Abstract
In Emergency Room, Point-of-care antigen testing for SARS-CoV-2 antigen can expedite clinical strategies for patient management. We tested 1,232 consecutive patients during Italian second wave peak using the recent LumiraDx microfluidic assay. This assay showed high concordance (96.9 %), sensitivity and specificity compared to molecular testing, being highly valuable.
Keywords: SARS-CoV-2, COVID-19, Antigen test, POCT, Emergency room
Development of rapid and easy-to-perform diagnostic tests is of high priority for COVID-19 pandemic, to shorten time to result-reporting and apply strategies for patients management and contact-tracing (Arons et al., 2020). The gold standard for SARS-CoV-2 diagnosis of infection relies on nucleic acid amplification testing (NAAT) that requires hours to result reporting (Dinnes et al., 2021), although some rapid molecular tests, like Abbott ID NOW and Roche Cobas Liat, are available (Blackall et al., 2021; Stokes et al., 2021). During the pandemic, proper patient allocation in Emergency Room (ER) is helped by the extensive use of COVID-19 antigen point-of-care-testing (POCT) (Cerutti et al., 2020), primarily based on lateral flow immunochromatography and visual result-reporting (https://www.finddx.org). The evaluation of these tests by the scientific community outlines a major concern of false-negative results in case of low viral loads. A recent Cochrane review (Dinnes et al., 2021) summarizes evidences on 48 POCT for SARS-CoV-2, with the final conclusion that they correctly identified COVID-19 infection in 72 % of symptomatic (78 % within the first week from symptoms onset) and 58 % of asymptomatic patients. These data are certainly not satisfying for the clinical management of COVID-19 affected patients in ER during the pandemic.
A new generation of POCT for SARS-CoV-2 antigen based on the microfluidic technology integrating sample preparation, reaction and detection into a miniaturized chip (Whitesides, 2006) has recently demonstrated a significant higher sensitivity compared with lateral flow POCT (Bianco et al., 2021; Cento et al., 2021; Dinnes et al., 2021; Drain et al., 2021; Kohmer et al., 2021).
The microfluidic technology has become available during the second wave of COVID-19 pandemic, and showed a very high sensitivity (ranging from 50.0 %–97.6 % according to different rate of COVID-19 prevalence), specificity (96.6 %–100 %) and concordance with molecular testing (63 %–96.9 %). The recent SARS-CoV-2 Ag Test LumiraDx (LDT-Ag, MA, USA) microfluidic assay is one of the most evaluated (Table 1 ).
Table 1.
Reported sensitivity and specificity of microfluidic-based rapid point-of-care tests for detecting SARS CoV-2 antigen October 2020-March 2021.
| References | SARS CoV-2 Microfluidic Antigen Test | Type of patients | COVID-19 prevalence | RT-PCR concordance | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|---|
| Cento et al., Viruses, 2021 | LumiraDx SARS-CoV-2 Ag Test, UK |
Adults | 36 % | 85.6 % | 85 % | 97 % | 94 % | 92 % |
| Bianco et al., Journal of Clinical Virology, 2021 | LumiraDx SARS-CoV-2 Ag Test, UK |
Adults +pediatric |
33 % | 91.5 % | 90.3 % | 92.1 % | 85 % | 95 % |
| Drain et al., Infectious Diseases and Therapy, 2021 | LumiraDx SARS-CoV-2 Ag Test, UK |
Adults +pediatric |
24 % | NA | 97.6 % | 96.6 % | NA | NA |
| Kohmer et al., Journal of Clinical Medicine, 2021 | LumiraDx SARS-CoV-2 Ag Test, UK |
NA | 74 % | 63 % | 50 % | 100 % | 100 % | 41 % |
| Orsi A et al., Journal Virol Methods, 2021 | FREND COVID-19 Ag assay, South Korea |
NA | 54 % | 96.3 % | 93.3 % | 100 % | 100 % | 92.5 % |
| This work | LumiraDx SARS-CoV-2 Ag Test, UK |
Adults | 25.3 % | 96.9 % | 89.6 % | 99.4 % | 97.9 % | 96.7 % |
NA: Not Available.
