ABSTRACT
Objectives:
Pediatric functional constipation (PFC) affects up to 30% of children. Current treatments often do not sustain symptomatic relief. Lubiprostone is a locally acting chloride channel activator that promotes fluid secretion into the small bowel without affecting serum electrolyte concentrations. We assessed the safety/tolerability of oral lubiprostone as treatment for PFC in a 24-week study.
Methods:
This phase 3 open-label safety trial conducted from April-November 2016 at 13 US sites included patients (ages 6–17 years) diagnosed with PFC (Rome III criteria). Patients <50 and ≥50 kg received lubiprostone 12 or 24 mcg twice daily, respectively, for 24 weeks. Safety endpoints included incidence of treatment-emergent adverse events (TEAEs) and changes from baseline in clinical laboratory parameters and vital signs.
Results:
Overall, 87 patients receiving lubiprostone, 64.3% (36/56) in the 12-mcg group and 54.8% (17/31) in the 24-mcg group, completed the study. Of 12 TEAEs leading to discontinuation, only upper abdominal pain occurred in >1 patient. TEAEs were mostly mild in intensity, with gastrointestinal disorders (diarrhea, vomiting) most frequently reported. No safety concerns were found in vital signs, abbreviated physical examinations, and laboratory tests. Subgroup analyses assessed an impact of age, sex, and race categories on TEAEs and treatment-related adverse events. Mean investigators’ assessments of treatment effectiveness (scale of 0–4) for lubiprostone 12- and 24-mcg groups, respectively, were 2.8 and 2.9 at week 12, and 2.7 and 2.2 at week 24.
Conclusions:
Lubiprostone was well tolerated in the pediatric population. The incidence of TEAEs was comparable to that observed in previous clinical trials and in adults.
Keywords: children, chloride channel agonists, constipation, lubiprostone, safety
What Is Known/What Is New
What Is Known
Despite the chronic nature of pediatric functional constipation, no trials have examined the long-term benefits of drug therapy in children.
Lubiprostone was approved for chronic idiopathic constipation in adults in 2006 in the United States.
What Is New
This study provided 24-week exposure data to complement the safety data from a separate randomized controlled phase 3 trial with open-label extension.
Lubiprostone was well tolerated in the pediatric population and the incidence of treatment-emergent adverse events was comparable to that observed in previous clinical trials and in adults.
Pediatric functional constipation (PFC) is a common disorder, affecting between 0.7% and 29.6% of children worldwide and accounting for 95% of childhood constipation cases (1,2). It is associated with painful or hard bowel movements, stool retention, and fecal incontinence (3), and remains a challenge for patients, families, and healthcare providers, causing significant distress to the child and having a significant impact on healthcare costs (3,4). When constipation continues into adulthood, the diminished quality of life leads to negative social consequences in 20% of patients (5). A systematic review showed that prolonged symptoms were observed in approximately 40% of constipated children after 6–12 months of treatment (6).
Although laxatives are commonly used for the management of chronic childhood constipation, there is a paucity of data addressing their efficacy, safety, and tolerability (7). The commonly used laxative polyethylene glycol has come under scrutiny, with gastrointestinal (GI) and neuropsychiatric adverse events (AEs) reported (8,9). Despite the chronic nature of the disease, no trials have examined the long-term benefits of drug therapy in PFC (7). Prucalopride, a high-affinity 5-hydroxytryptamine receptor 4 agonist, has been shown to be generally safe and well tolerated, but only comparable to placebo in efficacy (10,11).
Lubiprostone is a locally acting prostone analogue that activates chloride channels. By promoting fluid secretion into the small intestine, it accelerates overall colonic transit without affecting serum electrolyte concentrations (12,13). Trials of lubiprostone have confirmed its safety and efficacy in treating constipation (12,14–16), including one trial in patients with PFC (14). Lubiprostone was approved for chronic idiopathic constipation in adults in 2006 in the United States (17). This trial investigated the safety of lubiprostone as treatment for children and adolescents with PFC; however, it should be noted that lubiprostone is not approved for use in this population. This study was conducted to provide 24-week exposure data and to complement the safety data from a separate randomized controlled phase 3 trial with open-label extension (SAG/0211PFC-1131 and SAG/0211PFC-11S1) in this population.
METHODS
Study Design
This was a phase 3, multicenter, open-label safety trial conducted from April 12, 2016, to November 4, 2016, at 13 sites in the United States to assess the safety and tolerability of oral lubiprostone capsules at 12 or 24 mcg twice daily (BID) administered for 24 weeks in patients with PFC. The trial included a 1-day screening period, 24-week treatment period, and 1-week follow-up period. See Methods, Supplemental Digital Content 1 for the list of investigators and study sites.
