Abstract
Objectives:
To evaluate whether liver and spleen magnetic resonance elastography (MRE) can measure the severity of congenital hepatic fibrosis (CHF) and portal hypertension (pHTN) in individuals with autosomal recessive polycystic kidney disease (ARPKD), and to examine correlations between liver MRE and ultrasound (US) elastography.
Methods:
Cross-sectional study of 9 individuals with ARPKD and 14 healthy controls. MRE was performed to measure mean liver and spleen stiffness (kPa); US elastography was performed to measure point shear wave speed (SWS) in both liver lobes. We compared: (1) MRE liver and spleen stiffness between controls vs. ARPKD; and (2) MRE liver stiffness between participants with ARPKD without vs. with pHTN, and examined correlations between MRE liver stiffness, spleen length, platelet counts, and US elastography SWS. Receiver operating characteristic (ROC) analysis was performed to examine diagnostic accuracy of liver MRE.
Results:
Participants with ARPKD (median age 16.8 [IQR 13.3, 18.9] years) had higher median MRE liver stiffness than controls (median age 14.7 [IQR 9.7, 16.7 years) (2.55 vs. 1.92 kPa, p=0.008), but MRE spleen stiffness did not differ. ARPKD participants with pHTN had higher median MRE liver stiffness than those without (3.60 kPa vs 2.49 kPa, p=0.05). Liver MRE and US elastography measurements were strongly correlated. To distinguish ARPKD vs. control groups, liver MRE had 78% sensitivity and 93% specificity at a proposed cut-off of 2.48 kPa [ROC area 0.83 (95% CI: 0.63–1.00)].
Conclusion:
Liver MRE may be a useful quantitative method to measure the severity of CHF and pHTN in individuals with ARPKD.
Keywords: Autosomal recessive polycystic kidney disease, congenital hepatic fibrosis, magnetic resonance elastography, portal hypertension, ultrasound elastography
Introduction
Liver involvement in autosomal recessive polycystic kidney disease (ARPKD) includes biliary duct dilatation, congenital hepatic fibrosis (CHF) and portal hypertension (pHTN), a triad often referred to as Caroli syndrome [1]. Clinical complications can include ascending cholangitis and consequences of pHTN such as hypersplenism and esophageal varices. Variceal bleeding is potentially life-threatening, and a subset of patients may require portosystemic shunting or liver transplantation due to severe pHTN or recurrent cholangitis [2–4].
Although no targeted disease-modifying therapies are currently approved for clinical use, several therapies have shown promise in slowing progression of both liver and kidney disease in ARPKD animal models [5–7]. However, a significant barrier to advancing clinical trials in patients with ARPKD is the lack of quantitative, non-invasive measures of disease progression. Standard blood markers of liver inflammation and synthetic function are generally normal in patients with ARPKD, and are thus uninformative [2]. As liver fibrosis and pHTN progress, splenomegaly and thrombocytopenia can develop. However, since these are relatively late findings, spleen size and platelet counts are less useful measures in earlier stages of disease. Liver fibrosis could theoretically be quantified using liver biopsy, but this is not routinely performed in patients with ARPKD due to invasiveness and risk for sampling error [1, 8]. Novel non-invasive imaging methods are therefore needed to quantify liver disease progression and potentially serve as surrogate endpoints in clinical trials of disease-modifying therapies. We have previously reported that ultrasound (US) elastography with acoustic radiation force impulse (ARFI) appears to be useful to quantify the severity of ARPKD-related liver disease [9]. However, US-based methods have limitations such as limited sampling, operator dependency, and inconsistency of post-processing algorithms between vendors. In the current study, we sought to examine whether another imaging method, magnetic resonance elastography (MRE), can quantify liver stiffness in individuals with ARPKD, which we hypothesized would correlate with the severity of liver fibrosis and pHTN.
MRE measures tissue parenchymal stiffness by assessing the propagation of low-frequency mechanical waves [10]. Advantages of MRE over other elastographic techniques include the ability to evaluate the entire liver parenchyma, and standardization across manufacturer platforms and field strengths, since the vast majority of MRE hardware and software currently comes from a single company (Resoundant Inc., Rochester, MN)[11–13]. In diseases such as chronic viral hepatitis, non-alcoholic fatty liver disease (NAFLD), and autoimmune hepatitis, MRE liver stiffness has been shown to correlate strongly with biopsy-proven fibrosis score in both adults [14] and children [15]. In addition, MRE liver stiffness correlated well with histologic fibrosis in a mouse model of ARPKD [16]. To our knowledge, MRE has not previously been systematically evaluated to quantify the severity of ARPKD liver disease in children and young adults with ARPKD.
Our specific objectives were to determine if MRE liver and spleen stiffness can distinguish healthy controls from individuals with ARPKD, and to examine the relationship of MRE liver stiffness with the severity of pHTN in individuals with ARPKD. We also sought to examine correlations between liver stiffness measured by MRE and US ARFI elastography.
Methods
Study design and population
Participants in this prospective, cross-sectional study were recruited at a single pediatric tertiary care center between August 2014 and May 2018 under an Institutional Review Board-approved protocol. Written informed consent, including HIPAA authorization, was obtained from all participants/guardians, and child assent was obtained as appropriate. Children and young adults with a clinical diagnosis of ARPKD were recruited from the center’s nephrology practice, and were eligible to undergo liver imaging if they had not received a liver transplant. Participants with ARPKD who had a portosystemic shunt were excluded from this analysis. Healthy individuals with no personal history of hypertension, obesity, hematologic or rheumatologic disease, and no family history of kidney or liver disease, were recruited from the institution’s primary care practices. Eligibility criteria for MRI included the ability to lie still for up to 1 hour (generally age >7–8 years) and absence of standard contraindications such as claustrophobia or metal implants.
Previously reported data
US ARFI elastography data for all participants included in the current study have been previously published [9]; the prior manuscript also included data from additional participants (total n=25 ARPKD and n=24 healthy controls) who were enrolled in the overall study protocol but underwent only US elastography and not MRE elastography due to ineligibility for MRI (e.g. due to age or claustrophobia). MRE data was not reported in that manuscript.
MRE data from a subset of the participants in the current study (n=4 controls and n=4 ARPKD) was included in a manuscript that included 52 children with various liver diseases, comparing results from GRE and SE-EPI MRE sequences [17]. The focus of that paper was a technical comparison of performance between the two types of MRE sequences, and did not focus specifically on performance of MRE to quantify liver disease severity in participants with ARPKD.
