Abstract
Despite demonstrated efficacy of vaccines against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the causative agent of coronavirus disease-2019 (COVID-19), widespread hesitancy to vaccination persists. Improved knowledge regarding frequency, severity, and duration of vaccine-associated symptoms may help reduce hesitancy. In this prospective observational study, we studied 1032 healthcare workers who received both doses of the Pfizer-BioNTech SARS-CoV-2 mRNA vaccine and completed post-vaccine symptom surveys both after dose 1 and after dose 2. We defined appreciable post-vaccine symptoms as those of at least moderate severity and lasting at least 2 days. We found that symptoms were more frequent following the second vaccine dose than the first (74% vs. 60%, P < 0.001), with >80% of all symptoms resolving within 2 days. The most common symptom was injection site pain, followed by fatigue and malaise. Overall, 20% of participants experienced appreciable symptoms after dose 1 and 30% after dose 2. In multivariable analyses, female sex was associated with greater odds of appreciable symptoms after both dose 1 (OR, 95% CI 1.73, 1.19–2.51) and dose 2 (1.76, 1.28–2.42). Prior COVID-19 was also associated with appreciable symptoms following dose 1, while younger age and history of hypertension were associated with appreciable symptoms after dose 2. We conclude that most post-vaccine symptoms are reportedly mild and last <2 days. Appreciable post-vaccine symptoms are associated with female sex, prior COVID-19, younger age, and hypertension. This information can aid clinicians in advising patients on the safety and expected symptomatology associated with vaccination.
Keywords: SARS-CoV-2, Vaccination, Vaccine-associated symptoms
1. Introduction
Coronavirus disease 2019 (COVID-19) has affected over 100 million individuals worldwide and led to more than 3 million deaths across 223 countries, as of April 2021 (Rothwell et al., 2010; Zhu et al., 2020). In attempts to curb the global effects of the pandemic, appreciable resources are being invested to rapidly deploy vaccinations against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Notwithstanding these intense efforts, vaccine hesitancy persists and may stand as a key barrier to timely vaccination across multiple regions and subpopulations (Lin et al., 2020; Sherman et al., 2021; Schwarzinger et al., 2021; Dror et al., 2020). Despite strong endorsements by national and international health agencies for SARS-CoV-2 vaccines (Oliver et al., 2020; Dooling et al., 2020; Oliver et al., 2021), a not insignificant portion of the population continues to defer or completely decline vaccination (Schwarzinger et al., 2021; Fisher et al., 2020). Hesitant individuals have cited concerns regarding safety, side effects, and the perception that vaccination may cause symptomatic illness or even acute viral infection (Lin et al., 2020; Sherman et al., 2021; Sallam, 2021; Lennon et al., 2021).
Given the rapid development of SARS-CoV-2 vaccinations and the known immunogenicity underlying their mechanisms of action, a widely expressed need exists – even among willing vaccine recipients – for more information regarding the expected symptoms and side effect profiles (Dror et al., 2020; Thaker, 2021). Although clinical trials and surveillance studies have provided general data regarding potential vaccine side effects, limited information is currently available on the timing, duration, and severity for different symptom types, and the extent to which certain side effect profiles are more or less common for important at-risk subgroups (Polack et al., 2020; Baden et al., 2021; Folegatti et al., 2020). To this end, we conducted a prospective study to directly address existing information gaps regarding local and systemic symptoms experienced by a large cohort of recipients of a currently available and widely used SARS-CoV-2 messenger ribonucleic acid (mRNA) vaccine.
2. Methods
2.1. Participant recruitment
We conducted a comprehensive survey of symptoms in healthcare workers who received their first and second doses of the Pfizer-BioNTech vaccine between December 17, 2020 and February 10, 2021 at a large academic medical institution. Participation was voluntary and all self-reported symptoms data were collected using a survey instrument administered via Research Electronic Data Capture (REDCap) (Harris et al., 2019). Open study enrollment was made available through newsletters, flyers, and study staff present at institutional vaccination sites. Recruitment began prior to vaccination, and participants for the current analysis were enrolled up through day 8 following the first dose of vaccination in order to minimize potential time-dependent recall bias in assessments of post-vaccination symptoms. Each study participant completed a baseline survey including standardized questions on demographic, exposure, and medical history. Participants also completed a standardized survey of post-vaccination symptoms between 8 and 21 days after each of the two vaccine doses received (21 days apart) (Supplemental Fig. 1). Participants enrolled in vaccine clinical trials were excluded. Of the 1632 participants enrolled with baseline survey data collected, a total of 1032 participants completed post-vaccine symptom surveys both after dose 1 and then again after dose 2 of vaccination. All study participants provided written informed consent, and all study protocols were approved by the Cedars-Sinai Medical Center institutional review board (CORALE Study00000621).
2.2. Data collection
The survey was designed to assess demographic and clinical information in addition to self-reported post-vaccination symptoms pertaining to not only symptom type but also timing (i.e. onset in relation to administration of the vaccine dose), severity (mild, moderate, or severe), and duration (i.e. days since administration of the vaccine dose). For symptom type, the survey assessed injection site-related symptoms distinctly from systemic symptoms as in the original SARS-CoV-2 vaccine clinical trials (Polack et al., 2020; Baden et al., 2021; Folegatti et al., 2020). For potentially localizing symptoms, the survey instrument also queried location (e.g. if swelling was reported, the instrument queried if localized or widespread relative to injection site). The following localizing symptom variables were obtained: pain at injection site, redness at injection site, and swelling at injection site. Additionally, the following systemic symptom variables were recorded: fever (mild 99 °F, moderate 100–101 °F, or severe ≥102 °F), chills, fatigue or malaise, headache, dizziness, or lightheadedness, eye, ear, mouth, or throat changes, swollen lymph nodes, skin/nail or facial changes, cough, chest, or breathing symptoms, nausea, vomiting, diarrhea, or other digestive symptoms, urinary or genital symptoms, muscle, bone, joint, or nerve symptoms, memory or mood changes, sleep changes, and reports of all other symptoms. Open access to the full survey instrument is available at: corale-study.org/postvaxsxsurvey.