Study Population
Patients were recruited from a mix of primary care practices and gastroenterology specialty practices. Eligibility criteria included the following: age ≥6 years to <18 years at the time of enrollment and written informed consent from the patient and/or guardian; meeting Rome III criteria for childhood functional constipation; and discontinuation of any medication affecting gastrointestinal (GI) motility. Exclusion criteria included: constipation attributed to a physical/mental/cognitive condition or to inflammatory bowel disease, medication, or anatomic, neurologic, or endocrine/metabolic factors; prior abdominal surgery, including bowel resection, colectomy, or gastric bypass surgery; and conditions affecting GI motility. See Methods, Supplemental Digital Content 1 for the complete list of inclusion/exclusion criteria.
The institutional review board of each study center reviewed and approved the study protocol and other materials before use. All aspects of the study were conducted in accordance with the U.S. Code of Federal Regulations (CFR) governing the protection of human patients (21 CFR 50), institutional review boards (21 CFR 56), and the obligations of clinical investigators (21 CFR 312). The study was registered on clinicaltrials.gov (NCT02766777). An independent data and safety monitoring board reviewed safety data on a regular basis throughout the study, and Good Publication Practice guidelines were followed.
Study Assessments
Study visits included: screening, interim telephone visit, interim clinic examination, end-of-treatment visit, and follow-up visit on day 1 and at weeks 1, 12, 24, and 25, respectively. AEs were reported by the patient and/or parent/legal guardian from the time of informed consent through the end of the follow-up period. Occurrence of any AEs was provided verbally by either the patient or parent/caregiver during site visits in response to open-ended questions by site personnel. For AEs that were ongoing at any point during the study, site personnel always asked for an update on the outcome during subsequent visits or phone calls. AEs were followed until they were resolved, stabilized, or until 30 days after the end of treatment exposure. See Study Protocol, Supplemental Digital Content text for additional details on study procedures.
Dosing
Patients received lubiprostone at a dose based on weight at the time of enrollment: 12 mcg BID for <50 kg, and 24 mcg BID for ≥50 kg. If AEs continued for ≥3 days, the dose was reduced to a once daily evening dose and these patients could resume the BID dose regimen at the investigator's discretion.
If necessary, rescue medication was used to help induce a bowel movement. Each patient (along with a guardian) was educated about the protocol-specified use of rescue medications at screening and throughout the study.
Safety Endpoints
The safety endpoints were incidences of AEs grouped by Medical Dictionary of Regulatory Activities (MedDRA) version 19.1, system organ class and preferred term; changes from baseline in clinical laboratory parameters (hematology, serum chemistry, and urinalysis); changes from baseline in an abbreviated physical examination; and changes from baseline in vital sign measurements, including height and weight.
Exploratory Endpoint
Investigators’ assessments of treatment effectiveness were collected by investigators at weeks 12 and 24 (visits 3 and 4) using a 5-point rating scale: 0 = not at all effective; 1 = a little bit effective; 2 = moderately effective; 3 = quite a bit effective; 4 = extremely effective.
Statistical Analysis
Statistical analyses were performed for the safety population, which consisted of all enrolled patients who took ≥1 dose of study medication. The sample size was calculated to have at least 100 patients complete the full 24-week treatment period (based on expected attrition). Patient demographic data and assessments of actual study exposure were summarized with descriptive statistics.
Original terms used by investigators to identify AEs were coded to the MedDRA system organ class as preferred terms and summarized by incidence. Results of clinical laboratory parameters were summarized with mean changes from pretreatment to post-treatment visits using descriptive statistics; cross-tabulation analyses were also performed for laboratory parameters using the normal reference ranges provided. Descriptive statistics were provided to evaluate the changes from baseline in vital signs, including height and weight, during the study.
RESULTS
Patient Disposition
Of the 87 patients who received lubiprostone, 64.3% in the 12-mcg group (n = 36 of 56) and 54.8% in the 24-mcg group (n = 17 of 31) completed the study (Figure 1, Supplemental Digital Content 2).
Demographics and Baseline Characteristics
Demographic and baseline characteristics are summarized for the safety population in Table 1. The proportion of patients with a history of failed constipation treatment was comparable in the two treatment groups: 76.8% of patients in the lubiprostone 12-mcg BID group and 77.4% of patients in the 24-mcg BID group.
