Abstract
The TT virus (TTV) is a recently discovered DNA virus which was first identified in patients with non-A to -G hepatitis following blood transfusion. In this study, we tested 150 attendees of two hemodialysis (HD) units of the public hospitals of Marseilles, France, for the presence of TTV genome by using a PCR-based methodology. The overall prevalence of TTV viremia was 28% (compared to 5.3% in blood donors from the same region). We demonstrated the existence of chronic infections and superinfections by strains belonging to different genotypes. The prevalence of infection was higher in patients originating from Africa, in patients with previous blood transfusion or organ transplantation, in patients with antibody to hepatitis B core antigen, and in those with diabetes mellitus. A high prevalence of TTV infection (50%) was also observed in a population of patients with diabetes mellitus but without renal disease. No significant relationship was found between TTV viremia and hepatitis C virus or GB virus C, transaminases, age, sex, and duration of HD treatment. The PCR amplification products (located in open reading frame 1 of the TTV genome) were sequenced. These genomic sequences were submitted to phylogenetic analysis by using the Jukes-Cantor algorithm for distance determination and the neighbor-joining method for tree building. In several instances, sequences from viruses isolated in a HD unit were grouped in the same phylogenetic cluster. These results together with the different distribution of cases in the two HD units suggest there is viral transmission within each.
Recently, a new virus designated by the letters TT (TTV) has been identified by representational difference analysis (5). Initially, the virus was detected in sera of patients with posttransfusion hepatitis associated with elevated transaminases. The current data concerning the biological and molecular properties of this virus are incomplete. Preliminary studies demonstrated that the viral genome was approximately 3.7 kb long and had a single-stranded DNA chemistry. The analysis of the nucleic acid sequence revealed the existence of several open reading frames (ORFs) and suggested that TTV is a member of the Circoviridae family.
Epidemiological studies have shown the virus to be widely distributed in different populations with parenteral risk exposure: hemodialysis (HD) patients (46%) (6), intravenous drug users (19 to 40%) (2, 6) and hemophiliacs (27.4 to 68%) (8). TTV was also detected at a lower prevalence in voluntary blood donors (1.9 to 12%) (2, 6, 8). Recently, Prescott and Simmonds reported, in rural populations, a TTV prevalence ranging from 7% in Sudan to 83% in Gambia (7). Although it was observed that the prevalence of TTV infection was high in patients with liver diseases (6), i.e., hepatocellular carcinoma (39%), non-A to -G liver infection (46%), non-A to -G fulminant hepatitis (47%), and cirrhosis (48%), at this point in time, there are no definitive data providing a cause and effect relationship between TTV and any specific human pathology.
We report herein a study of TTV infection in two HD units of the public hospitals of Marseilles, France. Possible relationships to medical, biological, and epidemiological markers were investigated. We also examined the possibility of viral transmission within HD units and some features concerning the natural history of TTV infection.
MATERIALS AND METHODS
Populations studied. (i) HD patients.
One hundred fifty attendees of the HD units of Sainte Marguerite Hospital and La Conception Hospital (two of the public hospitals of Marseilles, France) were studied.
In unit 1, 45 men and 36 women with end-stage renal disease (ESRD) were included in the study (sex ratio [male/female], 1.25; mean ± standard deviation [SD] age, 59.9 ± 15.6 years). The mean duration of HD was 81.3 ± 82.6 months.
In unit 2, 34 men and 35 women were included in the study (sex ratio [male/female], 0.97; mean ± SD age, 63.4 ± 14.2 years). The mean duration of HD was 62.5 ± 58.8 months. In the case of three patients from this unit (HD unit 2 patients 1, 2, and 3), it was possible to test biological samples collected every 6 months over a period of 29 (patients 1 and 2) to 37 (patient 3) months.
Patients were divided into seven groups according to the disease responsible for their ESRD. Group I included patients with diabetes mellitus (DM) (13 in unit 1 and 10 in unit 2). Group II included patients with chronic glomerulonephritis (21 in unit 1 and 6 in unit 2). Group III included patients with inherited nephropathy, including polycystic kidney disease (6 in unit 1 and 11 in unit 2). Group IV included patients with monoclonal gammapathies (4 in unit 1 and 1 in unit 2). Group V included patients with chronic interstitial nephropathy (12 in unit 1 and 11 in unit 2). Group VI included patients with vascular kidney diseases (13 in unit 1 and 17 in unit 2). Group VII included patients with ESRD of indeterminate etiology (12 in unit 1 and 13 in unit 2).
