Abstract
Objective: Schwannomas are the most common type of neoplasm of the peripheral nerves. Enucleation is a standard surgical procedure; however, it occasionally results in iatrogenic nerve injury, even with atraumatic procedures. Herein, we present the clinical characteristics of schwannoma arising in the extremities and discuss the clinical outcomes of extra- and intra-capsular enucleation.
Patients and Methods: We reviewed 122 schwannomas treated at our institute. Schwannomas arising from the minor nerve (n=30) or intramuscularly (n=15) were operated using the extra-capsular technique. Of the 77 major nerve schwannomas, 62 schwannomas were treated using the intra-capsular technique and 15 schwannomas using the extra-capsular technique.
Results: Neurological deficits following enucleation were significantly lower using the intra-capsular technique than with the extra-capsular technique. The patient age, duration of symptoms, maximum tumor diameter, and site of occurrence were not associated with subsequent neurological deficits. With both techniques, no tumor recurrence was observed at the final follow-up.
Conclusion: These results support the use of intra-capsular micro-enucleation as a safe and reliable treatment for every type of schwannoma. To minimize the risk of nerve injury, en bloc resection should not be used because the main purpose of schwannoma surgery is the relief of symptoms, not tumor resection.
Keywords: Schwannoma, peripheral nerve, extremity, enucleation, microsurgery
Introduction
Schwannomas are the most common type of neoplasm of the peripheral nerves. They present as a benign tumor within the nerve sheaths and consist of hypertrophied Schwann cells1). Schwannomas are usually solitary (95%); however, some authors have reported multiple tumors in the extremities, which is referred to as schwannomatosis2, 3).
Surgical enucleation is the established treatment modality. However, some schwannomas cannot be easily enucleated; this occasionally results in an iatrogenic nerve injury, even with atraumatic procedures.
In this review, we present the clinical characteristics and diagnosis of a schwannoma arising in the extremities and discuss the clinical outcomes of the extra- and intra-capsular enucleation techniques.
Anatomy of Schwannoma and the Peripheral Nerve
Schwannomas are derived from the myelinating cells of the peripheral nervous system and are composed almost entirely of Schwann cells1).
Two distinct patterns of architecture were evident in these tumors. The Antoni-A component is a fibrillary, intensely polar tissue with an elongated appearance, while the Antoni-B component is composed of distinct, loose microcystic tissue adjacent to the Antoni-A regions4).
The tumor is well encapsulated by the following, 1) the epineurium, 2) the perineurium from the nerve bundle of origin, and 3) the pericapsular tissue that may contain nerve fibers and a tumor capsule that is often covered by tortuous blood vessels. The remaining nerve fascicles are spread over the capsule of the tumor; however, axons are not usually present within the tumor. This structure suggests that schwannomas can be removed without causing damage to the underlying nerve fascicles5, 6).
Diagnosis and Classification of Schwannoma: Which Nerve Does It Arise From?
An accurate diagnosis is the first essential step in the treatment of schwannomas. However, a preoperative diagnosis can be difficult, and there is a high risk of misdiagnosis due to the relatively low incidence of schwannomas and because the clinical signs and symptoms are often unclear. An isolated, palpable, slow-growing mass with a positive Tinel-like sign often leads to a suspicion of schwannoma; however, these findings and symptoms are not always typical.
Schwannomas are classified into three types depending on the nerve of origin (Table 1). The first type originates in the major nerve located intermuscularly, the second originates in the minor superficial sensory branch located subcutaneously, while the third originates from bundles of the intramuscular motor nerve7,8,9). This classification is useful for preoperative diagnoses and the choice of an operative procedure. The frequency of tinel-like signs in schwannomas has been reported to be between 43% and 94%9, 10). Tinel-like signs are usually positive in major nerve schwannomas because this nerve consists of several mixed bundles of sensory and motor nerve fibers. The clinical characteristics of the three types of schwannomas are summarized in Table 1.
