ABSTRACT
Objective:
This review examined the effectiveness of telemonitoring versus usual care on self-care behaviors among community-dwelling adults with heart failure.
Introduction:
Heart failure is a global health crisis. There is a body of high-level evidence demonstrating that telemonitoring is an appropriate and effective therapy for many chronic conditions, including heart failure. The focus has been on traditional measures such as rehospitalizations, length of stay, cost analyses, patient satisfaction, quality of life, and death rates. What has not been systematically evaluated is the effectiveness of telemonitoring on self-care behaviors. Involving patients in self-care is an important heart failure management strategy.
Inclusion criteria:
This review included studies on adult participants (18 years and older), diagnosed with heart failure (New York Heart Association Class I – IV), who used telemonitoring in the ambulatory setting. Studies among pediatric patients with heart failure, adult patients with heart failure in acute care settings, or those residing in a care facility were excluded.
Methods:
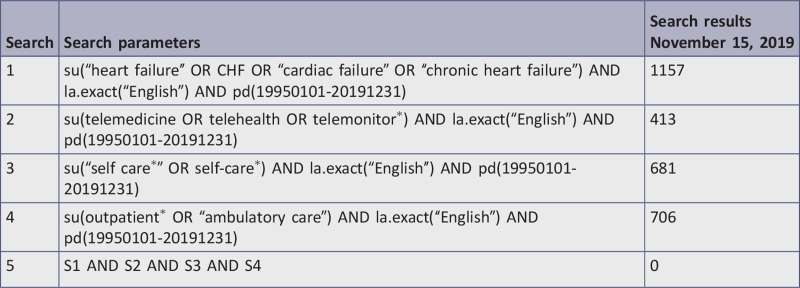
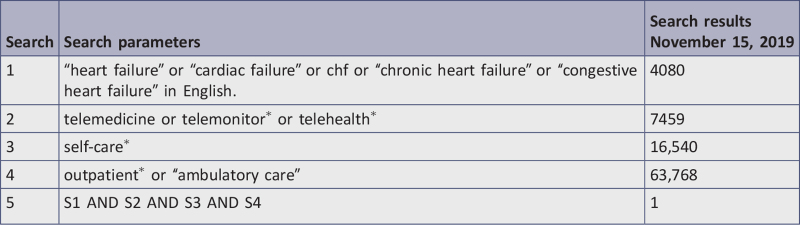
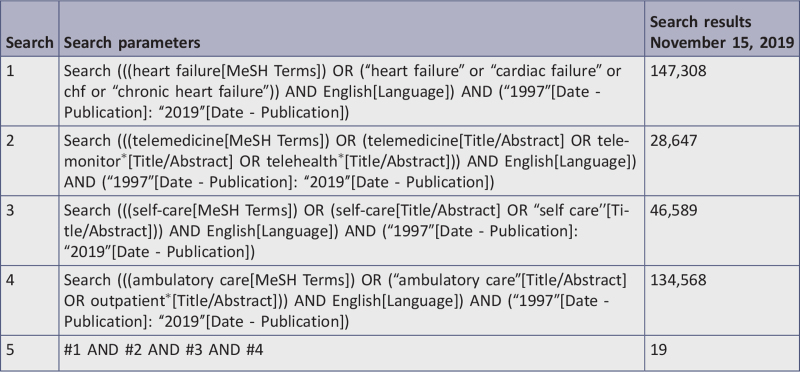
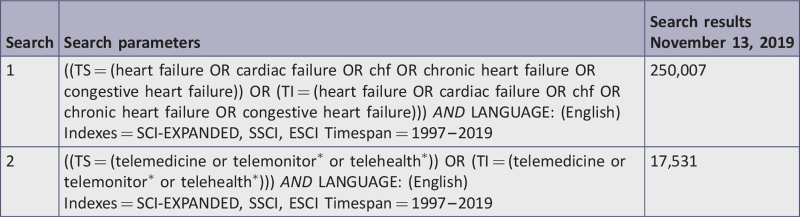
Eight databases, including CINAHL, Cochrane Central Register of Controlled Trials, Embase, MEDLINE, Epistemonikos, ProQuest Dissertations and Theses, PsycINFO, and Web of Science were systematically searched for English-language studies between 1997 and 2019. Studies selected for retrieval were assessed by two independent reviewers for methodological quality using critical appraisal checklists appropriate to the study design. Those meeting a priori quality standards of medium or high quality were included in the review.
Results:
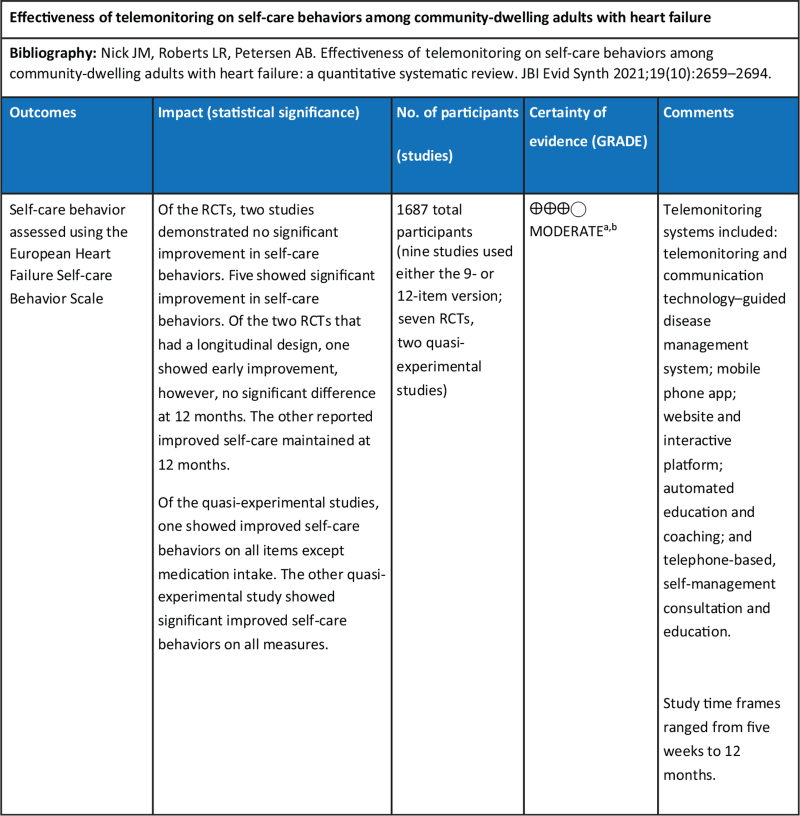
Twelve publications were included in this review (N = 1923). Nine of the 12 studies were randomized controlled trials and three were quasi-experimental studies. Based on appropriate JBI critical appraisal tools, the quality of included studies was deemed moderate to high. In a majority of the studies, a potential source of bias was related to lack of blinding of treatment assignment. Telemonitoring programs ranged from telephone-based support, interactive websites, and mobile apps to remote monitoring systems and devices. Self-care outcomes were measured with the European Heart Failure Self-care Behaviour Scale in nine studies and with the Self-care of Heart Failure Index in three studies. Telemonitoring improved self-care behaviors across 10 of these studies, achieving statistical significance. Clinical significance was also observed in nine of the 12 studies. All studies utilized one of two validated instruments that specifically measure self-care behaviors among patients with heart failure. However, in some studies, variation in interpretation and reporting was observed in the use of one instrument.
Conclusions:
Overall, telemonitoring had a positive effect on self-care behavior among adult, community-dwelling patients with heart failure; however, there is insufficient and conflicting evidence to determine how long the effectiveness lasts. Longitudinal studies are needed to determine the sustained effect of telemonitoring on self-care behaviors. In addition, the limitations of the current studies (eg, inadequate sample size, study design, incomplete statistical reporting, self-report bias) should be taken into account when designing future studies. This review provides evidence for the use of telemonitoring, which is poised for dramatic expansion given the current clinical environment encouraging reduced face-to-face visits.
Systematic review registration number:
PROSPERO CRD42019131852
Keywords: cardiovascular disease, home care, monitoring devices, telehealth, treatment adherence
Summary of Findings
Introduction
Heart failure (HF) represents a global public health burden that has increased dramatically and is associated with decreased quality of life due to excess morbidity and a high mortality rate.1,2 In high-resource countries, HF is the leading cause of hospitalization among adults and older persons.2 With this chronic, progressive disease, patients experience numerous symptoms including dyspnea, fatigue, activity intolerance, and fluid retention. Evidence-based recommendations for treatment of HF include interventions that aim to enhance self-care adherence, promote a partnership between patient and provider, and individualize treatment plans that take into consideration disease progression.3 Involving clients in self-care shows that timing is critical to this success; there is a window of opportunity when patients are symptomatic and are motivated and able to work with their provider, but once the patient becomes more debilitated, motivation decreases and so does self-care.4 Telemonitoring interventions have been associated with improvements in HF outcomes, including reduced mortality and hospitalization, increased health-related quality of life, and increased HF knowledge.5,6 A number of qualitative and quantitative studies have examined the effect of telemonitoring on self-care behaviors among adult HF populations using validated scales specifically developed to measure self-care among patients living with HF.7-17
Since the late 1990s, original research reports have been documenting the impact telemedicine has on a wide range of physical, social, and mental disorders. For example, since 2016, over 350 systematic reviews have been published summarizing the effectiveness of telemonitoring (TM). This intervention has been shown to improve disease management, reduce costs, enhance the patient experience, and increase satisfaction, indicating feasibility, acceptability, and effectiveness.18-29 At the same time, it is important to note that the evidence on TM also showed under-representation of African Americans,30-32 underscoring the need for greater representation. Under-representation in research may contribute to identified health disparities; therefore, sensitivity to this social issue and responding with a commitment to increase ethnic/racial representation in future research is essential. This caveat notwithstanding, there were sufficient studies to inform this review.
Effect of telemonitoring programs on managing heart failure
In the literature, telemonitoring, mHealth, telecare, and telehealth are often used interchangeably. For the purpose of this review, the definition of TM is the transmission of information related to patient health status (eg, physiological data and symptom scores) from home to a respective health care setting via automated invasive or non-invasive electronic devices or by web-based or telephone-based data entry.33-35 Telemonitoring modalities differ in terms of whether they send the information in real time or at specified intervals.34 Telemonitoring programs have historically utilized information and communication technologies to extend access to health care for HF patients, with the intent of decreasing the frequency of travel to facilities, patient burden, and associated costs, while simultaneously supporting self-care.10,36 These TM systems fall on a spectrum of patient interactivity – from completely passive to highly interactive. For example, telephone support used to monitor and manage HF symptoms may be as simple as telephone calls that provide educational support and symptom management.37-39 Smartphone apps constitute TM when used by patients to enter physiological data and symptoms that are reviewed by the provider.11 More advanced technology has also been used successfully, such as an interactive voice response system, in which patients respond to questions or input physiological data, which prompts tailored self-management advice and coaching.12-16Some technology systems depend on patients self-reporting. The information may be transmitted wirelessly or over a landline connection to the device, allowing for access in both rural and urban locations.13,37
Computer support for HF patients in the community has also included automated emails that provide information on a patient's health status and resources to support disease management,14 as well as more sophisticated virtual visits. Using a laptop to connect, patients can confer with their health care provider via video conferencing, a system that has gained popularity in recent months. With the assistance of the home health nurse, a peripheral stethoscope or portable imaging equipment can be connected to computers and used to monitor and document data.36,37
Other devices used for TM that support HF patients include wireless remote monitoring systems, which are software platforms linked via Wi-Fi to other devices. Commonly linked devices include stationary electronic devices external to the patient, such as an electronic blood pressure monitor, weighing scale, pulse oximeter, heart rate monitor, and electrocardiogram (ECG) recorder. The wireless sensor collects and uploads data to the remote monitoring system, which transmits the information to the health care system. The health care provider views the data using a web-based application.10,17,37 Invasive monitoring, such as implanted devices, can be used with a remote monitoring system for patients in their homes. Implanted devices transmit physiological measurements such as intrathoracic impedance, right-sided cardiac pressures, left atrial pressure, and pulmonary artery pressure.37
Effect of telemonitoring programs on self-care behavior
Systematic reviews of studies conducted among the adult population show that TM improves self-care for common chronic disorders. The synthesized research on TM programs for mental health conditions has indicated varying degrees of effectiveness.23-26 There is, however, strong evidence demonstrating the effectiveness of TM programs for improving self-care for social issues such as substance abuse,27,28 workplace stress reduction,29 and social isolation.40 However, to date, systematic reviews have not documented the effect of TM on self-care among the adult HF population.41-45 Telemonitoring improves self-care in other conditions and other HF outcomes; therefore, it is important to determine the impact of TM on self-care among adults with HF.
Self-care for adults with heart failure
In general, self-care in the HF population is defined as a “decision making process involving the choice of behaviors that maintain physiologic stability (maintenance) and the response to symptoms when they occur (management).”46(p.486) It is a process whereby patients recognize a change, evaluate the change, decide to take action, implement a treatment strategy, and evaluate the response to the treatment. According to the definition, adherence or compliance to prescribed treatment is a component of self-care but is not synonymous. The literature fairly consistently includes HF outcomes related to the following treatment recommendations: low-salt intake, healthy diet, daily weight monitoring, diuretic and other medication adherence, monitoring blood pressure, patient education to identify early warning signs, stress reduction, physical exercise, and consistent use of TM equipment.9-11,14-17,41 While TM has improved HF outcomes, synthesized evidence regarding the effects of TM on self-care is lacking. Fortunately, there are theoretical constructs and validated tools to measure self-care behaviors among adults with HF.
