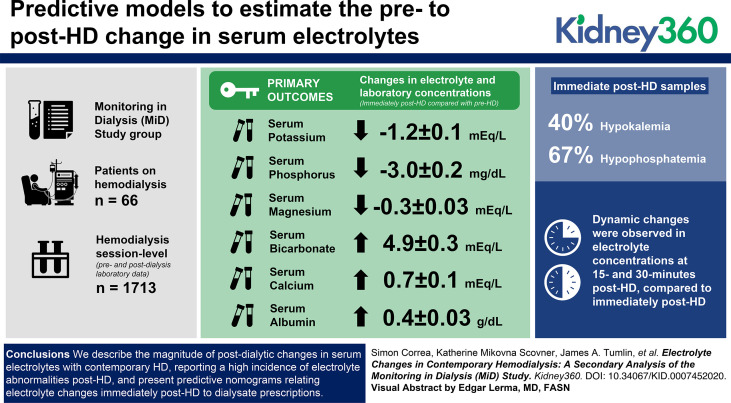
Visual Abstract
Keywords: dialysis, albumin, bicarbonate, BUN, calcium, dialysis, electrolyte, magnesium, phosphate, potassium, sodium
Key Points
Electrolyte fluxes after contemporary HD and the relationship between serum electrolytes and dialysate prescription remain understudied.
HCO3, Ca, and albumin increased, whereas K, Mg, and PO4 decreased immediately post-HD. Dynamic changes followed at 15- and 30-minutes post-HD.
We provide predictive models to estimate the pre- to post-HD change in serum electrolytes. Validation of models is warranted.
Abstract
Background
There is a paucity of contemporary data examining electrolyte changes during and immediately after hemodialysis (HD), and their relationship with dialysate prescriptions. This study examines these relationships.
Methods
We analyzed patient (n=66) and HD session–level pre and postdialysis laboratory data (n=1713) over a 6-month period from the Monitoring in Dialysis Study. We fit mixed-effects regression models to analyze electrolyte, BUN, creatinine, and albumin levels immediately post-HD, accounting for pre-HD and dialysate prescriptions. In a subset of US patients (n=40), 15-minute post-HD and 30-minute post-HD values were available at one session. Predictive models were fit to estimate electrolyte levels immediately post-HD, accounting for pre-HD concentrations and dialysate prescriptions.
Results
Serum bicarbonate, calcium, and albumin increased (mean increase 4.9±0.3 mEq/L, 0.7±0.1 mEq/L, and 0.4±0.03 g/dl, respectively), whereas potassium, magnesium, and phosphate decreased immediately post-HD (mean −1.2±0.1 mEq/L, −0.3±0.03 mEq/L, and −3.0±0.2 mg/dl, respectively). Hypokalemia and hypophosphatemia were present in 40% and 67% of immediate post-HD samples, respectively. Dynamic changes were observed in electrolyte concentrations at 15- and 30-minutes post-HD, compared with immediately post-HD.
Conclusions
We describe the magnitude of postdialytic changes in serum electrolytes with contemporary HD, reporting a high incidence of electrolyte abnormalities post-HD, and present predictive nomograms relating electrolyte changes immediately post-HD to dialysate prescriptions. Our results may be useful for clinical care and provide insights for future research on dialysate prescriptions.
Introduction
Over 450,000 patients in the United States are dependent on maintenance hemodialysis (HD) for the control of serum electrolyte concentrations and acid-base parameters (1). Traditionally offered as a thrice-weekly therapy, HD utilizes the processes of diffusion and convection to ensure adequate and safe removal of some molecules, while maintaining or replenishing others (2,3). The dialysate prescription is a critical component in this process and requires a detailed understanding of the dynamic changes and rebound in electrolyte concentrations that occur as a result of HD treatments. This is particularly important, because emerging data implicate the dialysis electrolyte prescription, and both lower and higher serum electrolyte concentrations, as important factors associated with the high incidence of sudden death in patients on maintenance HD (4).
Over the last few decades, several technological advances and changes in clinical practice have been implemented for HD therapy, including the use of higher-efficiency and higher-flux membranes, avoidance of membrane reuse, and shorter treatment times (2). These may have important implications for the expected peridialytic changes in serum electrolytes in modern HD practice. Despite this, a relative paucity of data exists in contemporary practice related to pre- and post-HD electrolyte changes, shifts in electrolytes during the postdialysis period, and the influence of the dialysis prescription on such changes.
The aim of this study was two-fold: (1) to describe the postdialytic changes in standardly assessed serum electrolyte and biochemical parameters; and (2) to determine the association of the dialysate prescription with electrolyte changes. These analyses harness the wealth of pre- and post-HD laboratory data from the Monitoring in Dialysis Study (MiD) study, a prospective, multicenter cohort study that used implantable loop recorders to determine the frequency of cardiac arrhythmias over a 6-month period.
Materials and Methods
Study Design and Population
This is a secondary analysis of the MiD study (5). MiD was a prospective cohort study that enrolled 66 patients on maintenance HD (n=43 from the United States; n=23 from India) from ten centers, and used implantable loop recorders to record continuous electrocardiographic readings over a 6-month primary observation period. Subjects were enrolled from January 2013 to January 2014 in the United States and from March 2014 to December 2015 in India. The primary eligibility criteria were age 21 or older, thrice-weekly in-center HD or eGFR <15 ml/min per 1.73 m2, with expected HD initiation within 2 months, although no patients were enrolled before their HD initiation. Key exclusion criteria were unsuitability for implantation, expected survival <6 months, left-sided HD catheter interfering with implantation, thoracic surgery within 6 months, bacteremia within 60 days or nonbacteremic infection within 14 days, hemoglobin <10 g/dl on consecutive measurements within prior 2 months, end-stage liver failure, transplantation or modality transfer expected within 6 months, existing pacemaker, or implantable cardioverter defibrillator. The design and main results of MiD have been reported elsewhere (5,6). Dialysate prescriptions were not dictated by protocol, but rather as deemed clinically indicated by the patient's nephrologist.
Exposures and Outcomes
The primary outcomes of this study were the changes in electrolyte and laboratory concentrations immediately post-HD, compared with pre-HD. Additionally, we analyzed the association of individual dialysate electrolyte concentrations with changes in serum electrolyte concentrations. As there were differences in several baseline characteristics according to country of origin, we also examined the changes in electrolyte concentration in these subgroups. In a subset of 40 participants (all from the United States), we analyzed electrolyte changes at 15-minutes post-HD, and 30-minutes post-HD, compared with the immediate post-HD measurements.
Laboratory Analysis
In MiD, per the prespecified protocol (5), blood samples were obtained before and after dialysis twice weekly for the first 4 weeks, and then once weekly through the remaining 5 months. Per protocol, additional samples were obtained at 15 and 30 minutes after dialysis on the first session after implantable loop recorder placement in a subgroup of the US participants (n=40). Blood samples were collected at study sites by trained personnel, centrifuged, refrigerated, and then shipped to a certified Central Laboratory (DaVita Total Renal Laboratories Inc. in the United States and a central laboratory in India) for measurement, using standard techniques.
Statistical Analyses
Continuous variables were examined graphically and recorded as mean±SD for normally distributed data, or medians (25th–75th percentile) for non-normally distributed data. Categorical variables were examined by frequency distribution and recorded as proportions. Pre-HD and post-HD electrolyte and laboratory assessments were described as mean±SEM, accounting for repeated measures across subjects via the use of mixed models with random intercept. The mean differences between post-HD and: (1) pre-HD measurements, (2) 15-minute post-HD, and (3) 30-minute post-HD were estimated and compared using mixed effects regression models. Additionally, as the differences between the predialysis serum and dialysate concentration may influence the postdialysis serum concentration of any given electrolyte, unadjusted and adjusted models were fit to determine the association of corresponding dialysate prescriptions with change (pre-HD to post-HD) in serum electrolyte concentrations. Model 1 adjusted for the corresponding pre-HD serum electrolyte concentrations; Model 2 additionally adjusted for the dialysis session length. Further, using the linear mixed-effect regression models described previously, we predict (and plot) the change (pre-HD to post-HD) in serum electrolyte concentration, according to the pre-HD serum and dialysate concentrations of the electrolyte of interest. These analyses were carried out for serum sodium, potassium, bicarbonate, and calcium separately. Subgroup analyses were also performed according to country of origin for these plots. Missing data were not imputed. Overall, there was <4% missing for any parameter. All analyses were carried out using the statistical software package SAS version 9.4 (Cary, NC). Two-sided P values of <0.05 were considered statistically significant.