TABLE 1.
Patients’ demographic and baseline∗ characteristics
| Treatment group | |||
| Category | Lubiprostone 12 mcg BID (n = 56) | Lubiprostone 24 mcg BID (n = 31) | Total lubiprostone (N = 87) |
| Sex, no. (%) | |||
| Female | 32 (57.1) | 17 (54.8) | 49 (56.3) |
| Male | 24 (42.9) | 14 (45.2) | 38 (43.7) |
| Age, y | |||
| Mean (SD) | 8.8 (1.81) | 13.2 (2.65) | 10.3 (3.03) |
| Age group, no. (%) | |||
| 6–9 y | 39 (69.6) | 3 (9.7) | 42 (48.3) |
| 10–13 y | 16 (28.6) | 14 (45.2) | 30 (34.5) |
| 14–17 y | 1 (1.8) | 14 (45.2) | 15 (17.2) |
| Race, no. (%) | |||
| White | 43 (76.8) | 25 (80.6) | 68 (78.2) |
| Black or African American | 11 (19.6) | 5 (16.1) | 16 (18.4) |
| Other | 2 (3.6) | 1 (3.2) | 3 (3.4) |
| American Indian or Alaska Native | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Ethnicity, no. (%) | |||
| Not Hispanic or Latino | 50 (89.3) | 27 (87.1) | 77 (88.5) |
| Hispanic or Latino | 6 (10.7) | 4 (12.9) | 10 (11.5) |
| Height, cm | |||
| Mean (SD) | 135.8 (10.53) | 159.9 (11.17) | 144.4 (15.78) |
| Weight, kg | |||
| Mean (SD) | 33.0 (7.75) | 66.4 (17.82) | 44.9 (20.19) |
| Body weight group, no. (%) | |||
| <50 kg | 56 (100.0) | 0 (0.0) | 56 (64.4) |
| ≥50 kg | 0 (0.0) | 31 (100.0) | 31 (35.6) |
| BMI, kg/m2 | |||
| Mean (SD) | 17.7 (2.43) | 26.0 (6.42) | 20.7 (5.85) |
BID = twice daily; BMI = body mass index; n = subgroup of total population; N = total population; SD = standard deviation.
Baseline was defined as the last non-missing measurement recorded before the date and time of the first dose of study medication.
The most common concomitant medications were antihistamines for systemic use (26.4% of total patients), drugs for obstructive airway diseases (25.3% of total patients), and psychoanaleptics (24.1% of total patients). A somewhat larger proportion of patients used antihistamines in the 12-mcg BID group (32.1%) than in the 24-mcg BID group (16.1%). The use of drugs for obstructive airway diseases and of psychoanaleptics was comparable between the two treatment groups (obstructive airway diseases 26.8% and 22.6% and psychoanaleptics 21.4% and 29.0% in the 12-mcg BID and 24-mcg BID groups, respectively).
In total, 13.8% of patients used rescue medication (16.1% in the lubiprostone 12-mcg BID group and 9.7% in the lubiprostone 24-mcg BID group). The most commonly received rescue medications were sennoside A + B (5.7% of total patients) and bisacodyl (3.4% of total patients).
Safety
Treatment exposure was similar in both study groups. In the lubiprostone 12-mcg BID group and 24-mcg BID group, the median study medication exposure was 169.5 and 169.0 days, respectively; in the same treatment groups, total mean doses were 221.7 capsules and 206.1 capsules, respectively. Mean compliance with study medication was 73.3% in the lubiprostone 12-mcg BID group and 63.2% in the 24-mcg BID group.
