Abstract
Study design:
Prospective longitudinal study of novel growth modulation system for early adolescent idiopathic scoliosis (AIS), consecutive case series from first human use to study endpoint at skeletal maturity, fusion, or five years postoperation.
Objectives:
Determine adverse events and curvature changes to end of study; examine factors most likely to explain variability in curve changes.
Summary of background data:
Pilot clinical safety study was performed under US Food and Drug Administration (FDA) Investigational Device Exemption (IDE). Safety and radiographic results were previously reported to 24 months postoperation.
Methods:
Subjects with early AIS underwent thoracoscopic placement of titanium clip-screw devices designed to modify growth asymmetrically. Eligibility was based on high risk of progression to 50°: single major thoracic curve 25°–40°, Risser 0, open triradiate cartilages, and premenarchal if female. Six subjects, the maximum allowed, enrolled. Adverse events (AEs), clinical outcomes, and curvatures were systematically collected. Disc heights, vertebral heights, and implant-bone contact areas were assessed.
Results:
Consecutive subjects enrolled, aged 12.1 years (±1.7), three were female. AEs from two to five years postoperation included deformity changes leading to a second surgery in three patients: two for posterior spinal fusion, and one for thoracoscopic removal of half the implants for overcorrection. One patient, whose curve exceeded 50° at 18 years, did not choose fusion. Major thoracic curves were 34° (±3°) preoperatively and 42° (±20°) at end of study.
Conclusions:
In a study of spine growth modulation in patients with early AIS with high risk of progression, at skeletal maturity or five years postoperation, major thoracic curves of half progressed to >50°, whereas curves of the other half remained <40°, below fusion indications. Removal of selected implants may halt overcorrection. The next, pivotal, study phase was approved by FDA.
Level of Evidence:
IV: Prospective case series under stringent regulatory controls
Keywords: Adolescent idiopathic scoliosis, Spine growth modulation, Safety, Skeletal maturity endpoint, Fusionless
Introduction
Current surgical methods of scoliosis correction are highly invasive. Skeletal immaturity and curve magnitude are widely considered to affect curve progression in adolescent idiopathic scoliosis (AIS) [1-6]. Bracing decreases frequency of progression to surgical indications, but not all curves respond. Curve type and magnitude, skeletal maturity, gender, and compliance are associated with bracing effectiveness [7-17]. When bracing fails, instrumented posterior spinal fusion (PSF) corrects the curve, but at the expense of spine flexibility and health of adjacent segments. Toward reducing rates of PSF in patients with late juvenile to early AIS, whose curves are most likely to progress and who are more likely to fail bracing, methods are under investigation to counteract progression by modulating spine growth using less invasive surgical methods.
The interaction between compressive stress and growth, that is, Hueter-Volkmann principle, has long been implicated in scoliosis progression, as well as suggested as the basis of potential treatments [18-23]. Several preclinical studies using different device types have induced curvatures in normal, straight, quadruped spines [24-29]. In one series, thoracic spine curvatures were created in porcine spines using an implant-screw construct [27]. Physeal hypertrophic zone and cell heights were asymmetrically reduced in a pattern that indicated coronal plane gradients in compression and vertebral growth [30].
After changes to implant and procedures for clinical use, a prospective safety study was conducted in six young adolescents with progressive AIS under a US Food and Drug Administration (FDA) Investigational Device Exemption (IDE) [31]. Safety and radiographic results 24 months postoperatively were previously reported [32]. These early results showed the method was safe. Consequently, approval was granted for the second phase, and IDE pivotal study, to survey probable benefit and humanitarian use. The purpose of the present investigation was to document adverse events and curvatures after 24 months to end of study at skeletal maturity or five years postoperation in the pilot study. Further, to help guide changes between this pilot and the next pivotal studies, the factors most likely to have affected final curve magnitudes were also examined.
Material and Methods
Prospective pilot safety study of a novel treatment was performed, consecutive case series from first human use, approved by IRB and US FDA under an IDE, with informed parental consent and child assent. Longitudinal clinical and radiographic review was conducted of six patients, the allowed maximum, with progressive AIS who underwent thoracoscopic implantation of titanium clip-screw devices (SpineForm, LLC, Cincinnati OH). Protocols from recruitment through 24 months were reported [32]. After 24 months, evaluations were scheduled biannually until end of study at either: 1) skeletal maturity, defined by height velocity <1 cm/year; and/or 2) PSF; or 3) 60 months postoperation. Study procedures complied with national and international standards (Good Clinical Practice; ISO 14155). A data monitoring committee (DMC) of three pediatric orthopaedic spine surgeons determined if patients qualified for the study, independently measured curve magnitudes, and adjudicated adverse events (AEs).
Eligibility criteria were designed to include only patients with curves most likely to progress. Inclusion criteria were idiopathic scoliosis, single major thoracic curve (Lenke 1A or B), magnitude 25°–40°, chronologic age ≥10 years, premenarchal if female, and skeletal immaturity (Risser 0, open triradiate cartilages, bone age by Atlas Matching method [33] between ≥8 years + 10 months and ≤13 years for females, and ≥10 years and ≤15 years for males). Exclusion criteria were any contraindication to thoracoscopy and previously reported factors [32]. Perioperative and operative procedures were reported [32,34,35].
Clinical and outcomes tests were administered biannually, with systematic assessment of adverse events. Patient-reported quality of life was measured with the Scoliosis Research Society questionnaire (SRS-22r). Major thoracic (MT), proximal thoracic (PT), lumbar/thoracolumbar (L/TL), and thoracic kyphosis (TK) curve magnitudes were measured using Cobb angles. Two measurements for each curve magnitude were averaged. For differences >8°, a measurement from a third reviewer was added. Final curve magnitudes were compared with preoperative values, with progression referring to an increase to surgical threshold, correction as a decrease from preoperative, and maintenance between the two. Skeletal maturity was defined by height velocity based on difference in heights between visits 6 months apart [14]. Summary statistics on clinical and radiographic outcomes were generated with the proviso that power to determine differences in any outcome variable is not part of the design of a pilot safety study.
Retrospectively, disc heights, vertebral heights, vertebral cross-sectional areas, and contact areas between implant blades and vertebral bone were measured radiographically. Disc heights were measured on concave and convex sides in posteroanterior (PA) radiographs using image analysis software (Photoshop, Adobe). Vertebral heights on concave and convex sides were measured independently from disc heights using a clinical digital radiographic system. Width and depth of each vertebra were measured, then total vertebral area was calculated assuming an elliptical cross-section. Blade length and width in contact with bone were measured, and relative contact area was determined as the ratio between the blade-to-bone contact area (Abc) and total vertebral cross-sectional area (Av). Correlations between side-to-side differences in disc heights and vertebral heights versus final MT curve magnitudes, and between contact area ratios and final MT curve magnitudes, were determined using Pearson correlation and regression (SAS 9.4; SAS, Cary NC).
Results
The first six eligible patients enrolled: three females (11.9 years ±1.5) and three males (12.4 years ±2.2). Major thoracic curvatures averaged 34° (±3°) preoperatively, SRS-22r score was 4.4, and VAS score was 1.25 (Table 1). End of study time points ranged from 24 to 60 months (Table 2). Reasons for end of study were skeletal maturity (2), PSF (n=2), skeletal maturity plus indications for PSF (n=1), and five years postoperation plus low curve magnitude (n=1). At end of study, SRS-22r score was 3.7 (range 3.2 – 4.1), and VAS score was 2.9 (range 0.3 - 5) (Table 2). Major thoracic curve outcomes ranged from progression, to maintenance, to correction and local overcorrection (Fig. 1). Removal of selected implants in the case of local overcorrection appeared to halt overcorrection (Fig. 1C, top right) in the 14 months between explant and end of study period.
Table 1.