(ii) Diabetic patients.
In addition to the 23 patients included in group I, seven HD patients (four in unit 1 and three in unit 2) were diabetic, but their diabetes was not considered to be the etiology of their ESRD. All of them had type 2 DM; three were treated with insulin injection, and four were treated with oral antidiabetic agents. In order to understand better the relationship between TTV infection, diabetes, and HD, a population of individuals with DM but without renal disease was also tested. This included 75 individuals (sex ratio, 1.34; mean age, 52.8 ± 15.9 years) treated in the endocrinology department of La Timone Hospital (one of the public hospitals of Marseilles): 26 with type 1 DM and 49 with type 2 DM; 48 were treated with insulin, and 27 were treated with an oral antidiabetic agent.
Serological markers and transaminases.
Sera from all HD patients were tested for serological markers of hepatitis B virus (HBV) infection (antibody to HBV core antigen [HBc]; HBc ELISA Test System; HBs antigen, antibody to HBs Ag ELISA test system 3 [both from Ortho Diagnostic Systems, Raritan, N.J.]). They were also tested for the presence of antibodies to human immunodeficiency virus (HIV) and hepatitis C virus (HCV) by using commercial enzyme-linked immunosorbent assay (ELISA) kits (HIV-1/HIV-2 Ab-capture ELISA Test System and HCV Third-Generation ELISA Test, respectively [both from Ortho Diagnostic Systems]).
Alanine aminotransferase (ALT) levels were analyzed every 3 months (Biotrol ALT/TGP bireactif; Merck, Nogent sur Marne, France), according to the manufacturer’s recommendations. Elevated ALT levels were defined as values greater than 1.5 times the value of normal range (men, 7 to 40 IU/liter; women, 5 to 37 IU/liter; people <20 years old, 5 to 25 IU/liter).
Markers of viremia.
Sera from all HD patients were tested for the presence of HCV RNA with a commercial reverse transcriptase-PCR assay (HCV Amplicor PCR Diagnostics; Roche Diagnostic Systems, Branchburg, N.J.). They were also tested for the presence of GB virus C/HGV (GBV-C/HGV) RNA by using a PCR protocol with the 5′ noncoding region of the viral genome (3).
Genomic DNA of TT virus was extracted from 200 μl of serum with the High Pure Viral Nucleic Acid Kit (Boehringer Mannheim, Meylan, France) according to the manufacturer’s recommendations, resuspended in 50 μl of nuclease-free water, and stored at −80°C until used. PCR amplification was performed as described elsewhere (1) with degenerate oligonucleotide primers located in ORF1 and derived from those described by Okamoto et al. (6) (sense primers, NG059d [WCAAGACAGAGGMGAAGGMAAYATG] and NG061d [GGMAAYATGYTRTGGATAGACTGG]; reverse primer, NG063d [CTGGCATYTTWCCRTTTCCAAART]). These primers were designed for the amplification of the most divergent variants described to date.
Molecular analysis of TT virus isolates.
All of the PCR products, except those from HD unit 2 patients 1, 2, and 3, were purified with the Qiagen PCR Purification Kit (Courtaboeuf, France) and submitted to direct sequencing with the DNA D-Rhodamine Sequencing Kit and an ABI Prism 377 sequence analyzer (both from Perkin-Elmer, Foster City, Calif.).
In the case of HD unit 2 patients 1, 2, and 3, amplification products were purified and cloned into the PGEM-T vector (Promega). The recombinant plasmids were transfected into Escherichia coli XL-Blue competent cells, and 25 clones of each reaction were sequenced on both strands by using universal M13 primers.
Sequence alignments were generated with the CLUSTAL W version 1.74 software program (10). Phylogenetic analysis of aligned sequences was performed with the Jukes-Cantor algorithm for distance determination and the neighbor-joining method for tree drawing, implemented in the software program MEGA (4).
Statistical analysis.
Statistical analysis was performed with the Epi Info program (version 6; Centers for Disease Control and Prevention, Atlanta, Ga.). Results were expressed as means ± standard deviation or as percentages. Means were compared between groups by using the t test, and frequency distributions were compared by using the χ2 test. The frequency distribution of TTV viremia in age groups was analyzed with the extended Mantel-Haenszel χ2 test.
