Purpose of review
Multiple myeloma is a disease of elderly adults. Improvement in survival has occurred because of biological insights and novel agents. Therapeutic options involve choices today, thus have become more complex. Demographics have led to an increased number of elderly patients and age may be associated with a poorer outcome but is not the only prognostic predictor today.
Recent findings
To evaluate patients’ health status rather than their chronological age alone, frailty scores and functional geriatric assessments are used to identify prognostic groups, avoid adverse events, compare clinical trials and tailor treatment. As most clinical trials exclude frail elderly patients, those enrolled therein are often younger and healthier than the typical multiple myeloma patient. This represents a challenge for frail cohorts because of their increased risk of adverse events, overtreatment and undertreatment and/or therapy discontinuation, which may lead to poorer survival and quality of life (QoL). Reassessing patients’ status via geriatric assessments is also relevant during treatment to adjust interventions appropriately.
Summary
Integrating geriatric assessments may lead to individual treatment decisions, dose adjustments, better clinical outcome and QoL. Prospective clinical trials that enroll elderly multiple myeloma patients with comorbidities, incorporate frailty scores/geriatric assessments and help with prognostication, adverse event avoidance and QoL maintenance, remain warranted.
Keywords: fitness, frailty scores/functional geriatric assessment, multiple myeloma
INTRODUCTION
Multiple myeloma is the second most common hematological malignant neoplasm driven by clonal proliferation of plasma cells in the bone marrow and increased production of monoclonal immunoglobulins [1▪]. The incidence of multiple myeloma is growing with aging of the general population, with an annual increase of older newly diagnosed multiple myeloma patients of 90% being expected by 2034 [2].
Box 1.
no caption available
With a median age of 70 years at the time of diagnosis, multiple myeloma is indeed a disease of elderly adults, with 30–40% of patients being older than 75 years and only less than 2% being very young (<40 years) [3]. The elderly group remains at higher risk for poorer outcome and early mortality, underlining the need for tools to optimize antimyeloma treatment especially in these patients [2].
EVALUATION OF FRAILTY TO IDENTIFY THE BEST TREATMENT OPTION
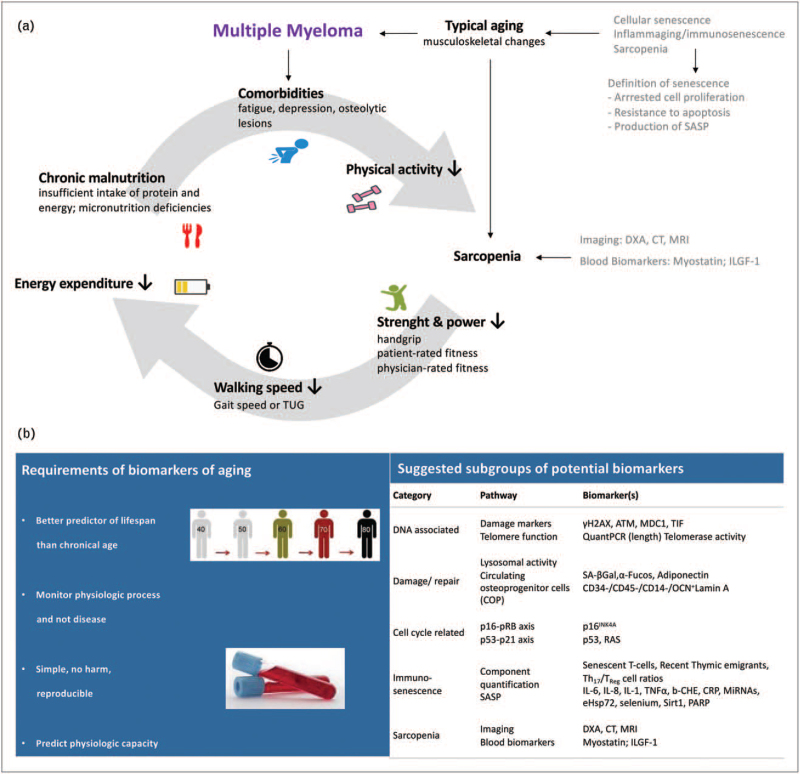
Age-related pathologies become more important when focusing on comprehensive treatment for older cancer patients. The biological process of aging, including immunosenescence in cancer and multiple myeloma patients, leads to sarcopenia [4], reduces strength, power and walking speed and causes less energy expenditure, chronic malnutrition and decreased physical activity. These factors cause a negative feed-back loop, which intensifies these processes and thereby clinical worsening ending in frailty. Moreover, multiple myeloma-specific comorbidities, like fatigue or osteolytic lesions, may boost this downward spiral (Fig. 1a). In summary, frailty is mainly driven by age-related biological changes [5].
FIGURE 1.
Biological aging and multiple myeloma. (a) Biological process of aging and senescence in multiple myeloma patients. CT, computer tomography; DXA, imaging like dual-energy X-ray absorptiometry; SASP, senescence-associated secretory phenotype; TUG, time up and go test. (b) Frailty measures and biomarkers of aging in multiple myeloma patients. Adapted with permission from Soto Perez de Celis, Lancet Oncol, 2018; Cook, Leukemia, 2020. Adapted with permission from Dr A. Rosko's pivotal work/slides and American Federation for Aging Research (AFAR).