To illustrate, the well-established Situation-Specific Theory of Heart Failure Self-Care provides an explanation of the impact and role of self-care behaviors in the context of HF and, as such, lends itself to the review question. This theory was first published by Riegel et al.46 in 2009 and recently revised in 2016.47 There are three constructs included in self-care in HF patients. A “naturalistic process” creates a situational awareness and influences self-care maintenance, symptom perception, and self-management of symptoms; each construct includes autonomous and consultative elements of providers and caregivers. In terms of patient behavior, TM could potentially influence all three self-care processes. For example, perhaps self-care maintenance may be enhanced by engagement with the TM therapy resulting in an autonomous decision-making process to follow the prescribed treatment. At the same time, it is possible that TM helps the patient recognize and evaluate changes (symptom perception) and respond to symptoms, thus affecting self-care. It is also plausible that TM may improve outcomes by enhancing self-care indirectly through the consultative contribution of the provider who is changing the treatment regime, based on the data received via the TM.
Validated tools that specifically measure self-care behaviors in the HF population have been developed. One, the Self-care of Heart Failure Index (SCHFI), first published in 2001, is a self-report scale that measures self-care maintenance, self-care management, and self-care confidence. It has undergone six revisions and has been translated and psychometrically tested in additional languages.46,47 Another major tool, The European Heart Failure Self-Care Behaviour Scale (EHFScBS) has been in existence since 2003 and has also been translated and psychometrically validated in multiple languages.48-60 This tool provides a measure of health maintenance behaviors that mature over time. In 2009, the instrument underwent revision and reduction, which resulted in a 9-item tool that carries the same validity as the original 12-item instrument.61 In an integrative review of the psychometric properties, both versions of the EHFScBS were shown to be psychometrically sound in multiple languages.62 As stated, both of these validated tools have been in use for almost two decades in primary research, and these studies were a rich source of information when searching literature for this review.
A search for systematic reviews and umbrella reviews using TM devices on this population, with the primary outcome of self-care behaviors, was performed. Two systematic reviews published in 2012 using the same population, intervention, and outcome as the current review were found; however, both the Ciere et al.63 and the Radhakrishnan et al.9 only included randomized controlled trials (RCTs) and are not current. Additional systematic reviews by Maric et al.,64 Inglis et al.,5 and Son65 explored multiple HF outcomes and included self-care as a secondary outcome with limited evidence provided. Two umbrella reviews were identified; one evaluated the effects of TM on chronic conditions,7 while the other specifically synthesized the effects of TM on HF patients.66 These umbrella reviews captured the systematic reviews above; however, neither had self-care as the primary outcome of interest. Therefore, we see a need to synthesize current primary evidence, and include various study designs in addition to RCTs, to determine the effectiveness of TM among adults with HF, with self-care behaviors as the primary outcome.
Review question
What is the effectiveness of TM versus usual care on self-care behaviors among community-dwelling adults with HF?
Inclusion criteria
The inclusion criteria were developed according to JBI guidance. Five considerations were used in the search strings to define the inclusion criteria for studies: i) type of participants, ii) type of intervention, iii) possible comparators, iv) the outcome of interest, and v) the type of primary studies.
Participants
This review considered studies that included adult participants (male and female; 18 years and older) with a diagnosis of HF. Studies needed to provide interventions in the community setting. Studies using pediatric patients with HF, adult patients with HF in acute care settings, or those residing in a care facility were excluded.
Interventions
This review considered studies that evaluated various word iterations of TM, such as telemedicine, mHealth, telehealth, and e-Health systems, that used technologies that remotely monitor and manage patients with HF in the community setting. Telemonitoring can monitor the condition, inform and educate the patient, and communicate physiological parameters and symptoms to the health care provider. There are four main approaches to TM in HF: i) structured telephone support, ii) stand-alone TM devices, iii) implantable/invasive remote monitoring systems, and iv) wearables.67 Studies reporting use of any of these TM systems were reviewed. Examples of stand-alone TM devices include smart apps, interactive voice-response systems, or web-based programs, and may or may not include physiological measurement tools (eg, blood pressure, heart rate, oxygen saturation, ECG). In addition to monitoring physiological data, implantable/invasive monitoring devices can monitor intrathoracic impedance, right-sided cardiac pressures, left atrial pressure, and pulmonary artery pressure. Wearables such as patches, watches, and textiles can monitor physiological parameters.67
Comparators
This review considered studies using comparators of usual or standard care, alternative treatments, or no intervention.
Outcomes
This review considered studies that measured self-care behaviors as the primary outcome with validated tools such as the EHFScBS or SCHFI. In addition, studies that measured specific self-care behaviors (eg, monitoring weight and blood pressure, modifying diet and self-managing diuretics, identifying early warning signs and reporting symptoms, engaging in stress reduction and physical activity) as a proxy for self-care were considered. To clarify, in this review the question is not whether a specific physiological parameter changed, but rather whether self-care behaviors changed as a result of TM; for example, did the patient measure their blood pressure more regularly rather than did their blood pressure improve. Therefore, studies that focused on changes in physiological parameters but did not use them as a proxy for self-care outcomes were not included in this review.
Types of studies
This review considered all data derived from both experimental and quasi-experimental study designs including blinded RCTs, RCTs, and non-randomized controlled studies (controlled clinical trials) that employed TM as the intervention and measured the primary outcome of self-care in the HF population. Observational studies, including prospective and retrospective cohort studies, case-control studies, and analytical cross-sectional studies, were also considered.
Methods
This quantitative systematic review was conducted in accordance with JBI methodology for systematic reviews of effectiveness.68 This review followed an a priori protocol.69 The title of this review is registered in PROSPERO (CRD42019131852).
Search strategy
The search strategy for the review aimed to locate published and unpublished studies. Words contained in titles and abstracts were analyzed to find alternate terms for the different elements on the topic. The searches used both MeSH and title/abstract for each element, and coupled expanded terms with Boolean operators, force phrasing, and truncation. The wildcard replacement strategy to find American and British spellings for self-care behavior was unnecessary as truncation yielded the same results. Whilst attempting to maintain consistency in search terms between databases, the strategy was tailored for individual databases during the review using the advanced search feature in each database.
The databases searched included: CINAHL (EBSCO), Cochrane Central Register of Controlled Trials, Embase (Elsevier), Epistemonikos, ProQuest Dissertations & Theses A&I: Health and Medicine, PsycINFO (EBSCO), MEDLINE (PubMed), and Web of Science. The reference lists of all reports and articles selected for critical appraisal were examined for additional salient studies and any reports included were added to “other data sources.” The search for gray literature included: conference proceedings, World Health Organization, Clinicaltrials.gov, and National Institute for Health and Care Excellence. The complete search strategy is displayed in Appendix I.
The date range for studies was from 1997 to 2019, as TM studies first emerged in the literature in 1997. The current review considered studies only in English due to lack of additional language skills among researchers who conducted this review.
Study selection
Studies obtained from the eight databases were imported into EndNote X9.0 (Clarivate Analytics, PA, USA), grouped by their database, and duplicates were removed. The Clinicaltrials.gov website and the National Institute for Health and Care Excellence did not provide results. The World Health Organization International Clinical Trials Registry Platform resulted in one applicable study, but the study was ongoing and had not published any results from patient data. The reports underwent three stages of screening that resulted in the final inclusion for the review.
In stage 1 screening, two reviewers examined titles and abstracts independently against the inclusion and exclusion criteria and made separate recommendations to retain or discard. Titles were divided into three groups so that two reviewers worked on a group: JN & LR; LR & ABP; ABP & JN. The two reviewers then discussed recommendations and made a final decision to retain or discard articles. Any disagreements that arose between the reviewers were resolved through discussion with the third reviewer.
Stage 2 screening involved importing the retained studies obtained from stage 1 into the JBI System for the Unified Management, Assessment, and Review of Information (JBI SUMARI; JBI, Adelaide, Australia). Two reviewers (pairs of either JN & LR; LR & ABP; or ABP & JN) independently assessed the full-text citations against the a priori inclusion and exclusion criteria. The two reviewers discussed recommendations and made a final decision to retain or discard articles. Any disagreements that arose between the reviewers were resolved through discussion with the third reviewer. Reasons for exclusion of full-text studies were recorded in JBI SUMARI, and are reported in Appendix II.
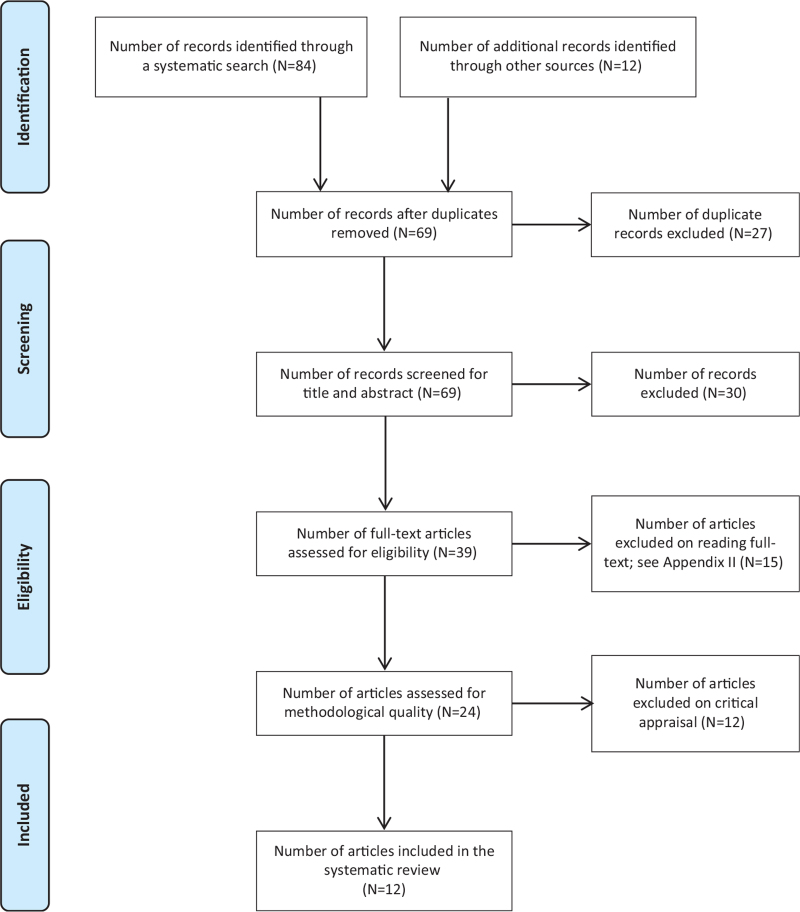
Stage 3 screening involved assessment of methodological quality of each study. Results from this process of screening and selecting studies are reported in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram70 in Figure 1.
Figure 1.
Search results, study selection, and inclusion process70
Assessment of methodological quality
Independently, two reviewers (pairs of either JN & LR; LR & ABP; or ABP & JN) critically appraised 24 studies for methodological quality using JBI critical appraisal tools for RCTs and quasi-experimental studies, analytic cross sectional, and cohort designs.68 The team sought clarification of study details on three studies; however, no replies were received from the authors. Therefore, the team evaluated the articles based on the information provided in the published reports and decisions were made to retain or discard. Any disagreements that arose between the two reviewers after critical appraisal of the articles were resolved by consulting the third reviewer, and consensus was reached through team discussion.
Once the team completed the critical appraisal of methodological quality on each study, a grading system was applied to determine the final inclusion or exclusion of individual studies. The three grades of study quality were: low quality (0% to 33% of criteria met), medium quality (34% to 66% of criteria met), or high quality (67% or more of criteria met). Studies reaching medium or high quality were included. The results of critical appraisals are reported in narrative form, and noted numerically in Tables 1 and 2.
Table 1.
