Abstract
Background
Two antifibrotic drugs, pirfenidone and nintedanib, are licensed for the treatment of patients with idiopathic pulmonary fibrosis (IPF). However, there is neither evidence from prospective data nor a guideline recommendation, which drug should be preferred over the other. This study aimed to compare pirfenidone and nintedanib-treated patients regarding all-cause mortality, all-cause and respiratory-related hospitalizations, and overall as well as respiratory-related health care costs borne by the Statutory Health Insurance (SHI).
Methods
A retrospective cohort study with SHI data was performed, including IPF patients treated either with pirfenidone or nintedanib. Stabilized inverse probability of treatment weighting (IPTW) based on propensity scores was applied to adjust for observed covariates. Weighted Cox models were estimated to analyze mortality and hospitalization. Weighted cost differences with bootstrapped 95% confidence intervals (CI) were applied for cost analysis.
Results
We compared 840 patients treated with pirfenidone and 713 patients treated with nintedanib. Both groups were similar regarding two-year all-cause mortality (HR: 0.90 95% CI: 0.76; 1.07), one-year all cause (HR: 1.09, 95% CI: 0.95; 1.25) and respiratory-related hospitalization (HR: 0.89, 95% CI: 0.72; 1.08). No significant differences were observed regarding total (€− 807, 95% CI: €− 2977; €1220) and respiratory-related (€− 1282, 95% CI: €− 3423; €534) costs.
Conclusion
Our analyses suggest that the patient-related outcomes mortality, hospitalization, and costs do not differ between the two currently available antifibrotic drugs pirfenidone and nintedanib. Hence, the decision on treatment with pirfenidone versus treatment with nintedanib ought to be made case-by-case taking clinical characteristics, comorbidities, comedications, individual risk of side effects, and patients’ preferences into account.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-021-01857-y.
Keywords: Idiopathic pulmonary fibrosis, Mortality, Hospitalization, Health care costs, Administrative data, Drugs, Statutory health insurance
Background
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, fibrosing interstitial lung disease (ILD) of unknown etiology [1]. The prognosis of survival is poor, with a reported median survival time of 3.8 years [2]. In recent years, two antifibrotic drugs, viz. pirfenidone and nintedanib, have been licensed for treatment of IPF patients. In Germany, pirfenidone was approved in 2012 and nintedanib in 2015. Both drugs have been shown to slow IPF progression [3, 4], to increase survival [5–7] and to reduce respiratory-related hospitalizations [8]. A recently published systematic review and meta-analysis of RCTs and cohort studies reported that antifibrotic treatment appears to reduce the risk of mortality and acute exacerbations [9]. So far, however, there is no guideline recommendation which drug should be preferred over the other [10], as no randomized controlled trials (RCT) comparing the two drugs have been conducted. Comparative observational studies, especially in real world settings are also rare and show inconclusive results on survival differences between the drugs [11–13]. A claims data-based study among Medicare enrollees suggested that pirfenidone treatment is associated with fewer all-cause and fewer respiratory-related hospitalizations and thus lower inpatient costs compared to nintedanib treatment [15]. Although the study included a large sample, it had a short observation period and did not compare mortality differences between the drugs. A recently published study with data from the French National Health System [13] also reported an association between pirfenidone treatment and lower respiratory-related hospitalizations. However, all-cause hospitalizations were not investigated.
Hence, given the inconclusive and sparse evidence, there is need to further investigate potential differences between both drugs regarding their effectiveness and related costs to guide clinicians with their treatment decisions. To broaden the pre-existing body of evidence, this study aimed to compare pirfenidone and nintedanib-treated patients regarding all-cause mortality, all-cause as well as respiratory-related hospitalization, and overall as well as respiratory-related health care costs.
Methods
Data set and sample selection
We performed a retrospective cohort study with health insurance claims data of the Allgemeine Ortskrankenkasse (AOK) provided by the AOK Research Institute. AOK provides statutory health insurance for roughly 32 percent of the German population [16]. Membership is open to anyone regardless of factors such as professional affiliation, income, age or comorbidities [17].
The initial data set included all individuals insured with an ICD-10 diagnosis of various ILDs, including IPF [J84.1], other fibrosing ILDs [J84.0, J84.8, J84.9, D48.1], sarcoidosis [D86.0-D86.9], drug-associated ILDs [J70.2-J70.4], pneumoconiosis [J62.0-J62.8, J63.0-J63.8], radiation-associated pneumonitis [J70.1], eosinophilic pneumonia [J82], hypersensitivity pneumonitis [J67.9] and connective tissue-associated ILD [J99.1] from January 1, 2013 to December 31, 2018. Survival information was available until the end of 2019. For our analysis, we selected a subsample with at least one IPF diagnosis [J84.1] combined with at least one prescription of pirfenidone (tradename: “Esbriet”) or nintedanib (tradename: “Ofev”) in the patient-individual observation period. Furthermore, selected patients needed to be at least 40 years old at the date of the therapy initiation.
To identify relevant patients for our analysis we used the ATC-Codes “L04AX05” for pirfenidone and “L01XE31” for nintedanib. For the latter, only “Ofev” was considered via the national product codes (Pharmazentralnummer—PZN), as nintedanib under the tradename “Vargatev” is also licensed for treating distinct forms of non-small-cell lung cancer. Furthermore, the license of nintedanib for systemic sclerosis-associated interstitial lung disease and progressive fibrosing interstitial lung disease is not considered in this study as the approval took place after the study period.
We subsequently excluded patients, who were not continuously insured with the AOK and those with a baseline period (pre-observational) or an outcome period (post-observational) of less than one year. Hence, we dropped cases with a first prescription before January 1, 2014 or after December 31, 2017.
Therapy initiation was set as the date the patient redeemed the first drug prescription with either pirfenidone or nintedanib. To perform an intention-to-treat analysis group assignment was based on the first prescription.
Outcome variables
We compared pirfenidone and nintedanib-treated patients regarding two-year all-cause mortality, one-year all-cause as well as respiratory-related hospitalization, and one year overall and respiratory-related health care costs. All outcomes were calculated starting at the date of the treatment initiation.
