Abstract
Nucleus accumbens-associated protein 1 (NACC1) has been reported to serve as an oncogenic role in several types of cancer; however, its role in nasopharyngeal carcinoma (NPC) remains to be determined. The present study aimed to investigate the role of NACC1 in NPC and elucidate the underlying mechanisms. Therefore, NACC1 expression in the normal nasopharyngeal epithelial cell line, NP69, and various NPC cell lines was determined by reverse transcription-quantitative PCR and western blot analyses. NACC1 expression was silenced in the NPC SUNE-1 cell line by transfection with a short hairpin RNA. Cell viability, proliferation, migration, invasion and epithelial-mesenchymal transition (EMT) were then evaluated using MTT, colony formation, wound healing, Transwell and western blot assays, respectively. SC79 was employed to activate AKT expression in NACC1-silenced SUNE-1 cells, and the aforementioned cellular processes were observed. The results revealed that NACC1 expression was upregulated in NPC cell lines. NACC1-knocdown inhibited SUNE-1 cell proliferation, migration, invasion and EMT. Moreover, the levels of phosphorylated AKT and mTOR were decreased upon NACC1 silencing. Mechanistically, the presence of SC79 significantly blocked all the effects of NACC1-knockdown on SUNE-1 cells. The findings of the present study demonstrated that NACC1-knockdown effectively suppressed NPC cell proliferation, migration and invasion by inhibiting the activation of the AKT/mTOR signaling pathway. NACC1 may thus serve as a potential target for the diagnosis and therapy of NPC.
Keywords: AKT/mTOR, nasopharyngeal carcinoma, nucleus accumbens-associated protein 1
Introduction
Nasopharyngeal carcinoma (NPC) is a common malignant tumor that originates in the epithelium of the nasopharyngeal mucosa. NPC has typical geographical distribution characteristics, with a high incidence in southern China and Southeast Asia (1). The pathogenesis of NPC is associated with multiple factors, such as genetics, environment and Epstein-Barr virus (EBV) infection; however, the specific pathogenic mechanisms underlying its development remain unclear (2). Due to the unique anatomical structure of the nasopharynx and the pathological type of NPC, radiotherapy is the main therapy for NPC. However, the majority of patients are initially diagnosed with advanced-stage NPC, and local or regional recurrence along with distant metastasis are prone to occur following radiotherapy and chemotherapy (3). Therefore, the exploration of the molecular mechanisms responsible for NPC cell invasion and metastasis is crucial for inhibiting or delaying NPC metastasis, and improving the prognosis of patients with NPC.
Nucleus accumbens-associated protein 1 (NACC1), encoded by the NACC1 gene, is a transcription factor that regulates several biological processes, including proliferation, apoptosis and epigenetic reprogramming (4,5). The oncogenic role of NACC1 has been reported in various types of cancer, such as urethral (6), ovarian (7) and lung cancer (8), as well as in melanocytic neoplasms (9); it facilitates tumor migration and invasion through epithelial-mesenchymal transition (EMT). In hepatocellular carcinoma, NACC1 expression has been found to be increased, and its knockdown inhibits cell proliferation and invasion, and enhances chemosensitivity to doxorubicin (10). NACC1 is expressed in urothelial carcinoma cells, contributing to cell proliferation, and is involved in cell migration and invasion (6). However, the role of NACC1 in NPC remains to be not elucidated.
mTOR functions as an oncogene in several types of cancer, including NPC, and the activation of the AKT/mTOR signaling pathway has been largely reported to be associated with cell activities, such as proliferation, migration and invasion (11,12). Notably, NACC1 can promote cell viability and inhibit the apoptosis of retinoblastoma cells via activating the AKT/mTOR signaling pathway (13). In the present study, NACC1 expression was examined in several NPC cell lines and the effects of NACC1-knockdown on NPC cell proliferation, migration, invasion and EMT were observed.
Materials and methods
Cell culture and treatment
The normal human nasopharyngeal epithelial cell line, NP69 (cat. no. MZ-3122), and NPC cell lines, including SUNE-1 (cat. no. MZ-3169), C666-1 (cat. no. MZ-1052), 5-8F (cat. no. MZ-0805) and 6-10B (cat. no. MZ-2187), were purchased from the agent company (Ningbo Mingzhou Biological Technology Co., Ltd.) of the American Type Culture Collection and cultured in RPMI-1640 medium (HyClone; Cytiva) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.) and 1% streptomycin and penicillin (Beyotime Institute of Biotechnology) in a 37°C, 5% CO2 incubator. For treatment with the AKT activator SC79 (cat. no. ab146428, Abcam), cells were exposed to 5 µg/ml SC79 for 48 h at 37°C (14).