2.3. Statistical analyses
In bivariate analyses, we examined the frequencies and duration of post-vaccine symptoms after the first and second dose of administered vaccine using Chi-square. In sensitivity analyses, we conducted the same analyses with groups stratified by age (<50 versus ≥50 years), sex, race and ethnicity, and history of prior COVID-19 diagnosis (infection naïve versus confirmed prior infection). We subsequently conducted multivariable-adjusted logistic regression analyses to examine associations of demographic (age, sex, race/ethnicity) and clinical characteristics with the outcomes of ‘appreciable symptomatology’ after each vaccine dose, with appreciable symptoms defined as any self-reported symptom experienced with at least moderate severity and lasting ≥2 days (i.e. at least 48 h). Initial models were deliberately sparse, adjusting for a limited number of key covariates (age and sex). Final models adjusted for those variables with associations meeting a significance threshold of P < 0.10 in the sparse regressions. Age was transformed to assess for associations with each additional decade of life. We performed all statistical analyses using R v3.5.1 and considered differences to be statistically significant at a threshold of P < 0.05.
2.4. Patient and public involvement
Patients and the public were not involved in the development of this study.
3. Results
3.1. Post-vaccine symptom characteristics and duration
A total of 1632 participants enrolled, of whom 1195 completed a symptoms survey after dose 1 and 1130 after dose 2. Four participants were part of vaccine trials and were excluded. A total of 1032 participants completed both surveys and were included in the analysis. The average age of the cohort was 43.3 ± 12.6 years, 691 (67.4%) were female, and 83 (8.0%) were previously diagnosed with COVID-19 (Table 1 ). Overall, 48.1% of the cohort identified as Non-Hispanic White, 27.3% as Asian, 12.3% as Hispanic/Latinx, and 3.2% as Non-Hispanic Black. Appreciable symptoms (symptoms of at least moderate severity lasting ≥2 days) were experienced by 207 (20.1%) participants after dose 1 and 311 (30.1%) after dose 2. Overall, only 20 (1.9%) participants reported symptoms lasting >7 days after dose 1, with 44 (4.3%) reporting symptoms lasting >7 days after their second dose. The most frequently reported appreciable symptoms were injection site symptoms (30.3%), followed by reports of fatigue or malaise (11.2%). There were no reports of acute bleeding, thrombotic events or severe allergic and/or anaphylactic reactions during or after vaccine administration.
Table 1.
Baseline characteristics and self-reported appreciable symptoms of participants who completed symptom surveys following each dose of the Pfizer-BioNTech SARS-CoV-2 mRNA vaccine.
| Characteristics | Total sample | Appreciable symptomsa after dose 1 |
Appreciable symptomsa after dose 2 |
||||
|---|---|---|---|---|---|---|---|
| None | Lasting 2–7 days | Lasting > 7 days | None | Lasting 2–7 days | Lasting > 7 days | ||
| N | 1032 | 825 | 187 | 20 | 721 | 267 | 44 |
| Age years, mean (SD) | 43.30 (12.61) | 43.48 (12.87) | 41.92 (11.25) | 48.93 (12.31) | 43.91 (13.28) | 41.68 (10.98) | 43.21 (9.54) |
| Female sex | 691 (67.4) | 536 (65.4) | 139 (75.1) | 16 (80.0) | 460 (64.0) | 193 (73.7) | 38 (86.4) |
| Race/Ethnicity | |||||||
| Non-Hispanic Asian | 280 (27.1) | 218 (26.4) | 57 (30.5) | 5 (25.0) | 206 (28.6) | 68 (25.5) | 6 (13.6) |
| Non-Hispanic Black | 33 (3.2) | 29 (3.5) | 3 (1.6) | 1 (5.0) | 23 (3.2) | 7 (2.6) | 3 (6.8) |
| Non-Hispanic White | 493 (47.8) | 400 (48.5) | 88 (47.1) | 5 (25.0) | 344 (47.7) | 131 (49.1) | 18 (40.9) |
| Hispanic/Latinx | 126 (12.2) | 97 (11.8) | 24 (12.8) | 5 (25.0) | 89 (12.3) | 23 (8.6) | 14 (31.8) |
| Other | 100 (9.7) | 81 (9.8) | 15 (8.0) | 4 (20.0) | 59 (8.2) | 38 (14.2) | 3 (6.8) |
| Medical conditions | |||||||
| Asthma | 163 (16.9) | 122 (15.8) | 35 (20.2) | 6 (33.3) | 106 (15.7) | 48 (19.2) | 9 (23.7) |
| Immune disorderb | 74 (7.2) | 53 (6.4) | 16 (8.6) | 5 (25.0) | 44 (6.1) | 21 (7.9) | 9 (20.5) |
| Cancerc | 35 (3.4) | 29 (3.5) | 6 (3.2) | 0 (0.0) | 26 (3.6) | 7 (2.6) | 2 (4.5) |
