ABSTRACT
Antigen-based rapid diagnostics tests (Ag-RDTs) are useful tools for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) detection. However, misleading demonstrations of the Abbott Panbio coronavirus disease 2019 (COVID-19) Ag-RDT on social media claimed that SARS-CoV-2 antigen could be detected in municipal water and food products. To offer a scientific rebuttal to pandemic misinformation and disinformation, this study explored the impact of using the Panbio SARS-CoV-2 assay with conditions falling outside manufacturer recommendations. Using Panbio, various water and food products, laboratory buffers, and SARS-CoV-2-negative clinical specimens were tested with and without manufacturer buffer. Additional experiments were conducted to assess the role of each Panbio buffer component (tricine, NaCl, pH, and Tween 20) as well as the impact of temperature (4°C, 20°C, and 45°C) and humidity (90%) on assay performance. Direct sample testing (without the kit buffer) resulted in false-positive signals resembling those obtained with SARS-CoV-2 positive controls tested under proper conditions. The likely explanation of these artifacts is nonspecific interactions between the SARS-CoV-2-specific conjugated and capture antibodies, as proteinase K treatment abrogated this phenomenon, and thermal shift assays showed pH-induced conformational changes under conditions promoting artifact formation. Omitting, altering, and reverse engineering the kit buffer all supported the importance of maintaining buffering capacity, ionic strength, and pH for accurate kit function. Interestingly, the Panbio assay could tolerate some extremes of temperature and humidity outside manufacturer claims. Our data support strict adherence to manufacturer instructions to avoid false-positive SARS-CoV-2 Ag-RDT reactions, otherwise resulting in anxiety, overuse of public health resources, and dissemination of misinformation.
IMPORTANCE With the Panbio severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigen test being deployed in over 120 countries worldwide, understanding conditions required for its ideal performance is critical. Recently on social media, this kit was shown to generate false positives when manufacturer recommendations were not followed. While erroneous results from improper use of a test may not be surprising to some health care professionals, understanding why false positives occur can help reduce the propagation of misinformation and provide a scientific rebuttal for these aberrant findings. This study demonstrated that the kit buffer’s pH, ionic strength, and buffering capacity were critical components to ensure proper kit function and avoid generation of false-positive results. Typically, false positives arise from cross-reacting or interfering substances; however, this study demonstrated a mechanism where false positives were generated under conditions favoring nonspecific interactions between the two antibodies designed for SARS-CoV-2 antigen detection. Following the manufacturer instructions is critical for accurate test results.
KEYWORDS: COVID-19, SARS-CoV-2, antigen, false positive, Panbio, clinical methods, diagnostics, epidemiology, virology
INTRODUCTION
High demand for diagnostic testing during the coronavirus disease 2019 (COVID-19) pandemic led to the development of various technologies for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) detection (1). Nucleic acid amplification tests (NAATs), like real-time PCR (RT-PCR), are considered the reference methods (1–3), but antigen-based rapid diagnostic tests (Ag-RDTs) have been widely used due to their ease of use, rapid results, and ability to be performed outside a laboratory setting (1). Many Ag-RDTs have been licensed as point-of-care (POC) devices for SARS-CoV-2 detection (4, 5), but their performance can vary between methods, testing frequency, and settings in which they are used (6–12). Ag-RDTs are well recognized to be less sensitive and specific than commercial NAATs, and false-positive results from Ag-RDTs are known to occur, particularly in settings of low disease prevalence (13, 14).
The intended use of the COVID-19 Ag rapid test device is qualitative detection of SARS-CoV-2 antigen (i.e., nucleocapsid protein) from nasal swabs (or nasopharyngeal [NP] swabs, depending on the formulation of the kit). The manufacturer kit insert states that instructions must be strictly followed by a trained health care professional to achieve accurate results, and the kit includes a buffer used for antigen extraction from the swabs used for specimen collection as well as viral inactivation. However, misleading demonstrations of a SARS-CoV-2 Ag-RDT (i.e., Panbio) on social media platforms have claimed that SARS-CoV-2 antigen can readily be detected in municipal water and commercial food and beverages if tested directly on the Panbio test device (15–18). Moreover, on social media, the misuse of an Ag-RDT was propagated by pupils in attempts to miss time in school (15–18). However, in both of these examples, the results are erroneous as direct testing of samples onto the Ag-RDT device is not recommended by the manufacturer. With misinformation and disinformation often perpetuated on social media and aberrant results obtained from improper use of the kit, unsubstantiated claims can undermine confidence in SARS-CoV-2 diagnostic testing and erode trust in public health efforts. As such, it is important to use science-based approaches to demonstrate that while nonspecific reactivity can occur when testing is performed under inappropriate conditions, SARS-CoV-2 is not truly present in food or potable water samples. For health care professionals, aberrant test results arising from procedures that deviate from the kit instructions would not be surprising. When manufacturer instructions are followed, the expected false-positivity rate would be very low (i.e., between 0.4 and 1.2%) (6–12), and the positive Ag-RDTs are often repeated using an alternative method, such as a NAAT (1–3). However, the cause of false-positive Ag-RDT reactions are rarely investigated.
This study deliberately evaluated conditions that fell outside those recommended by the manufacturer, which had the potential to generate aberrant Ag-RDT reactions, including unregulated buffering capacity or ionic strength and extremes of temperature, humidity, and pH. As expected from social media claims, direct testing of a wide variety of food products and water samples generated false-positive results with the Panbio Ag-RDT; however, this prompted further investigations into the underlying mechanism of artifact generation. Panbio kit extraction buffer was omitted, diluted, or reverse engineered to help demonstrate the importance of the buffer and each of its components. Overall, by identifying conditions that could favor artifact generation, this study not only helps provide evidence supporting the importance of following manufacturer instructions but also helps in the understanding of possible causes of false-positive reactions using Ag-RDTs, which can be informative to health care professionals, test manufacturers, and other users of the products.
RESULTS
False positives in food, water, buffers, media, and clinical specimens.
With the exception of soft drinks and some milk products with high fat content that produced negative or weak false-positive reactions, most of the food products that were tested directly onto the Panbio cassette (Fig. 1) produced a strong positive SARS-CoV-2 signal that resembled those obtained with the kit positive control (Table 1). It is interesting that milk products are often used as blocking agents in immunoassays to prevent nonspecific binding of antibodies (1, 19). Direct testing of known highly acidic samples caused invalid results for both Panbio and Veritor. All other products were Veritor negative. When nasal swabs were used to sample the various products and processing occurred with manufacturer buffer, no false positives or invalid results were observed.
