Abstract
Objective
Clinical trials evaluating pharmacological and non-pharmacological treatment of COVID-19, either excluded pregnant women or included very few women. Unlike the numerous systematic reviews on prevalence, symptoms and adverse outcomes of COVID-19 in pregnancy, there are very few on the effects of treatment on maternal and neonatal outcomes in pregnancy. We undertook a systematic review of all published and unpublished studies on the effects of pharmacological and non-pharmacological interventions for COVID-19 on maternal and neonatal pregnancy outcomes.
Data sources
We performed a systematic literature search of the following databases: Medline, Embase, Cochrane database, WHO (World Health Organization) COVID-19 database, China National Knowledge Infrastructure (CNKI), and Wanfang databases from 1 December 2019 to 1 December 2020.
Study eligibility criteria
Studies were only included if they involved pregnant or postnatal women who were exposed to pregnancy specific interventions like the mode of delivery and type of anaesthesia, pharmacological or non-pharmacological interventions.
Study appraisal and synthesis methods
We first screened the titles and abstracts of studies and then assessed the full text of the selected studies in detail for eligibility. Data on study design, population, type of screening for COVID-19, country, hospital, country status (high or low and middle income), treatment given (mode of delivery, type of anaesthesia, type of pharmacological and non-pharmacological treatment was extracted. The pre-defined maternal outcomes we collected were mode of delivery (vaginal or by caesarean section), severe or critical COVID-19 (as defined by the authors), symptomatic COVID-19, maternal death, maternal hospital admission, ICU admission, mechanical ventilation, ECMO and maternal pneumonia. The pre-defined neonatal outcomes we extracted were preterm birth (<37 weeks), stillbirth, neonatal death, NICU admission, neonatal COVID-19 positive, neonatal acidosis (pH < 7.0) and Apgar scores (<8 after 5 min). Study quality assessment was performed.
Results
From a total of 342 potential eligible studies, we included 27 studies in our systematic review, including 4943 pregnant women (appendix 3). Sixteen studies had a retrospective cohort design and 11 a prospective cohort design. There were no randomised controlled trials. There was a significant association between caesarean section and admission to ICU (OR 4.99, 95% CI 1.24 to 20.12; 4 studies, 153 women, I2 = 0%), and diagnosis of maternal COVID-19 pneumonia as defined by study authors (OR 3.09, 95% CI 1.52 to 6.28; 2 studies, 228 women, I2 = 0%). Women who had a preterm birth were more likely to have the baby via caesarean section (OR 3.03, 95% CI 1.71 to 5.36, 12 studies; 314 women, I2 = 0%). For pharmacological and non-pharmacological we provided estimates of the expected rates of outcomes in women exposed to various treatment of COVID-19. Comparative data for pregnant women, in particular for treatments proven to be effective in the general population, however, is lacking to provide clinically meaningful interpretation.
Conclusions
We found associations for pregnancy specific interventions, like mode of delivery and outcomes of the disease, but there were too few data on pharmacological and non-pharmacological treatments in pregnant women with COVID-19. We report the rates of complications found in the literature. We encourage researchers to include pregnant women in their trials and report the data on pregnant women separately.
Keywords: COVID-19, Pregnancy, Treatment, Systematic review, Meta-analysis, Neonatal
Introduction
Pregnant women with coronavirus (COVID-19) are more likely to have severe COVID-19 and complications than non-pregnant women with COVID-19 of similar age group, although symptoms and clinical presentation can be the same as in the general population [1]. In addition, adverse pregnancy outcomes, such as preterm delivery, maternal death and admission on intensive care unit (ICU), are seen in pregnant women with COVID-19 compared to those without the disease [1]. Many pharmacological interventions for treatment of COVID-19 have been used, but few, like Remdesivir and systemic steroids have been shown to be effective [2]. Non-pharmacological interventions like proning or invasive and non-invasive mechanical ventilation have been applied in pregnancy, but its impact on the course of the disease and on the pregnancy outcomes are not known in this specific group. Lastly, there are certain interventions that are specific to pregnancy, such as the mode of delivery or type of anaesthesia, which are only applicable to pregnant women. In pregnant women with COVID-19, these interventions have been used as forms of treatment, although their impact on COVID-19 and pregnancy related outcomes is not known.
Clinical trials evaluating pharmacological and non-pharmacological treatment of COVID-19, either excluded pregnant women or included very few women [3]. This has resulted in very little information on maternal and neonatal outcomes in pregnant women who have been exposed to these interventions. Unlike the numerous systematic reviews on prevalence, symptoms and adverse outcomes of COVID-19 in pregnancy, there are very few on the effects of treatment on maternal and neonatal outcomes in pregnancy.
To fill in the evidence gap, we undertook a systematic review and meta-analysis of all published and unpublished studies on the effects of pharmacological and non-pharmacological interventions for COVID-19 on maternal and neonatal pregnancy outcomes.
Methods
This systematic review is part of an ongoing set of living systematic reviews on COVID-19 in pregnancy, using a prospectively registered protocol (PROSPERO CRD42040178076; registered 22 April 2020) published elsewhere [4]. In this paper we specifically report the effects of pharmacological and non-pharmacological interventions on pregnancy outcomes. We also report on the complication rates of pregnancy specific interventions, such as mode of delivery and type of anaesthesia. We carried out our systematic review using the preferred reporting items for systematic reviews and meta-analysis (PRISMA) recommendations (see appendix 1).
Literature search
The PregCOV-19 Living Systematic Review Consortium performed a systematic literature search of the following databases: Medline, Embase, Cochrane database, WHO (World Health Organization) COVID-19 database, China National Knowledge Infrastructure (CNKI), and Wanfang databases from 1 December 2019 to 1 December 2020. The details of the search strategy are published elsewhere.