We evaluated the LDT-Ag for SARS-CoV-2 nucleocapsid detection in comparison to NAAT, in 1,232 consecutive patients referring to ER during the peak of phase-2 COVID-19 Italian epidemics (March 1st - March 31st, 2021) in a tertiary hospital in Turin. Nasal-pharyngeal swabs for NAAT and nasal swab for LDT-Ag were performed in each patient at the same time; for NAAT, the following methods were used: DiaSorin Simplexa® (n = 523, 42.3 %), Panther Hologic® (n = 254, 20.6 %), Cepheid Xpert® (n = 186, 15.0 %), Thermofisher TaqPath RT PCR® (n = 166, 13.4 %), Cobas 6800 Roche® (n = 87, 7.0 %), and Seegene Allplex® 2019 n-CoV Assay (n = 16, 1.3 %). Mean Cycle threshold (Ct) values through reactive genes was used as a proxy for viral load. SARS-CoV-2 culturing was performed in a subset of discordant LDT-Ag-/NAAT+ samples.
Comparison between continuous variables were done by the non parametric Mann-Whitney test. The degree of concordance between LDT-Ag and NAAT assays was tested by Cohen’s Kappa coefficient and logistic regression of LDT-Ag over NAAT as the reference variable was used to compute LDT-Ag performances (R statistical framework).
Out of 4,257 people who accessed the ER during the study period, 1,232 (28.9 %) couples of paired NAAT/LDT-Ag were available for comparison, with 312 (25.3 %) NAAT positive samples.
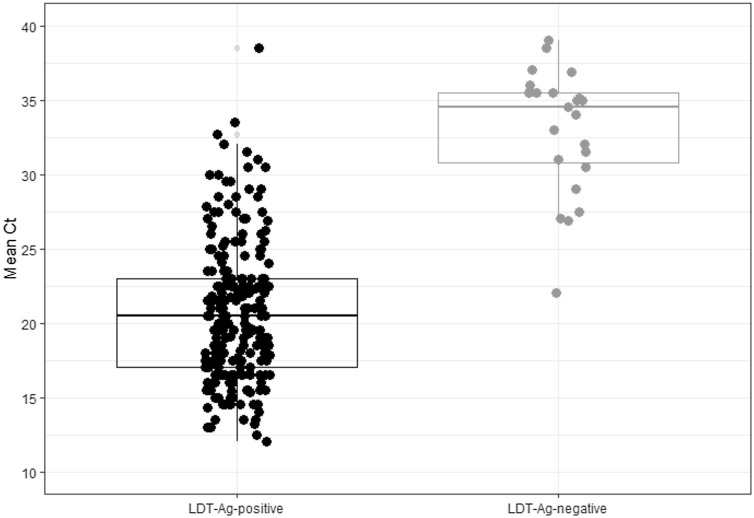
Concordance between the two methods was 97 % (1,195/1,232, Cohen’s k = 0.92, 95 % CI: 0.89−0.94, p < 0.001). We calculated LDT-Ag sensitivity (90.1 %, 95 % CI: 86.2–93.1), specificity (99.4 %, 95 % CI: 98.6–99.8), negative (96.5 %, 95 % CI: 95.0–97.5) and positive predictive value (98.2 %, 95 % CI: 95.7–99.3), negative (0.10, 95 % CI: 0.07−0.14) and positive likelihood ratio (138.1, 95 % CI: 62.1–306.5). Ct values (available for 77.0 % of NAAT positive samples) were significantly lower in concordant LDT-Ag+/NAAT+ samples (mean Ct = 20.2, CI 95 %: 20.1–21.3, Q1-Q3: 17.0–23.0) than in discordant LDT-Ag-/NAAT+ ones (mean Ct = 33.7, CI 95 %: 31.8–35.5, Q1-Q3: 30.7–36.4, p < 0.001) (Fig. 1 ).
