Abstract
Background
The association between vedolizumab (VDZ) exposure and treatment response is unclear and seems insufficiently explained by serum levels. The aim of this study was to assess the correlation between VDZ concentrations in serum and intestinal tissue and their association with mucosal inflammation and response to VDZ.
Methods
This prospective study included 37 adult patients with inflammatory bowel disease with endoscopic inflammation at baseline who started VDZ. At week 16, serum and biopsies were collected for VDZ measurement by enzyme-linked immunosorbent assay. Nonlinear mixed-effects modeling was used to calculate serum trough concentrations and to assess intestinal tissue concentrations. Validated clinical and endoscopic scores were used to define clinical and endoscopic response and remission, and fecal calprotectin levels were used to assess biochemical response. Histologic remission was determined by the Nancy score.
Results
A positive correlation was observed between VDZ concentrations in serum and tissue (r2 = 0.83; P < 0.0001). High mucosal rather than serum VDZ levels correlated with a reduced endoscopic (P = 0.06) grade of mucosal inflammation. Furthermore, patients with a positive biochemical and endoscopic outcome had higher tissue levels of VDZ than patients without biochemical and endoscopic response (P < 0.01 and P = 0.04, respectively).
Conclusions
Tissue levels of VDZ may provide a better marker than serum levels for mucosal inflammation and objective treatment outcome at week 16. The potential of VDZ tissue levels for therapeutic drug monitoring in inflammatory bowel disease warrants further exploration.
Keywords: vedolizumab, mucosal tissue, inflammatory bowel disease
Introduction
Vedolizumab (VDZ) is a humanized monoclonal anti-α4β7 integrin that has proven to be an effective treatment option for ulcerative colitis (UC) and Crohn disease (CD).1, 2 Optimization of biologic therapies through therapeutic drug monitoring (TDM) is evolving as a cornerstone in the management of inflammatory bowel disease (IBD).3, 4 Dose optimization in patients with loss of response who have low serum levels has been shown as an effective strategy in regaining response for patients on anti-tumor necrosis factor (TNF)α therapy.5 However, VDZ serum levels may be inadequate for this purpose, because data on the relationship between VDZ serum levels and treatment response rates remain conflicting and unclear. Analyses of the GEMINI data and real-world studies have indicated that higher VDZ serum concentrations are associated with higher clinical remission rates.6-12 However, other studies were unable to confirm this relationship.13, 14 Furthermore, approximately 30% of patients will not respond to VDZ despite having adequate drug serum levels and complete α4β7 receptor saturation.15, 16 A complicating factor is that VDZ is internalized upon binding to its target α4β7 integrin, which in turn may affect available VDZ levels.
The envisioned role of VDZ is to prevent the recruitment of α4β7 integrin-expressing memory T-cells to the inflamed mucosa by inhibiting their binding to MadCAM expressed on endothelial cells.17 However, other cells, including eosinophils, B-cells, monocytes, and dendritic cell precursors, also express α4β7 and may therefore affect VDZ levels.18-21 Although VDZ levels in serum are generally thought to be well in excess of available α4β7 levels, it is conceivable that mucosal VDZ levels are more affected by the presence of circulating α4β7-expressing cell types and may provide a more accurate measurement to assess a dose-response relationship in patients treated using VDZ. A similar finding was reported for anti-TNFα levels, where serum and intestinal mucosal anti-TNFα levels were found to be discordant in severely inflamed lesions.22 Thus, the investigation of mucosal tissue levels of VDZ may improve our understanding of the mechanism of action of VDZ in relation to its efficacy. In this study, we aimed to assess VDZ concentrations in the intestinal tissue and their correlation with serum levels and mucosal disease activity and treatment response in patients with IBD treated using VDZ.
METHODS
Adult patients with UC, IBD-unclassified (IBD-U), and CD who started VDZ between December 2016 and November 2018 at the Erasmus Medical Center (Rotterdam, the Netherlands) were prospectively included after obtaining written informed consent. The study protocol was approved by the Medical Ethics Committee Rotterdam (MEC 2004-168 2012) in the Netherlands. Inclusion criteria were active endoscopic disease at baseline, defined as a Mayo endoscopic score of ≥1, a simple endoscopic score for CD (SES-CD) ≥3, or a Rutgeerts score ≥2. Follow-up endoscopies for the assessment of endoscopic response and collection of biopsies for VDZ level measurements and histology were performed after 16 weeks. The concomitant use of corticosteroids and immunomodulators was allowed. The VDZ induction therapy consisted of four 300 mg infusions for patients with UC and was scheduled at baseline (week 0), week 2, week 6, and week 14. All patients with CD received an additional VDZ infusion at week 10. As maintenance therapy, an intravenous infusion of 300 mg VDZ every 8 weeks was scheduled.