| Cardiovasculard | 86 (8.3) | 70 (8.5) | 15 (8.0) | 1 (5.0) | 65 (9.0) | 17 (6.4) | 4 (9.1) |
| Chronic obstructive pulmonary disease | 6 (0.6) | 5 (0.7) | 1 (0.6) | 0 (0.0) | 3 (0.4) | 3 (1.2) | 0 (0.0) |
| Diabetes mellitus | 55 (5.7) | 45 (5.8) | 8 (4.7) | 2 (13.3) | 37 (5.5) | 14 (5.7) | 4 (11.8) |
| Hypertension | 162 (16.7) | 132 (16.9) | 24 (14.0) | 6 (35.3) | 106 (15.6) | 49 (19.7) | 7 (18.4) |
| Prior diagnosis of COVID-19 | 83 (8.0) | 56 (6.8) | 26 (13.9) | 1 (5.0) | 56 (7.8) | 25 (9.4) | 2 (4.5) |
| Appreciable symptomsa | 407 (39.4) | – | 187 (100.0) | 20 (100.0) | – | 267 (100.0) | 44 (100.0) |
| Appreciable non-injection site symptomsa | 188 (18.2) | 44 (23.5) | 19 (95.0) | 109 (40.8) | 42 (95.5) | ||
| Appreciable symptom typea | |||||||
| Injection site | 313 (30.3) | 165 (88.2) | 11 (55.0) | 202 (75.7) | 19 (43.2) | ||
| Fever or chills | 19 (1.8) | 5 (2.7) | 0 (0.0) | 11 (4.1) | 4 (9.1) | ||
| Fatigue or malaise | 116 (11.2) | 24 (12.8) | 10 (50.0) | 67 (25.1) | 27 (61.4) | ||
| Headache, dizziness or lightheadedness | 70 (6.8) | 19 (10.2) | 9 (45.0) | 29 (10.9) | 20 (45.5) | ||
| Eye, ear, mouth, or throat changes | 7 (0.7) | 1 (0.5) | 1 (5.0) | 3 (1.1) | 2 (4.5) | ||
| Swollen lymph node, skin/nail, or face changes | 22 (2.1) | 1 (0.5) | 2 (10.0) | 11 (4.1) | 8 (18.2) | ||
| Cough, chest, or breathing symptoms | 11 (1.1) | 2 (1.1) | 3 (15.0) | 3 (1.1) | 4 (9.1) | ||
| Nausea, vomiting, diarrhea, or other digestive symptoms | 19 (1.8) | 3 (1.6) | 2 (10.0) | 12 (4.5) | 5 (11.4) | ||
| Urinary or genital symptoms | 1 (0.1) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 0 (0.0) | ||
| Muscle, bone, joint, or nerve symptoms | 62 (6.0) | 9 (4.8) | 8 (40.0) | 36 (13.5) | 17 (38.6) | ||
| Memory or mood change | 14 (1.4) | 2 (1.1) | 2 (10.0) | 8 (3.0) | 4 (9.1) | ||
| Sleep changes | 21 (2.0) | 1 (0.5) | 1 (5.0) | 11 (4.1) | 10 (22.7) | ||
n (%); SD (standard deviation).
Appreciable symptoms are defined as any self-reported symptom of at least moderate severity and lasting at least 2 days.
Immune disorders include systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease and HIV.
Cancer includes malignancy of any type/stage, with survey questions specifically asking about colon, lung, liver, prostate and ‘other.’
Cardiovascular conditions include coronary artery disease, heart failure, atrial fibrillation or flutter, as well as a survey response ‘other.’
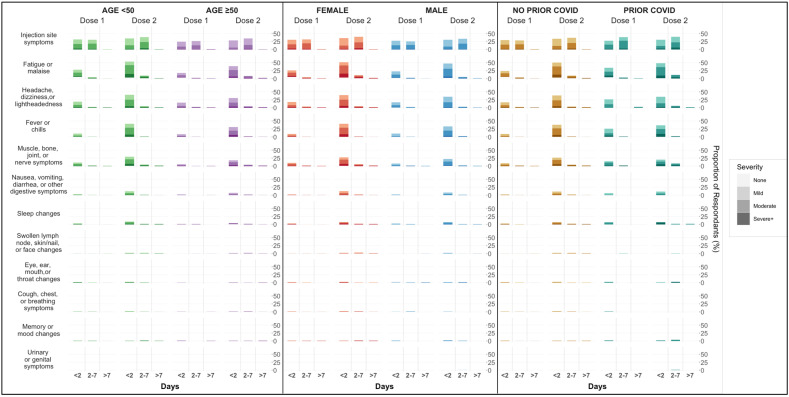
The most frequently reported symptom of any severity following each dose was injection site pain (Fig. 1 ), which was more frequently reported after the second vaccine dose (59.1% vs 71.2%, P < 0.001). Following dose 1, approximately half of participants who experienced injection site pain reported the pain lasting less than a day, with only 4 individuals (0.4%) reporting pain lasting longer than a week. Pain lasted longer following dose 2, with 368 (35.7%) reporting pain lasting up to a week (P < 0.001 for overall comparison between dose 1 and dose 2 symptoms). Reports of injection site redness and swelling were less common, but were still more frequently reported and lasted longer after the second dose compared to the first (Table 2 ).
Fig. 1.
Post-vaccine symptoms frequency, type, severity, and duration by age, sex and prior COVID-19 infection status.
Table 2.
Injection site symptom frequency and duration following first and second doses of the Pfizer-BioNTech SARS-CoV-2 mRNA vaccine.
| Post-dose 1 (n = 1032) | Post-dose 2 (n = 1032) | P-valuea | |
|---|---|---|---|
| Any injection site symptom | 610 (59.1) | 735 (71.2) | <0.001 |
| Pain | 604 (58.5) | 727 (70.4) | <0.001 |
| <1 h | 5 (0.8) | 7 (0.9) | <0.001 |
| <1 day | 309 (51.2) | 348 (47.9) | |
| <1 week | 286 (47.3) | 368 (50.6) | |
| >1 week | 4 (0.7) | 4 (0.6) | |
| Redness | 52 (5.0) | 94 (9.1) | <0.001 |
| <1 h | 1 (1.9) | 2 (2.1) | 0.005 |
| <1 day | 27 (51.9) | 40 (42.6) | |
| <1 week | 24 (46.1) | 50 (53.2) | |
| >1 week | 0 (0.0) | 2 (2.1) | |
| Swelling | 84 (8.1) | 132 (12.8) | <0.001 |
| <1 h | 3 (3.6) | 4 (3.0) | 0.027 |
| <1 day | 35 (41.6) | 57 (43.2) | |
| <1 week | 46 (54.8) | 70 (53.0) | |
| >1 week | 0 (0.0) | 1 (0.8) |
n (%).