Study selection
Two reviewers (SG and EG) independently selected studies using a two-stage process: they first screened the titles and abstracts of studies and then assessed the full text of the selected studies in detail for eligibility. Studies were included if they involved pregnant or recently pregnant women including postnatal women who were exposed to pregnancy specific interventions like the mode of delivery and type of anaesthesia, pharmacological or non-pharmacological interventions. Pharmacological intervention includes antiviral, immunotherapy, (systemic) corticosteroids, antibiotics or combinations of these interventions and non-pharmacological interventions comprised mechanical ventilation, extracorporeal membrane oxygenation (ECMO) or proning. The studies also needed to report on COVID-19 related pregnancy or neonatal outcomes. Pregnancy outcomes were severe or critical COVID-19 (as defined by the individual study authors), symptomatic COVID-19, maternal death, maternal hospital admission, ICU admission, mechanical ventilation, ECMO, maternal pneumonia, preterm birth (<37 weeks), caesarean section, stillbirth, neonatal death (up to 28 days), neonatal intensive care unit (NICU) admission, neonatal COVID-19 positive, neonatal acidosis (pH < 7.0) and Apgar scores (<8 after 5 min). Disagreements were resolved through discussion with a third reviewer (ST or JA). We included only cohort studies or case-series that reported on >10 women. We excluded studies that reported on duplicate data for the outcomes of interest when this was reported by the authors or when we found that the characteristics of the studies were similar to each other.
Quality assessment and data extraction
To assess the quality of comparative cohort studies for selection, comparability and outcome ascertainment bias we used the Newcastle Ottowa Scale [5]. At any time, two reviewers independently assessed the quality of the studies (SG, EG, TK). For internal validity we considered studies to be of low risk of bias when the data was collected directly from the subjects, the outcomes of interest were clearly defined, the data was collected from medical records and done in the same manner for all the subjects, the follow-up time was long enough to report the outcome and the numerators and denominators for the outcomes reported were appropriate. For external validity a study was considered to be of low risk when the studies target population closely represented the national population, universal testing was used, instead of selected testing, there was no form of random selection used to select the sample and the response rate for the study was higher than 90%.
Two reviewers (SG, EG) independently extracted data using a predefined format. In all studies we extracted data on the study design, the population, type of screening for COVID-19, country, hospital, country status (high or low and middle income), treatment given (mode of delivery, type of anaesthesia, type of pharmacological and non-pharmacological treatment and their definition. The pre-defined maternal outcomes we collected were severe or critical COVID-19 (as defined by the authors), symptomatic COVID-19, maternal death, maternal hospital admission, ICU admission, mechanical ventilation, ECMO and maternal pneumonia. The pre-defined neonatal outcomes we extracted were preterm birth (<37 weeks), mode of delivery (vaginal or by caesarean section), stillbirth, neonatal death, NICU admission, neonatal COVID-19 positive, neonatal acidosis (pH < 7.0) and Apgar scores (<8 after 5 min). We did a deduplication process by checking the data with other studies published by the same authors or where data was collected in the same hospitals. We contacted study authors if there were any inconsistency in their data or where data was missing. Disagreements were discussed with a third reviewer (JA)
Statistical analysis
The comparative dichotomous data assessing the association of exposures to outcomes were pooled using random-effects meta-analysis based on the Mantel-Haenszel estimation of between-study variance. The findings were summarized as odds ratios (OR) with 95% confidence intervals (CI). Heterogeneity was measured using the I-squared statistic. When the I2 is 80% or more its meant to have high heterogeneity, less then 50% low heterogeneity, 50–80% moderate heterogeneity.
Results
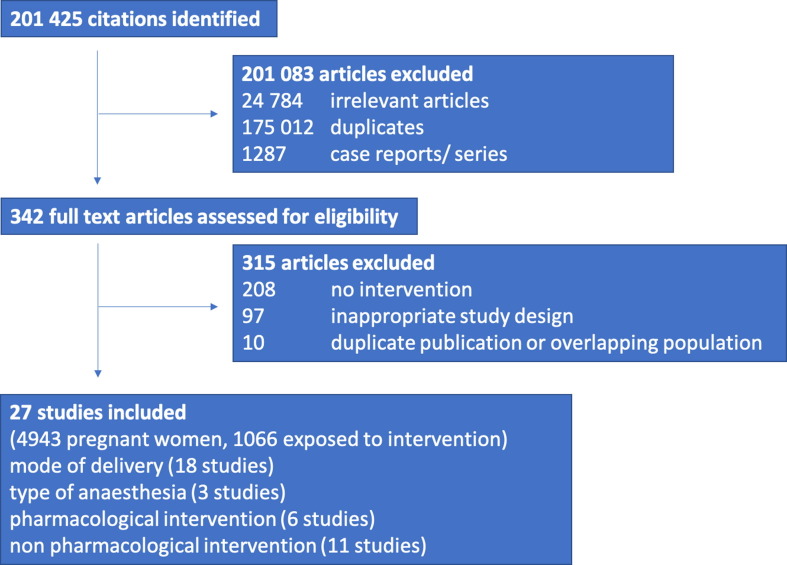
From a total of 342 potential eligible studies, we included 27 studies in our systematic review. (Fig. 1 ).
Fig. 1.
Study selection process.
Characteristics of the included studies
Of 27 studies, six (22%) were from China [6], [7], [8], [9], [10], [11]; five (19%) were from Italy [12], [13], [14], [15], [16]; three from Spain [17], [18], [19]; two each from Chile [20], [21], Turkey [22], [23] and the United States of America [24], [25]; one each from Brazil [26], France [27], India [28], Israel [29], Mexico [30], Panama [31] and Peru [32]. Fifteen were classified as high-income countries [24], [25], [27], [29], [31], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21] and 12 as low and middle-income countries [22], [23], [26], [28], [30], [32], [6], [7], [8], [9], [10], [11]. Sixteen studies had a retrospective cohort design [14], [24], [28], [29], [32], [6], [7], [8], [9], [10], [17], [18], [19], [20], [21], [22] and 11 a prospective cohort design [15], [16], [23], [30], [31], [11], [12], [13], [25], [26], [27]. There were no randomised controlled trials. Sixteen studies reported data on admitted women with COVID-19, eight reported data on all pregnant women and three reported data on a selected group of women, such as pregnant women with hypertension. Seven studies performed universal screening and testing to assess for COVID-19, nine studies did symptom-based testing, four studies did risk-based testing on the basis of epidemiological history and clinical manifestations by National Health Commission of China (NHCC) guidelines [33] and in seven studies the testing strategy was not known. From the 27 studies, 21 reported on pregnancy specific interventions, consisting of 18 studies reporting on mode of delivery and three on type of anaesthesia. Six studies reported on pharmacological interventions and 11 on non-pharmacological interventions. Detailed information can be found in appendix 3.