Cell transfection
For NACC1-knockdown, SUNE-1 cells were grown to 70% confluence, and then transfected with short hairpin RNA (shRNA)-NACC1 (shRNA-NACC1-1 or −2) or shRNA- negative control (NC, scrambled RNA) plasmids (100 ng; pGPU6/Neo; Shanghai GenePharma Co., Ltd.) using Lipofectamine 2000® at 37°C according to the manufacturer's instructions (Thermo Fisher Scientific, Inc.). At 48 h post-transfection, the cells were collected for use in subsequent functional experiments.
MTT assay
An MTT assay kit (Beyotime Institute of Biotechnology) was used to detect cell viability. Briefly, the control or transfected SUNE-1 cells were seeded in 96-well plates (2×103 cells/well). SC79-treated or untreated cells were incubated with 10 µl MTT solution at 37°C for 4 h. Subsequently, 100 µl formazan lysis solution was added to each well and incubated at 37°C until the purple crystals were all dissolved. Finally, the absorbance at 570 nm was measured using a microplate reader (Thermo Fisher Scientific, Inc.).
Colony formation assay
Transfected or untransfected SUNE-1 cells in the logarithmic growth phase were seeded in dishes at a density of 2,000 cells/dish. The cell culture medium of SC79-treated or untreated cells was replaced every 3 days and cell culture was terminated when macroscopic colonies could be observed. The cell colonies were then washed with PBS, fixed with 4% paraformaldehyde at room temperature for 15 min and stained with Giemsa solution at room temperature for 10–30 min. The number of colonies with >10 cells were photographed under a camera (Olympus Corporation; magnification, ×1).
Wound healing assay
For the wound healing assay, transfected or untransfected SUNE-1 cells were seeded in 6-well plates at a density of 5×105 cells/well. A pipette tip was used to create a wound in the cell monolayer of SC79-treated or untreated cells. The cells were then cultured in FBS-free medium and cell migration was determined by detecting the average distance of cells migrating into the wound surface under a light microscope at 0 and 48 h (Olympus Corporation; magnification, ×100). The cell migration rate was calculated using the following equation: (Initial width at 0 h-final width at 24 h)/width at 0 h. The relative migration rate (100%) was obtained by normalization to the control (untreated) group.
Transwell assay
Transwell chambers (8-µm; Corning, Inc.) pre-coated with Matrigel at 37°C for 30 min (Corning, Inc.) were placed in a 24-well plate. RPMI-1640 medium containing no serum (50 µl) was added to upper chamber, which were then seeded with control or transfected SUNE-1 cells at a density of 2×105 cells/well, with or without SC79 treatment. Normal RPMI-1640 medium containing 10% FBS was added to the lower chamber, followed by incubation at 37°C for 24 h. Finally, the invading cells in the lower chamber were fixed with 4% methanol at room temperature for 10 min and then stained with 0.1% crystal violet solution at room temperature for 15 min, and the number of invasive cells in three random fields (magnification, ×100) were counted under an inverted microscope (Olympus Coporation). The relative invasion rate (100%) was obtained by normalization to the control group.
Reverse transcription-quantitative (RT-q) PCR analysis
Total RNA was extracted from SUNE-1 cells using TRIzol® reagent (Thermo Fisher Scientific, Inc.). The TaqMan Reverse Transcription kit (Thermo Fisher Scientific, Inc.) was utilized to transcribe RNA into cDNA according to the manufacturer's protocol. qPCR was performed using a SYBR-Green kit (Thermo Fisher Scientific, Inc.) on an ABI 7500 Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The primers used in the present study are as follows: NACC1 forward, 5′-CTGGCTCCTACCACAATGAGG-3′ and reverse, 5′-TGGCCGACGTTCATCATGC-3′; and GAPDH forward, 5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse, 5′-GGCTGTTGTCATACTTCTCATGG-3′. The thermocycling conditions consisted of initial denaturation for 2 min at 94°C, followed by cycles of 35, denaturation for 30 sec at 94°C, annealing for 30 sec at 57°C and extension for 30 sec at 70°C. GAPDH was used as an internal control for mRNA expression. The relative expression levels of mRNA were calculated using the 2−ΔΔCq method (15).
