southeast Asia are recognized as the largest fruit because the fully grown fruit can yield more than 30 cm in diameter. 28 The fruit has a variety of name and is constituted of white or pinkish flesh and yellowish or greenish skin. The sweetish acidic flavor makes the citrus fruit delectable among people of different areas. 29 A photograph of a typical C. grandis fruit is presented in Figure 1.
Figure 1.
An illustrated photograph of a typical Citrus grandis (L.) Osbeck fruit.
Plant taxonomy is presented in Table 1.
Table 1.
Taxonomy of Citrus grandis (L.) Osbeck.
| Kingdom | Planteae |
|---|---|
| Subkingdom | Tracheobionta |
| Superdivision | Spermatophyta |
| Division | Magnoliophyta |
| Class | Magnoliopsida |
| Subclass | Rosidae |
| Order | Sapindales |
| Family | Rutaceae Rue family |
| Genus | Citrus L. |
| Species | Citrus grandis (L.) Osbeck |
Scientific Name
Citrus grandis (L.) Osbeck or Citrus maxima.
Common Names
Pomelo, Dangyuja. 30,31 Shaddock. 32,33 Pummelo, 34 –36 Jambola. 25,37,38 Chakota, 39 Mato Peiyu 40 Papanus, 39 Tomentosa, 41 Jamboa, Pompelmoes, Pamplemousse, Bali lemon, Betabi lebu, and Panis.
The edible citrusy fruit, widely cultivated in Bangladesh, India, and East Asia is enriched with different dietary minerals and vitamins. The tree is perennial and is used as a functional food as it is believed to have therapeutic values and it have been used traditionally for indigestion, cardiac diseases etc. 33,42 The pulp of this fruit was found to possess antioxidant, antihyperlipidemic, appetizing, antitoxic, stomach tonic and cardiac stimulant properties. 33,42 –46
Meanwhile, the leaf of Citrus grandis also was found to have antimicrobial, antioxidant. 40 and anticancer properties. 47 Furthermore, the fruit juice prepared from the pulp is rich in antioxidant and polyphenolic compounds such ferulic acid, hesperidin, caffeic acid, naringin, p-coumaric acid, and vanillic acid were reported to be present in the fruit juice. 33,44,48 –51 Current chemical, medical, and pharmacological literature has confirmed various pharmacological activities as well as nutritional value of the indigenous fruit and so a compendious review of the plant is established.
Traditional Uses
Since the beginning of human civilization, traditional medicine has been playing a key role in regulation of human health, prevention and treatment of illness. Even in this era of science and technology, it has become a topic of global interest. Citrus grandis has been preferable in traditional herbal medicine for its numerous healing purposes. In some Asian countries, the Citrus grandis fruits are not just used for consuming but also the other parts of this plants are practiced in folk culture such as leaves oil of Citrus grandis are applied to treat skin disorders, headache and abdominal pain. 40 The assorted sections of this plant are reported to be used extensively by diverse folk populations. The peel of the fruits is widely used in traditional medicines as a treatment of cough, swelling, epilepsy as well as beautification purposes. 52 Similarly, Citrus grandis flowers are used against anxiety and sleep disorders, fruits are used in case of mental abnormality, asthma, leprosy, hiccup, cough, and epilepsy. 41 Due to containing sweet aroma, Citrus grandis fruits are additionally used as food, cosmetics, perfume and flavoring or fragrance-enhancing agent in pharmaceutical industries. 52 –56 Apart from this, Citrus grandis also used to lower cholesterol and induce weight loss. 52,57
Phytochemistry
The fruit of Citrus grandis belongs to the family of citrus fruits which makes it really evident of being rich source of Vitamin C. Shao et al,(2017) determined the presence of Vitamin C by 2,6-dichlorophenolindophenol method. 58 Besides being a provenance for Ascorbic acid, Citrus grandis is also enriched with various active compounds which are very beneficial to health eg: carotenoids, flavonoids, acridone alkaloids, limonoids, minerals, essential oils and Vitamin B complex. 51,59 –61
The pulp and peel of Pomelo appears golden yellow to red or pink color due to the presence of carotenoids. 62 The peel and pulp of citrus fruits contains more than 115 different carotenoids. Singh et al (2016) reported the presence of Aurapte, Auraptene, carotene, 5-Geranyloxy-7-methoxy-coumarin, roseoside, bergamottin in the peel and also, 5-methyltodannol,6-hydroxymethylherniarin, 5-methoxy seselinin the stem bark and roots. 3 Different cultivars of Pomelo were reported to have numerous carotenoids. In “Chuzhou Early Red” and “Yuhuan” pomelo cultivars 11 carotenoids: lycopene, α-carotene, β-carotene, zeta-carotene, β-cryptoxanthin, phytoene, luteoxanthin, phytofluene, lutein, zeaxanthin and 9-cis-violaxanthin were detected. 63 In “Peiyou” and “Wendun” cultivars the major carotenoids detected were: β-carotene, β-cryptoxanthin, lutein and zeaxanthin. 64 Red-fleshed pomelo pulp juice had 7 times more β-carotene and lycopene than its peels. 63 Higher antioxidant, anticancer and hypolipidemic activity is observed due to the presence of high amount of Lycopene and β-carotene. 65
The aqueous ethanolic extract of Citrus grandis peel is mainly composed of flavonoids, Vitamin C and carotenoids, which plays a pivotal role in providing different biological activities like antioxidant, anti-inflammatory, and anti-atherogenic. 26,61 The flavonones present in the peel of Pomelo are mainly in the form of aglycones and glycosides and the frequently identified flavonone aglycones are hesperetin, naringenin and eriodictyol. Nobiletin, tangeretin, sinensetin, diosmetin, luteolin and apigenin are the 6 major frequently reported flavonone aglycones. The most abundant flavonones are naringin, naringenin, neoeriocitrin and neohesperidine. 31,64,66 –69 The presence of common flavonoids like: hesperidin, neohesperidin, naringenin, naringin and rutin are determined at high amount in Citrus grandis juice. 44,70 In an extraction condition for 1 and 3 hours at 50°C, the total yield of total flavonoid content from the peels of Citrus grandis extracted by aqueous methanol extract (80:20 v/v, methanol: water) were 149.05mg/L and 158.09mg/L. This was 12-26% higher when compared with the yield of aqueous ethanol extract (80:20 v/v, ethanol: water) i.e 118.33mg/L and 141.25mg/L respectively. On the other hand, for 1 and 3 hours at 80°C, the yield for aqueous ethanolic extract (80:20 v/v, ethanol: water) were 141.67mg/L and 199.17mg/L. This was 37%-49% higher than that of the yield of aqueous methanol extract (80:20 v/v, methanol: water) i.e. 103.10mg/L and 133.34mg/L respectively. This change in the yield was due to the different boiling points of methanol (65ºC) and ethanol (79ºC). 71 In another study carried out by Caengprasath et al (2013), characterized the presence of active components like neohesperidin (25.4± 0.12 mg/g dried extract), hesperidin, (12.04 ± 0.12 mg/g dried extract), naringin (11.90 ± 0.21 mg/g dried extract), and naringenin (9.20 ± 0.19 mg/g dried extract). 33,42,43
The total phenolic, flavonoids, and flavonoid contents in the peels of the plant and the concentration of total phenolic content ranged from 42.79 to 54.56 mg gallic acid equivalent/g and the total flavonoid content was found within the range of 26.70-13.43 mg of rutin/g. 72 by In the study carried out by Chang and Azrina (2017) the total phenolic content of different C. grandis tissue parts were derived where the amounts were given in descending order; albedo (63.11 ± 5.03 mg GAE g-1 FW) >flavedo (38.67 ± 4.27 mg GAE g-1 FW) >segment membrane (30.66 ± 1.29 mg GAE g-1 FW). 73 Abirami et al (2014) analyzed fresh fruit juices of C. grandis (red and white) and C. hystrix where 333.33-523.21 mg GAE of tannins and 769.05-909.52 mg GAE of phenols were detected and their amounts were highest in C. grandis (white) fruit juice and the amount decreased for C. hystrix followed by C. grandis (red) fruit juices. In addition, the study also provided with the information that C. grandis (white) fruit juice had the higher flavonoid content than the other 2 fruit juices incorporated. The result of the test provided a report that higher content of flavonoid was associated with the presence of higher phenolic content of the fruit juice. 74,75 Pomelo peel was detected to be comprised of various phenolic acids like chlorogenic acid, ferulic acid, caffeic acid, gallic acid, ρ-coumaric acid and sinapic acid. Among them gallic acid was most abundantly found in the peels of various Pomelo cultivars. 64,67 In HPLC-DAD analysis for phenolic compounds in ethanolic extract of C. grandis peel powder, a study performed by Chowdhury et al (2015) a sharp peak for caffeic acid and (-) epicatechin were seen in the chromatogram (concentration of 240.78 and 242.19 mg per 100 g of dry weight respectively) which showed potent antioxidant activity. It is noteworthy that phenolic compounds, for instance syringic acid, gallic acid, vanillic acid, and rutin hydrate were also observed. 50
Citrus grandis can exhibit different therapeutic activity for the presence of particular class of compounds in aqueous, methanolic, and petroleum ether extract, which were indicated by phytochemical screening of the plant. 59,62,76 The ethanolic extract of mesocarp, pericarp and segment membrane of Citrus grandis fruit were prepared, where phenols (25.71 mg CE/100 ml), saponins (345.39 mg CE/100 ml), flavonoids (31.48 mg GAE/100 ml) and tanins (40.47 mg CE/100 ml) were found in the highest amount in the pericarp extract. In the ethanolic extract of segment membrane, the amounts of phenols, saponins, flavonoids and tanins were 18.46 (mg CE/100 ml), 147.93 (mg CE/100 ml), 20.17 (mg GAE/100 ml) and 21.02 (mg CE/100 ml) and in the mesocarp extract the amounts were 13.94 (mg CE/100 ml), 79.03 (mg CE/100 ml), 11.65 (mg GAE/100 ml)) and 7.94 (mg CE/100 ml) respectively. 77 Terpenoids were seen present in mesocarp, pericarp and segment membrane but alkaloids were only present in pericarp and segment membrane. Researchers estimated that white Citrus grandis juice possessed 1-5 folds’ higher amount of terpenoids but lesser amount of total volatiles than pink Citrus grandis juice. 78
Different part of Citrus grandis plant contains different active components, which makes the fruit popular in having various effective biological activities. The 2 available cultivated varieties (cultivars) of Malaysian Citrus grandis juices; white Citrus grandis juice (cultivar PO 51) and pink Citrus grandis juice (cultivar PO 52) were analyzed using gas chromatography mass spectrometer/flame ionization detector to identify volatile and non-volatile compound found in the juice. 78
Polysaccharides are also present in C. grandis which is responsible for different biological activities. In a study of Yu et al (2018), polysaccharides were extracted from C. grandis peel by alcohol precipitation and hot water extraction. 79 Another method for extraction of Microcrystalline cellulose (MCC) and cellulose from C. grandis peel were done by hydrolysis of acid solution and mixed alkaline hydrogen peroxide (H2O2) liquor. 80 The extracted MCC functions as fat substitute, dietary fiber supplement and emulsion stabilizer. 81
Potassium, Phosphorous, vitamin B1, vitamin B2, folic acid, vitamin B12, sugar, water and pectin are also found in the citrus fruit which add more value to it. 25,44 Shao et al, (2017) determined the contents and concentrations of organic acid and soluble sugar by GC(Gas chromatography) and crude fiber by spectrophotometry, Calcium, potassium and magnesium were determined by an approach known as ICP (Inductively coupled plasma). 58 Beside the presence of bioactive compounds in various parts of citrus fruits, they are also rich in a number of mineral elements which can play role in upholding the homeostasis within the body. A study carried out by Czech et al (2020) in analyzing the mineral content in the peel and pulp of citrus fruits found out that Citrus grandis (600 g) contained 9-16% of DRI (dietary reference intake) for phosphorous and its rind (one Citrus grandis fruit of 320 g) contained 30-40% less DRI for phosphorous than pulp. On the other hand, the peel of Citrus grandis contained 7.5% calcium in its peel. Both Calcium and phosphorous are needed to maintain the health of bones as well as help in the bone growth. Sodium and potassium are required to provide balance to electrolyte and water within the body but from the study it was seen that citrus fruits contained less amount of sodium than potassium. Citrus grandis peel was 7-5 times more rich in sodium than its pulp and was found to have 20% more potassium in the peel than the pulp. 82
Apart from Citrus grandis pulp, peel and fruit, Citrus grandis seed also contains active compounds that exhibit biological action. Although Citrus grandis seed is considered as a by-product of the fruit but components like limolin and nomilin can be obtained from its seed that is significant in showing chemo preventive activity. By LC-MS/MS analysis, derivation of limonin (base peak at m/z 425.1) nomilin (base peak at m/z 515), isoobacunoic acid and naringin from segment membranes of C. grandis fruit were done where the elution process was carried out by using binary solvents: formic acid (solvent A) and acetonitrile (solvent B) and analytical identification was done by MRM (Multiple Reaction Monitor) and ESI (electrospray ionization) in negative mode for naringin and positive mode for limonoids. The analysis was done to further identify the already purified compounds obtained previously by semi preparative HPLC analysis where phosphoric acid was used instead of formic acid in the binary solvent system. From the study it was found that HPLC and LC-MS showed purity of 3 limonoids: limonin, nomilin and isoobacunoic acid over 95% and in each purification step the recovery of the target compound was more than 40%. This concludes that high purity limonoids can be obtained from segment membrane of the fruit. 83
In a study of deriving chemical profile of essential oil of C. grandis different components like α-Pinene (0.40%), β-Pinene (3.71%), Sabinine (0.93%), methyl heptenone (1.25%), β-myrcene (0.90%), hexanal (0.12%), t-Ocimine (1.19%), linalool (0.16%), 1-Hexene,3,3-dimethyl (0.67%), geranyl formate (1.83%), geranylacetate (0.82%) and β-farnesene (0.45%) were obtained from the leaves through GC-MS analysis, where the major components were: Z-citral (13.38%), 4-methyl-1-hexene (15.22%), E-citral (17.75%) and DL-limonene (31.83%). It was also found that the essential oil of Citrus grandis was efficacious against Aspergillus flavus. 3 The list of phytochemicals constituents with chemical structures are presented in Table 2.