Ethics
The MiD study was approved by the applicable institutional review boards or ethical review committees at each participating center and participants provided written informed consent before the beginning of the study.
Results
Baseline Characteristics
A total of 66 patients were included in this analysis, contributing a total of 1713 HD sessions. The mean age at baseline was 56±12 years, 70% were male, 53% were Black, and 35% were Asian. A total of 43% of participants had a history of diabetes, 26% had heart failure, and 11% had atrial fibrillation at baseline (Table 1).
Table 1.
Characteristics of the participants at baseline
| Baseline Characteristics | All Subjects (n=66) |
| Age, yr | 56±12 |
| Male, n (%) | 46 (70) |
| Race, n (%) | |
| Asian | 23 (35) |
| Black | 35 (53) |
| White | 7 (11) |
| Other | 1 (1) |
| ESKD vintage, yr | 2.4 (1.2, 5.3) |
| Vascular access, n (%) | |
| AV fistula | 46 (71) |
| AV graft | 16 (25) |
| Catheter | 3 (5) |
| Comorbid conditions, n (%) | |
| Diabetes mellitus | 28 (43) |
| Hyperlipidemia | 40 (61) |
| Hypertension | 56 (85) |
| Ischemic heart disease | 32 (48) |
| Congestive heart failure | 17 (26) |
| Arrhythmia | 21 (32) |
| Atrial fibrillation | 7 (11) |
| Systolic BP, mm Hg | 141±23 |
| Diastolic BP, mm Hg | 77±13 |
| Medication use, n (%) | |
| Aspirin | 30 (45) |
| Statin | 32 (48) |
| ACEI or ARB | 22 (33) |
| Beta blockers | 36 (55) |
| Predialysis serum laboratory values | |
| BUN, mg/dl | 60±18 |
| Creatinine, mg/dl | 10.0±3.4 |
| Sodium, mEq/L | 137±4 |
| Potassium, mEq/L | 5.0±1.0 |
| Calcium, mg/dl | 8.7±0.8 |
| Bicarbonate, mEq/L | 22±4 |
| Magnesium, mg/dl | 2.4±0.5 |
| Phosphate, mg/dl | 5.5±2.0 |
| Hemoglobin, g/dl | 11±1 |
| Serum albumin, g/dl | 3.9±0.3 |
Continuous variables are presented as mean±SD or median (25th, 75th percentiles). AV, arteriovenous; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Baseline characteristics by country of origin are presented in Supplemental Table 1.
Hemodialysis Treatment Characteristics
The median duration of HD was 4 hours, with a mean single-pool Kt/V of 1.5±0.4 and ultrafiltration rate of 10±4 ml/kg per hour across all participants (Table 2). In the subgroups according to country of origin, patients from the United States were heavier, had higher blood and dialysate flow rates, and tended to have a narrower range of dialysate prescriptions than those from India (Supplemental Table 2).
Table 2.
Characteristics of the dialysis prescription at baseline
| Baseline Characteristics | All Subjects (n=66) |
| Duration of hemodialysis, h | 4.0 (3.0, 6.0) |
| spKt/V | 1.5±0.4 |
| Predialysis weight, kg | 86.7±28.8 |
| Kg over dry weight target before dialysis | 4.2 (−0.4, 12.0) |
| UFR, ml/kg per h | 10±4 |
| Dialysate flow, ml/min | 600 (500, 800) |
| Blood flow, ml/min | 390 (324, 461) |
| High-flux dialyzer, n (%) | 42 (64) |
| Membrane reuse, n (%) | 18 (27) |
| Cellulose membrane, n (%) | 5 (8) |
| Dialysate temperature, n (%) | |
| 35.5° Celsius | 1 (2) |
| 36.0° Celsius | 3 (5) |
| 36.5° Celsius | 5 (8) |
| 37.0° Celsius | 57 (86) |
| Dialysate potassium, n (%) | |
| 1.0 mEq/L | 1 (2) |
| 2.0 mEq/L | 53 (80) |
| 3.0 mEq/L | 11 (17) |
| 4.0 mEq/L | 1 (2) |
| Dialysate calcium, n (%) | |
| 1.5 and 1.6 mEq/L | 13 (20) |
| 2.0 and 2.5 mEq/L | 39 (59) |
| 3.0 and 3.5 mEq/L | 14 (21) |
| Dialysate sodium, n (%) | |
| 135 mEq/L | 6 (10) |
| 138 mEq/L | 6 (10) |
| 140 mEq/L | 49 (80) |
| Dialysate bicarbonate, n (%) | |
| 24–32 mEq/L | 16 (24) |
| 33–36 mEq/L | 34 (52) |
| 37–40 mEq/L | 16 (24) |
Continuous variables are presented as mean±SD or median (25th, 75th percentiles). spKt/V, single-pool Kt/V; UFR, ultrafiltration rate.
Changes in Electrolyte Concentrations and Other Biochemical Parameters from Pre- to Post-HD: Descriptive Outcomes
The median (25th–75th percentile) number of pre-HD and post-HD sessions analyzed per patient was 28 (25–29) and 27 (24–29), respectively. Compared with predialysis concentrations, serum bicarbonate, calcium, and albumin increased immediately post-HD (mean increase 4.9±0.3 mEq/L, 0.7±0.1 mg/dl, and 0.4±0.03 g/dl, respectively; Figure 1, Table 3). Conversely, serum potassium, magnesium, and phosphate decreased immediately after dialysis (mean decrease −1.2±0.1 mEq/L, −0.3±0.03 mg/dl, and −3.0±0.2 mg/dl respectively). As expected, BUN and creatinine also declined, with mean decline of −39.9±1.2 mg/dl for BUN and −6.3±0.3 mg/dl for serum creatinine (Figure 1, Table 3). Post-HD values were frequently abnormal, with 311 out of 1685 (19%) having a post-HD sodium <135 mEq/L, 669 out of 1689 (40%) with post-HD potassium <3.5 mEq/L, 569 out of 1690 (34%) with post-HD bicarbonate >28 mEq/L, 190 out of 1685 (11%) with post-HD calcium >10.4 mg/dl, 162 out of 1690 (10%) with post-HD magnesium <1.7 mg/dl, and 1129 out of 1682 (67%) with post-HD phosphate <2.5 mg/dl (Figure 1, Table 3).
Figure 1.