To evaluate aging and frailty more objectively, it has been proposed that simple but suitable biomarkers, which predict physiological capacity, should be identified and used in clinical practice. These should ideally be better predictors of patients’ lifespan than their chronological age alone. In addition, biomarkers of aging and frailty should monitor physiological processes and not disease itself. Current biomarkers of inflammation, cellular senescence, endocrine, genomic and immune profiles are examined and displayed in Fig. 1b [6,7▪]. Additionally, imaging like dual-energy X-ray absorptiometry (DXA), computer tomography, MRI and bioelectrical impedance analysis are used to assess sarcopenia (Fig. 1a and b) [7▪].
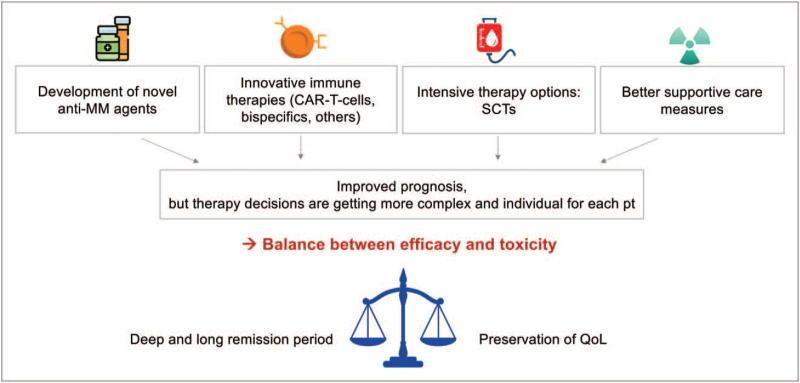
Overall survival (OS) in multiple myeloma patients has improved substantially in recent years because of ample biological insights, use of novel agents and innovative immunotherapies, autologous stem cell transplantation (ASCT) and better supportive care (Fig. 2) [8,9]. Nonetheless, aging remains a poor prognostic factor in cancer in general, including in multiple myeloma patients [1▪,8,10]. As therapy decisions have become more complex and involve multiple choices today, individual management of patients – ranging from fit to frail – should balance efficacy, toxicity and practicability (Fig. 2) [1▪]. Due to comorbidities and frailty, treating older patients can be challenging for physicians as these patients bear an increased risk of complications or adverse events leading to therapy discontinuation and treatment-related mortality (TRM) [8,11]. Patients who are identified as frail are, therefore, at risk of toxicity/severe adverse events and shorter progression-free survival (PFS) and OS [2]. Moreover, not only patient-related factors like advanced age or impaired Karnofsky Performance Status (KPS) but also myeloma-related factors, like advanced International Staging System (ISS) stage, high-risk cytogenetics, extramedullary involvement, no achieved partial response (PR) and short remission durations are associated with worse PFS/OS [12▪▪]. Both, over-treatment of frail patients and under-treatment of fit elderly patients are clinical challenges, which may induce poorer survival and reduce patients’ quality of life (QoL) [1▪,13,14▪▪]. Therefore, balancing efficacy and toxicity of therapy is highly relevant to obtain deep and long-lasting remission and preserve patients’ QoL (Fig. 2) [2]. The choice of the numerous available treatment options for older patients affected by frailty and comorbidities should consequently be individualized [1▪]. Indeed, adjusting for comorbidities induced significant differences in patients’ survival [10,11,14▪▪]. For example, clinical trials have shown feasibility and benefit of ASCT in older multiple myeloma patients, even with full-dose Melphalan (200 mg/m2) [15], highlighting that biologically fitter elderly patients can profit from intensive treatment as well as from interventions strengthening their performance and physical capabilities [10,14▪▪,16].
FIGURE 2.
Advances in antimyeloma therapies. MM, multiple myeloma; pt, patient; QoL, quality of life; SCT, stem cell transplantation.
GERIATRIC AND FRAILTY SCORES
Frailty is defined as increased vulnerability to stressors because of a multisystem reduction in reserve capacity, which can be associated with poor response to treatment, increased toxicity and worse survival [1▪]. About one-third of myeloma patients are frail at the time of diagnosis. There are several frailty scores that can be used to stratify patients’ fitness, albeit a standardized frailty score remains to be defined [5]. The initial International Myeloma Working Group (IMWG)-frailty index [17] was simplified by Facon [18] using only age, Eastern Cooperative Oncology Group (EGOC) performance status and Charlson comorbidity index (CCI) [19], both assessed in retrospectively scored multiple myeloma patients. Several others, such as the revised myeloma comorbidity index (R-MCI) [20,21,22▪▪], the Mayo risk score [17] and the UK myeloma research alliance risk profile [7▪,23] have additionally been proposed as valuable clinical tools.
The simple assessment of patient fitness is performed via KPS or ECOG and CCI. However, these tools, as illustrated in Fig. 3a, have been criticized to be prone to subjective judgement, as too simple, often with the KPS being notoriously overestimated by a median of 30% and/or impossible to be evaluated retrospectively for the CCI [7▪,22▪▪,24].