Table 2.
The List of Phytochemical Constituents in Citrus grandis L. (Osbeck) With Their Chemical Structures.
| IUPAC name | Common name | Chemical structure | Plant part | Reference |
|---|---|---|---|---|
| Tt | Roseoside |
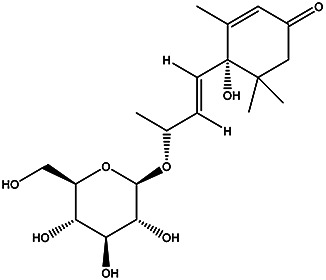
|
Peel | 84 |
| (E)-5-((3,7-dimethylocta-2,6-dien-1-yl)oxy)-7-methoxy-2H-chromen-2-one | 5-geranyloxy-7-methoxycoumarin |
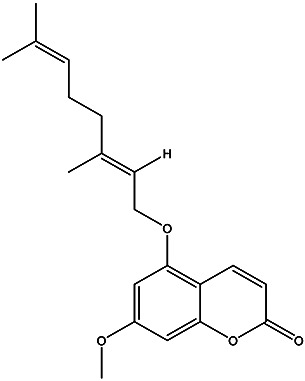
|
Peel | 85 |
| (S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-(((2S,3R,4S,5S,6S)-3,4,5-trihydroxy-6-((((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)methyl)tetrahydro-2H-pyran-2-yl)oxy)chroman-4-one | Hesperidin |
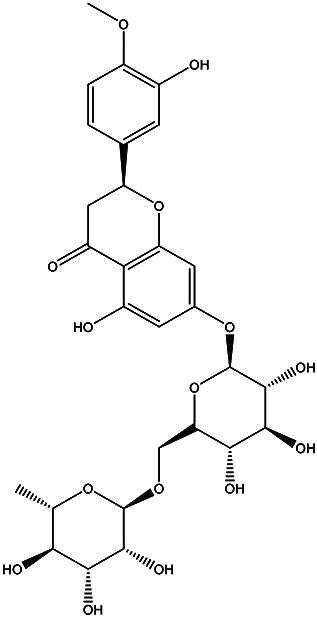
|
Pulp Juice | 86 |
| 1-(4-(((2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-(((2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-pyran-2-yl)oxy)-2,6-dihydroxyphenyl)-3-(3-hydroxy-4-methoxyphenyl)propan-1-one | Neohesperidin dihydrochalcone |
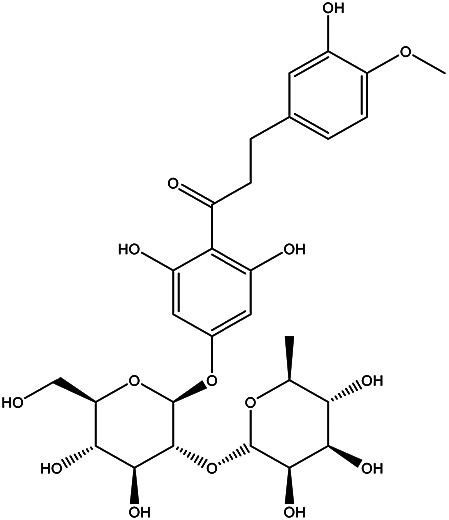
|
Pulp Juice | 87 |
| 8-(2,3-dihydroxy-3-methylbutyl)- 7-methoxy-2H-chromen-2-one |
Meranzin hydrate |
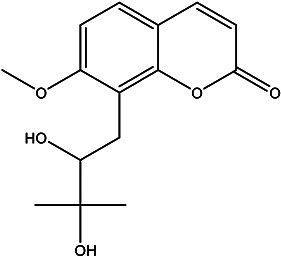
|
Peel | 88 |
| (S)-7-(((2S,3R,4S,5S,6R)-4,5- dihydroxy-6-(hydroxymethyl)-3-(((2S,3R,4R,5R,6S)-3,4,5- trihydroxy-6-methyltetrahydro -2H-pyran-2-yl)oxy)tetrahydro -2H-pyran-2-yl) oxy)-5-hydroxy-2-(4- hydroxyphenyl) chroman-4-one |
Naringin |
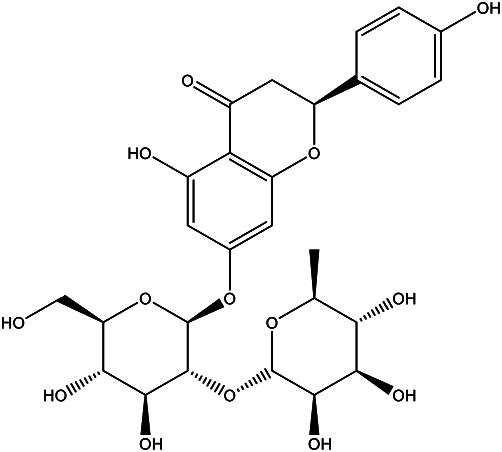
|
Juice | 89 |
| (4aS,6aR,8aR,8bR,9aS,12S,12a S,14aR,14bR)-12-(furan-3-yl) -6,6,8a,12a- tetramethyldecahydro-1H, 3H-oxireno[2,3-d]pyrano [4’,3’:3,3a] isobenzofuro[5,4-f]isochromene-3,8,10(6H,9aH)-trione |
Limonin |
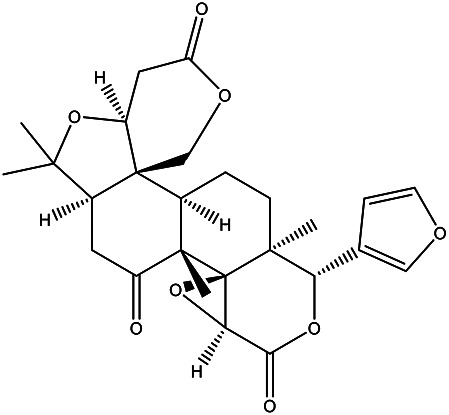
|
Essential oil | 90 |
| (6Z,8E,10E,12Z,14Z,16E,18E, 20E,22E,24E,26E)-2,6,10,14,19,23,27,31-octamethyldotriaconta-2,6,8,10,12,14,16,18,20, 22,24,26,30-tridecaene |
Lycopene |
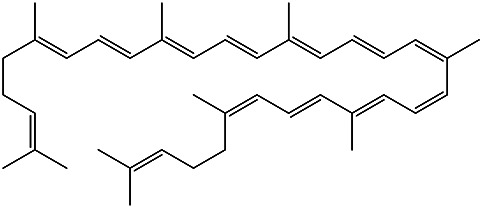
|
Pulp juice and Peel | 91 |
| 2,2’-((1E,3Z,5E,7E,9E,11E, 13E,15E,17E)-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaene-1,18-diyl)bis(1,3,3-trimethylcyclohex-1-ene) |
β-Carotene |
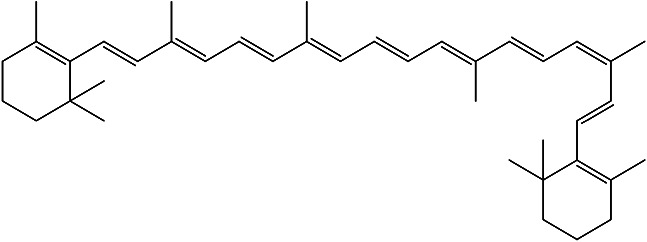
|
Pulp juice and Peel | 92 |
| (E)-7-((3,7-dimethylocta-2,6-dien-1-yl)oxy)-2H-chromen-2-one | Auraptene |
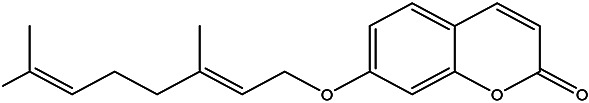
|
Peel | 93 |
| (S)-5-methoxy-6-(2-methoxy-3-methylbut-3-en-1-yl)-2H-chromen-2-one | Toddanone |
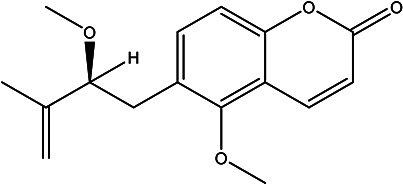
|
Peel | 94 |
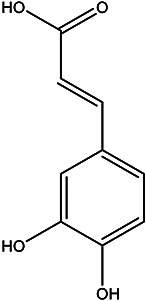
| (E)-3-(3,4-dihydroxyphenyl) acrylic acid |
Caffeic acid |
|
Pulp juice and peel | 95 |
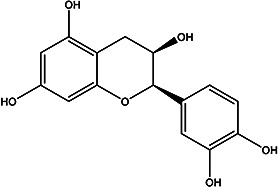
| (2R,3R)-2-(3,4-dihydroxyphenyl) chromane-3,5,7-triol |
Epicatechin |
|
Peel | 96 |
| acridin-9(10H)-one | Acridone (alkaloids) |
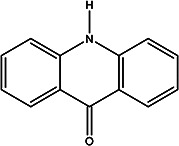
|
Pericarp and segment membrane | 97 |
| (3S,8S,9S,10R,13R,14S,17R)-17-((2S,5R) -5-ethyl-6-methylheptan-2-yl)-10,13- dimethyl-2,3,4,7,8,9,10,11,12,13, 14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-ol |
β-sitosterol |
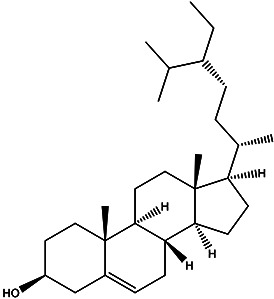
|
98 | |
| (E)-3,7-dimethylocta-2,6-dien-1-ol | Geraniol |
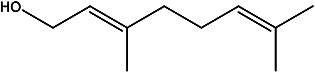
|
Essential oils from leaf | 99 |
| (E)-3-(4-hydroxyphenyl)acrylic acid | p-Coumaric acid |
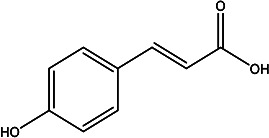
|
Fruit juice | 100 |
| (E)-3-(4-hydroxy-3-methoxyphenyl) acrylic acid |
Ferulic acid |
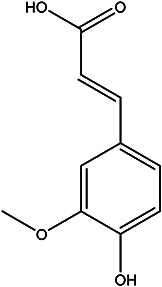
|
Pulp juice and peel | 101 |
| 4-hydroxy-3-methoxybenzoic acid | Vanillic acid |
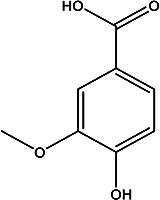
|
Fruit juice and peel | 102 |
| (1S,3aS,4aR,4bR,6aR,11S,11aR, 11bR,13aS)-1-(furan-3-yl)-4b,7,7,11a,13a-pentamethyl-3,5,9-trioxohexadecahydrooxepino [4’,3’:3,4]benzo[1,2-f]oxireno[2,3-d]isochromen-11-yl acetate |
Nomilin |
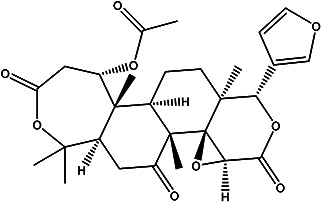
|
Seed | 103 |
| 1-methyl-4-(prop-1-en-2-yl)cyclohex-1-ene | Limonene |
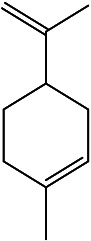
|
Essential oils from leaves | 104 |
| (E)-3,7-dimethylocta-2,6-dienal | E-Citral |
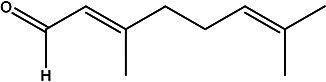
|
Essential oils from leaves | 105 |
| 4-methylhex-1-ene | 4-methyl-1-hexene |
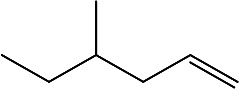
|
Essential oils from leaves | 106 |
| (Z)-3,7-dimethylocta-2,6-dienal | Z-Citral |
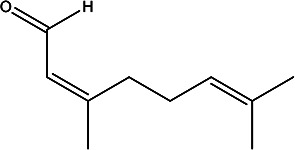
|
Essential oils from leaves | 107 |
| 4-hydroxy-3,5-dimethoxybenzoic acid | Syringic acid |
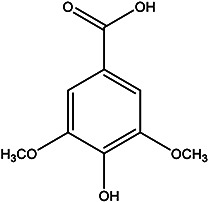
|
Peel | 108 |
| 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-(((2S,3R,4S,5S,6S)-3,4,5-trihydroxy-6-((((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)methyl)tetrahydro-2H-pyran-2-yl)oxy)-4H-chromen-4-one | Rutin hydrate |
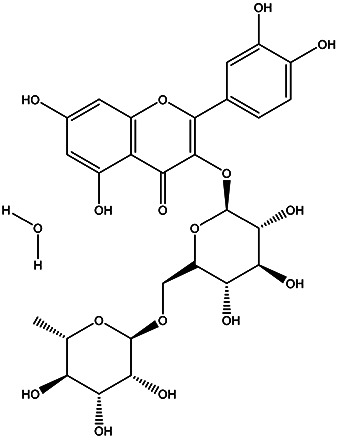
|
Peel | 109 |
| Benzoic acid | Benzoic acid |
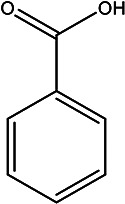
|
Peel | 110 |
| 5,6,7,8-tetramethoxy-2-(4-methoxyphenyl)chromen-4-one | Tangeretin |
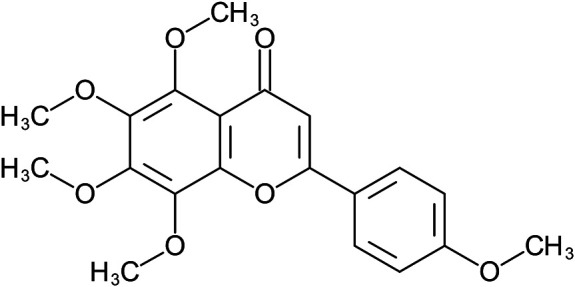
|
Peel | 111 |
| 5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one | Apigenin |
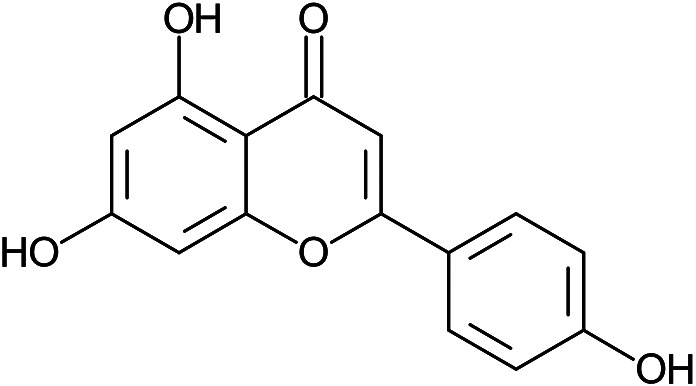
|
Peel | 112 |
| (E)-3-(4-hydroxy-3,5-dimethoxyphenyl)prop-2-enoic acid | Sinapic acid |
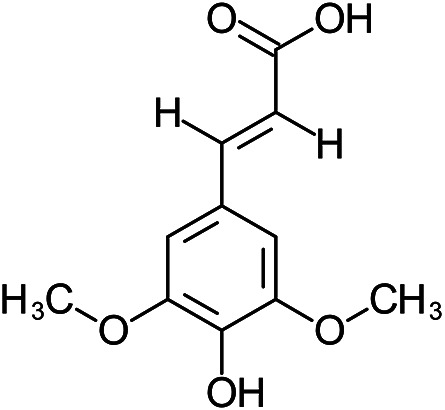
|
Peel | 113 |
| 3’,4’,5,6,7,8-Hexamethoxyflavone 5,6,7,8,3’,4’-Hexamethoxyflavone |
Nobiletin |
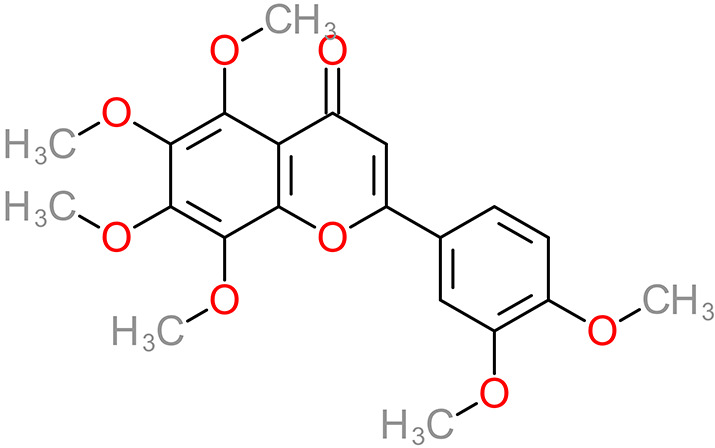
|
Peel | 114 |
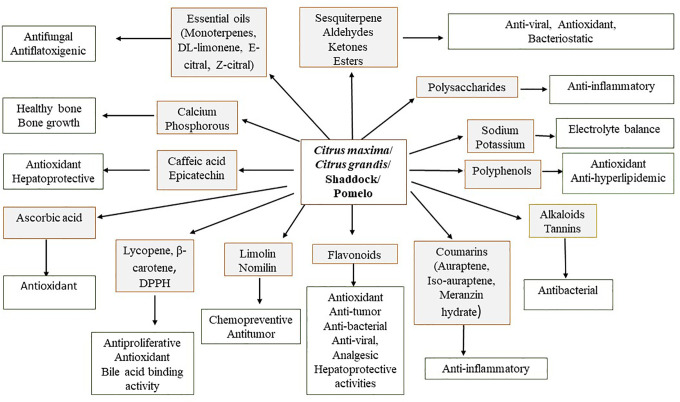
Pharmacological Activities of Citrus grandis
The pharmacological activities of Citrus grandis are presented in the Figure 2.
Figure 2.
Pharmacological activities of Citrus grandis (L.) Osbeck.
Antioxidant Potential
The unstable atoms known as free radicals that are commonly generated within the human body through various biochemical reactions can cause damage to the cells through a method called oxidation, which is linked to causing cancer, atherosclerosis, Parkinson’s disease, Alzheimer’s disease etc. Food products which contains OH (hydroxyl) functional group are considered to have antioxidant potential, prevents oxidative damage by donating their electrons to the free radicals. 115 Citrus grandis is a good source of antioxidants like flavonoids, polyphenols and ascorbic acid which are good neutralizers of free radicals, among which polyphenols are the vital components preventing oxidative damage. 51 Free radicals cause oxidative stress and this damage is majorly occluded by polyphenolic compounds as well as flavonoids and ascorbic acid found in C. grandis by counteracting the free reactive radicals. The pink Citrus grandis juice cultivar (8.3 mg/ml) was found to be richer in polyphenolic content, making it a potent antioxidant than the white Citrus grandis juice cultivar (5.6 mg/ml). 51 There are 4 accepted assays: DPPH, ABTS (2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid), FRAP and Total Phenolic content which help in evaluation of antioxidant potential. 25 By performing FRAP (Free Reducing Antioxidant Power) assay (for determining the conversion of ferric form of iron to ferrous form) and DPPH assay (for measuring percentages of radical scavenging activity), where both the assay gave high values, the high antioxidant potential of Citrus grandis was confirmed. 44 In a similar study by Kumar et al, 2019 25 and Kumar et al, 2017 115 significant potential antioxidant activity of Citrus grandis having the highest FRAP (1.92 mM L-1 Trolox) and ABTS (4.49 mM L-1 Trolox) activity in vitro was reported. Seven Citrus grandis cultivars: Pattavee (PV), Thong Dee (TD), Tha Khoi (TK), Kao Numpueng (KNP), Kao Pean (KP), Kao Tanggkya (KTG), Kao Yai (KY) were used to study the antioxidant activity by DPPH and FRAP assay. 116,117 It was observed that the pink cultivars (TD and TK) had better antioxidant capacity than the white ones (PV, KP, KNP, KTG and KY) which had higher flavonoids than the latter. The order of polyphenol content (TK, TD, PV, KY, KTG, KNP and KP) by cultivars was correspondent to the antioxidant activity. 116,117 The antioxidant activity of methanolic extract (25%) of Citrus grandis fruit was also studied in long Evan rats where the extract gave high DPPH and FRAP values. The total polyphenolic content of methanolic extract of Citrus grandis fruit in rat was 515.45 ± 4.62. 44 The in vitro study carried out by Abirami et al (2014) found out that highest ABTS scavenging activity was exhibited by the juice sample of C. grandis (red) (34,659.62 mmol TE/L juice) than C. grandis (white) (31,343.90 mmol TE/L juice). 75 Similarly, DPPH scavenging activity of C. grandis (red) fruit was higher than C. grandis (white) fruit. 75 The antioxidant activity of Citrus grandis was 7.92 ± 0.04 and 10.74 ± 0.32 respectively showed by DPPH and TPC assay. 25 The DPPH assay of Citrus grandis peel essential oil showed 26% higher free radical scavenging activity than the fruit pulp extracts. Relative activities of methanol extracts or water were seen to be higher significantly (p < 05) than ethyl acetate or 50% ethanol extracts and acetone. In another study that tested the Citrus grandis peel and pulp, the FRAP value (2.3 mM) of the Citrus grandis peel essential oil was higher than the extracts of fruit pulp. The highest FRAP value (1.2 mM) was observed for water extracts followed by the acetone, methanol, 50% ethanol and ethyl acetate extracts. 118
