Abstract
The coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), puts a heavy strain on healthcare systems around the globe with high numbers of infected patients. Pre-existing cardiovascular disease is a major risk factor for a severe clinical course of COVID-19 and is associated with adverse outcome. COVID-19 may directly exacerbate underlying heart disease and is frequently aggravated by cardiovascular complications, including arterial and venous thromboembolic events, malignant arrhythmia and myocardial injury. In addition to these direct cardiac manifestations of COVID-19, patients with cardiovascular disease face further indirect consequences of the pandemic, as the respective resources in the healthcare systems need to be redirected to cope with the high numbers of infected patients. Consecutively, a substantial decrease in cardiac procedures was reported during the pandemic with lower numbers of coronary angiographies and device implantations worldwide. As a consequence an increased number of out-of-hospital cardiac arrests, late-comers with subacute myocardial infarction and of patients presenting in cardiogenic shock or preshock were observed. Maintenance of high-quality cardiac care by avoiding a reduction of cardiac services is of utmost importance, especially in times of a pandemic.
Keywords: Venous thromboembolism, Myocarditis, Arrhythmia, Interventional procedure, Acute coronary syndrome
Introduction
The coronavirus disease 2019 (COVID-19) caused a global pandemic with more than 140 million patients infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in more than 200 countries worldwide [1]. Patients with a history of cardiovascular disease represent a particularly vulnerable group with a high risk of a severe clinical course of the disease [2, 3]. Virus-related cardiovascular complications, such as myocardial injury, cardiac arrhythmia and thromboembolic events are a frequent finding in patients with COVID-19 [4–6]. In addition, treatment of cardiac patients is further hampered by a significant reduction in diagnostic and therapeutic strategies during the peaks of the pandemic [7–9]. Regarding the substantial impact on cardiovascular medicine, this review focuses on national and international experience with cardiac complications in COVID-19 patients and pandemic-related collateral damage.
Pathogenesis and clinical course
SARS-CoV‑2 consists of four structural proteins and a positive-sense, single-stranded RNA with an approximate length of 30 kb [10, 11]. The structural proteins of the virus form a typical crown-like morphology with spike proteins on its surface [11]. The virus enters the host cells via binding of the spike proteins to the ACE‑2 (Angiotensin-converting enzyme 2) receptor, a membrane protein with a particularly high expression on the surface of alveolar epithelial type II cells in the human respiratory tract, causing the typical respiratory symptoms [12, 13]. In the early phase of the pandemic, concerns about the safety of treatment with ACE inhibitors in COVID-19 patients were raised due an upregulation of the ACE‑2 receptor [14]; however, several studies and randomized control trials demonstrated that ACE inhibitors can be safely continued despite a SARS-CoV‑2 infection [15–18].
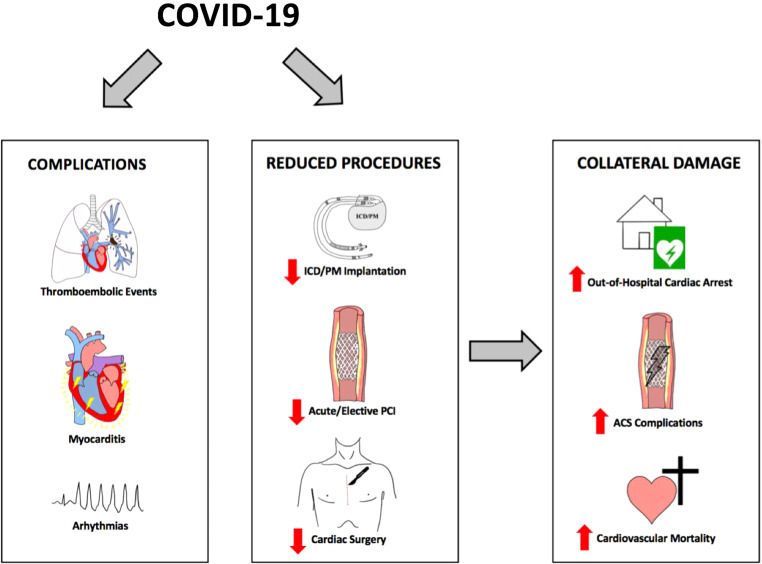
The clinical course of COVID-19 varies significantly with many patients experiencing no or only mild symptoms, including fever, fatigue, dry cough and gastrointestinal symptoms [13, 19]. Nevertheless, a substantial number of COVID-19 patients are affected by a more severe clinical course with viral pneumonia and acute respiratory distress syndrome [19]. Notably, infection with SARS-CoV‑2 often triggers an amplified inflammatory response with excessive cytokine release, platelet hyperactivity, endothelial dysfunction and systemic inflammation [20–23]. This hyperinflammatory response may provide a potential explanation for the high incidence rate of cardiovascular complications in patients with COVID-19 (Table 1; Fig. 1; [20]).
Table 1.
Direct cardiovascular complications and respective incidence rates associated with COVID-19
| Incidence rate of cardiovascular complications (%) |
|---|
|
Arterial thrombotic events |
|
Venous thrombotic events |
|
Myocardial inflammation |
|
Cardiac arrhythmia |
Fig. 1.