FIGURE 3.
Myeloma-specific risk scores. (a) Examples of geriatric assessment tools for myeloma patients. ADL, activity of daily living; BFI, Brief Fatigue Inventory; CCI, Charlson Comorbidity Index; ECOG, Eastern Cooperative Oncology Group; IADL, Instrumental Activities of Daily Living; KPS, Karnofsky Performance Status; MM, multiple myeloma; SF-2612, Short Form 36/12; TUG, Time Up and Go test. (b) Multivariable cox proportional hazard model and weights of 12 comorbidities of the revised Myeloma Comorbidity Index (R-MCI). eGFR, estimated glomerular filtration rate; HR, hazard ratio; KPS, Karnofsky Performance Status; n, number. (c) Examples of myeloma-specific risk scores. ADL, activity of daily living; AE, adverse event; CCI, Charlson Comorbidity Index; CG, cytogenetic; CRP, c-reactive protein; eGFR, estimated glomerular filtration rate; GAH, Geriatric Assessment in Hematology; IADL, Instrumental Activities of Daily Living; IMWG, International Myeloma Working Group; ISS, International Staging System; KPS, Karnofsky Performance Status; MRP, Myeloma Research Alliance Risk Profile; OS, overall survival; PFS, progression-free survival; PS, Performance Status; QoL, quality of life; UK, United Kingdom. (d) Examples of multidimensional functional tests. ADL, activity of daily living; IADL, Instrumental Activities of Daily Living; KPS, Karnofsky Performance Status; NRS, Numerical Rating Scale; SF-12, Short Form 12; TUG, Time Up and Go test. (e) Frailty index risk factors and dose adjustments with the aid of R-MCI. bw, bodyweight; cy, cycle; d, day; i.v., intravenous; R-MCI, Revised Myeloma Comorbidity Score; s.c., subcutaneous; wk, week.
Instead, geriatric assessments have proven to be more reliable tools to measure patients’ physical and psychological status [17,20,21,22▪▪,25–28]. As geriatric assessments are time-consuming and may be difficult to integrate in daily clinical practice, shorter and more objective assessments of frailty have been proposed. These involve the Time Up and Go (TUG) test, handgrip strength, the short Physical Performance Battery or self-reported items, like the Katz scale of basic activities of daily living (ADL), Lawton and Brody's instrumental ADL (IADL), Brief Fatigue Inventory (BFI) or Medical Outcomes Study Short Form 36/12 (SF-36/12; Fig. 3a). Albeit these provide more objective and self-reported metrics of patients‘ fitness, they are not myeloma-specific and have been used less frequently [14▪▪,24].
RISK-ASSESSMENT VIA REVISED MYELOMA COMORBIDITY INDEX
Objective assessment of patients’ fitness – at best over time – has been postulated as desirable [7▪,17,21,22▪▪,25–29]. Indeed, myeloma-specific geriatric assessments have been examined to objectively divide patients into fit, intermediate-fit and frail. Ideally, these assessments should be based on repeatedly tested and validated, multivariately determined and weighted risk factors [7▪,20,21,22▪▪,25–29]. This has led to the development of the R-MCI and IMWG-frailty index. The R-MCI has been evaluated in a large cohort of more than 1500 multiple myeloma patients and incorporates five risk factors, determined via multivariate Cox proportional hazard ratio model out of 12 meticulously assessed comorbidities (Fig. 3b). The five most relevant R-MCI risk factors were an impaired renal function [measured via estimated glomerular filtration rate (eGFR)], lung function, KPS, advanced age and frailty (according to Fried) [30], with cytogenetics, if available, being possible to include therein. The number of patients in the initial test analysis, with given risk groups, hazard ratio, P values and weights are depicted in Fig. 3b. A maximum of nine R-MCI points can be obtained, which generate risk groups of fit (0–3 points), intermediate-fit (4–6 points) and frail patients (7–9 points) with distinctly separating Kaplan–Meier curves for both PFS and OS [20,21], different TRM and risk of complications/adverse events. The side-by-side comparison of prospectively assessed multiple myeloma patients via R-MCI and IMWG-frailty index demonstrated that fit vs. frail patients were better distinguished with the R-MCI [20,21]. Of interest, Jackson et al.[10], Palumbo et al.[17] and Schinke et al.[12▪▪] demonstrated that similar to molecular markers being relevant predictors of outcome, clinical risk factors were equally important in elderly multiple myeloma patients, such as frailty, falls or nonresponsiveness, especially when repeatedly assessed over time [10,12▪▪]. Assessing frailty, with defined risk scores, should therefore, involve objectively weighted single risk factors, in order to most precisely describe a patient status, as the R-MCI or IMWG-frailty index do. Indeed, the R-MCI combines multivariate risk factors and has been prospectively used in clinical routine and clinical trials, both before therapy and at follow-up to assess patients’ improvement over time [14▪▪,20,31].