Western blot analysis
SUNE-1 cells were collected and total protein was extracted using RIPA buffer (Beyotime Institute of Biotechnology) containing protease inhibitors (Roche Diagnostics). Following protein quantification with the bicinchoninic acid assay kit (Thermo Fisher Scientific, Inc.), equal amounts of protein (20 µg/lane) were separated by 10% SDS-PAGE and then transferred onto PVDF membranes (EMD Millipore). The membranes were blocked with 5% skimmed milk at room temperature for 2 h, and incubated with primary antibodies against NACC1 (cat. no. ab29047, 1:250), Ki-67 (cat. no. ab92742, 1:1,000), proliferating cell nuclear antigen (PCNA; cat. no. ab92552, 1:5,000), MMP2 (cat. no. ab92536, 1:1,000), MMP9 (cat. no. ab76003, 1:1,000), E-cadherin (cat. no. ab40772, 1:5,000), N-cadherin (cat. no. ab18203, 1:5,000), vimentin (cat. no. ab45939, 1:2,000), Snail (cat. no. ab216347, 1:1,000), AKT (cat. no. ab18785, 1:500), phosphorylated (p)-AKT (cat. no. ab8933, 1:500), mTOR (cat. no. ab134903, 1:10,000), p-mTOR (cat. no. ab109268, 1:10,000) and GAPDH (cat. no. ab8245, 1:5,000; all from Abcam) overnight at 4°C. The membranes were then incubated with the corresponding horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (cat. no. ab7090, 1:5,000; Abcam) at room temperature for 2 h, followed by visualization with chemiluminescence detection reagent (Tanon Science and Technology Co., Ltd.) and quantitative analysis with ImageJ software 1.46r (National Institutes of Health).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 8 (GraphPad Software, Inc.). All experiments were performed at least three times and data are presented as the mean ± SD. Statistical differences between groups were determined using a one-way ANOVA followed by Tukey's post-hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
NACC1 expression is upregulated in NPC cell lines and its knockdown inhibits NPC cell proliferation
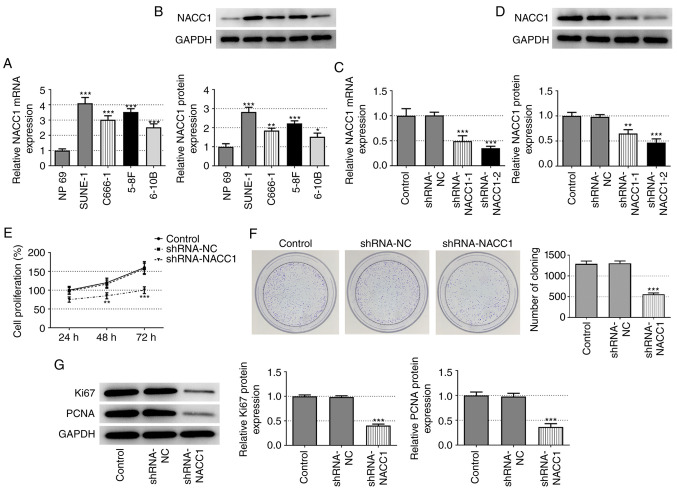
First, the differential expression of NACC1 was examined in the human normal nasopharyngeal epithelial cell line, NP69, and in the NPC cell lines, SUNE-1, C666-1, 5-8F and 6-10B. As shown in Fig. 1A and B, the NPC cell lines exhibited significantly higher mRNA and protein expression levels of NACC1 compared with the normal nasopharyngeal epithelial NP69 cell line, suggesting the possible role of NACC1 in the occurrence or progression of NPC. The SUNE-1 cell line was selected for use in subsequent experiments as it exhibited the highest NACC1 expression.
Figure 1.
Effect of NACC1-knockdown on NPC cell proliferation. (A) mRNA and (B) protein expression levels of NACC1 in the human normal nasopharyngeal epithelial NP69 cell line and NPC cell lines including SUNE-1, C666-1, 5-8F and 6-10B. *P<0.05, **P<0.01 and ***P<0.001 vs. NP69. (C) mRNA and (D) protein expression levels of NACC1 in control SUNE-1 cells or cells transfected with the indicated shRNAs. (E) Cell proliferation of control SUNE-1 cells or cells transfected with the indicated shRNAs was detected by MTT assay. (F) Representative colony formation assay of control SUNE-1 cells or cells transfected with the indicated shRNAs. (G) Protein expression levels of Ki67 and PCNA in SUNE-1 cells were measured by western blotting. **P<0.01 and ***P<0.001 vs. shRNA-NC. NPC, nasopharyngeal carcinoma; NACC1, nucleus accumbens-associated protein 1; shRNA, short hairpin RNA; NC, negative control; PCNA, proliferating cell nuclear antigen.