Selected subject demographics and preoperative clinical and radiographic parameters.*
| Subject ID | S1 | S2 | S3 | S4 | S5 | S6 |
|---|---|---|---|---|---|---|
| Demographics and pre-operative clinical parameters | ||||||
| Gender | F | F | M | M | F | M |
| Age (years) at surgery | 13.0 | 12.6 | 11.7 | 10.6 | 10.1 | 14.9 |
| Bone age at surgery (years) Site / DMC | 12 / 11 | 12 / 11 | 13.5 / 12 | 10 / 10 | 11.5 / 12 | 13.5 / 13 |
| Height (cm) | 151 | 141 | 174 | 134 | 139 | 164 |
| Weight (kg) | 40.2 | 33.9 | 54.0 | 26.5 | 52.7 | 50.9 |
| BMI | 17.7 | 17.0 | 17.9 | 14.7 | 27.4 | 19 |
| BMI (%-ile age, gender) | 35 | 28 | 55 | 8 | 98 | 39 |
| Menarchal status | Pre | Pre | NA | NA | Pre | NA |
| Risser grade | 0 | 0 | 0 | 0 | 0 | 0 |
| Triradiate status | Open | Open | Open | Open | Open | Open |
| Height velocity (cm/year), based on immediately pre-op to 6 month post-op visit | 8.8 | 5.8 | 4.8 | 8.0 | 7.8 | 10.8 |
| Clinical outcome score (SRS-22r) | 4.4 | 4.5 | 4.7 | 3.5 | 4.6 | 4.5 |
| Back pain, VAS (cm) | 0.8 | 4.3 | 0.2 | 0.2 | 1.5 | 0.5 |
| Preoperative curve parameters | ||||||
| Curve type (Lenke) | 1A | 1A | 1A | 1A | 1A | 1B |
| Major thoracic (MT) curvature (deg) | 35 | 30.5 | 35 | 36 | 31 | 38.5 |
| Levels, MT | T6 - L1 | T6 - T12 | T5 - T10, T6-T11 | T5 - T11, T6-T11 | T5 - T10, T5-T11 | T7 - T12 |
| Bend angle, MT, (deg), R/L | 15/38 | 2/25 | 18.5/40 | 4/37 | 10/29 | 12/36 |
| Flexibility (%) = 100 x [1-CobbRtBend/CobbStanding preop] | 57 | 93 | 47 | 89 | 68 | 69 |
| Thoracolumbar/lumbar (TL/L) curvature (deg) | 24 | 26 | 18.5 | 27.5 | 18.5 | 28 |
| Levels, TL/L | L1-L4, L1-L5 | T12-L4, L1-L4 | T10-L4, T11-L4 | T11-L4 | T10-L3, T11-L4 | T12-L4 |
| Proximal thoracic (PT) curvature (deg) | 14 | 12 | 25.5 | 17 | 19 | 22 |
| Levels, PT | T1-T5, T2-T6 | T2-T6 | T1-T5, T2-T5 | T2-T5, T2-T6 | T1-T5, T2-T5 | T3-T6, T3-T7 |
| Thoracic kyphosis (TK)(deg) | 33.5 | 27.7 | 10.5 | 22.5 | 25.5 | 18.0 |
| Levels, TK | T3-T12 | T3-T12, T1-T12, T3-L1 | T4-T12, T5-T12 | T4-T12, T3-T12 | T3-T12, T2-T12 | T3-T12, T2-T12 |
| Apical trunk rotation (deg) | 10 | 13 | 12 | 12 | 9 | 8 |
| Coronal balance (mm) | −9.5 | −4.5 | −9.0 | −3.0 | −13.0 | 21.5 |
BMI, body mass index; DMC, data monitoring committee; NA: not applicable; TK, thoracic kyphosis; VAS, visual analog scale.
Reprinted, in part, from Reference [32], with permission from Elsevier
Table 2.
Clinical and radiographic parameters at end of study time point for each subject.
| Subject ID | S1 | S2 | S3 | S4 | S5 | S6 |
|---|---|---|---|---|---|---|
| Demographics and clinical parameters at end of study or nearest previous visit | ||||||
| Reason for end of study | MT>50°, Height velocity <1 cm/year, PSF* at 39 mos | Height velocity <1 cm/year | MT>50°, PSF* | 5 years post-op | Height velocity <1cm/year | Height velocity <1cm/year, MT >50° |
| Postop time to end of study visit (months) | 36 | 48 | 24 | 60 | 42 | 48 |
| Age (years) | 16.1 | 16.6 | 13.5 | 15.5 | 14.2 | 18.8 |
| Height (cm) | 160 | 151 | 184 | 168 | 150 | 177 |
| Weight (kg) | 56 | 47.9 | 56.8 | 67.4 | 78.4 | 74.2 |
| BMI | 21.9 | 21.1 | 16.8 | 23.9 | 33 | 23.7 |
| BMI (%ile for age, gender) | 66 | 55 | 16 | 84.8 | 98 | 65 |
| Time from menarche (months) | 28 | 37 | NA | NA | 18 | NA |
| Risser grade, from radiologist report | 2 | 4 | 4-5 | 3 | 4 | 4 |
| Triradiates | Indeterminate / Closed | Closed | Closed | Closed | Closed | Closed |
| Height velocity, based on 6 months previous to end of study visit (cm/year) | 0 | 0.25 | 2.1 (prior to PSF) | 1.2 | 0.6 | 0 |
| Skeletally mature at end of study visit | Yes | Yes | No | No | Yes | Yes |
| Bracing | No | No | No | No | No | No |
| Other treatment | No | Massage/Manual therapy weekly | No | Shoe inserts | No | Physical therapy |
| Neurological impairment | No | No | No | No | No | No |
| Clinical outcome score (SRS-22r) | 3.6 | 3.9 | 3.4 | 4.1 | 4 (at last scoring at 36 mos) | 3.2 |
| Back pain, VAS (cm) | 4.6 | 5.5 | 0.3 | 0.8 | 1 (at last scoring at 36 mos) | 5 |
| Curve parameters | ||||||
| Major thoracic (MT) curvature (deg) | 59 | 32 | 61 | 9 | 39 | 53 |
| Change in MT curvature from pre-op (deg) | 24 | 1.5 | 26 | −27 | 8 | 14.5 |
| Thoracolumbar/lumbar (TL/L) curvature (deg) | 34 | 33 | 22 | 0 | 36 | 39 |
| Proximal thoracic (PT) curvature (deg) | 28 | 18 | 31 | −34 | 20 | 37 |
| Thoracic kyphosis (TK) (deg) | 33 | 32 | 17 | 46 | 25 | 21 |
| Levels, TK | T2-T12 | T3-T12 | T3-T12, T4-T12, T2-L2 | T2-T12 | T3-T12 | T3-T12 |
| Apical trunk rotation (deg) | 16 | 10 | 9 | 0 | 12 | 11 |
| Coronal balance (mm) | 17 | −1 | 9 | −14 | −5 | −2 |
| Rate of change of MT curvature from immediate postop to end of study visit (deg/year) | 8.0 | 0.4 | 13 | −5.4 | 2.3 | 3.6 |
BMI, body mass index; NA, not applicable; TK, thoracic kyphosis; VAS, visual analog scale.
PSF: Posterior spinal fusion after MT curve > 50°.
Fig. 1.