Respiratory-related hospitalizations included all hospital visits with the following ICD-10 codes as primary diagnosis [18]: IPF [J84.1], respiratory infection [A481, B250, J09-J22, J40], pneumothorax [J93], pulmonary embolism [I26], pulmonary hypertension and right heart disease [I50, I270, I272, I278, I279], respiratory insufficiency [J96], and other chronic and acute lung diseases [J40-J47].
All-cause and respiratory-related health care costs in the year after therapy initiation were calculated based on outpatient physician costs, inpatient costs, and pharmaceutical costs, which could be directly obtained from the claims data. Respiratory-related costs in the inpatient and outpatient sector were based on cases with the diagnoses mentioned above. For respiratory-related pharmaceutical costs we filed prescriptions of pirfenidone, nintedanib, glucocorticoids, corticosteroids, immunosuppressants, acetylcysteine, sildenafil, and antihypertensives for pulmonary arterial hypertension. The corresponding ATC-Codes are presented in Additional file 1: Table S1. Outpatient physician costs were available on a quarterly basis only. Therefore, we redistributed costs incurred in the quarter of the treatment initiation proportionally to the time before and after the date of treatment initiation. Accordingly, this was also carried out for the last observation quarter. Inpatient costs could be determined on a daily base, but if a hospital stay exceeded the one-year follow-up time, costs were also distributed proportionally to in hospital days within the observation period. Pharmaceutical costs were calculated based on the day they were retrieved by the patient.
Covariates and stabilized inverse probability of treatment weighting
Stabilized inverse probability of treatment weighting (IPTW) based on propensity scores [19, 20] was used to adjust for differences in covariates between both groups, as no randomization was performed. The advantage of IPTW in comparison to k:1 matching is that all eligible patients retain in the analysis [21]. Covariates included in the estimation of the propensity scores were selected a priori based on clinical expertise and pre-existing literature. These covariates comprised age, gender, and residential area in four district types (major city, urban, rural, remote rural) [22]. Furthermore, area deprivation according to the well-established “German Index of Multiple Deprivation” from the year 2010 (GIMD 2010) was incorporated, which usually serves as a proxy for socioeconomic background if corresponding individual data is not available [23, 24]. Additionally, the time (in years) between the first IPF diagnosis (left-censored at 2013) and treatment initiation was taken into account. Comorbidities were considered via the Elixhauser Index [25] using the ICD-10 coding algorithm of Quan et al. [26]. To capture IPF-relevant comorbidities more precisely, we modified the Elixhauser Index as described by Schwarzkopf et al. [27]. Accordingly, we separated pulmonary hypertension and lung cancer from the corresponding Elixhauser categories and analyzed them separately. The remaining Elixhauser categories were included in the propensity score model, if they had a prevalence of at least 5% in at least one treatment group. In addition, we included other IPF-specific comorbidities not covered by the Elixhauser Index [27], namely gastro-oesophageal reflux disease [K21], obstructive sleep apnoea syndrome [G47.3], ischemic heart disease [I20-I25], and thromboembolism [I80, I26]. To avoid false-positive comorbidity diagnoses, patients had to have at least two confirmed outpatient physician diagnosis in two separated quarters or one primary inpatient diagnosis in the year before therapy initiation. We accounted for the intake of IPF-related or comorbidity-specific drugs in the six months prior to therapy initiation. IPF-related drugs included immunosuppressants, acetylcysteine, glucocorticoids, and corticosteroids. Comorbidity-specific drugs followed the prescription patterns described by Schwarzkopf et al. [27] with corresponding ATC-Codes presented in Additional file 1: Table S1. Furthermore, we considered the use of health care services before therapy initiation by reflecting IPF-specific hospitalization (IPF as primary diagnosis), respiratory-related hospitalization, and all-cause hospitalization in the three months before therapy initiation by corresponding binary variables. Also included were the number of contacts to outpatient pulmonologists and the number of overall contacts to outpatient physicians in the year before therapy initiation.
Standardized Mean Differences (SMD) were used to assess balance of the covariates between the groups after IPTW, with differences less than 0.1 indicating a good balance [28].
Statistical analysis
Mortality was analyzed by using IPTW-weighted mortality rates per 100 person-years and Kaplan–Meier plots, while hospitalization was investigated with IPTW-weighted hospitalization rates per 100 person-years and cumulative probability curves. Additionally, we calculated weighted Cox Proportional Hazard models with sandwich estimators of the variance for mortality and hospitalization to obtain hazard ratios. In addition, we investigated the three most common reasons for all-cause and respiratory-related hospitalizations. To compare health care costs for the year after treatment initiation, we calculated IPTW-weighted means with bootstrapped 95% confidence intervals (CI). Weighted group differences were also calculated with bootstrapped 95% confidence intervals.
We performed two sensitivity analyses (SA). For SA1, we excluded patients with a first pirfenidone prescription before 2015. Thus, we cover only the period, when both drugs were approved and excluded pirfenidone-treated patients with treatment initiation before nintedanib was available. For SA2, we considered treatment discontinuation, which was defined as a treatment gap of more than 60 days, with day 60 as discontinuation date [15]. Hence, treatment discontinuation became an additional censoring event in the time-to-event analyses. Additionally, we investigated how many of the patients discontinuing the treatment switched medication within the treatment gap range of 60 days. In the cost analysis we excluded costs after the discontinuation date. To account for the discontinued observation time in the cost analysis, we calculated costs per month.
Results were defined as significant if the calculated CIs of the differences did not contain “1” in the Cox models and the “0” in the cost analyses. All analyses were conducted with R-Software version 4.0.3.