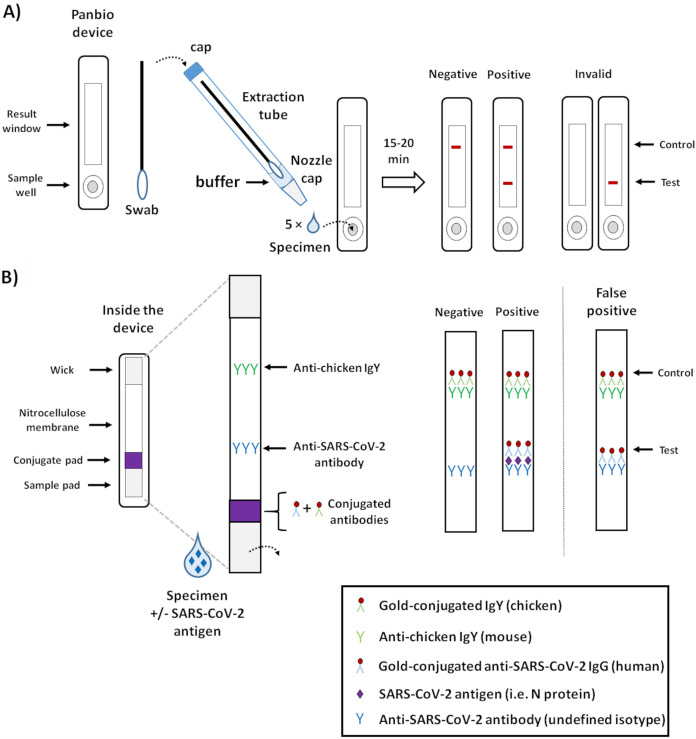
FIG 1.
Summary and principle of the Panbio COVID-19 Ag rapid test device. (A) Panbio kit components and summary of the test procedure. The Panbio kit is designed for detection of SARS-CoV-2 antigen (i.e., nucleocapsid protein or N protein). Following specimen collection, the swab is placed into an extraction tube prefilled with 11 to 12 drops (or 300 μl) of buffer, and the tube cap is added. The tube is pinched to help extract the respiratory secretions from the swab, which in turn is rotated into the buffer. The nozzle cap is removed from the extraction tube, and 5 drops are placed into the sample well of the Panbio lateral flow device. After 15 to 20 min, the results are read and interpreted as depicted. (B) The principle of the Panbio Ag-RDT relies on a nitrocellulose membrane precoated with anti-chicken IgY at the control line and an anti-SARS-CoV-2-specific antibody at the test line. When the buffer/specimen solution is added to the sample well, the liquid flows progressively through the device using capillary action and sequentially flows through the sample pad, the conjugate pad, the nitrocellulose membrane, and eventually into the wick. As the liquid comes into contact with the conjugate pad, both conjugated antibodies are resuspended (i.e., the gold-conjugated chicken IgY and the gold-conjugated human IgG specific to SARS-CoV-2). In the absence of SARS-CoV-2 antigen (i.e., N protein), the conjugated anti-SARS-CoV-2 antibody will not interact with the anti-SARS-CoV-2 capture antibody at the test line; however, the conjugated chicken IgY will be captured by the anti-chicken IgY immobilized at the control line. This generates a red-colored band that can be visualized. In the presence of SARS-CoV-2 antigen, a similar reaction occurs at the test line due to the interaction between the antigen and the conjugated and capture anti-SARS-CoV-2 antibodies. As seen in this study, false positives can occur with testing conditions falling outside the manufacturer instructions, depicted here as nonspecific interactions between the conjugated and capture anti-SARS-CoV-2 antibodies in the absence of antigen.
TABLE 1.
Samples tested by SARS-CoV-2 Ag-RDTs with and without manufacturer buffer
| Category | Branda | Description | Avg. pH (± SD)a | Panbio result |
Veritor result |
RT-PCR result | ||
|---|---|---|---|---|---|---|---|---|
| Sample, direct | Swab of sample in buffer | Sample, direct | Swab of sample in buffer | |||||
| Food products (n = 33) | Bragg | Apple cider vinegar | NA | INV | NEG | INV | NEG | NA |
| NA | Lemon, juice (fresh) | NA | INV | NEG | INV | NEG | NA | |
| NA | Lime, juice (fresh) | NA | INV | NEG | INV | NEG | NA | |
| Nakano | Rice vinegar | NA | INV | NEG | NEG | NEG | NA | |
| Natrel | Milk, lactose-free | NA | NEG | NEG | NEG | NEG | NA | |
| Scotsburn | 18% cream | NA | NEG | NEG | NEG | NEG | NA | |
| Scotsburn | Milk, 2% | NA | NEG | NEG | NEG | NEG | NA | |
| Farmer’s | Milk, 2% | NA | POSb | NEG | NEG | NEG | NA | |
| Farmer’s | Milk, 1% | NA | POSb | NEG | NEG | NEG | NA | |
| Farmer’s | Milk, fat-free skim | NA | POS | NEG | NEG | NEG | NA | |
| Bubbly | Soda | NA | POSb | NEG | NEG | NEG | NA | |
| Pepsi | Soda | NA | POSb | NEG | NEG | NEG | NA | |
| Coca-Cola | Soda | NA | POSb | NEG | NEG | NEG | NA | |
| Tim Horton’s | Apple juice, pure | NA | POSb | NEG | NEG | NEG | NA | |
| Tim Horton’s | Coffee, black | NA | POS | NEG | NEG | NEG | NA | |
| Simply | Apple juice, pure pressed | NA | POS | NEG | NEG | NEG | NA | |
| NA | Honeycrisp apple, juice (fresh) | NA | POS | NEG | NEG | NEG | NA | |
| NA | Tangerine, juice (fresh) | NA | POS | NEG | NEG | NEG | NA | |
| NA | Watermelon, juice (fresh) | NA | POS | NEG | NEG | NEG | NA | |
| NA | Red grapes, juice (fresh) | NA | POS | NEG | NEG | NEG | NA | |
| NA | Green grapes, juice (fresh) | NA | POS | NEG | NEG | NEG | NA | |
| NA | Peach, juice (fresh) | NA | POS | NEG | NEG | NEG | NA | |
| NA | Wild blueberries, juice (fresh) | NA | POS | NEG | NEG | NEG | NA | |
| NA | Tomato, juice (fresh) | NA | POS | NEG | NEG | NEG | NA | |
| NA | English cucumber, juice (fresh) | NA | POS | NEG | NEG | NEG | NA | |
| Heinz | Relish | NA | POS | NEG | NEG | NEG | NA | |
| Heinz | Ketchup | NA | POS | NEG | NEG | NEG | NA | |
| Heinz | Mustard | NA | POS | NEG | NEG | NEG | NA | |
| Stellenbosch | Wine, cabernet | NA | POS | NEG | NEG | NEG | NA | |
| Bud Light | Beer | NA | POS | NEG | NEG | NEG | NA | |
| Fisherman’s Helper | Rum, white | NA | POS | NEG | NEG | NEG | NA | |