Critical appraisal of eligible randomized controlled trials
| Citation | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Total | % |
| Boyne, et al.72 | Y | U | Y | N | N | U | Y | N | Y | Y | Y | Y | Y | 8/13 | 62a |
| Hägglund, et al.73 | U | U | Y | N | U | U | Y | N | Y | Y | Y | Y | Y | 7/13 | 54a |
| Lycholip, et al.10 | Y | N | Y | U | U | U | Y | N | Y | Y | Y | Y | U | 7/13 | 54a |
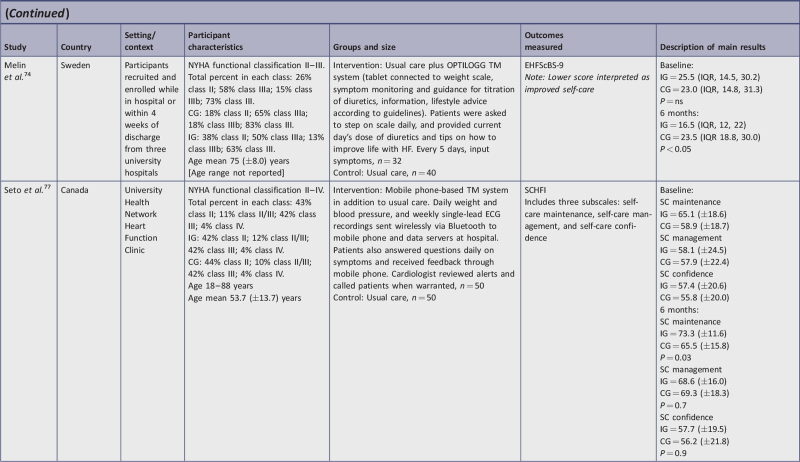
| Melin, et al.74 | U | N | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y | 9/13 | 69b |
| Seto, et al.77 | Y | Y | Y | N | N | N | Y | Y | Y | Y | Y | Y | Y | 10/13 | 71b |
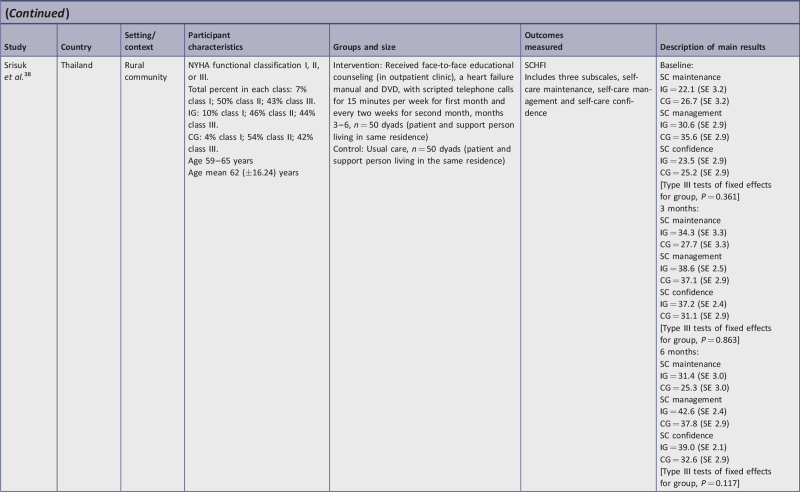
| Srisuk, et al.38 | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y | 11/13 | 85b |
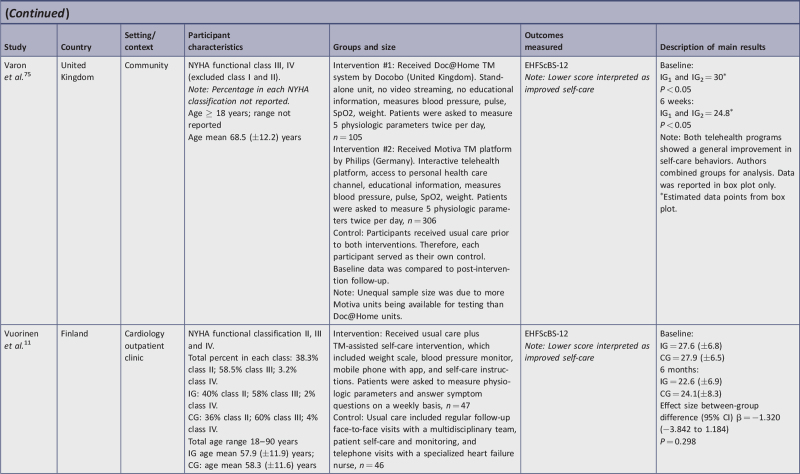
| Varon, et al.75 | U | U | Y | U | U | U | Y | N | Y | Y | Y | Y | Y | 7/13 | 54a |
| Vuorinen, et al.11 | U | N | Y | N | N | U | Y | Y | Y | Y | Y | Y | Y | 8/13 | 62a |
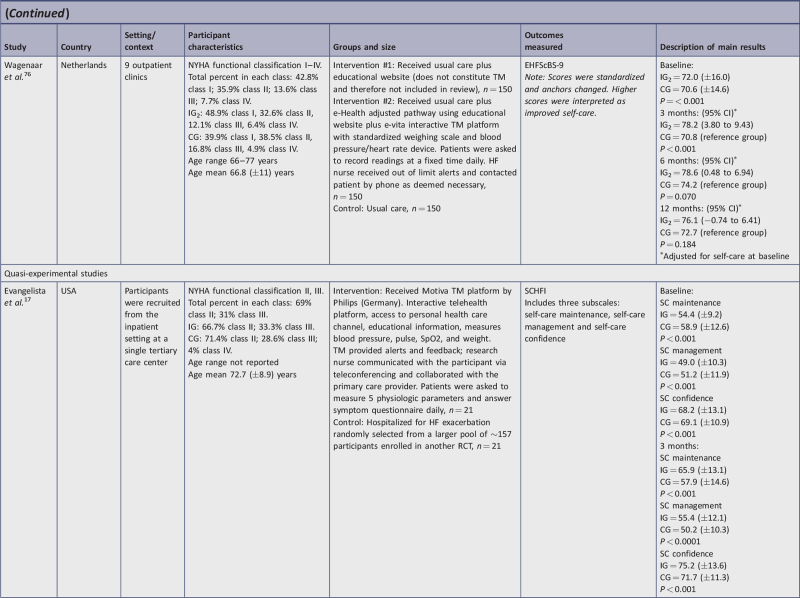
| Wagenaar, et al.76 | Y | U | N | N | N | U | Y | Y | Y | Y | Y | Y | Y | 8/13 | 62a |
| Total % | 56 | 22 | 89 | 0 | 0 | 22 | 100 | 56 | 100 | 100 | 100 | 100 | 89 |
Y, yes; N, no; U, unclear.
Medium quality.
High quality.
JBI critical appraisal checklist for randomized controlled trials (RCTs):
Q1 = was true randomization used for assignment of participants to treatment groups?
Q2 = was allocation to treatment groups concealed?
Q3 = were treatment groups similar at baseline?
Q4 = were participants blind to treatment assignment?
Q5 = were those delivering treatment blind to treatment assignment?
Q6 = were outcome assessors blind to treatment assignment?
Q7 = were treatment groups treated identically other than the intervention of interest?
Q8 = was follow-up complete, and if not, were strategies to address incomplete follow-up utilized?
Q9 = were participants analyzed in the groups to which they were randomized?
Q10 = were outcomes measured in the same way for treatment groups?
Q11 = were outcomes measured in a reliable way?
Q12 = was appropriate statistical analysis used?
Q13 = was the trial design appropriate, and any deviations from the standard RCT design (individual randomization, parallel groups) accounted for in the conduct and analysis of the trial?
Table 2.
Critical appraisal of eligible quasi-experimental studies (non-randomized experimental studies)
| Citation | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Total | % |
| Evangelista et al.17 | Y | Y | Y | Y | U | U | Y | Y | Y | 7/9 | 77b |
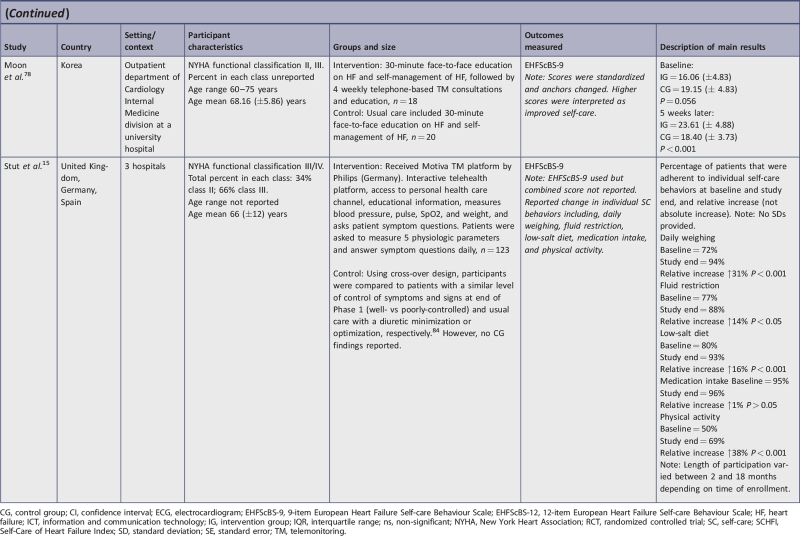
| Moon et al.78 | Y | Y | U | Y | Y | U | Y | Y | Y | 7/9 | 77b |
| Stut et al.15 | Y | Y | Y | N | Y | Y | Y | Y | Y | 8/9 | 89b |
| Total % | 100 | 100 | 67 | 67 | 67 | 33 | 100 | 100 | 100 |
Y, yes; N, no; U, unclear.
High quality.
JBI critical appraisal checklist for quasi-experimental studies:
Q1 = Is it clear in the study what is the “cause” and what is the “effect”?
Q2 = Were the participants included in any comparisons similar?
Q3 = Were the participants included in any comparisons receiving similar treatment/care, other than the exposure or intervention of interest?
Q4 = Was there a control group?
Q5 = Were there multiple measurements of the outcome both pre and post the intervention/exposure?
Q6 = Was follow-up complete, and if not, was follow-up adequately reported and strategies to deal with loss to follow-up employed?
Q7 = Were the outcomes of participants included in any comparisons measured in the same way?
Q8 = Were outcomes measured in a reliable way?
Q9 = Was appropriate statistical analysis used?
Data extraction
After finalizing the list of studies included in the review, the team extracted the data using the standardized data extraction tool in JBI SUMARI. The team extracted information separately, then came together and standardized the specific details about the study population, design and methods, sample size, interventions, statistical significance, time frames for single and repeated measures, and outcomes significant to the review question and specific objectives. The extracted data were checked and refined throughout the writing process by all three team members. Detailed characteristics of included studies are presented in Appendix III.
Data synthesis
The team calculated statistical significance for each study to determine the effectiveness of the outcome of interest for self-care behaviors. Statistical pooling of quantitative data and meta-analysis was not possible due to heterogeneity of the severity of HF in the population, the type and duration of intervention, varied time points of data collection, and variability in reporting of outcomes. Since the included studies used a range of statistical measures, when possible, the team converted these measures into standard deviations (SD) for the EHFScBS or SCHIFI scores to determine clinically significant changes in self-care behaviors. Clinical significance was defined in this review as ≥ 0.5 change in SD in the score from baseline to follow-up.71 New York Heart Association (NYHA) classification and age were also analyzed across studies. The findings are presented in narrative form and in tables and figures to aid in the data presentation where appropriate.
Assessing certainty in the findings
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach for determining the certainty of evidence was followed and a Summary of Findings (SoF) was created using GRADEPro GDT v.2015 (McMaster University, ON, Canada). The SoF provides a ranking of the quality of evidence based on absolute risks for the treatment and control, estimates of relative risk, study limitations, directness, consistency, heterogeneity, precision, and risk of publication bias. The outcome reported in the SoF includes self-care behaviors as measured by the EHFScBS and SCHFI instruments among community-dwelling adult patients with HF.