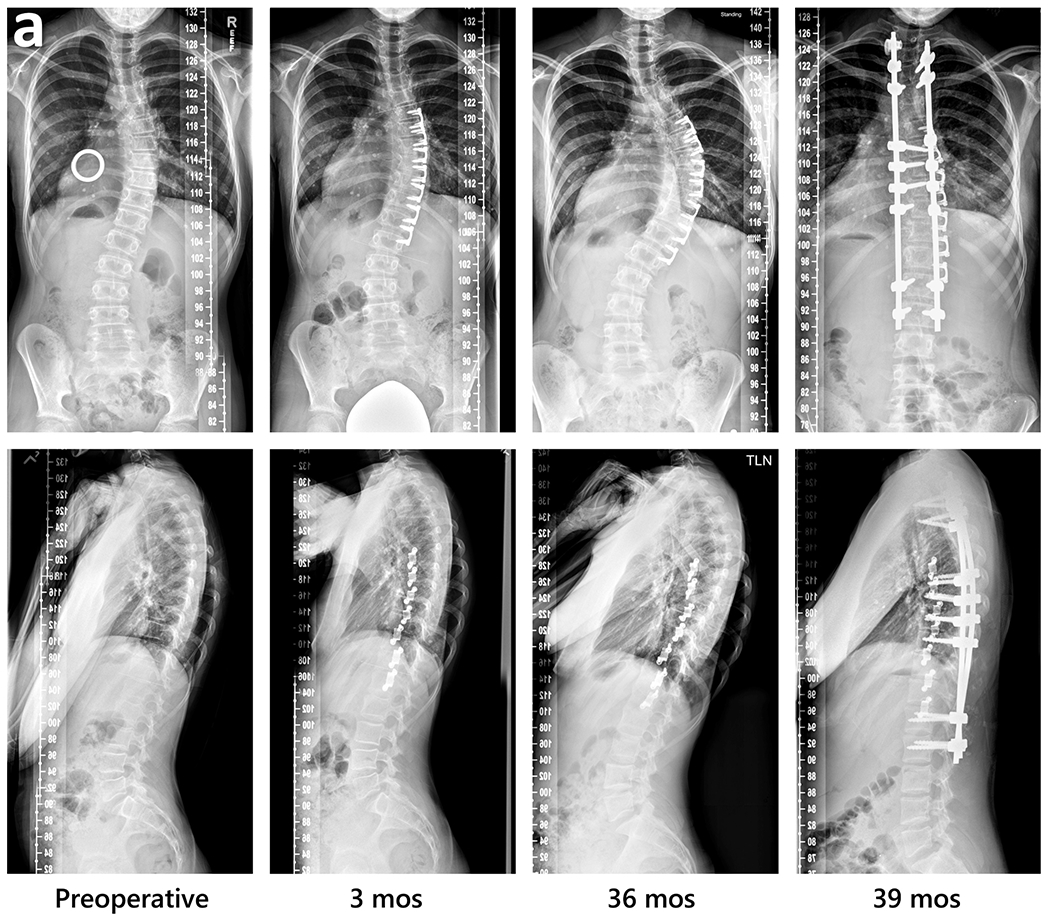
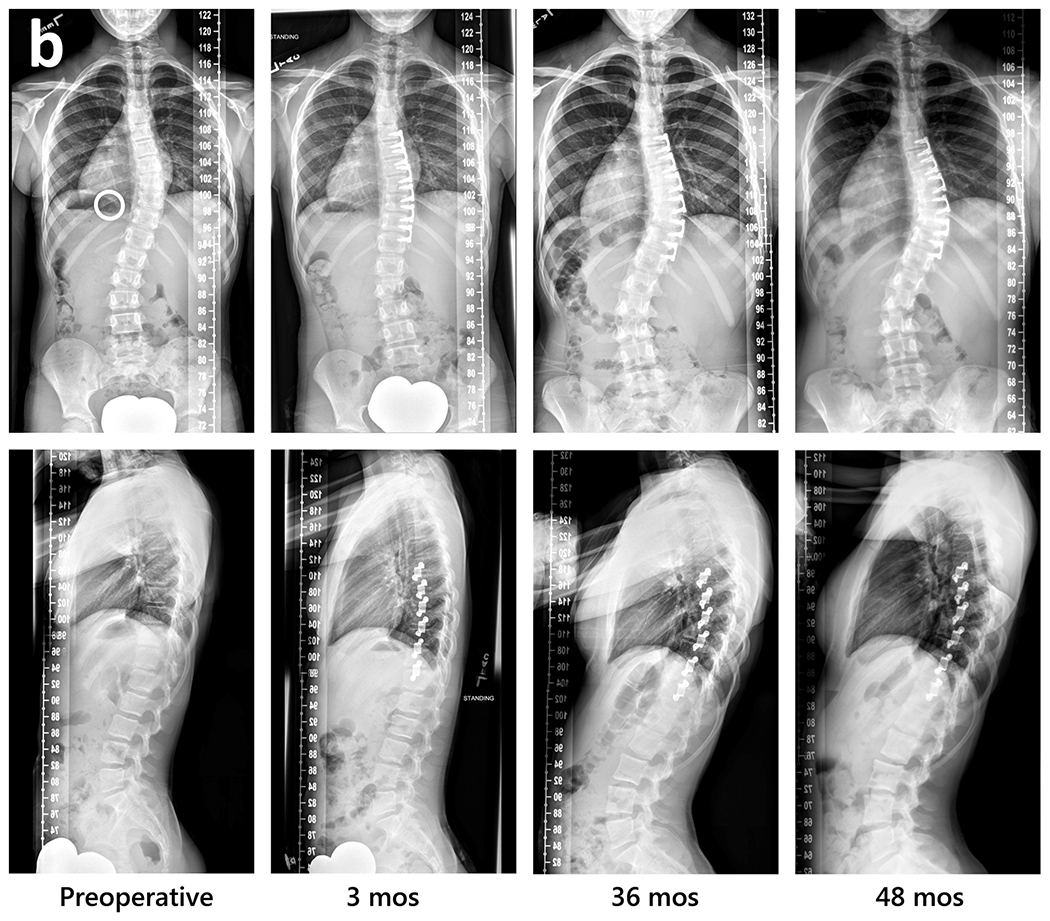
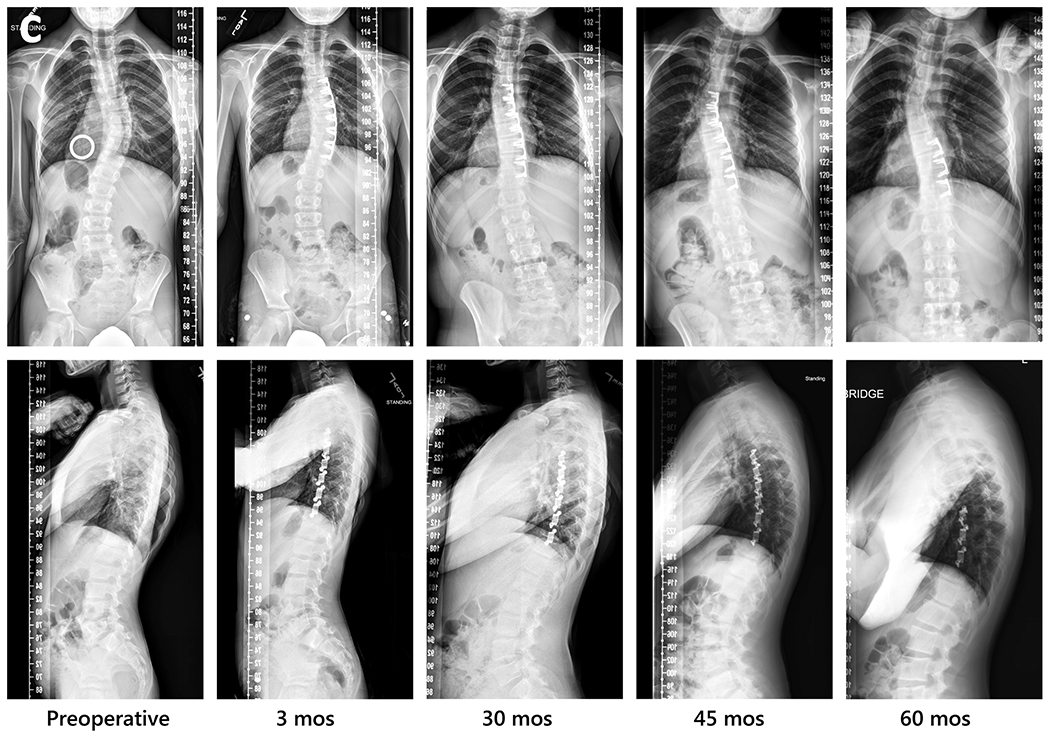
Radiographic sequences of three subjects, showing examples of major thoracic curve progression to surgical threshold, maintenance, and correction beyond preoperative curve magnitude followed by local overcorrection then selective implant removal.
a. Curve progression to PSF at three years (S1); Left to right: immediate preoperative (with calibration ring used for accurate measurement of linear spine dimensions to choose implant size), 3 months postoperative, 36 months, and 39 months. PSF, posterior spinal fusion.
b. Curve maintenance, with difference from preoperative magnitude <8° (S2). Left to right: preoperative, 3 months postoperative, 36 months, and 48 months.
c. Curve correction and overcorrection proximally, with second surgery at 46 months for selective removal of proximal 3 implants (S4). Left to right: preoperative, 3 months postoperative, 30 months, 45 months, and 60 months. In sagittal view sequence, note improvement in rib asymmetry with sustained reduction of rib hump. Reprinted in part (Fig. 1c preoperative): Posteroanterior (PA) view from Wall et al. [35], figure 45.9, with permission from Springer Nature; PA and lateral views from Wall et al [32], figure 7, with permission from Elsevier.
Adverse events in the early postoperative period were mostly pulmonary and resolved [32]. After 24 months, AEs classified as serious were spine deformity changes leading to second surgical procedures in three subjects. Two of the second surgeries were for PSF. The remaining one was for removal of three of six devices for curve overcorrection. Additionally, curvature increased to >50° in one subject who, then 18 years old, did not choose to undergo PSF. Other AEs, not categorized as serious, included pain (back, upper, lower, incision site, calves, shoulder), numbness at incision site, or muscle spasms. Mild device migration, noted in one subject prior to 24 months and PSF, was noted as no longer occurring at 36 months. No neurological deficits, and no device breakage or gross loosening were noted at any time point. During device explant, fluoroscopy was used to identify the implants; then thoracoscopic surgical procedures were used. Electrocautery and an elevator were used to clear the overlying bone. Bone reaction was estimated to occur over 50% of the implants to be removed, at a thickness of approximately 1 mm. The view of the area revealed no granulation tissue, bursal fluid, discoloration, implant debris, scar tissue, infection, or inflammation beyond standard. Estimated blood loss of 100 mL occurred during removal.