Subsequently, NACC1 was silenced in SUNE-1 cells by transfection with shRNAs (shRNA-NACC1-1 and −2). The transfection efficiency was verified by RT-qPCR and western blot analyses. shRNA-NACC1-2 was selected for NACC1 silencing based on its greater knockdown efficiency (Fig. 1C and D). The results of MTT and colony formation assays revealed that cell viability and colony formation ability were markedly decreased after NACC1 silencing (Fig. 1E and F). Consistently, NACC1-knockdown significantly decreased the protein expression levels of Ki-67 and PCNA (Fig. 1G). Collectively, these results demonstrated that NACC1-knockdown inhibited SUNE-1 cell proliferation.
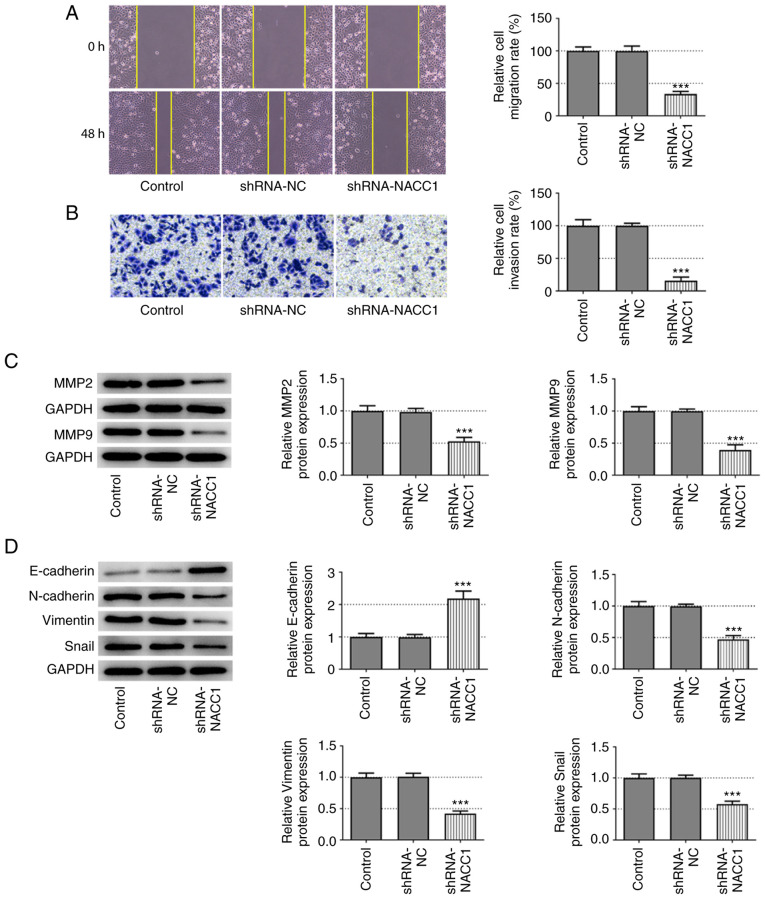
NACC1-knockdown inhibits NPC cell migration, invasion and EMT
The effects of NACC1-knockdown on cell migration and invasion were also evaluated. The results of wound healing and Transwell assays revealed that SUNE-1 cells in which NACC1 was silenced exhibited significantly decreased migratory and invasive abilities compared with the shRNA-NC group (Fig. 2A and B). Similarly, the protein expression levels of MMP2 and MMP9 were significantly decreased upon NACC1 silencing (Fig. 2C). In addition, NACC1-knockdown resulted in a significant upregulation of E-cadherin expression, whereas it led to a significant downregulation of N-cadherin, vimentin and Snail expression (Fig. 2D). Thus, these data illustrate the inhibitory effects of NACC1-knockdown on NPC cell migration, invasion and EMT.
Figure 2.
Effect of NACC1-knockdown on nasopharyngeal carcinoma cell migration and invasion. (A) Representative wound healing assay and quantitative analysis for control SUNE-1 cells or cells transfected with the indicated shRNAs (magnification, ×100). (B) Representative Transwell assay and quantitative analysis for control SUNE-1 cells or cells transfected with the indicated shRNAs (magnification, ×100). Protein expression levels of (C) MMP2 and MMP9, and (D) E-cadherin, N-cadherin, Vimentin and Snail in SUNE-1 cells were measured by western blot assay. ***P<0.001 vs. shRNA-NC. NACC1, nucleus accumbens-associated protein 1; shRNA, short hairpin RNA; NC, negative control.