Results
Study inclusion
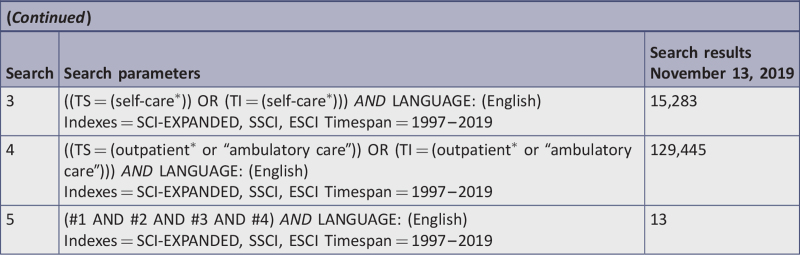
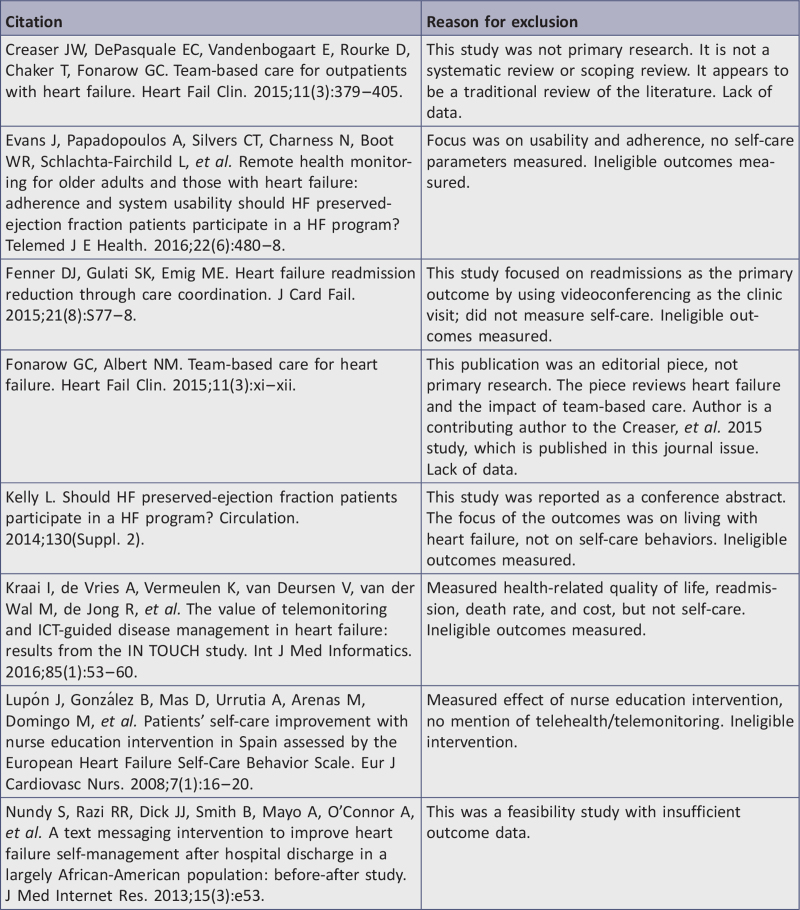
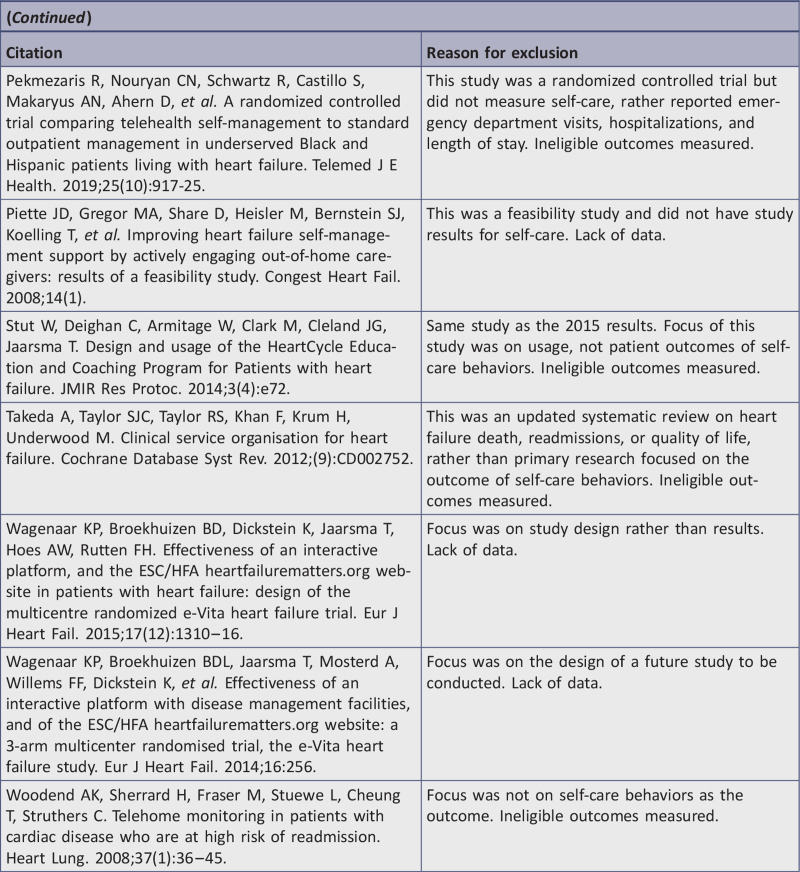
A comprehensive search of the literature returned 84 identified records, and 12 additional records were identified through other sources for a total sample size of 96 studies. Duplicates were removed, leaving 69 records, which were reviewed by title and abstract. After 30 records were excluded based on title and abstract, 39 remained. Fifteen of the 39 articles were excluded based on full-text review. Reasons for exclusion are detailed in Appendix II. Thus, a total of 24 articles required critical appraisal of methodological quality. Once critical appraisal was completed, 12 articles were excluded because, although they focused on the population of interest and used a TM intervention, the articles were either feasibility studies without sufficient data, did not report outcome data for self-care behaviors, or did not meet methodological rigor > 34% of study quality. The final analysis yielded 12 studies that were included in the systematic review; nine RCTs10,11,38,72-77 and three quasi-experimental studies.15,17,78
Figure 1 depicts the PRISMA flowchart search and review process for study selection and inclusion. Of note, two studies report on the same sample and intervention; findings, however, focused on different time points, specifically, three months73 and six months.74 Therefore, we included both publications to evaluate the ability of the intervention to sustain self-care behaviors.
Methodological quality
The 12 articles included in this review ranged from moderate to high quality, with low to moderate risk of bias. Sample size varied greatly across studies and results were not weighted, which could bias results. Out of nine RCTs (Table 1), three RCTs achieved high quality (≥ 67%) on critical appraisal,38,74,77 and six achieved between 54% and 62%, indicating moderate quality assessment.10,11,38,73,75,76 In the RCTs, true randomization was unclear in 44% (Q1),11,73-75 and only two studies clearly documented concealment of the allocation procedure (Q2).38,77 In all the RCTs, blinding of participants (Q4), those delivering the treatment (Q5), or outcome assessors (Q6) was either not possible due to the nature of the intervention, or not documented. Four of the RCTs did not clearly indicate follow-up procedures (Q8).10,72,73,75
All the quasi-experimental studies (Table 2) were of high quality (77% to 89%). In two studies it was not possible to determine follow-up strategies (Q6).17,78 A strength of all included studies was the use of validated scales.
Characteristics of included studies
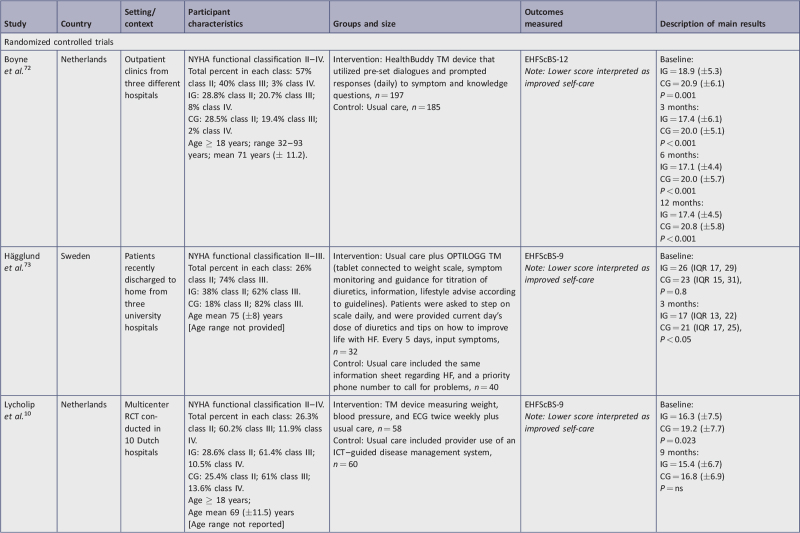
This review considered studies that investigated the effectiveness of TM on self-care behaviors among community-dwelling HF patients. The review aimed to compare the use of TM equipment and/or programs with usual care for the effect on self-care behaviors. Appendix III presents a detailed summary of salient characteristics of the research studies included in this review that used TM to influence self-care behaviors. Characteristics included setting, participants, description of the intervention, sample size, outcomes measured (specific instrument used), and main statistical results to indicate statistical significance or non-significance.
Country/setting
Of the 12 studies, six were conducted at a single site in the following countries: Canada,77 Finland,11 Korea,78 Thailand,38 the United Kingdom,75 and the USA.17 The other six studies were multi-center studies. One study15 was conducted in three countries (the United Kingdom, Germany, and Spain), while the remaining five studies were conducted across multi-center sites within single countries in either Netherlands10,72,76 or Sweden.73,74
Participants
The 12 studies considered in this review presented data obtained from 1923 participants. The total sample size for the nine studies using the EHFScBS was 1687 and for the three studies using the SCHFI tool was 236. The number of participants per study ranged from 36 to 450. Two studies had fewer than 50 participants,17,78 three studies had between 50 and 100,11,73,74 and seven studies had more than 100.10,15,38,72,75-77 One of the studies included a dyad interaction of a participant and a caregiver and measured the patient's perception of self-care as well as the caregiver's perception of the patient's self-care behaviors.38
All studies reported participants’ gender. Upon analysis, two studies included slightly more women (52% to 53%),17,38 while the other 10 studies showed a predominance of male participants ranging from 54% to 83% of the sample. Additionally, as HF is a chronic, progressive disease, severity of HF and age are important confounders in HF. Therefore, we considered the NYHA classification and age across studies to determine how variability in participant population may have affected self-care outcomes.
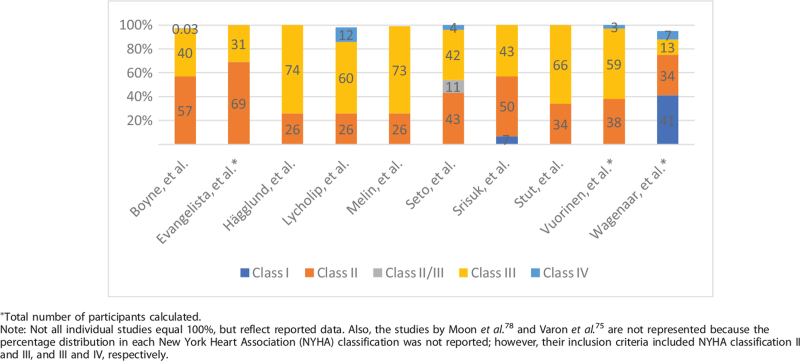
The included studies reported the NYHA classification to describe their participants. Ten studies provided detail as to the percentage of participants in each classification, while two studies simply stated the inclusion criteria was Class II or III,78 or Class III or IV75 without specifying the distribution. Class II and III constituted the majority of participants. Additionally, two studies included Class I,38,76 and five studies included Class IV.10,11,72,76,77Table 3 provides the breakdown of distributions in HF classification for each study.
Table 3.
Distribution of study participants by New York Heart Association classification
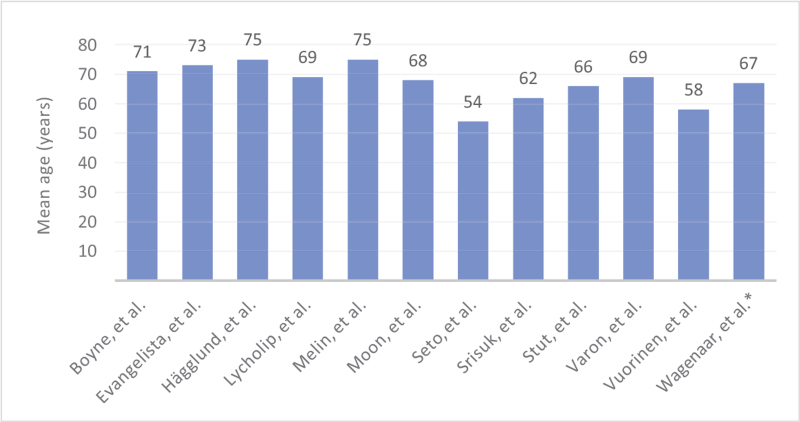
All studies included adult participants (age 18 years or older). The mean age across studies ranged from 54 to 75 years (Table 4). When mean ages between studies were analyzed, there were some notable differences. For two studies the mean age was less than 60 years old, six had a mean age between 60 and 70 years, and four studies had a mean age of 71 years or greater. Five studies provided the age range of participants: three studies had a narrow range 59 to 65,38 60 to 75,78 66 to 77,76 while the other two studies reported a very broad range: 32 to 93,72 and 18 to 90.11 Seven studies did not provide age range. Of note, the two related studies used the same participants; therefore, the age distribution was the same.73,74 The variation in the participants’ demographics was one factor that impacted the appropriateness of conducting a meta-analysis.
Table 4.
Distribution of study participants by mean age (years)
Interventions
The types of TM interventions included in this review fell into three broad categories: i) telephone or videoconference support, ii) interactive TM devices with physiological data collection, and iii) interactive TM devices without physiological data collection. The interactive TM devices that collected physiological data were the largest category. Several interventions combined modalities. Two interventions utilized telephone consultations as the primary modality:
telephone consultation and educational material in the form of HF manual and DVD38;
telephone consultation after an initial 30-minute face-to-face session.78
Eight different interventions involved the introduction of interactive TM devices that also collected physiological data. In these studies, treatment groups were provided devices that connected assessment equipment and facilitated electronic transmission of data.10,11,15,17,72-77 All data collected by the devices were automatically collected and transferred to the main system except for the mobile app utilized by Vuorinen et al.,11 which required manual input of physiological readings that were then uploaded and transferred. These interactive TM systems included:
a TM device that measured weight, blood pressure, and ECG10;
OPTILOGG health information system installed in the home via a tablet connected to weight scale, symptom monitor, medication guide, and lifestyle advice73,74;
Doc@Home unit measuring blood pressure, pulse, oxygen saturation, and weight75;
Motiva platform with interactive telehealth, access to personal health care channel, educational information, and measurement of blood pressure, pulse, oxygen saturation, and weight15,75;
a remote monitoring system that instructed participants to take weight, blood pressure, and heart rate daily, and provided feedback and alerts17;
access to Heartfailurematters.org website along with use of the e-Vita platform, which recorded weight, blood pressure, and heart rate76;
wireless uploading of weight, blood pressure, ECG recordings, and symptom questions on a mobile phone77;
a TM home-care package that included a weight scale, blood pressure monitor, a mobile phone with a pre-installed app, and self-care instructions, plus telephone follow-up from the HF nurse.11
One TM intervention provided an interactive HealthBuddy TM device that utilized pre-set dialogues and prompted responses to symptom and knowledge questions,72 but did not collect physiological data. In response to TM data, participants received feedback in a variety of formats, including autogenerated recommendations or a real-time telephone consultation with a health care provider. Most interventions that introduced a device utilized teleconferencing to allow provider response to data. Other differences between intervention modalities included the level of access to a nurse or other provider, frequency of user interface with device (twice daily to weekly), and level of individual tailoring (pre-set dialogues vs tailored messaging) based on patient self-reported measurements and assessment of symptoms. The variation in the type of intervention and timing of follow-up prevented construction of a suitable meta-analysis.