Radiographically, major thoracic curve magnitudes immediately preoperative, immediately postoperative, at 24 months, and at end of study were 34° (±3°), 24° (±8°), 39° (±18°), and 42° (±20°), respectively. Intrasubject change from preoperative to end of study was 7.7° (±19°). At end of study for each subject, MT curve magnitudes in order of increasing curvature were: 9°, 32°, 39°, 53°, 59°, and 61° (Fig. 2). For the latter two values, magnitudes just prior to the second surgical procedure were used for those who underwent PSF. Intrasubject changes in MT curvature from preoperative to the end of study time point for each subject were, respectively, [−27]°, 1.5°, 8°, 14.5°, 24°, and 26°. Rates of change of MT curvature from immediate postoperative to end of study visit were −5.4°/year, 0.4°/year, 2.3°/year, 3.6°/year, 8.0°/year, and 13°/year.
Fig. 2.
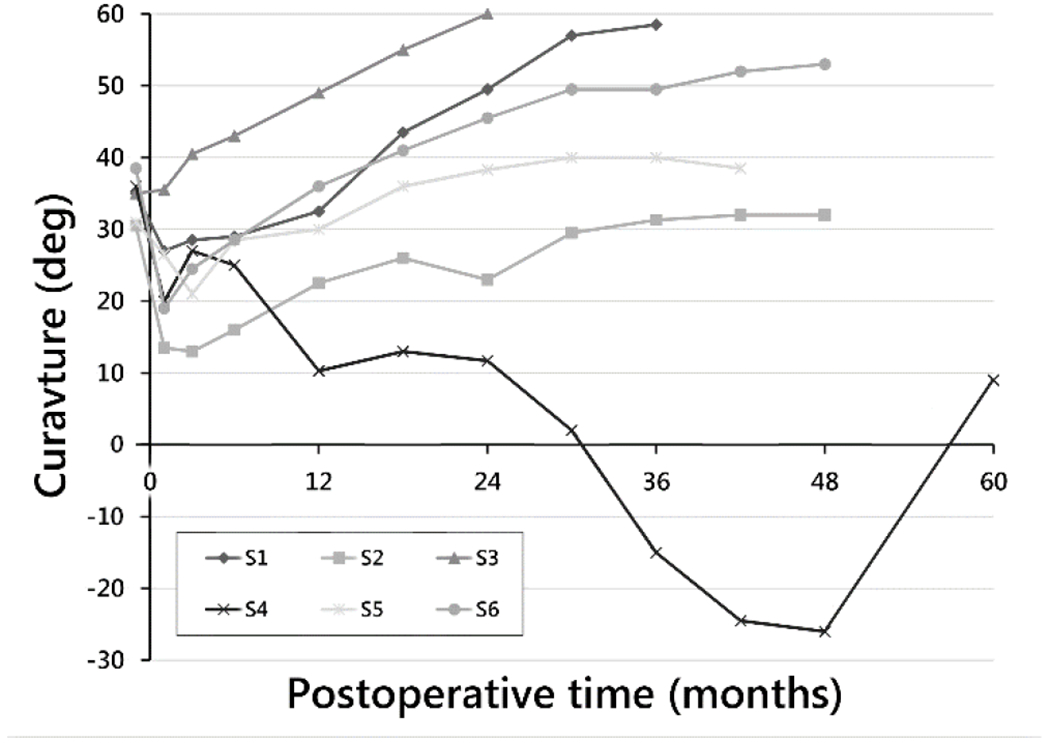
Major thoracic curve magnitude by postoperative time to end of study time point for each subject.
Lumbar curve magnitudes immediately preoperative, immediately postoperative, at 24 months, and at end of study were 24° (±4°), 17° (±4°), 28° (±7°), and 27° (±15°), respectively. L/TL curvatures (Fig. 3) at each subject’s end of study time point were, again in the order of increasing MT curvatures for comparison: 0°, 33°, 36°, 39°, 34°, and 22°. Intrasubject changes in L/TL curvature from preoperative to end of study time point were: [−27.5°], 7°, 17°, 11°, 10°, and 3.5°.
Fig. 3.
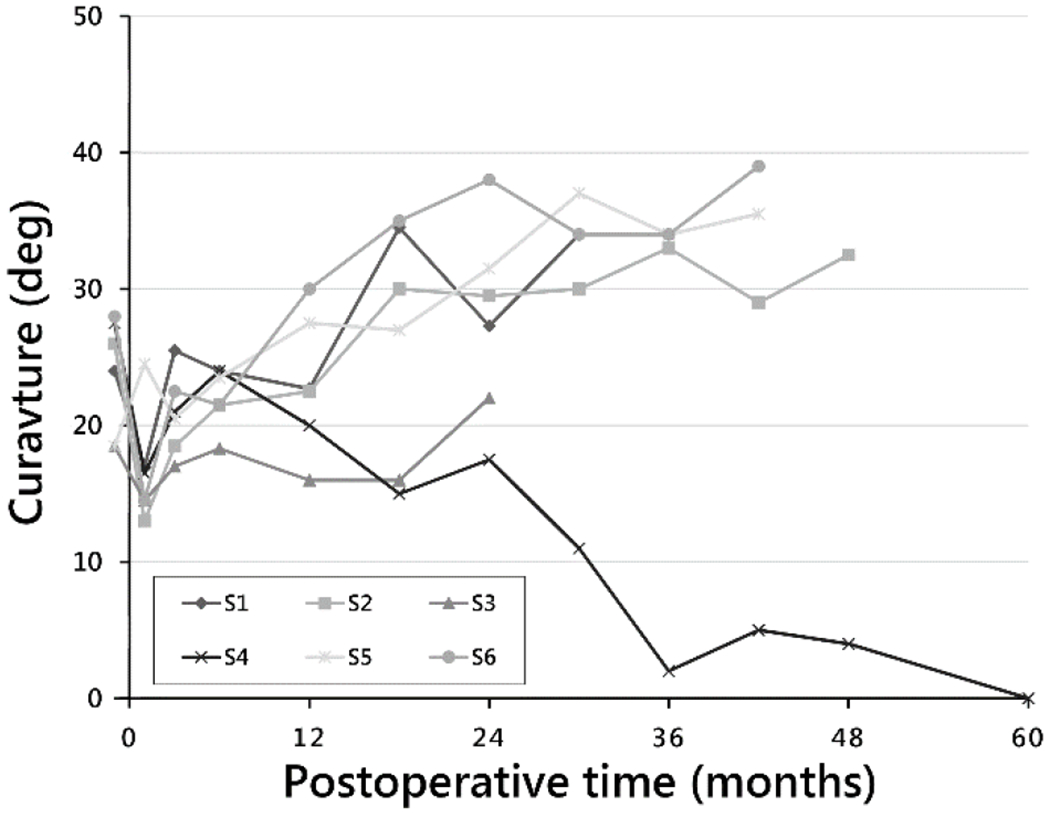
Lumbar curve magnitude by postoperative time to end of study time point for each subject.
In the sagittal plane, thoracic kyphosis curve magnitudes immediately preoperative, immediately postoperative, at 24 months postoperative, and at end of study were 23° (±8°), 19° (±11°), 27° (±6°), and 29° (±10°), respectively. TK magnitudes (Fig. 4) at end of study were, in order of increasing MT curvatures: 46°, 32°, 25°, 21°, 34°, and 17°. Intrasubject changes in TK from preoperation to end of study were 23.5°, 4.3°, [−0.5°], 3°, [−0.5°], and 6°.
Fig. 4.