RESULTS
Distribution of transaminases and HIV, HCV, HBV, and GBV-C/HGV markers.
Detailed results for transaminases and HIV, HCV, HBV, and GBV-C/HGV markers are presented in Table 1. During the year preceding this study, all patients were tested at least three times for transaminases. Only three had one or more abnormal ALT results: one of these patients was suffering from chronic hepatitis B (with positive HBV viremia), and two suffered from chronic hepatitis C (with positive HCV viremia). One patient was coinfected with HCV and TTV, but no specific symptomatology could be associated with this coinfection.
TABLE 1.
Distribution of transaminases and virological markers among HD patients
| Characteristic | No. (%) of patients
|
|
|---|---|---|
| Unit 1 (n = 81) | Unit 2 (n = 69) | |
| Transaminases >1.5 N | 2 (2.5) | 1 (1.4) |
| HBV markers | ||
| Anti-HBc antibody + HBs antigen | 6 (7.4) | 2 (2.9) |
| Anti-HBc antibody + anti-HBs antibody | 16 (19.7) | 12 (17.4) |
| Isolated anti-HBc antibody | 3 (3.7) | 1 (1.4) |
| Isolated anti-HBs antibody | 41 (50.6) | 36 (52.2) |
| None | 15 (18.5) | 18 (26.1) |
| HCV markers | ||
| Anti-HCV antibody | 16 (19.8) | 18 (26.1) |
| HCV RNA | 12 (14.8) | 12 (17.4) |
| GBV-C/HGV RNA marker | 18 (22.2) | 2 (2.9) |
| Anti-HIV antibody marker | 0 | 0 |
The distributions of age, sex ratio, dialysis duration (Table 2), transaminases, and HBV, HCV, and HIV markers (Table 1) were not significantly different between the two HD units. In contrast, there was a significant difference of prevalence of GBV-C/HGV viremia between the two HD units (22.2% in unit 1 and 2.9% in unit 2, P < 0.01), and more patients had a history of transfusion and/or graft in unit 2 than in unit 1 (70.4% versus 55%, P = 0.05; and 78.1% versus 51.6%, P = 0.01 [considering only antecedents of transfusion]).
TABLE 2.
Distribution of TTV infection among HD patients
| Parameter | Unit 1 (n = 81)
|
Unit 2 (n = 69)
|
||
|---|---|---|---|---|
| TTV DNA+(n = 30 [37.0%]) | TTV DNA−(n = 51 [63.0%]) | TTV DNA+(n = 13 [18.8%]) | TTV DNA−(n = 56 [81.2%]) | |
| Mean ± SD age (yr) | 61.7 ± 15.4 | 59.7 ± 15.8 | 57.1 ± 15.2 | 64.8 ± 13.7 |
| Sex ratio (male/female) | 15/15 | 30/21 | 4/9 | 30/26 |
| Mean ± SD dialysis duration (mo) | 76.5 ± 79.6 | 85.3 ± 85.6 | 58.7 ± 52.9 | 63.3 ± 60.7 |
| No. of patients with transfusion and/or graft | 24 | 33 | 8 | 30 |
| No. (%) of patients from: | ||||
| Africa | 7 (58.3) | 5 (41.7) | 8 (34.8) | 15 (65.2) |
| South Europea | 4 (40.0) | 6 (60.0) | 2 (33.3) | 4 (66.7) |
| France/North Europe | 19 (32.2) | 40 (67.8) | 2 (5.3) | 36 (94.7) |
| No. (%) of patients in disease groupb: | ||||
| I | 8 (61.5) | 5 (38.5) | 3 (30.0) | 7 (70.0) |
| II | 6 (28.6) | 15 (71.4) | 1 (16.7) | 5 (83.3) |
| III | 3 (50.0) | 3 (50.0) | 1 (9.1) | 10 (90.9) |
| IV | 1 (25.0) | 3 (75.0) | 1 (100) | 0 |
| V | 4 (33.3) | 8 (66.7) | 2 (18.2) | 9 (81.8) |
| VI | 5 (38.5) | 8 (61.5) | 5 (29.4) | 12 (70.6) |
| VII | 3 (25.0) | 9 (75.0) | 0 | 13 (100) |
| No. of patients with viral marker type: | ||||
| Anti-HBcc | 13 (2) | 12 (4) | 4 (1) | 11 (1) |
| Anti-HCV | 5 | 11 | 4 | 14 |
| HCV RNA | 5 | 7 | 2 | 10 |
| GBV-C/HGV RNA | 8 | 10 | 0 | 2 |
Spain, Italy, and Greece.
