Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines were developed, tested, and approved in record time. As patients with cancer were excluded from vaccine trials, some patients may be hesitant,1 given unanswered questions around safety and adverse reactions, especially those undergoing treatment. One study conducted among 134 patients receiving immune checkpoint inhibitors (ICIs) reported an 81% BNT162b2 vaccine uptake rate with similar rates of acute adverse reactions as reported among healthy individuals, except for higher frequency of myalgias among patients with cancer.2 Further research is needed by tumor type and treatment to better inform clinicians and patients during the vaccine decision-making process.
We report data on uptake and perspectives on SARS-CoV-2 vaccination and postvaccination adverse reactions in 208 recently diagnosed patients with cancer (median age 63 years, 52.4% women, 33.2% non-White minorities, Table 1 ) at a large healthcare system in Los Angeles spanning the timeline from limited vaccine availability to broader dissemination (November 2020 to July 2021). Vaccine hesitancy and perspectives were measured using a modified version of the World Health Organization Vaccine Hesitancy Scale (Supplementary Material, available at https://doi.org/10.1016/j.annonc.2021.10.005).3 A self-administered symptoms questionnaire was given to vaccinated recipients after dose 1 (D1) and D2 for messenger RNA (mRNA) SARS-CoV-2 vaccines. Electronic medical records provided correlative clinical information. Chi-square tests were used to assess differences for categorical variables and a Wilcoxon rank-sum test for continuous variables (Stata v. 15.1). All tests were two-sided and considered statistically significant at P < 0.05.
Table 1.
Demographic and clinical characteristics of patients by vaccination status
| Characteristicsa | Total (n = 208) | Unvaccinated (n = 59) | Vaccinated (n = 149) | P value |
|---|---|---|---|---|
| Age in years, median (IQR) | 63 (54-72) | 60 (51-69) | 66 (56-72) | 0.02 |
| Sex | 0.37 | |||
| Male | 99 (47.6) | 31 (52.5) | 68 (45.6) | |
| Female | 109 (52.4) | 28 (47.5) | 81 (54.4) | |
| Race/ethnicity | 0.39 | |||
| White | 135 (64.9) | 36 (61.0) | 99 (66.4) | |
| Black/African American | 12 (5.8) | 1 (1.7) | 11 (7.4) | |
| Asian | 19 (9.1) | 6 (10.2) | 13 (8.7) | |
| Hispanic/Latinx | 31 (14.9) | 12 (20.3) | 19 (12.8) | |
| Other/mixed | 7 (3.4) | 2 (3.4) | 5 (3.4) | |
| Declined to state | 4 (1.9) | 2 (3.4) | 2 (1.3) | |
| Education | 0.03 | |||
| High-school diploma/GED or less | 21 (10.1) | 5 (8.5) | 16 (10.7) | |
| Some college/2-year degree/4-year college | 85 (40.9) | 32 (54.2) | 53 (35.6) | |
| School beyond college/graduate/professional degree | 81 (38.9) | 16 (27.1) | 65 (43.6) | |
| Missing | 21 (10.1) | 6 (10.2) | 15 (10.1) | |
| Current employment status | 0.003 | |||
| Employed | 64 (30.8) | 18 (30.5) | 46 (30.9) | |
| Retired | 68 (32.7) | 10 (16.9) | 58 (38.9) | |
| Unable to work: disability/caregiver | 33 (15.9) | 16 (27.1) | 17 (11.4) | |
| Unemployed | 17 (8.2) | 8 (13.6) | 9 (6.0) | |
| Not sure/decline to state | 6 (2.9) | 1 (1.7) | 5 (3.4) | |
| Missing | 20 (9.6) | 6 (10.2) | 14 (9.4) | |
| Income last year before taxes | 0.91 | |||
| Less than $25 000 | 21 (10.1) | 7 (11.9) | 14 (9.4) | |
| $25 000-$49 999 | 18 (8.7) | 6 (10.2) | 12 (8.1) | |
| $50 000-$74 999 | 22 (10.6) | 5 (8.5) | 17 (11.4) | |
| $75 000 or more | 68 (32.7) | 18 (30.5) | 50 (33.6) | |
| Not sure/decline to state | 51 (24.5) | 15 (25.4) | 36 (24.2) | |
| Missing | 28 (13.5) | 8 (13.6) | 20 (13.4) | |
| ECOG score | 0.15 | |||
| 0: fully active | 94 (45.2) | 22 (37.3) | 72 (48.3) | |
| 1: restricted in physically strenuous activity but ambulatory | 100 (48.1) | 35 (59.3) | 65 (43.6) | |
| 2: ambulatory but unable to carry out work activities | 8 (3.8) | 2 (3.4) | 6 (4.0) | |
| Unknown/not available | 5 (2.4) | 0 (0.0) | 5 (3.4) | |
| Missing | 1 (0.5) | 0 (0.0) | 1 (0.7) |
ECOG, Eastern Cooperative Oncology Group; GED, general equivalency diploma; IQR, interquartile range.