Table 1. Three types of schwannoma: clinical features and treatment.
| Originating nerve | Symptom | Tinel-like sign | Location | Sensorydisturbance | Motordisturbance | Recommendedprocedure | Prognosis |
|---|---|---|---|---|---|---|---|
| Major nerve | painful mass | often positive | inter-muscular | sometimes positive | occasionally positive | intra-capsular enucleation | occasionally neurological deficit |
| Minor sensory branch | painful mass | sometimes negative | usually subcutaneous | usually negative | negative | extra-capsular enucleation or excision | good |
| Intramuscular motor branch | painless mass | often negative | intra-muscular | negative | negative | extra-capsular enucleation or excision | excellent |
The diagnosis of schwannoma arising in the extremities is usually straightforward and is based on physical and imaging findings7). Magnetic resonance imaging (MRI) is the most informative, with tumors often showing homogenous low signal intensity on T1-weighted images and high intensity on T2-weighted images5, 6). The “target signs” of central low-intensity signal and peripheral high-intensity signal are present in approximately 50% of patients9). A well-defined capsule is always present.
Why Does a Schwannoma Cause Pain?
A schwannoma is characterized by a slow and non-infiltrating pattern of growth that usually presents as a painless swelling for several years without any specific symptoms. When schwannomas become larger than 25 mm in diameter, patients frequently present with neurological symptoms, such as neuropathic pain, dysesthesia, sensory loss, and the presence of a tingling sensation due to local pressure exerted on the originating nerve8, 11, 12).
It is still unclear why major nerve schwannomas cause severe pain and neurological deficits in the originating nerve. The onset of symptoms correlates with progressive tumor growth and a sufficiently large tumor volume to compress the originating nerve. Long periods of compression are initially linked to venous insufficiency, with venous occlusion causing fatigue in the affected area, discomfort, and eventually pain. Additionally, mechanical compressive forces cause the cessation of retrograde and anterograde axonal transport, further aggravating nerve dysfunction and leading to structural damage, axonolysis, and finally Wallerian degeneration13).
Surgical Resection of Schwannoma: Indication and Purpose
Tumor resection is occasionally indicated for minor nerve schwannomas and intra-muscular schwannomas. If the diagnosis is not clear, a resection biopsy is recommended using the extra-capsular technique or simple excision.
The resection of a major nerve schwannoma is dependent on the patient’s symptoms. If the diagnosis is confirmed by MRI and the patient is asymptomatic, conservative treatment is recommended. However, Park et al.14) warned that large tumors are associated with a greater risk of major neurological deficit after surgery and therefore strongly recommend early surgical enucleation when a schwannoma is found.
We propose that early resection should not be performed because the main reason for schwannoma surgery is to relieve pain and tingling sensations, rather than resection of the tumor itself. Careful preoperative counseling is necessary to inform patients about the potential for immediate neurological deficit15, 16).
Techniques for Enucleation of Schwannoma: Extra-Capsular and Intra-Capsular
Schwannomas are located eccentrically in peripheral nerves and are well encapsulated by the epineurium, perineurium, and tumor capsule. Therefore, it is generally accepted that careful dissection under magnification can achieve complete enucleation without causing neurological deficit17, 18). Although many published studies support this concept19,20,21), this may not always hold true22,23,24,25,26). A recent study reported that some schwannomas cannot be completely enucleated26). Even when a schwannoma is dissected from the involved nerve using the atraumatic technique, incomplete nerve palsies can sometimes occur.
Two main approaches have been reported: extra-capsular and intra-capsular enucleation27). Till date, enucleation techniques have mostly been used for extracapsular resection along with the tumor capsule. Good results have been reported with both types of enucleation technique5, 6, 11). However, neurological deficits were reported in more than 70% of patients in more recent studies22,23,24,25,26). This notable and unacceptably high incidence of iatrogenic nerve injury following enucleation raises questions about the purpose of treatment for schwannoma. To minimize the risk of intraoperative injury, our preference for the treatment of schwannoma arising in the extremities is the modified microsurgical technique of palliative, intra-capsular enucleation27). The occurrence of neurological deficits following the extra-capsular and intra-capsular enucleation of schwannomas should be compared in future studies.