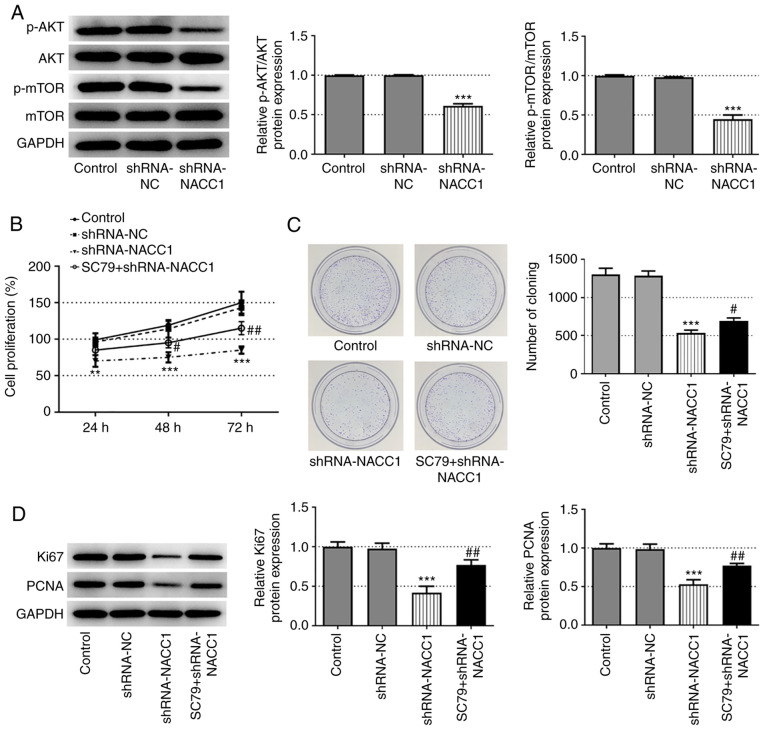
Activation of AKT blocks the inhibitory effects of NACC1-knockdown on NPC cell proliferation, migration and invasion
To further explore the mechanisms underlying the effects of NACC1 on NPC, the activation of AKT/mTOR signaling was investigated. As demonstrated in Fig. 3A, NACC1 silencing significantly inhibited the phosphorylation of AKT and mTOR. Subsequently, SC79, an activator of AKT (12), was used to treat cells in which NACC1 was knocked down. The results presented in Fig. 3B-D revealed that SC79 significantly recovered the cell proliferative ability, which was decreased in the shRNA-NACC1 group.
Figure 3.
Activation of AKT blocks the effect of NACC1-knockdown on nasopharyngeal carcinoma cell proliferation. (A) Protein expression levels of p-AKT, AKT, p-mTOR and mTOR in SUNE-1 cells were measured by western blot assay. (B) Proliferation of control SUNE-1 cells, transfected SUNE-1 cells or shRNA-NACC1-transfected cells plus SC79 treatment, was detected by MTT assay. (C) Representative colony formation assay of SUNE-1 cells in different groups. (D) Protein expression levels of Ki67 and PCNA in SUNE-1 cells were measured by western blotting. **P<0.01 and ***P<0.001 vs. shRNA-NC. #P<0.05 and ##P<0.01 vs. shRNA-NACC1. NACC1, nucleus accumbens-associated protein 1; shRNA, short hairpin RNA; NC, negative control; PCNA, proliferating cell nuclear antigen; p, phosphorylated.
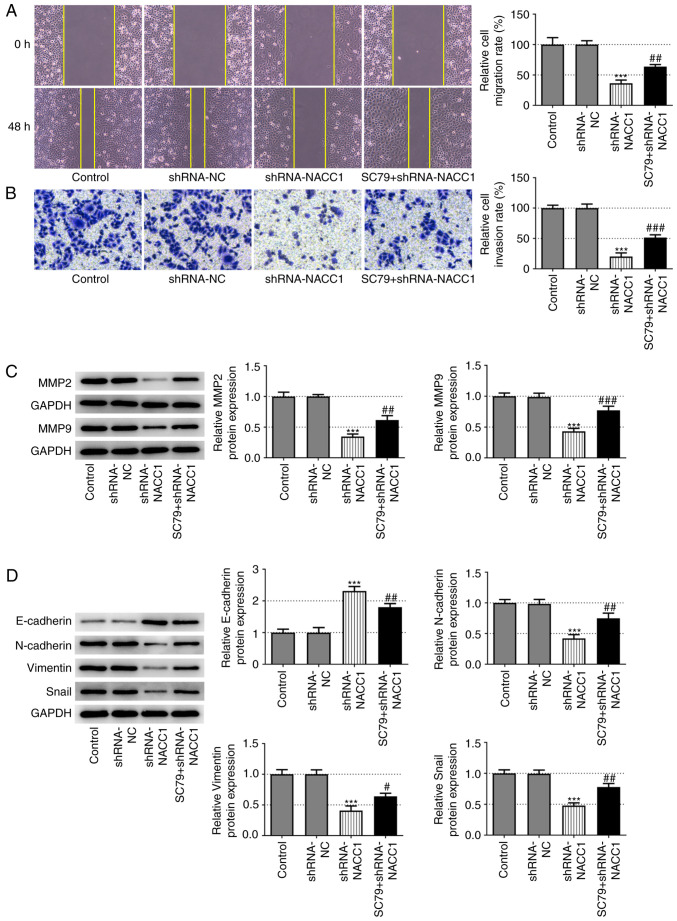
Furthermore, the decreased migration and invasion rates induced by NACC1-knockdown were significantly reversed upon SC79 treatment (Fig. 4A and B). Additionally, the application of SC79 effectively blocked the effects of NACC1 silencing on the protein expression levels of MMP2 and MMP9 (Fig. 4C), as well as on the expression levels of EMT-associated proteins, including E-cadherin, N-cadherin, vimentin and Snail (Fig. 4D).