Results
Population characteristics
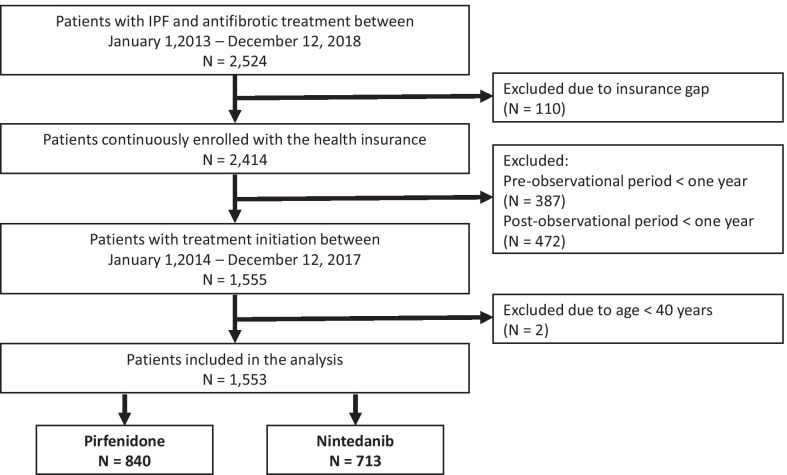
We identified 2524 patients with an IPF diagnosis who received an antifibrotic therapy with nintedanib or pirfenidone between January 1, 2013 and December 31, 2018. We excluded 110 patients with an insurance gap during the observation period and 859 patients with too short pre or post-observation period. In addition, two patients younger than 40 years were excluded. In total, eligibility criteria were met by 1553 patients, of whom 840 initially received pirfenidone (54.1%) (Fig. 1).
Fig. 1.
Participant flow of the study population
Even before the weighting process, both populations were balanced in most baseline variables (Table 1). Nintedanib-treated patients were slightly older than pirfenidone-treated patients. Pirfenidone-treated patients presented a shorter latency from diagnosis to treatment. Apart from few exceptions, the comorbidity profile was similar in both groups. Pirfenidone-treated patients had however more often cardiac arrhythmias and accordingly a higher probability of being treated with related drugs, while nintedanib-treated patients more often suffered from lung cancer. Furthermore, pirfenidone-treated patients were more often treated with acetylcysteine and anti-clotting drugs. After weighting SMDs for all variables were lower than 0.1 indicating no differences in the covariable structure.
Table 1.
Patients characteristics
| Unweighted | IPTW-weighted | |||
|---|---|---|---|---|
| Pirfenidone (N = 840) | Nintedanib (N = 713) | SMD | SMD | |
| Age (years), mean (SD) | 71.9 (8.6) | 73.0 (8.7) | 0.121 | 0.013 |
| Sex (female), n (%) | 207 (24.6) | 190 (26.6) | 0.046 | 0.005 |
| GIMD 2010 n (%) | ||||
| Q1 (least deprived quintile) | 202 (24.0) | 165 (23.1) | 0.075 | 0.015 |
| Q2 | 194 (23.1) | 170 (23.8) | ||
| Q3 | 155 (18.5) | 138 (19.4) | ||
| Q4 | 163 (19.4) | 129 (18.1) | ||
| Q5 (most deprived quintile) | 114 (13.6) | 95 (13.3) | ||
| Unknown | 12 (1.4) | 16 (2.2) | ||
| Residential area n (%) | ||||
| Major city | 176 (21.0) | 154 (21.6) | 0.076 | 0.018 |
| Urban districts | 341 (40.6) | 268 (37.6) | ||
| Rural districts | 165 (19.6) | 140 (19.6) | ||
| Remote rural districts | 155 (18.5) | 149 (20.9) | ||
| Unknown | 3 (0.4) | 2 (0.3) | ||
| Latency from diagnosis to treatment (years), mean (SD) | 0.8 (1.0) | 1.2 (1.3) | 0.358 | 0.002 |
| Comorbidities Elixhauser score, mean (SD) | 3.4 (2.2) | 3.4 (2.1) | 0.033 | 0.002 |
| Comorbidities modified Elixhauser categories, n (%) | ||||
| Congestive heart failure | 199 (23.7) | 157 (22.0) | 0.040 | < 0.001 |
| Cardiac arrhythmias | 207 (24.6) | 122 (17.1) | 0.186 | 0.001 |
| Valvular disease | 110 (13.1) | 90 (12.6) | 0.014 | 0.005 |
| Peripheral vascular disorders | 166 (19.8) | 140 (19.6) | 0.003 | 0.007 |
| Hypertension, uncomplicated | 452 (53.8) | 404 (56.7) | 0.057 | 0.006 |
| Hypertension, complicated | 107 (12.7) | 85 (11.9) | 0.025 | 0.006 |
| Chronic pulmonary disease | 352 (41.9) | 308 (43.2) | 0.026 | 0.005 |
| Diabetes, uncomplicated | 136 (16.2) | 91 (12.8) | 0.098 | 0.004 |
| Diabetes, complicated | 168 (20.0) | 156 (21.9) | 0.046 | 0.006 |
| Hypothyroidism | 73 (8.7) | 68 (9.5) | 0.029 | 0.009 |
| Renal failure | 120 (14.3) | 107 (15.0) | 0.020 | 0.020 |
| Liver disease | 126 (15.0) | 96 (13.5) | 0.044 | < 0.001 |
| Solid tumor without metastasis | 82 (9.8) | 61 (8.6) | 0.042 | 0.010 |
| Rheumatoid arthritis/collagen vascular diseases | 77 (9.2) | 63 (8.8) | 0.012 | 0.011 |
| Obesity | 154 (18.3) | 150 (21.0) | 0.068 | 0.005 |
| Depression | 160 (19.0) | 131 (18.4) | 0.017 | 0.006 |