Listed percentages for each symptom represent percent of entire respective subpopulation. Percentages for duration represent percent of symptomatic subpopulation in each timeframe.
Chi-Square or ANOVA for comparison of any presence of each symptom and duration of symptoms, respectively.
The majority of participants reported at least one non-injection site symptom following both doses of vaccine, with symptoms less common following the first dose than the second (60.0% vs 73.6%, P < 0.001) (Table 3 ). The most common non-injection site symptom following both doses was fatigue and malaise, again occurring more frequently after dose 2 than dose 1 (28.8% vs 60.8%, P < 0.001). Headache (21.6% vs 43.9%, P < 0.001) and fever or chills (10.5% vs 39.8%, P < 0.001) were the next most frequently reported symptoms, again reported more often after the second dose than the first. The vast majority non-injection site symptoms resolved in ≤2 days (84.0% after dose 1 and 83.4% after dose 2).
Table 3.
Symptom frequency and duration following first and second doses of the Pfizer-BioNTech SARS-CoV-2 mRNA vaccine.
| Post-dose 1 (n = 1032) | Post-dose 2 (n = 1032) | P-valuea | |
|---|---|---|---|
| Any symptom | 614 (60.0) | 752 (73.6) | <0.001 |
| Any non-injection site symptom | 428 (41.8) | 724 (70.8) | <0.001 |
| Fever or chills | 108 (10.5) | 410 (39.8) | <0.001 |
| ≤2 days | 103 (95.4) | 394 (96.1) | <0.001 |
| 2–7 days | 5 (4.6) | 15 (3.7) | |
| >7 days | 0 (0.0) | 1 (0.2) | |
| Fatigue or malaise | 297 (28.8) | 626 (60.8) | <0.001 |
| ≤2 days | 255 (85.9) | 516 (82.4) | <0.001 |
| 2–7 days | 36 (12.1) | 94 (15.0) | |
| >7 days | 6 (2.0) | 16 (2.6) | |
| Headache, dizziness or lightheadedness | 222 (21.6) | 451 (43.9) | <0.001 |
| ≤2 days | 185 (83.3) | 391 (86.7) | <0.001 |
| 2–7 days | 31 (14.0) | 46 (10.2) | |
| >7 days | 6 (2.7) | 14 (3.1) | |
| Eye, ear, mouth, or throat changes | 23 (2.2) | 34 (3.3) | 0.18 |
| ≤2 days | 17 (73.9) | 25 (73.5) | 0.50 |
| 2–7 days | 5 (21.7) | 6 (17.6) | |
| >7 days | 1 (4.3) | 3 (8.8) | |
| Swollen lymph node, skin/nail, or face changes | 10 (1.0) | 57 (5.5) | <0.001 |
| ≤2 days | 3 (30.0) | 22 (38.6) | <0.001 |
| 2–7 days | 3 (30.0) | 26 (45.6) | |
| >7 days | 4 (40.0) | 9 (15.8) | |
| Cough, chest, or breathing symptoms | 20 (1.9) | 32 (3.1) | 0.122 |
| ≤2 days | 13 (65.0) | 20 (62.5) | 0.41 |
| 2–7 days | 5 (25.0) | 9 (28.1) | |
| >7 days | 2 (10.0) | 3 (9.4) | |
| Nausea, vomiting, diarrhea, or other digestive symptoms | 32 (3.1) | 139 (13.5) | <0.001 |
| ≤2 days | 26 (81.3) | 118 (84.9) | <0.001 |
| 2–7 days | 4 (12.5) | 18 (12.9) | |
| >7 days | 2 (6.3) | 3 (2.2) | |
| Urinary or genital symptoms | 0 (0.0) | 3 (0.3) | – |
| ≤2 days | 0 (0.0) | 2 (66.7) | – |
| 2–7 days | 0 (0.0) | 1 (33.3) | |
| >7 days | 0 (0.0) | 0 (0.0) | |
| Muscle, bone, joint, or nerve symptoms | 120 (11.6) | 330 (32.0) | <0.001 |
| ≤2 days | 98 (81.7) | 271 (82.1) | <0.001 |
| 2–7 days | 14 (11.7) | 45 (13.6) | |
| >7 days | 8 (6.7) | 14 (4.2) | |
| Memory or mood change | 12 (1.2) | 26 (2.5) | 0.033 |
| ≤2 days | 8 (66.7) | 12 (46.2) | 0.08 |
| 2–7 days | 2 (16.7) | 10 (38.5) | |
| >7 days | 2 (16.7) | 4 (15.4) | |
| Sleep changes | 33 (3.2) | 101 (9.8) | <0.001 |
| ≤2 days | 29 (87.9) | 74 (73.3) | <0.001 |
| 2–7 days | 3 (9.1) | 18 (17.8) | |
| >7 days | 1 (3.0) | 9 (8.9) |
n (%).
Listed percentages for each symptom represent percent of entire respective subpopulation. Percentages for duration represent percent of symptomatic subpopulation in each timeframe.
Chi-Square or ANOVA for comparison of any presence of each symptom and duration of symptoms, respectively.