Incidence of Neurological Deficit Following Extra-Capsular Enucleation
The incidence of complications following the surgical treatment of peripheral schwannomas is extremely variable, ranging from 1.5% to 80% in the literature. Oberle et al.24) reported an immediate postoperative sensory deficit in 50% of the cases. Donner et al.7) reported that 13% of 85 schwannoma patients developed motor weakness after enucleation. In a series of schwannomas from the upper limb, Adani et al.22) reported postoperative paresthesia in 23 of 24 patients. Knight et al.2) reported significant complications in only 5 of 198 (2.5%) patients; however, these authors did not describe the detailed surgical technique. In a recent study, Park et al.14) reviewed 56 patients treated with enucleation and reported that 41 patients (73%) developed a new neurological deficit.
Sawada et al.26) suggested three possible explanations for the occurrence of nerve dysfunction even when careful enucleation was performed. First, some of the fascicles surrounding the tumor may be damaged by incision of the epineurium, although these may still be functional. Second, a longitudinal incision in the nerve sheath may divide a small number of fascicles running through the tumor mass. Third, intact fascicles that are not involved in the tumor may become compressed during or after the operation, resulting in neurapraxia injury.
It is unclear which of the above contributes the most to nerve dysfunction. Park et al.14) stated that the transection of fascicles running through the tumor is the major cause of postoperative neurological deficits. However, Donner et al.7) stimulated the fascicles entering the tumor substance and found that their division did not usually lead to increased neurological deficits because they were already non-functional.
From 2000 to 2004, our group performed enucleation using the extra-capsular technique. However, after 2005, we performed enucleation with the intra-capsular technique due to the high incidence of neurological deficits after surgery. To minimize the risk of nerve injury, we advocate several procedures, as summarized in Table 2.
Table 2. Factors to minimize neurological deficit after enucleation.
| Pre-operative | Avoid unnecessary biopsy |
| Intra-operative | Perform intra-capsular enucleation for major nerve schwannomaLimited incision of the epineuriumAtraumatic dissection, especially at proximal and distal polesNo en-bloc resection if traumatic |
| Post-operative | Drainage to prevent hematomaRaising the affected limb |
Surgical Technique for Intra-Capsular Enucleation Under the Microscope
We applied an air tourniquet, if possible, to prevent hemorrhage and to maintain good vision under the microscope. The skin incision was made in the center of the tumor and extended along the anatomic course of the involved nerve. Thus, the tumor was exposed, and the proximal and distal extents of the affected nerve were defined using vascular tapes (Figure 1). At this point, the location of the uninvolved fascicle splaying over the tumor surface was carefully noted. Using a sharp-pointed knife, a longitudinal incision was made in the epineurium distant from the fascicles, until the shiny surface of the tumor was exposed. This incision was always parallel to the nerve and was limited to within 75% of the tumor length. A small sample of the tumor was sent to the pathology department to confirm the histopathological diagnosis during the operation. A gentle dissection along the plane of the capsule and epineurium was performed using a curette (Figure 2). Usually, the central portion of the tumor is not adherent, thus allowing the separation of the tumor and epineurium. However, care must be taken not to ablate the fascicle and tumor, especially at the proximal and distal poles of the tumor, because traumatic ablation can injure the fascicle and damage any remaining nerve function. The tumor was resected piece-by-piece as much as possible (Figure 3). An en bloc resection of the schwannoma was never performed if the epineurium and capsule were tightly adhered. Intraoperative nerve conduction studies aimed at identifying functional and non-functional fascicles were not performed because it was impossible to obtain an accurate diagnosis.
Figure 1.