Quality of the included studies
Evaluation of study quality using the Newcastle Ottawa scale was overall low for 26 out of 27 studies. The risk of bias for study selection was low for 26 out of 27 studies, with one study scoring medium due to assessed outcome perceived to be present at study inception. Eleven out of 27 studies had a low risk of bias for the comparability of cohorts, on the basis of both design and selection. Thirteen studies had a medium and three a high risk of bias respectively for comparability. Risk of bias of study outcome was low in 25 out of 27 studies and medium in two out of 27 studies, due to either inadequate follow-up length or incomplete accountability of outcomes for all subjects at study termination.
Pregnancy specific interventions for COVID 19
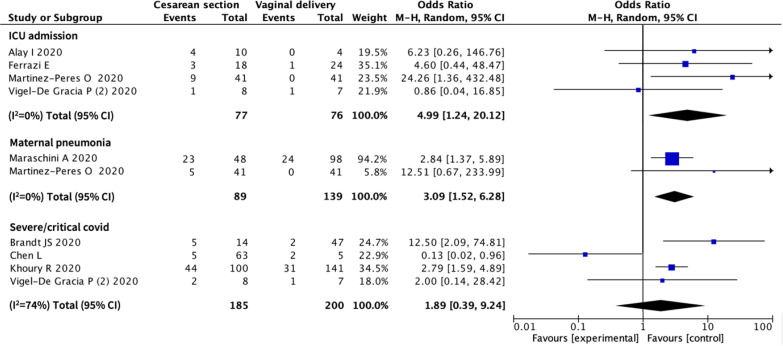
Eighteen studies provided data on mode of delivery and the relation with maternal and/ or neonatal outcomes (1020 women). (Fig. 2 ) There was a significant association between caesarean section and admission to ICU (OR 4.99, 95% CI 1.24 to 20.12; 4 studies, 153 women, I2 = 0%), and diagnosis of maternal COVID-19 pneumonia as defined by study authors (OR 3.09, 95% CI 1.52 to 6.28; 2 studies, 228 women, I2 = 0%), although in one of the two studies reporting on pneumonia it was not clear if this was COVID-19 pneumonitis. There were no associations between mode of delivery and severe COVID-19.
Fig. 2.
Association between mode of delivery and maternal outcomes in pregnant women with COVID-19. CI – Confidence Interval.
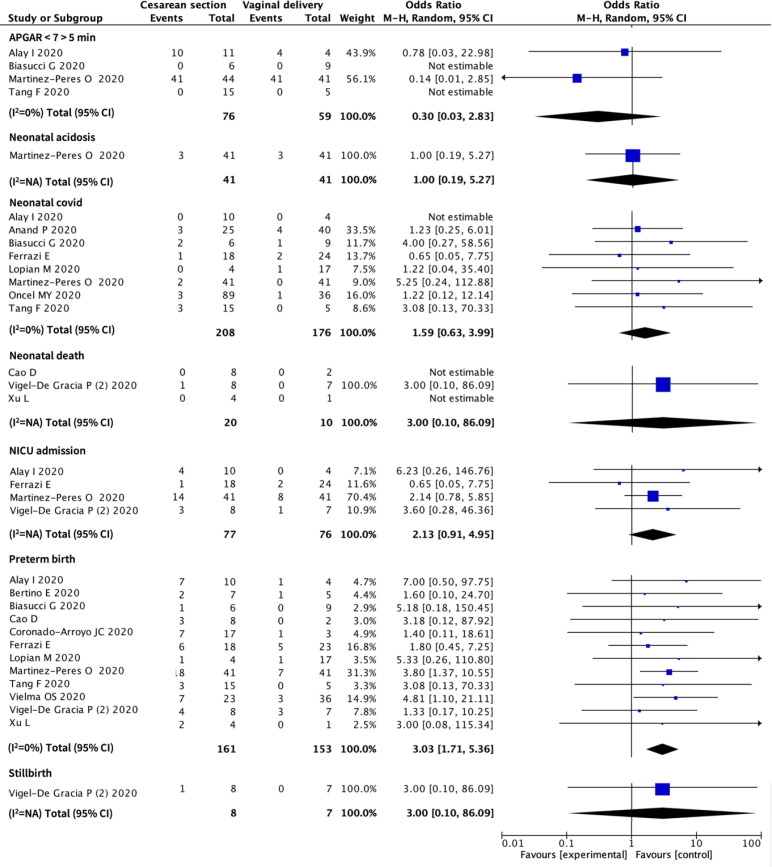
There was a significant association between mode of delivery and preterm birth. Women who had a preterm birth were more likely to have the baby via caesarean section (OR 3.03, 95% CI 1.71 to 5.36; 12 studies, 314 women, I2 = 0%). There were no associations between mode of delivery and a low Apgar score, neonatal acidosis, neonatal COVID-19, neonatal death, NICU admission or stillbirth. (Fig. 3 ).
Fig. 3.
Associations between mode of delivery and neonatal outcomes in pregnant women with COVID-19. CI – Confidence Interval.