| Crown Royal | Maple finished whisky | NA | POS | NEG | NEG | NEG | NA | |
| Jameson | Irish whiskey | NA | POS | NEG | NEG | NEG | NA | |
| Water samples (n = 24) |
Sigma Life Sciences | Double processed, tissue culture water, sterile filtered | 4.00 (±0.01) | POS | NEG | NEG | NEG | NEG |
| Montellier | Carbonated spring water | 4.68 (±0.00) | POS | NEG | NEG | NEG | NEG | |
| Invitrogen | Ultrapure distilled water, DNase- and RNase-free | 4.80 (±0.01) | POS | NEG | NEG | NEG | NEG | |
| Aquafina | Demineralized water, reverse osmosis | 5.05 (±0.01) | POS | NEG | NEG | NEG | NEG | |
| S. Pellegrino | Carbonated natural mineral water | 5.09 (±0.01) | POS | NEG | NEG | NEG | NEG | |
| Canadian Springs | Distilled water, ozonated | 5.31 (±0.01) | POS | NEG | NEG | NEG | NEG | |
| Dasani | Remineralized water, reverse osmosis treated | 5.68 (±0.01) | POS | NEG | NEG | NEG | NEG | |
| Big8 | Distilled water, ozonated | 6.25 (±0.01) | POS | NEG | NEG | NEG | NEG | |
| Big8 | Spring water, ozonated | 6.26 (±0.01) | POS | NEG | NEG | NEG | NEG | |
| Glaceau Smart | Vapor distilled water with added electrolytes | 6.71 (±0.02) | POS | NEG | NEG | NEG | NEG | |
| NA | Municipal water (Halifax, Nova Scotia, 12 May 2021) | 6.75 (±0.02) | POS | NEG | NEG | NEG | NEG | |
| Fiji | Natural spring water, tropical rain filtered through volcanic rock | 7.25 (±0.02) | POS | NEG | NEG | NEG | NEG | |
| Simple Drop | Natural spring water | 7.26 (±0.02) | POS | NEG | NEG | NEG | NEG | |
| Pathwater | Purified water, reverse osmosis treated, ozonated, and electrolytes added, pH balanced, pH 7.5+ | 7.27 (±0.01) | POS | NEG | NEG | NEG | NEG | |
| Art Life WTR | Purified water, mineralized and electrolytes added, pH balanced | 7.28 (±0.01) | POS | NEG | NEG | NEG | NEG | |
| Evian | Spring water, natural electrolytes, pH 7.2 | 7.39 (±0.02) | POS | NEG | NEG | NEG | NEG | |
| NA | Rainwater (Halifax, Nova Scotia, 12 May 2021) | 7.47 (±0.01) | POS | NEG | NEG | NEG | NEG | |
| Icelandic Glacial | Natural spring water | 7.58 (±0.02) | POS | NEG | NEG | NEG | NEG | |
| Nestle Pure Life | Natural spring water, ozonated | 7.75 (±0.02) | POS | NEG | NEG | NEG | NEG | |
| Earth Group | Spring water | 7.80 (±0.03) | POS | NEG | NEG | NEG | NEG | |
| Eska | Natural spring water, pH 7.4 | 7.81 (±0.00) | POS | NEG | NEG | NEG | NEG | |
| Smart Moodwater | Naturally alkaline spring water, pH 8+ | 7.91 (±0.01) | POS | NEG | NEG | NEG | NEG | |
| Flow | Naturally alkaline spring water, pH 8.1 | 8.02 (±0.00) | POS | NEG | NEG | NEG | NEG | |
| Glaceau Smart | Mineralized treated water, alkaline pH 9+ | 9.33 (±0.00) | POS | NEG | NEG | NEG | NEG | |
| Laboratory buffers and media (n = 14) |
Teligent | 0.9% saline | 5.62 (±0.02) | POS (weak) | NEG | NEG | NEG | NEG |
| Boston BioProducts | 0.5 M Pipes buffer, pH 6.8 | 6.66 (±0.01) | POSb | NEG | NEG | NEG | NEG | |
| Fisher Scientific | 10 mM Tris-HCl, 1 mM EDTA (TE) buffer, molecular grade, pH 7.4 | 7.14 (±0.01) | POS | NEG | NEG | NEG | NEG | |
| Sigma Life Sciences | Dulbecco’s phosphate buffered saline (PBS) | 7.18 (±0.01) | POSb | NEG | NEG | NEG | NEG | |
| LiofilChem | Viral transport media (VTM) | 7.24 (±0.01) | POSb | NEG | NEG | NEG | NEG | |
| Redoxica | Viral transport media (VTM) with fetal bovine serum (FBS) | 7.33 (±0.01) | POSb | NEG | NEG | NEG | NEG | |
| Becton Dickinson | Veritor sample buffer | 7.33 (±0.00) | NEG | NEG | NEG | NEG | NEG | |
| Copan Diagnostics | Universal transport medium (UTM) | 7.37 (±0.01) | NEG | NEG | NEG | NEG | NEG | |
| Yokon | Universal transport medium (UTM) | 7.44 (±0.01) | NEG | NEG | NEG | NEG | NEG | |
| Gibco | RPMI medium 1640, with HEPES | 7.48 (±0.01) | NEG | NEG | NEG | NEG | NEG | |
| Genesis | KaiBiLi extended ViralTrans, includes HEPES | 7.50 (±0.01) | NEG | NEG | NEG | NEG | NEG | |
| Gibco | Minimal essential media (MEM) | 7.83 (±0.01) | NEG | NEG | NEG | NEG | NEG | |
| Gibco | 1× phosphate buffered saline (PBS), pH 7.4 | 8.20 (±0.01) | NEG | NEG | NEG | NEG | NEG | |
| Abbott | Panbio sample buffer | 8.78 (±0.01) | NEG | NEG | NEG | NEG | NEG | |
| Clinical specimens (n = 120) |
NA | NP swabs in UTM (n = 30) | NA | POSb (28/30) | NEG | NEG | NEG | NEG |
| NA | OP/N swabs in PBS (n = 30) | NA | POSb (26/30) | NEG | NEG | NEG | NEG | |
| NA | BAL samples (n = 30) | NA | POSb (27/30) | NEG | NEG | NEG | NEG | |
| NA | Saline gargles (n = 30) | NA | POSb (27/30) | NEG | NEG | NEG | NEG | |
NA, not available; NEG, negative; POS, positive; INV, invalid.