Notably, almost all risk scores, as summarized in Fig. 3c, have determined age as a relevant risk factor, albeit age cut-offs were different with more than 70 years in the R-MCI vs. more than 80 years in the IMWG-frailty index [17,20,21]. All these scores have shown their relevance in multiple myeloma patients, reveal risk group allocation into fit vs. frail patients, with different PFS and OS, and with substantially differing therapy toxicity, adverse events and TRM [17,20,21,23,24,32▪▪,33–35]. Apart from age, some include impaired organ function (renal, lung, performance status), comorbidity scores (CCI), laboratory parameters (NT-pro-BNT, platelets, ß2-MG, albumin, CRP), ISS stage and the possibility to include adverse cytogenetics therein, thus are fairly different (Fig. 3c). Suggestions for their improvement or even fusion moving forward are discussed in the hematology–oncology/multiple myeloma community (G. Cook, A. Larocca, V. Goede, U. Wedding, H. Auner, K. Yong, S. Kumar, A. Brioli, S. Zweegman, and others; personal communication). Commentaries have suggested that using any of these scores in clinics and clinical trials should be better than none at all [22▪▪]. Moreover, as depicted in Fig. 3d, geriatric assessments may not only involve frailty scores but also multidimensional functional tests, including fitness measures, tests for cognitive function, self-sufficiency, pain, depression, QoL and nutrition. Not all of these factors have proven to be equally relevant in multiple myeloma, which was demonstrated in a large prospective multiple myeloma cohort, where functional tests were compared with frailty scores at the time of patients’ initial myeloma diagnosis and at follow-up [14▪▪].
As previous clinical trials have shown that frailty increases the risk of treatment-related adverse events and TRM, the US National Comprehensive Cancer Network, International Society of Geriatric Oncology and European Organization for Research and Treatment of Cancer recommended frailty assessment for older cancer patients to detect unrecognized health setbacks [5]. Risk scores, such as the R-MCI, include more objective fitness assessments that allow better patient prognostication than age alone, to allocate therapy intensity correctly and to avoid treatment toxicities, interruptions and early mortality. Risk scores are already used in tumor boards, follow-up assessments to determine improvement vs. deterioration, to compare trial cohorts but as yet less routinely in daily practice [7▪,17,22▪▪,28,31]. Recommended therapy doses for fit, intermediate-fit and frail multiple myeloma patients have been published in current guidelines and chemotherapy manuals (Fig. 3e) [20,36].
SELECTED CLINICAL TRIALS IMPLEMENTING FRAILTY ASSESSMENT IN MULTIPLE MYELOMA PATIENTS
As frailty is a known risk factor in older cancer patients, there are now more studies that evaluate the effectiveness of geriatric assessments to define risk groups, feasibility for dose-adjustments, effect on adverse events/TRM and whether a defined treatment schedule is equally superior in fit and frail cohorts (Table 1): most involve dose adjustments in intermediate-fit and frail patients, supportive interventions or the retrospectively performed analysis, whether patients rated fit vs. frail via simplified IMWG frailty score [18] profit equally from novel combination therapies, such as Daratumumab, Bortezomib, Melphalan and Prednisone (Dara-VMP), Isatuximab, Pomalidomide and Dexamethasone (Isa-Pd) or Selinexor, Bortezomib and Dexamethasone (SVd). The latter difficulty is, that the CCI was not available retrospectively and was estimated as low in both fit and frail groups. Thus, poorly separating multiple myeloma patients were defined as fit or frail rather via age and ECOG alone than via simplified IMWG frailty score [36,37▪,38].
Table 1.
Selected clinical trials with used frailty measures in multiple myeloma
| Author | Number of patients/study | Frailty measure | Results | Pros | Cons |
| Larocca (GIMEMA) NCT02215980 | 199/II | IMWG frailty score | Rd (n = 101) vs. Rd-R10 (n = 98) in intermediate-fit | Randomized | Intermediate, not frail pts, R10 later rather than initially = upfront reduced |
| Suvannasankha NCT04223661 | 44/Indiana/II | IMWG frailty score | Dara-Rd in fit: Len 10 mg → 15 mg Dara-Rd frail: Len 5 → 10 mg | Prospective data: intermediate/frail profit from dose-reductions | Nonrandomized, Small study → needs confirmation |
| Cook (UKMRA) NCT03720041 | 740/III | UK myeloma score and IMWG frailty score | IxRd w/o dose reduction, −1 and −2 dose reduction | Randomized, large study | Industry sponsor, no (i.e. exercise) intervention |
| Zweegman (HOVON) NCR6297 | 130/II | IMWG frailty score | IDd dose-adjustments feasible | Might translate in better outcome | Effect on early mortality not shown |
| Möller, …Engelhardt | 30/REAL-Fitness/II | R-MCI and others | Ongoing | Randomized, prospective data | Small → needs confirmation |
| Mateos | 706/Alcyone/III | Simplified IMWG frailty score | Dara-VMP vs. VMP | Frail and fit seem to profit | Frailty score was retrospectively assessed |
| Schjesvold | 307/Icaria/III | Simplified IMWG frailty score | Isa+Pd (n = 154) vs. Pd (n = 153) | Frail and fit seem to profit | Frailty score was retrospectively assessed |
| Auner | 402/Boston/III | Simplified IMWG frailty score | Selinexor (X)Vd (n = 195) vs. Vd (n = 207) | Frail and fit seem to profit | Frailty score was retrospectively assessed |
cons, disadvantages/issues to be considered; Dara, Daratumumab; IDd, Ixazomib, Daratumumab, Dexamethasone; IMWG, International Myeloma Working Group; Isa, Isatuximab; Pd, Pomalidomide, Dexamethasone; Pros, advantages of the study; pts, patients; Rd, Lenalidomide, Dexamethasone; R-MCI, Revised Myeloma Comorbidity Index; Vd, Bortezomib, Dexamethasone; VMP, Bortezomib, Melphalan, Prednisone.