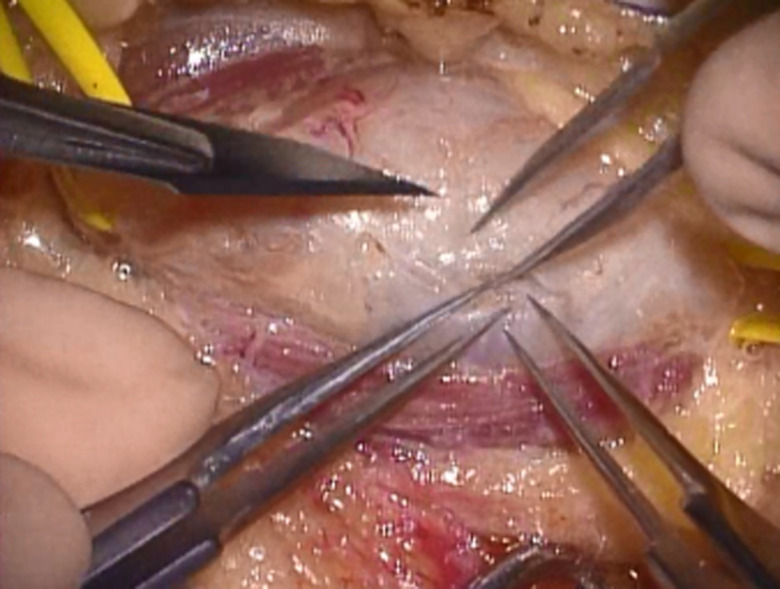
A schwannoma was seen inside the epineurium. The tumor was exposed, and the proximal and distal portions of the affected nerve were maintained with vascular tapes.
Figure 2.
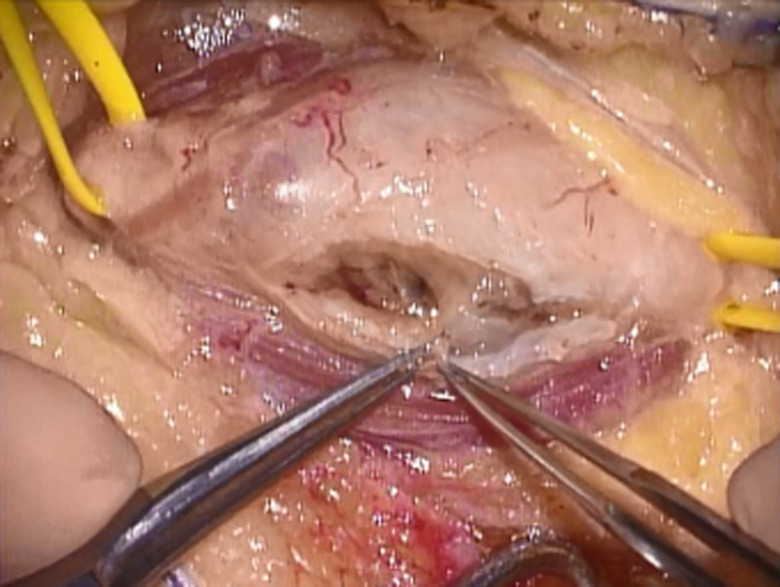
A longitudinal incision in the epineurium was made at a distance from the fascicles. This incision was parallel to the nerve and was limited to within 75% of the tumor length.
Figure 3.
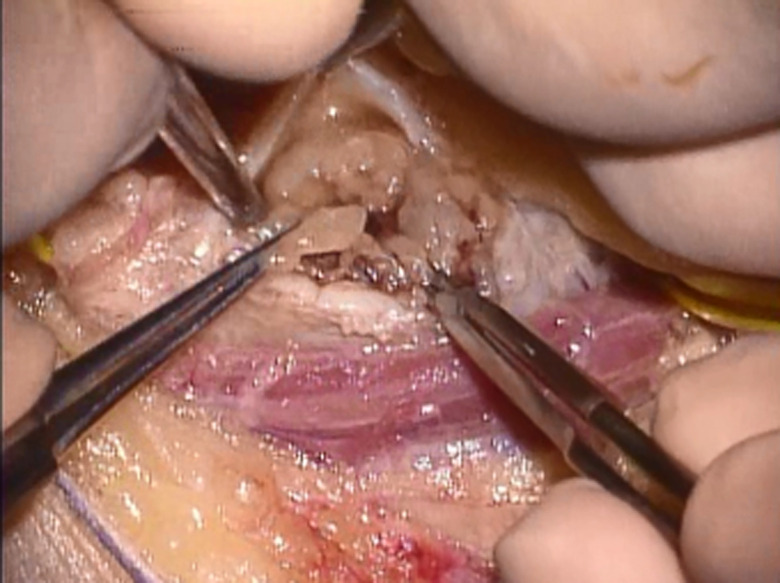
Gentle dissection along the plane of the capsule and epineurium was performed using a curette. The tumor was resected piece-by-piece. En block resection of schwannoma was not performed if the epineurium and capsule was tightly adhered.