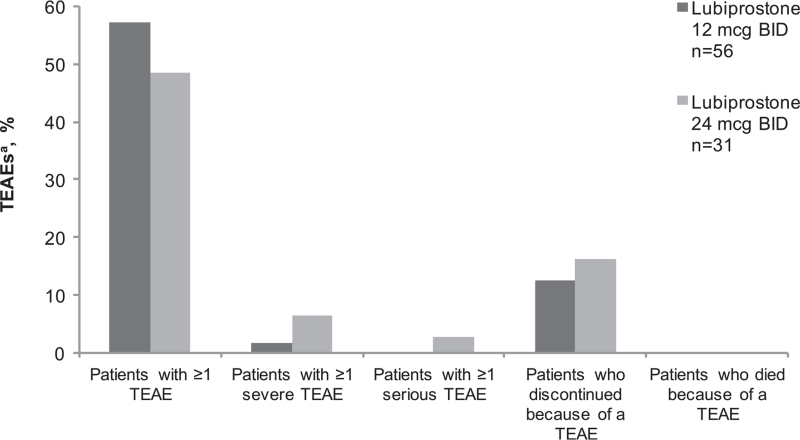
An overview of treatment-emergent AEs (TEAEs) is provided in Figure 1. Of patients in the lubiprostone 12-mcg BID and 24-mcg BID groups, 57.1% and 48.4% reported ≥1 TEAE, respectively. Two serious TEAEs (6.5%) were reported in the lubiprostone 24-mcg BID group, and neither one (ulcerative colitis or worsening constipation) was drug related. The proportions of patients discontinuing because of a TEAE were 12.5% and 16.1% in the lubiprostone 12-mcg BID group and 24-mcg BID group, respectively. Upper abdominal pain was the only TEAE leading to discontinuation (of 12 patients in total) that occurred in >1 patient. Most TEAEs in this study were of mild intensity and self-resolving.
FIGURE 1.
Overview of treatment-emergent adverse events (safety population). aTEAE is any event with an onset date on or after the first dose of study medication and with an onset date no more than 7 days after the last dose of study medication. BID = twice daily; n = subgroup of population; TEAE = treatment-emergent adverse event.
Treatment-Related Adverse Events Reported in ≥5% of Patients or Gastrointestinal Disorders Reported in ≥3% of Patients
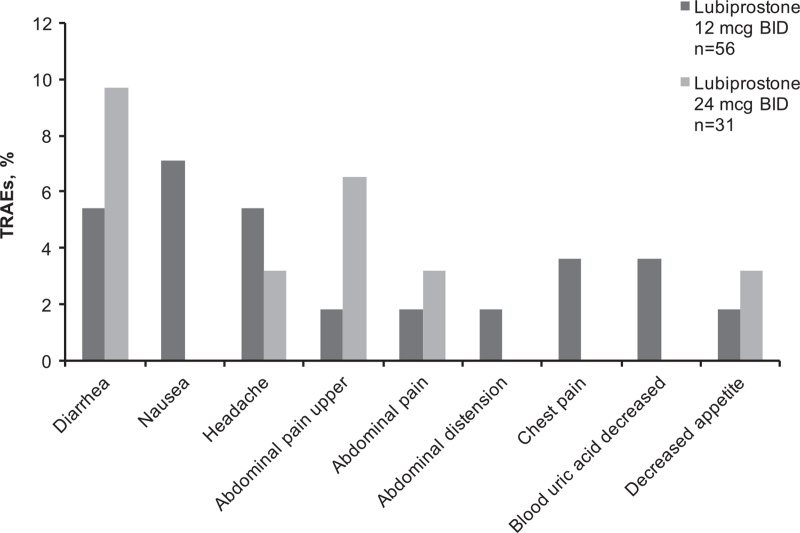
An overview of treatment-related adverse events (TRAEs) reported by ≥5% of patients is provided in Figure 2. Patients reported mostly GI disorders of diarrhea, followed by nausea, abdominal pain, and upper abdominal pain.
FIGURE 2.
Treatment-related adverse events reported by ≥5% of patients or GI disorders reported in ≥3% of patients in either lubiprostone treatment group (safety population). GI disorders: diarrhea, nausea, upper abdominal pain, abdominal pain, abdominal distension. BID = twice daily; GI = gastrointestinal; n = subgroup of population; TRAE = treatment-related adverse event.
Treatment-Related Adverse Events Occurring in >1 Patient
An overview of TRAEs occurring in >1 patient is provided in Table 2. Of patients in the lubiprostone 12-mcg BID and 24-mcg BID groups, 35.7% and 32.3%, respectively, reported TRAEs. Upper abdominal pain was the most commonly reported TRAE that led to discontinuation in the lubiprostone 12-mcg BID group (1.85%) and 24-mcg BID group (3.2%).
TABLE 2.
Summary of treatment-related adverse events occurring in >1 patient
| Preferred term, MedDRA dictionary 19.1 | Lubiprostone 12 mcg BID (n = 56), no. (%) | Lubiprostone 24 mcg BID (n = 31), no. (%) | Total (N = 87), no. (%) |
| Diarrhea | 3 (5.4) | 3 (9.7) | 6 (6.9) |
| Nausea | 4 (7.1) | 0 (0.0) | 4 (4.6) |
| Abdominal pain upper | 1 (1.8) | 2 (6.5) | 3 (3.4) |
| Abdominal pain | 1 (1.8) | 1 (3.2) | 2 (2.3) |
| Dyspepsia | 2 (3.6) | 0 (0.0) | 2 (2.3) |
| Chest pain | 2 (3.6) | 0 (0.0) | 2 (2.3) |
| Blood uric acid decreased | 2 (3.6) | 0 (0.0) | 2 (2.3) |
| Decreased appetite | 1 (1.8) | 1 (3.2) | 2 (2.3) |
| Headache | 3 (5.4) | 1 (3.2) | 4 (4.6) |
BID = twice daily; MedDRA = Medical Dictionary for Regulatory Activities; n = subgroup of total population; N = total population.