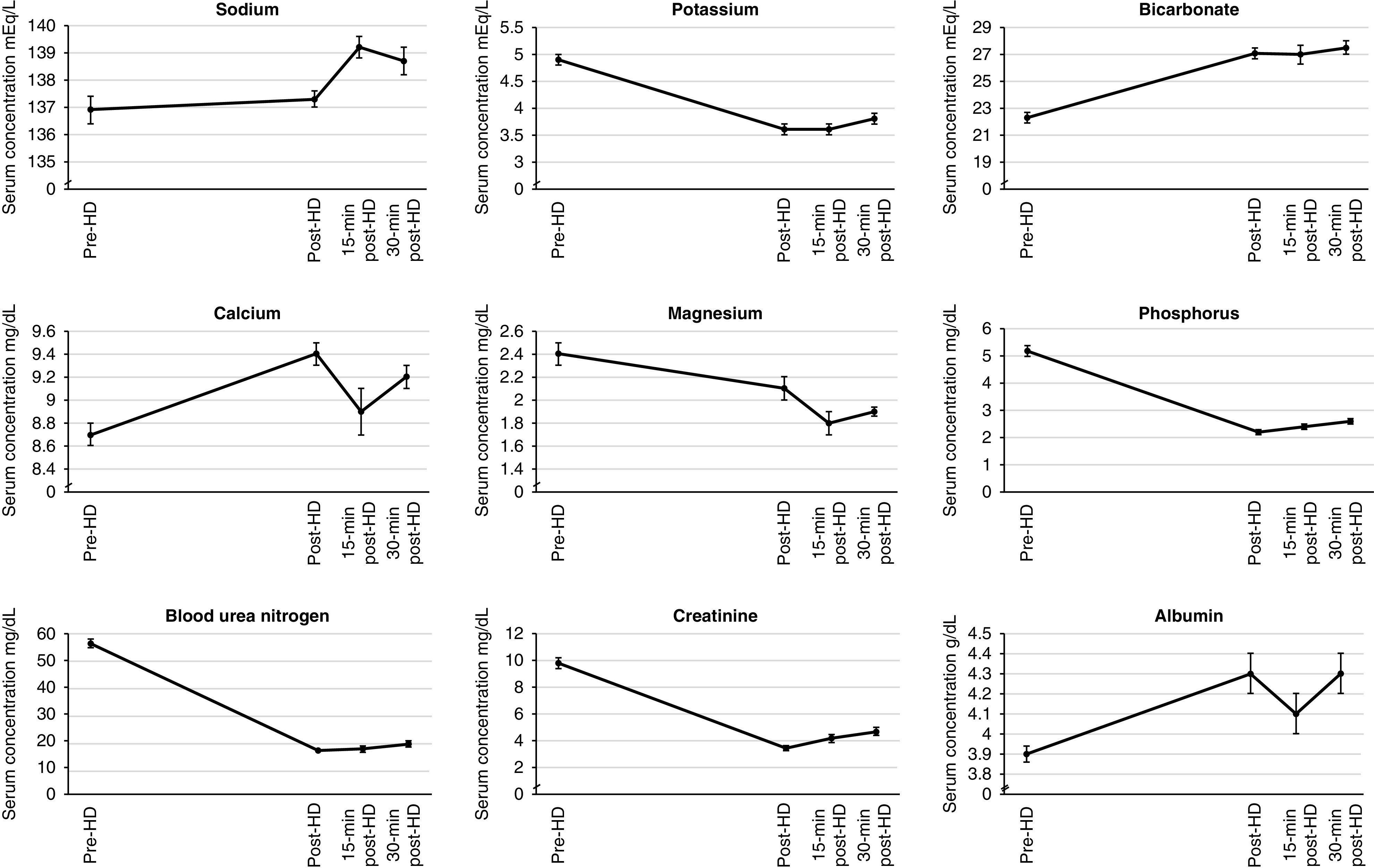
Serum electrolyte and laboratory parameter changes over time. HD, hemodialysis; min, minute.
Table 3.
Electrolyte and laboratory values pre- and immediately posthemodialysis
| Laboratory Parameter | Prehemodialysis Value, Mean (SEM), N Subjects, N Hemodialysis Sessions | Posthemodialysis Value, Mean (SEM), N Subjects, N Hemodialysis Sessions | Delta (Posthemodialysis Minus Pre hemodialysis), Mean (SEM), N Subjects, N Hemodialysis Sessions | P value (Post vs Prehemodialysis) | Lower Limit Normal | Frequency of Posthemodialysis Concentrations Below the Lower Limit Normal, N Subjects, N Hemodialysis Sessions (%) | Upper Limit Normal | Frequency of Posthemodialysis Concentrations Above the Upper Limit Normal, N Subjects, N Hemodialysis Sessions (%) |
| Sodium (mEq/L) | 136.9 (0.5) | 137.3 (0.3) | 0.5 (0.4) | 0.20 | <135 mEq/L | 38 (57.6) | >145 mEq/L | 2 (3.0) |
| 66 | 66 | 66 | 311 (18.5) | 2 (0.1) | ||||
| 1708 | 1685 | 1648 | ||||||
| Potassium (mEq/L) | 4.9 (0.1) | 3.6 (0.1) | −1.2 (0.1) | <0.001 | <3.5 mEq/L | 59 (89.4) | >5.0 mEq/L | 21 (31.8) |
| 66 | 66 | 66 | 669 (39.6) | 48 (2.8) | ||||
| 1699 | 1689 | 1645 | ||||||
| Bicarbonate (mEq/L) | 22.3 (0.4) | 27.1 (0.4) | 4.9 (0.3) | <0.001 | <22 mEq/L | 28 (42.4) | >28 mEq/L | 58 (87.9) |
| 66 | 66 | 66 | 141 (8.3) | 569 (33.7) | ||||
| 1707 | 1690 | 1651 | ||||||
| Calcium (mg/dl) | 8.7 (0.1) | 9.4 (0.1) | 0.7 (0.1) | <0.001 | <8.5 mg/dL | 28 (42.4) | >10.4 mg/dl | 29 (43.9) |
| 66 | 66 | 66 | 192 (11.4) | 190 (11.3) | ||||
| 1708 | 1685 | 1648 | ||||||
| Magnesium (mg/dl) | 2.4 (0.1) | 2.1 (0.1) | −0.3 (0.03) | <0.001 | <1.7 mg/dL | 29 (43.9) | >2.4 mg/dl | 18 (27.3) |
| 66 | 66 | 66 | 162 (9.6) | 286 (16.9) | ||||
| 1708 | 1690 | 1653 | ||||||
| Phosphate (mg/dl) | 5.2 (0.2) | 2.2 (0.1) | −3.0 (0.2) | <0.001 | <2.5 mg/dL | 65 (98.5) | >4.5 mg/dl | 8 (12.1) |
| 66 | 66 | 66 | 1129 (67.1) | 8 (0.5) | ||||
| 1706 | 1682 | 1645 | ||||||
| BUN (mg/dl) | 56.2 (1.6) | 16.2 (0.6) | −39.9 (1.2) | <0.001 | — | — | — | — |
| 66 | 66 | 66 | ||||||
| 1713 | 1695 | 1657 | ||||||
| Creatinine (mg/dl) | 9.8 (0.4) | 3.5 (0.2) | −6.3 (0.3) | <0.001 | — | — | — | — |
| 66 | 66 | 66 | ||||||
| 1713 | 1695 | 1657 | ||||||
| Albumin (g/dl) | 3.9 (0.04) | 4.3 (0.1) | 0.4 (0.03) | <0.001 | — | — | — | — |
| 66 | 66 | 66 | ||||||
| 1708 | 1691 | 1654 |
Mean differences (delta) were calculated using data from subjects who had both pre- and postmeasurement for each given electrolyte. Percent of sessions with a value above or below the limits of normal are calculated as nmeasurement outside of limits of normal/Ntotal measurements. HD, hemodialysis.
Post-dialytic Changes in Electrolyte Concentration and Other Biochemical Parameters: Descriptive Outcomes
Serum laboratory measurements were available at 15 and 30 minutes in a subgroup of patients from the United States (n=40). Compared with the immediate post-HD concentration, a post-HD increase at 15 minutes was noted only for serum phosphate (0.1±0.1 mg/dl), whereas declines were observed for bicarbonate, calcium, and albumin. However, compared with immediate post-HD concentrations, at 30-minutes post-HD increases were noted for potassium, (0.2±0.1 mEq/L), phosphate (0.4±0.1 mg/dl), BUN (2.5±0.4 mg/dl), and creatinine (0.6±0.1 mg/dl). Conversely, declines at 30-minutes post-HD were noted for bicarbonate (−0.7±0.2 mEq/L), calcium (−0.3±0.1 mg/dl), and albumin (−0.2±0.1 g/dl; Figure 1, Table 4). The proportion of sessions with abnormal serum electrolyte values at 30 minutes post-HD is presented in Table 5.
Table 4.