Review findings
Outcomes and instruments
Self-care behavior was the outcome of interest in this review. Measures, outcomes, and data collection intervals of each study are presented in Appendix III. Two validated instruments measured self-care behaviors using participants’ self-report. In studies using the EHFScBS-care Behavior scale, self-care behavior was denoted as a composite score in which the 12-item version encompasses adherence to regimen, consulting behaviors, and adaptation of behaviors,62 and in the 9-item version represents only adherence to regimen and consulting behaviors.79 Originally the EHFScBS (either 12- or 9-item version) was scored with a lower composite score indicating better self-care behaviors (possible score 12 to 60 for the 12-item version, and 9 to 45 for the 9-item version); however, some literature utilizes a standardized score of 0 to 100, with a higher composite score indicating better self-care behaviors. In this review, two of the included studies used the standardized scoring, as noted in Appendix III.76,78 In the three studies that used the SCHFI,17,38,77 self-care behavior was denoted in three components – self-care maintenance, self-care management, and self-care confidence – and results were reported for each subscale with higher scores indicating greater self-care behaviors. The variation in the scales and versions used, as well as scoring issues (eg, anchor reversal, standardized scores) precluded meta-analysis.
Measurement intervals
Eight studies conducted a one-time measurement of self-care behaviors at either five weeks, six weeks, three months, six months, or nine months.10,11,17,73-75,77,78 In the study by Stut et al.15 enrollment occurred at various times, but follow-up self-care behaviors were measured for all participants at “the end of the study.” As a result, this led to inconsistent intervention exposure ranging from two to 18 months. The remaining three studies conducted follow-up at multiple intervals (eg, three, six, and/or 12 months).38,72,76
Self-care behavior outcomes
In general, despite varied time frames and differences in intervention characteristics, studies indicated short-term effectiveness of TM for improved self-care, but long-term effectiveness was unclear. Ten15,17,38,72-78 of the 12 studies reported statistical significance for improved self-care behavior using EHFScBS and SCHFI instruments as shown in Appendix III and detailed below.
Self-care behavior measured with EHFScBS
Six studies used the 9-item version of EHFScBS. Three studies interpreted lower scores as improved self-care and measurements; all intervention group (IG) change scores improved and ranged from −2.4 points to −9 points lower, with an average of −6.8.10,73,74 Conversely, the control group (CG) change of scores in these studies showed little improvement (−0.75 lower range) or deterioration (+2.5 upper range); however, inconsistencies in one of these studies10 were observed between stated interpretation and reporting of results. Of the remaining three studies in this category, two used reverse anchoring and standardized the scoring, interpreting higher scores as improved self-care.76,78 In these studies, IG scores improved from 4.2 to 7.56 points higher. For Moon et al.,78 CG participants had worse self-care (−0.75). The study by Wagenaar et al.76 evaluated two IGs and self-care behavior improved similarly in both intervention groups. Results of the CG were not reported on follow-up. The final study using the EHFScBS-9 reported percentage change in five self-care behaviors rather than provide a score.15 In this study, all areas showed statistically improved self-care except for medication intake; there was no CG.
Three studies used the 12-item version of the EHFScBS11,72,75 and all three interpreted improved self-care with lower scores. The IG change scores ranged from −1.8 to −5.2 lower, indicating improved self-care behaviors. The final CG change score for Boyne et al.72 decreased by 0.1. The second study reported on the change scores for the two IGs but did not report on CG scores since they used a pre-post design.75 When testing one TM intervention against another TM system, there were no significant differences in the self-care improvement, as both interventions had a positive effect on self-care behaviors. In the third study,11 the IG and CG change score indicated similar improvement (−3.8 points lower).
When analyzing the studies using the EHFScBS by study design, there were seven RCTs and two quasi-experimental studies. Five RCTs were of moderate to high quality and showed significant improvement in self-care behaviors.72-76 Two RCTs, which were of moderate quality, showed no statistical difference in self-care behaviors.10,11 In actuality, in the study by Vuorinen et al.,11 both IG and CG groups showed improvement in self-care behaviors, diminishing statistical significance. In summary, of seven RCTs, only one study10 showed no improvement in self-care. The two quasi-experimental studies using the EHFScBS-9 version indicating improved self-care behavior were of high quality.15,78
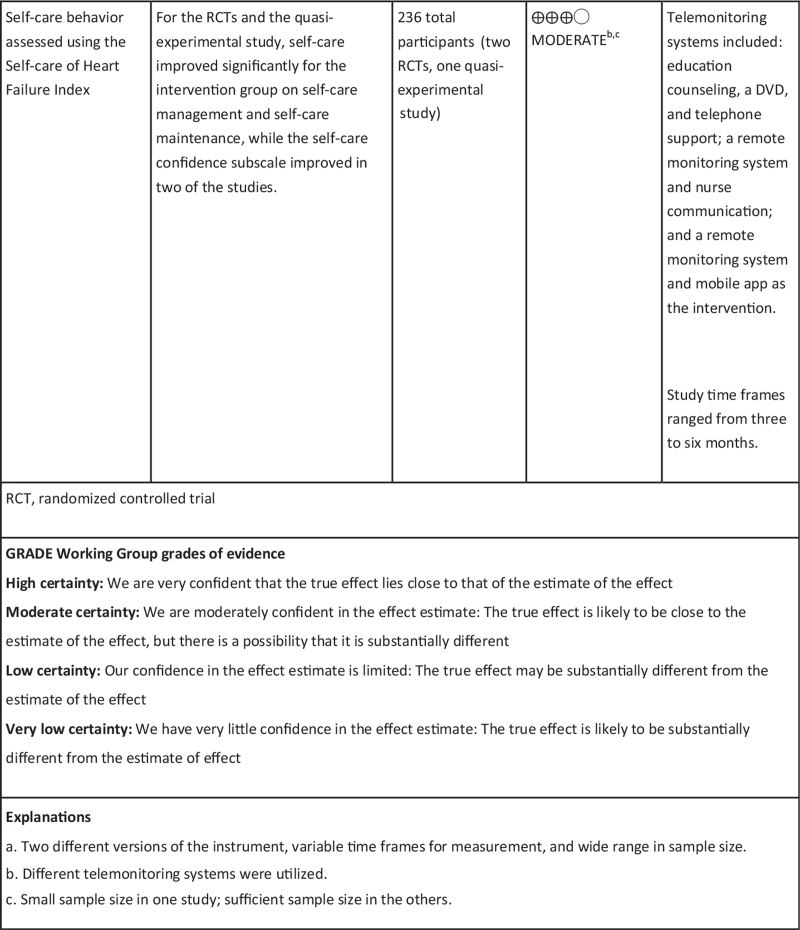
Self-care behavior measured with SCHFI
Three studies17,38,77 measured self-care behavior change using the SCHFI instrument, which has three sub-scales: self-care maintenance, management, and confidence, with higher change scores interpreted as improvement. One study measured self-care at three months,17 one study measured at three and six months,38 and one at six months only.77 At three months all IG subscales showed improvement. Self-care maintenance change scores ranged from +11.5 to 12.2; self-care management change scores ranged from +6.4 to 8; and self-care confidence change scores ranged from +7 to 13.7. At six months, self-care maintenance showed an 8.2 to 9.3 point positive change score; self-care management showed a 10.5 to 12 point positive change score; and self-care confidence showed a positive change score of 0.3 to 15.5.
For two studies,17,38 CG change scores at three months, namely self-care maintenance and management, changed negligibly; however, self-care confidence improved with a range of 2.6 to 5.9. At six-months CG change scores improved for both self-care management (+2.2 to 11.4) and confidence (+0.4 to 7.4) in both studies,38,77 whereas maintenance improved in one study (+6.6)77 but decreased (−1.4) in the other.38
Of the three studies using the SCHFI instrument, two were RCTs38,77 and one was a quasi-experimental study,17 which were all of high methodological quality. All three studies reported significant improvement in self-care management and maintenance, and one study reported significant improvement in self-care confidence at three months.17
Clinical significance
We analyzed change in SD for all included studies except for the study conducted by Stut et al.15 which reported only percentage improvement; for this study, we used the end point percentage change to evaluate clinical significance (see Table 5). If change in SD was not reported in the study, the team calculated SDs from the other statistical parameters provided such as interquartile ranges, standard errors, box plot and whiskers, and confidence intervals. Different equations were required depending on the original statistic reported in the study (equations used for each calculation of change in SD are included in Table 5). When we analyzed within intervention group changes, nine studies achieved clinical significance with change in SD10,17,38,72-77 and one study demonstrated clinical improvement with percentage change.15 However, when comparing between the IG and the CG, clinical significance was demonstrated in seven studies.11,17,38,72,74,77,78 Three studies did not report CG results post intervention,15,75,76 precluding evaluation of clinical significance, and two studies10,73 did not demonstrate clinical significance. Table 5 displays the change in SDs at study end points and indicates which studies achieved clinical significance.
Table 5.
Clinical significance of improvements in self-care behaviors in adults with heart failure using telemonitoring
| Within-group comparisons | Between-group comparisons | |||
| Citation | Final self-care behavior change in SD from baseline to follow-up | Clinical significance (change in SD ≥ 0.5) | Final self-care behavior change in SD between IG vs CG | Clinical significance (change in SD ≥ 0.5) |
| Boyne, et al.72 | 12 mo. = 0.8 | ✓ | 1.3 | ✓ |
| Evangelista, et al.17 | SC Maintenance 3 mo. = 3.9 SC Management 3 mo. = 1.8 SC Confidence 3 mo. = 0.5 |
✓ ✓ ✓ |
SC Maintenance = 1.5 SC Management = 1.8 SC Confidence = 2.3 |
✓ ✓ ✓ |
| Hägglund, et al.73a | 3 mo. = 0.8 | ✓ | 0.3 | X |
| Lycholip, et al.10 | 9 mo. = 0.8 | ✓ | 0.2 | X |
| Melin, et al.74a | 6 mo. = 1.5 | ✓ | 1.3 | ✓ |
| Moon, et al.78 | 5 weeks = 0.05 | X | 1.2 | ✓ |
| Seto, et al.77 | SC Maintenance 6 mo. = 7.0 SC Management 6 mo. = 8.5 SC Confidence 6 mo. = 1.1 |
✓ ✓ ✓ |
SC Maintenance = 7.1 SC Management = 2.3 SC Confidence = 2.3 |
✓ ✓ ✓ |
| Srisuk, et al.38b | SC Maintenance 6 mo. = 1.41 SC Management 6 mo. = 3.53 SC Confidence 6 mo. = 5.66 |
✓ ✓ ✓ |
SC Maintenance = 0 SC Management = 3.5 SC Confidence = 5.7 |
X ✓ ✓ |
| Stut, et al.15 | Daily weighing improved 31% Fluid restriction improved 14% Low-salt diet improved 16% Physical activity improved 38% |
Unable to calculate change in SD as only % changes reported. Improvements seen | No CG results reported | Unable to determine |
| Varon, et al.75c | 6 weeks = 2.5 | ✓ | No control group results reported | Unable to determine |
| Vuorinen, et al.11d | 6 mo. = 0.1 | X | 1.4 | ✓ |
| Wagenaar, et al.76e,f | 12 mo. = 6.15 | ✓ | Reported some results for CG, but unable to compare since CIs for CG were not reported at follow-up | Unable to determine |
CG, control group; CI, confidence interval; IG, intervention group; mo, months; SC, self-care; SD, standard deviation; SE, standard error; SQRT, square root.
SDs calculated from interquartile ranges using S ≈ (q3 – q1)/1.35 (http://www.math.hkbu.edu.hk/∼tongt/papers/median2mean.html).
SDs calculated from SEs using SD = SE x SQRT (N) (https://handbook-5-1.cochrane.org/chapter_7/7_7_3_2_obtaining_standard_deviations_from_standard_errors_and.htm).
Authors reported combined scores for both IGs. SDs estimated from box plot and whiskers as approximately equal to the range/4 (https://mathcs.org/statistics/course/Descriptives/box.html).
Significant differences between IG and CG at baseline.
SDs calculated from CIs of adjusted model SD = SQRT(N)∗(UpperCI – LowerCI)/(talpha,df∗2) (https://www.youtube.com/watch?v=lOnIPgCaspk).
IG 1 did not constitute telemonitoring, therefore only IG 2 is included.
Discussion
This review included 12 studies (nine RCTs and three quasi-experimental) of moderate to strong evidence. There is strong evidence showing both statistical and clinical significance with 11 different interventions tested. Overall, the findings of this review can be generalized to adult HF patients (age 18 years and above) and NYHA Class I–IV.