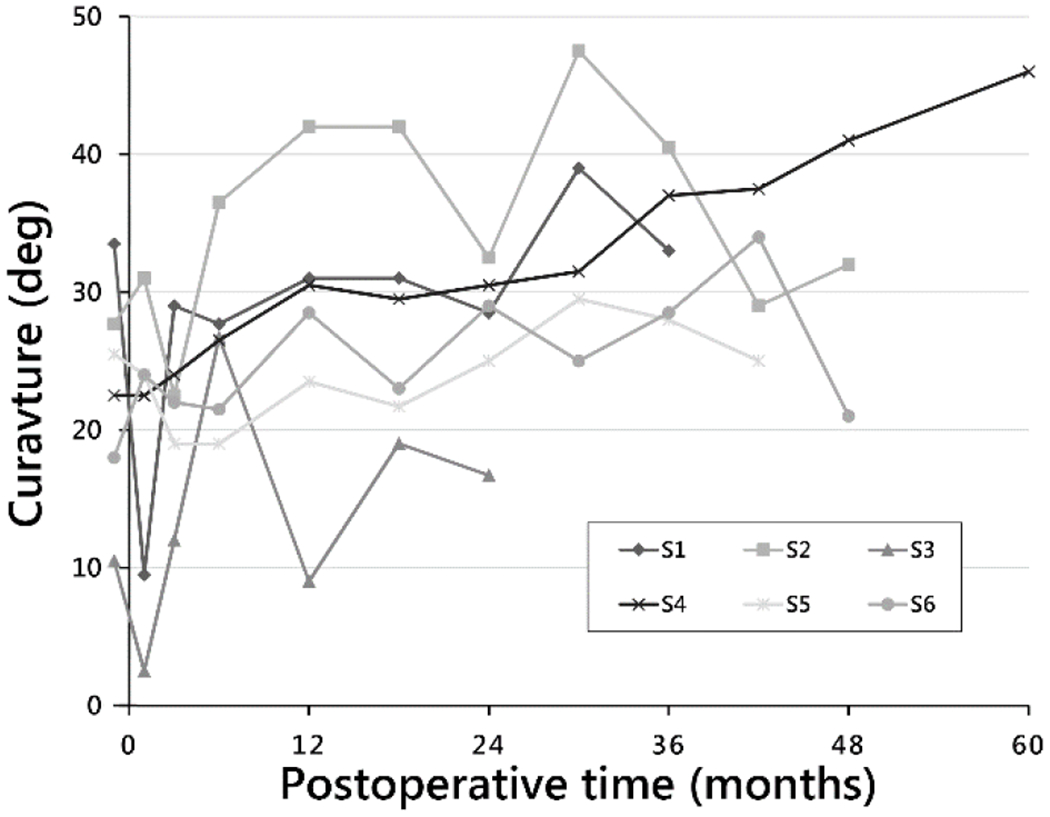
Thoracic kyphosis curve magnitudes by postoperative time to end of study time point for each subject.
Height velocities at 6 months postoperative, 24 months, and end of study were 7.7 cm/year (±2.1), 3.0 cm/year (±3.2), and 0.7 cm/year (±0.9), respectively (Fig. 5). For most subjects, height velocities were highest at time of surgery, or within 1 year postoperative, then decreased (Fig. 5). The exception was a sustained height velocity of 8–10 cm/year for nearly 3 years in the subject whose curve gradually corrected, then overcorrected at 30–36 months.
Fig. 5.
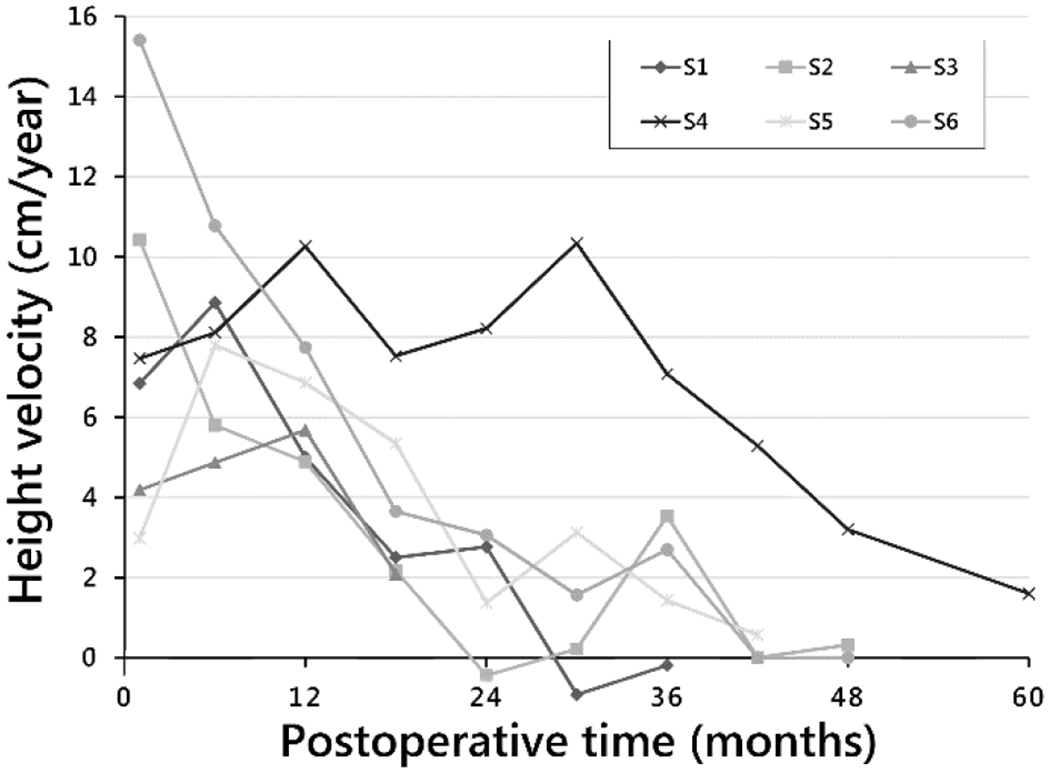
Height velocity by postoperative time to end of study time point for each subject.
Disc heights were measurable at most levels in five subjects. Discs were seldom visible in the subject with the greatest axial rotation and most rapidly progressive curve, so these could not be included in the analysis. Disc heights on convex and concave curve sides, respectively, were, preoperatively, 5.6 mm (±0.81) and 4.2 mm (±0.37), and at end of study, 5.9 mm (±1.8) and 3.9 mm (±0.50) (Fig. 6). Immediately postoperatively, paired disc height changes due to implantation were, on convex and concave sides, respectively, −0.24 mm (±0.18) and 0.4 mm (± 0.24). At end of study, paired disc height changes compared with preoperative were 0.26 mm (±1.1) on the convex side and −0.34 (±0.41) on the concave side. Side-to side differences in disc heights were at most weakly correlated with MT curvatures (r2=0.46, p=0.21) (Table 3).
Fig. 6.
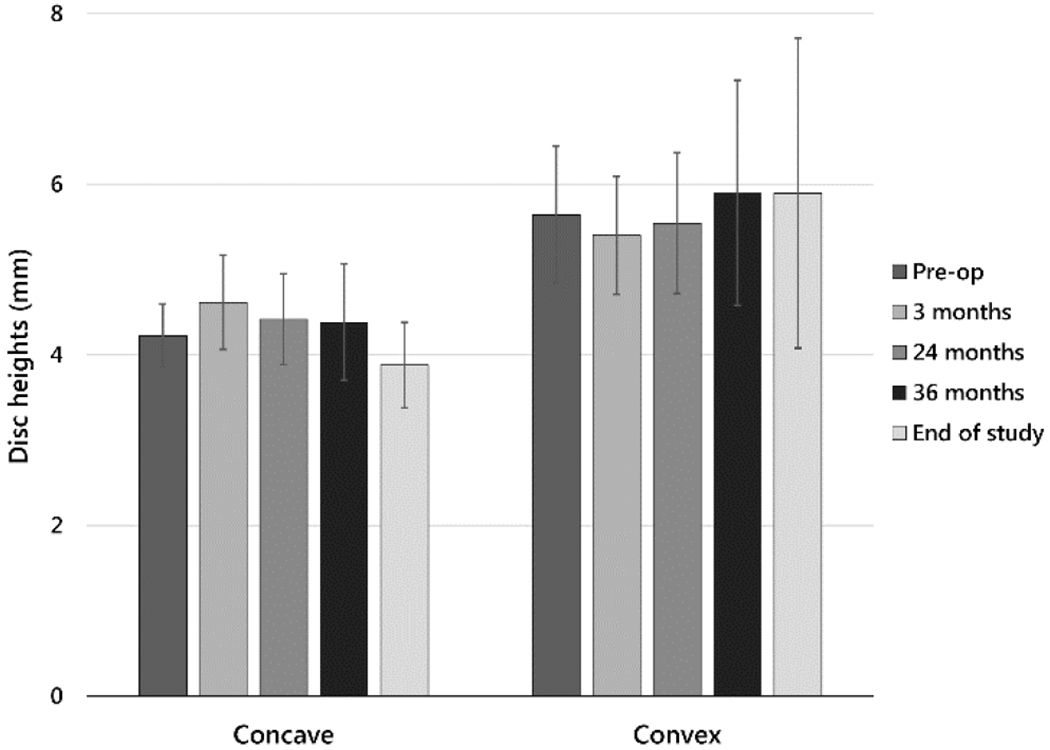
Disc heights on concave and convex sides (mean ± sample standard deviation) at five time points for those subjects in whom most discs were visible in radiographs.