Clinical measurements
Data collected during a single study visit included demographics, medical and family history, and measurements of height and weight. Laboratory studies, including complete blood counts and tests of kidney and liver function, were performed only in participants with ARPKD. Clinical signs of pHTN were defined as the presence of splenomegaly (sagittal spleen length measured on US of >90th percentile for height [18]) or low platelet counts (<150×103/μL)[1, 2]. Definitive clinical pHTN was defined as presence of both splenomegaly and low platelet counts, and absence of clinical pHTN was defined as the absence of both of these signs. Spleen length index was calculated as actual/90th percentile spleen length. Known varices were defined as esophageal or gastric varices diagnosed on upper endoscopy. Estimated glomerular filtration rate (eGFR) was calculated based on the bedside CKD in Children (CKiD) Study equation [19]. Participants fasted for age-appropriate durations (≥ 5 hours) prior to imaging.
MRE acquisition
MRE was performed on a 3T scanner (MAGNETOM Skyra, Siemens Medical Solutions, Malvern, PA, USA) equipped with commercially available MRE hardware consisting of an active and a passive driver system. The active driver is similar to an audio subwoofer and generates low-amplitude 60 Hz vibrations, which are transmitted via pneumatic pressure through a hollow plastic tube to a passive driver. The passive driver is strapped to the abdomen just under the radiofrequency (RF) coil, positioned in the right or left upper quadrant for liver or spleen stiffness measurements, respectively [10, 20, 21]. Two dimensional (2D) gradient-recalled-echo (GRE) image acquisition was completed in 4 breath-holds of up to 15 seconds each, to obtain 4 axial slices through the broadest part of the liver or spleen as identified on coronal localizer images. Scanning protocol parameters: TR 50 ms, TE 23.7 ms, matrix size 128×64 interpolated to 128×128, voxel size 1.2×1.2× 5 mm, slice thickness 5 mm, bandwidth 260 Hz/Px, averages: 1, motion encoding gradient (MEG) frequency 60 Hz, MEG direction Z axis; echo spacing 27.1 ms.
Anatomic T1 and T2 weighted images were reviewed by a board-certified radiologist to generate a written report of any clinically significant findings such as biliary duct dilatation; however, the sequences obtained in this study were not optimized for detailed evaluation of the biliary tract.
MRE image processing
Stiffness maps (elastograms) were generated automatically by the scanner software, including 95% confidence maps indicating areas of good wave propagation. Regions of interest (ROIs) were drawn manually within regions bound by the confidence maps, including the maximal amount of liver or spleen parenchyma in each slice and avoiding the capsule, biliary tree, and large vessels [22](Figure 1C). The mean stiffness (in kilopascals, kPa) and area (in cm2) of each slice was calculated. Overall organ mean stiffness was calculated as the average of stiffness measurements from each slice, weighted by the ROI area of each slice.
Figure 1.
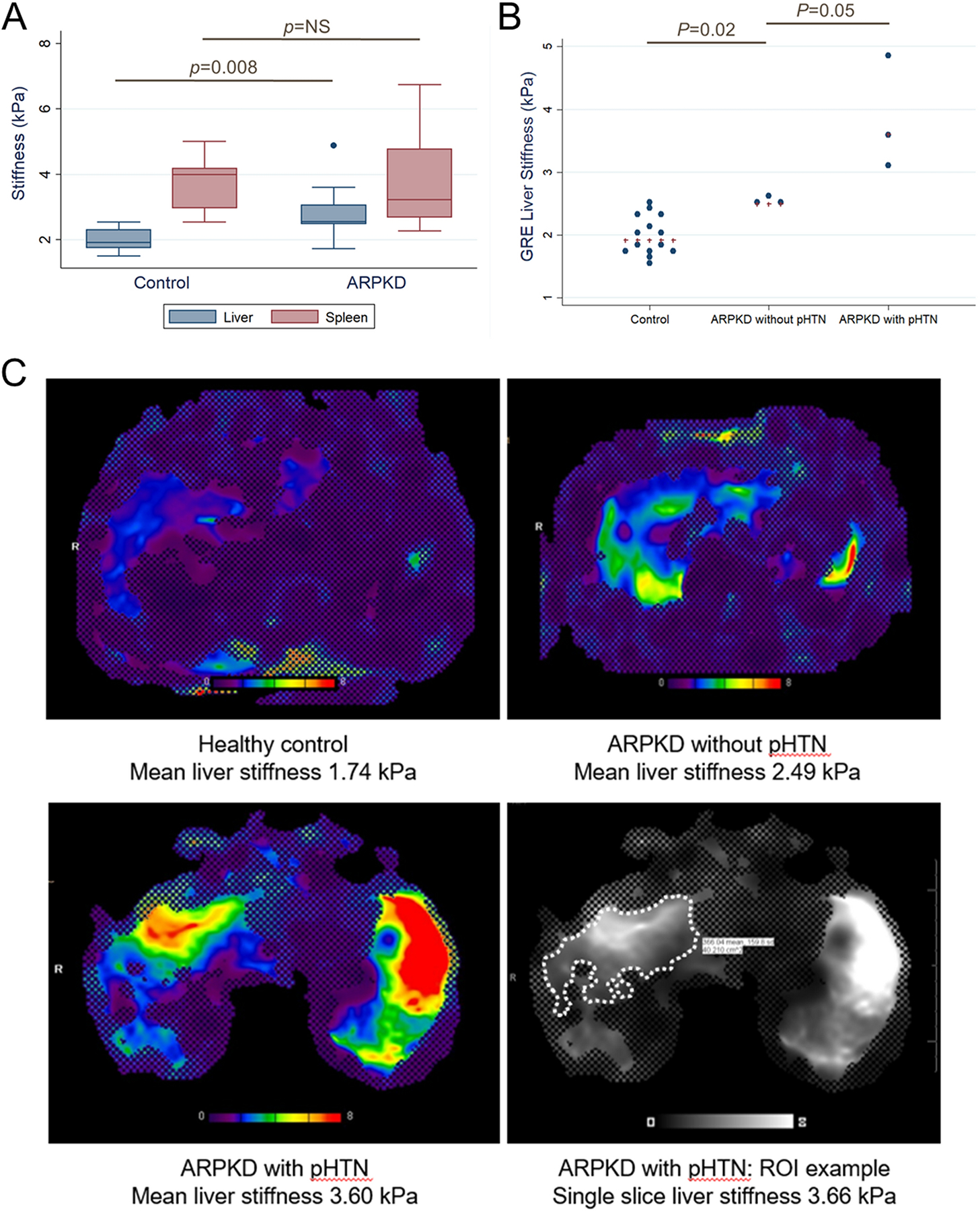
A. MRE liver and spleen stiffness in healthy controls and participants with ARPKD (n=14 and n=9, respectively, for liver measurements; n=10 and n=8, respectively, for spleen measurements); B. MRE liver stiffness in controls (n=14), participants with ARPKD without pHTN (normal spleen size and platelet counts, n=3), and participants with ARPKD with definitive pHTN (splenomegaly and low platelet counts, n=3). Blue dots show individual stiffness measurements, and red crosses indicate group medians. C. Representative liver elastograms with 95% confidence maps for an 18 year old control participant, a 13 year old with ARPKD without pHTN, and a 16 year old with ARPKD with pHTN, and an example of manually-drawn region of interest (ROI) for the latter participant (thicker line traced over original ROI outline for clarity).