Fig. 1.
Mean Ct across viral genes values for the LumiraDx SARS-CoV-2 Ag Test (LDT-Ag) positive and negative tests in the studied group.
According to different Ct classes (<25, 25–30, 30–35, >35), the detection rate was 98.4 %, 87.9 %, 43.8 % and 8.3 %, respectively. Fifteen discordant LDT-Ag-/NAAT+ samples (mean Ct = 34.8, CI 95 %: 33.0–36.8) were inoculated in Vero cells. After subculturing with sequential blind passages, no cytopathic effect was observed; supernatants tested negative for SARS-CoV-2 by NAAT after 21-day incubation.
High accurate, sensitive and specific POCTs for SARS-CoV-2 antigen allow timely and appropriate actions, with the potential of better patient management and mitigation of virus spreading. This turns crucial when ER are overwhelmed during the epidemic phase. However, major concern is the false-negative rate due to low viral load (Arons et al., 2020; Cento et al., 2021; Orsi et al., 2021) for antigen testing. A new generation of microfluidic-based POCT recently demonstrated a higher sensitivity than later flow immunochromatography-based POCT (Bianco et al., 2021; Dinnes et al., 2021; Drain et al., 2021; Kohmer et al., 2021; Orsi et al., 2021; Toptan et al., 2021). In our experience, we confirmed recent data showing that LumiraDx correctly identified the majority of NAAT positive samples and that when cell-culturing discordant antigen-negative/NAAT-positive samples, a negative result was reported, consistent with low viable virus and low infectiousness (Whitesides, 2006). In agreement with recently published works (Dinnes et al., 2021; Drain et al., 2021; Kohmer et al., 2021; Orsi et al., 2021; Toptan et al., 2021), our data confirm that microfluidic is highly effective with a significantly higher sensitivity than other POCTs, thus fulfilling the requirement of a rapid ER patient management.
In conclusion, a new generation of POCTs characterized by higher sensitivity, specificity and accuracy is now available, playing a central role as a replacement for NAAT in ER patient triaging and management. Confirmatory NAAT could be limited to high clinical risk patients with a negative rapid test result, thus lowering also diagnostic costs.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributions
Elisa Burdino: Writing - original draft, Conceptualization. Francesco Cerutti: Writing - original draft, Formal analysis. Francesco Panero: Writing - original draft, Investigation, Validation. Tiziano Allice: Investigation, Validation. Gabriella Gregori: Investigation, Validation. Maria Grazia Milia: Investigation, Validation. Giulia Cavalot: Investigation, Validation, Andrea Altavilla: Investigation, Validation. Franco Aprà: Conceptualization, Writing - review & editing, Supervision. Valeria Ghisetti: Conceptualization, Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The Authors acknowledge all the healthcare personnel, including nurses, laboratory technicians and health professionals, for the large efforts in the fight against the COVID-19 pandemics.