Only weak positive reactions were observed.
Multiple water samples were evaluated with tested pH values between 4.00 and 9.33 and differences in supplier-described purification methods and mineral and electrolyte composition (Table 1). Direct testing onto Panbio test devices showed strong false-positive SARS-CoV-2 signals, while samples diluted in Panbio buffer did not produce any artifacts. Notably, water samples near the pH of the Panbio buffer (pH 8.78) also displayed strong false-positive signals, suggesting that the mechanism behind artifact formation is not, or not solely, pH dependent. To investigate the possible roles of buffering capacity and ionic strength, commonly used laboratory buffers and buffer-containing viral transport medium spanning various pH values (5.62 to 8.78) were tested (Table 1). With the exception of Tris-EDTA (TE), all other buffers and media generated weakly positive or negative results (Table 1). All water samples, buffers, and media were RT-PCR and Veritor negative, suggesting absence of viral RNA and nucleocapsid antigen, respectively (Table 1).
Given that weak false-positive results were observed with universal transport medium (UTM), phosphate-buffered saline (PBS), and saline, direct testing was performed on clinical specimens containing these media and buffers. With direct testing onto Panbio cassettes, false-positive results were seen in 93.3% of NP swabs in UTM, 86.7% of oropharyngeal and bilateral nares (OP/N) swabs in PBS, 90.0% of bronchoalveolar lavage (BAL) specimens, and 90.0% of the saline gargles (Table 1). All specimens were negative when Panbio buffer was used, which was consistent with the Veritor and RT-PCR results.
Role of the Panbio buffer and its components.
Panbio buffer diluted in water at ratios greater than 1:8, and occasionally 1:10, resulted in artifact formation (Fig. 2A). Similarly, when buffering capacity was poor or lost when using low tricine concentrations (1 or 10 mM), strong false-positive signals were seen across a broad range of pH values (Fig. 2B). In contrast, high tricine concentrations (100 mM or 1 M) prevented artifact formation at a pH of 9 and above, which is consistent with the measured pH of Panbio buffer at 8.78 (Fig. 2B and Table 1). Similar to the buffering capacity, regulated ionic strength also played an important role, as 100 mM tricine solutions supplemented with high NaCl concentrations (100 mM or 1 M) reduced or prevented false-positive results, whereas the same solutions in the presence of lower NaCl concentrations (1 and 10 mM) (Fig. 2C) mirrored the results of NaCl-free 100 mM tricine solutions presented in Fig. 2B. Invalid results were sometimes obtained at pH values of 3 and 12 (Fig. 2B). Tween 20 (1%) was added to all tricine solutions but had no impact on results (data not shown). Antimicrobial agents in the Panbio buffer (i.e., ProClin 300 and sodium azide) were not investigated due to their unlikely contribution to artifact generation.
FIG 2.
False-positive SARS-CoV-2 Ag-RDT results can occur from Panbio buffer absence, dilution, or alterations. (A) Artifact generation by Panbio buffer dilution in PCR-grade water at different temperatures (4°C, 25°C, and 37°C). False-positive SARS-CoV-2 Ag-RDT result occurrence from uncontrolled pH and buffering conditions (B) or from changes in ionic strength from NaCl (C) are shown. Of note, results presented in Fig. 2C correspond to those obtained from solutions of 100 mM tricine, in which different concentrations of NaCl were prepared. All experiments were performed in the absence of SARS-CoV-2 antigen. False-positive reactions are indicated as POS (in red) under each Ag-RDT result; NEG, negative; INV, invalid.
Investigations into the mechanism of artifact generation.
Following conjugate pad transplantation (Fig. 3A), positive- and negative-control swabs displayed expected results after inoculation onto reassembled Panbio cassettes in which resuspended conjugated antibodies were reintroduced. Water-resuspended conjugated antibody generated a strong false-positive target signal, which was eliminated following proteinase K (PK) treatment (Fig. 3B). Removal of the gold-conjugated IgY antibody from the conjugate suspensions did not impair Panbio test performance, and the strong false-positive SARS-CoV-2 signal from water remained (Fig. 3C). These findings suggest that the gold-conjugated human anti-SARS-CoV-2 IgG is responsible for the nonspecific interactions with the immobilized anti-SARS-CoV-2 capture antibody on the test device nitrocellulose membrane. Thermal shift assays in 100 mM tricine solutions were used to compare structural differences of the anti-SARS-CoV-2 IgG at pH values consistent (i.e., pH of 5 to 7) or inconsistent (i.e., pH of 8 to 10) with artifact formation (Fig. 4A and B). Tm values were significantly different in tricine solutions between pH 5 and 7 (at 68.4°C ± 2.6°C, 71.4°C ± 1.2°C, and 72.5°C ± 1.0°C, respectively) compared to between pH 8 and 10 (at 75.6°C ± 1.0°C, 76.8°C ± 1.1°C, and 77.6°C ± 1.4°C, respectively) (Fig. 4A and B). Tm values at pH 4 and 11 were inconsistent, while no Tm values could be established at pH 3 and 12. Of note, the Panbio buffer could not be used directly for thermal shift experiments due to high background fluorescence with SYPRO orange. The cause of this background fluorescence was revealed in tricine solutions containing 1% Tween 20, which demonstrated similar interference.
FIG 3.