Study outcomes were measured with validated scales (two versions of the EHFScBS and the SCHFI instrument) to determine statistical significance of self-care behavior change. Not only did TM interventions and the type of follow-up vary, length of study time frames also varied from five weeks to 12 months, which has important implications for interpreting statistical and clinical significance. Ten of the 12 studies showed statistically significant improvement in self-care behaviors as a result of TM interventions.15,17,38,72-78 Additionally, clinicians should consider clinical significance as well as statistical significance71 in determining TM effectiveness. Seven of the 12 studies demonstrated TM effectiveness with clinical significance in improved self-care behaviors.11,17,38,72,74,77,78 We identified three categories of interventions: telephone-based consultation, interactive TM with physiological measurements, and interactive TM without physiological measurement.
Two TM interventions were primarily telephone-based consultations.38,78 The study by Moon et al.78 had a small sample size but still achieved statistical significance. It was difficult to analyze clinical significance due to the reverse scoring of EHFScBS-9, and based on the pre- and post-SDs presented, the reported change in SD was incorrect. Our calculation of change in SD, using the presented pre- and post-SDs, indicated no clinically significant change within IG, but did show between-group clinical significance.
Srisuk et al.38 found both statistical and clinically significant improvement was achieved within the IG. At both three months and six months, self-care behaviors improved; at three months, two SCHFI subscales statistically improved, and at six months all three SCHFI subscales statistically improved with the intervention when comparing IG and CG. At six months, clinical significance was partially demonstrated; only self-care management and confidence improved between groups. The inclusion of dyads was a unique component of this study, which could have affected outcomes as patients may have been prompted to complete self-care behaviors by their caregivers. This study was one of only two studies including patients from NYHA Class I in addition to Class II and III. With most patients classified as NYHA II or III at the start of the study and given the progressive nature of HF, continuing their level of self-care maintenance six months out could be considered clinically important.
While using different validated tools, these two telephone-based TM interventions demonstrated clinical effectiveness in improving self-care behaviors. Effectiveness is further substantiated by statistical significance demonstrated to varying degrees. Given both clinical and statistical significance, it appears that telephone-based consultation is an effective TM to improve self-care behaviors.
Eight interventions utilized interactive TM devices that either automatically uploaded physiological measurements or required the patient to enter physiological values.11,17,38,72,74,77,78 Despite small group size and quasi-experimental design, the study by Evangelista et al.17 found TM to be effective, showing both statistical and clinical significance. The engagement aspect of daily interaction with TM and additional interaction with a research nurse may have kept participants motivated and more attuned to their symptoms, which may explain why self-care maintenance and self-care confidence subscales were impacted the most. Because of the non-randomization in the design, the participants in the two groups were slightly different in terms of NYHA classification. However, the class IV comprised only 4% of the CG (ie, one patient more with severe HF than in the IG), and most likely did not affect results. Even with the above design challenges, the combination remote monitoring system plus alerts and feedback must have created enough magnitude in the TM intervention to make it effective.
Two related studies, Hägglund et al.73 and Melin et al.,74 used the same intervention and sample but reported outcomes at two different end points. Both studies found statistical and clinical significance with their intervention. While the TM device was similar to that used in other studies of this category of interventions, there was no additional human interaction. Having a sufficient and similar sample size between IG and CG, plus randomizing participants into the two groups, strengthened the validity of the results. The 9-point within-group improvement in the IG score at three months,73 and the 9-point improvement at six months for the Melin et al.74 study suggest that singular technology without human interaction can improve self-care behaviors. Of note, clinical significance was not demonstrated by Hägglund et al.73 at three months when comparing IG and CG, whereas at six months clinical significance was demonstrated favoring the IG compared to CG.74
Lycholip et al.10 found neither statistical nor clinical significance. Study design limitations may have precluded obtaining statistically significant improvement. Despite using an RCT design, there were significant between-group differences at baseline. Additionally, conflicting information was reported; in the abstract, the authors reported better self-care in the TM group at baseline, yet the results section reported that the control group had significantly better self-care behaviors at baseline. The authors report that over the course of the study, CG self-care behaviors improved, while the TM group did not significantly change. Thus, the authors report no difference between groups at the end of the study. However, the text of the abstract does not match the text of the results, and the pre-post bar graph in the results section does not match results text but does agree with the abstract. The discrepancies suggest that there was confusion regarding EHFScBS since interpretation is not intuitive as lower scores indicate better self-care. These inconsistencies preclude sound evaluation of TM effectiveness in this study.
Seto et al.77 conducted an RCT using SCHFI to measure self-care behavior. At six months, both statistical and clinical significance was demonstrated, indicating TM effectiveness. More specifically, only the self-care maintenance subscale was statistically improved between-groups. At the same time, clinical significance was demonstrated for all three subscales at six months. There are a number of features of this TM intervention that may have contributed to the observed statistical and clinical improvements in self-care behaviors. The intervention involved very close monitoring of the patients (eg, data were sent immediately and, when indicated, the cardiologist responded to alerts within minutes), which authors postulate led to improved ability to optimize patients’ medication regimen, which may have supported self-care maintenance. Furthermore, the real-time immediacy of the feedback capitalized on the “teachable moment” to help patients modify their lifestyle behaviors, or reinforce instructions they received in the clinic (eg, increase diuretics, decrease salt intake). In contrast, other interventions in this review provided delayed automated feedback at varied intervals. These findings further underscore the difficulty in distinguishing between the impact of changes in the patients’ self-care behaviors versus changes in clinical (provider) management. In addition, it is noted that the authors engaged in an extensive user-centered design process to develop and beta test the app, which is likely to have contributed to the higher rates of adherence observed in this study and, in turn, resulted in higher levels of engagement in self-care.
The study reported by Stut et al.15 was a quasi-experimental study using the Motiva TM device and measured self-care behavior using the EHFScBS-9. Despite using the EHFScBS to measure self-care behaviors, the authors only reported percentage changes on five domains of self-care, rather than EHFScBS scores. Statistically significant change was reported, and the study also appears to represent clinical significance by percent change. In this study, the percentage of adherence to self-care behaviors improved in four of the five areas. While there is no set threshold for percent change to indicate minimal clinically important difference specifically mentioned in the literature, we would argue that these improvements point to clinically significant change in self-care behavior, which aligns with other findings in this review. Medication intake was the only behavior that did not statistically or clinically change significantly. Outcomes may have been affected by inconsistent dose of the intervention since participant enrollment occurred over a period of time, but the study concluded on a set date. Therefore, the length of participation varied from 2 to 18 months (reported average: 9 months). The lack of EHFScBS scores limits conclusions drawn from this study.
Varon et al.75 tested two different TM devices (Motiva and Doc@Home) with a pre-post design that allowed participants to serve as their own control, comparing TM to usual care. The authors reported combined results from both groups; therefore, we are unable to determine if one intervention was better than the other. When analyzing the combined findings, both statistical and clinical significance (within-group) were observed in self-care behavior improvements. Interestingly, with both interventions, the authors reported general improvement in self-awareness, self-management, and assistance-seeking, all of which contribute to increases in perceived importance of self-management and self-care. The intensity of these interventions was greater than other studies included in this review in that it required measurement of four (vs one, two, or three) physiological parameters twice per day (vs daily, weekly, or biweekly). However, the study period was limited to six weeks, which was among the shortest. A longer trial period may have allowed for stronger conclusions on the effects of these telehealth platforms on self-care behaviors and other patient outcomes. Both this study and the previous study by Stut et al.15 using Motiva showed improvements in self-care behaviors.
Surprisingly, while the six-month study by Vuorinen et al.11 demonstrated no between-group statistical significance, it did demonstrate clinical significance in self-care behavior change, which is more important to the clinician. These disparate results may be explained by the fact that this study had a young age distribution of patients. As the authors note, this younger, more stable population may have derived less benefit from TM than older patients with worsening HF. Another factor to consider is that participants in the CG also showed increased interest in their health, which has positive implications for the maintenance of self-care behaviors. It is possible that a longer exposure to the TM would have resulted in statistical difference in self-care behaviors. Finally, achieving statistical significance may have been hampered by the Hawthorne effect, as the study nurse observed that the CG was more active in their care after study enrollment.
Wagenaar et al.76 compared a combined education website and a TM platform to usual care and demonstrated statistical significance at three months and clinically significant within-group differences in self-care behaviors at 12 months. However, the lack of confidence intervals prevented calculation of between-group clinical significance. In this study, attenuation of TM effect on self-care behaviors over time was of interest as there was no statistically significant difference observed at 12 months. Factors potentially impacting the longitudinal results may include the lower level of HF severity. The majority of participants were classified as NYHA Class I and II; it is possible that the effect of the TM intervention on self-care behaviors would be larger within a population experiencing more severe HF-related symptoms.4 At the same time, TM may hold the most promise among stable HF patients as it has the potential to decrease the need for, or replace, routine face-to-face clinic visits. This study, therefore, underscores the need to consider ways to sustain effect of TM.80,81
In summary, interactive TM devices that also collect physiological measurements were effective in improving self-care behaviors. Seven of the eight studies in this intervention category demonstrated statistical significance for improvement in self-care behaviors. Five studies showed between-group clinical significance, and a sixth, while only reporting percentages, also showed clinical improvement.
In a separate category, one TM, although interactive, did not collect physiological data.72 Using a commonly known smart app system, the HealthBuddy, Boyne et al.72 demonstrated both statistical and clinical significance at three, six, and 12 months’ measurement times. Three factors may have impacted the results. First, accessibility to the TM device may have promoted self-care behaviors since HealthBuddy is downloaded on to a mobile phone. Having ready access to the cell phone may have facilitated patient adherence. Secondly, using HealthBuddy for a long period of time (a year) also provided a large dose response. Finally, the impact of sample size must be considered, as it was quite large, and larger sample sizes can detect smaller effects. It appears that a TM intervention that simply increases awareness of HF symptoms and supports knowledge is also effective for improving self-care behaviors.
Overall, despite challenges such as unequal baseline groups, small sample sizes, and short study time frames, there is strong evidence showing both statistical and clinical significance with various forms of TM. The majority of patients in this review were NYHA Class II or III (ie, symptomatic but stable), which suggests that telemonitoring interventions may be more effective among patients within these classes rather than asymptomatic or unstable or with worsening HF disease. Like previous studies, we were unable to determine which TM is most effective.82 Systematic reviews have indicated TM effectiveness across various populations and disease conditions outcomes, including reduced rehospitalization, length of stay, and costs, as well as improved patient satisfaction and health-related quality of life.41-45 The current systematic review is unique in summarizing TM effectiveness on HF patients’ self-care behavior outcomes. The main findings support TM as an effective intervention to improve self-care behaviors as measured by either the EHFScBS or SCHFI. This review adds to the body of knowledge regarding the effective use of TM for chronic health conditions, including HF. This is encouraging as TM systems can be expensive initially, but may reduce costs in the long run.
Limitations of the included studies
The main limitations of the studies included in the review relate to the heterogeneity of the TM interventions, the length of intervention, the subjective nature of self-reporting, variation in sample sizes, and other design flaws. Telemonitoring systems included a range from simple telephone visits to more complex TM devices. Furthermore, each study reported data collected at variable times, thus hindering evaluation of dose response. All studies relied on self-reporting of self-care behavior, potentially introducing subjective recall bias. Additionally, some of the study designs included in the review were of medium quality and introduced risk of bias, thereby creating the possibility of alternate explanations for the conclusions.10,11,72,73,75,76 Two studies had a minimal sample size of just 18 to 21 participants per group,17,78 limiting generalizability despite apparent effectiveness. All of the RCTs either lacked blinding or a clear description of a blinding procedure (for participants or those delivering treatment),10,11,38,72-77 which could introduce bias. Two quasi-experimental studies lacked sufficient information on follow-up.17,78 Many studies did not report power analysis, effect size, or adequate information to determine appropriateness of sample size; therefore, questions remain regarding underpowered studies. Yet despite study design limitations and variability of treatment protocols, overall, there was a favorable effect of TM on self-care behaviors, thereby increasing generalizability. Moreover, the heterogeneity of the multinational study samples enhances clinical applicability to populations with varied characteristics.
Limitations of the review
A major limitation of this review is that meta-analysis was not possible, thus limiting the strength of conclusions. Factors that prohibited meta-analyses included inconsistent use of anchor scores resulting in disparate interpretations of high and low outcome score; outcomes reported with various statistics (mean score with SDs, interquartile ranges, standard errors, or confidence intervals, or percentage changes); different versions of the EHFScBS scale used; two different instruments measuring self-care behaviors; and different data collection periods. Without being able to determine overall effect size for TM, it is not possible to speak to the strength of the positive effect. As a result, this review cannot determine whether TM is superior to other interventions.
Heart failure is a progressive disease and as comorbidities increase with age, this variable could be a factor that impacts effectiveness of therapy. Comorbidities can negatively affect the ability to perform self-care behaviors.4 This team was unable to determine the confounding impact of comorbidities across studies as there was inconsistency in reporting. Some did not report presence of comorbidities,10,72,75,77 another only reported the presence of a comorbidity as a dichotomous variable (yes/no),78 while others listed frequency of specific diseases or risk behaviors (eg, smoking).11,15,17,73,74,76 However, the number and type of comorbidities reported varied considerably. One study reported comorbidities using an index score that reported either low, medium, or high.38 Without homogeneity of any of the variables, new statistical results could not be achieved through meta-analyses. Studies with older age ranges could be expected to have more comorbidities; however, in this review, age did not appear to negatively impact self-care outcomes.