Singh et al (2010) carried out a study on the essential oils (EO) of C. sinensis and C. grandis to find their activity against oxidation by free radical scavenging through spectrophotometer and DPPH radical scavenging assay on TLC (Thin layer chromatography). 119 The positive antioxidant activity of EOs’ were determined by recording the extent to which the purple colored DPPH solution was converted to yellow color. The IC50 (concentration of essential oil that neutralizes 50% 0f DPPH radicals) of C. grandis essential oil was 8.84µl/ml that indicated its strong antioxidant activity. 119 In another study carried out by He et al (2019), the total antioxidant activity, superoxide anion radical scavenging rate and DPPH free radical scavenging rate of Citrus grandis Peel Essential oil (PPEO) were determined in a concentration dependent manner, where the IC50 of PPEO was 70.12mg/ml. The study analyzed that PPEO at higher concentration exhibited high antioxidant activity. Superoxide anion radicals can also be removed by PPEO but with a lower scavenging ability than L-ascorbic acid at the same concentration. 54
Citrus flavonoids work as a scavenger of free radicals and exhibits anti-allergenic, analgesic, antimicrobial, anti-inflammatory, antiviral, antiulcer activities. In a comprehensive investigation by Makynen et al (2012) the antioxidant activity of 6 Citrus grandis cultivars were determined where the main flavonoids in the fruit hesperidin and neohesperidine dihydrochalone showed effectivity against superoxide formation as well as scavenging reactive oxygen species. 43 Thai Citrus grandis juice also reduced ferric ion to ferrous form that suggested the potential to repress the formation of Fenton reaction and impeded the generation of hydroxyl radical that are highly reactive in nature. 43 In a spectrophotometric analysis carried out by Zarina and Tan (2013) the flavonoid activity toward lipid peroxidation was evaluated in fish tissue treated with Citrus grandis peel where a reduction in peroxide value (PV) indicated its activity but there was evidence that flavonoid did not slow down the oxidation process. 71 Chang and Azrina (2017) studied the antioxidant capacity of different parts of Citrus grandis and its by-products where albedo was found to possess the highest Total Phenolic content and Total Flavonoid content while flavedo exhibited the highest antioxidant activity in both the DPPH and FRAP assays. 73 Barrion et al (2014) reported the antioxidant activity of the extract samples in the following order pericarp (29.64 expressed as % lipid peroxidation) >segment membrane >mesocarp. 77
Anti-Inflammatory Activity
Inflammation is body’s natural defense mechanism which protects body from the attack of foreign invaders and plays a role in the healing process. The presence of polysaccharides in Citrus grandis has potential anti-inflammatory activity. 33,120 –123 The anti-inflammatory activity of Citrus grandis was reported in a study carried out on both ammonia stimulated rabbits and clinical patients where polysaccharide present in the fruit relieved the symptoms as well as inhibited chronic inflammation. The therapy of 14 days with PCG (Polysaccharides of Citrus grandis)(p < 0.01) in 14 chronic pharyngitis patients relieved the inflammatory symptoms and further to study the relief of chronic inflammation with PCG, animal model was established by the combination of ammonia spray and subcutaneous injection of turpentine oil, where effect of oral intake of PCG was investigated. Treatment with PCGs inhibited various inflammatory mediators such as IFN-α, IL-2 and IL-4 present in an increased level in chronic pharyngitis rabbits. The exogenous administration of PCG (p < 0.01) showed inhibition of chronic inflammation in chronic pharyngitis animal model in a dose dependent manner which showed consistency with the results of clinical data. 120 In different studies it was determined that the activation of NF-kB leads to the increased release of inflammatory mediators. 120,124,125
Zhao et al (2019) manifested the anti-inflammatory effect of Citrus grandis by the methanolic (90%) and ethyl acetate extract (coumarin fraction, 32 g) of the peel of the fruit in animal model. 123 Anti-acute inflammation effect of the coumarins such as Auraptene, Isoauraptene, Meranzin hydrate obtained from Citrus grandis peels showed remarkably reduced (p < 0.05/ 0.01) auricular swelling after intraperitoneal injection for 3 consecutive days to the mice. 123 The inhibition ratios of the coumarins were 26.2% (Auraptene; 2 mg/kg), 23.5% (Auraptene; 1 mg/kg), 19.4% (Isoauraptene; 1 mg/kg), 35.6% (Isoauraptene; 0.5 mg/kg), 19.5% (Meranzin hydrate; 3 mg/kg), and 17.9% (Meranzin hydrate; 1.5 mg/kg), respectively. The compound Marmin (p > 0.05) didn’t show any significant inhibitory effect on xylene induced ear edema at the dose of 1 and 0.5 mg/kg in-vivo. In addition, Zhao et al (2019) treated mice with methanolic extract (90%), Ethyl acetate extract and the 4 bioactive coumarins (Auraptene, Marmin, Isoauraptene, Meranzin hydrate) of Citrus grandis peel, which showed significant inhibition of carrageenan-induced paw edema when given orally and intraperitoneally as a pre-treatment for 5 successive days before carrageenan injection. 123 The anti-subacute inflammatory effect was notable for EAC (p < 0.01) with an inhibitory rate of 60.3% and 62.3% at the dose of 60 and 30mg/kg and secondly ME gave the effect with an inhibitory rate of 49.9% and 51.4% at the dose of 2 and 1g/kg. The 4 coumarins (p < 0.05/ 0.01) of Citrus grandis peels showed effective results even at low doses. In vitro anti-inflammatory effect of the bioactive coumarins of Citrus grandis peel was due to the presence of 8-(6,7-Dihydroxy-3,7-dimethyl-2E-octenyloxy) psoralen, 5-(6-Hydroxy-3,7-dimethyl-2E,7-octadienyloxy) psoralen, Auraptene,7-(6-Hydroxy-3,7-dimethyl-2E,7-octadienyloxy) coumarin and Toddanone significantly when used as a pre-treatment. Also, exhibited their action by inhibiting pro-inflammatory cytokines like: IL-1β, PGE2, and TNF-α in LPS stimulated RAW264.7 cells at only 5µg/ml concentration and without any toxicity. 123 C. grandis functions as a good anti-inflammatory agent because of polysaccharides and bioactive coumarins present in the peel which blocks the inflammatory mediators and pro-inflammatory cytokines.
Antihyperlipidemic Activity
Hyperlipidemia is a type of metabolic disorder occurs due to elevated levels of triglycerides and cholesterol in the blood which can be prevented by the inhibition of pancreatic lipase that delays the digestion of triglyceride to absorbable free fatty acids. 126 It was stated in a study carried out by Makynen et al (2013) that phenolic compounds obtained from pulp of 6 cultivars of Citrus grandis played an important role as an antioxidant and antihyperlipidemic agent. 43 This study also provided us with the information that aqueous methanolic extract of Citrus grandis pulp prevented hyperlipidemia by the inhibition of the enzymes pancreatic lipase and cholesterol esterase. It also reduced the solubility of cholesterol into micelles by binding with primary bile acids. The interaction of the pulp extract of the fruit with the enzymes and solubility of cholesterol makes it a good anti-hyperlipdemic agent. 43 In a similar study carried out by Oyedepot and Babarinde (2012) it was found that administration of Citrus grandis fruit juice (50%) significantly reduced blood cholesterol, triglycerides and VLDL and increased HDL-C levels in Streptozin-induced diabetic rats. But in the study it was observed that administration of 25% C. grandis fruit juice showed no significant effects on lipid profile and blood glucose levels. 127 So it can be concluded from the observation of the study that higher concentration of the fruit juice is effective in exhibiting hypolipidemic activity.