3.2. Post-vaccine symptoms by age, sex, and race/ethnicity
In pre-specified stratified analyses, vaccine recipients age < 50 years were more likely than those over the age of 50 to report any symptoms after receiving both the first (62.8% vs. 52.0%, P = 0.002) and second dose (77.1% vs. 64.0%, P < 0.001) (Supplemental Table 1). Younger individuals specifically reported injection site pain and fatigue or malaise more frequently after both doses, and additionally more frequently reported fever or chills, headaches, and muscle aches after dose 2. Female participants reported the presence of any symptom more frequently than men after both doses (Dose 1 62.7% vs 53.8%, P = 0.008 and Dose 2 77.2% vs 66.3%, P < 0.001), though only reported non-injection site symptoms more frequently following dose 2 (Dose 1 43.4% vs 37.8%, P = 0.10 and Dose 2 74.5% vs 63.3%, P < 0.001) (Supplemental Table 2). Sex differences in specific reported symptoms were most appreciable after the second dose, with women more frequently reporting fevers or chills, fatigue or malaise, headache, swollen lymph nodes, breathing issues, gastrointestinal symptoms, and muscle aches. When stratified by prior COVID-19 diagnosis, there was no statistically significant difference in overall reported symptoms between those with and without prior COVID-19 infection after either dose (Dose 1 68.7% vs 59.2%, P = 0.12 and Dose 2 74.7% vs 73.5%, P = 0.91) (Supplemental Table 3). Of individual symptoms, fevers or chills were more frequently reported after dose 1 among those with prior COVID-19 infection than infection naïve individuals (26.5% vs 9.1%, P < 0.001), though this was not appreciated after dose 2. The overall frequency of injection site and non-injection site symptoms did not vary by race or ethnicity, though small differences were appreciated among specific symptoms; racial/ethnic minority participants reported fatigue and malaise more frequently after the first dose of vaccine, while non-Hispanic White participants reported slightly higher rates of fever and chills after their second dose (Supplemental Table 4).
3.3. Predictors of post-vaccine symptoms
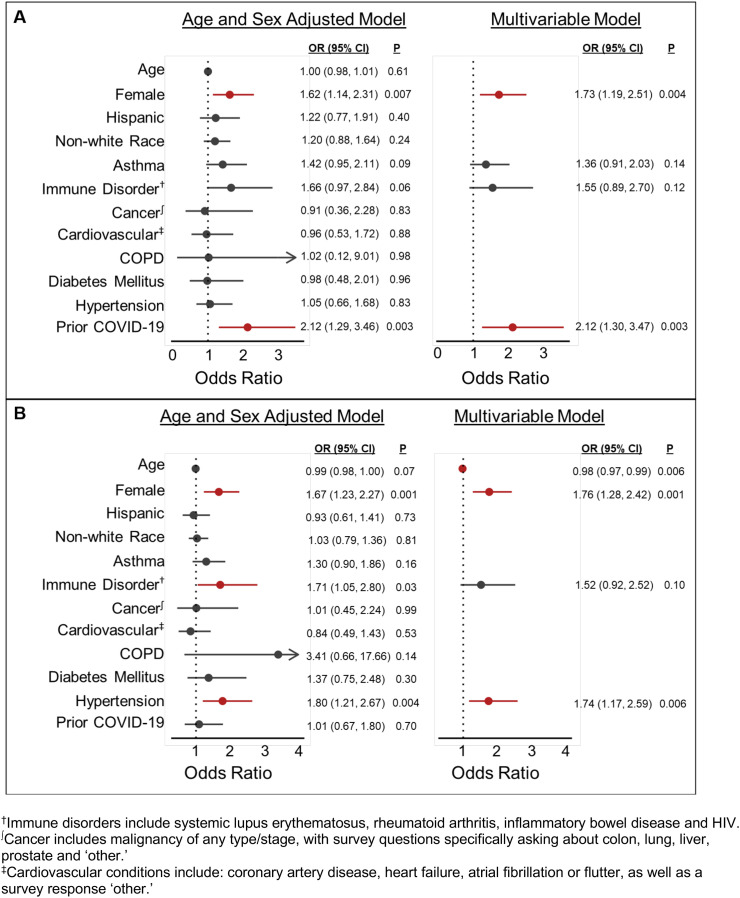
In age- and sex-adjusted analysis of predictors of appreciable symptoms following dose 1, female sex, history of asthma or an immune disorder and prior COVID-19 infection met the threshold for inclusion in the multivariable model. Following multivariable adjustment, only female sex (OR, 95% CI 1.73, 1.19–2.51) and prior COVID-19 diagnosis (2.12, 1.30–3.47) were statistically associated with appreciable symptoms following dose 1 (Fig. 2A). In age- and sex-adjusted analysis for predictors of appreciable symptoms following dose 2, additional decade of age, female sex and history of immune disorder and hypertension met criteria for inclusion in the multivariable model. Following multivariable adjustment, additional decade of age (0.98, 0.97–0.99), female sex (1.76, 1.28–2.42) and hypertension (1.74, 1.17–2.59) remained significantly associated with appreciable symptoms following a second vaccine dose (Fig. 2B).
Fig. 2.
Multivariable-adjusted correlates of appreciable post-vaccine symptoms following dose 1 (Panel A) and dose 2 (Panel B) of an mRNA SARS-CoV-2 vaccine. Appreciable symptoms are defined as any self-reported symptoms of at least moderate severity and lasting at least 2 days in duration. Multivariable-adjusted models included all covariates with P < 0.10 in the age- and sex-adjusted models.
4. Discussion
In this prospective study of over 1000 participants receiving both doses of a mRNA SARS-CoV-2 vaccine, the vast majority of post-vaccine symptoms were mild and short lasting, with temporary discomfort at the injection site being most common. Appreciable symptoms, those of at least moderate severity and lasting ≥2 days, occurred in less than a third of patients following either vaccine dose, with nearly all such symptoms resolving in less than 7 days, with no severe adverse events.