Following the release of the tourniquet, bleeding was carefully stopped to prevent secondary compression of the affected nerve by hematoma (Figure 4). Suction drainage was routinely performed for 3 days after surgery, and the dissected window of the epineurium was left open. It is to be noted that a tight bandage was never applied, and the affected extremity was kept in a raised position for at least one week after surgery (Table 2).
Figure 4.
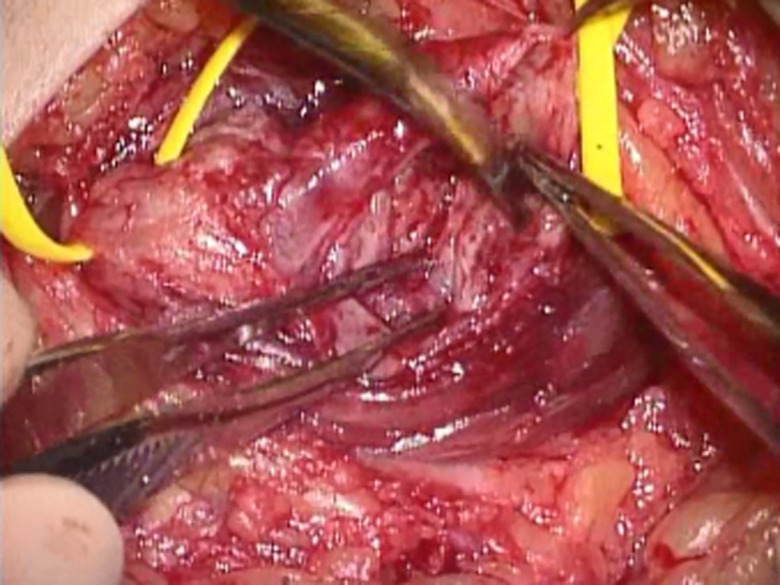
Following release of the tourniquet, bleeding was carefully stopped to prevent secondary compression of the affected nerve by hematoma.
Clinical Outcome Following Extra- or Intra-Capsular Enucleation in Our Patient Series
Beginning in 2000, we identified 122 patients from our institute who underwent surgical excision of schwannoma arising in the extremities (Table 3). This study was approved by the Regional Ethics Committee of Nagato General Hospital. All cases were histopathologically confirmed after the surgery. A single nerve was affected in 119 patients, while three patients exhibited multiple lesions. There were 59 men and 63 women with a mean age of 59.3 years (range, 22–85 years). The size of the schwannomas was estimated using MRI from their longitudinal extent along the affected nerve. The average tumor size was 31.7 mm (range, 10–100 mm). The interval between the onset of symptoms and surgical excision ranged from 1 month to 120 months (mean, 45.4 months). Tinel-like signs were noted in 83 patients and sensory disturbances in 23 patients, while 6 patients presented with motor weakness or palsy. The mean follow-up period after surgery was 11.2 months (range, 6–48 months).
Table 3. Our series of 122 patients with extremity schwannoma.
| Origin | Operation | N | Biopsy (%) | Location | Minor deficit (%) | Major deficit (%) | Transient deficit (%) | **FU (month) | Re-operated |
|---|---|---|---|---|---|---|---|---|---|
| Major nerve | Intra-capsular enucleation | 62 | 7 (11) | *Upper: 30Lower: 32 | 60 (97) | 2 (3) | 0 | 12 | 3 (6) |
| Extra-capsular E | 15 | 1 (7) | U: 6L: 9 | 2 (13) | 9 (60) | 4 (27) | 15 | 0 | |
| Minor nerve | Extra- or excision | 30 | 4 (13) | U: 8L: 22 | 30 (100) | 0 | 0 | 6 | 0 |
| Intra-muscular | Extra- or excision | 15 | 4 (27) | U: 6L: 9 | 15 (100) | 0 | 0 | 6 | 0 |
*Upper: upper extremity; Lower: lower extremity. **Minimum follow-up.