The lipid profile of Type-2 diabetic rat was observed in a study where administration of 50% Citrus grandis fruit juice significantly decreased serum cholesterol and triglyceride levels. In STZ-induced diabetic rats the HDL (High Density Lipoprotein)-cholesterol level was also reduced to a notable level possibly by controlling hydrolysis of certain lipoproteins and their selective uptake and metabolism by different tissues. It was also hypothesized that the strong antihyperlipidemic effect of Citrus grandis fruit juice could be through the control of hyperglycemia as it works as the major determinant of total and very low density lipoprotein and triglyceride concentration. 127
Oboh et al (2014) studied the hypocholesterolemic properties of grapefruit and shaddock juices; they found that both juices resulted in reduction of plasma triglyceride levels in a dose dependent manner which was thought to occur due to the ability of citrus flavonoids to inhibit hepatic apolipoprotein (apo)B secretion. 27 Gorinstein et al (2005) observed a reduction in the cholesterol levels in the rats fed with high cholesterol diet due to the administration of citrus juices as well as naringin, a major flavone found in citrus fruits like C. grandis juice. 128 It was also derived in a similar study that juices of C. grandis and C. paradisii increased plasma HDL levels and decreased the level of LDL (Low Density Lipoprotein) cholesterol. The mechanism was that the flavonoids in the citrus juice work as cellular signaling molecules which regulates the signal transduction pathways of the sterol regulatory element binding proteins. 27 Thus we can come to a conclusion from the studies carried out that, the antihyperlipidemic effect of the fruit juice of C. grandis could be due to the presence of flavonoid content.
Anticancer Potentials
The natural compounds of Citrus grandis is a part of active investigation to suppress the expansion of cancer. Citrus grandis was found to exhibit antioxidant potential which was evaluated via FRAP assays and DPPH radical-scavenging activity. Antioxidants are bioactive reducing agents which they neutralize radicals produced in the body through different complex biochemical reactions they are responsible for many life threating and serious diseases including cancer. 40,44,51 Limonoids from Citrus grandis seeds, particularly limonin and nomilin reported chemo preventive bioactivities, specifically free radical-scavenging. 31,129 and glutathione S-transferase-inducing activity. Methanolic extract of Citrus grandis administered by intraperitoneal route demonstrated to increase the longevity of non-viable tumor cell and their count count and decrease the volume of tumor. 130
To evaluate antitumor activity of Citrus grandis peel the S180 tumor-bearing mice model was established by Yu et al (2018) where Citrus grandis peel at 300mg/kg exhibited prophylactic and inhibitory effect on the formation of S180 solid tumor in a dose-dependent manner (p < 0.05). 81 In the same study it was showed that Citrus grandis peel can induce tumor cells apoptosis by blocking the cell cycle in S phase and down-regulating the Bcl-2 expressions and up-regulating Bax levels. All these results suggested that the in vivo antitumor effects treated with Citrus grandis peel might be attributed to the polysaccharides’ immunoregulatory properties and apoptosis-inducing characters on the tumor-bearing mice. 81
Sen and Samanta (2015) studied the antitumor activity of methanol extract of Citrus grandis (MECM) leaves against Ehrlich Ascites Carcinoma (EAC) cell line in Swiss albino mice. 131 The administration of MECM for 9 consecutive days decreased tumor parameters such as tumor volume, viable tumor cell count and increased body weight, hematological parameters and life span in respect of the EAC control mice in a dose dependent manner. Intraperitoneal administration of MECM at doses 200 and 400 mg/kg exhibited antitumor activity. The study showed that the presence of flavonoids and limonoids present in Citrus grandis mediated antitumor and anti-inflammatory activity. 131
In another study, Wang et al (2019) found that Citrus grandis juice contains lycopene, β-carotene, and 1,1-diphenyl-2-picrylhydrazyl (DPPH) that are found to provide antioxidant activity, decreasing power activity, higher bile-acid-binding activity and at the same time with better anti-proliferative activity in leukemia cells. It must be noted that lycopene and β-carotene found abundantly in red-fleshed (Citrus grandis) fruits may exert anticancer activity. 132
Also, Citrus grandis extract binds with secondary bile acids which is associated with developing colorectal cancer. Thus the result of the study provided the information that Citrus grandis extract may reduce the risk factor in decreasing colorectal cancer. 133
Antimicrobial Activity
Antifungal activity
An investigation was carried out by Singh and Navneet (2016) to detect activity of Citrus grandis and C. sinensis essential oils, along with their essential oil combination against fungus and aflatoxins. 3 The major components of the essential oils were DL-limonene, a cyclic monoterpene, carveoland E-citral, Z-citral (monoterpene aldehydes). 3,134 In the study carried out by Singh and Navneet (2016), it was found that according to ANOVA and Tukey’s comparison tests (p < 0.05), different concentrations (250,500,750 and 1000 ppm) of EOs were found significantly effective against fungal growth in vitro. Different species of Aspergillus like A. alternate, A. niger, A. fumigatus, A. terreus, and other filamentous fungi like F. oxysporum, H. oryzae, C. herbarum, C. lunata, and T. viride were taken where complete inhibition of A. flavus was found at 750 ppm by essential oil of both the fruits and their essential oil combination 3 . The essential oil of Citrus grandis showed more efficacy than that of both C. sinensis and their essential oil combination. A broad spectrum fungi toxic activity was seen by complete inhibition of the mycelium growth of A. alternata, A. terreus, F. oxysporum, A. fumigatus, H. oryzae and T. viride by the essential oil and their combination at 750 ppm. 3 At 500 ppm the essential oil of Citrus grandis inhibited A. flavus up to 48.1% and the combination of EOs inhibited the species up to 44.0% respectively. 3 This leads to a conclusion that essential oil of C. grandis can be a good candidate to perform as an antifungal agent. In a study carried out by Bijun (2004) the ethanolic extract of Citrus grandis peel was seen to be effective against some series of mold. 135 The extract was found to inhibit the growth of Aspergillus niger V, Tiegh, Penicillium and Aspergillus otyzae with inhibition rate of 60.5%, 59.5% and 34.3% respectively. 135 Antifungal activity of the seed extracts of Citrus grandis was observed by Singh and Navneet (2016) 3 against Aspergillus niger (MTCC 921) and Candida albicans (MTCC 227). The minimum inhibition zone against Candida albicans was 7.66 ± 0.32 mm and MICs was between 3.12 to 25 mg/mL for MeOH (1:3, 100 g seed powder into 300 ml methanol) extract of the fruit’s seed.
Antiflatoxigenic activity
Aflatoxin, a toxin produced by certain fungi (Aspergillus flavus and Aspergillus parasiticus) are poisonous carcinogen and mutagen. Singh et al, 2010 119 carried out a study where essential oils (EOs) of C. grandis and C. sinensis and their EO combination at 500 ppm showed a complete inhibition of Aflatoxin B1(AFB1) in SMKY broth (Sucrose, 200 g; MgSO47H2O, 0.5 g; KNO3, 0.3 g; Yeast extract, 7.0 g; distilled water, 1000 ml; pH, 5.6 ± 0.2). 119 Thus EO of C. grandis and its EO combination with C. sinensis EO can be used to activity against aflatoxin.