Direct cardiovascular complications of COVID-19 and indirect collateral damage during the pandemic. ACS acute coronary syndrome, ICD implantable cardioverter defibrillator, PM pacemaker, PCI percutaneous coronary intervention
In addition, SARS-CoV‑2 shows a propensity to genetic variations, leading to new variants that might differ in clinical presentation and characteristics [24]. In contrast to the previous year of the pandemic, the latest wave of infections in Europe is mainly driven by the emergence of the B.1.617.2 variant of the SARS-CoV‑2 virus, also referred to as delta variant [24]. Data from the European Center for Disease Prevention and Control show that B.1.617.2 accounted for more than 65% of all cases across Europe in the calendar week 28 of July 2021 [24]. The delta variant is associated with a higher transmissibility and might influence the effectiveness of vaccination [25].
The rapid emergence of new variants with a potentially higher risk for an aggravated course underlines the dynamic nature of the pandemic.
Virus-related thromboembolic complications
Thromboembolic events are a frequent complication in COVID-19 patients and contribute to increased morbidity and mortality [20]. Several studies demonstrated the high incidence of thromboembolism that affects both the arterial and the venous system [4, 26, 27]. A multinational observational cohort study of more than 14,000 patients reported that 1.1% of COVID-19 patients presented with acute ischemic stroke during initial hospital admission [28]. Similarly, a stroke rate of 0.9% was reported in a cohort study from New York [29] and COVID-19 was demonstrated to be an independent risk factor for the occurrence of ischemic stroke, with a significantly higher risk in patients with a severe clinical course [30, 31]. Of note, patients with COVID-19 appear to have an increased risk for cryptogenic and large vessel stroke [29, 32]. Patients presenting with ST elevation myocardial infarction (STEMI) and concurrent COVID-19 infection face an increased risk for a poor outcome [33, 34]. Several studies reported that STEMI patients with a concomitant SARS-CoV‑2 infection carry a higher thrombus burden and an increased risk of stent thrombosis [33, 34]; however, it remains controversial if SARS-CoV2 can trigger type 1 myocardial infarction itself, potentially via cytokine-related plaque instability [35, 36].
Venous thrombotic events and pulmonary embolism frequently occur in patients with COVID-19 [37–39]. In a Dutch cohort of 184 critically ill patients, the incidence of venous thrombotic complications was 27% despite routine thromboprophylaxis with low molecular-weight heparin (LMWH) [4]. A French multicenter cohort study demonstrated an incidence rate of pulmonary embolism of 8.3% in 1240 consecutive COVID-19 patients, as confirmed with computed tomography pulmonary angiography [40]. As evidence of the activated coagulation system, several studies reported elevated plasma levels of D‑dimer in a significant proportion of COVID-19 patients, associated with an adverse outcome [41, 42]. The high rates of thrombotic events make effective antithrombotic treatment essential in the management of COVID-19 patients. Hence, routine thromboprophylaxis with standard-dose LMWH should be established in all hospitalized patients [5, 43–45]. Selected high-risk patients may benefit from an intensified strategy with intermediate-dose or therapeutic-dose administration of LMWH [5, 43], although the evidence for such dosage is still limited [46].
Virus-related myocarditis
Acute myocarditis related to COVID-19 was reported by several case series and smaller studies [47, 48]. In an initial observational study from Wuhan, China, fulminant myocarditis was suspected as the immediate cause of death in 7% of all patients [49]. A prospective trial performed elective cardiac magnetic resonance imaging in 100 recently recovered COVID-19 patients and reported that 60 patients showed elevated T1 and T2 times, suggestive of myocardial inflammation with late gadolinium enhancement detected in 32 patients [50]. Endomyocardial biopsy was performed in patients with severe findings and showed diffuse active lymphocytic inflammation [50]. Similar findings were reported in a retrospective cohort study of patients with previous SARS-CoV‑2 infection who underwent cardiac magnetic resonance imaging, demonstrating that 31% of these patients had significant late gadolinium enhancement [51]. A prospective multicenter study in a cohort of 148 patients with a severe clinical course of COVID-19 detected myocarditis-like scars in 39 (26%) patients via cardiac magnetic resonance imaging [52]. On the contrary, Joy et al. investigated cardiovascular changes in a cohort of 74 patients with a mild clinical course of COVID-19 compared with 75 seronegative control patients 6 months after infection [53]. Thorough analysis of cardiovascular biomarkers and cardiac magnetic resonance imaging did not reveal any long-term cardiovascular abnormalities in COVID-19 patients with only mild symptoms [53].
This data might indicate that SARS-CoV‑2 infection frequently causes extensive myocardial inflammation, which may be dependent on disease severity. Potential long-term cardiac sequelae of COVID-19 in this respect still need to be investigated.
Virus-related cardiac arrhythmia
Cardiac rhythm disorders are frequently reported in COVID-19 patients with higher incidence rates among patients with elevated troponin levels or patients on an intensive care unit [54, 55]. Guo et al. reported in a retrospective cohort study that patients with elevated troponin T levels had a significantly higher incidence rate of ventricular arrhythmia compared to patients with normal troponin levels (17.3% vs. 1.5%, p < 0.001) [54]. Data from an American registry of consecutive COVID-19 cases reported an incidence rate of malignant ventricular arrhythmia of 11% in patients with a severe clinical course [56]. A meta-analysis of 17 observational cohort studies found an incidence rate of 10.4% of cardiac arrhythmia in COVID-19 patients, although the type of arrhythmia was not clearly defined in this analysis [57]. The propensity for the occurrence of potentially life-threatening ventricular arrhythmia may be the consequence of several contributing factors. In addition to myocardial injury, COVID-19 patients frequently develop metabolic disorders and electrolyte disorders on top of the hyperinflammatory state, creating a milieu that may favor the occurrence of cardiac arrhythmia [56, 58]. It has to be stressed that several drugs initially used to treat COVID-19 have proarrhythmic potential, e.g. hydroxychloroquine and azithromcycin [59, 60]. Although it might pose a logistic challenge in routine clinical practice, selected inpatients might benefit from continuous rhythm monitoring.