Only three studies reported on the type of anaesthesia and maternal or neonatal outcomes. (Zhang, Martinez and Chen R). Zhang et al described a Chinese multi-centre cohort of 89 COVID-19 positive women (90 neonates) who underwent a caesarean section with either locoregional or general anaesthesia [10]. Of the 90 neonates, 11 were born by general anaesthesia caesarean section of which five had an Apgar score lower than eight after five minutes. Seventy-nine neonates were born by caesarean section under locoregional anaesthesia of which one had an Apgar score lower than eight after five minutes. In the study by Martinez et al (41 women) more women who underwent a caesarean section under general anaesthesia had severe COVID-19 (2 out of 7) compared to women who had locoregional anaesthesia (2 out of 32) v. Chen R et al gives a retrospective study of 17 cases of which three women underwent general anaesthesia for caesarean section, however no relevant maternal outcomes were reported according to our outcome definitions [8]. There were no adverse neonatal outcomes, like neonatal death, neonatal acidosis, neonatal COVID-19 positive babies and low Apgar scores.
Pharmacological interventions for treatment of COVID-19 in pregnancy
Six studies (599 women) reported on pharmacological treatment of COVID-19 in pregnancy, which included: antiviral treatment, (systemic) corticosteroids, antibiotics and immunotherapy; however, the types and doses of the medications were not specified. The number of pregnant women exposed to intervention was small despite the big denominators, hence it was not possible to do a meta-analysis and make any conclusions about pharmacological interventions for treatment of pregnant women with COVID-19 (Table 1 ).
Table 1.
Pharmacological interventions for treatment of pregnant women with COVID-19.
| Pharmacological interventions | Drug type (reference) | Nr of studies (total nr of women) | Women exposed to intervention | Outcomes in exposed | Outcomes in non-exposed |
|---|---|---|---|---|---|
| Antiviral | lopinavir–ritonavir, remdesivir, or darunavir (12) | 1 (77) | 25 | Severe or critical COVID-19* (8/25) | Severe or critical COVID-19* (6/52) |
| Not specified (9, 13, 26) | 3 (439) | 118 | CS (4/5); preterm delivery (2/5); maternal death (11/112); maternal COVID-19 pneumonia (0/1) | CS (0/0); preterm delivery (0/0); maternal death (25/176); maternal COVID-19 pneumonia (47/145) | |
| Remdesivir (24) | 1 (61) | 2 | Severe or critical COVID-19† (2/2) | Severe or critical COVID-19† (5/59) | |
| Antibiotics | Not specified (Mostly penicillins or cephalosporins) (13) | 4 (289) | 50 | CS* (5/5); preterm delivery (2/5); maternal COVID-19 pneumonia (2/14); severe or critical COVID-19*† (14/31); neonatal death (0/5) | CS* (0/0); preterm delivery (0/0); maternal COVID-19 pneumonia (45/132); severe or critical COVID-19*† (8/107); neonatal death (0/0) |
| Corticosteroids | Dose and type not specified (9, 24) | 1 (66) | 7 | CS* (2/3); preterm delivery (2/3); severe/ critical COVID-19† (4/4); neonatal death (0/3) | CS* (2/2); preterm delivery (0/2); severe/ critical COVID-19† (3/57); neonatal death (0/2) |
| Antimalarials | Hydroxychloroquine (12, 13, 18, 24) | 4 (306) | 32 | CS* (0/1); preterm delivery (0/1); maternal COVID-19 pneumonia (2/8); ICU admission (0/1); severe/ critical COVID-19*† (10/23); neonatal COVID-19 positive (0/1); NICU admission (0/1) | CS* (4/21); preterm delivery (3/21); maternal COVID-19 pneumonia (45/138); ICU admission (1/21); severe/ critical COVID-19*† (11/115); neonatal COVID-19 positive (0/21); NICU admission (2/21) |
| Combination of interventions | Antivirals and antibiotics (13) | 1 (146) | 5 | Maternal COVID-19 pneumonia (4/5) | Maternal COVID-19 pneumonia (43/141) |
| Hydroxychloroquine and antibiotics (13, 18) | 2 (168) | 15 | CS* (1/1); preterm delivery (0/1); maternal COVID-19 pneumonia (8/14); ICU admission (0/1); neonatal COVID-19 positive (0/1); NICU admission (1/1) | CS* (4/21); preterm delivery (3/21); maternal COVID-19 pneumonia (39/132); ICU admission (1/21); neonatal COVID-19 positive (0/21); NICU admission (3/21) | |
| Hydroxychloroquine and antivirals (13, 18) | 2 (168) | 10 | CS* (0/2); preterm delivery (0/2); maternal COVID-19 pneumonia (7/8); ICU admission (0/2); neonatal COVID-19 positive (0/2); NICU admission (0/2) | CS* (3/20); preterm delivery (2/20); maternal COVID-19 pneumonia (40/138); ICU admission (1/20); neonatal COVID-19 positive (0/20); NICU admission (2/20) | |
| Hydroxychloroquine and antibiotics and antivirals (13) | 1 (146) | 20 | Maternal COVID-19 pneumonia (17/20) | Maternal COVID-19 pneumonia (30/126) | |
| Targeted antibiotics (13) | 1 (146) | 2 | Maternal COVID-19 pneumonia (0/2) | Maternal COVID-19 pneumonia (47/144) |
*CS = Caesarean Section ICU = Intensive Treatment Unit NICU = Neonatal Intensive Care Unit.
*severe or critical COVID-19 is defined as need for urgent delivery based on maternal respiratory function and/or ICU or subintensive care admission during pregnancy or the postpartum period.
†severe COVID-19 is defined as dyspnoea, respiratory frequency ≥ 30/min, blood oxygen saturation ≤ 93%, partial pressure of arterial oxygen to fraction of inspired oxygen ratio < 300, and lung infiltrates > 50% on chest X-ray; and critical COVID-19 was defined as respiratory failure, septic shock, and multiple organ failure.