| Comorbidities IPF-specific, n (%) | ||||
| Coronary heart disease | 318 (37.9) | 271 (38.0) | 0.003 | 0.003 |
| Gastro-oesophageal reflux disease | 167 (19.9) | 123 (17.3) | 0.068 | 0.008 |
| Obstructive sleep apnoea syndrome | 86 (10.2) | 59 (8.3) | 0.068 | 0.003 |
| Thrombosis | 31 (3.7) | 28 (3.9) | 0.012 | 0.020 |
| Lung cancer | 9 (1.1) | 16 (2.2) | 0.092 | 0.011 |
| Pulmonary hypertension | 43 (5.1) | 34 (4.8) | 0.016 | 0.011 |
| Drug treatments, n (%) | ||||
| Immunosuppressants | 35 (4.2) | 22 (3.1) | 0.058 | 0.002 |
| Acetylcysteine | 142 (16.9) | 71 (10.0) | 0.205 | 0.001 |
| Glucocorticoids, Corticosteroids | 323 (38.5) | 274 (38.4) | < 0.001 | 0.005 |
| Treatment with anti-clotting drugs | 250 (29.8) | 166 (23.3) | 0.147 | 0.002 |
| Treatment with anti-acid drugs | 476 (56.7) | 387 (54.3) | 0.048 | 0.004 |
| Treatment with anti-depressants | 99 (11.8) | 84 (11.8) | < 0.001 | 0.002 |
| Treatment with anti-diabetic drugs | 201 (23.9) | 160 (22.4) | 0.035 | 0.001 |
| Treatment with drugs against obstructive airway disease | 219 (26.1) | 210 (29.5) | 0.076 | 0.004 |
| Treatment of heart insufficiency/cardiac arrhythmia | 348 (41.4) | 244 (34.2) | 0.149 | 0.001 |
| Treatment of cardiovascular disease | 601 (71.5) | 483 (67.7) | 0.083 | 0.007 |
| Hospitalizations in three months before treatment, n (%) | ||||
| All cause | 611 (72.7) | 486 (68.2) | 0.100 | < 0.001 |
| IPF-specific | 473 (56.3) | 384 (53.9) | 0.049 | 0.005 |
| Respiratory-related | 79 (9.4) | 64 (9.0) | 0.015 | 0.007 |
| Use of outpatient services in the year before treatment, mean (SD) | ||||
| Contacts to physicians overall | 19.2 (8.5) | 18.6 (7.9) | 0.072 | 0.003 |
| Contact to pulmonologists | 2.0 (1.8) | 2.1 (1.9) | 0.077 | 0.009 |
GIMD 2010 German Index of Multiple Deprivation, year 2010, Q Quintile, SD Standard deviation, SMD Standardized mean difference
Mortality
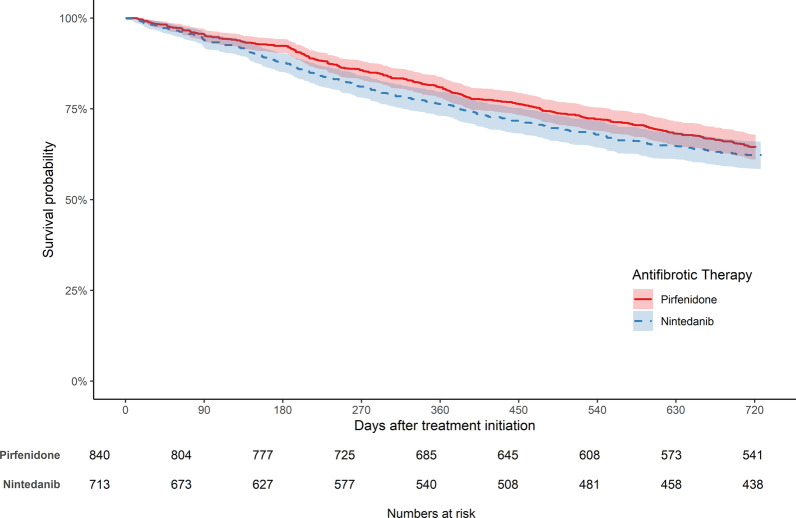
About 36% of pirfenidone-treated and 39% of the nintedanib-treated patients died during the two years after treatment initiation. Weighted mortality rates were 21.9 (95% CI: 19.5; 24.6) per 100 person-years for pirfenidone-treated and 24.4 (95% CI: 21.6; 27.5) for nintedanib-treated patients. Weighted two-year mortality after initiation of antifibrotic treatment was similar in both groups (Fig. 2). Accordingly, the weighted hazard ratio (HR) was not significant (0.90, 95% CI: 0.76; 1.07). The unweighted results are presented in Additional file 2: Tables S2, S3.
Fig. 2.
IPTW-weighted Kaplan–Meier plots for two-year all-cause mortality in patients treated with pirfenidone or nintedanib. The colored areas represent 95% confidence intervals
Hospitalization
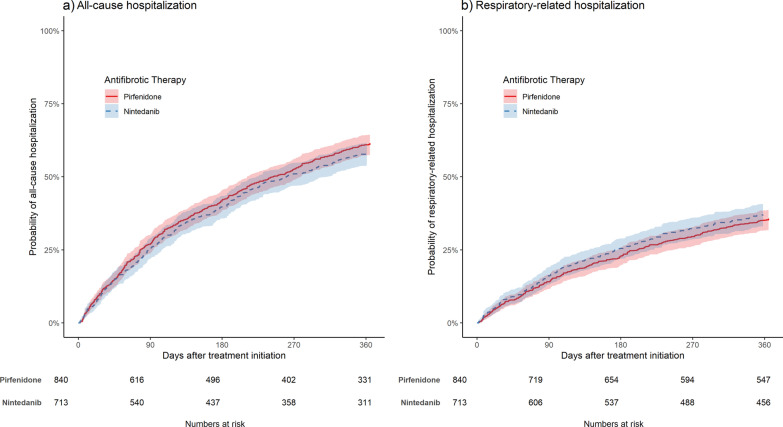
About 61% of pirfenidone-treated and 57% of nintedanib-treated patients were hospitalized during the first year after treatment initiation. Respiratory-related hospitalizations were experienced by 36% of pirfenidone-treated and 36% of nintedanib-treated patients. Weighted all-cause hospitalization rates were 101.3 (95% CI: 92.8; 110.4) per 100 person-years for pirfenidone-treated and 92.7 (95%-CI: 84.0; 102.0) for nintedanib-treated patients, while respiratory-related hospitalization rates were 45.7 (95% CI: 40.7; 51.2) and 48.6 (95% CI: 42.9; 54.8). There were no substantial differences regarding one-year all-cause as well as respiratory-related hospitalization between both groups (Fig. 3). Accordingly, the weighted HRs for all-cause (HR: 1.09, 95% CI: 0.95; 1.25) and respiratory-related hospitalization (HR: 0.89, 95% CI: 0.72; 1.08) were not significant.
Fig. 3.