Figure 4.
Activation of AKT blocks the effect of NACC1-knockdown on nasopharyngeal carcinoma cell migration and invasion. (A) Representative wound healing assay and quantitative analysis for SUNE-1 cells in different groups (magnification, ×100). (B) Representative Transwell assay and quantitative analysis for SUNE-1 cells in different groups (magnification, ×100). Protein expression levels of (C) MMP2 and MMP9, and (D) E-cadherin, N-cadherin, Vimentin and Snail in SUNE-1 cells were measured by western blot assay. ***P<0.001 vs. shRNA-NC. #P<0.05, ##P<0.01 and ###P<0.001 vs. shRNA-NACC1. NACC1, nucleus accumbens-associated protein 1; shRNA, short hairpin RNA; NC, negative control.
Discussion
NPC is a malignant tumor of the head and neck originating from the epithelial cells of the nasopharyngeal mucosa, which is highly malignant and prone to metastasis to the cervical lymph nodes and bones, liver and lungs; however, its pathogenesis is not yet fully understood (16). Therefore, there is an urgent need to elucidate the mechanisms underlying NPC metastasis and to identify effective strategies for inhibiting this process, so as to improve the prognosis of patients with NPC. It is generally considered that abnormal gene and protein expression levels are inherent changes in the early stages of cancer (17). Therefore, in recent years, continuous efforts have been made to explore gene markers associated with the onset and progression of NPC (18). NACC1 has been largely reported to exhibit abnormally increased expression levels in various types of cancer, including urothelial carcinoma, ovarian cancer and lung adenocarcinoma, and to be associated with poor patient prognosis (6–10). However, its role in NPC is not yet fully understood. The present study demonstrated that NACC1 expression was significantly upregulated in various NPC cell lines compared with that in normal human nasopharyngeal epithelial cells.
A previous study has revealed that NACC1 expression is upregulated in EBV-positive gastric tumor compared with that in EBV-negative gastric tumor (19). NACC1 expression in the poorly differentiated EBV-positive C666-1 cells was lower than in EBV-negative SUNE-1 cells in the present study, suggesting a potentially different role from that in gastric cancer, which expands the role of NACC1 in NPC. It was therefore hypothesized that NACC1 may serve a role in NPC development. To verify this hypothesis, NACC1 was silenced in NPC cells, and cell proliferation, migration and invasion were evaluated. It was revealed that NACC1 silencing effectively suppressed NPC cell proliferation, migration and invasion. In addition, NACC1 silencing increased E-cadherin expression, whereas it decreased N-cadherin, vimentin and Snail expression, suggesting the suppression of the EMT process in NPC cells induced by NACC1 silencing. EMT refers to the biological process of epithelial cells transforming into cells with a mesenchymal phenotype, characterized by a decrease in the expression levels of cell adhesion molecules (such as E-cadherin) and an increase in N-cadherin and vimentin expression (20). Through EMT, epithelial cells lose their polarity, their connection with the basement membrane and other epithelial phenotypes, and acquire mesenchymal phenotype characteristics, such as high migration and invasion, anti-apoptotic ability and the ability to degrade the extracellular matrix (21). EMT is a crucial biological process for malignant tumor cells to acquire the ability of migration and invasion (21). The migration and invasion of cancer cells into surrounding tissues and vasculature are crucial initial steps for cancer metastasis, which is the leading cause of cancer-associated mortality (22). The inhibition of cancer cell migration and invasion in vitro has been confirmed to be effective in preventing cancer metastasis in vivo (23). The results of the present study demonstrated that NACC1 silencing may be useful for preventing NPC metastasis by inhibiting NPC cell migration and invasion.