Electrolyte and laboratory changes 15 and 30 minutes posthemodialysis
| Laboratory Parameter | Mean (SEM), N Subjects, N Hemodialysis Sessions | P Value (Post Versus 15 Minutes Post) | Mean (SEM), N Subjects, N Hemodialysis Sessions | P Value (Post Versus 30 Minutes Post) | ||||
| Prehemodialysis | Posthemodialysis | 15 min Posthemodialysis | Delta (15 Minutes Post Minus Post) | 30 Minutes Posthemodialysis | Delta (30 Minutes Post Minus Post) | |||
| Sodium (mEq/L) | 136.9 (0.5) | 137.3 (0.3) | 139.2 (0.4) | 0.5 (0.3) | 0.10 | 138.7 (0.5) | −0.1 (0.3) | 0.80 |
| 66 | 66 | 40 | 40 | 38 | 38 | |||
| 1708 | 1685 | 40 | 40 | 38 | 38 | |||
| Potassium (mEq/L) | 4.9 (0.1) | 3.6 (0.1) | 3.6 (0.1) | −0.1 (0.1) | 0.26 | 3.8 (0.1) | 0.2 (0.1) | 0.03 |
| 66 | 66 | 40 | 40 | 38 | 38 | |||
| 1699 | 1689 | 40 | 40 | 38 | 38 | |||
| Bicarbonate (mEq/L) | 22.3 (0.4) | 27.1 (0.4) | 27.0 (0.7) | −1.1 (0.5) | 0.02 | 27.5 (0.5) | −0.7 (0.2) | <0.01 |
| 66 | 66 | 40 | 40 | 38 | 38 | |||
| 1707 | 1690 | 40 | 40 | 38 | 38 | |||
| Calcium (mg/dl) | 8.7 (0.1) | 9.4 (0.1) | 8.9 (0.2) | −0.6 (0.2) | <0.01 | 9.2 (0.1) | −0.3 (0.1) | <0.01 |
| 66 | 66 | 40 | 40 | 38 | 38 | |||
| 1708 | 1685 | 40 | 40 | 38 | 38 | |||
| Magnesium (mg/dl) | 2.4 (0.1) | 2.1 (0.1) | 1.8 (0.1) | −0.1 (0.04) | 0.08 | 1.9 (0.04) | 0.01 (0.02) | 0.59 |
| 66 | 66 | 40 | 40 | 38 | 38 | |||
| 1708 | 1690 | 40 | 40 | 38 | 38 | |||
| Phosphate (mg/dl) | 5.2 (0.2) | 2.2 (0.1) | 2.4 (0.1) | 0.1 (0.1) | 0.03 | 2.6 (0.1) | 0.4 (0.1) | <0.001 |
| 66 | 66 | 39 | 39 | 38 | 38 | |||
| 1706 | 1682 | 39 | 39 | 38 | 38 | |||
| BUN (mg/dl) | 56.2 (1.6) | 16.2 (0.6) | 16.9 (1.2) | 0.8 (0.6) | 0.19 | 18.9 (1.2) | 2.5 (0.4) | <0.001 |
| 66 | 66 | 24 | 24 | 38 | 24 | |||
| 1713 | 1695 | 24 | 24 | 24 | 24 | |||
| Creatinine (mg/dl) | 9.8 (0.4) | 3.5 (0.2) | 4.2 (0.3) | 0.2 (0.1) | 0.20 | 4.7 (0.3) | 0.6 (0.1) | <0.001 |
| 66 | 66 | 39 | 39 | 38 | 38 | |||
| 1713 | 1695 | 39 | 39 | 38 | 38 | |||
| Albumin (g/dl) | 3.9 (0.04) | 4.3 (0.1) | 4.1 (0.1) | −0.4 (0.1) | 0.001 | 4.3 (0.1) | −0.2 (0.1) | <0.001 |
| 66 | 66 | 40 | 40 | 38 | 38 | |||
| 1708 | 1691 | 40 | 40 | 38 | 38 | |||
Mean differences (delta) were calculated using data from subjects who had both pre- and postmeasurement for each given electrolyte. The total number of sessions with laboratory data may vary due to missing collections or data recording. Percentages may not add to 100 due to rounding. HD, hemodialysis.
Table 5.
Proportion of patients with abnormal serum electrolyte values at 30 minutes posthemodialysis
| Laboratory Parameter | Lower Limit Normal | Frequency of Posthemodialysis Concentrations Below the Lower Limit Normal (%) | Upper Limit Normal | Frequency of Posthemodialysis Concentrations Above the Upper Limit Normal (%) |
| Sodium (mEq/L) | <135 | 3/38 (7.9) | >145 | 0 (0) |
| Potassium (mEq/L) | <3.5 | 11/38 (29.0) | >5.0 | 1 (2.6) |
| Bicarbonate (mEq/L) | <22 | 0 (0) | >28 | 13 (34.2) |
| Calcium (mg/dl) | <8.5 | 4/38 (10.5) | >10.4 | 1 (2.6) |
| Magnesium (mg/dl) | <1.7 | 7/38 (18.4) | >2.4 | 0 (0) |
| Phosphate (mg/dl) | <2.5 | 17/38 (44.7) | >4.5 | 1 (2.6) |
A total of 38 sessions from 38 patients had 30-min posthemodialysis measurements.
Association of Dialysate Prescriptions with Changes in Pre- to Post-HD Electrolyte Concentrations
The dialysate sodium prescription was significantly associated with the change in serum sodium concentration from pre-HD to immediately post-HD. For example, adjusting for the pre-HD serum sodium, a dialysate sodium prescription of 135 mEq/L was associated with a post-HD decline of 1.4 mEq/L in serum sodium, whereas a dialysate sodium concentration of 140 mEq/L was associated with a post-HD increase of 0.6 mEq/L. As expected, lower dialysate potassium prescriptions were associated with a greater decrease in post-HD serum potassium, whereas higher dialysate bicarbonate and calcium prescriptions were associated with higher post-HD serum bicarbonate and calcium concentrations, respectively. Results were qualitatively unchanged when further adjusted for dialysis session duration (Table 6). Analogous models are presented in subgroup analyses according to the country of origin (Supplemental Table 3). Although there are some qualitative and quantitative differences, the precision and stability of these estimates are limited by the small sample size and low variability across dialysate prescriptions among the patients from India.
Table 6.
Unadjusted and adjusted changes in prehemodialysis to posthemodialysis serum electrolytes according to different dialysate prescriptions
| Dialysate Prescription | Change in Serum Electrolyte (Posthemodialysis Minus Prehemodialysis) in mEq/L or mg/dl,a Mean (SEM) | |||||
| Unadjustedb | P Value | Model 1b | P Value | Model 2b | P Value | |
| Dialysate sodium n/N (%) | ||||||
| 135 (mEq/L) | −0.6 (0.6) | −1.4 (0.5) | −1.5 (0.5) | |||
| 191/1511 (13%) | ||||||
| 138 (mEq/L) | −0.7 (1.2) | −0.4 (0.9) | −0.4 (0.9) | |||
| 146/1511 (10%) | ||||||
| 140 (mEq/L) | 0.5 (0.4) | 0.6 (0.3) | 0.6 (0.3) | |||
| 1174/1511 (78%) | ||||||
| 0.10 | <0.001 | <0.001 | ||||
| Dialysate potassium | ||||||
| 1 and 2 (mEq/L) | −1.3 (0.1) | −1.3 (0.04) | −1.3 (0.04) | |||
| 1336/1630 (82%) | ||||||
| 3 and 4 (mEq/L) | −0.9 (0.1) | −0.9 (0.1) | −0.9 (0.1) | |||
| 294/1630 (18%) | ||||||
| <0.001 | <0.001 | <0.001 | ||||
| Dialysate bicarbonate | ||||||
| 24–32 (mEq/L) | 4.5 (0.5) | 3.7 (0.4) | 3.5 (0.5) | |||
| 351/1610 (22%) | ||||||
| 33–36 (mEq/L) | 4.8 (0.3) | 4.4 (0.3) | 4.5 (0.3) | |||
| 803/1610 (50%) | ||||||
| 37–40 (mEq/L) | 5.1 (0.4) | 6.7 (0.4) | 6.7 (0.4) | |||
| 456/1610 (28%) | ||||||
| 0.57 | <0.001 | <0.001 | ||||
| Dialysate calcium | ||||||
| 1.5 and 1.6 (mEq/L) | 1.2 (0.2) | 0.8 (0.2) | 0.8 (0.2) | |||
| 359/1647 (22%) | ||||||
| 2 and 2.5 (mEq/L) | 0.4 (0.1) | 0.4 (0.1) | 0.4 (0.1) | |||
| 926/1647 (22%) | ||||||
| 3.0 and 3.5 (mEq/L) | 1.1 (0.1) | 1.4 (0.1) | 1.4 (0.1) | |||
| 362/1647 (22%) | ||||||
| <0.001 | <0.001 | <0.001 | ||||
Model 1 adjusted for the pre-dialysis serum concentration; Model 2 adjusted for the predialysis serum concentration and dialysis session length.