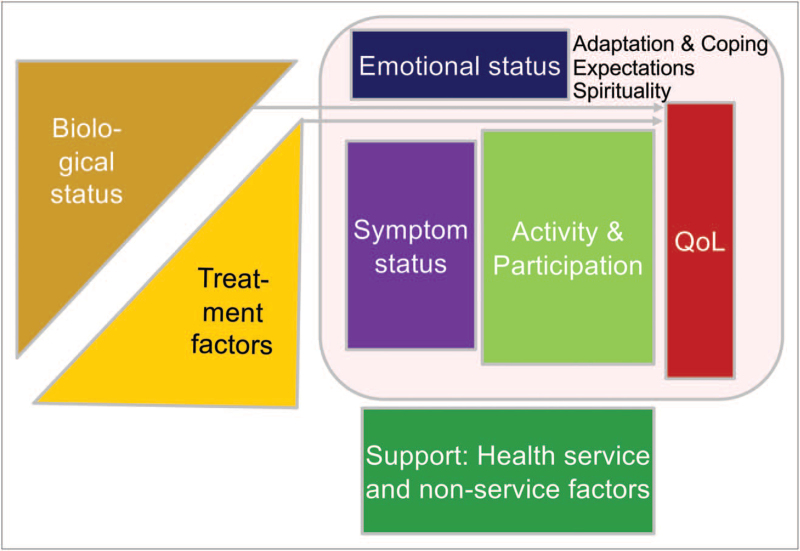
As shown in Fig. 4, both biological and treatment factors influence patients’ symptoms and emotional status, their activity in daily life and QoL. Frailty scores and geriatric assessments may help to assess the complex patient status before and during therapeutic interventions, leading at best to supportive steps that allow patients to cope with their frailty, comorbidity burden and therapy. With an increasing number of elderly multiple myeloma patients, frailty needs should be identified and addressed in clinical trials, some representative of those being described in Table 1[11].
FIGURE 4.
Theoretical model of quality of life and fitness preservation.
Most oncologic procedures are based on findings of multicenter, randomized clinical trials but the patients enrolled in these studies are generally younger and healthier than the typical elderly and eventually frail multiple myeloma patient with comorbidities [9]. Therefore, physicians may administer novel agents to elderly and frail patients rather restrained, until clinical trials have shown efficacy and safety in elderly patients as much as in younger patients (Table 1) [11]. The simplified IMWG-frailty index [18] incorporating age, ECOG performance status and CCI, suggested an easier applicability than the original IMWG-frailty index with ADL and IADL inclusion. Here, the CCI was based on the retrospectively assessed comorbidity enumeration within the FIRST trial [18]. This led to low CCI values, therefore, the simplified IMWG-frailty index heavily relies on age and performance status alone [27]. Consequently, retrospectively performed ‘ad hoc’ analyses of the Alcyone, Icaria and Boston trials have suggested alike to the FIRST study [40], that fit and frail multiple myeloma patients profit from Dara-VMP, Isa-Pd and SVd similarly, albeit differences in the risk groups were small and prospective frailty assessment should have preferably been incorporated upfront into these clinical trials (Table 1) [39–41,42▪,43▪]. Frailty scores, should therefore, be prospectively planned to be included in research projects to get a much better understanding of how novel agents and treatment interventions can serve both fit and frail multiple myeloma patients [9], like ongoing representative studies do as summarized in Table 1.
FRAILTY SCORES AND QUALITY OF LIFE
Frailty may often be associated with lower QoL [20]. QoL is a complex model, influenced by the balance of symptoms, comorbidities, treatment-related toxicity and response to therapy. This model includes additional issues involving the biological status, emotional well being, possible physical activity, support by family, health service and nonservice factors at and during the disease course (Fig. 4).
Among cancer survivors, multiple myeloma patients have shown low QoL scores, highlighting the importance of the periodic assessment and interventions to improve QoL [1▪,20]. In principle, treatment response may induce or be associated with improved QoL as pain, anemia and organ impairment may subside, and strength, energy and physical activity may increase again, which we had indeed observed in follow-up analyses, specifically in responding (≥PR) and more strikingly in 70 year- or less vs. more than 70 year-old multiple myeloma patients [14▪▪].