Subgroup Analyses of Treatment-Related Adverse Events
Female patients reported numerically higher TEAE rates (≥1 TEAE) than male patients in the 12-mcg BID group (21 females [65.6%] vs 11 males [45.8%]) as well as the 24-mcg BID group (10 females [58.8%] vs 5 males [35.7%]). See Tables 1 and 2, Supplemental Digital Content 3 and 4 for TEAEs categorized by sex.
White patients reported numerically higher TEAE rates (≥1 TEAE) than Black or other race patients in the 12-mcg BID group (White, 27 [62.8%]; Black, 4 [36.4%]; other race, 1 [50.0%]) as well as the 24-mcg BID group (White, 13 [52.0%]; Black, 2 [40.0%]; other race, none). See Tables 3, 4, and 5, Supplemental Digital Content 5, 6, and 7 for TEAEs categorized by race.
In the 12-mcg BID group, 24 patients (61.5%), 7 patients (43.8%), and 1 patient (100.0%) reported ≥1 TEAE in the 6–9, 10–13, and 14–17 years age groups, respectively. In the 24-mcg BID group, one patient (33.3%), seven patients (50.0%), and seven patients (50.0%) reported ≥1 TEAE in the 6–9, 10–13, and 14–17 years age groups, respectively. See Tables 6, 7, and 8, Supplemental Digital Content 8, 9, and 10 for TEAEs categorized by age.
Additionally, among patients who weighed ≥50 kg (ie, patients receiving the 24-mcg BID dose), the rates of TEAEs were numerically similar between patients weighing ≥50 to ≤60, >60 to <80 kg, and ≥80 kg, and did not indicate any clinically meaningful trends (see Table 9, Supplemental Digital Content 11).
Subgroup Analyses of Treatment-Related Adverse Events
Female patients reported numerically higher TRAE rates (≥1 TRAE) than male patients in the 12-mcg BID group (13 females [40.6%] vs 7 males [29.2%]), as well as the 24-mcg BID group (7 females [41.2%] vs 3 males [21.4%]). In male patients, the most commonly reported TRAEs were: diarrhea, decreased blood uric acid, and dyspepsia, each in 8.3% of 12-mcg BID group patients; and diarrhea, upper abdominal pain, and irregular heartbeat, each in 7.1% of the 24-mcg group patients. In female patients, the most commonly reported TRAEs were nausea and diarrhea in 12.5% and 11.8% of patients in the 12-mcg and 24-mcg BID groups, respectively.
In the 12-mcg BID and 24-mcg BID groups, TRAEs ≥1 were reported in 17 (39.5%) and 8 (32.0%) of White patients, and 3 (27.3%) and 2 (40.0%) of Black patients, respectively. In the 24-mcg group, the most commonly reported TRAE in White patients was diarrhea (12.0%). The most commonly reported TRAE by Black patients in the 12-mcg BID group was headache (18.2%); however, Black patients in the 24-mcg BID group reported abdominal pain, upper abdominal pain, decreased appetite, and back pain, each in 20.0% of patients. None of the other race patients reported ≥1 TRAE.
In the 12-mcg BID group, 17 (43.6%), 3 (18.8%), and none of the patients in the 6–9, 10–13, and 14–17 years age groups, respectively, reported ≥1 TRAE. In the 24-mcg BID group, no patients, 5 patients (35.7%), and 5 patients (35.7%) in the 6–9, 10–13, and 14–17 years age groups, respectively, reported ≥1 TRAE. The most commonly reported TRAE in the 6–9 years age category was nausea (10.3%) in the 12-mcg BID group. TRAEs in the 12-mcg BID group in patients ages 10–13 years were reported by one patient (6.3%), and included diarrhea, abdominal distension, upper abdominal pain, dyspepsia, headache, and epistaxis; in the 24-mcg BID 10–13 years age group, TRAEs were reported by one patient (7.1%), and included diarrhea, upper abdominal pain, anaphylactoid reaction, increased alanine aminotransferase, and headache. The most commonly reported TRAEs in patients ages 14–17 years were upper abdominal pain and diarrhea, each reported in 14.3% of patients in the 24-mcg group.