Review findings may not be generalizable to all populations due to under-representation of subgroups, other forms of TM not reviewed, and differences in health care systems. Additionally, variation in personal and community characteristics (eg, social support and living conditions), which were not reflected in these studies, could impact TM effectiveness. While self-care behaviors improved with TM, it does not ensure better HF outcomes and was not the focus of this review.
Search limitations must be noted because search terms may have prevented the capture of additional publications. Due to language constraints, important studies were potentially excluded that would have helped broaden the extant literature for inclusion. Since both the EHFScBS and SCHFI instruments have been translated into multiple languages and have undergone psychometric validation, there are likely studies using either of the two instruments that were published in other languages. While we used the broadest MeSH terms for TM and self-care, if published studies did not link their definition to the MeSH terms we used, this could also have impacted the results. For instance, authors may not have identified their telephone intervention as a TM. Finally, the searches were completed in November 2019, and there may be new primary research studies published that have not been included in this review.
Conclusions
The evidence from this review provides support for using TM as an effective therapy for increasing self-care management in adult community-dwelling HF populations. Effective TM interventions ranged from simple telephone-based support to sophisticated remote monitoring devices. Heart failure is a prevalent chronic condition globally; therefore, it was important to determine effectiveness of this specific intervention on increasing patients’ involvement in their own care. The importance of improving self-care behaviors cannot be underestimated as involving patients in their own care improves disease outcomes.
Recommendations for practice
Given the moderate to strong quality of the studies, and the largely consistent findings of statistical and clinical significance, TM should be considered a valid intervention to improve self-care behavior among adult community-dwelling HF patients. Care providers can choose from a variety of TM options to enhance patients’ engagement in self-care behaviors. However, due to the possibility of attenuated effect over time, health care providers must be alert to the possibility of declining self-care. Re-motivation strategies may be needed to sustain benefits gained during early periods of TM.
Originally TM was used to extend health care access to rural settings, with limited expansion to specific settings lacking specialists.80 Insurance reimbursement was also a limiting factor in the use of TM (eg, Medicare limited to use in US rural settings, poor reimbursement rates). However, the public health emergency from the COVID-19 pandemic and resultant social sequestration has forced expansion of TM use and temporary full reimbursement across all areas of health care.81 Local, state, and federal policy-makers can use the results of this systematic review to refine reimbursement policies and procedures to maintain the expanded use and level of remuneration for TM. Greater health care access and achievement of primary care milestones through use of TM may help mitigate the gap caused by social determinants of health. Professional organizations can also use this systematic review to increase support for this practice.
Recommendations for research
As the use of TM increases, there is great potential to reduce gaps in the science of TM. Research is needed to determine which components of TM are most critical and whether there is value-added with layered or multi-modal approaches. Since there were a variety of TM systems included in this review, replication studies are needed to compare multiple systems to determine specificity and sensitivity in the science of TM as therapy. In the current culture of cost containment, head-to-head comparisons and cost-effectiveness analyses are needed. Despite strict security and privacy regulations, the explosive use of telehealth was made possible through temporary relaxation of government restrictions and additional funding.81 Given the current climate, measuring the effect of telehealth office visits in conjunction with TM versus face-to-face office visits will be a popular and useful topic to study.
In general, diverse populations are under-represented in research, and telehealth studies are no exception. A priority would be to test effectiveness of TM equipment among participants with demographics mirroring the HF patient population. A pivotal question remains: Is the effectiveness of TM due to increased provider interaction in response to alerts or due to patients’ increased ability to adjust self-care behaviors, or both? There is still a need for greater understanding of the mechanism by which TM improves outcomes.83 Existing theoretical models may provide a structure to test hypotheses and could explain the mechanism of self-care behavior in HF patients.37,46,47 Additionally, since the two instruments used by the study authors in this review rely on self-report of behaviors, subjectivity is built in to results. Future studies using the EHFScBS or SCHFI with parallel objective measures would provide new knowledge on the impact of self-care on disease outcome. Finally, we are unable to make a statement regarding sustained effectiveness of this therapy. All studies administered treatment and measured the effect at short intervals. We now know short-term TM is effective; however, the use of TM for 12 months had conflicting results. Studies of longer duration must be conducted to see if TM provides a sustained effect or whether other factors, such as novelty of equipment wearing off, increased self-care burden, or progressively worsening HF, attenuate effects. The possibility of long-term effectiveness for TM is an exciting prospect.
Appendix I: Search strategy
Search conducted in June 2019 and updated November 2019.
CINAHL (EBSCO)
Cochrane Central Register of Controlled Trials
Embase (Elsevier)
Epistemonikos
ProQuest Dissertations & Theses A&I: Health and Medicine
PsycINFO (EBSCO)
MEDLINE (PubMed)
Web of Science (Clarivate Analytics)
Appendix II: Studies ineligible following full-text review
Appendix III: Characteristics of included studies
Footnotes
The authors declare no conflict of interest.
References
- 1.Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev 2017;3 (1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziaeian B, Fonarow G. Epidemiology and aetiology of heart failure. Nature Rev Cardiol 2016;13 (6):368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers C, Bush N. Heart failure: pathophysiology, diagnosis, medical treatment guidelines, and nursing management. J Nursing Clinics 2015;50 (4):787–799. [DOI] [PubMed] [Google Scholar]
- 4.Carlson B, Riegel B, Moser DK. Self-care abilities of patients with heart failure. Heart Lung 2001;30 (5):351–359. [DOI] [PubMed] [Google Scholar]
- 5.Inglis SC, Clark RA, Dierckx R, Prieto-Merino D, Cleland JG. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst Rev 2015; (10):CD007228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schnell-Hoehn KN, Naimark BJ, Tate RB. Determinants of self-care behaviors in community-dwelling patients with heart failure. J Card Nurs 2009;24 (1):40–47. [DOI] [PubMed] [Google Scholar]
- 7.Hanlon P, Daines L, Campbell C, McKinstry B, Weller D, Pinnock H. Telehealth interventions to support self-management of long-term conditions: a systematic metareview of diabetes, heart failure, asthma, chronic obstructive pulmonary disease, and cancer. J Med Internet Res 2017;19 (5):e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riley JP, Gabe JP, Cowie MR. Does telemonitoring in heart failure empower patients for self-care? A qualitative study. J Clin Nurs 2013;22 (17–18):2444–2455. [DOI] [PubMed] [Google Scholar]
- 9.Radhakrishnan K, Jacelon C. Impact of telehealth on patient self-management of heart failure: a review of literature. J Cardiovasc Nurs 2012;27 (1):33–43. [DOI] [PubMed] [Google Scholar]
- 10.Lycholip E, Thon Aamodt I, Lie I, Simbelyte T, Puronaite R, Hillege H, et al. The dynamics of self-care in the course of heart failure management: data from the IN TOUCH study. Patient Pref Adherence 2018;12:1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vuorinen A-L, Leppänen J, Kaijanranta H, Kulju M, Heliö T, van Gils M, et al. Use of home telemonitoring to support multidisciplinary care of heart failure patients in Finland: randomized controlled trial. J Med Internet Res 2014;16 (12):e282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Austin LS, Landis C, Hanger KH, Jr, Bakitas M, Dionne-Odom JN, Pamboukian SV, et al. An interactive technology self-management solution engaging patients and families to create a feasible clinical trial integrating palliative and heart failure care: results of the ENABLE CHF-PC pilot clinical trial. J Nurs Admin 2012;42 (9):442–446. [Google Scholar]
- 13.Kenealy TW, Parsons MJ, Rouse APB, Doughty RN, Sheridan NF, Hindmarsh JKH, et al. Telecare for diabetes, CHF or COPD: effect on quality of life, hospital use and costs. A randomised controlled trial and qualitative evaluation. PLoS One 2015;10 (3):e0116188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piette JD, Striplin D, Marinec N, Chen J, Trivedi RB, Aron DC, et al. A mobile health intervention supporting heart failure patients and their informal caregivers: a randomized comparative effectiveness trial. J Med Internet Res 2015;17 (6): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stut W, Deighan C, Cleland JG, Jaarsma T. Adherence to self-care in patients with heart failure in the HeartCycle study. Patient Pref Adherence 2015;9:1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagenaar KP, Broekhuizen BDL, Dickstein K, Jaarsma T, Hoes AW, Rutten FH. Effectiveness of an interactive platform, and the ESC/HFA website in patients with heart failure: design of the multicentre randomized e-Vita heart failure trial. Eur J Heart Fail 2015;17 (12):1310–1316. [DOI] [PubMed] [Google Scholar]
- 17.Evangelista LS, Jung-Ah L, Moore AA, Motie M, Ghasemzadeh H, Sarrafzadeh M, et al. Examining the effects of remote monitoring systems on activation, self-care, and quality of life in older patients with chronic heart failure. J Card Nurs 2015;30 (1):51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nanevicz T, Piette J, Zipkin D, Serlin M, Ennis S, De Marco T, et al. The feasibility of a telecommunications service in support of outpatient congestive heart failure care in a diverse patient population. Congest Heart Fail 2002;6 (3):140–145. [DOI] [PubMed] [Google Scholar]
- 19.Heynsbergh N, Heckel L, Botti M, Livingston PM. Feasibility, useability and acceptability of technology-based interventions for informal cancer carers: a systematic review. BMC Cancer 2018;18 (1):244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kazemi DM, Borsari B, Levine MJ, Li S, Lamberson KA, Matta LA. A systematic review of the mHealth interventions to prevent alcohol and substance abuse. J Health Comm 2017;22 (5):413–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gentry MT, Lapid MI, Clark MM, Rummans TA. Evidence for telehealth group-based treatment: a systematic review. J Telemed Telecare 2019;25 (6):327–342. [DOI] [PubMed] [Google Scholar]
- 22.Michaud TL, Zhou J, McCarthy MA, Siahpush M, Su D. Costs of home-based telemedicine programs: a systematic review. Int J Technol Assess Health Care 2018;34 (4):410–418. [DOI] [PubMed] [Google Scholar]
- 23.Brearly TW, Shura RD, Martindale SL, Lazowski RA, Luxton DD, Shenal BV, et al. Neuropsychological test administration by videoconference: a systematic review and meta-analysis. Neuropsychol Rev 2017;27 (2):174–186. [DOI] [PubMed] [Google Scholar]
- 24.Dham P, Colman S, Saperson K, McAiney C, Lourenco L, Kates N, et al. Collaborative care for psychiatric disorders in older adults: a systematic review. Can J Psychiatry 2017;62 (11):761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langarizadeh M, Tabatabaei MS, Tavakol K, Naghipour M, Rostami A, Moghbeli F. Telemental health care, an effective alternative to conventional mental care: a systematic review. Acta Inform Med 2017;25 (4):240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sztein DM, Koransky CE, Fegan L, Himelhoch S. Efficacy of cognitive behavioural therapy delivered over the internet for depressive symptoms: a systematic review and meta-analysis. J Telemed Telecare 2018;24 (8):527–539. [DOI] [PubMed] [Google Scholar]
- 27.Gilmore AK, Wilson SM, Skopp NA, Osenbach JE, Reger G. A systematic review of technology-based interventions for co-occurring substance use and trauma symptoms. J Telemed Telecare 2017;23 (8):701–709. [DOI] [PubMed] [Google Scholar]
- 28.Hollis C, Falconer CJ, Martin JL, Whittington C, Stockton S, Glazebrook C, et al. Annual research review: digital health interventions for children and young people with mental health problems–a systematic and meta-review. J Child Psychol Psychiatry 2017;58 (4):474–503. [DOI] [PubMed] [Google Scholar]