4.1. Post-vaccine symptoms and vaccine hesitancy
The development, testing and emergency authorization of mRNA vaccines against SARS-CoV-2 has dramatically shifted the fight against the ongoing pandemic. As mass distribution and administration efforts continue around the globe, an accurate understanding of potential post-vaccine symptoms is vital to allow individuals to make informed personal decisions around vaccination. Multiple analyses have identified vaccine hesitancy as a major hurdle, with approximately 40% of US adults expressing either hesitancy or non-intent to receive SARS-CoV-2 vaccination (Fisher et al., 2020; Nguyen et al., 2021). Concerns around safety, efficacy and adverse events were consistent themes raised by participants. Importantly, a study of nearly 2000 US adults found that reductions in reported major adverse vaccine side effects were associated with increased willingness to receive vaccination (Fisher et al., 2020; Kreps et al., 2020). While the United States has made incredible early strides in vaccination, with over 200 million vaccine doses administered by the end of April 2021, rate of vaccination has already begun to decline, with thousands of vaccination appointments remaining unfilled every day (Center for Disease Control and Prevention COVID Data Tracker, 2021; Taylor and Coleman, 2021; Kluck, 2021; Lin-Fisher and Mills, 2021). As such, addressing patients' concerns around identified issues such as post-vaccine symptoms is vital in order to obtain sufficient vaccine uptake to curb the pandemic.
4.2. Prior studies of post-vaccine symptoms
Early studies reported similar frequencies of post-vaccine symptoms, though lacked granularity regarding symptom severity and duration, and failed to adjust for important patient-level characteristics. As in our analysis, pain at the injection site has been the most frequently reported symptom across studies, with fatigue being the most frequently reported systemic symptom (Sallam, 2021; Baden et al., 2021). The Center for Disease Control and Prevention's V-Safe Program has monitored for signals of adverse events following mRNA SARS-CoV-2 vaccination and found increased reactogenicity following the second dose of vaccine, similar to our findings (Chapin-Bardales et al., 2021). Our data also supports early work demonstrating that women, younger individuals and those with prior COVID-19 infection tend to report symptoms more frequently following vaccination (Chapin-Bardales et al., 2021; Ebinger et al., 2021; Mathioudakis et al., 2021; Ou et al., 2021). Importantly, these prior analyses are limited by their lack of detailed information on symptom severity, duration and detailed associations with patient-specific characteristics.
Our analysis focuses on appreciable symptoms, those of at least moderate severity, lasting 2 or more days. This differentiation between appreciable and mild symptoms helps patients and clinicians understand the clinical and functional implications of post-vaccine reactogenicity. To this end, appreciable non-injection site symptoms were low, occurring in only 18% of participants. The overall frequency of appreciable symptoms did not differ by race or ethnicity. Further, when appreciable symptoms did occur, they tended to resolve between 2 and 7 days after vaccination. When duration of symptoms, regardless of severity, were examined, a vast majority resolved in less than 2 days.
To provide more individualized information to patients and clinicians, we assessed the association of multiple demographic and clinical characteristics with the development of appreciable symptoms. Female sex was associated with an increased odds of reporting one or more appreciable symptoms following both doses of mRNA vaccine. This finding is supported by other analyses and importantly, this association persists even with multivariable adjustment for baseline medical conditions and when limited to appreciable symptoms that are more likely to impact a patient's decision to receive vaccination.
As in other analyses, prior COVID-19 diagnosis was associated with increased odds of appreciable symptoms, but only after the first dose of vaccine (Tré-Hardy et al., 2021). Specifically in female patients, our analysis helps previously infected patients better understand the potential expected severity and duration of symptoms. Additionally, this analysis provides reassurance that these symptoms are not more likely to occur after a second dose and are unrelated to prior infection status. Multiple potential mechanisms have been postulated to drive this finding. Our team has previously reported on higher anti-Spike protein IgG levels following single-dose vaccination for those with a history of COVID-19 compared with COVID-19 naïve vaccine recipients (Ebinger et al., 2021). This robust immunologic response following single vaccination in those with prior COVID-19 infection may contribute to their increased reactogenicity, with ‘normalization’ of their reactogenicity following second dose when the COVID-19 naïve recipients immunologic response approximates that of those with prior infection (Lennon et al., 2021; Baden et al., 2021; Kluck, 2021; Lin-Fisher and Mills, 2021).
Interestingly, compared with prior studies, older age was associated with only slightly lower odds of vaccine reactogenicity and only after the second dose (Chapin-Bardales et al., 2021; Ou et al., 2021). In unadjusted analyses, 13% more participants under age 50 years compared to older participants experienced post-vaccine symptoms. In multivariable-adjusted analyses the age association was modest and significant only after dose 2, suggesting that factors other than chronologic age are more important for determining degree of reactogenicity to vaccine.
The finding of hypertension as a marker of increased odds of appreciable vaccine reactogenicity after dose 2 of vaccine is a novel finding, raising questions of pathophysiologic effects from both natural- and vaccine-mediated immune responses to SARS-CoV-2. Multiple studies have found associations between hypertension and more severe COVID-19, though the degree to which hypertension may contribute to worse outcomes or simply reflect limited physiologic reserve due to underlying chronic disease and end organ dysfunction remains unknown (Wu et al., 2020; Cummings et al., 2020; Guan et al., 2020; Tadic et al., 2020; Drager et al., 2020). Initial hypotheses on how hypertension may affect COVID-19 severity focused on the role of ACE2 receptor expression; given its role in cellular entry, however, this has not proven to be a major factor in disease severity (Liu et al., 2020). Further, how hypertension may contribute to increased reactogenicity to a vaccine that produced SARS-CoV-2 spike protein remains unclear. While experimental data have suggested an elevated propensity for pro-inflammatory cytokine production in the setting of hypertension and COVID-19 infection (Trump et al., 2021; Watanabe et al., 2021), there are no prior clinical data relating hypertension with reactogenicity to vaccines for COVID-19 or other infectious agents.