Clinical Laboratory Parameters
Minimal to no variations were observed in blood chemistry or hematology or urinalysis parameters. No noticeable change from baseline to week 25 was observed for sodium (ranged from mean 137.9 mmol/L, 95% confidence interval (CI) [137.4, 138.37] to 139.4 mmol/L, 95% CI [138.8, 140.1]; reference range 133–145 mmol/L) or potassium (ranged from mean 4.0 mmol/L, 95% CI [3.9, 4.1] to 4.2 mmol/L, 95% CI [4.1, 4.3]; reference range 3.4–5.2 mmol/L). There were no cases of electrolyte abnormalities in the study participants, even in children who reported having diarrhea, and no actions were necessary for any electrolyte derangements. In addition, the liver function markers—albumin, alkaline phosphatase, alanine aminotransferase, aspartate transaminase, and bilirubin (direct and total)—did not indicate any clinically meaningful trends.
Minimal to small variations were observed from baseline in physical examinations and vital sign parameters that included height, weight, body mass index, heart rate, systolic blood pressure, and diastolic blood pressure; there were no TEAEs related to variations in systolic or diastolic blood pressure observed.
Exploratory Efficacy Assessment
The efficacy of lubiprostone in PFC was assessed by the investigators as moderately to quite a bit effective. At week 12, the mean lubiprostone 12-mcg BID group grading was 2.8 and the mean 24-mcg BID group grading was 2.9 (on a scale of 0 to 4). At week 24, the mean lubiprostone 12-mcg BID group grading was 2.7 and the mean 24-mcg BID group grading was 2.2 (Figure 2, Supplemental Digital Content 12).
DISCUSSION
This phase 3, multicenter, open-label trial assessed the safety and tolerability of oral lubiprostone over 24 weeks at two doses for the treatment of PFC in children ages ≥6 to <18 years. Lubiprostone was shown to be well tolerated in patients with PFC.
Most TEAEs in this study were of mild intensity and self-resolving. Those TEAEs leading to discontinuation of study drug were also mostly GI disorders, primarily abdominal pain, and very few were serious or related to the study drug; most resolved upon discontinuation. The differences in incidence of TEAEs observed between the treatment groups may not reflect a true clinically meaningful effect because dosing assignment was weight based and not randomized across comparable patients.
The incidence of TEAEs in this trial was generally lower than previous lubiprostone clinical trials (12,15,16), except for one trial (14). The most frequently reported TEAE in both treatment groups was diarrhea, a TEAE that has been observed in prior lubiprostone clinical trials, and also in trials of polyethylene glycol, a commonly used laxative (12,14–16,18–20).
The incidence of diarrhea observed in our study was either comparable to or higher than reported in prior trials investigating the same lubiprostone doses (12,14–16); the difference was most pronounced when compared with two other trials in adult constipation (which, for instance, reported diarrhea incidences of 5.0% and 3.4% with the 24-mcg dose) (12,16).
In addition, vomiting and nausea occurred in >5% of patients in this trial; interestingly, vomiting is an event that has not been reported in adult trials investigating the same lubiprostone doses (12,15,16), but it has been reported at higher rates in a previous PFC trial (9.2% and 15.6% at 12-mcg BID and 24-mcg BID doses, respectively) (14). The incidences of nausea in our trial were markedly lower than those reported by previous PFC or adult trials (range 18.5−31.7%) (12,14,15,16).
Although the physiological mechanisms responsible for the GI symptoms are unknown, a previous study in normal healthy volunteers showed that lubiprostone treatment resulted in a modest delay in gastric emptying, which may contribute to the observed GI TEAEs (21). Taking into account the gastric effect, uneven compliance with the direction to take lubiprostone with food could explain the differences in the incidence of GI symptoms across trials. Alternatively, there might be an impact of age, as the majority of trials were conducted in adult patients (12,14,15,16). Our subgroup analysis assessed the impact of age categories (6–9, 10–13, and 14–17 years) on the type of TEAEs, with the expectation of a general age-related effect on tolerability, although statistical differences between subgroups were not calculated. Our subgroup analysis showed numerical trends for sex and race, albeit without statistical significance, but a recent clinical trial has shown no effect of sex, race, or age on the efficacy of lubiprostone (22). Validating any potential relationship between demographic factors and lubiprostone tolerability and/or efficacy would require analyses conducted in larger data sets.