Non-pharmacological interventions for COVID-19 in pregnancy
Eleven studies reported on non-pharmacological interventions, of which mechanical ventilation was reported in 6 studies and oxygen administration in 8 studies. In total 1738 women were included in these studies and 240 were exposed to interventions, 28 patients had mechanical ventilation and 212 had oxygen administration. (Table 2 ).
Table 2.
Non-pharmacological interventions for treatment of pregnant women with COVID-19.
| Non-pharmaco-logical interventions | Type | Nr of studies (total nr of women) | Women exposed to intervention | Outcomes in exposed | Outcomes in non-exposed |
|---|---|---|---|---|---|
| Mechanical ventilation | Invasive respiratory support (13, 26) | 2 (434) | 40 | Maternal death (21/27); maternal COVID-19 pneumonia (10/13) | Maternal death (15/261); maternal COVID-19 pneumonia (37/133) |
| Not specified (20, 23, 24, 30) | 4 (531) | 15 | Preterm delivery (1/2); maternal death (1/4); severe/ critical COVID-19† (1/1); neonatal death (0/2); neonatal COVID-19 positive (1/8); NICU admission (1/2) | Preterm delivery (3/35); maternal death (6/304); severe/ critical COVID-19† (6/60); neonatal death (1/35); neonatal COVID-19 positive (3/117); NICU admission (4/35) | |
| Oxygen administration | Nasal cannula (9) | 1 (5) | 1 | CS (1/1); preterm delivery (1/1); neonatal death (0/1) | CS (1/1); preterm delivery (1/1); neonatal death (0/1) |
| Oxygen support or non-invasive ventilation (9, 12, 14, 26, 27) | 5 (1133) | 215 | CS (26/30); preterm delivery (15/30); maternal death (5/166); maternal COVID-19 pneumonia (22/28); severe/ critical COVID-19* (11/20); NICU admission (14/30); stillbirth (0/29) | CS (65/156); preterm delivery (37/156); maternal death (32/739); maternal COVID-19 pneumonia (25/118); severe/ critical COVID-19* (3/57); NICU admission (23/160); stillbirth (7/152) | |
| Not specified (19, 20, 24) | 3 (130) | 26 | Preterm delivery (0/1); ICU admission (2/18); severe/ critical COVID-19† (7/7); neonatal death (0/1); NICU admission (0/1) | Preterm delivery (4/36); ICU admission (0/14); severe/ critical COVID-19† (0/54); neonatal death (1/36); NICU admission (5/36) | |
| Nasal cannula, CPAP (14) | 1 (42) | 7 | CS (5/7) | CS (13/35) |
*CS = Caesarean Section ICU = Intensive Treatment Unit NICU = Neonatal Intensive Care Unit.
*severe/critical COVID-19 is defined as need for urgent delivery based on maternal respiratory function and/or ICU or subintensive care admission during pregnancy or the postpartum period.
†Dyspnoea, respiratory frequency ≥ 30/min, blood oxygen saturation ≤ 93%, partial pressure of arterial oxygen to fraction of inspired oxygen ratio < 300, and lung infiltrates > 50% on chest X-ray; and critical disease was defined as respiratory failure, septic shock, and multiple organ failure.
There were no studies reporting on proning during pregnancy and no studies were found that report on extracorporeal membrane oxygenation (ECMO), haemodialysis or inotropic treatment.
Discussion
Summary of findings
All interventions, either pregnancy specific or COVID-19 related interventions in pregnant women diagnosed with the disease were poorly reported. None of the randomised trials reported outcomes specific for pregnant women. The RECOVERY Trial included outcomes however this has yet to be published.
Pregnancy-specific interventions, such as the mode of delivery or the type of anaesthesia appears to be related with severity of disease, but not with perinatal outcomes. We are unable to ascertain the temporality on all cases. There is an association between caesarean section and the increased likelihood of being admitted to ICU and increased odds of COVID-19-related maternal pneumonia.
We provide estimates of the rates of outcomes in women exposed to various pharmacological treatment for COVID-19 and non-pharmacological interventions such as oxygen administration and ventilation. Comparative data for pregnant women, in particular for treatments proven to be effective in the general population, however is lacking to provide clinically meaningful interpretation.
Strengths and limitations of this review
To our knowledge this is the first systematic review looking into management and treatment of pregnant women with COVID-19. We did this in a structured manner and included not only pharmacological treatment, but also non-pharmacological and pregnancy specific interventions, such as mode of delivery. We also assessed at the quality of the studies. We refrained conducting a meta-analyses for non-comparative cohorts. We set strict inclusion and exclusion criteria for the selection of papers. The studies we have included are clear about the included women, the treatment given, and the outcomes reported. If the relationship between treatment and outcome was not clear, the paper would be excluded. Extensive collaboration and capturing of data through different databases allowed for a big pool of studies to be reviewed. There were no language restrictions.
We were limited by the paucity of the data and the heterogeneity in the studies. We could not establish the temporality for some of the interventions such as caesarean section as it is possible that some women admitted to ICU for severe COVID-19 might have had caesarean section for maternal reasons. There were no randomised controlled trials, and it is very likely that the intervention was influenced by the characteristics of the participant, the setting and the availability of resources as most of the trials were in high income settings. There is also the issue of generalisability, and therefore the rate of outcomes may not reflect the effect of treatment, but it could be more indicative of the underlying severity of the disease of the mother. We were also restricted with performing meta-analyses for most of the data due to the small number of reported treatment and outcomes. We could only provide narrative reviews for those interventions.
Comparison with existing evidence
Pregnant women are known to be more often affected by severe COVID-19 than women in the general population [1]. In our systematic review, we found an association between having a caesarean section and being admitted to ICU or having COVID-19 pneumonia. This, however, may be influenced by pregnancy. By performing a caesarean section, the concern of impaired lung capacity due to the gravid uterus is diminished. Delivery allows for more postpartum treatment options and minimises the risk of causing harm to the fetus in-utero and ventilation is also easier postpartum.