Impact of proteinase K (PK) and heat treatment on the conjugated SARS-CoV-2 antibody. (A) Conjugate pad transplantation was used to access and investigate properties of the proprietary Panbio conjugated antibodies. Each step was followed as depicted, leading to treatment of the conjugated antibodies with proteinase K (PK) or heat (T°C), and comparisons were made with untreated controls (none). In some experiments (dashed arrows), the gold-conjugated antibody suspensions were pretreated with mouse anti-chicken IgY (obtained from a fragment of the nitrocellulose membrane at the control line) to purify the gold-conjugated human IgG specific for SARS-CoV-2 conjugated antibody. This suspension was used for subsequent lateral flow and thermal shift assays; RT, room temperature. (B) PK and heat treatments of the conjugated antibodies. Using conjugate pad transplantation, gold-conjugated antibody suspensions in Panbio buffer or water were treated for 1 h with PK at 56°C, followed by heat inactivation of PK at 70°C for 10 min. Following reintroduction into Panbio cassettes of conjugated antibodies that were untreated (none), heat treated (T°C), or PK treated, water was inoculated in the sample well. (C) Removal of the conjugated chicken IgY from the conjugated antibody suspensions to purify the conjugated SARS-CoV-2-specific antibody. Untreated (none) or pretreatment (−IgY) are depicted for reassembled Panbio cassettes containing the purified conjugated SARS-CoV-2-specific antibody, which was then inoculated with PCR-grade water. For B and C, similar reactions as performed for water were undertaken with a positive- or negative-control swab to demonstrate that the method did not impact conjugate antibody function.
FIG 4.
Thermal shift profiles for the Panbio gold-conjugated human IgG specific to SARS-CoV-2 at different pH values. All reactions were performed in 100 mM tricine, and representative thermal shift profiles are presented (A). Melting temperature (Tm) changes based on pH are summarized (B) as well as which conditions were consistent (i.e., pH 5 to 7) or inconsistent (i.e., pH 8 to 10) with the generation of false-positive results when tested by Ag-RDT.
Impact of heat and humidity on Panbio kit function.
In all test conditions evaluated (Table S1 in the supplemental material), no deleterious effects on test sensitivity or specificity were observed concerning temperature or humidity. In a complementary series of experiments, Panbio buffer dilutions showed similar findings, regardless of operating temperature (Fig. 2A).
DISCUSSION
False-positive and false-negative results occur with any diagnostic test but are increasingly likely when manufacturer recommendations are not followed (1, 13, 14). This study demonstrated that in the absence of manufacturer buffer, a variety of food, water, laboratory buffer, specimen transport media, and clinical specimens resulted in false-positive reactions with the Panbio Ag-RDT. These data are consistent with others (20) who recognized the importance of Ag-RDT kit buffers. However, false-negative results would typically be expected with aberrant test conditions. The generation of false-positive signals demonstrated in this study prompted an investigation into the underlying causes of this phenomenon. Uncontrolled conditions of pH, buffering capacity, and ionic strength all favored artifact generation, whereas temperature and humidity were not contributory under the tested parameters. In review of the literature, possible causes of false-positive Ag-RDT results include cross-reactions (21), interfering substances (19), and improper operating or storage conditions for temperature or humidity (22). Cross-reacting or interfering substances common to all samples tested in the study are unlikely. Temperature extremes have been shown to induce conformational changes in SARS-CoV-2 antibodies, leading to nonspecific binding (23); however, in this study, Panbio was unaffected by the temperature and humidity conditions evaluated. As described below, the most likely cause of Panbio false-positive results was aberrant protein-protein interactions faced with improper buffer conditions, ionic strength, or pH.
In a previous study (20), 20 of 27 of the malaria Ag-RDT brands evaluated showed false-positive reactions when the manufacturer buffer was replaced with saline, tap water, or distilled water. Distilled water alone generated false-positive reactions (20), similar to what was observed in this study with Panbio (Table 1). Possible explanations for their findings included inefficient resuspension of blocking agents, altered capillary flow rates, decreased flushing of contaminating substances, and finally nonspecific interactions between the conjugated and capture antibodies faced with uncontrolled buffering and ionic strength conditions (20). Tricine is a zwitterionic amino acid with a pKa of 8.26 and would be negatively charged at the measured pH of 8.78 in the Panbio buffer. Therefore, under recommended testing conditions, tricine may mask positively charged residues on the SARS-CoV-2-specific conjugated and capture antibodies, while uncontrolled buffer conditions would favor aberrant electrostatic or hydrophobic interactions between the two antibodies, resulting in false-positive results. Supporting this theory, PK treatment eliminated the false-positive Panbio results generated by water, and the propensity to generate this artifact varied with buffering capacity, pH, and ionic strength. Removal of the gold-conjugated chicken IgY (used for control band detection) did not alter formation of the SARS-CoV-2 target artifact formation, suggesting the conjugated SARS-CoV-2-specific IgG alone is responsible for artifact formation through nonspecific binding to the SARS-CoV-2-specific capture antibody immobilized on the nitrocellulose membrane (Fig. 1B). Finally, thermal shift assays were performed on the conjugated anti-SARS-CoV-2 IgG, and pH-dependent conformation changes were observed under conditions causing false-positive results or not (Fig. 4). This study was not able to further investigate pH-dependent binding interactions between the conjugated and capture anti-SARS-CoV-2 antibodies, as the latter is immobilized on the nitrocellulose membrane and not available in an unfixed formulation due to the proprietary nature of the Panbio assay.
False-positive reactions with the Panbio Ag-RDT have the potential to cause a significant impact to Public Health. To date, over 200 million Panbio Ag-RDT tests have been distributed to over 120 countries worldwide for use in health care settings, businesses, or home self-testing. In low-prevalence populations, positive Ag-RDTs are typically confirmed by clinical laboratories with NAATs, thereby limiting the overall public health impact of the possible artifacts described in this study (1–3). However, in programs where home self-testing kits are deployed (24–26), it is important to educate users on the importance of strict adherence to manufacturer instructions. Another area for consideration is outdoor testing strategies (e.g., drive-through testing), where the Panbio kit supplies may be exposed to precipitation and fluctuations in temperature and humidity (26). While temperature and humidity did not alter the Panbio performance in this study, rainwater was shown to cause false-positive reactions if processed without buffer. Haage et al. (22) demonstrated that prolonged exposure to elevated temperatures affected the sensitivity of SARS-CoV-2 detection by some Ag-RDTs, whereas low temperatures impaired the specificity of assays, including Panbio (20). However, an alternative explanation for the false positives observed at low temperatures by these investigators could be the use nonvalidated specimen types (i.e., NP swabs in PBS) (20). In this study, PBS alone caused false-positive results in the absence of buffer. The quantity of PBS material (i.e., 20 μl in approximately 300 μl of buffer) used by Haage et al. (20) was inconsistent with the limit of tolerability of Panbio to dilution in water observed in this study of between 1:8 and 1:10.