There are a number of cell signaling pathways that are considered to be involved in tumor carcinogenesis, such as the NF-κB, JAK/STAT, PI3K/AKT/mTOR and Wnt signaling pathways, among which the AKT/mTOR signaling pathway is one of the main signaling pathways in tumor research (24–28). In NPC, the AKT/mTOR signaling pathway is considered to induce EMT, and serves a key role in tumor cell proliferation, invasion and migration, as well as in resistance to radiotherapy and chemotherapy (29–31). In the present study, it was demonstrated that NACC1-knockdown significantly inhibited the activation of AKT/mTOR signaling, indicating that NACC1 may exert its effects on NPC by regulating AKT/mTOR signaling. NACC1 has been reported to activate AKT/mTOR signaling in retinoblastoma (13). In the present study, an AKT activator was subsequently added to NPC cells in which NACC1 was silenced. As expected, the activation of AKT markedly blocked the inhibitory effects of NACC1 silencing on NPC cell proliferation, migration and invasion. Therefore, NACC1 silencing may inhibit NPC cell proliferation, migration and invasion via inactivating AKT/mTOR signaling.
However, there were certain limitations to the present study. First, the differential NACC1 expression in NPC clinical samples, which would provide more substantial evidence, was not examined. Thus, the current findings must be validated in animal models and human samples. Second, SC79 did not completely abolish the effects of NACC1 silencing; thus, whether NACC1 serves a role in NPC via other signaling pathways requires further investigation.
In conclusion, the present study demonstrated that NACC1 was highly expressed in NPC cell lines. The results of in vitro experiments indicated that NACC1 silencing inhibited NPC cell proliferation, migration and invasion via inactivating AKT/mTOR signaling.
Acknowledgements
No applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
ZC, HC, XM and XL made substantial contributions to the conception and design of the study, and performed the experiments and interpreted the data, drafted and revised the manuscript for important intellectual content. ZC and XL confirm the authenticity of the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394:64–80. doi: 10.1016/S0140-6736(19)30956-0. [DOI] [PubMed] [Google Scholar]
- 2.Tsao SW, Tsang CM, Lo KW. Epstein-Barr virus infection and nasopharyngeal carcinoma. Philoso Trans R Soc Lond B Biol Sci. 2017;372:20160270. doi: 10.1098/rstb.2016.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee AWM, Ng WT, Chan JYW, Corry J, Mäkitie A, Mendenhall WM, Rinaldo A, Rodrigo JP, Saba NF, Strojan P, et al. Management of locally recurrent nasopharyngeal carcinoma. Cancer Treat Rev. 2019;79:101890. doi: 10.1016/j.ctrv.2019.101890. [DOI] [PubMed] [Google Scholar]
- 4.Ishibashi M, Nakayama K, Yeasmin S, Katagiri A, Iida K, Nakayama N, Miyazaki K. Expression of a BTB/POZ protein, NAC1, is essential for the proliferation of normal cyclic endometrial glandular cells and is up-regulated by estrogen. Clin Cancer Res. 2009;15:804–811. doi: 10.1158/1078-0432.CCR-08-2134. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Ji C, Zhang H, Shan Y, Ren Y, Hu Y, Shi L, Guo L, Zhu W, Xia Y, et al. Zhang, Identification of a small-molecule compound that inhibits homodimerization of oncogenic NAC1 protein and sensitizes cancer cells to anticancer agents. J Biol Chem. 2019;294:10006–10017. doi: 10.1074/jbc.RA119.007664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morita K, Fujii T, Itami H, Uchiyama T, Nakai T, Hatakeyama K, Sugimoto A, Miyake M, Nakai Y, Tanaka N, et al. NACC1, as a target of MicroRNA-331-3p, regulates cell proliferation in urothelial carcinoma cells. Cancers (Basel) 2018;10:347. doi: 10.3390/cancers10100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du W, Feng Z, Sun Q. LncRNA LINC00319 accelerates ovarian cancer progression through miR-423-5p/NACC1 pathway. Biochem Biophys Res Commun. 2018;507:198–202. doi: 10.1016/j.bbrc.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Sun Z. Circular RNA hsa_circ_0001588 promotes the malignant progression of lung adenocarcinoma by modulating miR-524-3p/NACC1 signaling. Life Sci. 2020;259:118157. doi: 10.1016/j.lfs.2020.118157. [DOI] [PubMed] [Google Scholar]
- 9.Jiao H, Jiang S, Wang H, Li Y, Zhang W. Upregulation of LINC00963 facilitates melanoma progression through miR-608/NACC1 pathway and predicts poor prognosis. Biochem Biophys Res Commun. 2018;504:34–39. doi: 10.1016/j.bbrc.2018.08.115. [DOI] [PubMed] [Google Scholar]