Changes in serum sodium, potassium and bicarbonate are presented in mEq/L; changes in serum calcium are presented in mg/dl. P values for the association between categorical dialysate prescription with electrolyte change.
Variability in number of sessions may be due to missing collections or data recording.
Negative sign (−) indicates a decrease.
We developed predictive models to estimate the immediate post-HD serum electrolyte concentration, according to the pre-HD serum concentration and dialysate prescription. In these models, dialysate sodium, potassium, bicarbonate, and calcium were significantly associated with pre- to post-HD changes in the serum concentration of the corresponding electrolyte (Figure 2, Supplemental Tables 4–8). Subgroup analyses according to country of origin are presented in Supplemental Figure 1.
Figure 2.
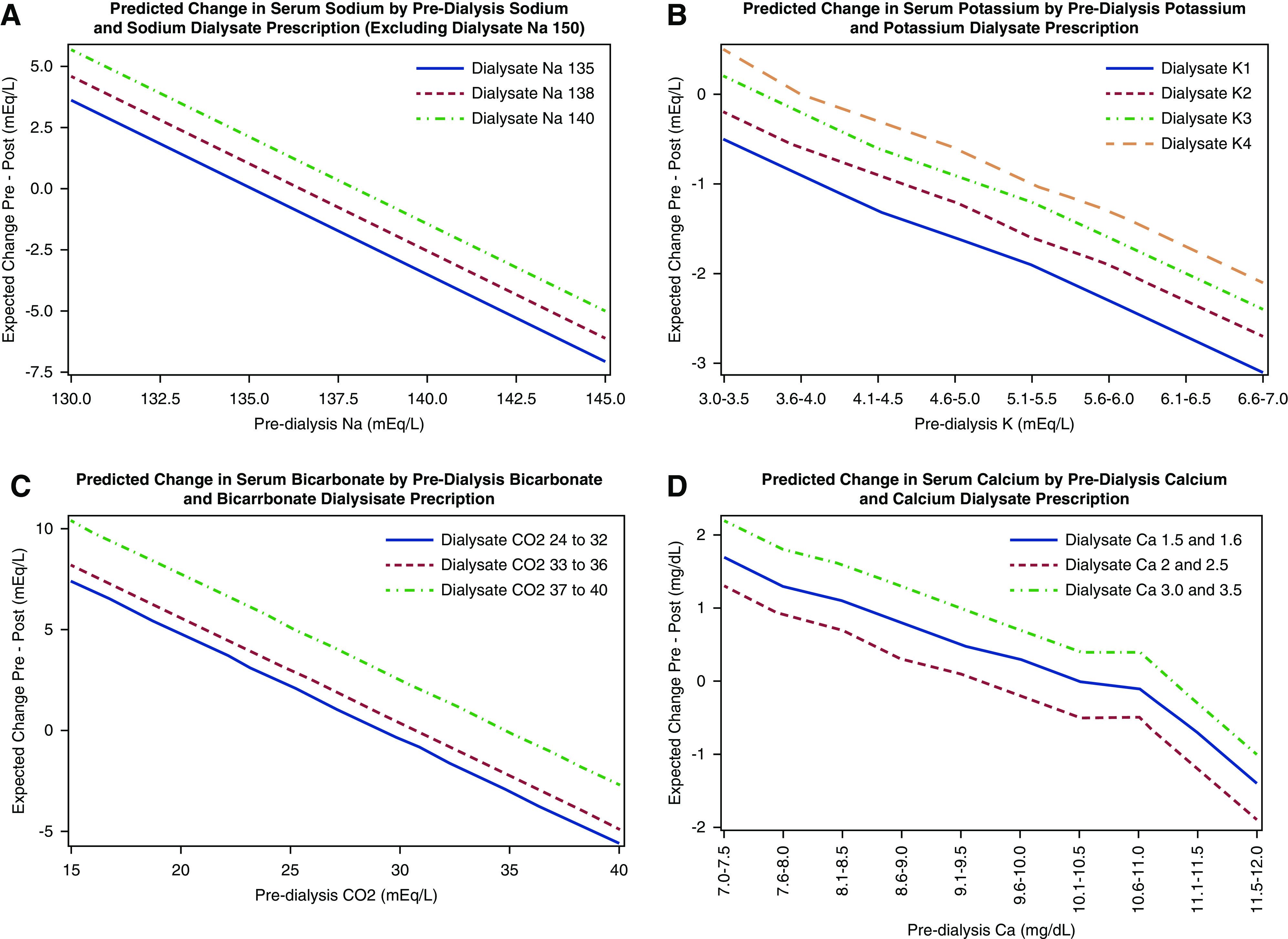
Predicted change in (A) serum sodium, (B) serum potassium, (C) serum bicarbonate, and (D) serum calcium according to baseline serum electrolyte and dialysate prescription (mEq/L). Each graph is on the basis of a model that adjusted for the prehemodialysis (HD) concentration of the electrolyte of interest. For instance, the predictive graph for serum sodium is adjusted for pre-HD serum sodium.
Discussion
Our secondary analysis of the MiD study describes the immediate and post-HD changes in standardly assessed electrolytes, analyzes the association of dialysate prescription with such changes, and develops initial predictive models to estimate the pre- to post-HD change in serum electrolytes. We observed significant increases in serum bicarbonate, calcium, and albumin, and significant decreases in serum potassium, magnesium, and phosphate immediately after contemporary HD sessions. As post-HD measurements are infrequently performed in clinical practice, our results provide important information on temporal changes and highlight that a significant proportion of values fall outside standard laboratory reference ranges.
Maintenance HD provides a lifesaving therapy that has become increasingly available to patients with ESKD across the world. Despite familiarity with this process from over 50 years of clinical use, descriptions of the magnitude of change in serum electrolytes with contemporary practice remain necessary to augment current clinical understanding of the biochemical changes during HD on the one hand, while providing important data for dialysate prescription research on the other.
Dialysate potassium is typically prescribed with the goal of lowering serum (and total body) potassium concentrations that have risen in the interdialytic period. Recent research has called particular attention to the observation that post-HD hypokalemia, especially in the setting of pre-HD hypokalemia, is associated with higher all-cause mortality (7). Although the use of dialysate potassium concentrations <2 mEq/L were uncommon in our study, and have become less common in clinical practice more generally, our data clearly outline a greater magnitude of decline in serum potassium with lower dialysate potassium concentrations, with 40% of sessions having an immediate post-HD potassium below the lower reference limit and 29% with hypokalemia at 30 minutes post-HD. Although serum potassium continues to “rebound” when measured 6 hours post-HD (8), these data support the concept of a higher-risk period peri- and immediately post-HD, which temporally aligns with the periods of highest risk for clinically significant arrhythmia in the primary analyses of the MiD study (6,9). Our findings demonstrate that post-HD hypokalemia is common. Although prior research demonstrates that rebound in serum potassium continues beyond the post-HD timepoints assessed in our study, more proximal post-HD hypokalemia may partially explain the increased risk of arrhythmia in the immediate post-HD period.