No concerning signals emerged from vital signs, abbreviated physical examinations, and laboratory tests that contributed to the assessment of safety. In addition, no evidence of any electrolyte abnormality was observed.
Although our trial was not powered to assess efficacy, the investigators’ assessments of treatment effectiveness were determined by investigators at week 12 and week 24 to be moderately to quite a bit effective, a finding supported by the efficacy data of the one prior pediatric trial and the adult trials (12,14,15,16).
In a separate lubiprostone study in PFC, following a 12-week treatment period, there was no statistically significant difference in the primary endpoint of overall spontaneous bowel movement (SBM) response rate between the lubiprostone and placebo groups (18.5% for lubiprostone vs 14.4% for placebo; treatment difference = 4.1%; P = 0.2245). Some of the secondary endpoints deemed clinically important in the FDA review (abdominal pain, painfulness of SBM, and frequency of incontinence episodes) were also assessed, but no statistically significant differences were observed after adjusting for multiple comparisons (23).
The limitations of this trial include lack of statistical power to assess between group differences due to low sample size, nonblinded and nonrandomized design, and the lack of a placebo/comparator arm. However, it has to be considered that this study merely complements the existing data on the safety and tolerability of lubiprostone; these findings are amply supported by previous clinical trials in adults (12,15,16) and one trial in pediatric patients (14).
In conclusion, this study in pediatric patients has shown that both lubiprostone 12 mcg BID and lubiprostone 24 mcg BID administered orally were well tolerated, with no evidence of clinically significant safety concerns.
Amitiza_303_Study protocol_redacted.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
The authors would like to extend their sincere thanks to all the patients, caregivers, and investigators of the 303 study, and to those from Sucampo Pharmaceuticals who provided biostatistics and clinical operations support.
Footnotes
Source of Funding: This study was supported by Takeda Development Center Americas, Inc, Lexington, MA, USA, and Mallinckrodt Pharmaceuticals, Bedminster, NJ, USA. Although the sponsors were involved with study design, analysis, and interpretation of the data, the decision to submit the manuscript was the sole decision of the authors.
www.clinicaltrials.gov registration number: NCT02766777.
Conflicts of Interest: S.Z.H. was a consultant for Sucampo Pharmaceuticals (now Mallinckrodt Pharmaceuticals). S.M. is a former employee of Sucampo Pharmaceuticals (now Mallinckrodt Pharmaceuticals); had stock options in Sucampo Pharmaceuticals before acquisition by Mallinckrodt Pharmaceuticals. The other authors report no conflict of interest.
Writing Assistance: Medical writing assistance conducted in accordance with Good Publication Practice (GPP3) and the International Committee of Medical Journal Editors (ICMJE) guidelines was provided by Ian J. Phillips, PhD, and Nik Sanyal, PhD, on behalf of Syneos Health Medical Communications, LLC, and supported by Takeda Development Center Americas, Inc, and Mallinckrodt Pharmaceuticals. All authors had access to the study data and take responsibility for the accuracy and integrity of this manuscript's content.
Author Contributions: S.Z. Hussain: conceptualization, data curation, formal analysis, investigation, methodology, review and editing.
S. Mareya: conceptualization, data curation, formal analysis, funding acquisition, methodology, project administration, supervision, review and editing.
R.A. Clifford: conceptualization, data curation, formal analysis, investigation, methodology, review and editing.
B. Labrum: conceptualization, data curation, formal analysis, investigation, methodology, review and editing.
S. Stripling: conceptualization, data curation, investigation, methodology, review and editing.
Prior presentations: Interim data were presented at the Pediatric Academic Societies (PAS) Meeting, May 5–8, 2018, held in Toronto, Canada.
Supplemental digital content is available for this article.