Nevertheless, receiving the most effective treatment may not always guarantee patients’ well being as emotional and socioeconomic factors influence patients likewise [44]. It is known, that anxiety and pain decrease QoL more than clinical characteristics [45▪▪]. Therefore, if QoL in any multiple myeloma patient does not improve during therapy, even though treatment response has been achieved, the evaluation of the R-MCI has shown to facilitate decisions on treatment adaptations or supportive interventions a patient might need [20,31].
CONCLUSION
Advanced age, frailty/myeloma-specific comorbidities and vulnerability/treatment tolerability are challenging to balance and may increasingly affect our patient management. Integrating frailty scores and geriatric assessments to support individual treatment decisions and dose adjustments may allow to improve clinical outcome and enhance patients’ QoL to an even larger extend. To date, objective frailty and senescence markers remain to be exactly defined and it has to be determined, which can be reliably implemented into clinical practice to identify possible risks. Furthermore, reassessing patients’ status during the treatment course seems important to determine, if patients’ QoL improves, remains the same or deteriorates, therefore if the patient may need therapy adjustments [14▪▪]. Multifactorial interventions of comprehensive cancer centers today, combining physical activity, nutrition, cognitive training and other supportive measures seem necessary tools to preserve or at best improve patients’ physical function. More prospective studies that include frailty scores and geriatric assessments in antimyeloma treatment and broader range of multicenter clinical trials that allow to enroll elderly multiple myeloma patients with various comorbidities are eagerly awaited and clinically needed. These should further determine, if and to what extend multiple myeloma patient and disease management have indeed improved and what can be done to foster this in the future.
Acknowledgements
The authors thank distinguished IMWG, EMN, DSMM and GMMG myeloma experts for their advice, recommendations and insightful, inspiring comments. M.E. and all authors also thank all German, Austrian, Swiss, European and international elderly task forces for their support, and especially Professor Dr Justus Duyster (Freiburg, UKF) and the CCCF. We are also very thankful to all AG Engelhardt & Wäsch group members, especially Drs Heike Reinhardt, Amelie Rösner, Magdalena Braun and Stefanie Adebola Ajayi for their chemotherapy surveillance work, including their MM enthusiasm. We also thank the members of our MM-tumorboard group, MM self-help group Freiburg and Center for biobanking (FREEZE-Biobank) for their support. We are very grateful for the advice of numerous MM experts, that is, from Gordon Cook, Alessandra Larocca, Valentin Goede, Ulrich Wedding, Holger Auner, Kwee Yong, Shaji Kumar, Annamaria Brioli and Sonja Zweegman, who we discussed earlier thoughts, projects and future trial ideas with. This work was supported by the DKH.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Mandy-Deborah Möller and Monika Engelhardt contributed equally.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪.Antoine-Pepeljugoski C, Braunstein MJ. Management of newly diagnosed elderly multiple myeloma patients. Curr Oncol Rep 2019; 21:64. [DOI] [PubMed] [Google Scholar]; This is a well structured overview how to deal with elderly myeloma patients.
- 2.Wildes TM, Campagnaro E. Management of multiple myeloma in older adults: gaining ground with geriatric assessment. J Geriatr Oncol 2017; 8:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mian HS, Wildes TM, Fiala MA. Development of a medicare health outcomes survey deficit-accumulation frailty index and its application to older patients with newly diagnosed multiple myeloma. JCO Clin Cancer Inform 2018; 2:CCI.18.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelke C, Dziewas R, Minnerup J, et al. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine 2019; 49:381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee H, Lee E, Jang I-Y. Frailty and comprehensive geriatric assessment. J Korean Med Sci 2019; 35:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soto-Perez-de-Celis E, Li D, Yuan Y, et al. Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol 2018; 19:e305–e316. [DOI] [PubMed] [Google Scholar]
- 7▪.Cook G, Larocca A, Facon T, et al. Defining the vulnerable patient with myeloma-a frailty position paper of the European Myeloma Network. Leukemia 2020; 34:2285–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is an accomplished review of risks, including biomarkers of aging in the treatment of frail myeloma patients.
- 8.Pulte D, Redaniel MT, Lowry L, et al. Age disparities in survival from lymphoma and myeloma: a comparison between US and England. Br J Haematol 2014; 165:824–831. [DOI] [PubMed] [Google Scholar]
- 9.Diamond E, Lahoud OB, Landau H. Managing multiple myeloma in elderly patients. Leuk Lymphoma 2018; 59:1300–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson GH, Pawlyn C, Cairns DA, et al. UK NCRI Haemato-oncology Clinical Studies Group. Optimising the value of immunomodulatory drugs during induction and maintenance in transplant ineligible patients with newly diagnosed multiple myeloma: results from Myeloma XI, a multicentre, open-label, randomised, phase III trial. Br J Haematol 2021; 192:853–868. [DOI] [PubMed] [Google Scholar]
- 11.Jones A, Bowcock S, Rachet B. Survival trends in elderly myeloma patients. Eur J Haematol 2021; 106:126–131. [DOI] [PubMed] [Google Scholar]
- 12▪▪.Schinke M, Ihorst G, Duyster J, et al. Risk of disease recurrence and survival in patients with multiple myeloma: a German Study Group analysis using a conditional survival approach with long-term follow-up of 815 patients. Cancer 2020; 126:3504–3515. [DOI] [PubMed] [Google Scholar]; This is the first analysis of conditional survival in multiple myeloma patients using both baseline and follow-up risk parameters, demonstrating that regular risk assessment throughout the course of disease and complete follow-up provide a more reliable conditional survival estimation than via baseline assessment alone.