Table 3.
Correlation and regression coefficients, and p values testing the hypotheses that the slopes of the linear regression curves are zero for three retrospectively measured variables* vs. major thoracic curve magnitudes at end of study.
| Disc height difference (mm), vertebral height difference (mm), and initial contact area ratio (%) versus MT curve magnitude (deg) at end of study | Pearson correlation coefficient, r2 | Slope, a (mm/deg, or %/deg) | Intercept, b (mm, or %) | p-value |
|---|---|---|---|---|
| Disc height difference (mm) | 0.46 | 0.05 | −0.03 | 0.21 |
| Vertebral height difference (mm) | 0.85 | 0.10 | −2.4 | <0.01 |
| Contact area ratio (100 x Abc/Av) immediately postop (%) | 0.84 | −0.05 | 13 | 0.01 |
Abc, area of blade in contact with bone (one side of each blade); Av, total cross-sectional area of vertebra.
Difference between disc height on convex and concave sides at end of study, difference between vertebral heights on convex and concave sides at end of study, and contact area ratios (100 x Abc/Av (%)) immediately postoperative.
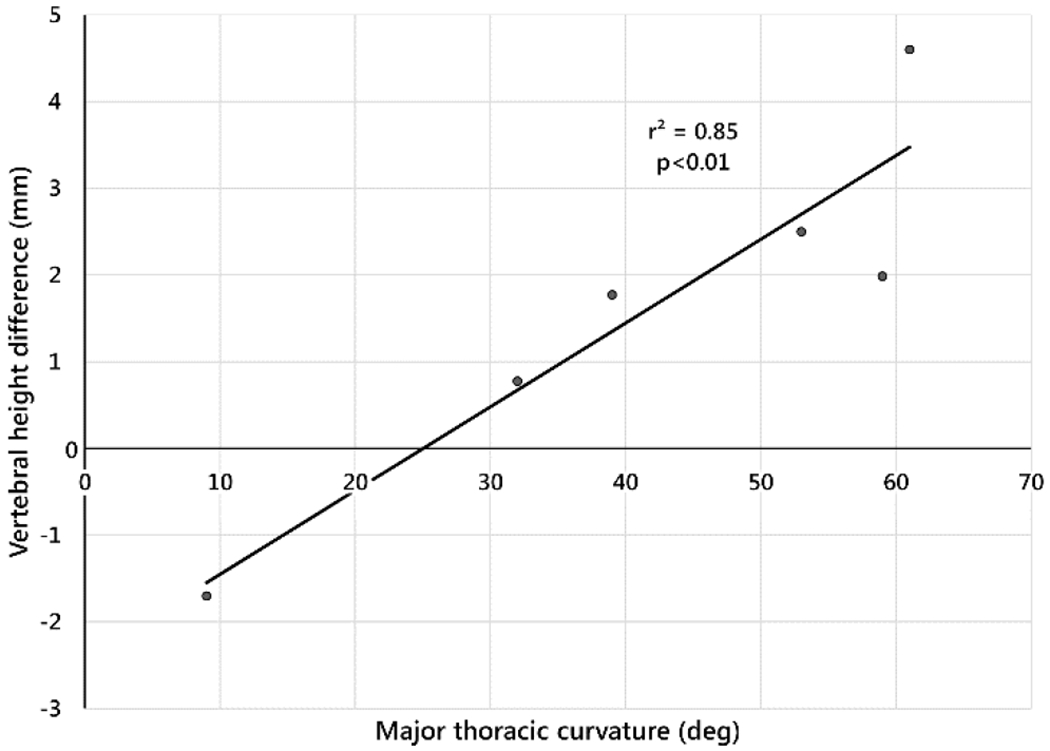
Vertebral heights immediately postoperatively on convex and concave sides were 18.7 mm (±1.2) and 18.0 mm (±1.1), respectively. At end of study, these values were 23.6 mm (±1.5) and 22.5 mm (±1.5) (Fig. 7a). Paired side-to-side differences (convex – concave) were 0.76 mm (±0.37) immediately postoperatively, and 1.1 mm (±1.7) at end of study. Paired side-to-side differences in vertebral heights and final MT curvatures were correlated (Fig. 7b, r2=0.85, p<0.01) (Table 3).
Fig. 7.
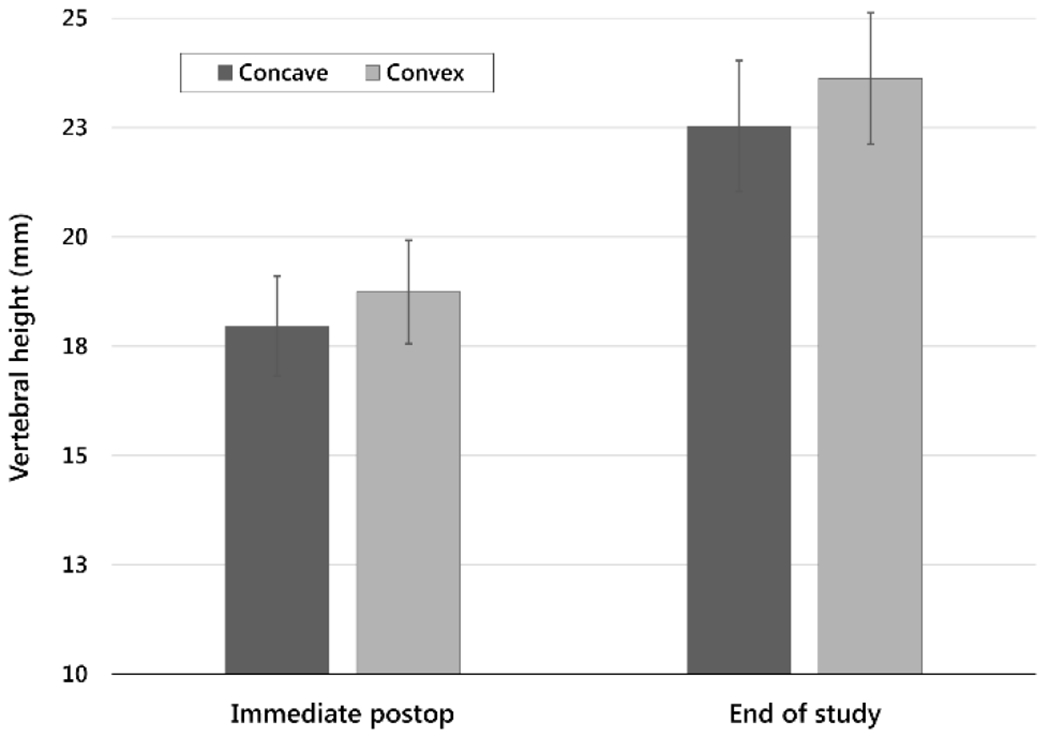
a. Vertebral heights on concave and convex sides (mean ± sample standard deviation) at two time points, immediately postoperative and end of study.
b. Side-to-side difference in vertebral height (convex – concave) at end of study versus final major thoracic curve magnitude. Each point is the average of the vertebral height differences at the treated levels for each subject.
Contact area ratios immediately postoperatively ranged, on average by subject, from 10-13% (Fig. 8). At end of study, this range was 7-13%. Initial contact area ratios were correlated with MT curve magnitudes (r2=0.84, p=0.01) (Table 3).
Fig. 8.