Antibacterial activity
Barrion et al (2014) studied the antibacterial activity of the ethanolic extract (90%) of the phytochemical constituents of the pericarp, mesocarp, and segment membrane of Citrus grandis against 2 gram negative bacteria Escherichia coli and Salmonella typhimurium. 77 At 100% concentration the highest zone of inhibition was observed for mesocarp extract against S. typhimurium (18 mm), followed by the extract of pericarp (17.10 mm) and the lowest zone of inhibition was observed for the segment membrane extract (17.03 mm). 77 On the other hand, in all the 3 extracts E.coli was seen to be inactive at 100% concentration. 77 Antibacterial activity of ethanolic extract of Citrus grandis peel against Escherichia coli, Staphylococcus aureus and Salmonella was also examined. The minimum inhibitory concentration (MIC) of the extracts against different strains of bacteria were 2.5% for E.coli, S. aureus and Salmonella respectively. The extract was found to be effective more in acidic condition and the activity decreased if the temperature was elevated to 80 o C. 135 The crude ethyl acetate extract of flavedo which is the peripheral surface of Citrus grandis peel inhibited the growth of Gram-positive bacteria. 136 Das et al (2013) used ethanolic (90%) leaf extract of Citrus grandis to study its antibacterial activity against Pseudomonas aeruginosa and E.coli where maximum zone of inhibition was reported for P. aeruginosa. 137 E.coli showed higher MIC value for the extract than P. aeruginosa (0.312mg/ml) but the Minimum bactericidal concentration value (1.25mg/ml) was same for both the bacteria. 137 In another study of antibacterial activity of methanolic (175 ml of methanol) extract of leaves, peel and pulp of Citrus grandis against Escherichia coli (MTCC 40), P. aeruginosa (MTCC 424), Salmonella typhi (MTCC 3215), Staphylococcus aureus (MTCC 3160), Klebsiella pneumoniae (MTCC 3384). 74,75 It was found that the maximum activity against all microorganisms were remarkable for the extracts of leaves and pulp than the peel of the fruit. The least activity was 8-9 mm shown by the peel extract of Citrus grandis (red) and its pulp extract showed a moderate activity of 12-8 mm followed by the Citrus grandis (White) against the pathogenic bacteria. In a similar study carried out by Singh and Navneet (2016), it was observed that the seed extracts of Citrus grandis showed antibacterial activity against common respiratory tract pathogens like Pseudomonas aeruginosa (MTCC 2474), Haemophillus influenzae (MTCC 3826), Staphylococcus aureus (MTCC 1144), Streptococcus pneumoniae (MTCC 655), Streptococcus pyogens (MTCC 442). The highest antibacterial activity was seen by methanolic extract followed by acetone, water and petroleum ether. 3
Cytoprotective Effect
In 2009 Chularojmontri et al established a study about using Citrus grandis as a functional food in order to protect the cells from Doxorubicin induced cytotoxicity, increasing the phase 2 enzyme activity and accelerating the metabolism of carcinogenic/ cytotoxic agents in rat model. It was seen in the study that Doxorubicin treated mice decreased GST (Glutathione S Transferase) which is needed to protect cellular macromolecules from the attack of reactive electrophiles. But the incorporation of C. grandis fruit juice reversed Doxorubicin effect by enhancing enzyme activity. 138
Reactive oxygen species (ROS) accumulation along with endothelial injury and damage can be perilous to cardiovascular health. The presence of antioxidant compounds like: phenolics, flavonoids, vitamins, carotenoids, anthocyanins helps in reducing oxidative stress on the biomolecules. 25,139 In vivo studies of Citrus grandis fruit extract (10.1% w/v) on human umbilical vein endothelial cell migration and aging indicated an positive changes in cell migration and population doubling level. In addition, the fruit extract also delayed the onset of aging phenotype by senescence-associated β-galactosidase (SA-β-gal) staining and attenuated increased ROS level in aged cells. 140
Anti-Glycation
Various studies investigated anti-glycation compounds from natural sources. Several groups examined extract of Citrus grandis and found antiglycation effect against oxidation-dependent damage and fructose-mediated non-enzymatic glycation to bovine serum albumin (BSA) which determined the effects of this extract during the early stage (amadori products), middle stage (protein oxidation) and last stage (the cross-linking compound) of AGE (Advanced glycation end product) formation. 141,142 A study showed that the methanolic extract of Citrus grandis pulp decrease the fructosamine level with reduction in production of AGE and CML (Chronic myelogenous leukemia). Similarly, the methanolic extract of Citrus grandis pulp inhibited proteinoxidation and prevented decreased protein carbonyl formation with the loss of thiol groups which demonstrate the potentiality of Citrus grandis pulp extract to be an antiglycation agent and prevent of pathogenesis conditions associated with diabetic complication and aging. 42
Effect of Citrus grandis on Metabolic Disorders
Several groups observed that Citrus grandis peel or its ethanolic (70% ethanol; v/w 10/1) extracts of the whole fruit which includes both pulp and peel of the fruit, have therapeutic potential in case of metabolic disorders by improving glucose metabolism, preventing body weight gain, and lessening dyslipidemia. 143,144 Peel extract of Citrus grandis was found to reduce body weight and blood TC, TG, LDL-c in a study with obese mice by activating biochemical enzymes responsible for lipid metabolism, such as carnitine palmitoyl-transferase and lipase. Another study suggested in high-fat-diet induced obese rats, Citrus grandis can recover lipid profiles and prevent hypertension. 143 Additionally, ethanolic (90%) extracts (100 g was extracted with 90% ethanol (2 L) twice at 80°C for 2 h of Citrus grandis peel has been reported to contain numerous valuable effects on Hf diet-fed mice-with the activation of GLUT4 and PPARα. It was found to prevent the advance of obesity that was promoted by a HF diet and also it had the potential of augmentation of dyslipidemia and hyperglycemia and reducing glucose resistance. Apart from this, therapeutic potential of Citrus grandis peels extract as a traditional remedy for metabolic disorders has been reported in a study using high fat diet induced rats. 72
Hepatotoprotective and Nephroprotective Activities
Citrus grandis have been used as hepatoprotective and nephroprotective agent. Citrus grandis peel powder supplementation was tested in CCl4 treated rats which reported to prevent the hepatic inflammation and fibrosis. CCl4 is the most used hepatotoxic agent that induce large inflammatory cell infiltration and oxidative stress in rat liver which has similar symptoms of varied human dysfunctional liver. 145 A study administrated by Oboh et al (2014) determined that Citrus grandis juice in a dose dependent manner, reduced plasma triglyceride level. 27 It was because of the presence of flavonoid which inhibits secretion of hepatic apolipoprotein (apo) B, thus providing a hepatoprotective effect. 146 Another study performed by Chowdhury et al (2015) to determine the hepatoprotective activity of powder of Citrus grandis peel (0.5% of powder food) in CCl4 treated rat model. 50 They examined liver damage in rats by measuring the alanine transaminase (ALT), aspartate transaminase (AST) and Alkaline phosphatase (ALP) enzyme activities. This group also exhibited lipid peroxidation product (MDA), nitric oxide (NO), advanced protein oxidation products level (APOP), and catalase activities. Also included are histological profiling for the inflammatory cell infiltration, collagen, and iron deposition in liver. 50 In addition, reduction of serum AST, ALT and ALP activities in carbon tetrachloride treated rats was observed after dietary supplementation of Citrus grandis. Its powder of peel (0.5%) was found to significantly reduce oxidative stress markers- Nitric Oxide (NO), Malondialdehyde (MDA), APOP level and also found to restore catalase activity in CCl4 treated rats. Furthermore, effectiveness of the peel powder against liver disorders can be due to the presence of antioxidant constituents such as caffeic acid and epicatechin. 50 Likewise, methanolic leaf extract (25 g leaf powder mixed with 175 ml methanol) of Citrus grandis also exhibited significant improvement in paracetamol induced hepatotoxicity in rat/mice. 74,75 Besides, when compare to control group Citrus grandis fruit methanolic extracts were found to improve renal function biomarkers in another study. Renal function of its methanolic extract (25%) were examined by measuring the uric acid, serum urea, creatinine levels and electrolytes such as K+, Na+, Mg2+, PO3+, Cl− and Ca2+ level where the group found improved uric acid, serum creatinine and urea levels which established the renal-protective efficacy of the extract. 44
Circulatory Problems
Citrus grandis juice have been studied for inhibiting angiotensin converting enzyme (ACE) and hypocholesterolemic activity by various activities. A study was designed by administering 3 different doses of methanolic extract (25%) of Citrus grandis in rats, which showed significant serum triglyceride (TG) level reduction, and all atherogenic indices. In addition, rats were found to have significantly increased high-density lipoprotein cholesterol (HDL-C) level and decreased total cholesterol(TC) and low-density lipoprotein cholesterol (LDL-C) level when administered with 1000 mg/kg dose which exhibited that Citrus grandis has preventive effect against cardiovascular disease. 44 Besides, a study was carried out on Citrus grandis and Citrus paradisii juices to observe the interaction of ACE with citrus fruit juices which showed in a dose dependent manner inhibition of ACE and Citrus grandis was found to provide superior ACE inhibitory activity than Citrus paradisii (p < 0.05). In addition, there also was a significant decrease (p < 0.05) in total cholesterol (87.32 mg/dL vs. 60.32 mg/dL) with increased quantities of citrus fruit juices. Also, significant increase in HDL-C and decrease (p < 0.05) in LDL-C atherogenic index in rats were observed when compared to the control, which can inhibit the key enzyme related hypertension and hypocholesterolemia. 27 The juice extracts of C. grandis showed potent activity in ameliorating cardiac condition by inhibiting the ACE enzyme and also maintain the balance of LDL-C and HDL-C in blood.
Conclusion
Citrus grandis have been used for years as a part of traditional medicine but now the fruit has been recognized in the era of modern medicine for its beneficiary uses. This study summarizes significant activities of the citrus fruit against hyperlipidemia, inflammation, microorganisms, cancer, glycation, metabolic disorders, oxidation etc. The presence of various chemical components like steroids, phenols, alkaloids, glycosides, flavonoids, saponins, cardiac glycosides, carbohydrate and amino acid aids in exhibiting therapeutic activity of the fruit. Besides the therapeutic activity, the fruit is famous for having different nutritional value and health benefits. The chemical components like carotenoids, flavonoids, limonoids, essential oils, acridone alkaloids, minerals and Vitamin B complex makes it a beneficial fruit for the improvement of health conditions. This article valorizes the effects that Citrus grandis provides to improve health condition and further studies on various parts of fruit, including pulp, peel, leaf, seed and it essential oil can help us in discovering new activities which can be beneficial to the mankind.