- 29.Stratton E, Lampit A, Choi I, Calvo RA, Harvey SB, Glozier N. Effectiveness of eHealth interventions for reducing mental health conditions in employees: a systematic review and meta-analysis. PLoS One 2017;12 (12):e0189904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James DC, Harville C, Sears C, Efunbumi O, Bondoc I. Participation of African Americans in e-Health and m-Health studies: a systematic review. Telemed e-Health 2017;23 (5):351–364. [DOI] [PubMed] [Google Scholar]
- 31.Hawkins NM, Jhund PS, McMurray JJ, Capewell S. Heart failure and socioeconomic status: accumulating evidence of inequality. Eur J Heart Fail 2012;14 (2):138–146. [DOI] [PubMed] [Google Scholar]
- 32.Hughes HA, Granger BB. Racial disparities and the use of technology for self-management in blacks with heart failure: a literature review. Curr Heart Fail Rep 2014;11 (3):281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathew J, Lail J, Chang AC, Jefferies JL. Jefferies JL, Chang AC, Rossano JW, Shaddy RE, Towbin JA. Chapter 58 - Outpatient monitoring and self-care. Heart failure in the child and young adult. Boston: Academic Press; 2018. 755–772. [Google Scholar]
- 34.Pandor A, Thokala P, Gomersall T, Baalbaki H, Stevens JW, Wang J, et al. Home telemonitoring or structured telephone support programmes after recent discharge in patients with heart failure: systematic review and economic evaluation. Health Technol Assess 2013;17 (32):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paré G, Jaana M, Sicotte C. Systematic review of home telemonitoring for chronic diseases: the evidence base. J Am Med Inform Assoc 2007;14 (3):269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monza K, Harris D, Shaw C. The role of the nurse navigator in the management of the heart failure patient. Crit Care Nurs Clin 2015;27 (4):537–549. [DOI] [PubMed] [Google Scholar]
- 37.Andrikopoulou E, Abbate K, Whellan DJ. Conceptual model for heart failure disease management. Can J Cardiol 2014;30 (3):304–311. [DOI] [PubMed] [Google Scholar]
- 38.Srisuk N, Cameron J, Ski CF, Thompson DR. Randomized controlled trial of family-based education for patients with heart failure and their carers. J Adv Nurs 2017;73 (4):857–870. [DOI] [PubMed] [Google Scholar]
- 39.Wells R, Stockdill ML, Dionne-Odom JN, Ejem D, Burgio KL, Durant RW, et al. Educate, nurture, advise, before life ends comprehensive heartcare for patients and caregivers (ENABLE CHF-PC): study protocol for a randomized controlled trial. Trials 2018;19 (1):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Góngora Alonso S, Hamrioui S, de la Torre Díez I, Motta Cruz E, López-Coronado M, Franco M. Social robots for people with aging and dementia: a systematic review of literature. Telemed e-Health 2019;25 (7):533–540. [DOI] [PubMed] [Google Scholar]
- 41.Adamse C, Dekker-Van Weering MG, van Etten-Jamaludin FS, Stuiver MM. The effectiveness of exercise-based telemedicine on pain, physical activity and quality of life in the treatment of chronic pain: a systematic review. J Telemed Telecare 2018;24 (8):511–526. [DOI] [PubMed] [Google Scholar]
- 42.Chongmelaxme B, Lee S, Dhippayom T, Saokaew S, Chaiyakunapruk N, Dilokthornsakul P. The effects of telemedicine on asthma control and patients’ quality of life in adults: a systematic review and meta-analysis. J Allergy Clin Immunol Pract 2019;7 (1):199–216.e11. [DOI] [PubMed] [Google Scholar]
- 43.Faruque LI, Wiebe N, Ehteshami-Afshar A, Liu Y, Dianati-Maleki N, Hemmelgarn BR, et al. Effect of telemedicine on glycated hemoglobin in diabetes: a systematic review and meta-analysis of randomized trials. CMAJ 2017;189 (9):E341–E364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hui CY, Walton R, McKinstry B, Jackson T, Parker R, Pinnock H. The use of mobile applications to support self-management for people with asthma: a systematic review of controlled studies to identify features associated with clinical effectiveness and adherence. J Am Med Inform Assoc 2017;24 (3):619–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jackson BD, Gray K, Knowles SR, De Cruz P. EHealth technologies in inflammatory bowel disease: a systematic review. J Crohns Colitis 2016;10 (9):1103–1121. [DOI] [PubMed] [Google Scholar]
- 46.Riegel B, Lee CS, Dickson VV, Carlson B. An update on the self-care of heart failure index. J Cardiovasc Nurs 2009;24 (6):485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riegel B, Dickson VV, Faulkner KM. The situation-specific theory of heart failure self-care: revised and updated. J Cardiovasc Nurs 2016;31 (3):226–235. [DOI] [PubMed] [Google Scholar]
- 48.Doris S, Lee DT, Thompson DR, Jaarsma T, Woo J, Leung EM. Psychometric properties of the Chinese version of the European heart failure self-care behaviour scale. Int J Nurs Stud 2011;48 (4):458–467. [DOI] [PubMed] [Google Scholar]
- 49.Shuldham C, Theaker C, Jaarsma T, Cowie MR. Evaluation of the European Heart Failure Self-care Behaviour Scale in a United Kingdom population. J Adv Nurs 2007;60 (1):87–95. [DOI] [PubMed] [Google Scholar]
- 50.Lee CS, Lyons KS, Gelow JM, Mudd JO, Hiatt SO, Nguyen T, et al. Validity and reliability of the European Heart Failure Self-care Behavior Scale among adults from the United States with symptomatic heart failure. Eur J Cardiovasc Nurs 2013;12 (2):214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jaarsma T, Strömberg A, Mårtensson J, Dracup K. Development and testing of the European heart failure self-care behaviour scale. Eur J Heart Fail 2003;5 (3):363–370. [DOI] [PubMed] [Google Scholar]
- 52.Pulignano G, Del Sindaco D, Minardi G, Tarantini L, Cioffi G, Bernardi L, et al. Translation and validation of the Italian version of the European Heart Failure Self-care Behaviour Scale. J Cardiovasc Med 2010;11 (7):493–498. [DOI] [PubMed] [Google Scholar]
- 53.González B, Lupón J, Parajón T, Urrutia A, Herreros J, Valle V. Use of the European Heart Failure Self-care Behaviour Scale (EHFScBS) in a heart failure unit in Spain. Rev Esp Cardiol (Engl Ed) 2006;59 (2):166–170. [PubMed] [Google Scholar]
- 54.Jaarsma T, Strömberg A, Franzén Årestedt K. The 9-item European Heart Failure Self-Care Behavior Scale tested with data from 6 countries [paper presentation]. Heart Failure 2008 Conference, Milano; 2008. [Google Scholar]
- 55.Kato N, Ito N, Kinugawa K, Kazuma K. Validity and reliability of the Japanese version of the European Heart Failure Self-care Behavior Scale. Eur J Cardiovasc Nurs 2008;7 (4):284–289. [DOI] [PubMed] [Google Scholar]
- 56.Feijó MK, Ávila CW, Souza ENd, Jaarsma T, Rabelo ER. Cross-cultural adaptation and validation of the European Heart Failure Self-care Behavior Scale for Brazilian Portuguese. Rev Lat Am Enfermagem 2012;20 (5):988–996. [DOI] [PubMed] [Google Scholar]
- 57.Baydemir C, Özdamar K, Ünalir A. Validity of the Turkish version of the European heart failure self-care behavior scale. Anadolu Kardiyol Derg 2013;13 (6):573e9. [DOI] [PubMed] [Google Scholar]
- 58.Uchmanowicz I, Łoboz-Rudnicka M, Jaarsma T, Łoboz-Grudzień K. Cross-cultural adaptation and reliability testing of Polish adaptation of the European Heart Failure Self-care Behavior Scale (EHFScBS). Patient Pref Adherence 2014;8:1521–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Østergaard B, Mahrer-Imhof R, Lauridsen J, Wagner L. Validity and reliability of the Danish version of the 9-item European Heart Failure Self-care Behavior Scale. Scand J Caring Sci 2017;31 (2):405–412. [DOI] [PubMed] [Google Scholar]
- 60.Lin C-Y, Pakpour AH, Broström A, Fridlund B, Årestedt K, Strömberg A, et al. Psychometric properties of the 9-item European Heart Failure Self-Care Behavior Scale using confirmatory factor analysis and Rasch analysis among Iranian patients. J Cardiovasc Nurs 2018;33 (3):281–288. [DOI] [PubMed] [Google Scholar]
- 61.Jaarsma T, Årestedt KF, Mårtensson J, Dracup K, Strömberg A. The European Heart Failure Self-care Behaviour scale revised into a nine-item scale (EHFScB-9): a reliable and valid international instrument. Eur J Heart Fail 2009;11 (1):99–105. [DOI] [PubMed] [Google Scholar]
- 62.Sedlar N, Socan G, Farkas J, Mårtensson J, Strömberg A, Jaarsma T, et al. Measuring self-care in patients with heart failure: a review of the psychometric properties of the European Heart Failure Self-Care Behaviour Scale (EHFScBS). Patient Educ Couns 2017;100 (7):1304–1313. [DOI] [PubMed] [Google Scholar]
- 63.Ciere Y, Cartwright M, Newman SP. A systematic review of the mediating role of knowledge, self-efficacy and self-care behaviour in telehealth patients with heart failure. J Telemed Telecare 2012;18 (7):384–391. [DOI] [PubMed] [Google Scholar]
- 64.Maric B, Kaan A, Ignaszewski A, Lear SA. A systematic review of telemonitoring technologies in heart failure. Eur J Heart Fail 2009;11 (5):506–517. [DOI] [PubMed] [Google Scholar]
- 65.Son Y-J, Lee Y, Lee H-J. Effectiveness of mobile phone-based interventions for improving health outcomes in patients with chronic heart failure: a systematic review and meta-analysis. Int J Environ Res Public Health 2020;17 (5):1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bashi N, Karunanithi M, Fatehi F, Ding H, Walters D. Remote monitoring of patients with heart failure: an overview of systematic reviews. J Med Internet Res 2017;19 (1):e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brahmbhatt DH, Cowie MR. Remote management of heart failure: an overview of telemonitoring technologies. Card Fail Rev 2019;5 (2):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tufanaru C, Munn Z, Aromataris E, Campbell J, Hopp L. Chapter 3: Systematic reviews of effectiveness. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis [internet]. 2020 [cited 2020 Jul 17]. Available from: https://synthesismanual.jbi.global. [Google Scholar]
- 69.Nick JM, Petersen AB, Roberts LR. Effect of telemonitoring on self-care behaviors among community-dwelling adults with heart failure: a quantitative systematic review protocol. JBI Evid Synth 2020;18 (5):1091–1099. [DOI] [PubMed] [Google Scholar]
- 70.Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151 (4):264–269. [DOI] [PubMed] [Google Scholar]
- 71.Rai SK, Yazdany J, Fortin PR, Aviña-Zubieta JA. Approaches for estimating minimal clinically important differences in systemic lupus erythematosus. Arthritis Res Ther 2015;17 (1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boyne J, Vrijhoef HJM, Spreeuwenberg M, Deweerd GJ, Kragten J, Gorgels APM. The effects of tailored telemonitoring on heart failure patients’ knowledge, self-care, self-efficacy and adherence: a randomized controlled trial. Eur J Cardiovasc Nurs 2014;13 (3):243–252. [DOI] [PubMed] [Google Scholar]
- 73.Hägglund E, Lyngå P, Frie F, Ullman B, Persson H, Melin M, et al. Patient-centred home-based management of heart failure: findings from a randomised clinical trial evaluating a tablet computer for self-care, quality of life and effects on knowledge. Scand Cardiovasc J 2015;49 (4):193–199. [DOI] [PubMed] [Google Scholar]
- 74.Melin M, Hägglund E, Ullman B, Persson H, Hagerman I. Effects of a tablet computer on self-care, quality of life, and knowledge a randomized clinical trial. J Cardiovasc Nurs 2018;33 (4):336–343. [DOI] [PubMed] [Google Scholar]
- 75.Varon C, Alao M, Minter J, Stapleton M, Thomson S, Jaecques S, et al. Telehealth on heart failure: results of the Recap project. J Telemed Telecare 2015;21 (6):340–347. [DOI] [PubMed] [Google Scholar]
- 76.Wagenaar KP, Broekhuizen BDL, Jaarsma T, Kok I, Mosterd A, Willems FF, et al. Effectiveness of the European Society of Cardiology/Heart Failure Association website ’heartfailurematters.org’ and an e-health adjusted care pathway in patients with stable heart failure: results of the ’e-Vita HF’ randomized controlled trial. Eur J Heart Fail 2019;21 (2):238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seto E, Leonard KJ, Cafazzo JA, Barnsley J, Masino C, Ross HJ. Mobile phone-based telemonitoring for heart failure management: a randomized controlled trial. J Med Internet Res 2012;14 (1):e31–e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moon MK, Yim J, Jeon MY. The effect of a telephone-based self-management program led by nurses on self-care behavior, biological index for cardiac function, and depression in ambulatory heart failure patients. J Asian Nurs Res 2018;12 (4):251–257. [DOI] [PubMed] [Google Scholar]
- 79.Wagenaar KP, Broekhuizen BD, Rutten FH, Strömberg A, van Stel HF, Hoes AW, et al. Interpretability of the European heart failure self-care behaviour scale. Patient Pref Adherence 2017;11:1841–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mann DM, Chen J, Chunara R, Testa PA, Nov O. COVID-19 transforms health care through telemedicine: evidence from the field. J Am Med Informatics Assoc 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Contreras CM, Metzger GA, Beane JD, Dedhia PH, Ejaz A, Pawlik TM. Telemedicine: patient-provider clinical engagement during the COVID-19 pandemic and beyond. J Gastrointest Surg 2020;24 (7):1692–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmidt S, Schuchert A, Krieg T, Oeff M. Home telemonitoring in patients with chronic heart failure: a chance to improve patient care? Dtsch Arztebl Int 2010;107 (8):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kitsiou S, Paré G, Jaana M. Effects of home telemonitoring interventions on patients with chronic heart failure: an overview of systematic reviews. J Med Internet Res 2015;17 (3):e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stut W, Deighan C, Armitage W, Clark M, Cleland JG, Jaarsma T. Design and usage of the Heartcycle education and coaching program for patients with heart failure. JMIR Res Protocol 2014;3 (4):e72. [DOI] [PMC free article] [PubMed] [Google Scholar]