As defined in Materials and Methods.
Values in parentheses represent the number of patients with positive HBs antigen.
TTV viremia.
The TTV genome was detected in 30 patients undergoing HD in unit 1 and 13 patients undergoing HD in unit 2. The prevalence was 28.7% in the entire population, with a significant difference (37% versus 18.8%, P = 0.014) between the two units.
The percentages of prevalence of TTV infection were 61.5% for group I in unit 1 (30% in unit 2), 28.6% for group II (16.7% in unit 2), 50% for group III (9.1% in unit 2), 25% for group IV (100% in unit 2), 33.3% for group V (18.2% in unit 2), 38.5% for group IV (29.4% in unit 2), and 25% for group VII (0% in unit 2).
The relevance of demographic and virological parameters was investigated in the population tested for TTV DNA (Table 2). Out of 95 patients with a history of transfusion and/or transplantation, 32 tested positive for TTV DNA. This prevalence was found to be significantly higher than that found in the 41 patients never transfused nor transplanted (33.7% versus 17.1%, P = 0.049; 35.2% versus 14.9%, P = 0.01 [considering only antecedents of transfusion]). Similarly, the prevalence of positive TTV DNA was found to be significantly higher in the population of patients with anti-HBc antibody (42.5% versus 23.8% in patients testing negative for anti-HBc antibody, P = 0.026).
The presence of TTV DNA was found to be related to the geographical origin of the patients. In particular, the prevalence was higher in patients originating from Africa (42.8% versus 24.3% in European patients, P = 0.034). Overall, a North-to-South frequency gradient was observed: 21.6% in French or North European patients, 37.5% in South European patients, and 42.8% in African patients (including 83% of patients from North African countries).
The prevalence of TTV viremia was also found to be related to the origin of the ESRD. In group I (ESRD due to DM), 48.0% of the patients tested positive versus 25.2% in groups I to VII (P = 0.027). The prevalence of TTV infection in HD diabetic patients was not significantly higher than that observed in a population of 75 diabetic patients without renal disease (Table 3). No significant relationship was found between TTV infection and the type of DM or antidiabetic treatment (oral or by insulin injections).
TABLE 3.
Distribution of TTV among diabetic patients
| Parameter | TTV DNA+ | TTV DNA− | Total |
|---|---|---|---|
| No. (%) of patients | 52 (49.5) | 53 | 105 |
| Mean age (yr) | 56.2 | 54.4 | 55.3 |
| Sex ratio (male/female) | 1.12 | 1.21 | 1.17 |
| No. (%) of patients: | |||
| Type 1 DM | 11 (40.7) | 16 | 27 |
| Type 2 DM | 41 (52.6) | 37 | 78 |
| Insulin treatment | 34 (52.3) | 31 | 65 |
| Oral antidiabetic treatment | 18 (45.0) | 22 | 40 |
| HD | 16 (53.3) | 14 | 30 |
| Non-HD | 36 (48.0) | 39 | 75 |
The four epidemiological or biological markers listed above (transfusion and/or graft, anti-HBc antibody, geographical origin, and DM) were found to be independently related to the prevalence of TTV viremia. The distribution of these markers in the two HD departments was analyzed to determine if they could account for the difference in prevalence of TTV viremia between units 1 and 2. There was no significant difference between the frequency of ESRD group 1 in the two units (16% in unit 1 versus 14.5% in unit 2) and that of anti-HBc antibody (30.9% in unit 1 versus 21.7% in unit 2). The percentage of patients originating from Africa was higher in the unit in which the lower prevalence of TTV infection was found (14.8% in unit 1 versus 34.3% in unit 2). The only data that could possibly explain the higher prevalence of TTV in unit 1 were those showing the higher frequency of antecedents of transfusion and/or graft in this unit (see above).