The association we found between mode of delivery and preterm birth is also likely to reflect the needs for more intensive treatment. The PregCOV-19 Living Systematic Review estimated the risk of preterm birth at approximately 17%, of which approximately 94% were iatrogenic [1].
The Royal College of Anaesthetists (RCoA) issued recommendations for types of anaesthesia to be offered to pregnant women with COVID-19. To date however, no trials have been conducted in regards to management or outcomes for such women. The RCoA recommends epidural analgesia in labour to minimise the need for general anaesthesia if urgent delivery is required [34]. In the event of a caesarean delivery, it is recommended to avoid general anaesthesia unless absolutely necessary guided by clinical indications.
With regards to pharmacological interventions for treatment of COVID-19, corticosteroids, in particular betamethasone, are beneficial when people are admitted in hospital and require oxygen support [35]. To date there is no subgroup analysis performed on pregnant women, but the RCOG and WHO made the statement that no harm is expected from steroid use, although the first choice should be prednisolone, instead of dexamethasone, because prednisolone is extensively metabolised in the placenta resulting in minimal transfer to the fetus [2], [36].
The use of antiviral medication, such as remdesivir, is currently not recommended by WHO in patients with COVID-19 [2]. The RCOG guideline, like the WHO guideline, recommends avoiding remdesivir, unless clinicians believe the benefit of the treatment outweighs the risks, although no randomised controlled data for pregnant women have been published [2], [36]. There is sufficient data that tocilizumab, an interleukine-6 antagonist improves outcomes, including survival, in hospitalised patients with hypoxia with evidence of systemic inflammation [37]. NICE guidance recommends using tocilizumab in hospitalized patients that are having or had completed a course of steroids, an increase of C-reactive protein (CRP) > 75, the need for supplemental oxygen, or within 48 h of initiating mechanical ventilation. The data for the use of tocilizumab in pregnancy are scarce, but there are no adverse effects reported to date. The RCOG advice is to offer tocilizumab to pregnant women when they fit the criteria and the decision is taken by a multi-disciplinary team (MDT) and given the benefits outweigh the risks. Although there were some studies included which looked at the effects of hydroxychloroquine and antibiotics, this has now been shown to not bebeneficial and is not recommended for either non-pregnant patients or pregnant women [2], [38].
The same treatment principles apply to pregnant women as to non-pregnant patients with regards to non-pharmacological interventions, such as oxygen supplementation or mechanical ventilation. A very gravid uterus can cause difficul ventilation and the need for supplemental oxygen. This is due to the increased demand of oxygen in pregnancy due to the higher metabolic rate and the increased consumption of oxygen [39]. Mechanical ventilation is more difficult when a woman is pregnant, due to the gravid uterus, lung capacity can be impaired and it can be difficult to get the required volumes to support adequate ventilation [40], [41]. In the presence of ARDS, proning has been proven to help ventilate patients [42]. This cannot be practiced when a patient has a wound of a caesarean section or when she is over 34 weeks pregnant, the heavily pregnant uterus can make this position more difficult. Furthermore, after 24–28 weeks there is the risk of aortocaval compression when proning a pregnant woman. Although there are no trials performed with pregnant woman, there are techniques describing how proning can be done in this particular group of patients [41]. The authors of this article describe how they place pillows in a specific way to place the pregnant woman in a comfortable position without compromising the pregnancy.
Relevance for clinical practice and research
A hurdle which was acknowledged and acted upon during the pandemic was that of recruitment of COVID-19 positive pregnant womeninto trials. Traditionally, pregnant women have been excluded from clinical trials. There are concerns of the effect of the drugs on fetus both in short term and long term which has led to the reluctance in evaluating the use of drugs in pregnancy. Researchers have previously highlighted their concerns regarding the issue of pregnant women being excluded from trials during various endemics and pandemics and COVID-19 has highlighted this issue even more [43]. To date only three international trials worldwide have involved pregnant women [44]. The SOLIDARITY trial now includes pregnant women, and the RECOVERY trial allows pregnant and lactating women to participate with informed consent. Though the numbers are small, the acceptance and initiative are a step forward in health sciences.
In summary, there is a need for more data involving pregnant women in clinical trials. Where trials are not available, more data is needed on the outcomes when drugs are given in clinical practice. Interventions and outcomes as shown in this article do appear to be associated with the severity of the disease. There is a paucity of data with regards to pregnant and postnatal women; clinical trials needs to include pregnant women.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejogrb.2021.10.007.
Contributor Information
PregCOV-19 Group:
Shaunak Chatterjee, Andrea Gae, Elena Stallings, Magnus Yap, Jameela Sheikh, Heidi Lawson, Dyuti Coomar, Anushka Dixit, Dengyi Zhou, Rishab Balaji, Megan Littmoden, Yasmin King, Luke Debenham, Anna Clavé Llavall, Kehkashan Ansari, Gurimaan Sandhu, Adeolu Banjoko, Helen Fraser, Tanisha Rajah, Anoushka Ramkumar, Alya Khashaba, Shruit Attarde, Kate Walker, Jim Thornton, Madelon van Wely, Elizabeth van Leeuwen, Elena Kostova, Asma Khalil, Simon Tiberi, Nathalie Broutet, Caron Rahn Kim, Anna Thorson, Olufemi T. Oladapo, Javier Zamora, and Lynne Mofenson
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. [DOI] [PMC free article] [PubMed]
- 2.Organization WH. Therapeutics and COVID-19: living guideline 2021 [Available from: https://apps.who.int/iris/bitstream/handle/10665/340374/WHO-2019-nCoVtherapeutics-2021.1-eng.pdf. [PubMed]
- 3.Taylor M.M., Kobeissi L., Kim C., Amin A., Thorson A.E., Bellare N.B., et al. Inclusion of pregnant women in COVID-19 treatment trials: a review and global call to action. Lancet Glob Health. 2021;9(3):e366–e371. doi: 10.1016/S2214-109X(20)30484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yap M., Debenham L., Kew T., Chatterjee S.R., Allotey J., Stallings E., et al. Clinical manifestations, prevalence, risk factors, outcomes, transmission, diagnosis and treatment of COVID-19 in pregnancy and postpartum: a living systematic review protocol. BMJ Open. 2020;10(12):e041868. doi: 10.1136/bmjopen-2020-041868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wells GA SB, O’Connell D, Peterson J, Welch V, Tugwell P, editor The NewcastleOttawa Scale (NOS) for assessing the quality of nonrandomised studies in metaanalyses [abstract]. 3rd Symposium on Systematic Reviews: Beyond the Basics; 2000; Oxford.