It should be noted that the findings of this study with false-positive results observed with Panbio when tested outside manufacturer claims should not be extrapolated to other Ag-RDTs without supporting evidence, as a second SARS-CoV-2 antigen test (i.e., BD Veritor) did not show similar findings. Other Ag-RDTs could rely on different assay principles and would need to be investigated independently.
Overall, we provide rigorous scientific evidence that erroneous false-positive SARS-CoV-2 results can occur with improper test conditions with the Panbio Ag-RDT, resulting in nonspecific interaction between the SARS-CoV-2-specific conjugated and capture antibodies. While generation of false-positive results from direct testing of products onto Panbio Ag-RDT devices may not surprise some health care professionals, having a better understanding of the importance of the buffer and its components, as well as knowing the mechanism of false positive generation, can help dispute unsound demonstrations on social media and help inform users on the value of following the manufacturer instructions.
MATERIALS AND METHODS
Sample types.
Ag-RDT samples included food products (Table 1), water, laboratory buffers, specimen transport media, and four different clinical specimen types previously tested negative by RT-PCR in routine diagnostic testing: (i) 30 nasopharyngeal (NP) swabs in universal transport medium (UTM), (ii) 30 oropharyngeal and bilateral nares (OP/N) swabs in phosphate-buffered saline (PBS) (24, 27), (iii) 30 bronchoalveolar lavages (BAL), and (iv) 30 saline gargles (Table 1) (25).
Antigen and molecular testing.
SARS-CoV-2 nucleocapsid antigen detection was performed using the Abbott Panbio COVID-19 rapid antigen test (Fig. 1) and the BD Veritor system for rapid detection of SARS-CoV-2. Each kit’s nasal swabs were dipped into the test samples and placed in the appropriate kit buffers, and 3 or 5 drops were used to inoculate the sample wells of the Veritor and Panbio cassettes, respectively, as per the manufacturer recommendations. Each sample was also tested without manufacturer buffer (i.e., direct sample testing), mirroring the test procedure recommended for clinical specimens. Results were visualized by the unaided eye after 15 min, and Veritor readouts also included automated detection using a BD Veritor Plus instrument. Panbio test results were based on the kit insert in which the presence of the control band alone was considered a negative result, the presence of both the control and target bands was considered positive, and the presence of the target band alone or absence of bands was considered invalid (Fig. 1). RT-PCR testing was performed for all specimens except food products using the Roche Diagnostics cobas SARS-CoV-2 test on the cobas 6800 instrument.
Assessing the role of the Panbio buffer and its components.
PCR-grade water (Invitrogen) was chosen as a representative matrix to generate false-positive Panbio results (Table 1). To assess the assay tolerability to buffer dilution, Panbio buffer was subjected to 2-fold serial dilutions in PCR-grade water, and testing was performed at 4°C, 20°C, and 37°C (Fig. 2A). The exact composition of Panbio buffer is proprietary, yet according to the product insert, it consists of tricine, sodium chloride (NaCl), Tween 20, ProClin 300, and sodium azide (<0.1%). To assess the role of these components, the buffer was reverse engineered. Solutions of tricine (1 mM to 1 M) from pH 3 to 12 were prepared (Fig. 2B) with or without 1% Tween 20. The contribution of ionic strength was assessed using NaCl (1 mM to 1 M) in 100 mM tricine solutions from pH 3 to 12 (Fig. 2C).
Effect of temperature and humidity on Panbio performance.
According to manufacturer specifications, PanBio kits should be stored between 2 and 30°C, and all kit components should be brought to room temperature (15 to 30°C) for 30 min before use. To assess the impact of storage temperature, sealed PanBio test devices were incubated for 1 h at 4°C, 20°C, or 45°C (Table S1 in the supplemental material). The 45°C incubations were performed with 90% relative humidity using a Binder constant climate chamber (model KBF 115) (Table S1). Test devices were removed from their packaging, and incubations were repeated under the same conditions. Testing was performed using 20 μl of gamma-irradiated SARS-CoV-2 into 280 μl of Panbio buffer. Viral stocks (at 1.2 × 106 PFU/ml) were diluted in PBS (pH 7.4) to concentrations spanning 1.2 × 105 to 1.1 × 103 PFU/ml (Table S1). Panbio buffer was used as a negative control. Freeze-thaw effects were investigated by incubation of test components at –20°C for 16 h before thawing and testing.
Investigations into possible causes of false-positive results.
Using “conjugate pad transplantation” (Fig. 3A), the proprietary gold-conjugated antibodies of the Panbio device (i.e., the SARS-CoV-specific human IgG and the chicken IgY used for the control) were accessed from disassembled Panbio cassettes. Each conjugate pad was resuspended with 100 μl of Panbio buffer, PCR-grade water, or tricine solutions, and the suspensions were subjected to various treatments. Proteinase K (PK) (Qiagen GmbH., Hilden, Germany) was used at 100 μg/reaction for 1 h at 56°C, followed by enzyme inactivation at 70°C for 10 min (Fig. 3B). Untreated and heat treatment controls were included as controls (Fig. 3B). The remaining conjugate-free pads were washed three times with 1 ml of water or buffer, dried using a Whatman number 1 filter, and reintroduced into the Panbio cassettes. For testing, 25 μl of each water- or buffer-derived conjugated antibody suspension was added onto the conjugate pads of reassembled cassettes, followed by addition of 5 drops into the sample well of either positive or negative controls processed in water or the kit buffer.
In a second set of experiments (Fig. 3A, dashed lines), the control chicken IgY was removed from the SARS-CoV-2-specific IgG by pretreatment of the conjugate suspensions with a fragment of the nitrocellulose membrane from the Panbio test device containing the immobilized mouse monoclonal anti-chicken IgY. Fragments were excised at approximately 3 mm on each side of the control line indicated on the Panbio cassette. For each 100 μl of conjugate suspension, one fragment was added, followed by a 15-min incubation at room temperature. Then, SARS-CoV-2-specific conjugated antibody was removed and subjected to lateral flow and thermal shift assays to explore possible pH-induced conformational changes (Fig. 4).
Conjugated SARS-CoV-2 IgG thermal shift assays.