References
- Arons M.M., Hatfield K.M., Reddy S.C., Kimball A., James A., Jacobs J.R., Taylor J., Spicer K., Bardossy A.C., Oakley L.P., Tanwar S., Dyal J.W., Harney J., Chisty Z., Bell J.M., Methner M., Paul P., Carlson C.M., McLaughlin H.P., Thornburg N., Tong S., Tamin A., Tao Y., Uehara A., Harcourt J., Clark S., Brostrom-Smith C., Page L.C., Kay M., Lewis J., Montgomery P., Stone N.D., Clark T.A., Honein M.A., Duchin J.S., Jernigan J.A. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N. Engl. J. Med. 2020;382:2081–2090. doi: 10.1056/nejmoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco G., Boattini M., Barbui A.M., Scozzari G., Riccardini F., Coggiola M., Lupia E., Cavallo R., Costa C. Evaluation of an antigen-based test for hospital point-of-care diagnosis of SARS-CoV-2 infection. J. Clin. Virol. 2021;139 doi: 10.1016/j.jcv.2021.104838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackall D., Moreno R., Jin J., Plotinsky R., Dworkin R., Oethinger M. Performance characteristics of the Roche diagnostics cobas liat PCR system as a COVID-19 screening tool for hospital admissions in a regional health care delivery system. J. Clin. Microbiol. 2021;59 doi: 10.1128/JCM.01278-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cento V., Renica S., Matarazzo E., Antonello M., Colagrossi L., Di Ruscio F., Pani A., Fanti D., Vismara C., Puoti M., Scaglione F., Perno C., Alteri C. Frontline screening for SARS-CoV-2 infection at emergency department admission by third generation rapid antigen test: can we spare RT-qPCR? Viruses. 2021;13:818. doi: 10.3390/v13050818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti F., Burdino E., Milia M.G., Allice T., Gregori G., Bruzzone B., Ghisetti V. Urgent need of rapid tests for SARS CoV-2 antigen detection: evaluation of the SD-Biosensor antigen test for SARS-CoV-2. J. Clin. Virol. 2020;132 doi: 10.1016/j.jcv.2020.104654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinnes J., Deeks J.J., Berhane S., Taylor M., Adriano A., Davenport C., Dittrich S., Emperador D., Takwoingi Y., Cunningham J., Beese S., Domen J., Dretzke J., Ferrante di Ruffano L., Harris I.M., Price M.J., Taylor-Phillips S., Hooft L., Leeflang M.M.G., McInnes M.D.F., Spijker R., Van den Bruel A., Arevalo-Rodriguez I., Buitrago D.C., Ciapponi A., Mateos M., Stuyf T., Horn S., Salameh J.P., McGrath T.A., van der Pol C.B., Frank R.A., Prager R., Hare S.S., Dennie C., Jenniskens K., Korevaar D.A., Cohen J.F., van de Wijgert J., Damen J.A.A.G., Wang J., Agarwal R., Baldwin S., Herd C., Kristunas C., Quinn L., Scholefield B. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2021 doi: 10.1002/14651858.CD013705.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drain P.K., Ampajwala M., Chappel C., Gvozden A.B., Hoppers M., Wang M., Rosen R., Young S., Zissman E., Montano M. A rapid, high-sensitivity SARS-CoV-2 nucleocapsid immunoassay to aid diagnosis of acute COVID-19 at the point of care: a clinical performance study. Infect. Dis. Ther. 2021;10:753–761. doi: 10.1007/s40121-021-00413-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohmer N., Toptan T., Pallas C., Karaca O., Pfeiffer A., Westhaus S., Widera M., Berger A., Hoehl S., Kammel M., Ciesek S., Rabenau H.F. The comparative clinical performance of four SARS-CoV-2 rapid antigen tests and their correlation to infectivity in vitro. J. Clin. Med. 2021;10:328. doi: 10.3390/jcm10020328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsi A., Pennati B.M., Bruzzone B., Ricucci V., Ferone D., Barbera P., Arboscello E., Dentone C., Icardi G. On-field evaluation of a ultra-rapid fluorescence immunoassay as a frontline test for SARS-CoV-2 diagnostic. J. Virol. Methods. 2021;295 doi: 10.1016/j.jviromet.2021.114201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes W., Berenger B.M., Singh T., Adeghe I., Schneider A., Portnoy D., King T., Scott B., Pabbaraju K., Shokoples S., Wong A.A., Gill K., Turnbull L., Hu J., Tipples G. Acceptable performance of the Abbott ID NOW among symptomatic individuals with confirmed COVID-19. J. Med. Microbiol. 2021;70 doi: 10.1099/JMM.0.001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toptan T., Eckermann L., Pfeiffer A.E., Hoehl S., Ciesek S., Drosten C., Corman V.M. Evaluation of a SARS-CoV-2 rapid antigen test: potential to help reduce community spread? J. Clin. Virol. 2021;135 doi: 10.1016/j.jcv.2020.104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesides G.M. The origins and the future of microfluidics. Nature. 2006 doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]