US ARFI elastography
US ARFI elastography measurements were acquired per published methods [9] using the Siemens Acuson S3000 in Virtual Touch quantification mode (Siemens Medical Solutions USA, Inc, Malvern, Pennsylvania). Mean point shear wave speed (SWS), in meters per second (m/s), of the left and right liver lobes was calculated from ten valid SWS measurements at each site.
Statistical Analysis
Continuous variables were reported as median and interquartile range (IQR), and binary variables were reported as frequency and percentage. Group differences were compared using Wilcoxon rank sum test for continuous variables and Fisher’s exact test for binary variables. MRE liver and spleen stiffness (in kPa) were compared between healthy controls and all participants with ARPKD. MRE liver stiffness was also compared between healthy controls, participants with ARPKD without clinical pHTN (neither splenomegaly nor low platelets), and participants with ARPKD with definitive clinical pHTN (both splenomegaly and low platelets). Linear fit plots and Spearman correlation were performed to examine relationships between MRE liver stiffness and the following variables: spleen length index, platelet count, age, and SWS in both liver lobes measured using US elastography. Receiver operating characteristic (ROC) analysis was performed to evaluate the diagnostic performance of MRE liver stiffness to distinguish between participants with ARPKD and healthy controls. Diagnostic value of MRE liver stiffness cut-offs was evaluated using the sensitivity, specificity, and percent of subjects correctly classified, with the cut-off chosen to maximize the percent of subjects correctly classified. Statistical analyses were performed using Stata 13.1 (StataCorp, College Station, TX).
Results
Clinical and demographic data
Ten participants with ARPKD and 14 healthy controls underwent MRE. One participant with ARPKD was excluded from this analysis due to history of a portosystemic shunt, because shunts alter spleen size and platelet count and would therefore affect our ascertainment of clinical pHTN [23], and because shunts are known to affect MRE parameters [24]. This left n=9 individuals with ARPKD in the final analysis. Two of the participants with ARPKD had previously received a kidney transplant. All participants had evaluable liver MRE data; 8/9 (89%) of participants with ARPKD and 10/14 (71%) of controls (71%) had evaluable spleen MRE data. Unusable spleen MRE data was due to inadequate wave propagation through the spleen (i.e. no spleen parenchyma within 95% confidence maps).
Clinical and demographic characteristics of the participants are shown in Table 1. Median age was similar in controls and participants with ARPKD (14.7 vs. 16.8 years, p=0.3). Splenomegaly was more common in the ARPKD group compared to controls (63% vs. 7%, p=0.01), with median spleen length index of 1.06 in the ARPKD group vs. 0.84 in controls (p=0.05). Within the ARPKD group, 5/9 (63%) participants had splenomegaly; of these, 3 had low platelet counts and were thus considered to have definitive pHTN (33% of total ARPKD group), and 2 had normal platelet counts and were considered to have possible pHTN. Three participants (33%) with ARPKD had no signs of pHTN (neither splenomegaly nor low platelets). One participant with ARPKD had missing spleen length data because US could not be obtained during the study visit; this individual had a normal platelet count. Participants with ARPKD who had definitive pHTN (n=3) were older than those with no signs of pHTN (n=3) (18.9 vs. 8.5 years, p=0.05). One participant with ARPKD had a history of varices; this individual had both splenomegaly and low platelets. Two participants with ARPKD had MRI findings consistent with biliary duct dilatation, both of whom also had low platelets and splenomegaly. None of the participants with ARPKD had a history of ascending cholangitis.
Table 1.
Comparison of clinical and demographic characteristics of healthy controls vs. participants with ARPKD, and of participants with ARPKD without portal hypertension (i.e. normal platelet count and spleen size) vs. with definitive portal hypertension (i.e. both low platelet count and splenomegaly).
| Characteristic | Healthy controls (n = 14) | All ARPKD (n = 9) | p | ARPKD without pHTN (n = 3) | ARPKD with definitive pHTN (n = 3) | p |
|---|---|---|---|---|---|---|
| Age, years | 14.7 [9.7, 16.7] | 16.8 [13.3, 18.9] | 0.3 | 8.5 [8.5, 13.3] | 18.9 [16.8, 20.9] | 0.05 |
| Male sex | 6 (43%) | 6 (67%) | 0.4 | 0 (0%) | 2 (67%) | >0.99 |
| eGFR* (mL/min/1.73m2) | - | 59 [52, 66] | - | 82.7 [55.1, 90.4] | 58.9 [32.9, 65.2] | 0.3 |
| History of kidney transplant | - | 2 (22%) | 0 (0%) | 1 (33%) | ||
| WBC count (×103/μL) | - | 4.4 [4.1, 6.3] | - | 4.2 [3.3, 6.3] | 4.1 [3.1, 4.4] | 0.5 |
| Platelets | ||||||
| Spleen length+ | ||||||
| History of varices | - | 1 (11%) | - | 0 (0%) | 1 (33%) | - |
| History of ascending cholangitis | - | 0 (0%) | - | - | - | - |
Continuous variables given as median [IQR]; binary variables as count (%).
includes 2 participants with kidney transplant
Spleen length data missing in 1 participant with ARPKD.
ARPKD, autosomal recessive polycystic kidney disease; eGFR, estimated glomerular filtration rate; pHTN, pHTN; WBC, white blood cell count
Liver and spleen stiffness in control vs. ARPKD groups
Participants with ARPKD (n=9) had higher liver stiffness than healthy controls (n=14), with median liver stiffness of 2.55 (range 1.72–4.88) vs. 1.92 (range 1.51–2.54) kPa (p=0.008). Spleen stiffness did not differ significantly between the two groups (Figure 1A). Given the lack of association between spleen stiffness and ARPKD status, further analyses examined only liver stiffness.