Footnotes
Authors’ Note: Rusat Jahin Anmol, Shabnam Marium, Md. Moklesur Rahman Sarker, Siok Yee Chan, and Long Chiau Ming: wrote the manuscript draft; Fei Tsong Hiew, Wan Chien Han, Lee Kuan Kwan, Alicia Khai Yeen Wong, Nurolaini Kifli: revised the manuscript; Md. Moklesur Rahman Sarker, Siok Yee Chan, Long Chiau Ming: conceived and designed the study, supervised the work and revised the manuscript; All the authors read the manuscript and agreed to be accountable for all aspects of the work and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Long Chiau Ming  https://orcid.org/0000-0002-6971-1383
https://orcid.org/0000-0002-6971-1383
References
- 1. Kamarudin MNA, Sarker MMR, Kadir HA, Ming LC. Ethnopharmacological uses, phytochemistry, biological activities, and therapeutic applications of Clinacanthus nutans (Burm. f.) Lindau: a comprehensive review. J Ethnopharmacol. 2017;206:245–266. [DOI] [PubMed] [Google Scholar]
- 2. Tilburt JC, Kaptchuk TJ. Herbal medicine research and global health: an ethical analysis. Bull World Health Organ. 2008;86(8):594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh A, Navneet Evaluation of antimicrobial potential and phytochemical assessment of Citrus maxima Burm. Seeds extracts against respiratory tract pathogens. New Y Sci J. 2016;9(9):4–10. [Google Scholar]
- 4. Cordenonsi LM, Sponchiado RM, Campanharo SC, et al. Study of flavonoids presente in Pomelo (Citrus máxima) by DSC, UV-VIS, IR, 1 H and 13C NMR and MS. Drug Analyt Res Porto Alegre. 2017;1(1):31–37. [Google Scholar]
- 5. Chen Y, Liu Y, Sarker MMR, et al. Structural characterization and antidiabetic potential of a novel heteropolysaccharide from Grifola frondosa via IRS1/PI3K-JNK signaling pathways. Carbohydr Polym. 2018;198:452–461. doi:10.1016/j.carbpol.2018.06.077 [DOI] [PubMed] [Google Scholar]
- 6. Chen Y, Liu D, Wang D, et al. Hypoglycemic activity and gut microbiota regulation of a novel polysaccharide from Grifola frondosa in type 2 diabetic mice. Food Chem Toxicol. 2019;126:295–302. doi:10.1016/j.fct.2019.02.034 [DOI] [PubMed] [Google Scholar]
- 7. Munira S, Nesa L, Islam MS, et al. Antidiabetic activity of Neolamarckia cadamba (Roxb.) Bosser flower extract in alloxan-induced diabetic rats. Clin Phytosci. 2020;6:1–6. doi:10.1186/s40816-020-00183-y [Google Scholar]
- 8. Sarker MMR, Soma MA. Updates on clinical study reports of phytotherapy in the management of type 2 diabetes mellitus. In: Eddouks M, ed. Phytotherapy in the Management of Diabetes and Hypertension. Vol. 4. Bentham Science Publishers; 1–60:2020. doi:10.2174/9789811480515120040003 [Google Scholar]
- 9. Sheikh BY, Sarker MMR, Kamarudin MNA, Mohan G. Antiproliferative and apoptosis inducing effects of citral via p53 and ROS-induced mitochondrial-mediated apoptosis in human colorectal HCT116 and HT29 cell lines. Biomed Pharmacother. 2017;96:834–846. [DOI] [PubMed] [Google Scholar]
- 10. Sheikh BY, Sarker MMR, Kamarudin MNA, Ismail A. Prophetic medicine as potential functional food elements in the intervention of cancer: a review. Biomed Pharmacother. 2017;95:614–648. [DOI] [PubMed] [Google Scholar]
- 11. Shajib M, Rashid RB, Ming LC, et al. Polymethoxyflavones from Nicotiana plumbaginifolia (Solanaceae) exert antinociceptive and neuropharmacological effects in mice. Front Pharmacol. 2018;9:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sarker MMR, Nahar S, Shahriar M, Seraj S, Choudhuri MSK. Preliminary study of the immunostimulating activity of an Ayurvedic preparation, Kanakasava, on the splenic cells of BALB/c mice in vitro. Pharm Biol. 2012;50(11):1467–1472. doi:10.3109/13880209.2012.681329 [DOI] [PubMed] [Google Scholar]
- 13. Sarker MMR, Choudhuri MSK, Zhong M. Effect of Chandanasav, an Ayurvedic formulation, on mice whole splenocytes for the production of polyclonal IgM and proliferation of cells: a preliminary study. Int J Pharm Sci Res. 2012; 3: 1294–1299. doi:10.13040/IJPSR.0975-8232.3(5).1294-99 [Google Scholar]
- 14. Sarker MMR. Induction of humoral immunity through the enhancement of IgM production in murine splenic cells by ethanolic extract of seed of Piper nigrum L. J Sci Res. 2012;4(3):751–756. doi:10.3329/jsr.v4i3.10485 [Google Scholar]
- 15. Sarker MMR, Imam H, Bhuiyan MS, Choudhuri KMS. In vitro assessment of prasarani sandhan, a traditional polyherbal Ayurvedic medicine, for immunostimulating activity in splenic cells of balb/c mice. Int J Pharm Sci. 2014;6(9):531–534. https://innovareacademics.in/journals/index.php/ijpps/article/view/1315 [Google Scholar]
- 16. Sarker MMR, Mazumder MEH, Rashid MH. In vitro enhancement of polyclonal IgM production by ethanolic extract of Nigella sativa L. seeds in whole spleen cells of female BALB/c Mice. Bangladesh Pharm J. 2011;14(1):73–77. [Google Scholar]
- 17. Sarker MMR, Ming LC, Sarker MZI, Choudhuri MSK. Immunopotentiality of Ayurvedic polyherbal formulations “Saribadi” and “Anantamul Salsa” with augmentation of IgM production and lymphocytes proliferation: a preliminary study. Asian Pac J Tropic Biomed. 2016;6(7):568–573. doi:10.1016/j.apjtb.2016.05.004 [Google Scholar]
- 18. Goto T, Sarker MMR, Zhong M, Tanaka S, Gohda E. Enhancement of immunoglobulin M production in B cells by the extract of red bell pepper. J Health Sci. 2010;56(3):304–309. doi:10.1248/jhs.56.304 [Google Scholar]
- 19. Rouhi SZT, Sarker MMR, Rahmat A, Alkahtani SA, Othman F. The effect of pomegranate fresh juice versus pomegranate seed powder on metabolic indices, lipid profile, inflammatory biomarkers, and the histopathology of pancreatic islets of Langerhans in streptozotocin-nicotinamide induced type 2 diabetic Sprague–Dawley rats. BMC Complement Altern Med. 2017;17(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Imam H, Mahbub NU, Khan MF, Hana HK, Sarker MM. Alpha amylase enzyme inhibitory and anti-inflammatory effect of Lawsonia inermis. Pak J Biol Sci. 2013;16(23):1796–1800. doi:10.3923/pjbs.2013.1796.1800 [DOI] [PubMed] [Google Scholar]
- 21. Nesa ML, Karim SS, Api K, et al. Screening of Baccaurea ramiflora (Lour.) extracts for cytotoxic, analgesic, anti-inflammatory, neuropharmacological and antidiarrheal activities. BMC Complement Altern Med. 2018;18(1):35. doi:10.1186/s12906-018-2100-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sarker MMR, Zihad MATR, Islam M, et al. Antihyperglycemic, insulin-sensitivity and anti-hyperlipidemic potential of Ganoderma lucidum, a dietary mushroom, on alloxan-and glucocorticoid-induced diabetic Long-Evans rats. Funct Foods Health Dis. 2015;5(12):450–466. doi:10.31989/ffhd.v5i12.220 [Google Scholar]
- 23. Kazemipoor M, Cordell GA, Sarker MMR, et al. Alternative treatments for weight loss: safety/risks and effectiveness of anti-obesity medicinal plants. Int J Food Prop. 2015;18(9):1942–1963. doi:10.1080/10942912.2014.933350 [Google Scholar]
- 24. Das SK, Sengupta P, Mustapha MS, Sarker MMR. An experimental evaluation of adaptogenic potential of standardized Epipremnum aureum leaf extract. J. Pharm. Bioallied Sci. 2017;9(2):88. doi:10.4103/0975-7406.183227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kumar D, Ladaniya MS, Gurjar M. Underutilized Citrus sp. pomelo (Citrus grandis) and Kachai lemon (Citrus jambhiri) exhale in phytochemicals and antioxidant potential. J Food Sci Technol. 2019;56(1):217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vijayalakshmi P, Radha R. Pharmacognostical and phytochemical screening of the peels of Citrus maxima . Res J Pharmacog Phytochem. 2016;8(1):25–31. [Google Scholar]
- 27. Oboh G, Bello FO, Ademosun AO. Hypocholesterolemic properties of grapefruit (Citrus paradisii) and shaddock (Citrus maxima) juices and inhibition of angiotensin-1-converting enzyme activity. J Food Drug Anal. 2014;22(4):477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shah N, Supian NAM, Hussein NA. Disinfectant of pummelo (Citrus grandis L. Osbeck) fruit juice using gaseous ozone. J Food Sci Technol. 2019;56(1):262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang M, Li L, Wu Z, et al. Volatile composition in two pummelo cultivars (Citrus grandis L. Osbeck) from different cultivation regions in China. Molecules. 2017;22(5):716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Herath KHINM, Bing SJ, Cho J, et al. Anti-inflammatory activities of Dangyuja (Citrus grandis Osbeck) in concanavalin a stimulated murine splenocytes and 12-O-tetradecanoylphorbol-13-acetate-induced murine skin edema. Biomed Pharmacother. 2016;83:1353–1364. [DOI] [PubMed] [Google Scholar]
- 31. Yu EA, Kim GS, Lee JE, et al. Flavonoid profiles of immature and mature fruit tissues of Citrus grandis Osbeck (Dangyuja) and overall contribution to the antioxidant effect. Biomed Chromatogr. 2015;29(4):590–594. doi:10.1002/bmc.3318. [DOI] [PubMed] [Google Scholar]
- 32. Puglisi I, De Patrizio A, Schena L, et al. Two previously unknown Phytophthora species associated with brown rot of pomelo (Citrus grandis) fruits in Vietnam. PLoS One. 2017;12(2):e0172085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shah NN, Rahman RA, Shamsuddin R, Adzahan NM. Effects of pectinase clarification treatment on phenolic compounds of pummelo (Citrus grandis L. Osbeck) fruit juice. J Food Sci Technol. 2015;52(8):5057–5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang XY, Qiu JY, Hui QL, et al. Systematic analysis of the basic/helix-loop-helix (bHLH) transcription factor family in pummelo (Citrus grandis) and identification of the key members involved in the response to iron deficiency. BMC Genomics. 2020;21(1):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao XJ, Guo PM, Pang WH, et al. A rapid UHPLC-QqQ-MS/MS method for the simultaneous qualitation and quantitation of coumarins, furocoumarins, flavonoids, phenolic acids in pummelo fruits. Food Chem. 2020;325:126835. [DOI] [PubMed] [Google Scholar]
- 36. Chen C, Nie Z, Wan C, Gan Z, Chen J. Suppression on postharvest juice sac granulation and cell wall modification by chitosan treatment in harvested pummelo (Citrus grandis L. Osbeck) stored at room temperature. Food Chem. 2021;336:127636. [DOI] [PubMed] [Google Scholar]
- 37. Toh JJ, Khoo HE, Azrina A. Comparison of antioxidant properties of pomelo [Citrus grandis (L) Osbeck] varieties. Int Food Res J. 2013;20(4):1661–1668. [Google Scholar]
- 38. Tocmo R, Pena-Fronteras J, Calumba KF, Mendoza M, Johnson JJ. Valorization of pomelo (Citrus grandis Osbeck) peel: a review of current utilization, phytochemistry, bioactivities, and mechanisms of action. Compr Rev Food Sci Food Saf. 2020;19(4):1969–2012. [DOI] [PubMed] [Google Scholar]
- 39. Sawant T, Panhekar D. A brief review on recent advances of Citrus maxima (chakota). Int J Recent Sci Res. 2017;8:19400–19416. [Google Scholar]
- 40. Tsai ML, Lin CD, Khoo KA, et al. Composition and bioactivity of essential oil from Citrus grandis (L.) Osbeck “Mato Peiyu” leaf. Molecules. 2017;22(12):2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Duan L, Guo L, Dou LL, et al. Comparison of chemical profiling and antioxidant activities of fruits, leaves, branches, and flowers of Citrus grandis “Tomentosa”. J Agric Food Chem. 2014;62(46):11122–11129. [DOI] [PubMed] [Google Scholar]
- 42. Caengprasath N, Ngamukote S, Makynen K, Adisakwattana S. The protective effects of pomelo extract (Citrus grandis L. Osbeck) against fructose-mediated protein oxidation and glycation. EXCLI J. 2013;12:491–502. [PMC free article] [PubMed] [Google Scholar]
- 43. Makynen K, Jitsaardkul S, Tachasamran P, et al. Cultivar variations in antioxidant and antihyperlipidemic properties of pomelo pulp (Citrus grandis [L.] Osbeck) in Thailand. Food Chem. 2013;139(1-4):735–743. [DOI] [PubMed] [Google Scholar]
- 44. Ali MY, Rumpa NN, Paul S, et al. Antioxidant potential, subacute toxicity, and beneficiary effects of methanolic extract of pomelo (Citrus grandis L. Osbeck) in long evan rats. J Toxicol. 2019;2019:2529569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mokbel MS, Suganuma T. Antioxidant and antimicrobial activities of the methanol extracts from pummelo (Citrus grandis Osbeck) fruit albedo tissues. Eur Food Res Technol. 2006;224(1):39–47. [Google Scholar]