REFERENCES
- 1.van den Berg MM, Benninga MA, Di Lorenzo C. Epidemiology of childhood constipation: a systematic review. Am J Gastroenterol 2006; 101:2401–2409. [DOI] [PubMed] [Google Scholar]
- 2.Xinias I, Mavroudi A. Constipation in childhood: an update on evaluation and management. Hippokratia 2015; 19:11–19. [PMC free article] [PubMed] [Google Scholar]
- 3.Tabbers MM, DiLorenzo C, Berger MY, et al. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr 2014; 58:258–274. [DOI] [PubMed] [Google Scholar]
- 4.Choung RS, Shah ND, Chitkara D, et al. Direct medical costs of constipation from childhood to early adulthood: a population-based birth cohort study. J Pediatr Gastroenterol Nutr 2011; 52:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bongers ME, Benninga MA, Maurice-Stam H, et al. Health-related quality of life in young adults with symptoms of constipation continuing from childhood into adulthood. Health Qual Life Outcomes 2009; 7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pijpers MA, Bongers ME, Benninga MA, et al. Functional constipation in children: a systematic review on prognosis and predictive factors. J Pediatr Gastroenterol Nutr 2010; 50:256–268. [DOI] [PubMed] [Google Scholar]
- 7.van Wering HM, Tabbers MM, Benninga MA. Are constipation drugs effective and safe to be used in children? A review of the literature. Expert Opin Drug Saf 2012; 11:71–82. [DOI] [PubMed] [Google Scholar]
- 8. US Food and Drug Administration. October−December 2011: Potential signals of serious risks/new safety information identified by the Adverse Event Reporting System (AERS). US Food and Drug Administration Website. Available at: https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm295585.htm. Accessed April 26, 2020. [Google Scholar]
- 9.Hussain SZ, Belkind-Gerson J, Chogle A, et al. Probable neuropsychiatric toxicity of polyethylene glycol: roles of media, internet and the caregivers. GastroHep 2019; 1:118–123. [Google Scholar]
- 10.Mugie SM, Korczowski B, Bodi P, et al. Prucalopride is no more effective than placebo for children with functional constipation. Gastroenterology 2014; 147:1285.e1–1295.e1. [DOI] [PubMed] [Google Scholar]
- 11.Winter HS, Di Lorenzo C, Benninga MA, et al. Oral prucalopride in children with functional constipation. J Pediatr Gastroenterol Nutr 2013; 57:197–203. [DOI] [PubMed] [Google Scholar]
- 12.Johanson JF, Morton D, Geenen J, et al. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of lubiprostone, a locally-acting type-2 chloride channel activator, in patients with chronic constipation. Am J Gastroenterol 2008; 103:170–177. [DOI] [PubMed] [Google Scholar]
- 13.Rao SSC, Lichtlen P, Habibi S. Effects of lubiprostone, an intestinal secretagogue, on electrolyte homeostasis in chronic idiopathic and opioid-induced constipation. J Clin Gastroenterol 2021; 55:512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyman PE, Di Lorenzo C, Prestridge LL, et al. Lubiprostone for the treatment of functional constipation in children. J Pediatr Gastroenterol Nutr 2014; 58:283–291. [DOI] [PubMed] [Google Scholar]
- 15.Lembo AJ, Johanson JF, Parkman HP, et al. Long-term safety and effectiveness of lubiprostone, a chloride channel (ClC-2) activator, in patients with chronic idiopathic constipation. Dig Dis Sci 2011; 56:2639–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barish CF, Drossman D, Johanson JF, et al. Efficacy and safety of lubiprostone in patients with chronic constipation. Dig Dis Sci 2010; 55:1090–1097. [DOI] [PubMed] [Google Scholar]
- 17.Wilson N, Schey R. Lubiprostone in constipation: clinical evidence and place in therapy. Ther Adv Chronic Dis 2015; 6:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katelaris P, Naganathan V, Liu K, et al. Comparison of the effectiveness of polyethylene glycol with and without electrolytes in constipation: a systematic review and network meta-analysis. BMC Gastroenterol 2016; 16:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammer HF, Santa Ana CA, Schiller LR, et al. Studies of osmotic diarrhea induced in normal subjects by ingestion of polyethylene glycol and lactulose. J Clin Invest 1989; 84:1056–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Attar A, Lemann M, Ferguson A, et al. Comparison of a low dose polyethylene glycol electrolyte solution with lactulose for treatment of chronic constipation. Gut 1999; 44:226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camilleri M, Bharucha AE, Ueno R, et al. Effect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory, and motor functions in healthy volunteers. Am J Physiol Gastrointest Liver Physiol 2006; 290:G942–G947. [DOI] [PubMed] [Google Scholar]
- 22.Joswick T, Dolecek G, Lichtlen P, et al. PTH-197 long-term efficacy of lubiprostone demonstrated in patients with constipation regardless of age, gender or race. Gut 2013; 62:A292. [Google Scholar]
- 23.Benninga MA, Hussain SZ, Sood MR, et al. Lubiprostone for pediatric functional constipation: randomized, controlled, double-blind study with long-term extension. Clin Gastroenterol Hepatol 2021; S1542-3565(21)00393-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.