Takotsubo cardiomyopathy and COVID-19
In the COVID-19 era, governments worldwide were repeatedly forced to impose drastic public health measures to control and reduce transmission rates of SARS-CoV‑2. Strict social distancing rules, self-isolation, quarantine, economic and social stress, in addition to fear of virus infection, exerted significant psychosocial distress on broad parts of the population [61, 62]. Emotional and physical distress are common triggers of the takotsubo syndrome, referred to as stress cardiomyopathy [63]. During the COVID-19 pandemic, a significant increase in the incidence rate of takotsubo syndrome was found [64]. Jabri et al. reported in a North American cohort that the incidence rate of takotsubo syndrome was 4.5 times higher during the COVID-19 pandemic compared to corresponding time frames of prepandemic years [65]. Interestingly, the vast majority of patients presenting with takotsubo syndrome in this study tested negative for SARS-CoV‑2, potentially associated with an increased level of stress in the general population [65].
Indirect consequences of the pandemic—Reduction in interventional procedures
Several studies demonstrated that patients with a history of cardiovascular comorbidities have a higher risk for an aggravated clinical course of COVID-19 [41, 42, 54, 66].
In addition to the described direct cardiovascular complications induced by a COVID-19, the pandemic might have several indirect consequences due to a pandemic-related reduction in diagnostic and therapeutic strategies for patients with cardiovascular disease ([67]; Fig. 1). National healthcare systems in several countries had to relocate resources to cope with the high numbers of infected patients [68, 69]. Consequently, a substantial decrease in elective cardiac procedures was observed during the peaks of the COVID-19 pandemic, with significantly lower numbers of patients undergoing routine coronary angiography, pacemaker implantation or cardiac surgery ([9, 70, 71]; Fig. 1). Importantly, the numbers of acute procedures showed a comparable decline [7, 72–74]. An Italian study from 15 cardiac centers reported a reduction of 30% in patients admitted with acute coronary syndrome in February and March 2020, compared to the previous year’s respective period [7]. Data from a French registry of 21 participating centers showed a reduction of 24% in hospital admissions for STEMI during the first lockdown, irrespective of the regional prevalence of COVID-19 [72]. Similar numbers were reported in studies from the USA and England [8, 73, 75, 76]. Recent data from Austria have shown that the numbers of interventional cardiac procedures were significantly lower in the whole year of 2020 compared to the previous 12 months of 2019 [77]. While a significant reduction of 8% in elective percutaneous coronary interventions (PCI) was observed, the rate of acute PCI showed an even greater decline of 12% [77]. The number of cardiac device implantations and cardiac surgeries were also significantly affected by the pandemic-related lockdown measures [70, 78]. A nationwide analysis from England reported a substantial decline of 44% in pacemaker implantation and of 45% in implantable cardioverter defibrillator implantations during the first wave of the pandemic [78]. Patients admitted for coronary artery bypass craft surgery were markedly reduced by 64% and for surgical aortic valve replacement by 41% compared with the respective period in 2019 [78]. Similar numbers were reported from an Italian multicenter analysis and an international quantitative survey of 60 cardiac surgery centers [70, 79].
Pandemic-related cardiac collateral damage
Reduced capacities for cardiac patients and interventional procedures may have led to significant collateral damage (Fig. 1). An Italian multicenter study reported a threefold increase in STEMI case fatality rate (risk ratio, RR = 3.3, p < 0.001) and a substantial increase in complications (RR = 1.8, p = 0.009) during the COVID-19 pandemic [80]. These complications included cardiac rupture, ventricular septal defect and severe mitral regurgitation, which may be attributed to prolonged patient delay due to neglected symptoms [80]. Accordingly, the delay from symptom onset to wire-crossing was substantially increased by 39.2% compared to the previous year’s respective period [80]. In addition, a profound impact on out-of-hospital cardiac arrest was observed [81]. In the region of Lombardy, Italy, a 58% point increase in out-of-hospital cardiac arrests during the first peak of the pandemic compared to the same period of the previous year was noted [82]. Bystander cardiopulmonary resuscitation was performed significantly less often [82]. Likewise, an analysis from the Paris metropolitan area reported a transient two-times increased incidence rate of out-of-hospital cardiac arrest in the period from March to April 2020 [83]. Furthermore, several studies found a significant decline in admission rates for patients with acute heart failure during the peaks of the pandemic [84, 85]. Patients presenting with worsening or new onset heart failure were in aggravated clinical conditions and had more severe symptoms [84]. Data from England and Germany suggested an increased mortality in heart failure patients during the pandemic [86, 87], although these findings were not confirmed in a recent study from Denmark [88]. Importantly, a potential undertreatment of patients with heart failure during the pandemic may influence long-term prognosis of these patients [89]. Overall, an increased cardiovascular mortality in the COVID-19 era was observed in several studies. Wu et al. reported in a national cohort analysis of England and Wales a proportional increase in cardiovascular mortality of 8% compared with the average of the previous years [90]. An excess in cardiovascular death was also found in studies from the USA and Brazil [91, 92]; however, a recent analysis from Danish nationwide registries did not identify an increased mortality of patients with established cardiovascular diseases during the pandemic [93]. In this context, it needs to be acknowledged that although increasing evidence for a profound pandemic-related impact on cardiovascular care is available, these data were mostly generated from observational studies [94]. Jung et al. demonstrated in a systematic evaluation that COVID-19 clinical research has lower methodological quality than comparable control studies and should be interpreted with appropriate care [94].