All values are frequencies and column percentages unless otherwise specified. Missing categories do not contribute to P value estimates.
Overall, 128 individuals had solid tumors and 80 hematological malignancies [median time since diagnosis 2.5 (interquartile range 1.0-5.5) years]. The majority of patients (n = 156, 75%) were within 6 months of diagnosis and receiving one or more treatments [ICI: n = 77 (37.0%); chemotherapy (CT): n = 48 (23.1%); B-cell targeted therapies (BCT): n = 64 (30.8%); aromatase inhibitor/hormone therapy: n = 38 (18.3%); allogeneic hematopoietic stem cell transplant: n = 16 (7.7%)]. Approximately 6% (n = 13) of participants had a prior SARS-CoV-2 infection. Overall, 7.3% reported intention to delay or abstain from vaccination (hematologic malignancy: 7.4% versus solid tumor: 7.3%, P value = 0.99). Among unvaccinated patients, women were more hesitant than men (35.7% versus 12.9%, P = 0.06). The most common concerns among hesitant patients were fear of adverse reactions (n = 8, 57.1%), rushed vaccine development (n = 6, 42.9%), and insufficient knowledge (n = 9, 64.3%).
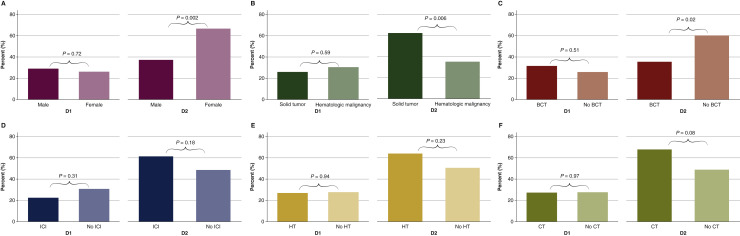
Eighty-three participants (39.9%) received Pfizer-BioNTech-BNT162b2, 66 (31.7%) received Moderna-mRNA-1273, and 59 were unvaccinated (28.4%) at the time of the survey. Older (P = 0.02), highly educated (P = 0.03), and retired (P = 0.003) individuals were more likely to be vaccinated (Supplementary Material, available at https://doi.org/10.1016/j.annonc.2021.10.005). Adverse reactions were common (D1: 27.6% and D2: 53.5%). There were no significant differences in adverse reactions by age, mRNA vaccine type, or prior SARS-CoV-2 infection. We found that the Eastern Cooperative Oncology Group (ECOG) score was not associated with adverse reactions after D1 (P = 0.72), but being fully active was associated with a greater number of reported adverse reactions after D2 (P = 0.005). Women experienced more pain compared with men (D2: 66.7% versus 37.3%; P = 0.002), including pain (P = 0.02) and fatigue/malaise (P = 0.003; Figure 1 ). Patients with solid tumors also reported more symptoms compared with those with hematologic malignancies (D2: 62.3% versus 35.1%; P = 0.006); more specifically, symptoms were less frequent among those receiving BCTs compared with those not on BCTs (D2: 35.5% versus 60.2%; P = 0.02). Fevers were more common among patients receiving ICIs compared with those not on ICIs (D2: 34.1% versus 11.4%, P = 0.003). Women on hormone therapy frequently reported muscle/bone/joint or nerve symptoms (D2: 11.5% versus 2.0%; P = 0.025). Patients on CT reported muscle/bone/joint/nerve (D1: 15.2% versus 0%; P < 0.001) and cough/chest or breathing symptoms (D2: 10.7% versus 1.2%; P = 0.02).