- 6.Cao D., Yin H., Chen J., Tang F., Peng M., Li R., et al. Clinical analysis of ten pregnant women with COVID-19 in Wuhan, China: A retrospective study. Int J Infect Dis. 2020;95:294–300. doi: 10.1016/j.ijid.2020.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L., Li Q., Zheng D., Jiang H., Wei Y., Zou L.i., et al. Clinical Characteristics of Pregnant Women with Covid-19 in Wuhan, China. N Engl J Med. 2020;382(25):e100. doi: 10.1056/NEJMc2009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen R., Zhang Y., Huang L., Cheng B.-H., Xia Z.-Y., Meng Q.-T. Safety and efficacy of different anesthetic regimens for parturients with COVID-19 undergoing Cesarean delivery: a case series of 17 patientsSécurité et efficacité de différents modes d’anesthésie pour des parturientes infectées par la COVID-19 accouchant par césarienne : une série de 17 cas. Can J Anaesth. 2020;67(6):655–663. doi: 10.1007/s12630-020-01630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu L., Yang Q., Shi H., Lei S., Liu X., Zhu Y., et al. Clinical presentations and outcomes of SARS-CoV-2 infected pneumonia in pregnant women and health status of their neonates. Sci Bull (Beijing) 2020;65(18):1537–1542. doi: 10.1016/j.scib.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Chen R, Wang J, Gong Y, Zhou Q, Cheng H-h, et al. Anaesthetic managment and clinical outcomes of parturients with COVID-19: a multicentre, retrospective, propensity score matched cohort study. medRxiv. 2020:2020.03.24.20042176.
- 11.F. Tang W. Luo X. Wang Z. Chen H. Li W. Liu et al. Hui and Liu, Weiyong and Zheng, Nannan and Hu, Xijiang and Li, Ran and Liu, Jie and Shao, Jianbo and Song, Qifa., An Observational Study of Intrauterine Vertical Transmission of SARS-CoV-2 in 20 Neonates 10.2139/ssrn.3618210
- 12.V.M. Savasi F. Parisi L. Patanè E. Ferrazzi L. Frigerio A. Pellegrino et al. Clinical Findings and Disease Severity in Hospitalized Pregnant Women With Coronavirus Disease 2019 (COVID-19) 136 2 2020 252 258 [DOI] [PubMed]
- 13.Maraschini A., Corsi E., Salvatore M.A., Donati S., ItOSS COVID-19 Working Group Coronavirus and birth in Italy: results of a national population-based cohort study. Ann Ist Super Sanita. 2020;56(3):378–389. doi: 10.4415/ANN_20_03_17. [DOI] [PubMed] [Google Scholar]
- 14.Ferrazzi E., Frigerio L., Savasi V., Vergani P., Prefumo F., Barresi S., et al. Vaginal delivery in SARS-CoV-2-infected pregnant women in Northern Italy: a retrospective analysis. BJOG. 2020;127(9):1116–1121. doi: 10.1111/1471-0528.16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertino E., Moro G.E., De Renzi G., Viberti G., Cavallo R., Coscia A., et al. Detection of SARS-CoV-2 in Milk From COVID-19 Positive Mothers and Follow-Up of Their Infants. Front Pediatr. 2020;8 doi: 10.3389/fped.2020.597699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biasucci G., Cannalire G., Raymond A., Capra M.E., Benenati B., Vadacca G., et al. Safe Perinatal Management of Neonates Born to SARS-CoV-2 Positive Mothers at the Epicenter of the Italian Epidemic. Front Pediatr. 2020;8 doi: 10.3389/fped.2020.565522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martínez-Perez O., Vouga M., Cruz Melguizo S., Forcen Acebal L., Panchaud A., Muñoz-Chápuli M., et al. Association between mode of delivery among pregnant women With COVID-19 and maternal and neonatal outcomes in Spain. JAMA. 2020;324(3):296. doi: 10.1001/jama.2020.10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pereira A., Cruz‐Melguizo S., Adrien M., Fuentes L., Marin E., Perez‐Medina T. Clinical course of coronavirus disease-2019 in pregnancy. Acta Obstet Gynecol Scand. 2020;99(7):839–847. doi: 10.1111/aogs.13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.San-Juan R., Barbero P., Fernández-Ruiz M., López-Medrano F., Lizasoáin M., Hernández-Jiménez P., et al. Incidence and clinical profiles of COVID-19 pneumonia in pregnant women: A single-centre cohort study from Spain. EClinicalMedicine. 2020;23:100407. doi: 10.1016/j.eclinm.2020.100407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Díaz-Corvillón P., Mönckeberg M., Barros A., Illanes S.E., Soldati A., Nien J.-K., et al. Routine screening for SARS CoV-2 in unselected pregnant women at delivery. PLoS ONE. 2020;15(9):e0239887. doi: 10.1371/journal.pone.0239887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vielma O SLA, Marcia; Bustos V, Juan Carlos; Assar, Rodrigo; Valdés P, Fernanda. Premature delivery in COVID-19 patients at San Juan de Dios Hospital / Parto prematuro en pacientes COVID-19 en Hospital San Juan de Dios. Rev chil obstet ginecol (En línea). 2020;85(supl.1): S59-S66, set. 2020. tab.