- 13.DuMontier C, Loh KP, Bain PA, et al. Defining undertreatment and overtreatment in older adults with cancer: a scoping literature review. J Clin Oncol 2020; 38:2558–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14▪▪.Scheubeck S, Möller M-D, Ihorst G, et al. Comparison of the prognostic significance of 5 comorbidity scores and 12 functional tests in a prospective multiple myeloma patient cohort. Cancer 2021; 127:3422–3436. [DOI] [PubMed] [Google Scholar]; This is a comparison of existing myeloma scores with their advantages and disadvantages and of retrospectively and prospectively assessed risk scores, clearly showing that, that is for the CCI and others, retrospectively assessed data lead to underscored results.
- 15.Munshi PN, Vesole D, Jurczyszyn A, et al. Age no bar: A CIBMTR analysis of elderly patients undergoing autologous hematopoietic cell transplantation for multiple myeloma. Cancer 2020; 126:5077–5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Möller M-D, Ihorst G, Pahl A, et al. Physical activity is associated with less comorbidity, better treatment tolerance and improved response in patients with multiple myeloma undergoing stem cell transplantation. J Geriatr Oncol 2020; 12:521–530. [DOI] [PubMed] [Google Scholar]
- 17.Palumbo A, Bringhen S, Mateos M-V, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood 2015; 125:2068–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Facon T, Dimopoulos MA, Meuleman N, et al. A simplified frailty scale predicts outcomes in transplant-ineligible patients with newly diagnosed multiple myeloma treated in the FIRST (MM-020) trial. Leukemia 2020; 34:224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383. [DOI] [PubMed] [Google Scholar]
- 20.Engelhardt M, Dold SM, Ihorst G, et al. Geriatric assessment in multiple myeloma patients: validation of the International Myeloma Working Group (IMWG) score and comparison with other common comorbidity scores. Haematologica 2016; 101:1110–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engelhardt M, Domm A-S, Dold SM, et al. A concise revised Myeloma Comorbidity Index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica 2017; 102:910–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22▪▪.Engelhardt M, Ihorst G, Duque-Afonso J, et al. Structured assessment of frailty in multiple myeloma as a paradigm of individualized treatment algorithms in cancer patients at advanced age. Haematologica 2020; 105:1183–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]; An informative review, addressing the role of geriatric assessments and providing guidelines for the management of elderly myeloma patients.
- 23.Redder L, Klausen TW, Vangsted AJ, et al. Validation of the UK myeloma research alliance risk profile, a new clinical prediction model for outcome in patients with newly diagnosed multiple myeloma not eligible for autologous stem cell transplantation; a population-based study from the Danish national multiple myeloma registry. Br J Haematol 2021; 193:119–124. [DOI] [PubMed] [Google Scholar]
- 24.Schoeller K, Ihorst G, Scheubeck S, et al. The Revised Myeloma Comorbidity Index (R-MCI) as a promising approach for predicting overall (os)- and progression-free (pfs) survival and optimizing therapy strategies in multiple myeloma (mm) patients (pts) - comparative analysis of 5 comorbidity indices (ci), including retro- and prospective applicability. Blood 2019; 134:3474–13474. [Google Scholar]
- 25.Kleber M, Ihorst G, Terhorst M, et al. Comorbidity as a prognostic variable in multiple myeloma: comparative evaluation of common comorbidity scores and use of a novel MM–comorbidity score. Blood Cancer J 2011; 1:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleber M, Ihorst G, Udi J, et al. Prognostic risk factor evaluation in patients with relapsed or refractory multiple myeloma receiving lenalidomide treatment: analysis of renal function by eGFR and of additional comorbidities by comorbidity appraisal. Clin Lymphoma Myeloma Leuk 2012; 12:38–48. [DOI] [PubMed] [Google Scholar]
- 27.Kleber M, Ihorst G, Gross B, et al. Validation of the Freiburg Comorbidity Index in 466 multiple myeloma patients and combination with the international staging system are highly predictive for outcome. Clin Lymphoma Myeloma Leuk 2013; 13:541–551. [DOI] [PubMed] [Google Scholar]
- 28.Zweegman S, Engelhardt M, Larocca A, et al. EHA SWG on ‘Aging and Hematology’. Elderly patients with multiple myeloma: towards a frailty approach? Curr Opin Oncol 2017; 29:315–321. [DOI] [PubMed] [Google Scholar]
- 29.Larocca A, Dold SM, Zweegman S, et al. Patient-centered practice in elderly myeloma patients: an overview and consensus from the European Myeloma Network (EMN). Leukemia 2018; 32:1697–1712. [DOI] [PubMed] [Google Scholar]
- 30.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 31.Küchlin S, Duffner J, Scheubeck S, et al. Kidney embolization induces prompt organ response in a 86-year-old patient with MGRS-related AL-amyloidosis. Hemodial Int 2019; 23:E59–E64. [DOI] [PubMed] [Google Scholar]
- 32▪▪.Rajkumar SV. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematol 2020; 95:548–567. [DOI] [PubMed] [Google Scholar]; Excellent review on state-of-the-art diagnostics, risks and treatment in multiple myeloma patients by Mayo Clinic.