Contributors and implications of reduced capacities for cardiac care
The substantial decrease in hospital admissions and interventional cardiac procedures may have several causes. During the peaks of the pandemic, strict social distancing rules were imposed, avoidance of personal contact was encouraged and the risks of COVID-19 were broadly propagated through public media and the political discourse. Potentially, many patients opted to avoid hospitals and emergency services out of fear of contagion. Even patients with acute medical conditions, including typical signs of an ACS may try to endure symptoms for as long as possible before seeking adequate medical attention [95]. Such changes in patients’ behavior led to a significant increase in patient delay and time to wire-crossing in STEMI patients [80, 95]. In addition, relocation of resources to deal with the high number of infected patients may have contributed to the declined numbers of cardiac procedures during the COVID-19 pandemic. Under the continuous pressure of the pandemic, healthcare providers were obliged to establish specialized COVID-19 wards on normal care and intensive care units. Resources from wards of cardiology departments were not excluded from such relocations, as the limited resources of bed capacities and medical staff were partly redirected from the treatment of cardiac patients to the high numbers of COVID-19 patients. As a consequence of these relocations, elective, non-emergency cardiac procedures had to be postponed during the peaks of the pandemic, which led to significantly lower numbers of interventional procedures [70, 71, 77].
Regarding the significant impact of the pandemic on cardiovascular treatment, countermeasures should be considered [96, 97]. Maintenance of well-functioning cardiac care and specialized tertiary hospitals is of utmost importance, especially in times of a global pandemic. Considering the importance of timely primary PCI in the setting of STEMI, patients need to be adequately educated and encouraged to immediately seek medical attention if symptoms suggestive of an ACS are present [98, 99]. It should be emphasized that patients may not avoid contacting emergency services due to fear of potential nosocomial SARS-CoV‑2 infections but rather focus on the importance of timely treatment in cases of an acute coronary event. In this context, maintenance of a well-functioning STEMI network is of great importance. Guideline-compliant, timely interventional treatment of patients with a STEMI or high-risk NSTEMI and continued appropriate treatment on specialized cardiac units should not be compromised despite shortages in personnel or resources. Thus, relocation of resources towards the appropriate care of COVID-19 patients may exclude cardiac departments, which carry the responsibility of continued cardiovascular treatment of a population at risk. Finally, it is of utmost need for governments and healthcare systems to provide public information and reassurance to the people to seek medical attention in case of acute or emergency conditions.
Conclusion
The COVID-19 pandemic poses a severe and persistent challenge to national healthcare systems. Patients with pre-existing heart diseases are a vulnerable patient group that may face both direct and indirect consequences of the COVID-19 pandemic. Cardiovascular complications occur frequently and aggravate the clinical course of the disease. While the high numbers of infected patients repeatedly forced healthcare systems to relocate the limited resources towards an adequate treatment of COVID-19 patients, appropriate high-quality care for patients with cardiovascular disease needs to be maintained to avoid collateral damage to this patient group.
Acknowledgments
Funding
The authors report no specific funding for this research article.
Conflict of interest
A.L. Burger, C.C. Kaufmann, B. Jäger, E. Pogran, A. Ahmed, J. Wojta, S. Farhan and K. Huber declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Johns Hopkins Coronavirus Resource Center. COVID-19 dashboard. 2021. https://coronavirus.jhu.edu/map.html. Accessed 1 Sept 2021.