Differential scanning fluorometry (DSF), also known as thermal shift assays, relies on monitoring temperature-dependent unfolding of a protein in the presence of a fluorescent dye that is quenched in water but fluoresces when bound to hydrophobic residues (28–30). As a native protein is unfolded with heat, different hydrophobic residues are exposed, and the melting temperature (Tm) can be calculated for various test conditions. In this study, a 25-μl reaction mixture containing 10× SYPRO orange (Invitrogen, Eugene, OR, USA) was added to the gold-conjugated human IgG specific to SARS-CoV-2 resuspended in Panbio buffer or 100 mM tricine at pH values consistent (i.e., pH 5 to 7) and inconsistent (i.e., pH 8 to 10) with artifact formation (Fig. 4A and B). A similar set of experiments was performed with the addition of 1% Tween 20. Melting curve analysis was performed by increasing the temperature from 25°C to 99.9°C at a ramp rate of 1% with continuous fluorescence at 610 nm using an Applied Biosystems 7500 Fast instrument. Tm values were calculated by manufacturer software (Fig. 4B).
Ethics.
This evaluation was deemed exempt from Nova Scotia Health Research Ethics Board approval, as the activities described were conducted in fulfillment of ongoing verification of SARS-CoV-2 diagnostic assays used in Nova Scotia and are therefore considered a quality assurance initiative. Clinical specimens tested were obtained from anonymized residual samples collected for routine diagnostic testing for SARS-CoV-2 from consenting participants, and all data related to clinical specimens were provided anonymized and deidentified and were used solely with the intent to evaluate the potential for false positives in these clinical specimen types for rapid antigen testing programs used in Nova Scotia.
ACKNOWLEDGMENTS
This work received no private or public funding, with the exception of the Panbio kits that were provided in-kind from the Public Health Agency of Canada (PHAC). We thank the Special Pathogens Program of the National Microbiology Laboratory (NML) (Winnipeg, Manitoba) for the gamma-irradiated SARS-CoV-2 virus used in this study. We would also like to recognize the ongoing efforts of all the NML and NSH staff for their dedication and exceptional services throughout the pandemic, including the help with RT-PCR testing during this evaluation. We also thank all the volunteers and health care professionals who dedicate their time at popup clinics and other settings that use Ag-RDTs or NAATs. You are all instrumental for our pandemic responses and safety in our communities.
J.H., A.B., N.S., and J.R. identified this phenomenon and were involved in food sample testing. J.J.L., R.J.D., G.P., and T.F.H. designed and undertook testing of water, buffer, media, and gargles. J.J.L. designed and performed the buffer reverse engineering, conjugate pad transplantation, PK experiments, and DSF experiments. M.G.B., A.M., E.M., B.M.H., and P.S. designed and performed the temperature and humidity experiments. G.P., J.J.L., and T.F.H. wrote the initial draft of the manuscript with all authors contributing to the final version.
We declare no conflicts of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Jason J. LeBlanc, Email: jason.leblanc@nshealth.ca.
Rebekah M. Martin, Labcorp
REFERENCES
- 1.Safiabadi Tali SH, LeBlanc JJ, Sadiq Z, Oyewunmi OD, Camargo C, Nikpour B, Armanfard N, Sagan SM, Jahanshahi-Anbuhi S. 2021. Tools and techniques for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/COVID-19 detection. Clin Microbiol Rev 34:e00228-20. doi: 10.1128/CMR.00228-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Public Health Agency of Canada (PHAC). 2020. Interim guidance on the use of rapid antigen detection tests for the identification of SARS-CoV-2 infection. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/guidance-documents/use-rapid-antigen-detection-tests.html. Accessed 9 September 2021.
- 3.World Health Organization (WHO). 2020. Antigen-detection in the diagnosis of SARS-CoV-2 infection using rapid immunoassays. Interim guidance. https://www.who.int/publications/i/item/antigen-detection-in-the-diagnosis-of-sars-cov-2infection-using-rapid-immunoassays. Accessed 9 September 2021.
- 4.US Food and Drug Administration. 2020. In vitro diagnostics EUAs. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/vitro-diagnostics-euas#individual-molecular. Accessed 9 September 2021.
- 5.Health Canada. 2020. Authorized medical devices for uses related to COVID-19: list of authorized testing devices. https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/medical-devices/authorized/list.html. Accessed 9 September 2021.
- 6.Patriquin G, LeBlanc JJ. 2021. SARS-CoV-2 sensitivity limbo—how low can we go? Int J Infect Dis 103:23–24. doi: 10.1016/j.ijid.2020.11.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linares M, Pérez-Tanoira R, Carrero A, Romanyk J, Pérez-García F, Gómez-Herruz P, Arroyo T, Cuadros J. 2020. Panbio antigen rapid test is reliable to diagnose SARS-CoV-2 infection in the first 7 days after the onset of symptoms. J Clin Virol 133:104659. doi: 10.1016/j.jcv.2020.104659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheiblauer H, Filomena A, Nitsche A, Puyskens A, Corman VM, Drosten C, Zwirglmaier K, Lange C, Emmerich P, Müller M, Knauer O, Nübling CM. 2021. Comparative sensitivity evaluation for 122 CE-marked SARS-CoV-2 antigen rapid tests. medRxiv. doi: 10.1101/2021.05.11.21257016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gremmels H, Winkel BMF, Schuurman R, Rosingh A, Rigter NAM, Rodriguez O, Ubijaan J, Wensing AMJ, Bonten MJM, Hofstra LM. 2021. Real-life validation of the Panbio COVID-19 antigen rapid test (Abbott) in community-dwelling subjects with symptoms of potential SARS-CoV-2 infection. EClinicalMedicine 31:100677. doi: 10.1016/j.eclinm.2020.100677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merino P, Guinea J, Muñoz-Gallego I, González-Donapetry P, Galán JC, Antona N, Cilla G, Hernáez-Crespo S, Díaz-de Tuesta JL, Gual-de Torrella A, González-Romo F, Escribano P, Sánchez-Castellano MÁ, Sota-Busselo M, Delgado-Iribarren A, García J, Cantón R, Muñoz P, Folgueira MD, Cuenca-Estrella M, Oteo-Iglesias J, Spanish Panbio™ COVID-19 validation group. 2021. Multicenter evaluation of the Panbio COVID-19 rapid antigen-detection test for the diagnosis of SARS-CoV-2 infection. Clin Microbiol Infect 27:758–761. doi: 10.1016/j.cmi.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albert E, Torres I, Bueno F, Huntley D, Molla E, Fernández-Fuentes MÁ, Martínez M, Poujois S, Forqué L, Valdivia A, Solano de la Asunción C, Ferrer J, Colomina J, Navarro D. 2021. Field evaluation of a rapid antigen test (Panbio COVID-19 Ag Rapid Test Device) for COVID-19 diagnosis in primary healthcare centres. Clin Microbiol Infect 27:472.e7–472.e10. doi: 10.1016/j.cmi.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stokes W, Berenger BM, Portnoy D, Scott B, Szelewicki J, Singh T, Venner AA, Turnbull L, Pabbaraju K, Shokoples S, Wong AA, Gill K, Guttridge T, Proctor D, Hu J, Tipples G. 2021. Clinical performance of the Abbott Panbio with nasopharyngeal, throat, and saliva swabs among symptomatic individuals with COVID-19. Eur J Clin Microbiol Infect Dis 40:1721–1726. doi: 10.1007/s10096-021-04202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Food and Drug Administration (FDA). 2020. Potential for false positive results with antigen tests for rapid detection of SARS-CoV-2—letter to clinical laboratory staff and health care providers. https://www.fda.gov/medical-devices/letters-health-care-providers/potential-false-positive-results-antigen-tests-rapid-detection-sars-cov-2-letter-clinical-laboratory. Accessed 21 June 2021.