Conversely, no significant correlation was found between TTV DNA positivity and any of the following parameters: sex, age, duration of HD treatment, diseases of groups II to VII, ALT level, anti-HCV or anti-HIV antibody, HCV RNA, and GBV-C/HGV RNA positivity.
Molecular analysis of TTV isolates.
During a follow-up of 37 months for HD unit 2 patient 3, the TTV genome was detected in all of the samples that could be tested (seven samples collected in November 1995; July 1996; March and November 1997; and April, June, and November 1998). The molecular analysis of these samples showed that HD unit 2 patient 3 was infected with a type 2 strain. During a follow-up of 29 months for HD unit 2 patients 1 and 2 (six samples collected from July 1996 to November 1998), the TTV was also detected in all samples tested. HD unit 2 patient 2 was infected with a type 1b strain throughout the follow-up. HD unit 2 patient 1 was initially found to be infected with a type 1a strain, but clone-based sequencing revealed that he was superinfected with a type 2 strain during the first months of 1998. Both type 1a and 2 strains could be detected in the three samples collected between April and November 1998.
For HD unit 2 patients 1, 2, and 3, the comparison of the viral sequences obtained from the different samples collected showed that the nucleotide sequence of a given strain remained unaltered throughout the follow-up.
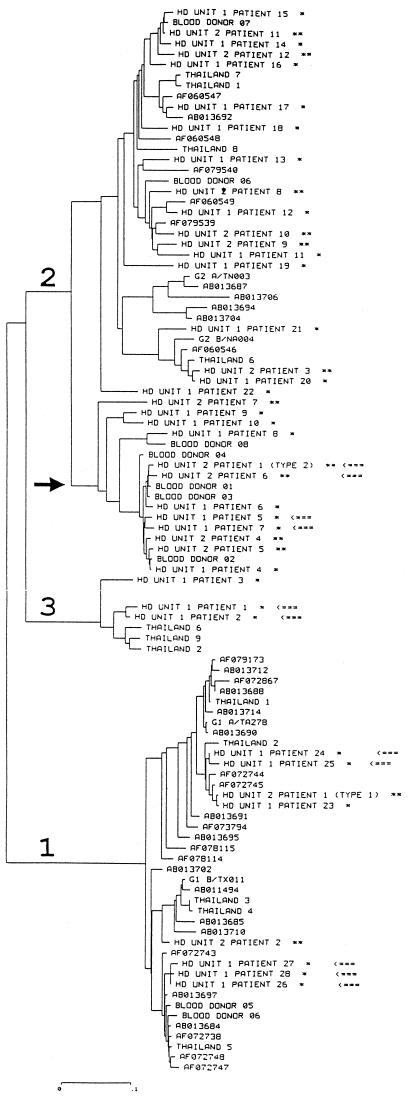
Viral sequences of isolates from our patients were compared to those available from databases, and a phylogenetic tree was constructed (Fig. 1). Isolates from Marseilles were distributed in the previously defined groups 1, 2, and 3 (1). Interestingly, it was found that viral sequences from certain patients treated in the same HD unit formed distinct phylogenetic clusters. This was the case in unit 1 for patients 1 and 2 and patients 5 and 7 and in unit 2 for patients 26, 27, and 28 and patients 1 (type 2 strain) and 6. In the case of HD unit 2 patients 26 and 28, it was established that they had been treated in the same cubicle and at the same time for several months.
FIG. 1.
Unrooted phylogenetic tree from TTV sequence alignment, including 10 isolates from French blood donors (Blood donors 01 to 10), 38 sequences retrieved from databases (identified by their GenBank accession numbers), 9 sequences from Thai isolates (Thailand 1 to 9) (9), and 41 isolates collected from HD patients (HD unit 1 patients 1 to 28, HD unit 2 patient 1 types 1a and 2, and HD unit 2 patients 2 to 12 [labeled with ∗ for patients treated in unit 1 or with ∗∗ for patients treated in unit 2]). Arrows indicate isolates with very similar sequences collected in the same HD unit. The three major branches corresponding to genotypes 1, 2, and 3 are identified, and an arrow indicates a cluster within type 2 including only French isolates. The horizontal scale bar indicates the number of nucleotide substitutions per site (0 to 0.1).