- 2.Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, Bi Z, Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McBane RD, Torres Roldan VD, Niven AS, et al. Anticoagulation in COVID-19: a systematic review, meta-analysis, and rapid guidance from mayo clinic. Mayo Clin Proc. 2020;95(11):2467–2486. doi: 10.1016/j.mayocp.2020.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auer J, Huber K. COVID-19 – Kardiologische Aspekte. J. Kardiol. Austrian J. Cardiol. 2020;27(5):152–155. [Google Scholar]
- 7.De Filippo O, D’Ascenzo F, Angelini F, et al. Reduced rate of hospital admissions for ACS during Covid-19 outbreak in northern Italy. N Engl J Med. 2020;383(1):88–89. doi: 10.1056/NEJMc2009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia S, Albaghdadi MS, Meraj PM, Schmidt C, Garberich R, Jaffer FA, Dixon S, Rade JJ, Tannenbaum M, Chambers J, Huang PP, Henry TD. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75(22):2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ullah A, Fraser DGW, Fath-Ordoubadi F, Holt CM, Malik N. Decrease in cardiac catheterization and MI during COVID pandemic. Am Heart J Plus. 2021;1:100001. doi: 10.1016/j.ahjo.2020.100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML, Zhang YL, Dai FH, Liu Y, Wang QM, Zheng JJ, Xu L, Holmes EC, Zhang YZ. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soler MJ, Barrios C, Oliva R, Batlle D. Pharmacologic modulation of ACE2 expression. Current Science Inc. 2008;10(5):410–414. doi: 10.1007/s11906-008-0076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopes RD, Macedo AVS, de Barros E Silva PGM, et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA. 2021;325(3):254–264. doi: 10.1001/jama.2020.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen JB, Hanff TC, William P, et al. Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir Med. 2021;9(3):275–284. doi: 10.1016/s2213-2600(20)30558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauer A, Schreinlechner M, Sappler N, et al. Discontinuation versus continuation of renin-angiotensin-system inhibitors in COVID-19 (ACEI-COVID): a prospective, parallel group, randomised, controlled, open-label trial. Lancet Respir Med. 2021;9(8):863–872. doi: 10.1016/s2213-2600(21)00214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, Hausvater A, Newman JD, Berger JS, Bangalore S, Katz SD, Fishman GI, Kunichoff D, Chen Y, Ogedegbe G, Hochman JS. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382(25):2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epidemiology Working Group for NCIP Epidemic Response. Chinese Center for Disease Control and Prevention The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Gu SX, Tyagi T, Jain K, Gu VW, Lee SH, Hwa JM, Kwan JM, Krause DS, Lee AI, Halene S, Martin KA, Chun HJ, Hwa J. Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat Rev Cardiol. 2021;18(3):194–209. doi: 10.1038/s41569-020-00469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA, da Silva LFF, de Oliveira EP, Saldiva PHN, Mauad T, Negri EM. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost. 2020;18(6):1517–1519. doi: 10.1111/jth.14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, Pesenti A, Peyvandi F, Tripodi A. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ECDC. Weekly surveillance summary. 2021. https://covid19-country-overviews.ecdc.europa.eu/#Weekly_surveillance_summary. Accessed 25 July 2021.
- 25.Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, Myers R, Campbell CNJ, Amirthalingam G, Edmunds M, Zambon M, Brown KE, Hopkins S, Chand M, Ramsay M. Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021 doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Bertuzzi A, Sandri MT, Barco S. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zakeri A, Jadhav AP, Sullenger BA, Nimjee SM. Ischemic stroke in COVID-19-positive patients: an overview of SARS-CoV-2 and thrombotic mechanisms for the neurointerventionalist. J NeuroIntervent Surg. 2021;13(3):202–206. doi: 10.1136/neurintsurg-2020-016794. [DOI] [PubMed] [Google Scholar]
- 28.Siegler JE, Cardona P, Arenillas JF, et al. Cerebrovascular events and outcomes in hospitalized patients with COVID-19: the SVIN COVID-19 multinational registry. Int J Stroke. 2020 doi: 10.1177/1747493020959216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yaghi S, Ishida K, Torres J, Mac Grory B, Raz E, Humbert K, Henninger N, Trivedi T, Lillemoe K, Alam S, Sanger M, Kim S, Scher E, Dehkharghani S, Wachs M, Tanweer O, Volpicelli F, Bosworth B, Lord A, Frontera J. SARS-coV-2 and stroke in a New York healthcare system. Stroke. 2020;51(7):2002–2011. doi: 10.1161/strokeaha.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belani P, Schefflein J, Kihira S, Rigney B, Delman BN, Mahmoudi K, Mocco J, Majidi S, Yeckley J, Aggarwal A, Lefton D, Doshi AH. COVID-19 is an independent risk factor for acute Ischemic stroke. AJNR Am J Neuroradiol. 2020;41(8):1361–1364. doi: 10.3174/ajnr.A6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. Jama Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, De Leacy RA, Shigematsu T, Ladner TR, Yaeger KA, Skliut M, Weinberger J, Dangayach NS, Bederson JB, Tuhrim S, Fifi JT. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382(20):e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choudry FA, Hamshere SM, Rathod KS, Akhtar MM, Archbold RA, Guttmann OP, Woldman S, Jain AK, Knight CJ, Baumbach A, Mathur A, Jones DA. High thrombus burden in patients with COVID-19 presenting with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2020;76(10):1168–1176. doi: 10.1016/j.jacc.