Notably, 77 schwannomas arose from the major nerve, whereas 30 schwannomas arose from the minor sensory nerve branch present mainly in the subcutaneous tissue. Fifteen schwannomas exited intra-muscularly and probably arose from the small motor nerve. Of the 77 major nerve schwannomas, 62 were treated using the intra-capsular technique and 15 using the extra-capsular technique. Schwannomas arising from the minor nerve (n=30) or intramuscularly (n=15) were managed using the extra-capsular technique or by simple excision.
The severity of postoperative neurological deficit was classified into 3 grades: grade 1, no or minor deficit experienced, or development of only minor and temporary sensory changes that recovered within 6 months; grade 2, developed major new neurological deficits that took more than 6 months to recover; and grade 3, transiently developed new motor deficits or paresthesia after surgery that had not recovered at the final follow-up.
After surgery, pain relief was satisfactory in all the patients with major nerve schwannoma. Tinel-like signs were strongly positive shortly after surgery but decreased thereafter. Of the 15 patients with major nerve schwannoma, who underwent surgery using the extra-capsular technique, nine patients showed a major neurological deficit (grade 2), four patients showed transient deficit (grade 3), and only two patients had minor deficits (grade 1) after surgery. In contrast, of the 62 cases with major nerve schwannoma operated using the intra-capsular technique, only two patients (3%) showed major deficits and the remainder of patients (97%) showed minor deficits. All 45 patients with minor nerve or intramuscular schwannomas experienced only minor deficits after surgery. One patient with schwannoma arising from the median nerve showed no neurological deficit immediately after surgery, but later developed numbness in the middle finger caused by hematoma and the use of a tight bandage. This symptom was relieved after bandage removal. Three patients with major nerve schwannoma required re-exploration due to a residual palpable mass. However, none of the patients showed recurrence of the tumor, and intra-capsular seroma or hematoma was not observed in each case. No recurrence of the tumor was observed with either technique up to the final follow-up.
Postoperative neurological deficits for the two surgical procedures of extra-capsular and intra-capsular enucleation were compared statistically. Fisher’s exact test was used to compare resection with the two enucleation methods. The level of significance was set at P<0.05. The occurrence of neurological deficits following tumor excision was significantly lower with the intra-capsular technique than with the extra-capsular technique.
Influence of Tumor Location, Duration, and Size on the Neurological Deficit
It is important to consider the possible risk factors associated with nerve deficits following enucleation, although these remain controversial. Oberle et al.24) reported that postoperative neurological deficits occur mostly in patients with large tumors or those with long-standing symptoms. Sawada et al.26) suggested that schwannomas arising from the brachial plexus or the proximal part of the upper arm may present a higher risk of injury during surgery. Takase et al.28) reported that the period required for recovery of neurological deficits was significantly correlated with the duration of the pretreatment symptoms, but was not influenced by the tumor size. Conversely, Park et al.14) reported that the age and duration of symptoms were not significantly linked to neurological deficits. Instead, they suggested that larger tumors were associated with a greater risk of major neurological deficits following surgery and therefore strongly recommend early surgical enucleation of schwannoma.
In our series treated with palliative intra-capsular enucleation (n=62), we found no significant correlation between the postoperative neurological deficit and tumor location and size, or onset of symptoms by the Mann-Whitney U test. The present results demonstrate that our technique is safe for every type of schwannoma. Careful preoperative counseling is still important to inform patients about the potential for immediate neurological deficit.