In patients on maintenance HD, the intradialytic delivery of bicarbonate facilitates buffering of acidic byproducts of metabolism, which occurs in the interdialytic period. However, this may come at the expense of rapid increases in serum bicarbonate during HD. It has thus been proposed that the optimal dialysate bicarbonate concentration is one that prevents both acidosis between sessions and alkalosis during an HD session (10). As expected, our results demonstrate a substantial increase in serum bicarbonate concentrations during HD sessions, and are consistent with other reports that describe higher post-HD serum concentrations with the use of higher dialysate bicarbonate baths. Interestingly, our data suggest a rebound decrease in serum bicarbonate in the 30 minutes post-HD, which may reflect some element of redistribution. As it is known that patients on HD are 1.7-fold more likely to develop sudden death in the 12 hours after HD (11), more data are required to fully understand the changes in acid-base parameters and associated changes in electrolytes that occur during and between HD sessions.
In these analyses, on average, serum calcium appeared to increase during HD sessions, with higher post-HD serum concentrations noted with use of higher dialysate calcium baths. In recent years, there has been a movement toward the use of lower dialysate calcium concentrations, on the basis of Kidney Disease Improving Global Outcomes guidelines, in an attempt to minimize vascular calcification (12). As with serum bicarbonate, there seems to be a rebound decrease in serum calcium concentrations in the 30 minutes post-HD. Conversely, serum calcium and phosphate exist in biochemical equilibrium. Phosphate levels tended to decrease dramatically during HD and, on the basis of our data, we are unable to fully determine the extent to which changes in serum calcium reflect the influence of dialysate calcium concentration, compared with reduction in serum phosphate. Additionally, we observed changes in serum albumin, suggesting the corrected calcium may not change significantly. The sawtooth pattern of changes in serum albumin and calcium in the postdialytic period is unusual; whether this reflects true changes or artifact in these samples is unclear and requires further investigation. Measurement of ionized calcium concentrations would likely provide a better physiologic measurement for future studies in this regard.
The development of hyperphosphatemia is a common manifestation of progressive CKD and is associated with several adverse outcomes in patients with ESKD on HD (13–15). However, it is less well appreciated that serum phosphate is rather efficiently removed by HD, leading to rapid decline in serum concentrations at the end of an HD session. It is notable that the most rapid decline in serum phosphate occurs early in an HD session (16), which corresponds to the timing of the largest decline in cardiac output and BP (17). Additionally, hypophosphatemia is associated with the development of ventricular arrhythmias (18). Although phosphate-enriched dialysate baths have been used in the setting of hypophosphatemia (19), to our knowledge no research has assessed this association of this practice with outcomes, such as arrhythmia and mortality (20). Our data clearly show significant decreases in phosphate levels during HD, with an average decline in serum phosphate of 3 mg/dl, whereas 67% of immediate post-HD values were below the lower reference limit. Whether this phenomenon predisposes to arrhythmia or other adverse effects during HD, and further, whether the addition of phosphate to the dialysate may mitigate this rapid change, is uncertain, but clearly warrants further study. Our data also confirm the presence of a previously reported rebound increase in phosphate concentrations in the post-HD period (21,22). This rebound is of course expected, as phosphate is a largely intracellular cation (23), with the rebound likely occurring as a result of redistribution from the intracellular stores.
Lastly, our data highlight that serum magnesium is lowered during HD, but does not appear to have the same degree of rebound toward pre-HD concentrations observed for some other electrolytes. Although perhaps less appreciated in the HD population, it has been shown that both extremes of higher (>2.1 mg/dl) and lower (<1.7 mg/dl) serum magnesium concentrations are associated with worse clinical outcomes, including in-hospital mortality (24), whereas other studies have reported an association of hypomagnesemia with all-cause mortality (25). Further, it has been demonstrated that patients on contemporary HD are generally normo- or hypomagnesemic (26), whereas magnesium supplementation and higher magnesium concentrations are associated with less vascular calcification (27,28), and, in MiD, higher serum magnesium was associated with a lower incidence rate of arrhythmia (9). Our data confirm that, with current HD practices, a significant proportion of post-HD magnesium concentrations are abnormal (10% below the lower reference limit and 17% above the upper reference limit), and suggest that greater attention to the choice of magnesium bath may be warranted, although, again, future studies to assess the associations of changes of dialysate bath with clinical outcomes are warranted. Unfortunately, data on the dialysate magnesium concentration were not recorded in MiD.
Although prior studies have assessed intra- and post-HD electrolyte changes (29,30), and some have even generated predictive models for these changes, our analyses extend earlier studies by generating predictive models of multiple post-HD serum electrolytes on the basis of their respective pre-HD serum and dialysate concentrations in the contemporary MiD cohort. We believe our models provide important data that might be used prospectively and tested in clinical trials to assess whether post-HD electrolyte abnormalities can be prevented and whether arrhythmias can be reduced on that basis. Although the subgroup analyses according to country of origin should be interpreted with caution due to their small size and a narrower range of dialysate and serum chemistry values, they do highlight the deficiencies of a “one-size-fits-all” approach to dialysate prescription. Future studies are warranted to confirm and refine such predictive models with the goal of providing personalized HD prescriptions that will minimize adverse cardiovascular outcomes and mortality.
There are several strengths to our analyses. This study uses data from the MiD study, a multicenter prospective cohort with detailed session-level information on pre- and post-HD serum chemistries that were measured at a central laboratory and mandated by protocol. Although prior research has described changes in electrolyte concentrations postdialysis, the relatively large number of samples in our study allowed prediction models to be developed. Additionally, 15- and 30-minute post-HD measurements were available for a subset of patients, allowing analysis of delayed changes. However, some limitations deserve consideration. The modest sample size and concerns of model overfitting precluded extensive adjustment for other variables, such as body weight and ultrafiltration volume. In this respect, although our overall goal was to provide primarily descriptive analyses, the possibility of residual confounding remains, and our results may be underpowered and should be considered with caution. Another limitation is the lack of intradialytic measurements and short duration of post-HD laboratory follow-up (laboratories only obtained the 15- and 30-minute post-HD time points, in comparison to other studies, which drew levels up to the 6-hour post-HD time point) (8). Future studies should assess electrolyte changes during HD and for a longer duration after HD, and should assess the associations of these electrolyte changes with intra- and post-HD arrhythmia for a longer post-HD time interval. Data regarding the association of intra- and post-HD electrolytes with clinical outcomes will be necessary for understanding the implications of abnormal electrolytes specific to the population of patients on HD. Neither ionized calcium nor dialysate magnesium were recorded or measured in MiD, precluding further analyses of these parameters. Further, dialysis baths reflected ordered, rather than measured, concentrations of electrolytes. In a typical “three-stream” dialysis system, manipulation of dialysate bicarbonate, for example, may affect dialysate sodium slightly. However, this would not be expected to induce a clinically relevant difference in the sodium concentration delivered. Although it is unlikely the relationship between pre-Kt/Vs and dialysate chemistry varies widely, our cohort included a modest number of patients from a few centers in the United States and India that may not fully represent the broad range of predialysis concentrations or prescription choices utilized throughout the world. Furthermore, the inclusion and exclusion criteria in this study may have selected potentially patients who are “healthier,” further limiting the generalizability of our findings to those with more extreme pre-HD electrolyte abnormalities.
In conclusion, this secondary analysis of the MiD study provides a detailed assessment of the changes in electrolytes from pre- to post-HD with contemporary HD treatments. Although the sample size is modest, these data provide information for practicing clinicians who wish to better understand the implications of differing dialysate prescriptions on the changes in serum electrolytes from pre- to immediately post-HD; however, prospective validation of these findings is required. This study highlights several areas of unmet need, which are ripe for development of future interventional studies involving the dialysate prescription.