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamadeh A, Aldujeli A, Briedis K, Tecson KM, Sanz-Sánchez J, Al Dujeili M, Al-Obeidi A, Diez JL, Žaliūnas R, Stoler RC, McCullough PA. Characteristics and outcomes in patients presenting with COVID-19 and ST-segment elevation myocardial infarction. Am J Cardiol. 2020;131:1–6. doi: 10.1016/j.amjcard.2020.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyle JJ. Association of coronary plaque rupture and atherosclerotic inflammation. J Pathol. 1997;181(1):93–99. doi: 10.1002/(SICI)1096-9896(199701)181:1<93::AID-PATH696>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 36.Naghavi M, Wyde P, Litovsky S, Madjid M, Akhtar A, Naguib S, Siadaty MS, Sanati S, Casscells W. Influenza infection exerts prominent inflammatory and thrombotic effects on the atherosclerotic plaques of apolipoprotein E-deficient mice. Circulation. 2003;107(5):762–768. doi: 10.1161/01.cir.0000048190.68071.2b. [DOI] [PubMed] [Google Scholar]
- 37.Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324(8):799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piazza G, Campia U, Hurwitz S, Snyder JE, Rizzo SM, Pfeferman MB, Morrison RB, Leiva O, Fanikos J, Nauffal V, Almarzooq Z, Goldhaber SZ. Registry of arterial and venous thromboembolic complications in patients with COVID-19. J Am Coll Cardiol. 2020;76(18):2060–2072. doi: 10.1016/j.jacc.2020.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fauvel C, Weizman O, Trimaille A, et al. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J. 2020;41(32):3058–3068. doi: 10.1093/eurheartj/ehaa500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oldenburg J, Klamroth R, Langer F. Aktualisierte Empfehlungen zur Thromboseprophylaxe bei SARS-CoV-2 (COVID-19) 2020. [Google Scholar]
- 44.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spyropoulos AC, Levy JH, Ageno W, Connors JM, Hunt BJ, Iba T, Levi M, Samama CM, Thachil J, Giannis D, Douketis JD. Scientific and standardization committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Talasaz AH, Sadeghipour P, Kakavand H, et al. Recent randomized trials of antithrombotic therapy for patients with COVID-19: JACC state-of-the-art review. J Am Coll Cardiol. 2021;77(15):1903–1921. doi: 10.1016/j.jacc.2021.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim IC, Kim JY, Kim HA, Han S. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J. 2020;41(19):1859. doi: 10.1093/eurheartj/ehaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sawalha K, Abozenah M, Kadado AJ, Battisha A, Al-Akchar M, Salerno C, Hernandez-Montfort J, Islam AM. Systematic review of COVID-19 related myocarditis: insights on management and outcome. Cardiovasc Revasc Med. 2021;23:107–113. doi: 10.1016/j.carrev.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M, Nagel E. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) Jama Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang L, Zhao P, Tang D, Zhu T, Han R, Zhan C, Liu W, Zeng H, Tao Q, Xia L. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. Jacc Cardiovasc Imaging. 2020;13(11):2330–2339. doi: 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kotecha T, Knight DS, Razvi Y, et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J. 2021;42(19):1866–1878. doi: 10.1093/eurheartj/ehab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joy G, Artico J, Kurdi H, et al. Prospective case-control study of cardiovascular abnormalities 6 months following mild COVID-19 in healthcare workers. Jacc Cardiovasc Imaging. 2021 doi: 10.1016/j.jcmg.2021.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Si D, Du B, Ni L, Yang B, Sun H, Jiang N, Liu G, Massé S, Jin L, Nanthakumar J, Bhaskaran A, Yang P, Nanthakumar K. Death, discharge and arrhythmias among patients with COVID-19 and cardiac injury. CMAJ. 2020;192(28):E791–e8. doi: 10.1503/cmaj.200879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turagam MK, Musikantow D, Goldman ME, et al. Malignant arrhythmias in patients with COVID-19: incidence, mechanisms, and outcomes. Circ Arrhythm Electrophysiol. 2020;13(11):e008920. doi: 10.1161/circep.120.008920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kunutsor SK, Laukkanen JA. Cardiovascular complications in COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):e139–e141. doi: 10.1016/j.jinf.2020.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen D, Li X, Song Q, Hu C, Su F, Dai J, Ye Y, Huang J, Zhang X. Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou, China. JAMA Netw Open. 2020;3(6):e2011122. doi: 10.1001/jamanetworkopen.2020.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chorin E, Wadhwani L, Magnani S, et al. QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm. 2020;17(9):1425–1433. doi: 10.1016/j.hrthm.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diaz-Arocutipa C, Brañez-Condorena A, Hernandez AV. QTc prolongation in COVID-19 patients treated with hydroxychloroquine, chloroquine, azithromycin, or lopinavir/ritonavir: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf. 2021;30(6):694–706. doi: 10.1002/pds.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531–542. doi: 10.1016/j.bbi.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N, Rubin GJ. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912–920. doi: 10.1016/s0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghadri JR, Wittstein IS, Prasad A, et al. International expert consensus document on Takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39(22):2032–2046. doi: 10.1093/eurheartj/ehy076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giustino G, Croft LB, Oates CP, Rahman K, Lerakis S, Reddy VY, Goldman M. Takotsubo cardiomyopathy in COVID-19. J Am Coll Cardiol. 2020;76(5):628–629. doi: 10.1016/j.jacc.2020.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jabri A, Kalra A, Kumar A, Alameh A, Adroja S, Bashir H, Nowacki AS, Shah R, Khubber S, Kanaa NA, Hedrick DP, Sleik KM, Mehta N, Chung MK, Khot UN, Kapadia SR, Puri R, Reed GW. Incidence of stress cardiomyopathy during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3(7):e2014780. doi: 10.1001/jamanetworkopen.2020.14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shi S, Qin M, Cai Y, Liu T, Shen B, Yang F, Cao S, Liu X, Xiang Y, Zhao Q, Huang H, Yang B, Huang C. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41(22):2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burger AL, Kaufmann CC, Jaeger B, Wojta J, Farhan S, Huber K. Verlauf undTherapie kardiovaskulärer Erkrankungen im Rahmen der Covid-19-Pandemie. J. Kardiol. Austrian J. Cardiol. 2021;28:1–2. [Google Scholar]
- 68.Senni M. COVID-19 experience in Bergamo, Italy. Eur Heart J. 2020;41(19):1783–1784. doi: 10.1093/eurheartj/ehaa279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohamed Abdel Shafi A, Hewage S, Harky A. The impact of COVID-19 on the provision of cardiac surgical services. J Card Surg. 2020;35(6):1295–1297. doi: 10.1111/jocs.14631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boriani G, Palmisano P, Guerra F, Bertini M, Zanotto G, Lavalle C, Notarstefano P, Accogli M, Bisignani G, Forleo GB, Landolina M, D’Onofrio A, Ricci R, De Ponti R. Impact of COVID-19 pandemic on the clinical activities related to arrhythmias and electrophysiology in Italy: results of a survey promoted by AIAC (Italian association of arrhythmology and cardiac pacing) Intern Emerg Med. 2020;15(8):1445–1456. doi: 10.1007/s11739-020-02487-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Casey L, Khan N, Healy DG. The impact of the COVID-19 pandemic on cardiac surgery and transplant services in Ireland’s national centre. Ir J Med Sci. 2020 doi: 10.1007/s11845-020-02292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mesnier J, Cottin Y, Coste P, et al. Hospital admissions for acute myocardial infarction before and after lockdown according to regional prevalence of COVID-19 and patient profile in France: a registry study. Lancet Public Health. 2020;5(10):e536–e42. doi: 10.1016/s2468-2667(20)30188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJ. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020;41(19):1852–1853. doi: 10.1093/eurheartj/ehaa314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bugger H, Gollmer J, Pregartner G, Wünsch G, Berghold A, Zirlik A, von Lewinski D. Complications and mortality of cardiovascular emergency admissions during COVID-19 associated restrictive measures. PLoS ONE. 2020;15(9):e0239801. doi: 10.1371/journal.pone.0239801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reinstadler SJ, Reindl M, Lechner I, Holzknecht M, Tiller C, Roithinger FX, Frick M, Hoppe UC, Jirak P, Berger R, Delle-Karth G, Laßnig E, Klug G, Bauer A, Binder R, Metzler B. Effect of the COVID-19 pandemic on treatment delays in patients with ST-segment elevation myocardial infarction. J Clin Med. 2020 doi: 10.3390/jcm9072183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mafham MM, Spata E, Goldacre R, Gair D, Curnow P, Bray M, Hollings S, Roebuck C, Gale CP, Mamas MA, Deanfield JE, de Belder MA, Luescher TF, Denwood T, Landray MJ, Emberson JR, Collins R, Morris EJA, Casadei B, Baigent C. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396(10248):381–389. doi: 10.1016/s0140-6736(20)31356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mühlberger V, Alber H, Bonner G, et al. Herzkathetereingriffsdaten im COVID-Pandemiejahr 2020 im Vergleich zu 2019 aus dem österreichischen ANCALAR-Register. J Kardiol. 2021;28:5–6. [Google Scholar]
- 78.Leyva F, Zegard A, Okafor O, Stegemann B, Ludman P, Qiu T. Cardiac operations and interventions during the COVID-19 pandemic: a nationwide perspective. Europace. 2021 doi: 10.1093/europace/euab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gaudino M, Chikwe J, Hameed I, Robinson NB, Fremes SE, Ruel M. Response of cardiac surgery units to COVID-19: an internationally-based quantitative survey. Circulation. 2020;142(3):300–302. doi: 10.1161/circulationaha.120.047865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Rosa S, Spaccarotella C, Basso C, Calabrò MP, Curcio A, Filardi PP, Mancone M, Mercuro G, Muscoli S, Nodari S, Pedrinelli R, Sinagra G, Indolfi C. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J. 2020;41(22):2083–2088. doi: 10.1093/eurheartj/ehaa409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hassager C, Price S, Huber K. Cardiac arrest in the COVID-19 era. Eur Heart J Acute Cardiovasc Care. 2020;9(3):239–240. doi: 10.1177/2048872620922789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baldi E, Sechi GM, Mare C, Canevari F, Brancaglione A, Primi R, Klersy C, Palo A, Contri E, Ronchi V, Beretta G, Reali F, Parogni P, Facchin F, Bua D, Rizzi U, Bussi D, Ruggeri S, Oltrona Visconti L, Savastano S. Out-of-hospital cardiac arrest during the Covid-19 outbreak in Italy. N Engl J Med. 2020;383(5):496–498. doi: 10.1056/NEJMc2010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marijon E, Karam N, Jost D, Perrot D, Frattini B, Derkenne C, Sharifzadehgan A, Waldmann V, Beganton F, Narayanan K, Lafont A, Bougouin W, Jouven X. Out-of-hospital cardiac arrest during the COVID-19 pandemic in Paris, France: a population-based, observational study. Lancet Public Health. 