DISCUSSION
The first data of Nishizawa and colleagues (5), resulting from the study of several posttransfusion contaminations by the TTV, suggested that patients could recover from infection after a few weeks. In contrast, Simmonds and colleagues suggested that TTV was able to establish a long-term infection, since hemophiliacs were found to be infected 10 years after their last exposure to non-virally-inactivated clotting factor concentrates (8). Our study demonstrated the existence of chronic TTV infection in HD patients, since an identical viral strain could be detected in a given individual over a period lasting up to 37 months. Our data did not support the hypothesis that constant reexposure to viral infection may be misinterpreted as chronic infection. When multiple samples were available from specific patients, these yielded indistinguishable TTV sequences, whereas sequences obtained from different patients exhibited a high level of variability. However, it was shown in the case of HD unit 2 patient 1 that reexposure to viral infection could occur. This patient was initially infected by a type 1a strain and secondarily superinfected by a strain belonging to type 2. This superinfection did not result in the disappearance of the first strain nor in the alteration of its genomic sequence. These results suggest that the infection by a strain of a given genotype is not protective against the superinfection by another type. Such cases of coinfection by strains belonging to distinct viral genotypes have also been reported by Tanaka et al. (9) in Thai patients.
The prevalence of TTV infection was found to be higher in HD patients than among European blood donors. The overall prevalence in the two units studied was 28%, compared to 5.3% among French blood donors from the region of Marseilles (2) and 1.9% in United Kingdom blood donors (8). Among our patients, several factors were associated with an increased frequency of TTV infection. First, we identified a North-to-South geographical gradient of prevalence, with the highest frequency of TTV infection in HD patients originating from Africa (>40%). These data are concordant with those of Prescott and Simmonds (7), who reported a high prevalence of TTV infection in various African rural populations.
Transfusion and/or transplantation was also found to be associated with a higher prevalence of infection. This confirms that TT virus could be transmitted by blood transfusion, as suggested by the initial study of Nishizawa et al. (5). Similarly, the prevalence was found to be elevated in patients with positive anti-HBc antibody, a classical marker of parenteral exposure in HD patients. However, TTV infection was not found to be more prevalent in patients infected with other parenterally transmitted viruses, such as HCV and GBV-C/HGV, despite the fact that there was a significant correlation between HBV and HCV infections.
The discovery of a significantly higher prevalence of TTV infection in patients with DM was unexpected. This relationship was found independently in both units studied with relative risks of TTV infection in diabetic patients of 1.9 in unit 1 and 1.8 in unit 2. The relationship between TTV infection and DM was found to be independent of the other identified risk factors (geographical origin, transfusion or transplantation, and anti-HBc antibody). Our investigation of diabetics without renal disease suggested that a high prevalence of TTV infection is not specific to diabetic ESRD, but that a large proportion of diabetic patients are also infected by the virus. The significance of the high rate of TTV infection in patients with DM remains to be elucidated. Several hypotheses can be proposed. (i) TTV sets off a train of events that eventually result in diabetes. However, because the prevalence of TTV infection was elevated in both type 1 and 2 diabetes, diseases for which the underlying physiopathological mechanisms are markedly different, this hypothesis is unlikely. (ii) the correlation between TTV infection and DM is due to an unidentified common epidemiological risk factor. However, it could be argued again that the epidemiological distributions of type 1 and 2 diabetes are very different. (iii) There is a nosocomial spread of TTV in diabetic patients due to the use of plastic lancet devices, as previously suggested for HCV and HBV. (iv) As previously demonstrated for other pathogens, opportunistic infections by TTV in DM patients occur more frequently than in the general population, since their underlying illness leads to partial immunodeficiency. This could be responsible for a higher incidence of TTV infection and/or for a higher frequency and duration of chronic infections in diabetic patients.
The discovery of high rates of TTV infection in diabetic patients constitutes the first report of a potential association between DM and a chronic viral infection. Further studies are needed to clarify the mechanisms of this association and determine its potential medical implications.
In contrast, no significant relationship could be identified between TTV infection and either biological markers of hepatic cytolysis (transaminases) or duration of HD treatment. It is noteworthy that PCR methodology permits the detection of only the active forms of infection and that some patients may have been infected by TTV and have recovered. Infection in such patients could only be confirmed by a serological method, but these have not yet become available.