IPTW-weighted cumulative probability curves in patients treated with pirfenidone or nintedanib. a comparison of risk for all-cause hospitalization, b comparison of risk for respiratory-related hospitalization. The colored areas represent 95% confidence intervals
The most common primary diagnoses for all-cause hospitalizations based on the ICD-10 chapters were diseases of the respiratory system [J00-J99], which accounted for 48.2% of hospitalizations of pirfenidone-treated patients and 51.5% of hospitalizations of nintedanib-treated patients. Further key reasons were diseases of the circulatory system [I00-I99] (pirfenidone: 15.8%, nintedanib: 16.6%) and diseases of the digestive system [K00-K93] (pirfenidone: 6.1%, nintedanib: 8.3%). The three most common primary diagnosis for respiratory-related hospitalizations were IPF (pirfenidone: 62.8%, nintedanib: 64.6%), respiratory infection (pirfenidone: 19.8%, nintedanib: 16.3%), and pulmonary hypertension/right heart disease (pirfenidone: 10.7%, nintedanib: 9.7%).
The unweighted results are presented in Additional file 2: Tables S2, S3.
Costs
We observed no differences in overall (€− 807; 95% CI: €− 2977; €1220) and respiratory-related costs (€− 1282; 95% CI: €− 3423; €534), but at the level of the distinct cost components, outpatient costs were significantly lower for pirfenidone-treated patients (€− 164; 95% CI: €− 280; €− 55) (Table 2). The unweighted results are presented in Additional file 2: Table S4.
Table 2.
IPTW-weighted one-year costs after treatment initiation in patients treated with pirfenidone or nintedanib and related cost differences with bootstrapped 95% confidence intervals
| Pirfenidone (N = 840) | Nintedanib (N = 713) | ||
|---|---|---|---|
| Costs (in €) | Costs (in €) | Difference (in €) | |
| Overall | |||
| Total | 33,893 (32,559; 35,251) | 34,700 (33,407; 36,636) | − 807 (− 2977; 1220) |
| Inpatient | 5991 (5247; 7125) | 5590 (4739; 7376) | 400 (− 1130; 1849) |
| Outpatient | 1107 (1065; 1160) | 1271 (1187; 1410) | − 164 (− 280; − 55) |
| Pharmaceuticals | 26,796 (25,706; 28,008) | 27,839 (26,626; 28,880) | − 1043 (− 2638; 476) |
| Respiratory-related | |||
| Total | 29,085 (27,826; 30,344) | 30,366 (29,245; 32,082) | − 1282 (− 3423; 534) |
| Inpatient | 2867 (2369; 3717) | 3,096 (2398; 5163) | − 229 (− 1661; 884) |
| Outpatient | 690 (666; 717) | 693 (666; 726) | − 3 (− 42; 37) |
| Pharmaceuticals | 25,528 (24,416; 26,587) | 26,578 (25,443; 27,710) | − 1050 (− 2549; 473) |
Statistically significant results are marked in bold
Bootstrapping with 1000 repetitions, bias-corrected and accelerated bootstrap method
Sensitivity analysis 1
After excluding pirfenidone-treated patients before 2015, 556 pirfenidone-treated patients were compared to the 713 nintedanib-treated patients. The results of SA1 mirrored the results of the main analysis (See Additional file 3: Tables S5–S7).
Sensitivity analysis 2
Treatment was discontinued by 50.7% of pirfenidone-treated patients and by 44.0% of nintedanib-treated patients. Among those who discontinued treatment, 14.6% of pirfenidone-treated patients and 5.9% of nintedanib-treated patients switched medications within the treatment gap range of 60 days. Due to the censoring, mortality rates were lower compared to the main analysis (See Additional file 4: Table S8). There were no other noteworthy differences regarding mortality, hospitalization, and costs compared to the main analysis (See Additional file 4: Tables S9, S10).
Discussion
In Germany, two drugs are currently licensed for the treatment of IPF. However, data on a direct comparison of both drugs in terms of effectiveness and associated costs are sparse. Hence, our study compared pirfenidone and nintedanib-treated IPF patients by using a large claims data set. We found no differences between both drugs regarding two-year all-cause mortality, one-year all-cause and respiratory-related hospitalization, and overall as well as respiratory-related health care costs.
Our finding on similar risk of all-cause mortality within both groups is in line with most published studies comparing pirfenidone and nintedanib-treated patients. Two Network Meta-Analysis reported that pirfenidone and nintedanib-treated patients do not differ in survival [29, 30]. However, these studies did not compare the drugs in one study sample, but merged information from different RCTs to indirectly compare pirfenidone and nintedanib. In an Italian study, a retrospective analysis with 263 IPF patients treated between 2011 and 2019 was conducted, which also showed no differences in survival between the groups [11]. However, the study relied on a small sample and included data only from one specialized Italian center for rare lung diseases. In contrast, our study included information from all over Germany and was not limited to specialized institutions. In another real-world study in the United States with data from 2014 to 2018 comparing 662 pirfenidone with 593 nintedanib-treated patients, no differences in survival were observed between the two drugs [12]. The population included was comparable to ours in terms of space of time, data source (insurance data base), and age of patients. However, a recently published study with data from the French National Health System reported a greater risk of all-cause mortality in nintedanib-treated patients [13]. The study covered a similar time horizon, sample size, population’s age, and sex distribution as our study. Furthermore, the drugs became available at different time points as it was the case in Germany. The study reported a HR of 1.8 (CI 95%: 1.3–2.6) to the disadvantage of nintedanib. Contrary to our analyses, the authors did not perform an intention-to treat analysis, but treatment discontinuation was considered as censoring event. Higher discontinuation rates in pirfenidone-treated patients increased censoring compared to nintedanbib-treated patients, which might to some extent explain the more favorable results in pirfenidone-treated patients. In our SA 2, we also considered treatment discontinuation, which was more frequent in pirfenidone-treated patients. The HR became slightly lower in favor of pirfenidone, but the difference was not significant. Previous evidence already demonstrated the effectiveness of pirfenidone [8, 12, 31] and nintedanib [12, 31] regarding reductions of respiratory-related and all-cause hospitalization. A direct comparison between both drugs in regard of all-cause hospitalization was so far only conducted by Corral et al. [15]. Contrary to our study, these analyses unveiled an advantage of pirfenidone treatment. This might be partially explained by a different study population. Indeed the claims data based study by Corral and colleagues included only patients aged 67 years and older, whereas in our study 24.5% of the patients were younger than 67 years. Also, the proportion of women in their study was higher by approximately 10% compared to our study. Additionally, in their main analysis they considered treatment discontinuation in a similar way as we did in the SA2. Nevertheless, we also found no significant group differences in SA2. One possible explanation is that the study by Corral et al. did not include the hospitalization rate prior to the index date as a covariate in the propensity score model. Although they descriptively reported respiratory-related hospitalization in the three months prior to the index date, they did not report the all-cause hospitalization and did not include the variable in the propensity score model. Also, Corral et al. reported a high rate of administrative censoring due to various reasons, such as discontinuation of the treatment or end of enrollment. In our study, all patients included in the analysis had a post-observation period after treatment initiation of at least one year. Hence, both study designs differ and thus a direct comparison of the results obtained is a sensitive issue. Furthermore, previous evidence suggests an association between hospitalization and mortality in patients with IPF [8, 32, 33]. Therefore, reduced hospitalization rates should be associated with lower mortality rates. However, Corral et al. did not investigate survival differences between drugs and we therefore cannot compare the results in this regard.