2020;5(8):e437–e43. doi: 10.1016/s2468-2667(20)30117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bromage DI, Cannatà A, Rind IA, Gregorio C, Piper S, Shah AM, McDonagh TA. The impact of COVID-19 on heart failure hospitalization and management: report from a heart failure unit in London during the peak of the pandemic. Eur J Heart Fail. 2020;22(6):978–984. doi: 10.1002/ejhf.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Doolub G, Wong C, Hewitson L, Mohamed A, Todd F, Gogola L, Skyrme-Jones A, Aziz S, Sammut E, Dastidar A. Impact of COVID-19 on inpatient referral of acute heart failure: a single-centre experience from the south-west of the UK. ESC Heart Fail. 2021;8(2):1691–1695. doi: 10.1002/ehf2.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cannatà A, Bromage DI, Rind IA, Gregorio C, Bannister C, Albarjas M, Piper S, Shah AM, McDonagh TA. Temporal trends in decompensated heart failure and outcomes during COVID-19: a multisite report from heart failure referral centres in London. Eur J Heart Fail. 2020;22(12):2219–2224. doi: 10.1002/ejhf.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bollmann A, Hohenstein S, König S, Meier-Hellmann A, Kuhlen R, Hindricks G. In-hospital mortality in heart failure in Germany during the Covid-19 pandemic. ESC Heart Fail. 2020;7(6):4416–4419. doi: 10.1002/ehf2.13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Andersson C, Gerds T, Fosbøl E, Phelps M, Andersen J, Lamberts M, Holt A, Butt JH, Madelaire C, Gislason G, Torp-Pedersen C, Køber L, Schou M. Incidence of new-onset and worsening heart failure before and after the COVID-19 epidemic lockdown in Denmark: a nationwide cohort study. Circ Heart Fail. 2020;13(6):e007274. doi: 10.1161/circheartfailure.120.007274. [DOI] [PubMed] [Google Scholar]
- 89.Moayedi Y, Alba AC, Lee DS, Wijeysundera HC, Ross HJ. The next wave of health care strain related to COVID-19: heart failure patients coming back in force-we must not fail them. Can J Cardiol. 2020;36(7):993–994. doi: 10.1016/j.cjca.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu J, Mamas MA, Mohamed MO, Kwok CS, Roebuck C, Humberstone B, Denwood T, Luescher T, de Belder MA, Deanfield JE, Gale CP. Place and causes of acute cardiovascular mortality during the COVID-19 pandemic. Heart. 2021;107(2):113–119. doi: 10.1136/heartjnl-2020-317912. [DOI] [PubMed] [Google Scholar]
- 91.Bhatt AS, Moscone A, McElrath EE, Varshney AS, Claggett BL, Bhatt DL, Januzzi JL, Butler J, Adler DS, Solomon SD, Vaduganathan M. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76(3):280–288. doi: 10.1016/j.jacc.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brant LCC, Nascimento BR, Teixeira RA, Lopes M, Malta DC, Oliveira GMM, Ribeiro ALP. Excess of cardiovascular deaths during the COVID-19 pandemic in Brazilian capital cities. Heart. 2020;106(24):1898–1905. doi: 10.1136/heartjnl-2020-317663. [DOI] [PubMed] [Google Scholar]
- 93.Butt JH, Fosbøl EL, Gerds TA, Andersson C, Kragholm K, Biering-Sørensen T, Andersen J, Phelps M, Andersen MP, Gislason G, Torp-Pedersen C, Køber L, Schou M. All-cause mortality and location of death in patients with established cardiovascular disease before, during, and after the COVID-19 lockdown: a Danish nationwide cohort study. Eur Heart J. 2021;42(15):1516–1523. doi: 10.1093/eurheartj/ehab028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jung RG, Di Santo P, Clifford C, Prosperi-Porta G, Skanes S, Hung A, Parlow S, Visintini S, Ramirez FD, Simard T, Hibbert B. Methodological quality of COVID-19 clinical research. Nat Commun. 2021;12(1):943. doi: 10.1038/s41467-021-21220-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Primessnig U, Pieske BM, Sherif M. Increased mortality and worse cardiac outcome of acute myocardial infarction during the early COVID-19 pandemic. ESC Heart Fail. 2021;8(1):333–343. doi: 10.1002/ehf2.13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huber K, Goldstein P. Covid-19: implications for prehospital, emergency and hospital care in patients with acute coronary syndromes. Eur Heart J Acute Cardiovasc Care. 2020;9(3):222–228. doi: 10.1177/2048872620923639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chieffo A, Stefanini GG, Price S, Barbato E, Tarantini G, Karam N, Moreno R, Buchanan GL, Gilard M, Halvorsen S, Huber K, James S, Neumann FJ, Möllmann H, Roffi M, Tavazzi G, Mauri Ferré J, Windecker S, Dudek D, Baumbach A. EAPCI position statement on invasive management of acute coronary syndromes during the COVID-19 pandemic. Eur Heart J. 2020;41(19):1839–1851. doi: 10.1093/eurheartj/ehaa381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scholz KH, Maier SKG, Maier LS, Lengenfelder B, Jacobshagen C, Jung J, Fleischmann C, Werner GS, Olbrich HG, Ott R, Mudra H, Seidl K, Schulze PC, Weiss C, Haimerl J, Friede T, Meyer T. Impact of treatment delay on mortality in ST-segment elevation myocardial infarction (STEMI) patients presenting with and without haemodynamic instability: results from the German prospective, multicentre FITT-STEMI trial. Eur Heart J. 2018;39(13):1065–1074. doi: 10.1093/eurheartj/ehy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European society of cardiology (ESC) Eur Heart J. 2018;39(2):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]