To ensure that the slight age difference between the control and ARPKD groups was not contributing to the observed differences in MRE liver stiffness, we examined correlations between MRE liver stiffness and age in both groups. There was no correlation between MRE liver stiffness and age in healthy controls (rho=−0.02, p=0.9). There was a weak positive correlation between liver stiffness and age in the ARPKD group, but this was not statistically significant (rho=0.13, p=0.7) (Figure, Supplemental Digital Content 1).
Relationship of liver stiffness and severity of pHTN in participants with ARPKD
To explore whether MRE liver stiffness could distinguish different severities of ARPKD liver disease, we compared liver stiffness between healthy controls, participants with ARPKD without pHTN, and participants with ARPKD with definitive pHTN (splenomegaly and low platelets). Median liver stiffness for controls (n=14) was 1.92 (range 1.51–2.54) kPa; for participants with ARPKD without pHTN (n=3) was 2.49 (range 2.48–2.66) kPa; and for participants with ARPKD with definitive pHTN (n=3) was 3.60 (range 3.07–4.89) kPa (p=0.02 for controls vs. ARPKD without pHTN; p=0.05 for ARPKD without vs. with pHTN; Figure 1B). Representative MRE stiffness maps for each of these groups are shown in Figure 1C. Of the remaining participants with ARPKD, the two individuals with possible pHTN (splenomegaly with normal platelet counts) had liver stiffness measurements of 1.72 kPa and 2.55 kPa respectively, and the individual with missing spleen length data but normal platelet count had liver stiffness of 1.91 kPa. Statistical comparisons of these individuals with the other groups was not possible due to small sample size.
We next examined linear correlations of MRE liver stiffness with clinical markers of pHTN in participants with ARPKD, namely spleen length and platelet count. Liver stiffness showed a moderately strong positive correlation with spleen length index, but did not reach statistical significance (rho=0.67, p=0.07, Figure 2A). Liver stiffness showed a weaker negative correlation with platelet count (rho=−0.48, p=0.2, Figure 2B).
Figure 2.
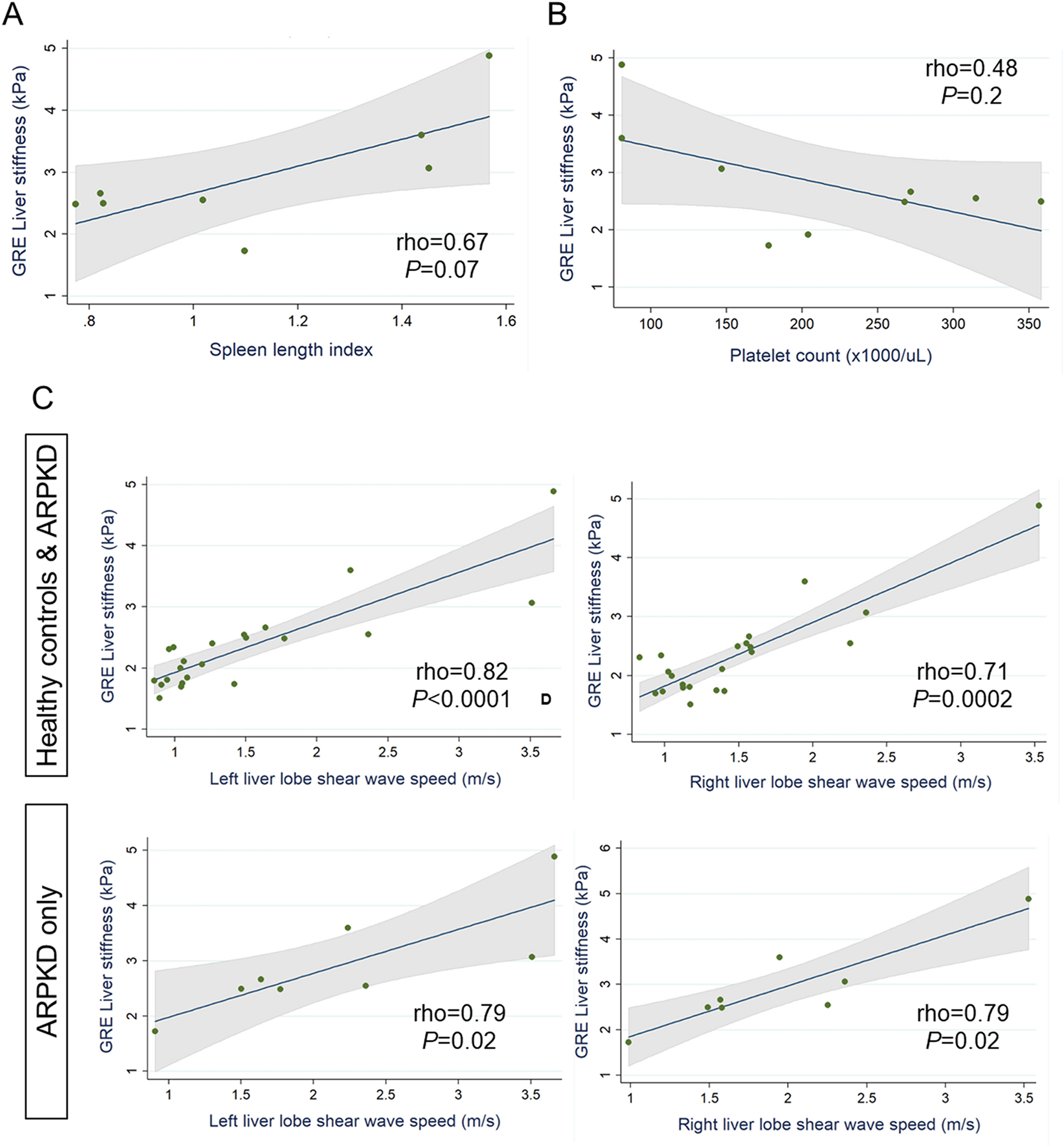
Relationship of MRE liver stiffness with A. spleen length index and B. platelet count in participants with ARPKD. C. Relationship of liver stiffness measured by GRE MRE (whole liver) vs. US ARFI elastography (left and right lobes) in all healthy and ARPKD participants combined (C, upper panels) and in the ARPKD group only (C, lower panels).