Corral et al. also described a lower respiratory-related hospitalization risk for pirfenidone-treated patients with a reported HR of 0.71 (95% CI: 0.57; 0.90). Similarly, Belhassen et al. also reported a greater risk of respiratory-related hospitalizations for nintedanib-treated patient with an HR of 1.3 (95% CI: 1.0; 1.7) [13]. The different results for respiratory-related hospitalizations compared to our study might be explained by different definitions of the outcome, as different conditions were considered to be respiratory-related in each study. Other possible reasons were already mentioned in the discussion above.
Concerning health care expenditures, we did not observe differences between pirfenidone and nintedanib-treated patients in overall and also in respiratory-related costs. Regarding the subcategories, a significant difference in outpatient costs to the benefit of pirfenidone was observed. Nevertheless, since the absolute difference (€164) is rather low when compared to total costs of care, its practical relevance is marginal. Pharmaceutical costs were the main cost drivers for both groups. In our SAs, where we adjusted the time period and excluded those patients who discontinued treatment, also no differences between the groups were observed. The study conducted by Corral et al. [15] was the only other real-world study comparing costs between pirfenidone and nintedanib-treated patients. Their study unveiled significantly lower costs in patients treated with pirfenidone, which was driven by lower inpatient costs, due to lower hospitalization rates. Our study does not support this finding, as we observed no differences in hospitalizations and, consequently, no differences in inpatient costs.
Our sensitivity analyses revealed no substantial differences compared to the main analysis. In SA2 the treatment discontinuation was rather high, with 50.7% of pirfenidone-treated and 44.0% of nintedanib-treated patients. Similar high discontinuation rates were also reported in other claims data based studies [12, 13, 34]. One possible explanation for the difference in the discontinuation rates between the drugs in our study is that nintedanib was approved during the study period. Drug switching was more common in pirfenidone-treated patients with 14.6% compared to 5.9% in nintedanib-treated patients, which could be due to the fact that the new treatment with nintedanib was considered more promising.
When interpreting our results, some limitations need to be considered. First, we used the ICD-10 code J84.1 to identify patients with IPF, which might lead to misidentification. Although pirfenidone was approved only for the treatment of IPF and we used only nintedanib with the tradename “Ofev” to identify relevant patients, some uncertainty regarding the coding remains. Second, we applied the stabilized IPTW approach to adjust for observed confounding, but we could not adjust for important variables such as severity of the disease, socio-economic parameters, and lifestyle factors including smoking history. We assume that the severity of the disease is similar between both groups since there is no guideline recommendation as to which of the drugs should be preferred. When looking into real-world studies, forced vital capacity (FVC) or oxygen use were mostly balanced between pirfenidone and nintedanib-treated patients [12, 15, 34, 35]. As a rare example in the opposite direction, differences were reported in one Italian study showing worse FVC baseline values for nintedanib [11]. Against this background, group differences regarding concomitant oxygen therapy or FVC values in our sample cannot be fully excluded. Third, we could not distinguish between IPF-related and all-cause mortality. When comparing the drugs, it would have been important to investigate IPF-related mortality.
There are also several strength of our study. Claims data are less prone to selection bias compared to primary data, which in the context of IPF often is collected in registers and may include predominantly patients treated in specialized hospitals. IPF is a very rare disease and data sources like ours are needed to achieve a sufficiently large population. Furthermore, this is one of the very few studies comparing pirfenidone and nintedanib directly in a large, widely unselected IPF population.
Conclusion
Pirfenidone and nintedanib appear to be associated with equivalent mortality, hospitalization, and SHI-borne costs. These effect parameters however disregard patient-relevant outcomes such as quality of life and clinically relevant aspects such as lung function and adverse effects. Therefore, the decision to treat with pirfenidone and nintedanib should be made on a case-by-case basis taking into account clinical characteristics including comorbidities and comedication, individual risk for adverse events, and patient preferences.
Supplementary Information
Additional file 1: Table S1. Drug-related ATC-Codes for coding of respiratory-related outcomes and covariables.
Additional file 2: Table S2. Unweighted incidence rates for mortality and hospitalization of the main analysis. Table S3. Unweighted Cox Proportional Hazard models for two-year mortality and one-year hospitalization of the main analysis. Table S4. Unweighted one-year costs and cost differences with bootstrapped 95% confidence intervals of the main analysis.
Additional file 3: Table S5. Sensitivity analysis 1 with unweighted and IPTW-weighted incidence rates for mortality and hospitalization. Table S6. Sensitivity analysis 1 with unweighted and IPTW-weighted Cox Proportional Hazard models for 2 year mortality and 1-year hospitalization. Table S7. Sensitivity analysis 1 with unweighted and IPTW-weighted cost differences with bootstrapped 95% confidence intervals.
Additional file 4: Table S8. Sensitivity analysis 2 with unweighted and IPTW-weighted incidence rates for mortality and hospitalization. Table S9. Sensitivity analysis 2 with unweighted and IPTW-weighted Cox Proportional Hazard models for 2 year mortality and 1-year hospitalization. Table S10. Sensitivity analysis 2 with unweighted and IPTW-weighted cost differences with bootstrapped 95% confidence intervals.
Acknowledgements
We are grateful to Andrea Wulff and Harald Hänsch for their support in the data preparation. Moreover, we thank Adina Ismagambetova for her support in the literature search.