- 10.Yin L, Sun T, Liu R. NACC-1 regulates hepatocellular carcinoma cell malignancy and is targeted by miR-760. Acta Biochim Biophys Sin (Shanghai) 2020;52:302–309. doi: 10.1093/abbs/gmz167. [DOI] [PubMed] [Google Scholar]
- 11.Zhao M, Luo R, Liu Y, Gao L, Fu Z, Fu Q, Luo X, Chen Y, Deng X, Liang Z, et al. miR-3188 regulates nasopharyngeal carcinoma proliferation and chemosensitivity through a FOXO1-modulated positive feedback loop with mTOR-p-PI3K/AKT-c-JUN. Nat Commun. 2016;7:11309. doi: 10.1038/ncomms11309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang ML, Qi CL, Zou Y, Yang R, Jiang Y, Sheng JF, Kong YG, Tao ZZ, Chen SM. Plac8-mediated autophagy regulates nasopharyngeal carcinoma cell function via AKT/mTOR pathway. J Cell Mol Med. 2020;24:7778–7788. doi: 10.1111/jcmm.15409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Yu H, Ren Q. MiR-218-5p suppresses the progression of retinoblastoma through targeting NACC1 and inhibiting the AKT/mTOR signaling pathway. Cancer Manag Res. 2020;12:6959–6967. doi: 10.2147/CMAR.S246142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang Y, Liu J, Hong W, Fei X, Liu R. Arctigenin inhibits glioblastoma proliferation through the AKT/mTOR pathway and induces autophagy. BioMed Res Int. 2020;2020:3542613. doi: 10.1155/2020/3542613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet. 2016;387:1012–1024. doi: 10.1016/S0140-6736(15)00055-0. [DOI] [PubMed] [Google Scholar]
- 17.Soria F, Krabbe LM, Todenhöfer T, Dobruch J, Mitra AP, Inman BA, Gust KM, Lotan Y, Shariat SF. Molecular markers in bladder cancer. World J Urol. 2019;37:31–40. doi: 10.1007/s00345-018-2503-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma BBY, Lim WT, Goh BC, Hui EP, Lo KW, Pettinger A, Foster NR, Riess JW, Agulnik M, Chang AY, et al. Antitumor activity of nivolumab in recurrent and metastatic nasopharyngeal carcinoma: An international, multicenter study of the mayo clinic phase 2 consortium (NCI-9742) J Clin Oncol. 2018;36:1412–1418. doi: 10.1200/JCO.2017.77.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edwards RH, Dekroon R, Raab-Traub N. Alterations in cellular expression in EBV infected epithelial cell lines and tumors. PLoS Pathog. 2019;15:e1008071. doi: 10.1371/journal.ppat.1008071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Weinberg RA. Epithelial-to-mesenchymal transition in cancer: Complexity and opportunities. Front Med. 2018;12:361–373. doi: 10.1007/s11684-018-0656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedl P, Alexander S. Cancer invasion and the microenvironment: Plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 23.Duff D, Long A. Roles for RACK1 in cancer cell migration and invasion. Cell Signal. 2017;35:250–255. doi: 10.1016/j.cellsig.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Krishnamurthy N, Kurzrock R. Targeting the wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat Rev. 2018;62:50–60. doi: 10.1016/j.ctrv.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koni M, Pinnarò V, Brizzi MF. The wnt signalling pathway: A tailored target in cancer. Int J Mol Sci. 2020;21:7697. doi: 10.3390/ijms21207697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calses PC, Crawford JJ, Lill JR, Dey A. Hippo pathway in cancer: Aberrant regulation and therapeutic opportunities. Trends Cancer. 2019;5:297–307. doi: 10.1016/j.trecan.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Owen KL, Brockwell NK, Parker BS. JAK-STAT signaling: A double-edged sword of immune regulation and cancer progression. Cancers. 2019;11:2002. doi: 10.3390/cancers11122002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Populo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13:1886–1918. doi: 10.3390/ijms13021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lyu X, Wang J, Guo X, Wu G, Jiao Y, Faleti OD, Liu P, Liu T, Long Y, Chong T, et al. EBV-miR-BART1-5P activates AMPK/mTOR/HIF1 pathway via a PTEN independent manner to promote glycolysis and angiogenesis in nasopharyngeal carcinoma. PLoS Pathog. 2018;14:e1007484. doi: 10.1371/journal.ppat.1007484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Q, Zheng W, Zhu L, Yao D, Wang C, Song Y, Hu S, Liu H, Bai Y, Pan Y, et al. ANXA6 contributes to radioresistance by promoting autophagy via inhibiting the PI3K/AKT/mTOR signaling pathway in nasopharyngeal carcinoma. Front Cell Dev Biol. 2020;8:232. doi: 10.3389/fcell.2020.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.