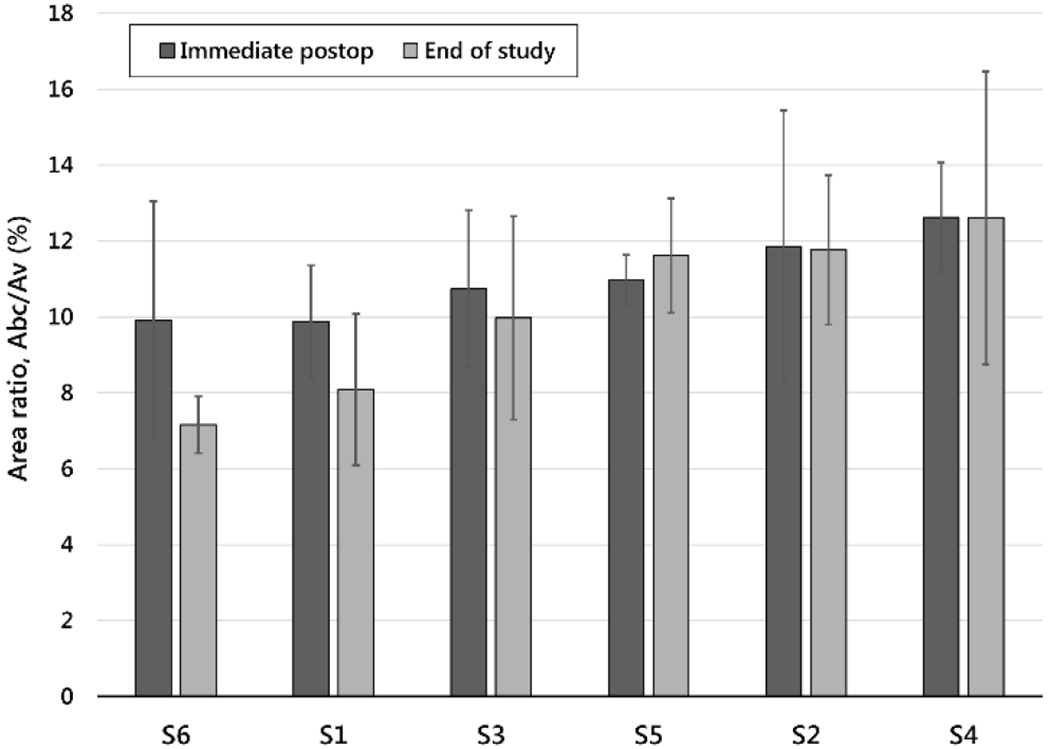
Ratio of the area of the blade in contact with bone divided by the total cross-sectional area of the vertebra for each subject at two time points, immediately postoperative and at end of study (mean ± sample standard deviation). Subjects are ordered from lowest contact area ratio to highest. This order largely corresponds to the order of major thoracic curve outcome from progression, to maintenance, to correction and overcorrection.
Abc = Area of blade in contact with bone (one side of each blade)
Av = Total cross-sectional area of vertebra
Discussion
In a study of a novel growth modulation technique conducted under stringent regulatory protocols in patients with early AIS at very high risk of progression to PSF indications, adverse events consisted primarily of second surgeries for conversion to PSF in 2 of 6 subjects, and for selective removal of implants for overcorrection in one. Curvature results were variable. At skeletal maturity or five years postoperation, three curves progressed to >50° and three were <40°. Evidence of spinal growth modulation was most clearly provided by a patient whose curve gradually decreased. In addition, deformity progression was likely halted in one and slowed in at least one other. In half the patients, curve magnitudes remained below PSF indications throughout the study. To date, four of six patients did not undergo fusion surgery.
Safety characteristics of the system included constructs fixed to bone with two screws which were entirely implanted, and selectively retrieved, using thoracoscopic procedures. As titanium is nonferrous, MRI is not precluded. Blades and screws were short relative to vertebral body width and distance to spinal canal. Bicortical fixation was not necessary to retain the implants, nor was it possible, precluding any chance of breech of major vessels near the opposite cortex. Divergently angled blades aim away from disc and growth plates as they are driven in, so direct physeal injury was not likely a mechanism of growth modulation.
Vertebral heights increased on both sides of the curve. The increase on the ipsilateral side indicated that a mechanism of progression included migration of blades through bone, likely due to micro-motion between blades and bone. The strong correlation between the side-to-side differences in vertebral heights and final curvature implies that vertebral growth modulation occurred in both positive and negative directions. Disc height changes were, on average, <0.5 mm at three to five years postoperation.
To guide changes, it is incumbent to discern as well as possible from early evidence which factors were more or less likely associated with curve changes. Factors may relate to subject, implant design, and/or surgical processes. Several surgical factors were discussed [32]. Considering gender, as well as high versus low levels of curve flexibility, thoracic hypokyphosis, and axial rotation, each single factor had at least one case of both high and low final MT curve magnitude. By contrast, high sustained height velocities and high relative contact area ratios were each associated with lower final curve magnitudes. Notably, both the highest mean contact area ratio, 12.6% (Fig. 8), and a high sustained height velocity occurred in the subject whose curve overcorrected. Moreover, the highest area ratio at any single level, 19%, occurred proximally in this patient, the location of the overcorrection. Only in the two most successful cases did contact area ratios reach or exceed 15%, a stated design criteria in the preclinical study [27]. Results suggest that the target contact area ratio is 15% (±2) in humans as well as in the quadrupedal model.
Other factors that may affect initial conditions and final outcomes include blade angle, bridge flexibility, implant surface characteristics, and instrumented levels. Divergent blades decrease disc height on the ipsilateral side and increase it on the contralateral side at insertion. Initial changes in disc height were maintained for the first year [32], but then gradually decreased with time. Blade angle and bridge length affect initial disc height asymmetry changes, which affect both the magnitude of the immediate postoperative curve change and the applied compression gradient. Optimized blade angle, bridge length, and relative blade size may allow for greater initial, and sustained, disc height and curve changes. Other factors likely exist, for example, how the extent of surgical procedures such as electrocautery alter vascular or other biological changes around periosteum and physes. However, at this pilot stage, one subject factor, height velocity, which cannot be controlled, and one implant-subject interaction factor, contact area ratio, which can be better controlled, were identified as factors that likely affect the efficacy of growth modulation using this type of implant.
Limitations include that this was an early-phase clinical safety case series, albeit followed to skeletal maturity. The purpose of a pilot FDA IDE safety study is systematic collection of adverse events. The smallest implants with likelihood of success were used as a starting point. Reporting of curvatures was nonetheless essential. Variability in curvature outcomes allowed for a limited number of correlations with the caveat that these were tertiary variables collected retrospectively. These preliminary results may serve as the basis for the next hypothesis tests. The strengths of the protocol include that it was a prospective study with stringent eligibility criteria designed to include only subjects most likely to progress, who were then followed longitudinally to skeletal maturity. Comparisons may now be made to studies of observed, braced, or surgically treated patients with the same eligibility criteria conducted under similar protocols.
Conclusions
In a prospective initial safety study of a novel spinal growth modulation system, in a cohort of six late juvenile to early adolescent idiopathic scoliosis patients at very high risk of progression, adverse events consisted primarily of second surgical procedures in three cases: instrumented PSF in two, and removal of half the implants for curve overcorrection in one. Major thoracic curvatures at end of study were <40° in half the subjects and >50° in the other half. Evidence of growth modulation was provided by a curve that corrected and overcorrected, and by cessation of overcorrection after selective implant removal. Possible evidence may also be afforded by two cases of slow to no curve progression. A proposed study of 30 patients, previously approved by the US FDA and using a modified device design, would be expected to provide for initial subgroup analyses to determine patients who may benefit most from this procedure. If eventually proven efficacious, this type of system may allow select patients with late juvenile to early adolescent idiopathic scoliosis to avoid brace wear and instrumented posterior spinal fusion.
Key Points.
In a pilot safety study of a novel method of spine growth modulation for early AIS in subjects at very high risk of progression, adverse events consisted primarily of second surgeries, either for conversion to PSF or for overcorrection. At skeletal maturity or 5 years postoperation, major thoracic curve magnitudes of 3 of 6 subjects progressed to >50°, whereas those of the other 3 remained below 40°.
The curve of one subject overcorrected proximally; selective removal of half of the implants halted overcorrection.
Mean changes in disc height at end of study compared to preoperative values were an increase of <0.5 mm on the convex side, and a decrease of <0.5 mm on the concave side.
Side-to-side vertebral height differences at end of study were correlated with changes in curvature, which implies that vertebral growth modulation occurred during both progression and correction.
Initial contact area ratios between implant blades and vertebral bone were correlated with changes in curvature, which suggests a range of relative implant sizes that are most likely to avoid both progression and overcorrection.
Acknowledgments:
The authors thank: Rich Grant, BSE, Kevyn Irving, BSE, Rena Irving, David L. Glos, BSE, George H. Thompson, MD, Paul J. Samuels, MD, Sean J. Barnett, MD, Jose A. Herrera-Soto, MD, Courtney W. Brown, MD, Mark A. Erickson, MD, Robert M. Campbell Jr., MD (deceased), Richard E. McCarthy, MD, Peter F. Sturm, MD, and the CCHMC Office of Clinical and Translational Research (OCTR).
Funding sources:
State of Ohio, Ohio Third Frontier TECH 11-042B (2010-13); USA FDA 1-R01 FD004144-01 (2012-13)
Footnotes
Device Status Statement: The devices that are the subject of this manuscript were evaluated as part of a US FDA Investigational Device Exemption (IDE) for the intended use of guided spinal growth treatment of progressive idiopathic scoliosis (IS). The test article is intended for anterior-lateral Cobb to Cobb fixation across the growth plates from T3 to L1 with placement through thoracoscopic surgery.