Figure 1.
Acute side-effects after vaccination among patients with cancer by (A) Sex: male versus female. (B) Cancer type: solid versus hematologic malignancies. (C) Treatment: B-cell targeted therapy (BCT) versus no BCT. (D) Treatment: immune checkpoint inhibitors (ICI) versus no ICI. (E) Treatment: hormone therapy (HT) versus no HT. (F) Treatment: cytotoxic chemotherapy (CT) versus no CT.
Our study shows that postvaccination adverse events varied by tumor type and treatment. The report of adverse events in this study may be subject to recall bias and the potential influence of the media. Previous studies have suggested that patients receiving combined ICI therapy (anti-PD-1, anti-PD-L1, anti-CTLA-4) should be closely monitored for increased immune-related adverse reactions during vaccine administration.4 , 5
Addressing specific vaccine concerns among patients with cancer early may ensure high compliance, in particular among women. The findings discussed here may be generalizable to patients with cancer in other metropolitan, multiethnic populations. Future research is needed on patient and provider concerns regarding immunogenicity of SARS-CoV-2 vaccines among patients on various cancer therapies, especially as booster vaccinations are now recommended for individuals with underlying medical conditions including cancer.
Acknowledgements
We sincerely appreciate all SeroNet-CORALE participants and each member of the study (www.corale-study.org) and biospecimen management group. We are also grateful to all the front-line health care workers in our health care system who continue to be dedicated to delivering the highest quality care for all patients.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health [grant number U54CA260591].
Disclosure
JG holds a consultant or advisory role at EMD Serono; Elsevier; Exelixis; QED Therapeutics; Natera, Basilea, HalioDx, Eisai, Janssen. KLR holds a consultant or advisory role at Amgen, AstraZeneca, Blueprint, Boehringer Ingelheim, Daiichi Sankyo, EMD Serono, Genentech, GSK, Janssen, Lilly, Merck KGA, Mirati, Seattle Genetics, Takeda. JD holds a consultant or advisory role at Kite Pharma and MorphoSys. RB holds a consultant or advisory role at Genomic Health, Astra Zeneca, Biotheranostics, Pfizer; she is on the Speakers Bureau for Genentech, Seattle Genetics; she also has research funding from Seattle Genetics, Ichnos Biosciences, Merck. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Barriere J., Gal J., Hoch B., et al. Acceptance of SARS-CoV-2 vaccination among French patients with cancer: a cross-sectional survey. Ann Oncol. 2021;32:673–674. doi: 10.1016/j.annonc.2021.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waissengrin B., Agbarya A., Safadi E., et al. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021;22:581–583. doi: 10.1016/S1470-2045(21)00155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domek G.J., O’Leary S.T., Bull S., et al. Measuring vaccine hesitancy: field testing the WHO SAGE Working Group on Vaccine Hesitancy survey tool in Guatemala. Vaccine. 2018;36:5273–5281. doi: 10.1016/j.vaccine.2018.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo B., Li J., Hou X., et al. Indications for and contraindications of immune checkpoint inhibitors in cancer patients with coronavirus disease 2019 vaccination. Future Oncol. 2021;17:3477–3484. doi: 10.2217/fon-2021-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maio M., Hamid O., Larkin J., et al. Immune checkpoint inhibitors for cancer therapy in the COVID-19 era. Clin Cancer Res. 2020;26:4201–4205. doi: 10.1158/1078-0432.CCR-20-1657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.