- 22.Alay I., Yildiz S., Kaya C., Yasar K.K., Aydin O.A., Karaosmanoglu H.K., et al. The clinical findings and outcomes of symptomatic pregnant women diagnosed with or suspected of having coronavirus disease 2019 in a tertiary pandemic hospital in Istanbul, Turkey. J Obstet Gynaecol Res. 2020 doi: 10.1111/jog.14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oncel M.Y., Akın I.M., Kanburoglu M.K., Tayman C., Coskun S., Narter F., et al. A multicenter study on epidemiological and clinical characteristics of 125 newborns born to women infected with COVID-19 by Turkish Neonatal Society. Eur J Pediatr. 2021;180(3):733–742. doi: 10.1007/s00431-020-03767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandt J.S., Hill J., Reddy A., Schuster M., Patrick H.S., Rosen T., et al. Epidemiology of coronavirus disease 2019 in pregnancy: risk factors and associations with adverse maternal and neonatal outcomes. Am J Obstet Gynecol. 2021;224(4) doi: 10.1016/j.ajog.2020.09.043. 389 e1-e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R. Khoury P.S. Bernstein C. Debolt J. Stone D.M. Sutton L.L. Simpson et al. Characteristics and Outcomes of 241 Births to Women With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection at Five New York City Medical Centers 136 2 2020 273 282 [DOI] [PubMed]
- 26.Health TMo. Profile of pregnant and children and adolecents with COVID-19. 2020.
- 27.Kayem G., Lecarpentier E., Deruelle P., Bretelle F., Azria E., Blanc J., et al. A snapshot of the Covid-19 pandemic among pregnant women in France. J Gynecol Obstet Hum Reprod. 2020;49(7):101826. doi: 10.1016/j.jogoh.2020.101826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anand P., Yadav A., Debata P., Bachani S., Gupta N., Gera R. Clinical profile, viral load, management and outcome of neonates born to COVID 19 positive mothers: a tertiary care centre experience from India. Eur J Pediatr. 2021;180(2):547–559. doi: 10.1007/s00431-020-03800-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopian M., Kashani-Ligumsky L., Czeiger S., Cohen R., Schindler Y., Lubin D., et al. Safety of vaginal delivery in women infected with COVID-19. Pediatr Neonatol. 2021;62(1):90–96. doi: 10.1016/j.pedneo.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lumbreras‐Marquez M.I., Campos‐Zamora M., Lizaola‐Diaz de Leon H., Farber M.K. Maternal mortality from COVID-19 in Mexico. Int J Gynaecol Obstet. 2020;150(2):266–267. doi: 10.1002/ijgo.13250. [DOI] [PubMed] [Google Scholar]
- 31.Vigel - de Gracia PV, Caballero LC, Sanchez J, Espinosa J, Campana S, Quintero A, et al. Pregnancies recovered from SARS-CoV-2 infection in second or third trimester: obstetric evolution. Ultrasound Obstet Gynecol. 2020;56(5):777-8. [DOI] [PMC free article] [PubMed]
- 32.Coronado-Arroyo J.C., Concepción-Zavaleta M.José., Zavaleta-Gutiérrez F.E., Concepción-Urteaga L.A. Is COVID-19 a risk factor for severe preeclampsia? Hospital experience in a developing country. Eur J Obstet Gynecol Reprod Biol. 2021;256:502–503. doi: 10.1016/j.ejogrb.2020.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7). Chin Med J (Engl). 2020;133(9):1087-95. [DOI] [PMC free article] [PubMed]
- 34.ICM Anaesthesia COVID-19. Management of pregnant women with known or suspected COVID-19 2020 [Available from: https://icmanaesthesiacovid-19.org/management-of-pregnant-women-with-known-or-suspected-covid-19.
- 35.Group RC, Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Royal College of Obstetricians and Gynaecologists. Coronavirus (COVID-19) infection and pregnancy 2021 [updated 19-02-2021. Available from: https://www.rcog.org.uk/en/guidelines-research-services/guidelines/coronavirus-pregnancy/.
- 37.Abani O., Abbas A., Abbas F., Abbas M., Abbasi S., Abbass H., et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh B, Ryan H, Kredo T, Chaplin M, Fletcher T. Chloroquine or hydroxychloroquine for prevention and treatment of COVID-19. Cochrane Database Syst Rev. 2021;2:CD013587. [DOI] [PMC free article] [PubMed]
- 39.Mockridge A., Maclennan K. Physiology of pregnancy. Anaes Intens Care Med. 2019;20(7):397–401. [Google Scholar]
- 40.Pacheco L.D., Saade G.R., Hankins G.D. Mechanical ventilation during pregnancy: sedation, analgesia, and paralysis. Clin Obstet Gynecol. 2014;57(4):844–850. doi: 10.1097/GRF.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 41.Tolcher M.C., McKinney J.R., Eppes C.S., Muigai D., Shamshirsaz A., Guntupalli K.K., et al. Prone positioning for pregnant women with hypoxemia due to coronavirus disease 2019 (COVID-19) Obstet Gynecol. 2020;136(2):259–261. doi: 10.1097/AOG.0000000000004012. [DOI] [PubMed] [Google Scholar]
- 42.Guérin C., Reignier J., Richard J.-C., Beuret P., Gacouin A., Boulain T., et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 43.Costantine M.M., Landon M.B., Saade G.R. Protection by exclusion: another missed opportunity to include pregnant women in research during the coronavirus disease 2019 (COVID-19) pandemic. Obstet Gynecol. 2020;136(1):26–28. doi: 10.1097/AOG.0000000000003924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith D.D., Pippen J.L., Adesomo A.A., Rood K.M., Landon M.B., Costantine M.M. Exclusion of pregnant women from clinical trials during the coronavirus disease 2019 Pandemic: A review of international registries. Am J Perinatol. 2020;37(08):792–799. doi: 10.1055/s-0040-1712103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.