Disclosures
The MiD study was conducted by the MiD Investigators, funded by Medtronic, and designed by Medtronic in collaboration with an advisory committee that included the authors. D.M. Charytan participated in the MiD study as investigator and advisory committee member; reports receiving expert witness fees related to dialysate composition from Fresenius; reports receiving research support from Amgen and Medtronic; reports receiving research support and consulting fees related to services as national investigator, trial steering committee or Data Monitoring Committee of Allena Pharmaceuticals, AstraZeneca (modest), Janssen Pharmaceuticals, Gilead Pharmaceuticals, Novo Nordisk, and Zoll Medical; and reports receiving consulting fees and travel support from Amgen, AstraZeneca, Daichi Sankyo, Fresenius (modest), GlaxoSmithKline, Medtronic/Covidien, and Merck. A.I. Costea reports receiving speaker's bureau for Biotronik and Biosense Webster. V. Kher reports receiving research funding from Astellas India, Novartis India, and Sanofi Aventis India; reports receiving honoraria from Astellas India, Novartis India, Roche India, Torrent, and Reddy's India; reports being a scientific advisor for Biocon India, Medtronic, Novartis India, Reddy's India, Roche India, Sanofi Aventis, and Torrent India; reports receiving speakers bureau from Biocon India, Intas India, Medtronic, Novartis India, Panacea India Roche India, Pfizer, and Sanofi Aventis India. P. Roy-Chaudhury reports being a consultant or on the advisory board of Akebia, Bayer, BD-Bard, Cormedix, Humacyte, Medtronic, WL Gore, and Vifor-Relypsa. D. Williamson reports being COO for American Renal Associates. All remaining authors have nothing to disclose.
Funding
All of the authors, with the exception of S. Correa, K. Scovner, and F.R. Mc Causland, received significant research support and/or consulting fees from Medtronic in relation to the design and conduct of the study. K. Scovner is supported by the American Society of Nephrology Ben J. Lipps Research Fellowship grant. F.R. Mc Causland was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants U01DK096189, R03DK122240, and K23DK102511.
Acknowledgments
The authors would like to thank Mr. Ven Manda, Dr. John Burnes, and Ms. Amy Roettger from Medtronic for support and collaboration on MiD, and Ms. Candace McClure from North American Science Associates for statistical support.
Author Contributions
D.M. Charytan, S. Correa, and F.R. McCausland designed the study; D.M. Charytan acquired the study data; C.K. McClure conducted statistical analyses; S. Correa and K.M. Scovner drafted the manuscript; D.M. Charytan and F.R. Mc Causland provided supervision or mentorship; and all authors analyzed and interpreted the results. Medtronic was responsible for design of the study database, the data collection instruments, and funding the statistical analysis. The authors were responsible for data interpretation and writing the manuscript. Each author contributed important intellectual content during manuscript drafting or revision, and accepts accountability for the overall work by ensuring questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Footnotes
S.C. and K.M.S. contributed equally to this manuscript as first authors.
D.M.C. and F.R.M.C. contributed equally to this manuscript as senior authors.
Contributor Information
Collaborators: MiD Investigators and Committees, Don Williamson, Prabir Roy-Chaudhury, James A. Tumlin, Vijay Kher, Vikranth Reddy, Kowdle Chandrasekhar Prakash, David Charytan, Suresh Chandra Tiwari, Saurabh Pokhariyal, Amber Podoll, Sanjeev Jasuja, G. Leslie Walters, Kraig Wangsnes, Alexandru I. Costea, Selcuk Tombul, Balbir Singh, Brajesh Mishra, Sachin Yalagudri, Abhijeet Shelke, Calambur Narasimhan, A.M. Karthigesan, Abraham Oomman, K P Pramod Kumar, Bruce A. Koplan, Upendra Kaul, Tapan Ghose, Ripen Gupta, Arvind Sethi, Nikhil Kumar, Ramesh Hariharan, Rajnish Sardana, Arif Wahab, N.N Khanna, Mark Smith, Suresh Kamath, Claude Galphin, Puneet Sodhi, Rajsekara Chakravarthy, Subba Rao Budithi, Finnian R. McCausland, Sanjeev Gulati, Munawer Dijoo, Upendra Singh, Salil Jain, Vishal Saxena, Gaurav Sagar, David M. Charytan, Rachel Fissell, Robert Foley, Charles A. Herzog, Peter McCullough, John D. Rogers, James A. Tumlin, Peter Zimetbaum, Manish Assar, Mark Kremers, and Wolfgang C. Winkelmayer
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0007452020/-/DCSupplemental.
Characteristics of the participants at baseline according to country of origin. Download Supplemental Table 1, PDF file, 522 KB
Characteristics of the dialysis prescription at baseline, according to country of origin. Download Supplemental Table 2, PDF file, 522 KB
Changes in pre-HD to post-HD serum electrolytes according to country of origin. Download Supplemental Table 3, PDF file, 522 KB
Fixed-effect estimates from models predicting pre- to immediately post-HD electrolyte change from dialysate concentration and pre-dialysis electrolyte concentration in the MiD Study. Download Supplemental Table 4, PDF file, 522 KB
Predicted change in serum sodium concentration from pre-HD to post-HD measurement. Download Supplemental Table 5, PDF file, 522 KB
Predicted change in serum potassium concentration from pre-HD to post-HD measurement. Download Supplemental Table 6, PDF file, 522 KB
Predicted change in serum bicarbonate concentration from pre-HD to post-HD measurement. Download Supplemental Table 7, PDF file, 522 KB
Predicted change in serum calcium concentration from pre-HD to postHD measurement. Download Supplemental Table 8, PDF file, 522 KB
Predicted change in serum sodium (A, India; B, US), serum potassium (C, India; D, US), serum bicarbonate (E, India; F, US), and serum calcium (G, India; H, US), according to baseline serum electrolyte and dialysate prescription. Each graph is based on a model that adjusted for the pre-HD concentration of the corresponding electrolyte of interest. Download Supplemental Figure 1, PDF file, 522 KB
References
- 1.United States Renal Data System: End-stage Renal Disease (ESRD) in the United States, Chapter 1 incidence, prevalence, patient characteristics, and treatment modalities. In: USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Health, 2017 [Google Scholar]
- 2.National Kidney Foundation: KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update [published correction appears in Am J Kidney Dis 67: 534, 2016]. Am J Kidney Dis 66: 884–930, 2015. 10.1053/j.ajkd.2015.07.015 [DOI] [PubMed] [Google Scholar]
- 3.Richard RJ, John F, Floege J, Tonelli M: Hemodialysis: Principles and techniques. In: Comprehensive Clinical Nephrology, 6th Ed., edited by Kotanko P, Kuhlmann MK, Chan C, Levin NW, United Kingdom, Elsevier Health Sciences, 2019 [Google Scholar]
- 4.Rhee CM, Chou JA, Kalantar-Zadeh K: Dialysis prescription and sudden death. Semin Nephrol 38: 570–581, 2018. 10.1016/j.semnephrol.2018.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charytan DM, Foley R, McCullough PA, Rogers JD, Zimetbaum P, Herzog CA, Tumlin JA; MiD Investigators and Committees: Arrhythmia and sudden death in hemodialysis patients: Protocol and baseline characteristics of the monitoring in dialysis study. Clin J Am Soc Nephrol 11: 721–734, 2016. 10.2215/CJN.09350915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roy-Chaudhury P, Tumlin JA, Koplan BA, Costea AI, Kher V, Williamson D, Pokhariyal S, Charytan DM; MiD investigators and committees: Primary outcomes of the Monitoring in Dialysis Study indicate that clinically significant arrhythmias are common in hemodialysis patients and related to dialytic cycle. Kidney Int 93: 941–951, 2018. 10.1016/j.kint.2017.11.019 [DOI] [PubMed] [Google Scholar]