Relationship between MRE and US ARFI elastography measurements of liver stiffness
Given our previously published data showing that liver US ARFI elastography, particularly of the left liver lobe, has high sensitivity and specificity for detecting ARPKD liver disease [9], we examined the correlation between MRE and US elastography measures of liver stiffness. We found that MRE liver stiffness was strongly correlated with US elastography SWS in the left and right liver lobes, both in analyses of control and ARPKD groups combined (rho 0.82, p<0.0001 left lobe; rho 0.71, p=0.0002 right lobe) and in analyses of the ARPKD group only (rho 0.79, p=0.02 for left and right lobes) (Figure 2C).
ROC analysis
ROC analysis showed that MRE liver stiffness had high accuracy for differentiating participants with ARPKD from healthy controls, with area under the ROC curve (AUROC) of 0.83 (95% CI: 0.63–1.00) (Figure 3). A proposed MRE liver stiffness cut-off of 2.48 kPa had 78% sensitivity and 93% specificity, resulting in correct classification of 87% of individuals as ARPKD vs. healthy controls. The number of participants within the ARPKD group who did vs. did not have definitive pHTN was too small to allow valid ROC analysis (n=3 in each group). However, we note that all 3 (100%) participants with ARPKD who had definitive pHTN had liver stiffness ≥3.07 kPa.
Figure 3.
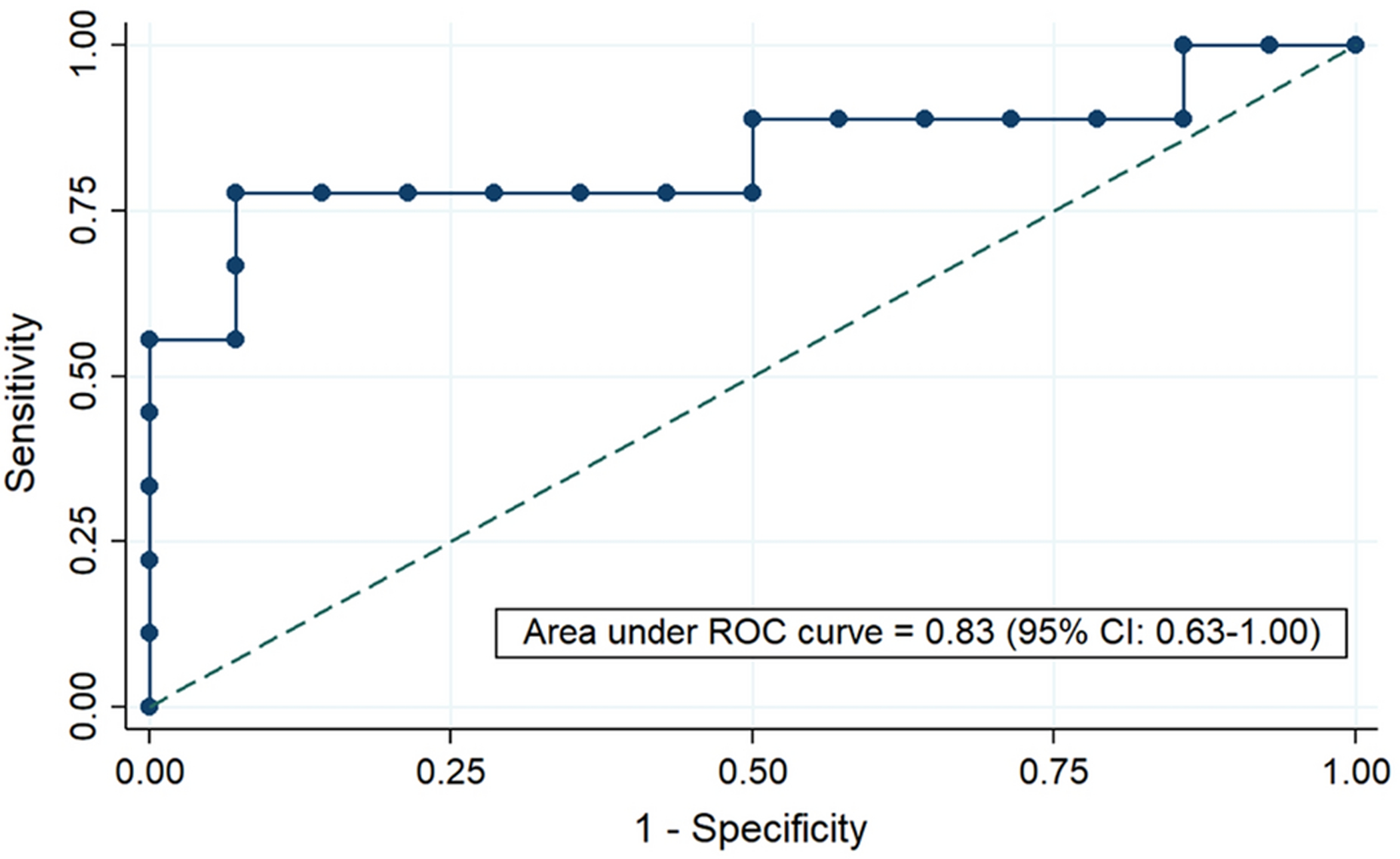
Receiver operating characteristic (ROC) curve to evaluate diagnostic performance of MRE liver stiffness to distinguish between healthy controls and participants with ARPKD. Area under the ROC curve with 95% confidence interval (CI) is as shown.
Discussion
This study sought to examine the performance of MRE of the liver and spleen as measures of liver fibrosis and pHTN in children and young adults with ARPKD. We found that MRE liver stiffness was significantly higher in individuals with ARPKD compared to healthy controls, and ROC analysis showed that a proposed cut-off of 2.48 kPa had high sensitivity and specificity for distinguishing these groups. In addition, MRE liver stiffness was able to distinguish healthy controls from individuals with ARPKD who did not have clinical evidence of pHTN, suggesting that MRE may be useful to detect early stages of liver fibrosis. Within the ARPKD group, we found that a cut-off of 3.07 kPa completely differentiated individuals with clinical evidence of pHTN (splenomegaly and low platelets) from those without pHTN. Although linear correlations of MRE liver stiffness with spleen length and platelet count did not reach statistical significance in this small study, the trends observed suggest that MRE liver stiffness may track with severity of pHTN. MRE spleen stiffness did not appear to be a helpful marker of ARPKD liver disease severity, as it did not differ significantly between individuals with ARPKD and healthy controls. MRE spleen stiffness measurements also had a higher rate of technical failure due to poor wave propagation. This problem predominantly occurred in healthy control participants recruited early in the study, and was likely due to the passive driver not being secured tightly enough with the strap. It is also possible that because a GRE-based MRE acquisition was performed, that the smaller spleen size in healthy controls contributed to decreased efficiency of wave propagation compared to participants with ARPKD. Future prospective studies with a spin-echo echo planar imaging (SE-EPI) based acquisition, which has a lower rate of technical failure than GRE,[25] may be needed to further investigate differences in spleen stiffness.[17, 26]
Overall, our results suggest that liver MRE may be a useful quantitative measure of ARPKD-related liver fibrosis and pHTN. If these results are validated in larger studies, it could allow MRE to potentially serve as a surrogate endpoint in future clinical trials of targeted ARPKD therapies. Several agents such as octreotide, pasireotide, and tesevatinib, have appeared promising for ARPKD-associated liver disease in orthologous rodent models [5, 7], and a Phase 1 clinical trial for tesevatinib has been completed [27]. However, the ability to perform any future efficacy trials will depend on the availability of reliable, non-invasive measures of liver disease progression and response to therapy. Since liver MRE appears to be able to detect elevated liver stiffness in individuals with ARPKD even before they have clinical evidence of pHTN, it may be more useful than current clinical measures (e.g. platelet count and spleen size) to detect early signs of liver disease progression.