Several limitations to this study merit consideration. First, our cohort consists of healthcare workers from a single center who volunteered and responded to sequential surveys about post-vaccine symptoms, potentially limiting generalizability. Fortunately, study subjects were relatively diverse and reported post-vaccine symptoms consistent with univariate and bivariate analyses from prior studies. Second, all participants in this study received the Pfizer-BioNTech mRNA SARS-CoV-2 vaccine; thus, additional studies are needed to determine the extent to which any of our findings are generalizable to other vaccine manufacturers and platforms. Third, although we collected self-reported information on prior COVID-19 infections, we were unable to verify the timing of or circumstances associated with prior infection given these details were not consistently available; thus, we considered prior COVID-19 infection as a binary variable and further studies are needed to examine whether post-vaccine symptomatology varies based on the timing or severity of prior COVID-19 infection. Finally, while this was a prospective study, the surveys required participants to report symptoms up to 3 weeks after vaccination, contributing to potential recall bias. Further, self-reports of symptoms and, to a lesser extent, of comorbidities are inherently subjective; nonetheless, this approach can provide a granular assessment of symptom experience that may be applicable to future vaccine recipients.
In summary, this study found that mild, time-limited, symptoms are common after mRNA SARS-CoV-2 vaccination; more appreciable symptoms of at least moderate severity (including fatigue, headache, or fever) are less frequent and tend to resolve in less than a week. Female sex increases the odds of appreciable reactogenicity after both doses of vaccine, while prior COVID-19 infection increases these odds after only the first dose; odds of appreciable reactogenicity are also greater among hypertensives and younger individuals following the second dose. This information may be used to help clinicians and the public make informed decisions around vaccination against SARS-CoV-2, by specifically addressing concerns surrounding the type and timing of vaccine side effects – which are generally mild or self-limited.
Competing interests
None declared.
Funding
This work was supported in part by the Cedars-Sinai Medical Center (no award number), the Erika J Glazer Family Foundation (no award number), the F. Widjaja Family Foundation (no award number), the Helmsley Charitable Trust (no award number), and NIH grants U54-CA260591, R01-AI072726, and K23-HL153888.
Acknowledgments
We are grateful to all the front-line healthcare workers in our healthcare system who continue to be dedicated to delivering the highest quality care for all patients. We would like to thank the following people for their collective effort: Andrew Aguila; Francesca Paola Aguirre, MD; Kawsar Ahmad; Christine M. Albert, MD, MPH; Mona Alotaibi, MD; Allen Andres, PhD; Ani Balmanoukian, MD; Courtney Becker; Diana Benliyan; Anders H. Berg, MD, PhD; Eva Biener-Ramanujan; Aleksandra Binek, PhD; David Casero; Jose Chavez, DNP, CNS, Blandine Chazarin Orgel; Mingtian Che; Peter Chen, MD; Vi Chiu, MD, PhD; Dain Choi; Melanie Chow; Cathie Chung; MD; Cailin Climer; Sarah Cooley, MBA; Rachel Coren, MPH; Donna Costales, RN; Tahir Dar; Tod Davis; Jessica Dos Santos, Keren R. Dunn, CIP; Rebecca Ely, RN; Mark Faries; Barbara Fields, RN; Lucia Florindez, PhD; Joslyn Foley; Norma Fontelera; Sarah Francis, RN; Jeffrey A. Golden, MD; Alma Gonzalez; Helen S. Goodridge, PhD; Jeanette Gonzalez; Jonathan D. Grein, MD; Gena Guidry, MSc; Ismar Hadziabdulahovic; Omid Hamid, MD; Alyssa Haley; Melissa Hampton, RN; Mary Hanna; Shima Hashemzadeh; Jorge Hernandez; Veronica Hollister; Lilith Huang; Khalil Huballa; Quyen Hurlburt, RN; Justina Ibrahim; Ugonna Ihenacho, MPH; Mohit Jain, MD, PhD; Harneet Jawanda, MD; Mary Jordan, Ashley Jose-Isip; Elizabeth H. Kim, MHDS; Edward Kowalewski; Catherine N. Le, MD; Nicole A. Leonard, JD, MBA; Yin Li; Anzhelya Makaryan; Danica-Mae Manalo, David Marshall, DNP, JD; Angela McArdle; Inderjit Mehmi, MD; Darlene Mejia; Larry Mendez; Emebet Mengesha; Kathrin S. Michelsen, PhD; Gail Milan, RN; Jordan Miller; Romalisa Miranda-Peats, MPH; Seyedeh Elnaz Mirzadeh; April Moore; Pamela Moore; Janette Moreno, DNP; Magali Noval Rivas, PhD; Fleury Nsole Bitghe, PhD; Michelle Offner, NP; Jillian Oft, MD; Elmar Park; Eunice Park; Vipul Patel, PharmD; Isabel Pedraza, MD; Connor Phebus; André Pile; Lawrence Piro, MD; Lauren R. Polak, JD; Ashley Porter; Matthew Puccio; V. Krishnan Ramanujan, PhD; Rocio Ramirez; Gerardo Ramirez; Celine E. Riera, PhD; Richard V. Riggs, MD; Alejandro Rivas; Jackie Robertson; Maria Salas; Michelle Schafieh, MS; Lorraine Sheffield, MSN, RN; Cristina Simons, RN; Muhammad Soomar; Clive Svendsen, PhD; Brian Tep; Warren G. Toutellotte, MD, PhD; Debra Valdes; Christy Velasco; Mectabel Velasquez; Kirstin Washington; Kristopher Wentzel, MD; Benjamin Wong; Mahendra Yatawara, MBA; Rachel Zabner, MD; Cindy Zamudio, MD; Yi Zhang, MD, MS; Lisa Zhou; Patrick Zvara, MS.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ypmed.2021.106860.
Appendix A. Supplementary data
Supplementary material
References
- Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention COVID Data Tracker 2021. https://covid.cdc.gov/covid-data-tracker/#datatracker-home Accessed April 22, 2021.
