Abstract
Vaccine administration may be involved in the development of some central nervous system demyelinating diseases. The COVID-19 vaccine is being administered to the entire population, but to date, little association between vaccination and the risk of developing multiple sclerosis (MS) has been suggested, and only a few case reports have been published. Here, we present a 40-year-old woman who developed cervical myelitis after receiving the COVID-19 vaccine. Myelitis was considered the initial clinical manifestation of MS. Our case suggests a possible link between the vaccination and the clinical MS attack.
Keywords: Multiple sclerosis, Myelitis, COVID-19, Vaccine
1. Introduction
Vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is generally recommended for multiple sclerosis (MS), as is vaccination against other infectious agents (Reyes et al., 2020). It is thought that vaccine-induced protection from infection far outweighs the risk of autoimmune exacerbation (Havla et al., 2021). Regarding vaccination against SARS-CoV-2, three cases of reactivation or new-onset demyelinating disease have been reported after vaccination with the Oxford-AstraZeneca COVID-19 recombinant adenovirus (ChAdOx1 nCoV-19; AstraZeneca) (Voysey et al., 2021). Recently, a case of initial MS manifestation after vaccination with the Pfizer-BioNTech COVID-19 vaccine (BNT162b2, Comirnaty, BioNTech/Pfizer) was also reported (Havla et al., 2021). Herein, we report an additional Japanese case who presented with the first manifestation of MS after immunization with the BNT162b2 vaccine.
2. Case description
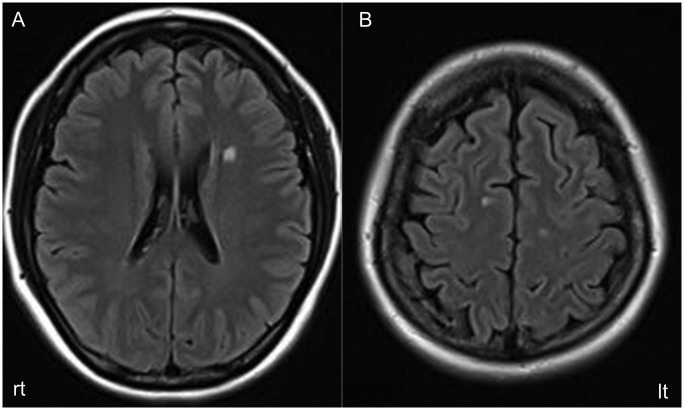
A 40-year-old woman was referred to the Department of Neurology, Tohoku Medical and Pharmaceutical University Hospital after she developed numbness and sensory disturbance in her right hand that gradually ascended to her right shoulder over a week. She had received the second dose of the BNT162b2 vaccine two weeks before the onset. After vaccination, she suffered from transient high-grade fever (38.5 degrees) without other symptoms. Her past history revealed that she was treated with steroids for left peripheral facial nerve palsy with full recovery 4 years ago. On admission, neurological examination revealed sensory disturbance in the dermatome of the right cervical 5th to 8th area. A brain MRI showed several periventricular or subcortical T2 hyperintense white matter lesions but no brainstem lesions (Fig. 1 ). These white matter lesions did not show abnormal gadolinium enhancement. A cervical spine MRI revealed a T2 hyperintense right spinal cord lesion with gadolinium enhancement at the level of C5/C6 (Fig. 2A, B). Cerebrospinal fluid (CSF) analysis showed slightly elevated leukocytes (4 cells/ml; 100% mononuclear cells), normal protein levels (27 mg/dl) and normal glucose levels (60 mg/dl). The oligoclonal IgG band, which was examined using an automatic electrophoresis processor based on a high-sensitive isoelectric focusing technique on agarose gel followed by immunofixation with peroxidase labelled anti-IgG, was positive. The IgG index was 1.04. The myelin basic protein (MBP) in the CSF was 146 pg/ml (below 102). The CSF interleukin-6 level was 2.4 pg/ml (below 4.0). Examinations for infectious CNS diseases, including herpes infection, vasculitis, collagen diseases, and sarcoidosis, were unremarkable. Serum aquaporin 4 (AQP4)-IgG and myelin oligodendrocyte glycoprotein (MOG)-IgG were assayed and turned out to be negative by live-cell-based assay (CBA) using full-length human AQP4 or MOG-transfected HEK 293 cells with IgG gamma-specific secondary antibodies as performed in our previous reports (Sato et al., 2014) (Takahashi et al., 2007). SARS-CoV-2 infection was excluded on the basis of a negative PCR test and clinical symptoms. After relevant differential diagnoses were excluded, we diagnosed her with MS based on the 2017 McDonald criteria (Thompson et al., 2018). Subsequent treatment with high-dose IV methylprednisolone (HIMP) (1000 mg methylprednisolone i.v. for three days) led to recovery (Fig. 2C).
Fig. 1.
Axial fluid-attenuated inversion recovery (FLAIR) imaging (A, B) (TR, 9000 ms; TE, 111 ms) MRI scan of the head on admission revealed left periventricular and bilateral frontal subcortical high-intensity lesions.
Fig. 2.

Midsagital T2-weighted image (T2WI) (1.5 T; TR 3800 ms, TE 95 ms) (A) on admission showing a high-intensity lesion, while a gadolinium-enhanced T1-weighted image (Gd T1WI) (TR 560 ms, TE 11 ms) (B) showing a faintly enhanced lesion at the C5/C6 level. Axial T2WI (TR 4000 ms, TE 100 ms) (a) on admission showing a high intensity and Gd T1WI (TR 430 ms, TE 12 ms) (b) showing an enhancement on the right side of the spinal cord at the C5/C6 level.