We have previously shown that liver stiffness measured by US ARFI elastography is a sensitive and specific measure of ARPKD liver disease severity [9]. In this study, we found strong correlations between liver stiffness measurements obtained by MRE and US ARFI elastography, both in healthy individuals and in those with ARPKD. Prior studies in other patient populations have similarly found good correlation between MR and US elastography stiffness measurements [28, 29]. This suggests that US elastography and MRE may have complementary roles as measures of ARPKD liver disease severity, both in clinical and research settings. Although US has the benefit of being less expensive and feasible in patients of all ages without the need for sedation, MRE has the advantage of capturing the entire organ parenchyma and being able to be combined with other MRI sequences to provide more detailed anatomic evaluations. In other patient populations, MRE also appears to have higher diagnostic performance than US elastography for prediction of histologic fibrosis [30, 31]. MRE may therefore be useful in patients who require more comprehensive evaluations of liver and biliary tract anatomy (e.g. patients with biliary tract dilatation requiring magnetic resonance cholangiopancreatography) or as a confirmatory method in patients suspected to have liver fibrosis based on US elastography. The GRE MRE sequence used in this study requires four breath-holds, which restricts its usefulness to patients who are able to comply. However, a newer SE-EPI based sequence is available that has a much faster acquisition time and can be completed in a single breath-hold [17]. We have previously shown that SE-EPI has excellent agreement with GRE measures of liver stiffness in children with various liver diseases including ARPKD [17]. Therefore the use of SE-EPI sequences may expand the feasibility of MRE to wider range of patients.
The liver stiffness cut-off values obtained in this study appear to be consistent with the published literature. We identified thresholds of 2.48 kPa to differentiate participants with ARPKD from healthy controls, and 3.07 kPa to differentiate participants with pHTN within the ARPKD group. In comparison, a pooled analysis of >200 adults with NAFLD who underwent MRE paired with liver biopsy identified thresholds of 2.61 kPa to differentiate stage ≥1 from stage 0 fibrosis, and 2.97 kPa to differentiate stage ≥2 from stage ≤1 fibrosis [32]. Similarly, in pediatric studies of NAFLD and other liver diseases, Xanthakos et al. [15] reported a threshold of 2.71 kPa to differentiate stage ≥2 from stage≤1 fibrosis, and Schwimmer et al. [33] reported a threshold of 2.69–2.77 kPa to differentiate stage ≥1 from stage 0 fibrosis. Liver stiffness measurements in our healthy controls were also similar to those in the literature, with median liver stiffness of 1.92 (range 1.51–2.54) kPa. In comparison, a study of 102 healthy young adults (aged 20–28 years) by Obrzut et al. [34] reported a mean liver stiffness of 2.14 (range 1.37–2.66) kPa (GRE sequences, 1.5T Optima GE). In another study of 24 healthy adults, Trout et al. [13] reported a mean liver stiffness of 1.95 ± 0.27 kPa (3T GE 750W scanner). Similar values have also been reported in several other studies of healthy adults using both 1.5T and 3T scanners from different manufacturers [35, 36]. However, one study in 81 healthy children (aged 8–17 years) by Sawh et al. [37] reported higher mean liver stiffness of 2.45 kPa (standard deviation 0.35 kPa; 95th percentile 3.19 kPa; GRE sequences, 3T Signa HDxt GE) [37]. This resulted in ~20% of their healthy cohort being classified as having higher than normal liver stiffness when applying published cut-off values [37]. It is unclear whether this study’s higher values were due to true physiologic differences between children and adults or possibly due to technical factors. However, similar to the healthy participants in our study, Sawh et al. [37] did not find any correlation between liver stiffness measurements and age within their cohort, making it less likely that there are true differences in liver stiffness between children and adults [37].
There are several limitations to our study, most notably the small sample size. Larger multicenter studies are therefore needed to validate the MRE liver stiffness cut-off values reported here. Since liver biopsies are not part of the standard of care in individuals with ARPKD, we cannot determine whether the differences we observed in MRE liver stiffness measurements correlate with histologic fibrosis. However, the similarities in our cut-off values to those obtained in studies that included histologic correlations in other patient populations supports the validity of our findings. Although we presumed that elevated liver stiffness in our participants with ARPKD correlated with liver fibrosis, we cannot rule out an effect of biliary duct dilatation on liver stiffness measurements. For example, in a study of primary sclerosis cholangitis, segmental liver stiffness was positively correlated with segmental bile duct strictures (but not with dilatation)[38]. Since only two of the ARPKD participants in this cohort had biliary dilatation, we could not ascertain its effect on liver stiffness measures. Given this study’s cross-sectional nature, we cannot yet determine how MRE liver stiffness measurements change over time with progression of liver fibrosis and pHTN. Our ongoing longitudinal study in this ARPKD cohort will help to address this question.
In summary, this study shows that liver MRE may be a promising non-invasive method to measure the severity of liver fibrosis and pHTN in children and young adults with ARPKD.
Supplementary Material
Relationship of MRE liver stiffness with age in A. healthy controls and B. participants with ARPKD.
Funding information
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (NIH), through grant 5-K23-DK109203 (PI: Hartung) and by the National Center for Advancing Translational Sciences (NCATS), NIH, through grant 5-KL2-TR-000139. The Clinical and Translational Research Center at the CHOP is supported by the National Center for Research Resources and NCATS, NIH, through grants UL1RR024134 and UL1TR000003 and UL1TR001878. Funders did not have a role in study design, data collection, analysis, reporting, or the decision to submit for publication.