- 14.Surkova E, Nikolayevskyy V, Drobniewski F. 2020. False-positive COVID-19 results: hidden problems and costs. Lancet Respir Med 8:1167–1168. doi: 10.1016/S2213-2600(20)30453-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The British Broadcasting Corporation (BBC). 5 July 2021. How children are spoofing Covid-19 tests with soft drinks. https://www.bbc.com/future/article/20210705-how-children-are-spoofing-covid-19-tests-with-soft-drinks. Accessed 9 September 2021.
- 16.The Guardian. 2 July 2021. UK pupils use orange juice to fake ‘positive’ Covid test results. https://www.theguardian.com/world/2021/jul/02/uk-pupils-orange-juice-fake-positive-covid-test-results. Accessed 9 September 2021.
- 17.Newsweek. 6 June 2021. School kids using orange juice to get false positive COVID test results. https://www.newsweek.com/school-kids-orange-juice-get-false-positive-covid-tests-1604118?amp=1. Accessed 9 September 2021.
- 18.iNews. 1 July 2021. Teenagers are figuring out how to fake positive Covid tests using lemon juice and hacks from TikTok. https://inews.co.uk/news/technology/tiktok-fake-covid-positive-test-schools-1079693. Accessed 9 September 2021.
- 19.Selby C. 1999. Interference in immunoassay. Ann Clin Biochem 36:704–721. doi: 10.1177/000456329903600603. [DOI] [PubMed] [Google Scholar]
- 20.Gillet P, Mori M, Van den Ende J, Jacobs J. 2010. Buffer substitution in malaria rapid diagnostic tests causes false-positive results. Malar J 9:215. doi: 10.1186/1475-2875-9-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wanji S, Amvongo-Adjia N, Njouendou AJ, Kengne-Ouafo JA, Ndongmo WP, Fombad FF, Koudou B, Enyong PA, Bockarie M. 2016. Further evidence of the cross-reactivity of the Binax NOW Filariasis ICT cards to non-Wuchereria bancrofti filariae: experimental studies with Loa and Onchocerca ochengi. Parasit Vectors 9:267. doi: 10.1186/s13071-016-1556-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haage V, Ferreira de Oliveira-Filho E, Moreira-Soto A, Kühne A, Fischer C, Sacks JA, Corman VM, Müller MA, Drosten C, Drexler JF. 2021. Impaired performance of SARS-CoV-2 antigen-detecting rapid diagnostic tests at elevated and low temperatures. J Clin Virol 138:104796. doi: 10.1016/j.jcv.2021.104796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin J, Dai W, Li W, Xiao L, Luo T, Guo Y, Yang Y, Han Y, Zhu P, Wu Q, He B, Wu J, Xia X. 2020. Potential false-positive and false-negative results for COVID-19 IgG/IgM antibody testing after heat-inactivation. Front Med (Lausanne) 7:589080. doi: 10.3389/fmed.2020.589080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patriquin G, Davis I, Heinstein C, MacDonald J, Hatchette TF, LeBlanc JJ. 2020. Exploring alternative swabs for use in SARS-CoV-2 detection from the oropharynx and anterior nares. J Virol Methods 285:113948. doi: 10.1016/j.jviromet.2020.113948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LeBlanc JJ, Pettipas J, Di Quinzio M, Hatchette TF, Patriquin G. 2021. Reliable detection of SARS-CoV-2 with patient-collected swabs and saline gargles: a three-headed comparison on multiple molecular platforms. J Virol Methods 295:114184. doi: 10.1016/j.jviromet.2021.114184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siegler AJ, Hall E, Luisi N, Zlotorzynska M, Wilde G, Sanchez T, Bradley H, Sullivan PS. 2020. Willingness to seek diagnostic testing for SARS-CoV-2 with home, drive-through, and clinic-based specimen collection locations. Open Forum Infect Dis 7:ofaa269. doi: 10.1093/ofid/ofaa269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LeBlanc JJ, Heinstein C, MacDonald J, Pettipas J, Hatchette TF, Patriquin G. 2020. A combined oropharyngeal/nares swab is a suitable alternative to nasopharyngeal swabs for the detection of SARS-CoV-2. J Clin Virol 128:104442. doi: 10.1016/j.jcv.2020.104442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davey L, Cohen A, LeBlanc J, Halperin SA, Lee SF. 2016. The disulfide oxidoreductase SdbA is active in Streptococcus gordonii using a single C-terminal cysteine of the CXXC motif. Mol Microbiol 99:236–253. doi: 10.1111/mmi.13227. [DOI] [PubMed] [Google Scholar]
- 29.Gao K, Oerlemans R, Groves MR. 2020. Theory and applications of differential scanning fluorimetry in early-stage drug discovery. Biophys Rev 12:85–104. doi: 10.1007/s12551-020-00619-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramu T, Prasad ME, Connors E, Mishra A, Thomassin JL, LeBlanc JJ, Rainey JK, Thomas NA. 2013. A novel C-terminal region within the multicargo type III secretion chaperone CesT contributes to effector secretion. J Bacteriol 195:740–756. doi: 10.1128/JB.01967-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download Spectrum.00683-21-s0001.pdf, PDF file, 0.2 MB (191.8KB, pdf)