- 46. Quynh NTT, Phong VC, Huong PT. Effect of pomelo (Citrus grandis (L). Osbeck) peel extract on lipid-carbohydrate metabolic enzymes and blood lipid, glucose parameters in experimental obese and diabetic mice. VNU J Sci Nat Sci Technol. 2010;26(4):224–232. [Google Scholar]
- 47. Kim H, Moon JY, Mosaddik A, Cho SK. Induction of apoptosis in human cervical carcinoma HeLa cells by polymethoxylated flavone-rich Citrus grandis Osbeck (Dangyuja) leaf extract. Food Chem Toxicol. 2010;48(8-9):2435–2442. [DOI] [PubMed] [Google Scholar]
- 48. Kore VT, Chakraborty I. Efficacy of various techniques on biochemical characteristics and bitterness of pummelo juice. J Food Sci Technol. 2015;52(9):6073–6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lin LY, Huang BC, Chen KC, Peng RY. Integrated anti-hyperlipidemic bioactivity of whole Citrus grandis [L.] Osbeck fruits-multi-action mechanism evidenced using animal and cell models. Food Funct. 2020;11(4):2978–2996. [DOI] [PubMed] [Google Scholar]
- 50. Chowdhury MR, Sagor MA, Tabassum N, et al. Supplementation of Citrus maxima peel powder prevented oxidative stress, fibrosis, and hepatic damage in carbon tetrachloride (CCl4) treated rats. Evid Based Complement Alternat Med. 2015;2015:598179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tsai HL, Chang SK, Chang SJ. Antioxidant content and free radical scavenging ability of fresh red pummelo [Citrus grandis (L.) Osbeck] juice and freeze-dried products. J Agric Food Chem. 2007;55(8):2867–2872. [DOI] [PubMed] [Google Scholar]
- 52. Thavanapong N, Wetwitayaklung P, Charoenteeraboon J. Comparison of essential oils compositions of Citrus maxima merr. Peel obtained by cold press and vacuum stream distillation methods and of its peel and flower extract obtained by supercritical carbon dioxide extraction method and their antimicrobial activity. J Essent Oil Res. 2010;22(1):71–77. [Google Scholar]
- 53. Gonzalez-Mas MC, Rambla JL, Lopez-Gresa MP, Blazquez MA, Granell A. Volatile compounds in citrus essential oils: a comprehensive review. Front Plant Sci. 2019;10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. He W, Li X, Peng Y, He X, Pan S. Anti-oxidant and anti-melanogenic properties of essential oil from peel of pomelo cv. Guan Xi. Molecules. 2019;24(2):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Misni N, Mohamed Nor Z, Ahmad R. Microencapsulation of Citrus grandis peel oil using interfacial precipitation chemistry technique for repellent application. Iran J Pharm Res. 2019;18(1):198–209. [PMC free article] [PubMed] [Google Scholar]
- 56. Van Hung P, Chi PT, Phi NT. Comparison of antifungal activities of Vietnamese citrus essential oils. Nat Prod Res. 2013;27(4-5):506–508. [DOI] [PubMed] [Google Scholar]
- 57. Sidana J, Saini V, Dahiya S, Nain P, Bala S. A review on citrus-“the boon of nature.” Int J Pharm Sci Rev Res. 2013;18(2):20–27. [Google Scholar]
- 58. Shao SY, Xu WJ, Tao J, et al. Glycemic index, glycemic load, and glycemic response to pomelo in patients with type 2 diabetes. J Huazhong Univ Sci Technolog Med Sci. 2017;37(5):711–718. [DOI] [PubMed] [Google Scholar]
- 59. He JZ, Shao P, Liu JH, Ru QM. Supercritical carbon dioxide extraction of flavonoids from pomelo (Citrus grandis (L.) Osbeck) peel and their antioxidant activity. Int J Mol Sci. 2012;13(10):13065–13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shi M, Liu X, Zhang H, et al. The IAA- and ABA-responsive transcription factor CgMYB58 upregulates lignin biosynthesis and triggers juice sac granulation in pummelo. Hortic Res. 2020;7:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Abudayeh ZH, Al Khalifa II, Mohammed SM, Ahmad AA. Phytochemical content and antioxidant activities of pomelo peel extract. Pharmacog Res. 2019;11(3):244. [Google Scholar]
- 62. Liu Y, Ren C, Cao Y, et al. Characterization and purification of bergamottin from Citrus grandis (L.) Osbeck cv. Yongjiazaoxiangyou and its antiproliferative activity and effect on glucose consumption in HepG2 cells. Molecules. 2017;22(7):1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xu CJ, Fraser PD, Wang WJ, Bramley PM. Differences in the carotenoid content of ordinary citrus and lycopene-accumulating mutants. J Agric Food Chem. 2006;54(15):5474–5481. doi:10.1021/jf060702t [DOI] [PubMed] [Google Scholar]
- 64. Wang YC, Chuang YC, Hsu HW. The flavonoid, carotenoid and pectin content in peels of citrus cultivated in Taiwan. Food Chem. 2008;106(1):277–284. doi:10.1016/j.foodchem.2007.05.086 [Google Scholar]
- 65. Wang F, Lin J, Xu L, et al. On higher nutritional and medical properties of a carotenoid-rich mutant pomelo (Citrus maxima (L.) Osbeck). Ind Crops Prod. 2019;127:142–147. [Google Scholar]
- 66. Li PL, Liu MH, Hu JH, Su WW. Systematic chemical profiling of Citrus grandis “Tomentosa” by ultra-fast liquid chromatography/diode-array detector/quadrupole time-of-flight tandem mass spectrometry. J Pharm Biomed Anal. 2014;90:167–179. doi:10.1016/j.jpba.2013.11.030 [DOI] [PubMed] [Google Scholar]
- 67. Lü Z, Zhang Z, Wu H, Zhou Z, Yu J. Phenolic composition and antioxidant capacities of Chinese local pummelo cultivars’ peel. Hortic Plant J. 2016;2(3):133–140. doi:10.1016/j.hpj.2016.05.001 [Google Scholar]
- 68. Xi W, Fang B, Zhao Q, Jiao B, Zhou Z. Flavonoid composition and antioxidant activities of Chinese local pummelo (Citrus grandis Osbeck.) varieties. Food Chem. 2014;161:230–238. doi:10.1016/j.foodchem.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 69. Nogata Y, Ohta H, Yoza KI, Berhow M, Hasegawa S. High-performance liquid chromatographic determination of naturally occurring flavonoids in citrus with a photodiode-array detector. J Chromatogr A. 1994;667(1-2):59–66. doi:10.1016/0021-9673(94)89051-X [Google Scholar]
- 70. Kawaii S, Tomono Y, Katase E, Ogawa K, Yano M. Quantitation of flavonoid constituents in citrus fruits. J Agric Food Chem. 1999;47(9):3565–3571. [DOI] [PubMed] [Google Scholar]
- 71. Zarina Z, Tan SY. Determination of flavonoids in Citrus grandis (Pomelo) peels and their inhibition activity on lipid peroxidation in fish tissue. Int Food Res J. 2013;20(1):313–317. [Google Scholar]
- 72. Ding X, Guo L, Zhang Y, et al. Extracts of pomelo peels prevent high-fat diet-induced metabolic disorders in c57bl/6 mice through activating the PPARalpha and GLUT4 pathway. PLoS One. 2013;8(10):e77915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chang S, Azrina A. Antioxidant content and activity in different parts of pomelo [Citrus grandis (L.) Osbeck] by-products. Acta Hortic. 2017;1152:27–34. doi:10.17660/ActaHortic.2017.1152.4 [Google Scholar]
- 74. Abirami A, Nagarani G, Siddhuraju P. Antimicrobial activity of crude extract of Citrus hystrix and Citrus maxima . Int J Pharm Sci Res. 2013;4:1–5. [Google Scholar]
- 75. Abirami A, Nagarani G, Siddhuraju P. In vitro antioxidant, anti-diabetic, cholinesterase and tyrosinase inhibitory potential of fresh juice from Citrus hystrix and C. maxima fruits. Food Sci Hum Well. 2014;3(1):16–25. [Google Scholar]
- 76. Wu B, Liu X, Xu K, Zhang B. Genome-wide characterization, evolution and expression profiling of UDP-glycosyltransferase family in pomelo (Citrus grandis) fruit. BMC Plant Biol. 2020;20(1):459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Barrion ASA, Hurtada WA, Papa IA, Zulayvar TO, Yee MG. Phytochemical composition, antioxidant and antibacterial properties of pummelo (Citrus maxima (Burm.)) merr. against Escherichia coli and Salmonella typhimurium. Food Nutr Sci. 2014;5:749–758. [Google Scholar]
- 78. Cheong MW, Liu SQ, Zhou W, Curran P, Yu B. Chemical composition and sensory profile of pomelo (Citrus grandis (L.) Osbeck) juice. Food Chem. 2012;135(4):2505–2513. [DOI] [PubMed] [Google Scholar]
- 79. Yu J, Ji H, Liu A. Preliminary structural characteristics of polysaccharides from pomelo peels and their antitumor mechanism on S180 tumor-bearing mice. Polymers (Basel). 2018;10(4):419. doi:10.3390/polym10040419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Liu Y, Liu A, Ibrahim SA, Yang H, Huang W. Isolation and characterization of microcrystalline cellulose from pomelo peel. Int J Biol Macromol. 2018;111:717–721. doi:10.1016/j.ijbiomac.2018.01.098 [DOI] [PubMed] [Google Scholar]
- 81. Poli A, Anzelmo G, Fiorentino G, Nicolaus B, Tommonaro G, Di Donato P. Polysaccharides from wastes of vegetable industrial processing: new opportunities for their eco-friendly re-use. Biotechnol Biopolym. 2011; 33–56. [Google Scholar]
- 82. Czech A, Zarycka E, Yanovych D, et al. Mineral content of the pulp and peel of various citrus fruit cultivars. Biol Trace Elem Res. 2020;193(2):555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Xiang Y, Cao J, Luo F, et al. Simultaneous purification of limonin, nomilin and isoobacunoic acid from pomelo fruit (Citrus grandis) segment membrane. J. Food Sci. 2014;79(10):C1956–C1963. [DOI] [PubMed] [Google Scholar]
- 84. Hong EY, Kim TY, Hong GU, et al. Inhibitory effects of roseoside and icariside e4 isolated from a natural product mixture (No-ap) on the expression of angiotensin II receptor 1 and oxidative stress in angiotensin II-stimulated H9C2 Cells. Molecules. 2019;24(3):414. doi:10.3390/molecules24030414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Patil JR, Jayaprakasha GK, Kim J, et al. 5-Geranyloxy-7-methoxycoumarin inhibits colon cancer (SW480) cells growth by inducing apoptosis. Planta Med. 2013;79(3-4):219–226. doi:10.1055/s-0032-1328130 [DOI] [PubMed] [Google Scholar]
- 86. Hajialyani M, Hosein Farzaei M, Echeverría J, et al. Hesperidin as a neuroprotective agent: a review of animal and clinical evidence. Molecules. 2019;24(3):648. doi:10.3390/molecules24030648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Servant G, Kenakin T, Zhang L, Williams M, Servant N. The function and allosteric control of the human sweet taste receptor. Adv Pharmacol. 2020;88:59–82. doi:10.1016/bs.apha.2020.01.002 [DOI] [PubMed] [Google Scholar]
- 88. Xie W, Qiu X, Huang X, et al. Comparison between the pharmacokinetics of meranzin hydrate in a rat model of chronic depression and in controls following the oral administration of Chaihu-Shugan-San. Exp Ther Med. 2013;6(4):913–918. doi:10.3892/etm.2013.1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chen R, Qi QL, Wang MT, Li QY. Therapeutic potential of naringin: an overview. Pharm Biol. 2016;54(12):3203–3210. doi:10.1080/13880209.2016.1216131 [DOI] [PubMed] [Google Scholar]
- 90. Fan S, Zhang C, Luo T, et al. Limonin: a review of its pharmacology, toxicity, and pharmacokinetics. Molecules. 2019;24(20):3679. doi:10.1080/13880209.2016.1216131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mozos I, Stoian D, Caraba A, et al. Lycopene and vascular health. Front Pharmacol. 2018;9:521. doi:10.3389/fphar.2018.00521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Grune T, Lietz G, Palou A, et al. β-Carotene is an important vitamin A source for humans. J Nutr. 2010;140(12):2268S–2285S. doi:10.3945/jn.109.119024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Razavi BM, Arasteh E, Imenshahidi M, Iranshahi M. Antihypertensive effect of auraptene, a monoterpene coumarin from the genus citrus, upon chronic administration. Iran J Basic Med Sci. 2015;18(2):153–158. [PMC free article] [PubMed] [Google Scholar]
- 94. Takomthong P, Waiwut P, Yenjai C, et al. Structure–activity analysis and molecular docking studies of Coumarins from Toddalia Asiatica as multifunctional agents for Alzheimer’s disease. Biomedicines. 2020;8(5):107. doi:10.3390/biomedicines805010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yoncheva K, Tzankova V, Yordanov Y, et al. Evaluation of antioxidant activity of caffeic acid phenethyl ester loaded block copolymer micelles. Biotechnol Biotechnol Equip. 2019;33(1):64–74. doi:10.1080/13102818.2018.1537753 [Google Scholar]