Footnotes
Conflicts of interest
No relevant conflicts of interest.
Ethics approval
The study protocol was approved by the Children’s Hospital of Philadelphia Institutional Review Board (protocol #14–010785).
Consent to participate
Written informed consent, including HIPAA authorization, was obtained from all participants/guardians, and child assent was obtained as appropriate.
Availability of data and material
Primary data is available from the Corresponding Author on request.
References
- 1.Wehrman A, Kriegermeier A, Wen J (2017) Diagnosis and Management of Hepatobiliary Complications in Autosomal Recessive Polycystic Kidney Disease. Front Pediatr 5:124. 10.3389/fped.2017.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gunay-Aygun M, Font-Montgomery E, Lukose L, Tuchman Gerstein M, Piwnica-Worms K, Choyke P, Daryanani KT, Turkbey B, Fischer R, Bernardini I, Sincan M, Zhao X, Sandler NG, Roque A, Douek DC, Graf J, Huizing M, Bryant JC, Mohan P, Gahl WA, Heller T (2013) Characteristics of congenital hepatic fibrosis in a large cohort of patients with autosomal recessive polycystic kidney disease. Gastroenterology 144:112–121. e2. 10.1053/j.gastro.2012.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srinath A, Shneider BL (2012) Congenital hepatic fibrosis and autosomal recessive polycystic kidney disease. J Pediatr Gastroenterol Nutr 54:580–587. 10.1097/MPG.0b013e31824711b7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shneider BL, Magid MS (2005) Liver disease in autosomal recessive polycystic kidney disease. Pediatr Transplant 9:634–639. 10.1111/j.1399-3046.2005.00342.x [DOI] [PubMed] [Google Scholar]
- 5.Masyuk TV, Radtke BN, Stroope AJ, Banales JM, Gradilone SA, Huang B, Masyuk AI, Hogan MC, Torres VE, Larusso NF (2013) Pasireotide is more effective than octreotide in reducing hepatorenal cystogenesis in rodents with polycystic kidney and liver diseases. Hepatology 58:409–21. 10.1002/hep.26140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Constans MM, Chebib FT, Torres VE, Pellegrini L (2019) Effect of a Vasopressin V2 Receptor Antagonist on Polycystic Kidney Disease Development in a Rat Model. Am J Nephrol 49:487–493. 10.1159/000500667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sweeney WE, Frost P, Avner ED (2017) Tesevatinib ameliorates progression of polycystic kidney disease in rodent models of autosomal recessive polycystic kidney disease. World J Nephrol 6:188–200. 10.5527/wjn.v6.i4.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guay-Woodford LM, Bissler JJ, Braun MC, Bockenhauer D, Cadnapaphornchai MA, Dell KM, Kerecuk L, Liebau MC, Alonso-Peclet MH, Shneider B, Emre S, Heller T, Kamath BM, Murray KF, Moise K, Eichenwald EE, Evans J, Keller RL, Wilkins-Haug L, Bergmann C, Gunay-Aygun M, Hooper SR, Hardy KK, Hartung EA, Streisand R, Perrone R, Moxey-Mims M (2014) Consensus expert recommendations for the diagnosis and management of autosomal recessive polycystic kidney disease: report of an international conference. J Pediatr 165:611–7. 10.1016/j.jpeds.2014.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartung EA, Wen J, Poznick L, Furth SL, Darge K (2019) Ultrasound Elastography to Quantify Liver Disease Severity in Autosomal Recessive Polycystic Kidney Disease. J Pediatr. 10.1016/j.jpeds.2019.01.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serai SD, Towbin AJ, Podberesky DJ (2012) Pediatric liver MR elastography. Dig Dis Sci 57:2713–9. 10.1007/s10620-012-2196-2 [DOI] [PubMed] [Google Scholar]
- 11.Serai SD, Obuchowski NA, Venkatesh SK, Sirlin CB, Miller FH, Ashton E, Cole PE, Ehman RL (2017) Repeatability of MR elastography of liver: A meta-analysis. Radiology 285:92–100. 10.1148/radiol.2017161398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serai SD, Yin M, Wang H, Ehman RL, Podberesky DJ (2015) Cross-vendor validation of liver magnetic resonance elastography. Abdom Imaging 40:789–94. 10.1007/s00261-014-0282-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trout AT, Serai S, Mahley AD, Wang H, Zhang Y, Zhang B, Dillman JR (2016) Liver Stiffness Measurements with MR Elastography: Agreement and Repeatability across Imaging Systems, Field Strengths, and Pulse Sequences. Radiology 281:793–804. 10.1148/radiol.2016160209 [DOI] [PubMed] [Google Scholar]
- 14.Lefebvre T, Wartelle-Bladou C, Wong P, Sebastiani G, Giard J-M, Castel H, Murphy-Lavallée J, Olivié D, Ilinca A, Sylvestre M-P, Gilbert G, Gao Z-H, Nguyen BN, Cloutier G, Tang A (2019) Prospective comparison of transient, point shear wave, and magnetic resonance elastography for staging liver fibrosis. Eur Radiol. 10.1007/s00330-019-06331-4 [DOI] [PubMed] [Google Scholar]
- 15.Xanthakos SA, Podberesky DJ, Serai SD, Miles L, King EC, Balistreri WF, Kohli R (2013) Use of magnetic resonance elastography to assess hepatic fibrosis in children with chronic liver disease. J Pediatr 164:186–8. 10.1016/j.jpeds.2013.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin M, Woollard J, Wang X, Torres VE, Harris PC, Ward CJ, Glaser KJ, Manduca A, Ehman RL (2007) Quantitative assessment of hepatic fibrosis in an animal model with magnetic resonance elastography. Magn Reson Med 58:346–53. 10.1002/mrm.21286 [DOI] [PubMed] [Google Scholar]
- 17.Calle-Toro JS, Serai SD, Hartung EA, Goldberg DJ, Bolster BD, Darge K, Anupindi SA (2019) Magnetic resonance elastography SE-EPI vs GRE sequences at 3T in a pediatric population with liver disease. Abdom Radiol (New York). 10.1007/s00261-018-1884-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Megremis SD, Vlachonikolis IG, Tsilimigaki AM (2004) Spleen length in childhood with US: normal values based on age, sex, and somatometric parameters. Radiology 231:129–34. 10.1148/radiol.2311020963 [DOI] [PubMed] [Google Scholar]