Incidence of Schwannoma Recurrence or Malignant Change
If the frequency of tumor recurrence or malignant change is high, intra-capsular resection cannot be justified; additionally, the extra-capsular technique is recommended for major nerve schwannoma. However, it is clear from previous studies that schwannoma recurrence is very rare after surgery, even when it is only partially resected15). In our series, we observed no obvious recurrences after partial enucleation27). Malignant transformation has yet to be reported for benign schwannoma. Three of our cases required re-exploration; however, these showed only intra-capsular hematoma or cystic changes, with no recurrence of schwannoma.
Informed consent
Informed consent was obtained from all patients.
Funding
This research was not funded by any individual or organization.
Conflict of interest
All authors have no conflicts of interest to declare.
References
- 1.Weiss SW, Goldblum JR, Enzinger FM. Benign tumors of the peripheral nerves. In: Enzinger and Weiss’s soft tissue tumors. 4th ed. Weiss S, Goldblum JR, Eds. St. Louis, Mosby, 2001; 1111–1208.
- 2.Knight DM, Birch R, Pringle J. Benign solitary schwannomas: a review of 234 cases. J Bone Joint Surg Br 2007; 89: 382–387. doi: 10.1302/0301-620X.89B3.18123 [DOI] [PubMed] [Google Scholar]
- 3.Saito S, Suzuki Y. Schwannomatosis affecting all three major nerves in the same upper extremity. J Hand Surg Eur Vol 2010; 35: 592–594. doi: 10.1177/1753193410369284 [DOI] [PubMed] [Google Scholar]
- 4.Wippold FJ, 2nd, Lubner M, Perrin RJ, et al. Neuropathology for the neuroradiologist: Antoni A and Antoni B tissue patterns. AJNR Am J Neuroradiol 2007; 28: 1633–1638. doi: 10.3174/ajnr.A0682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim DH, Murovic JA, Tiel RL, et al. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg 2005; 102: 246–255. doi: 10.3171/jns.2005.102.2.0246 [DOI] [PubMed] [Google Scholar]
- 6.Kim DH, Murovic JA, Tiel RL, et al. A series of 146 peripheral non-neural sheath nerve tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg 2005; 102: 256–266. doi: 10.3171/jns.2005.102.2.0256 [DOI] [PubMed] [Google Scholar]
- 7.Donner TR, Voorhies RM, Kline DG. Neural sheath tumors of major nerves. J Neurosurg 1994; 81: 362–373. doi: 10.3171/jns.1994.81.3.0362 [DOI] [PubMed] [Google Scholar]
- 8.Phalen GS. Neurilemmomas of the forearm and hand. Clin Orthop Relat Res 1976; 219–222. [PubMed] [Google Scholar]
- 9.Shimose S, Sugita T, Kubo T, et al. Major-nerve schwannomas versus intramuscular schwannomas. Acta Radiol 2007; 48: 672–677. doi: 10.1080/02841850701326925 [DOI] [PubMed] [Google Scholar]
- 10.Ujigo S, Shimose S, Kubo T, et al. Therapeutic effect and risk factors for complications of excision in 76 patients with schwannoma. J Orthop Sci 2014; 19: 150–155. doi: 10.1007/s00776-013-0477-z [DOI] [PubMed] [Google Scholar]
- 11.Das Gupta TK, Brasfield RD, Strong EW, et al. Benign solitary Schwannomas (neurilemomas). Cancer 1969; 24: 355–366. doi: [DOI] [PubMed] [Google Scholar]
- 12.Strickland JW, Steichen JB. Nerve tumors of the hand and forearm. J Hand Surg Am 1977; 2: 285–291. doi: 10.1016/S0363-5023(77)80128-7 [DOI] [PubMed] [Google Scholar]
- 13.Siqueira MG, Socolovsky M, Martins RS, et al. Surgical treatment of typical peripheral schwannomas: the risk of new postoperative deficits. Acta Neurochir (Wien) 2013; 155: 1745–1749. doi: 10.1007/s00701-013-1818-6 [DOI] [PubMed] [Google Scholar]