Regulatory status: IRB approved, FDA Investigational Device Exemption (IDE) and Humanitarian Use Device (HUD); http://clinicaltrials.gov /show/ NCT01465295. US Food and Drug Administration. Evaluate Initial Safety of the HemiBridge System in Guided Spinal Growth Treatment of Progressive Idiopathic Scoliosis. 2013 [First posted November 4, 2011; Current update posted February 5, 2018]. Device approved for use in European Union (EU CE Marking) for the labeled indications.
References
- 1.Ponseti IV, Friedman B. Prognosis in idiopathic scoliosis. J Bone Joint Surg Am 1950:32:381–95. [PubMed] [Google Scholar]
- 2.Lonstein JE, Carlson JM. The prediction of curve progression in untreated idiopathic scoliosis during growth. J Bone Joint Surg Am 1984;66:1061–71. [PubMed] [Google Scholar]
- 3.Charles YP, Daures JP, de Rosa V, et al. Progression risk of idiopathic juvenile scoliosis during pubertal growth. Spine 2006;31:1933–42. [DOI] [PubMed] [Google Scholar]
- 4.Sanders JO, Browne RH, McConnell SJ, et al. Maturity assessment and curve progression in girls with idiopathic scoliosis. J Bone Joint Surg Am 2007;89:64–73. [DOI] [PubMed] [Google Scholar]
- 5.Sanders JO, Khoury JG, Kishan S, et al. Predicting scoliosis progression from skeletal maturity: a simplified classification during adolescence. J Bone Joint Surg Am 2008;90:540–553. [DOI] [PubMed] [Google Scholar]
- 6.Sitoula P, Verma K, Holmes L Jr, et al. Prediction of curve progression in idiopathic scoliosis: validation of the Sanders skeletal maturity staging system. Spine 2015;40(13):1006–13. [DOI] [PubMed] [Google Scholar]
- 7.Karol LA, Johnston CE, Browne RH, et al. Progression of the curve in boys who have idiopathic scoliosis. J Bone Joint Surg Am 1993;75:1804–1810. [DOI] [PubMed] [Google Scholar]
- 8.Nachemson AL, Peterson LE. Effectiveness of treatment with a brace in girls who have adolescent idiopathic scoliosis. A prospective, controlled study based on data from the Brace Study of the Scoliosis Research Society. J Bone Joint Surg Am 1995;77:815–22. [DOI] [PubMed] [Google Scholar]
- 9.Emans JB, Kaelin A, Bancel P, et al. The Boston bracing system for idiopathic scoliosis. Follow-up results in 295 patients. Spine 1986;11:792–801. [DOI] [PubMed] [Google Scholar]
- 10.Little DG, Song KM, Katz D, et al. Relationship of peak height velocity to other maturity indicators in idiopathic scoliosis in girls. J Bone Joint Surg Am 2000;82(5):685–93. [DOI] [PubMed] [Google Scholar]
- 11.Song KM, Little DG. Peak height velocity as a maturity indicator for males with idiopathic scoliosis. J Pediatr Orthop 2000. May 1 ;20(3):286–8. [PubMed] [Google Scholar]
- 12.Ryan PM, Puttler EG, Stotler WM, et al. Role of the triradiate cartilage in predicting curve progression in adolescent idiopathic scoliosis. J Pediatr Orthop 2007. 1 ;27(6):671–6. [DOI] [PubMed] [Google Scholar]
- 13.Karol LA. Effectiveness of bracing in male patients with idiopathic scoliosis. Spine 2001. ;26:2001–2005. [DOI] [PubMed] [Google Scholar]
- 14.Richards BS, Bernstein RM, D’Amato CR, et al. Standardization of criteria for adolescent idiopathic scoliosis brace studies: SRS Committee on Bracing and Nonoperative Management. Spine 2005;30:2068–75. [DOI] [PubMed] [Google Scholar]
- 15.Katz DE, Herring JA, Browne RH, et al. Brace wear control of curve progression in adolescent idiopathic scoliosis. J Bone Joint Surg Am 2010;92:1343–1352. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein SL, Dolan LA, Wright JG, et al. Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med 2013;369:1512–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karol LA, Virostek D, Felton K, et al. The effect of the Risser stage on bracing outcome in adolescent idiopathic scoliosis. J Bone Joint Surg Am 2016;98:1253–9. [DOI] [PubMed] [Google Scholar]
- 18.Arkin AM, Katz JF. The effects of pressure on epiphyseal growth; the mechanism of plasticity of growing bone. J Bone Joint Surg Am 1956; 38A:1056–1076. [PubMed] [Google Scholar]
- 19.Roaf R Vertebral growth and its mechanical control. J Bone Joint Surg Br 1960;42-B:40–59. [DOI] [PubMed] [Google Scholar]
- 20.Harrington PR. Is Scoliosis Reversible? In vivo observations of reversible morphological changes in the production of scoliosis in mice. Clin Orthop Relat Res 1976;116:103–11. [PubMed] [Google Scholar]
- 21.Stokes IAF, Aronsson DD, Urban JPG. Biomechanical factors influencing progression of angular skeletal deformities during growth. Eur J Exp Musculoskel Res 1994;3:51–60. [Google Scholar]
- 22.Stokes IA, Burwell RG, Dangerfield PH, IBSE: Biomechanical spinal growth modulation and progressive adolescent scoliosis—a test of the ‘vicious cycle’ pathogenic hypothesis: summary of an electronic focus group debate of the IBSE. Scoliosis 2006;1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bylski-Austrow DI, Glos DL, Wall EJ, Crawford AH. Scoliosis vertebral growth plate histomorphometry: Comparisons to controls, growth rates, and compressive stresses. J Orthop Res 2018; EPub ahead of print. DOI 10.1002/jor.23900 [DOI] [PubMed] [Google Scholar]
- 24.Mente PL, Aronsson DD, Stokes IAF, Iatridis JC. Mechanical modulation of growth for the correction of vertebral wedge deformities. J Orthop Res 1999; 17.4:518–24. [DOI] [PubMed] [Google Scholar]
- 25.Stokes IA, Mente PL, Iatridis JC, et al. Enlargement of growth plate chondrocytes modulated by sustained mechanical loading. J Bone Joint Surg Am 2002;84:1842–8. [DOI] [PubMed] [Google Scholar]
- 26.Braun JT, Ogilvie JW, Akyuz E, et al. Fusionless scoliosis correction using a shape memory alloy staple in the anterior thoracic spine of the immature goat. Spine 2004;29:1980–89. [DOI] [PubMed] [Google Scholar]
- 27.Wall EJ, Bylski-Austrow DI, Kolata RJ, Crawford AH. Endoscopic mechanical spinal hemiepiphysiodesis modifies spine growth. Spine 2005; 30:10:1148–1153. [DOI] [PubMed] [Google Scholar]
- 28.Stokes IA, Aronsson DD, Dimock AN, et al. Endochondral growth in growth plates of three species at two anatomical locations modulated by mechanical compression and tension. J Orthop Res 2006; 24: 1327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newton PO, Upasani VV, Farnsworth CL, et al. Spinal growth modulation with use of a tether in an immature porcine model. J Bone Joint Surg Am 2008;90:2695–706. [DOI] [PubMed] [Google Scholar]
- 30.Bylski-Austrow DI, Wall EJ, Glos DL, et al. Spinal hemiepiphysiodesis decreases the size of vertebral growth plate hypertrophic zone and cells. J Bone Joint Surg Am 2009;91:584–93. [DOI] [PubMed] [Google Scholar]
- 31.Clinicaltrials.gov [Internet], Identifier: NCT01465295: Evaluate initial safety of the HemiBridge™ System in guided spinal growth treatment of progressive idiopathic scoliosis. [Updated 2018. February 5]. Available from: http://clinicltrials.gov/ct2/show/NCT01465295.
- 32.Wall EJ, Reynolds JE, Jain VV, et al. Spine growth modulation in early adolescent idiopathic scoliosis: Two year results of prospective US FDA IDE pilot clinical safety study of titanium clip-screw implant. Spine Deform 2017;5:314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. Stanford, Stanford University Press; 21, 1959. p. 266–270. [Google Scholar]
- 34.Jain V, Lykissas M, Trobisch P, et al. Surgical aspects of spinal growth modulation in scoliosis correction. Instr Course Lect, Am Acad Orthop Surg 2014;63:335–44. [PubMed] [Google Scholar]
- 35.Wall EJ, Bylski-Austrow DI, Reynolds JE, et al. Growth modulation techniques: titanium clip-screw implant system (HemiBridge). In: The Growing Spine. Springer, Berlin, Heidelberg, 2016. 769–781. [Google Scholar]