- Chapin-Bardales J., Gee J., Myers T. Reactogenicity following receipt of mRNA-based COVID-19 vaccines. JAMA. 2021;325(21):2201–2202. doi: 10.1001/jama.2021.5374. [DOI] [PubMed] [Google Scholar]
- Cummings M.J., Baldwin M.R., Abrams D., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooling K., McClung N., Chamberland M., et al. The advisory committee on immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine – United States, 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69(49):1857–1859. doi: 10.15585/mmwr.mm6949e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drager L.F., Pio-Abreu A., Lopes R.D., Bortolotto L.A. Is hypertension a real risk factor for poor prognosis in the COVID-19 pandemic? Curr. Hypertens. Rep. 2020;22(6):43. doi: 10.1007/s11906-020-01057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dror A.A., Eisenbach N., Taiber S., et al. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur. J. Epidemiol. 2020;35(8):775–779. doi: 10.1007/s10654-020-00671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebinger J.E., Fert-Bober J., Printsev I., et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021;27(6):981–984. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K.A., Bloomstone S.J., Walder J., Crawford S., Fouayzi H., Mazor K.M. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of U.S. adults. Ann. Intern. Med. 2020;173(12):964–973. doi: 10.7326/M20-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folegatti P.M., Ewer K.J., Aley P.K., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Liang W.H., Zhao Y., et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur. Respir. J. 2020;55(5):2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P.A., Taylor R., Minor B.L., et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluck K. WJHL | Tri-Cities News & Weather. WJHL News Channel. Vol. 11. April 7, 2021. Majority of available vaccine appointments go unfilled in Northeast Tennessee as cases, hospitalizations rise. [Google Scholar]
- Kreps S., Prasad S., Brownstein J.S., et al. Factors associated with US Adults’ likelihood of accepting COVID-19 vaccination. JAMA Netw. Open. 2020;3(10) doi: 10.1001/jamanetworkopen.2020.25594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon R.P., Small M.L., Smith R.A., et al. Unique predictors of intended uptake of a COVID-19 vaccine in adults living in a rural college town in the United States. Am. J. Health Promot. 2021 doi: 10.1177/08901171211026132. Online ahead of print, 08901171211026132. [DOI] [PubMed] [Google Scholar]
- Lin C., Tu P., Beitsch L.M. Confidence and receptivity for COVID-19 vaccines: a rapid systematic review. Vaccines (Basel). 2020;9(1):16. doi: 10.3390/vaccines9010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin-Fisher B., Mills E. 3 in 4 appointments filled at weekend Summit County COVID-19 vaccine clinics. Akron Beacon Journal. April, 20, 2021 [Google Scholar]
- Liu P.P., Blet A., Smyth D., Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020;142(1):68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- Mathioudakis A.G., Ghrew M., Ustianowski A., et al. Self-reported real-world safety and reactogenicity of COVID-19 vaccines: a vaccine recipient survey. Life (Basel). 2021;11(3):249. doi: 10.3390/life11030249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen K.H., Srivastav A., Razzaghi H., et al. COVID-19 vaccination intent, perceptions, and reasons for not vaccinating among groups prioritized for early vaccination – United States, September and December 2020. MMWR Morb. Mortal. Wkly Rep. 2021;70(6):217–222. doi: 10.15585/mmwr.mm7006e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S.E., Gargano J.W., Marin M., et al. The advisory committee on immunization practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine – United States, December 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69(50):1922–1924. doi: 10.15585/mmwr.mm6950e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S.E., Gargano J.W., Marin M., et al. The advisory committee on immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine – United States, December 2020. MMWR Morb. Mortal. Wkly Rep. 2021;69(5152):1653–1656. doi: 10.15585/mmwr.mm695152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou M.T., Boyarsky B.J., Motter J.D., et al. Safety and reactogenicity of 2 doses of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 2021;105(10):2170–2174. doi: 10.1097/TP.0000000000003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell P.M., Howard S.C., Dolan E., et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375(9718):895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- Sallam M. COVID-19 vaccine hesitancy worldwide: a concise systematic review of vaccine acceptance rates. Vaccines. 2021;9(2):160. doi: 10.3390/vaccines9020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzinger M., Watson V., Arwidson P., Alla F., Luchini S. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. Lancet Public Health. 2021;6(4):e210–e221. doi: 10.1016/S2468-2667(21)00012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman S.M., Smith L.E., Sim J., et al. COVID-19 vaccination intention in the UK: results from the COVID-19 vaccination acceptability study (CoVAccS), a nationally representative cross-sectional survey. Hum. Vaccin. Immunother. 2021;17(6):1612–1621. doi: 10.1080/21645515.2020.1846397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadic M., Cuspidi C., Grassi G., Mancia G. COVID-19 and arterial hypertension: hypothesis or evidence? J. Clin. Hypertens. (Greenwich). 2020;22(7):1120–1126. doi: 10.1111/jch.13925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor E., Coleman A. Thousands of vaccination appointments go unfilled at Pipkin building, health officials says. WREG News Memphis. April 8, 2021 [Google Scholar]
- Thaker J. The persistence of vaccine hesitancy: COVID-19 vaccination intention in New Zealand. J. Health Commun. 2021;26(2):104–111. doi: 10.1080/10810730.2021.1899346. [DOI] [PubMed] [Google Scholar]
- Tré-Hardy M., Cupaiolo R., Papleux E., et al. Reactogenicity, safety and antibody response, after one and two doses of mRNA-1273 in seronegative and seropositive healthcare workers. J. Inf. Secur. 2021;83(2):237–279. doi: 10.1016/j.jinf.2021.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trump S., Lukassen S., Anker M.S., et al. Hypertension delays viral clearance and exacerbates airway hyperinflammation in patients with COVID-19. Nat. Biotechnol. 2021;39(6):705–716. doi: 10.1038/s41587-020-00796-1. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Balena A., Tuccinardi D., et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab. Res. Rev. 2021 doi: 10.1002/dmrr.3465. e3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material