- 7.Ohnishi T, Kimachi M, Fukuma S, Akizawa T, Fukuhara S: Postdialysis hypokalemia and all-cause mortality in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol 14: 873–881, 2019. 10.2215/CJN.07950718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blumberg A, Roser HW, Zehnder C, Müller-Brand J: Plasma potassium in patients with terminal renal failure during and after haemodialysis; relationship with dialytic potassium removal and total body potassium. Nephrol Dial Transplant 12: 1629–1634, 1997. 10.1093/ndt/12.8.1629 [DOI] [PubMed] [Google Scholar]
- 9.Tumlin JA, Roy-Chaudhury P, Koplan BA, Costea AI, Kher V, Williamson D, Pokhariyal S, Charytan DM; MiD investigators and Committees: Relationship between dialytic parameters and reviewer confirmed arrhythmias in hemodialysis patients in the monitoring in dialysis study. BMC Nephrol 20: 80, 2019. 10.1186/s12882-019-1212-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.John Gennari F: Very low and high predialysis serum bicarbonate levels are risk factors for mortality: What are the Appropriate Interventions? Semin Dial 23: 253–257, 2010. 10.1111/j.1525-139X.2010.00737.x [DOI] [PubMed] [Google Scholar]
- 11.Bleyer AJ, Hartman J, Brannon PC, Reeves-Daniel A, Satko SG, Russell G: Characteristics of sudden death in hemodialysis patients. Kidney Int 69: 2268–2273, 2006. 10.1038/sj.ki.5000446 [DOI] [PubMed] [Google Scholar]
- 12.Uhlig K, Berns JS, Kestenbaum B, Kumar R, Leonard MB, Martin KJ, Sprague SM, Goldfarb S: KDOQI US commentary on the 2009 KDIGO clinical practice guideline for the diagnosis, evaluation, and treatment of CKD-Mineral and Bone Disorder (CKD-MBD). Am J Kidney Dis 55: 773–799, 2010. 10.1053/j.ajkd.2010.02.340 [DOI] [PubMed] [Google Scholar]
- 13.Kraśniak A, Drozdz M, Pasowicz M, Chmiel G, Michałek M, Szumilak D, Podolec P, Klimeczek P, Konieczyńska M, Wicher-Muniak E, Tracz W, Khoa TN, Souberbielle JC, Drueke TB, Sulowicz W: Factors involved in vascular calcification and atherosclerosis in maintenance haemodialysis patients. Nephrol Dial Transplant 22: 515–521, 2007. 10.1093/ndt/gfl564 [DOI] [PubMed] [Google Scholar]
- 14.Young EW, Albert JM, Satayathum S, Goodkin DA, Pisoni RL, Akiba T, Akizawa T, Kurokawa K, Bommer J, Piera L, Port FK: Predictors and consequences of altered mineral metabolism: The Dialysis Outcomes and Practice Patterns Study. Kidney Int 67: 1179–1187, 2005. 10.1111/j.1523-1755.2005.00185.x [DOI] [PubMed] [Google Scholar]
- 15.Slinin Y, Foley RN, Collins AJ: Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: The USRDS waves 1, 3, and 4 study. J Am Soc Nephrol 16: 1788–1793, 2005. 10.1681/ASN.2004040275 [DOI] [PubMed] [Google Scholar]
- 16.DeSoi CA, Umans JG: Phosphate kinetics during high-flux hemodialysis. J Am Soc Nephrol 4: 1214–1218, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Buchanan C, Mohammed A, Cox E, Köhler K, Canaud B, Taal MW, Selby NM, Francis S, McIntyre CW: Intradialytic cardiac magnetic resonance imaging to assess cardiovascular responses in a short-term trial of hemodiafiltration and hemodialysis. J Am Soc Nephrol 28: 1269–1277, 2017. 10.1681/ASN.2016060686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christopoulou EC, Filippatos TD, Megapanou E, Elisaf MS, Liamis G: Phosphate imbalance in patients with heart failure. Heart Fail Rev 22: 349–356, 2017. 10.1007/s10741-017-9615-6 [DOI] [PubMed] [Google Scholar]
- 19.Ing TS, Chebrolu SB, Cheng YL, Yu AW, Choi P, Kjellstrand CM: Phosphorus-enriched hemodialysates: Formulations and clinical use. Hemodial Int 7: 148–155, 2003. 10.1046/j.1492-7535.2003.00023.x [DOI] [PubMed] [Google Scholar]
- 20.Ayus JC, Achinger SG, Mizani MR, Chertow GM, Furmaga W, Lee S, Rodriguez F: Phosphorus balance and mineral metabolism with 3 h daily hemodialysis. Kidney Int 71: 336–342, 2007. 10.1038/sj.ki.5002044 [DOI] [PubMed] [Google Scholar]
- 21.Haas T, Hillion D, Dongradi G: Phosphate kinetics in dialysis patients. Nephrol Dial Transplant 6[Suppl 2]: 108–113, 1991 [PubMed] [Google Scholar]
- 22.Mucsi I, Hercz G: Control of serum phosphate in patients with renal failure--new approaches. Nephrol Dial Transplant 13: 2457–2460, 1998. 10.1093/ndt/13.10.2457 [DOI] [PubMed] [Google Scholar]
- 23.Kemp GJ, Meyerspeer M, Moser E: Absolute quantification of phosphorus metabolite concentrations in human muscle in vivo by 31P MRS: A quantitative review. NMR Biomed 20: 555–565, 2007. 10.1002/nbm.1192 [DOI] [PubMed] [Google Scholar]
- 24.Cheungpasitporn W, Thongprayoon C, Qian Q: Dysmagnesemia in hospitalized patients: Prevalence and prognostic importance. Mayo Clin Proc 90: 1001–1010, 2015. 10.1016/j.mayocp.2015.04.023 [DOI] [PubMed] [Google Scholar]
- 25.Ishimura E, Okuno S, Yamakawa T, Inaba M, Nishizawa Y: Serum magnesium concentration is a significant predictor of mortality in maintenance hemodialysis patients. Magnes Res 20: 237–244, 2007 [PubMed] [Google Scholar]
- 26.Alhosaini M, Leehey DJ: Magnesium and dialysis: The neglected cation. Am J Kidney Dis 66: 523–531, 2015. 10.1053/j.ajkd.2015.01.029 [DOI] [PubMed] [Google Scholar]
- 27.Turgut F, Kanbay M, Metin MR, Uz E, Akcay A, Covic A: Magnesium supplementation helps to improve carotid intima media thickness in patients on hemodialysis. Int Urol Nephrol 40: 1075–1082, 2008. 10.1007/s11255-008-9410-3 [DOI] [PubMed] [Google Scholar]
- 28.Tzanakis I, Virvidakis K, Tsomi A, Mantakas E, Girousis N, Karefyllakis N, Papadaki A, Kallivretakis N, Mountokalakis T: Intra- and extracellular magnesium levels and atheromatosis in haemodialysis patients. Magnes Res 17: 102–108, 2004 [PubMed] [Google Scholar]
- 29.Agar BU, Akonur A, Lo YC, Cheung AK, Leypoldt JK: Kinetic model of phosphorus mobilization during and after short and conventional hemodialysis. Clin J Am Soc Nephrol 6: 2854–2860, 2011. 10.2215/CJN.03860411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudnicki M, Frølich A, Haaber A, Tvedegaard E, Thode J: Serum ionized calcium, parathyroid hormone and phosphate in uremic patients during and between hemodialysis. Clin Nephrol 40: 225–229, 1993 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of the participants at baseline according to country of origin. Download Supplemental Table 1, PDF file, 522 KB
Characteristics of the dialysis prescription at baseline, according to country of origin. Download Supplemental Table 2, PDF file, 522 KB
Changes in pre-HD to post-HD serum electrolytes according to country of origin. Download Supplemental Table 3, PDF file, 522 KB
Fixed-effect estimates from models predicting pre- to immediately post-HD electrolyte change from dialysate concentration and pre-dialysis electrolyte concentration in the MiD Study. Download Supplemental Table 4, PDF file, 522 KB
Predicted change in serum sodium concentration from pre-HD to post-HD measurement. Download Supplemental Table 5, PDF file, 522 KB
Predicted change in serum potassium concentration from pre-HD to post-HD measurement. Download Supplemental Table 6, PDF file, 522 KB
Predicted change in serum bicarbonate concentration from pre-HD to post-HD measurement. Download Supplemental Table 7, PDF file, 522 KB
Predicted change in serum calcium concentration from pre-HD to postHD measurement. Download Supplemental Table 8, PDF file, 522 KB
Predicted change in serum sodium (A, India; B, US), serum potassium (C, India; D, US), serum bicarbonate (E, India; F, US), and serum calcium (G, India; H, US), according to baseline serum electrolyte and dialysate prescription. Each graph is based on a model that adjusted for the pre-HD concentration of the corresponding electrolyte of interest. Download Supplemental Figure 1, PDF file, 522 KB