- 14.Park MJ, Seo KN, Kang HJ. Neurological deficit after surgical enucleation of schwannomas of the upper limb. J Bone Joint Surg Br 2009; 91: 1482–1486. doi: 10.1302/0301-620X.91B11.22519 [DOI] [PubMed] [Google Scholar]
- 15.Bakkouri WE, Kania RE, Guichard JP, et al. Conservative management of 386 cases of unilateral vestibular schwannoma: tumor growth and consequences for treatment. J Neurosurg 2009; 110: 662–669. doi: 10.3171/2007.5.16836 [DOI] [PubMed] [Google Scholar]
- 16.Kim P, Ebersold MJ, Onofrio BM, et al. Surgery of spinal nerve schwannoma. Risk of neurological deficit after resection of involved root. J Neurosurg 1989; 71: 810–814. doi: 10.3171/jns.1989.71.6.0810 [DOI] [PubMed] [Google Scholar]
- 17.Russell SM. Preserve the nerve: microsurgical resection of peripheral nerve sheath tumors. Neurosurgery 2007; 61(Suppl): 113–117, discussion 117–118. [DOI] [PubMed] [Google Scholar]
- 18.Uchida K, Nakajima H, Sato R, et al. Microsurgical intraneural extracapsular resection of neurinoma around the cervical neuroforamen: a technical note. Minim Invasive Neurosurg 2009; 52: 271–274. doi: 10.1055/s-0029-1241849 [DOI] [PubMed] [Google Scholar]
- 19.Kim SM, Seo SW, Lee JY, et al. Surgical outcome of schwannomas arising from major peripheral nerves in the lower limb. Int Orthop 2012; 36: 1721–1725. doi: 10.1007/s00264-012-1560-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai CS, Chen IC, Lan HC, et al. Management of extremity neurilemmomas: clinical series and literature review. Ann Plast Surg 2013; 71(Suppl 1): S37–S42. doi: 10.1097/SAP.0000000000000042 [DOI] [PubMed] [Google Scholar]
- 21.Tang CY, Fung B, Fok M, et al. Schwannoma in the upper limbs. BioMed Res Int 2013; 2013: 167196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adani R, Baccarani A, Guidi E, et al. Schwannomas of the upper extremity: diagnosis and treatment. Chir Organi Mov 2008; 92: 85–88. doi: 10.1007/s12306-008-0049-0 [DOI] [PubMed] [Google Scholar]
- 23.Kang HJ, Shin SJ, Kang ES. Schwannomas of the upper extremity. J Hand Surg [Br] 2000; 25: 604–607. doi: 10.1054/jhsb.2000.0472 [DOI] [PubMed] [Google Scholar]
- 24.Oberle J, Kahamba J, Richter HP. Peripheral nerve schwannomas—an analysis of 16 patients. Acta Neurochir (Wien) 1997; 139: 949–953. doi: 10.1007/BF01411304 [DOI] [PubMed] [Google Scholar]
- 25.Ozdemir O, Ozsoy MH, Kurt C, et al. Schwannomas of the hand and wrist: long-term results and review of the literature. J Orthop Surg (Hong Kong) 2005; 13: 267–272. doi: 10.1177/230949900501300309 [DOI] [PubMed] [Google Scholar]
- 26.Sawada T, Sano M, Ogihara H, et al. The relationship between pre-operative symptoms, operative findings and postoperative complications in schwannomas. J Hand Surg [Br] 2006; 31: 629–634. doi: 10.1016/J.JHSB.2006.06.008 [DOI] [PubMed] [Google Scholar]
- 27.Date R, Muramatsu K, Ihara K, et al. Advantages of intra-capsular micro-enucleation of schwannoma arising from extremities. Acta Neurochir (Wien) 2012; 154: 173–178, discussion 178. doi: 10.1007/s00701-011-1213-0 [DOI] [PubMed] [Google Scholar]
- 28.Takase K, Yamamoto K, Imakiire A. Clinical pathology and therapeutic results of neurilemmoma in the upper extremity. J Orthop Surg (Hong Kong) 2004; 12: 222–225. doi: 10.1177/230949900401200216 [DOI] [PubMed] [Google Scholar]