- 33.Binder M, Rajkumar SV, Ketterling RP, et al. Substratification of patients with newly diagnosed standard-risk multiple myeloma. Br J Haematol 2019; 185:254–260. [DOI] [PubMed] [Google Scholar]
- 34.Cook G, Royle K-L, Pawlyn C, et al. A clinical prediction model for outcome and therapy delivery in transplant-ineligible patients with myeloma (UK Myeloma Research Alliance Risk Profile): a development and validation study. Lancet Haematol 2019; 6:e154–e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonanad S, De la Rubia J, Gironella M, GAH Group, et al. Development and psychometric validation of a brief comprehensive health status assessment scale in older patients with hematological malignancies: the GAH Scale. J Geriatr Oncol 2015; 6:353–361. [DOI] [PubMed] [Google Scholar]
- 36.Engelhardt M, Berger DP, Mertelsmann R, Non-Hodgkin-lymphoma. Engelhardt M, Berger DP, Mertelsmann R, et al. Das Blaue Buch: Chemotherapie-Manual Hämatologie und, Onkologie. Berlin, Heidelberg: Springer; 2017. 121–286. [Google Scholar]
- 37▪.Bonello F, Boccadoro M, Larocca A. Diagnostic and therapeutic challenges in the management of intermediate and frail elderly multiple myeloma patients. Cancers (Basel) 2020; 12:3106. [DOI] [PMC free article] [PubMed] [Google Scholar]; Recommendation paper on today's challenges in elderly multiple myeloma patients by Turin's multiple myeloma experts.
- 38.Schjesvold FH, Richardson PG, Facon T, et al. Isatuximab plus pomalidomide and dexamethasone in elderly patients with relapsed/refractory multiple myeloma: ICARIA-MM subgroup analysis 2021; 106:1182–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Auner HW, Gavriatopoulou M, Delimpasi S, et al. Effect of age and frailty on the efficacy and tolerability of once-weekly selinexor, bortezomib, and dexamethasone in previously treated multiple myeloma. Am J Hematol 2021; 96:708–718. [DOI] [PubMed] [Google Scholar]
- 40.Benboubker L, Dimopoulos MA, Dispenzieri A, et al. FIRST Trial Team. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med 2014; 371:906–917. [DOI] [PubMed] [Google Scholar]
- 41.Cavo M, Dimopoulos MA, San-Miguel J, et al. Comparative efficacy of bortezomib, melphalan, and prednisone (VMP) with or without daratumumab versus VMP alone in the treatment of newly diagnosed multiple myeloma: propensity score matching of ALCYONE and VISTA phase III studies. Clin Lymphoma Myeloma Leuk 2020; 20:480–489. [DOI] [PubMed] [Google Scholar]
- 42▪.Dimopoulos MA, Leleu X, Moreau P, et al. Isatuximab plus pomalidomide and dexamethasone in relapsed/refractory multiple myeloma patients with renal impairment: ICARIA-MM subgroup analysis. Leukemia 2021; 35:562–572. [DOI] [PMC free article] [PubMed] [Google Scholar]; Subgroup analysis of ICARIA trial results in RRMM patients with renal impairment, demonstrating the advantage of Isatuximab-Pd vs. Pd likewise in patients with renal impairment.
- 43▪.Dimopoulos MA, Delimpasi S, Simonova M, et al. Weekly selinexor, bortezomib, and dexamethasone (SVd) versus twice weekly bortezomib and dexamethasone (Vd) in patients with multiple myeloma (MM) after one to three prior therapies: initial results of the phase III BOSTON study. JCO 2020; 38:8501–18501. [Google Scholar]; Comparative trial of a once-per-week regimen of selinexor, bortezomib, and dexamethasone (SVd) vs. Vd. This demonstrated to be more effective with the triplet and suggested SVd as a novel, effective and convenient treatment option for patients with multiple myeloma who have received one to three prior lines of therapy.
- 44.Seitzler S, Finley-Oliver E, Simonelli C, et al. Quality of life in multiple myeloma: considerations and recommendations. Expert Rev Hematol 2019; 12:419–424. [DOI] [PubMed] [Google Scholar]
- 45▪▪.Zaleta AK, Miller MF, Olson JS, et al. Symptom burden, perceived control, and quality of life among patients living with multiple myeloma. J Natl Compr Canc Netw 2020; 18:1087–1095. [DOI] [PubMed] [Google Scholar]; The results highlight substantial concern among patients with multiple myeloma about physical symptoms and function. Additionally, greater symptom burden significantly accounted for poorer QoL, and lower perceived control over illness was linked to depression and anxiety, all this pointing to the critical need for comprehensive symptom management, integrated palliative care and enhancement of social and emotional support for individuals with multiple myeloma.