Abbreviations
- AOK
Allgemeine Ortskrankenkasse
- CI
Confidence interval
- FVC
Forced vital capacity
- GIMD
German Index of Multiple Deprivation
- HR
Hazard ratio
- ILD
Interstitial lung disease
- IPF
Idiopathic pulmonary fibrosis
- IPTW
Inverse probability of treatment weighting
- RCT
Randomized controlled trials
- SA
Sensitivity analysis
- SHI
Statutory Health Insurance
- SMD
Standardized Mean Difference
Authors' contributions
All authors were involved in the conception of the study. MK and Larissa Schwarzkopf (LS) initiated the project. TR prepared the health insurance claims data of the AOK Research Institute on anonymized data sets and executed the secure file exchange to the Helmholtz Zentrum München. PM conducted the data preparation and analysis in close coordination with MK, LS, LSt, and FT. PM wrote the first draft of the manuscript and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The project is funded by the foundation Stiftung Oskar-Helene-Heim (OHH).
Availability of data and materials
The authors confirm that the data utilized in this study cannot be made available in the manuscript, the supplemental files, or in a public repository due to German data protection laws (‘Bundesdatenschutzgesetz’, BDSG). Therefore, they are stored on a secure drive in the AOK Research Institute (WIdO) to facilitate replication of the results. Generally, access to data of statutory health insurance funds for research purposes is possible only under the conditions defined in German Social Law (SGB V § 287). Requests for data access can be sent as a formal proposal specifying the recipient and purpose of the data transfer to the appropriate data protection agency. Access to the data used in this study can only be provided to external parties under the conditions of the cooperation contract of this research project and after written approval by the health insurance. For assistance in obtaining access to the data, please contact wido@wido.bv.aok.de.
Declarations
Ethics approval and consent to participate
We performed a retrospective analysis of completely anonymized data. According to the German Guidelines for Secondary Data Analysis, ethical approval and consent to participate is not required for this type of study.
Consent for publication
Not applicable.
Competing interests
Michael Kreuter received grants and fees for consultation and speeches from Galapagos, Boehringer Ingelheim, and Roche either for himself or his institution. Larissa Schwarzkopf received fees for consultation from Galapagos. The other authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. Diagnosis of idiopathic pulmonary fibrosis an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 2.Raghu G, Chen S-Y, Yeh W-S, Maroni B, Li Q, Lee Y-C, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001–11. Lancet Respir Med. 2014;2(7):566–572. doi: 10.1016/S2213-2600(14)70101-8. [DOI] [PubMed] [Google Scholar]
- 3.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 4.King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 5.Nathan SD, Albera C, Bradford WZ, Costabel U, Glaspole I, Glassberg MK, et al. Effect of pirfenidone on mortality: pooled analyses and meta-analyses of clinical trials in idiopathic pulmonary fibrosis. Lancet Respir Med. 2017;5(1):33–41. doi: 10.1016/S2213-2600(16)30326-5. [DOI] [PubMed] [Google Scholar]
- 6.Richeldi L, Cottin V, du Bois RM, Selman M, Kimura T, Bailes Z, et al. Nintedanib in patients with idiopathic pulmonary fibrosis: combined evidence from the TOMORROW and INPULSIS((R)) trials. Respir Med. 2016;113:74–79. doi: 10.1016/j.rmed.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Behr J, Prasse A, Wirtz H, Koschel D, Pittrow D, Held M, et al. Survival and course of lung function in the presence or absence of antifibrotic treatment in patients with idiopathic pulmonary fibrosis: long-term results of the INSIGHTS-IPF registry. Eur Respir J. 2020 doi: 10.1183/13993003.02279-2019. [DOI] [PubMed] [Google Scholar]
- 8.Ley B, Swigris J, Day BM, Stauffer JL, Raimundo K, Chou W, et al. Pirfenidone reduces respiratory-related hospitalizations in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2017;196(6):756–761. doi: 10.1164/rccm.201701-0091OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petnak T, Lertjitbanjong P, Thongprayoon C, Moua T. Impact of antifibrotic therapy on mortality and acute exacerbation in idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Chest. 2021 doi: 10.1016/j.chest.2021.06.049. [DOI] [PubMed] [Google Scholar]
- 10.Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;378(19):1811–1823. doi: 10.1056/NEJMra1705751. [DOI] [PubMed] [Google Scholar]
- 11.Cameli P, Refini RM, Bergantini L, d'Alessandro M, Alonzi V, Magnoni C, et al. Long-term follow-up of patients with idiopathic pulmonary fibrosis treated with pirfenidone or nintedanib: a real-life comparison study. Front Mol Biosci. 2020;7:581828. doi: 10.3389/fmolb.2020.581828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dempsey TM, Sangaralingham LR, Yao X, Sanghavi D, Shah ND, Limper AH. Clinical effectiveness of antifibrotic medications for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2019;200(2):168–174. doi: 10.1164/rccm.201902-0456OC. [DOI] [PubMed] [Google Scholar]
- 13.Belhassen M, Dalon F, Nolin M, Van Ganse E. Comparative outcomes in patients receiving pirfenidone or nintedanib for idiopathic pulmonary fibrosis. Respir Res. 2021;22(1):135. doi: 10.1186/s12931-021-01714-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bargagli E, Piccioli C, Rosi E, Torricelli E, Turi L, Piccioli E, et al. Pirfenidone and Nintedanib in idiopathic pulmonary fibrosis: Real-life experience in an Italian referral centre. Pulmonology. 2019;25(3):149–153. doi: 10.1016/j.pulmoe.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Corral M, Chang E, Broder MS, Gokhale S, Reddy SR. Healthcare use and costs among Medicare enrollees on pirfenidone versus nintedanib for idiopathic pulmonary fibrosis. J Comp Eff Res. 2020;9(13):933–943. doi: 10.2217/cer-2020-0084. [DOI] [PubMed] [Google Scholar]
- 16.Bundesministerium für Gesundheit (BMG). Gesetzliche Krankenversicherung—Mitglieder, mitversicherte Angehörige und Krankenstand – Monatswerte Jahresdurchschnitt 2018: BMG; 2019. https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/Statistiken/GKV/Mitglieder_Versicherte/KM1_JD_2018.pdf. Accessed 20 June 2021.