Because we found a significant difference in prevalence of TTV infection between unit 1 and unit 2, we studied the distribution of the markers found to be related to TTV viremia in the two departments. The percentages of patients with DM or anti-HBc antibody were similar in the two units and therefore could not account for the observed difference. The ethnic origin of the patients also could not underlie the higher prevalence of TTV infection in unit 1, because the proportion of individuals originating from Africa was twofold lower than that in unit 2. Thus, among the identified risk factors, only blood transfusion could be associated with the disparity in TTV prevalence between the two units.
However, an alternative hypothesis explaining this disparity could be nosocomial transmission of TTV in unit 1. Such an explanation is supported by epidemiological and molecular findings. First, the prevalence of not only TTV infection, but also GBV-C/HGV infection was significantly higher in unit 1, despite the fact that GBV-C/HGV infection was not significantly related to antecedents of blood transfusion. Second, the high degree of homology between viral strains isolated from certain patients treated in the same unit may have resulted from patient-to-patient transmission or from the contamination of several patients from a common source located within the unit.
The involvement of TTV in human pathology and its relationship with the host immune system remain unclear and warrant further investigations. We have demonstrated that the prevalence of TTV infection was elevated in French HD units and that some HD patients were chronically infected. Infection could be related to parenteral exposure for some patients, but also to unit-dependent factors and to the diabetic etiology of chronic renal failure.
ACKNOWLEDGMENT
We thank R. Khayat de Chesse for excellent technical assistance.
REFERENCES
- 1.Biagini P, Gallian P, Attoui H, Cantaloube J F, de Micco P, de Lamballerie X. Determination and phylogenetic analysis of partial sequences from TT virus isolates. J Gen Virol. 1999;80:419–424. doi: 10.1099/0022-1317-80-2-419. [DOI] [PubMed] [Google Scholar]
- 2.Biagini P, Gallian P, Cantaloube J F, De Micco P, de Lamballerie X. Presence of TT virus in French blood donors and intravenous drug users. J Hepatol. 1998;29:684–685. doi: 10.1016/s0168-8278(98)80167-0. [DOI] [PubMed] [Google Scholar]
- 3.Cantaloube J F, Charrel R N, Attoui H, Biagini P, De Micco P, de Lamballerie X. Evaluation of four PCR systems amplifying different genomic regions for molecular diagnosis of GB virus C infections. J Virol Methods. 1997;6:131–135. doi: 10.1016/s0166-0934(96)02154-4. [DOI] [PubMed] [Google Scholar]
- 4.Kumar, S., K. Tamura, and M. Nei. MEGA: Molecular Evolutionary Genetics Analysis, version 1.02. Pennsylvania State University, University Park.
- 5.Nishizawa T, Okamoto H, Konishi K, Yoshizawa H, Miyakawa Y, Mayumi M. A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem Biophys Res Commun. 1997;241:92–97. doi: 10.1006/bbrc.1997.7765. [DOI] [PubMed] [Google Scholar]
- 6.Okamoto H, Nishizawa T, Kato N, Ukita M, Ikeda H, Iizuka H, Miyakawa Y, Mayumi M. Molecular cloning and characterisation of a novel DNA virus (TTV) associated with posttransfusion hepatitis of unknown etiology. Hepatol Res. 1998;10:1–16. [Google Scholar]
- 7.Prescott L E, Simmonds P. Global distribution of transfusion-transmitted virus. N Engl J Med. 1998;339:776. doi: 10.1056/NEJM199809103391118. [DOI] [PubMed] [Google Scholar]
- 8.Simmonds P, Davidson F, Lycett C, Prescott L E, MacDonald D M, Ellender J, Yap P L, Ludlam C A, Haydon G H, Gillon J, Jarvis L M. Detection of a novel DNA virus (TT virus) in blood donors and blood products. Lancet. 1998;352:191–195. doi: 10.1016/s0140-6736(98)03056-6. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka H, Okamoto H, Luengrojanakul P, Chainuvati T, Tsuda F, Tanaka T, Miyakawa Y, Mayumi M. Infection with an unenveloped DNA virus (TTV) associated with posttransfusion non-A to G hepatitis in hepatitis patients and healthy blood donors in Thailand. J Med Virol. 1998;56:234–238. doi: 10.1002/(sici)1096-9071(199811)56:3<234::aid-jmv10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 10.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]