Sagittal (C) (1.5 T; TR, 3800 ms, TE, 95 ms) and axial C5/C6 level. (c) T2-weighted MRI (TR, 4000 ms, TE, 100 ms) of the cervical spine one month after admission showing a decreased high-intensity lesion.
3. Discussion
In the course of the current COVID-19 pandemic, a growing number of neurological manifestations and complications have been described. In this context, an increasing number of reports on spinal cord affection and transverse myelitis associated with COVID-19 have been published since 2020. Most cases represent postinfectious immune-mediated myelitis, whereas only a few are considered parainfectious or infectious. On the other hand, the onset of myelitis temporally associated with vaccination has been observed (Pagenkopf and Südmeyer, 2021).
In this study, we reported a patient who experienced the initial clinical manifestation of MS with a previously unknown background but likely with pre-existing subclinical inflammatory CNS disease after immunization with the BNT162b2 vaccine. We assumed that the patient had pre-existing subclinical inflammatory CNS disease before vaccination, since the patient already had several asymptomatic non‑gadolinium-enhanced brain lesions and oligoclonal IgG band on admission. Although she had a past history of left peripheral facial nerve palsy that resolved after steroid therapy, we could not confirm the episode as her initial clinical manifestation of MS.
To our knowledge, this is the second report in the literature that describes developing the first manifestation of MS after immunization with the BNT162b2 vaccine. In contrast to the previous report that described developing myelitis 6 days after the first immunization with the vaccine and required two cycles of glucocorticoid therapy (2000 mg methylprednisolone i.v. for five days) and plasma exchange, our patient developed myelitis 2 weeks after the second immunization with the vaccine and showed a good response to IVMP.
To date, national vigilance boards have already received several spontaneous reports on myelitis following COVID-19 vaccines. For instance, in the UK, there have been 17 cases reported for the BNT162b2 vaccine and 45 cases reported for the ChAdOx1 nCoV-19 vaccine. In Germany, there was one case for the BNT162b2 vaccine, and there were 2 cases for the ChAdOx1 nCoV-19 vaccine. In the US, there were 9 cases reported without mentioning a specific vaccine (Pagenkopf and Südmeyer, 2021). However, no further data on diagnostic findings are available, and generally no distinction is made between infectious and other aetiologies (Pagenkopf and Südmeyer, 2021).
Although in our case, the onset of MS might have been triggered by the vaccination, it is impossible to decide whether this occurrence is causally linked to the vaccination or a mere coincidence. Moreover, while a diagnosis of MS is possible on the basis of the McDonald criteria, we are not sure whether there is a chance that the condition in this patient remains monophasic. However, careful long-term observation is required, since vaccination might lead to attack in MS patients who are not administered disease-modifying drugs.
However, a recently published study involving approximately 500 MS patients showed that the relapse rate after vaccination with the BNT162b2 vaccine was similar (approximately 2%) to the relapse rate in a comparative time period without vaccination (Achiron et al., 2021). Therefore, as has been suggested in a previous report, the rarity of case reports, such as ours, may support the view that the benefits of vaccination against SARS-CoV-2 far outweigh the potential risks (Havla et al., 2021).
Consent statement
The patient provided written informed consent.
Conflicts of interest
IN is serving on scientific advisory boards for Biogen Japan and Novartis Pharma and is receiving honoraria for speaking engagements with Biogen Japan, Mitsubishi Tanabe Pharma, Novartis Pharma, Takeda Pharmaceutical, and Eisai. IN is receiving research support from LSI Medience and is funded by MHLW Program Grant Number 20FC1030 and JSPS KAKENHI Grant Number 20K07892. JF and KM report no disclosures.
Acknowledgements
None.
References
- Achiron A., Dolev M., Menascu S., Zohar D.N., Dreyer-Alster S., Miron S., et al. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by February 2021. Mult. Scler. 2021;27:864–870. doi: 10.1177/13524585211003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havla J., Schultz Y., Zimmermann H., Hohlfeld R., Danek A., Kümpfel T. First manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J. Neurol. 2021:1–4. doi: 10.1007/s00415-021-10648-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagenkopf C., Südmeyer M. A case of longitudinally extensive transverse myelitis following vaccination against Covid-19. J. Neuroimmunol. 2021;358:577606. doi: 10.1016/j.jneuroim.2021.577606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes S., Ramsay M., Ladhani S., Amirthalingam G., Singh N., Cores C., et al. Protecting people with multiple sclerosis through vaccination. Pract. Neurol. 2020;20:435–445. doi: 10.1136/practneurol-2020-002527. [DOI] [PubMed] [Google Scholar]
- Sato D.K., Callegaro D., Lana-Peixoto M.A., Waters P.J., de Haidar Jorge F.M., Takahashi T., et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology. 2014;82:474–481. doi: 10.1212/WNL.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Fujihara K., Nakashima I., Misu T., Miyazawa I., Nakamura M., et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain. 2007;130:1235–1243. doi: 10.1093/brain/awm062. [DOI] [PubMed] [Google Scholar]
- Thompson A.J., Banwell B.L., Barkhof F., Carroll W.M., Coetzee T., Comi G., et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]