- 19.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637. 10.1681/ASN.2008030287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mannelli L, Godfrey E, Joubert I, Patterson AJ, Graves MJ, Gallagher FA, Lomas DJ (2010) MR elastography: Spleen stiffness measurements in healthy volunteers--preliminary experience. AJR Am J Roentgenol 195:387–92. 10.2214/AJR.09.3390 [DOI] [PubMed] [Google Scholar]
- 21.Venkatesh SK, Ehman RL (2015) Magnetic resonance elastography of abdomen. Abdom Imaging 40:745–59. 10.1007/s00261-014-0315-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serai SD, Trout AT, Sirlin CB (2017) Elastography to assess the stage of liver fibrosis in children: Concepts, opportunities, and challenges. Clin. Liver Dis 9:5–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verbeeck S, Mekhali D, Cassiman D, Maleux G, Witters P (2018) Long-term outcome of transjugular intrahepatic portosystemic shunt for portal hypertension in autosomal recessive polycystic kidney disease. Dig Liver Dis 50:707–712. 10.1016/j.dld.2018.03.009 [DOI] [PubMed] [Google Scholar]
- 24.Guo J, Büning C, Schott E, Kröncke T, Braun J, Sack I, Althoff C (2015) In vivo abdominal magnetic resonance elastography for the assessment of portal hypertension before and after transjugular intrahepatic portosystemic shunt implantation. Invest Radiol 50:347–351. 10.1097/RLI.0000000000000136 [DOI] [PubMed] [Google Scholar]
- 25.Kim DW, Kim SY, Yoon HM, Kim KW, Byun JH (2019) Comparison of technical failure of MR elastography for measuring liver stiffness between gradient-recalled echo and spin-echo echo-planar imaging: A systematic review and meta-analysis. J Magn Reson Imaging. 10.1002/jmri.26918 [DOI] [PubMed] [Google Scholar]
- 26.Nedredal GI, Yin M, McKenzie T, Lillegard J, Luebke-Wheeler J, Talwalkar J, Ehman R, Nyberg SL (2011) Portal hypertension correlates with splenic stiffness as measured with MR elastography. J Magn Reson Imaging 34:79–87. 10.1002/jmri.22610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.A Safety, Pharmacokinetic, Single Ascending Dose Study of Tesevatinib in Pediatric Subjects With Autosomal Recessive Polycystic Kidney Disease (ARPKD). In: ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03096080 [Google Scholar]
- 28.Yoon JH, Lee JM, Joo I, Lee ES, Sohn JY, Jang SK, Lee KB, Han JK, Choi BI (2014) Hepatic fibrosis: Prospective comparison of MR elastography and us shear-wave elastography for evaluation. Radiology 273:772–781. 10.1148/radiol.14132000 [DOI] [PubMed] [Google Scholar]
- 29.Zhao H, Chen J, Meixner DD, Xie H, Shamdasani V, Zhou S, Robert J-L, Urban MW, Sanchez W, Callstrom MR, Ehman RL, Greenleaf JF, Chen S (2014) Noninvasive assessment of liver fibrosis using ultrasound-based shear wave measurement and comparison to magnetic resonance elastography. J Ultrasound Med 33:1597–604. 10.7863/ultra.33.9.1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barr RG, Ferraioli G, Palmeri ML, Goodman ZD, Garcia-Tsao G, Rubin J, Garra B, Myers RP, Wilson SR, Rubens D, Levine D (2015) Elastography assessment of liver fibrosis: Society of radiologists in ultrasound consensus conference statement. Radiology 276:845–861. 10.1148/radiol.2015150619 [DOI] [PubMed] [Google Scholar]
- 31.Yin M, Venkatesh SK (2018) Ultrasound or MR elastography of liver: which one shall I use? Abdom Radiol 43:1546–1551. 10.1007/s00261-017-1340-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu C, Caussy C, Imajo K, Chen J, Singh S, Kaulback K, Le M-D, Hooker J, Tu X, Bettencourt R, Yin M, Sirlin CB, Ehman RL, Nakajima A, Loomba R (2019) Magnetic Resonance vs Transient Elastography Analysis of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Pooled Analysis of Individual Participants. Clin Gastroenterol Hepatol 17:630–637. e8. 10.1016/j.cgh.2018.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwimmer JB, Behling C, Angeles JE, Paiz M, Durelle J, Africa J, Newton KP, Brunt EM, Lavine JE, Abrams SH, Masand P, Krishnamurthy R, Wong K, Ehman RL, Yin M, Glaser KJ, Dzyubak B, Wolfson T, Gamst AC, Hooker J, Haufe W, Schlein A, Hamilton G, Middleton MS, Sirlin CB (2017) Magnetic resonance elastography measured shear stiffness as a biomarker of fibrosis in pediatric nonalcoholic fatty liver disease. Hepatology 66:1474–1485. 10.1002/hep.29241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obrzut M, Atamaniuk V, Obrzut B, Ehman R, Cholewa M, Rzucidło M, Pozaruk A, Gutkowski K (2019) Normative values for magnetic resonance elastography-based liver stiffness in a healthy population. Polish Arch Intern Med 129:321–326. 10.20452/pamw.4456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin M, Talwalkar JA, Glaser KJ, Venkatesh SK, Chen J, Manduca A, Ehman RL (2011) Dynamic postprandial hepatic stiffness augmentation assessed with MR elastography in patients with chronic liver disease. AJR Am J Roentgenol 197:64–70. 10.2214/AJR.10.5989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee DH, Lee JM, Han JK, Choi BI (2013) MR elastography of healthy liver parenchyma: Normal value and reliability of the liver stiffness value measurement. J Magn Reson Imaging 38:1215–23. 10.1002/jmri.23958 [DOI] [PubMed] [Google Scholar]
- 37.Sawh MC, Newton KP, Goyal NP, Angeles JE, Harlow K, Bross C, Schlein AN, Hooker JC, Sy EZ, Glaser KJ, Yin M, Ehman RL, Sirlin CB, Schwimmer JB (2019) Normal range for MR elastography measured liver stiffness in children without liver disease. J Magn Reson Imaging jmri.26905. 10.1002/jmri.26905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bookwalter CA, Venkatesh SK, Eaton JE, Smyrk TD, Ehman RL (2018) MR elastography in primary sclerosing cholangitis: correlating liver stiffness with bile duct strictures and parenchymal changes. Abdom Radiol 43:3260–3270. 10.1007/s00261-018-1590-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relationship of MRE liver stiffness with age in A. healthy controls and B. participants with ARPKD.
Data Availability Statement
Primary data is available from the Corresponding Author on request.