- 17.Busse R, Blümel M, Knieps F, Bärnighausen T. Statutory health insurance in Germany: a health system shaped by 135 years of solidarity, self-governance, and competition. The Lancet. 2017;390(10097):882–897. doi: 10.1016/S0140-6736(17)31280-1. [DOI] [PubMed] [Google Scholar]
- 18.Walscher J, Witt S, Schwarzkopf L, Kreuter M. Hospitalisation patterns of patients with interstitial lung disease in the light of comorbidities and medical treatment—a German claims data analysis. Respir Res. 2020;21(1):73. doi: 10.1186/s12931-020-01335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. doi: 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- 20.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allan V, Ramagopalan SV, Mardekian J, Jenkins A, Li X, Pan X, et al. Propensity score matching and inverse probability of treatment weighting to address confounding by indication in comparative effectiveness research of oral anticoagulants. J Comp Eff Res. 2020;9(9):603–614. doi: 10.2217/cer-2020-0013. [DOI] [PubMed] [Google Scholar]
- 22.Federal Institute for Research on Building Urban Affairs and Spatial Development. Laufende Raumbeobachtung—Raumabgrenzungen. 2021. https://www.bbsr.bund.de/BBSR/DE/forschung/raumbeobachtung/Raumabgrenzungen/deutschland/kreise/siedlungsstrukturelle-kreistypen/kreistypen.html. Accessed 20 June 2021.
- 23.Maier W. Indizes multipler deprivation zur Analyse regionaler Gesundheitsunterschiede in Deutschland: Erfahrungen aus Epidemiologie und Versorgungsforschung. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2017;60(12):1455–6. [DOI] [PubMed]
- 24.Maier W, Fairburn J, Mielck A. Regionale Deprivation und Mortalitat in Bayern. Entwicklung eines “Index Multipler Deprivation” auf Gemeindeebene. Gesundheitswesen. 2012;74(7):416–25. [DOI] [PubMed]
- 25.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 27.Schwarzkopf L, Witt S, Waelscher J, Polke M, Kreuter M. Associations between comorbidities, their treatment and survival in patients with interstitial lung diseases - a claims data analysis. Respir Res. 2018;19(1):73. doi: 10.1186/s12931-018-0769-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canestaro WJ, Forrester SH, Raghu G, Ho L, Devine BE. Drug treatment of idiopathic pulmonary fibrosis: systematic review and network meta-analysis. Chest. 2016;149(3):756–766. doi: 10.1016/j.chest.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Rochwerg B, Neupane B, Zhang Y, Garcia CC, Raghu G, Richeldi L, et al. Treatment of idiopathic pulmonary fibrosis: a network meta-analysis. BMC Med. 2016;14:18. doi: 10.1186/s12916-016-0558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang J, Han M, Song JW. Antifibrotic treatment improves clinical outcomes in patients with idiopathic pulmonary fibrosis: a propensity score matching analysis. Sci Rep. 2020;10(1):15620. doi: 10.1038/s41598-020-72607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durheim MT, Collard HR, Roberts RS, Brown KK, Flaherty KR, King TE, et al. Association of hospital admission and forced vital capacity endpoints with survival in patients with idiopathic pulmonary fibrosis: analysis of a pooled cohort from three clinical trials. Lancet Respir Med. 2015;3(5):388–396. doi: 10.1016/S2213-2600(15)00093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown AW, Fischer CP, Shlobin OA, Buhr RG, Ahmad S, Weir NA, et al. Outcomes after hospitalization in idiopathic pulmonary fibrosis: a cohort study. Chest. 2015;147(1):173–179. doi: 10.1378/chest.13-2424. [DOI] [PubMed] [Google Scholar]
- 34.Corral M, DeYoung K, Kong AM. Treatment patterns, healthcare resource utilization, and costs among patients with idiopathic pulmonary fibrosis treated with antifibrotic medications in US-based commercial and Medicare Supplemental claims databases: a retrospective cohort study. BMC Pulm Med. 2020;20(1):188. doi: 10.1186/s12890-020-01224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holtze CH, Freiheit EA, Limb SL, Stauffer JL, Raimundo K, Pan WT, et al. Patient and site characteristics associated with pirfenidone and nintedanib use in the United States; an analysis of idiopathic pulmonary fibrosis patients enrolled in the Pulmonary Fibrosis Foundation Patient Registry. Respir Res. 2020;21(1):48. doi: 10.1186/s12931-020-1315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Drug-related ATC-Codes for coding of respiratory-related outcomes and covariables.
Additional file 2: Table S2. Unweighted incidence rates for mortality and hospitalization of the main analysis. Table S3. Unweighted Cox Proportional Hazard models for two-year mortality and one-year hospitalization of the main analysis. Table S4. Unweighted one-year costs and cost differences with bootstrapped 95% confidence intervals of the main analysis.
Additional file 3: Table S5. Sensitivity analysis 1 with unweighted and IPTW-weighted incidence rates for mortality and hospitalization. Table S6. Sensitivity analysis 1 with unweighted and IPTW-weighted Cox Proportional Hazard models for 2 year mortality and 1-year hospitalization. Table S7. Sensitivity analysis 1 with unweighted and IPTW-weighted cost differences with bootstrapped 95% confidence intervals.
Additional file 4: Table S8. Sensitivity analysis 2 with unweighted and IPTW-weighted incidence rates for mortality and hospitalization. Table S9. Sensitivity analysis 2 with unweighted and IPTW-weighted Cox Proportional Hazard models for 2 year mortality and 1-year hospitalization. Table S10. Sensitivity analysis 2 with unweighted and IPTW-weighted cost differences with bootstrapped 95% confidence intervals.
Data Availability Statement
The authors confirm that the data utilized in this study cannot be made available in the manuscript, the supplemental files, or in a public repository due to German data protection laws (‘Bundesdatenschutzgesetz’, BDSG). Therefore, they are stored on a secure drive in the AOK Research Institute (WIdO) to facilitate replication of the results. Generally, access to data of statutory health insurance funds for research purposes is possible only under the conditions defined in German Social Law (SGB V § 287). Requests for data access can be sent as a formal proposal specifying the recipient and purpose of the data transfer to the appropriate data protection agency. Access to the data used in this study can only be provided to external parties under the conditions of the cooperation contract of this research project and after written approval by the health insurance. For assistance in obtaining access to the data, please contact wido@wido.bv.aok.de.