- 96. Hollands WJ, Tapp H, Defernez M, et al. Lack of acute or chronic effects of epicatechin-rich and procyanidin-rich apple extracts on blood pressure and cardiometabolic biomarkers in adults with moderately elevated blood pressure: a randomized, placebo-controlled crossover trial. Am J Clin Nutr. 2018;108(5):1006–1014. doi:10.1093/ajcn/nqy139 [DOI] [PubMed] [Google Scholar]
- 97. Choi GS, Choo HJ, Kim BG, Ahn JH. Synthesis of acridone derivatives via heterologous expression of a plant type III polyketide synthase in Escherichia coli . Microb Cell Fact. 2020;19(1):73. doi:10.1186/s12934-020-01331-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lampronti I, Dechecchi MC, Rimessi A, et al. β-Sitosterol reduces the expression of chemotactic cytokine genes in cystic fibrosis bronchial epithelial cells. Front Pharmacol. 2017;8:236. doi:10.3389/fphar.2017.00236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Medeiros KAA, dos Santos JR, Melo TCDS, et al. Depressant effect of geraniol on the central nervous system of rats: behavior and ECoG power spectra. Biomed J. 2018;41(5):298–305. doi:10.1016/j.bj.2018.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Shen Y, Song X, Li L, et al. Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. Biomed Pharmacother. 2019;111:579–587. doi:10.1016/j.biopha.2018.12.074 [DOI] [PubMed] [Google Scholar]
- 101. Lin FH, Lin JY, Gupta RD, et al. Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. J Invest Dermatol. 2005;125(4):826–832. doi:10.1111/j.0022-202X.2005.23768.x [DOI] [PubMed] [Google Scholar]
- 102. Salau VF, Erukainure OL, Ibeji CU, et al. Vanillin and vanillic acid modulate antioxidant defense system via amelioration of metabolic complications linked to Fe 2+-induced brain tissues damage. Metab Brain Dis. 2020;35(5):727–738. doi:10.1007/s11011-020-00545-y [DOI] [PubMed] [Google Scholar]
- 103. Dea S, Plotto A, Manthey JA, et al. Interactions and thresholds of limonin and nomilin in bitterness perception in orange juice and other matrices. J Sens. 2013;28(4):311–323. doi:10.1111/joss.12046 [Google Scholar]
- 104. Miller JA, Thompson PA, Hakim IA, et al. Safety and feasibility of topical application of limonene as a massage oil to the breast. J Cancer Ther. 2012;3(5A):10.4236/jct.2012.325094. doi:10.4236/jct.2012.325094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kittler J, Krüger H, Ulrich D, et al. Content and composition of essential oil and content of rosmarinic acid in lemon balm and balm genotypes (Melissa officinalis). Genet Resour Crop Evol. 2018;65(5):1517–1527. [Google Scholar]
- 106. PubChem [Internet]. National Library of Medicine (US), National Center for Biotechnology Information; 2004. PubChem Compound Summary for CID 19589, 4-Methyl-1-hexene; Accepted March 9, 2021. https://pubchem.ncbi.nlm.nih.gov/compound/4-Methyl-1-hexene
- 107. Di Mola A, Massa A, De Feo V, et al. Effect of citral and citral related compounds on viability of pancreatic and human B-lymphoma cell lines. Med Chem Res. 2017;26:631–639. doi:10.1007/s00044-017-1779-z [Google Scholar]
- 108. Cikman O, Soylemez O, Ozkan OF, et al. Antioxidant activity of syringic acid prevents oxidative stress in L-arginine–induced acute pancreatitis: an experimental study on rats. Int Surg. 2015;100(5):891–896. doi:10.9738/INTSURG-D-14-00170.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mostafa DG, Khaleel EF, Badi RM, et al. Rutin hydrate inhibits apoptosis in the brains of cadmium chloride-treated rats via preserving the mitochondrial integrity and inhibiting endoplasmic reticulum stress. Neurol Res. 2019;41(7):594–608. doi:10.1080/01616412.2019.1596206 [DOI] [PubMed] [Google Scholar]
- 110. Indrayanto G, Syahrani A, Rahman A, et al. Benzoic acid. In: Brittain Harry G., ed. Analytical Profiles of Drug Substances and Excipients. Vol. 26. Academic Press; ; 1999: 1–46. doi:10.1016/S0099-5428(08)60620-6 [Google Scholar]
- 111. PubChem [Internet]. National Library of Medicine (US), National Center for Biotechnology Information; 2004. PubChem Compound Summary for CID 68077, Tangeretin; Accepted June 27, 2021. https://pubchem.ncbi.nlm.nih.gov/compound/Tangeretin
- 112. PubChem [Internet]. National Library of Medicine (US), National Center for Biotechnology Information; 2004. PubChem Compound Summary for CID 5280443, Apigenin. Accepted June 27, 2021. https://pubchem.ncbi.nlm.nih.gov/compound/Apigenin
- 113. PubChem [Internet]. National Library of Medicine (US), National Center for Biotechnology Information; 2004. PubChem Compound Summary for CID 637775, Sinapic acid; [Accepted June 27, 2021. https://pubchem.ncbi.nlm.nih.gov/compound/Sinapic-acid
- 114. PubChem [Internet]. National Library of Medicine (US), National Center forBiotechnology Information; 2004. PubChem Compound Summary for CID 72344, Nobiletin; [Accepted June 27, 2021. https://pubchem.ncbi.nlm.nih.gov/compound/Nobiletin
- 115. Koleva II, Van Beek TA, Linssen JP, et al. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal. 2002;13(1):8–17. [DOI] [PubMed] [Google Scholar]
- 116. Pichaiyongvongdee S, Rattanapun B, Haruenkit R. Total polyphenol content and antioxidant properties in different tissues of seven pomelo (Citrus grandis (L.) Osbeck) cultivars. Agric Nat Resour. 2014;48(6):989–996. [Google Scholar]
- 117. Pichaiyongvongdee S, Rattanapun B. Effect of chemical treatments to reduce the bitterness and drying on chemical physical and functional properties of dietary fiber pomelo powder from Citrus grandis (L.) Osbeck Albedo. Agric Nat Resour. 2015;49(1):122–132. [Google Scholar]
- 118. Jang H-D, Chang K-S, Chang T-C, Hsu C-L. Antioxidant potentials of buntan pumelo (Citrus grandis Osbeck) and its ethanolic and acetified fermentation products. Food Chem. 2010;118(3):554–558. [Google Scholar]
- 119. Singh P, Shukla R, Prakash B, et al. Chemical profile, antifungal, antiaflatoxigenic and antioxidant activity of Citrus maxima Burm. and Citrus sinensis (L.) Osbeck essential oils and their cyclic monoterpene, DL-limonene. Food Chem Toxicol. 2010;48(6):1734–1740. [DOI] [PubMed] [Google Scholar]
- 120. Chen L, Lai Y, Dong L, Kang S, Chen X. Polysaccharides from Citrus grandis L. Osbeck suppress inflammation and relieve chronic pharyngitis. Microb Pathog. 2017;113:365–371. [DOI] [PubMed] [Google Scholar]
- 121. Chen X, Lai Y, Song X, et al. Polysaccharides from Citrus grandis associate with luteolin relieves chronic pharyngitis by anti-inflammatory via suppressing NF-kappaB pathway and the polarization of M1 macrophages. Int J Immunopathol Pharmacol. 2018;32:2058738418780593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Fang J, Cao Z, Song X, et al. Rhoifolin alleviates inflammation of acute inflammation animal models and LPS-Induced RAW264.7 Cells via IKKbeta/NF-kappaB signaling pathway. Inflammation. 2020;43(6):2191–2201. [DOI] [PubMed] [Google Scholar]
- 123. Zhao YL, Yang XW, Wu BF, et al. Anti-inflammatory effect of pomelo peel and its bioactive Coumarins. J Agric Food Chem. 2019;67(32):8810–8818. [DOI] [PubMed] [Google Scholar]
- 124. Shukla S, Shankar E, Fu P, MacLennan GT, Gupta S. Suppression of NF-κB and NF-κB-regulated gene expression by apigenin through IκBα and IKK pathway in TRAMP mice. PLoS One 2015;10(9):e0138710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Suchal K, Malik S, Khan SI, et al. Molecular pathways involved in the amelioration of myocardial injury in diabetic rats by Kaempferol. Int J Mol Sci. 2017;18(5):1001. doi:10.3390/ijms18051001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Birari RB, Bhutani KK. Pancreatic lipase inhibitors from natural sources: unexplored potential. Drug Discov Today. 2007;12(19-20):879–889. [DOI] [PubMed] [Google Scholar]
- 127. Oyedepot A, Babarinde S. Effects of shaddock (Citrus maxima) fruit juice on glucose tolerance and lipid profile in type-II diabetic rats. Chem Sci Trans. 2013;2(1):19–24. [Google Scholar]
- 128. Gorinstein S, Leontowicz H, Leontowicz M, et al. Changes in plasma lipid and antioxidant activity in rats as a result of naringin and red grapefruit supplementation. J Agric Food Chem. 2005;53(8):3223–8228. [DOI] [PubMed] [Google Scholar]
- 129. Poulose SM, Harris ED, Patil BS. Citrus limonoids induce apoptosis in human neuroblastoma cells and have radical scavenging activity. J Nutr. 2005;135(4):870–877. [DOI] [PubMed] [Google Scholar]
- 130. Kundusen S, Gupta M, Mazumder UK, et al. Antitumor activity of Citrus maxima (Burm.) merr. leaves in Ehrlich’s ascites carcinoma cell-treated mice. ISRN Pharmacol. 2011;2011:138737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Sen T, Samanta SK. Medicinal plants, human health and biodiversity: a broad review. Adv Biochem Eng Biotechnol. 2015;147:59–110. [DOI] [PubMed] [Google Scholar]
- 132. Wang M, Liu B, Ruan R, et al. Genomic sequencing of Phyllosticta citriasiana provides insight into its conservation and diversification with two closely related Phyllosticta species associated with citrus. Front Microbiol. 2020;10:2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Aumeeruddy-Elalfi Z, Ismael IS, Hosenally M, Zengin G, Mahomoodally MF. Essential oils from tropical medicinal herbs and food plants inhibit biofilm formation in vitro and are non-cytotoxic to human cells. 3 Biotech. 2018;8(9):395. doi:10.1007/s13205-018-1413-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ruberto G, Baratta MT. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000;69(2):167–174. [Google Scholar]
- 135. Bijun LCDJX. Antimicrobial activity of shaddock peels extract [J]. Food Ferment Ind. 2004;1. [Google Scholar]
- 136. Suklampoo L, Thawai C, Weethong R, Champathong W, Wongwongsee W. Antimicrobial activities of crude extracts from pomelo peel of Khao-nahm-peung and Khao-paen varieties. Curr Appl Sci Technol. 2012;12(1):55–61. [Google Scholar]
- 137. Das S, Borah M, Ahmed S. Antibacterial activity of the ethanolic extract of leaves of Citrus maxima (Burm.) Merr. on Escherichia coli and Pseudomonas aeruginosa . Asian J Pharm Clin Res. 2013;6(4):136–139. [Google Scholar]
- 138. Chularojmontri L, Suwatronnakorn M, Wattanapitayakul SK. Pomelo fruit juice increased cell survival and enhanced Glutathione-S transferase (GST) activity in doxorubicin-induced rat cardiac cell cytotoxicity. Planta Med. 2009;75(09):PJ82. [Google Scholar]
- 139. Almeida MMB, de Sousa PHM, Arriaga ÂMC, et al. Bioactive compounds and antioxidant activity of fresh exotic fruits from northeastern Brazil. Food Res Int. 2011;44(7):2155–2159. [Google Scholar]
- 140. Buachan P, Chularojmontri L, Wattanapitayakul SK. Selected activities of Citrus maxima Merr. fruits on human endothelial cells: enhancing cell migration and delaying cellular aging. Nutrients. 2014;6(4):1618–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223–234. [DOI] [PubMed] [Google Scholar]
- 142. Wu C-H, Huang S-M, Lin J-A, Yen G-C. Inhibition of advanced glycation endproduct formation by foodstuffs. Food Funct. 2011;2(5):224–234. [DOI] [PubMed] [Google Scholar]
- 143. Hong HJ, Jin JY, Yang H, et al. Dangyuja (Citrus grandis Osbeck) peel improves lipid profiles and alleviates hypertension in rats fed a high-fat diet. Lab Anim Res. 2010;26(4):361–367. [Google Scholar]
- 144. Raasmaja A, Lecklin A, Li XM, et al. A water-alcohol extract of Citrus grandis whole fruits has beneficial metabolic effects in the obese Zucker rats fed with high fat/high cholesterol diet. Food Chem. 2013;138(2-3):1392–1399. [DOI] [PubMed] [Google Scholar]
- 145. Lee G-P, Jeong W-I, Jeong D-H, Do S-H, Kim T-H, Jeong K-S. Diagnostic evaluation of carbon tetrachloride-induced rat hepatic cirrhosis model. Anticancer Res. 2005;25(2A):1029–1038. [PubMed] [Google Scholar]
- 146. Lin SP, Chao PD, Tsai SY, Wang MJ, Hou YC. Citrus grandis peel increases the bioavailability of cyclosporine and tacrolimus, two important immunosuppressants, in rats. J Med Food. 2011;14(11):1463–1468. [DOI] [PubMed] [Google Scholar]