Data Collection
Clinical and demographic characteristics were collected at baseline (age, sex, smoking status, disease characteristics, and treatment history). Endoscopies were performed at baseline and week 16 (±2 weeks). Endoscopic inflammation was determined using the endoscopic Mayo score for patients with UC and IBD-U, the SES-CD for patients with CD, and the Rutgeerts score for postoperative patients with CD. During endoscopy at week 16, ileal and segmental colonic biopsies were collected (colon ascending, transverse, descending, sigmoid, and rectum) whenever possible from inflamed and noninflamed mucosa and stored in RNAlater at –20°C. Additional biopsies were formalin fixed and paraffin embedded for assessment of histologic inflammation by a blinded expert gastrointestinal pathologist. Because no validated/consensus histologic score for CD exists,23 and in this study both patients with UC and patients with CD were included, the Nancy score24 was used for both sets of patients. In this way, the results for the histology scores are reported homogeneously for the entire study population. At the day of the endoscopy at week 16, serum was collected and stored at –20°C. Four patients refused the endoscopy (only serum was collected), and 1 serum sample was not collected (only biopsies were available).
Clinical disease activity scores (Harvey-Bradshaw Index [HBI] for CD and Simple Clinical Colitis Activity Index [SCCAI] for UC) were collected at baseline and week 16. At baseline and week 16, fecal calprotectin (FC) was determined using a quantitative enzyme-linked immunosorbent assay (ELISA; Bühlmann Laboratories AG, Schönenbuch, Switzerland) or QuantOn cal (Preventis, Germany) FC home test.
Outcome Measures and Definitions
The primary outcome was the correlation between the levels of VDZ in serum and intestinal tissue (µg/mL) and their association with mucosal inflammation (measured by endoscopy and histology). As a secondary aim, VDZ levels were compared between patients with and without clinical, biochemical, and mucosal (endoscopic, histologic) response or remission at week 16.
The grade of inflammation at endoscopy was classified as depicted in Supplementary Table 1. The grade of inflammation at histologic examination was classified by the Nancy score. The classification of clinical disease activity is also depicted in Supplementary Table 1.
Response and Remission Definitions
Clinical response was defined as a decline of ≥3 points on the SCCAI or HBI as compared to baseline. Clinical remission was defined as an SCCAI score <3 or HBI score <5.25 Biochemical response was defined as a reduction of ≥50% in FC levels as compared to baseline and biochemical remission in FC levels < 250 µg/g at week 16. Endoscopic response was defined as a decline of 1 or more points in the endoscopic Mayo score, ≥50% decline in the SES-CD score, or a decline of 1 or more points in the Rutgeerts score. Endoscopic remission was defined as an endoscopic Mayo score of 0, an SES-CD score ≤2, and a Rutgeerts score of i0 or i1.26, 27 In patients with an ileostomy, ileoanal pouch anastomosis, or ileorectal anastomosis, endoscopic disease activity was classified as none, mild, moderate, or severe as judged by the endoscopist. In those patients, endoscopic response was defined as a decline of ≥1 point on the 4-grade scale described in Supplementary Table 1, and endoscopic remission was defined as “no endoscopic disease activity.” Histologic remission was defined as a zero Nancy score in all collected biopsies.
Laboratory Analysis
The VDZ serum level was measured by ELISA, according to the manufacturer’s protocol (LISA Tracker, Theradiag). Optical densities obtained for serum samples were calculated to the µg/mL of VDZ relative to the standard curve and considering the dilution factor of the sera as per the manufacturer’s instructions. Intestinal biopsies were removed from RNAlater and lysed in 150 μL lysis buffer (10 mM Tris, 150 mM sodium chloride, 0.2% Triton X-100, and 2 mM EDTA) containing a cocktail of protease and phosphatase inhibitors, followed by 1-minute vortexing before and after a 10-minute incubation on ice.22 After centrifugation at maximum speed for 10 minutes at 4°C, the supernatant was collected and the total protein content was measured using the DC Protein Assay (Biorad). The VDZ concentration per 25 µg of tissue sample was determined using the same ELISA kit used for the sera. Tissue samples from patients with IBD treated with ustekinumab (UST) were used as a negative control. The optical density obtained for the tissue samples was contrasted to the standard curve, yielding a concentration of VDZ in µg/µL normalized to µg tissue. In contrast to the biopsies taken from patients receiving ustekinumab, VDZ was reliably detected in biopsies obtained from patients receiving VDZ and did not differ between patients with CD and patients with UC (Supplementary Fig. 1). To allow a comparison of VDZ concentration between sera and tissue, VDZ tissue concentrations were subsequently calculated per µL, taking the dilution factor of tissues during workup into consideration and assuming a volume of 1 mm3 for all biopsies. The VDZ limit of detection reported by the manufacturer was 2 µg/mL. In all our samples, both serum and biopsy, VDZ concentrations were above the limit of quantification of the assay.
Population Pharmacokinetic Model
Because pharmacokinetic (PK) sampling was randomly timed after VDZ administration, trough serum and intestinal tissue concentrations were generated to assess interindividual PK data. A previously established population PK model, in which extensive phase I-III PK data were included, was used as a reference model.28 This model had 2 compartments with parallel linear and nonlinear Michaelis-Menten elimination. In this model, the minor but significant effects of IBD type (UC or CD) and albumin level on the linear clearance from the central compartment were included. For 1 patient, the albumin level was missing and the median value of the dataset was imputed. For population PK analysis, the albumin level was centered on its median level. Because no data on weight were available in the dataset, this factor was not considered. The Bayesian estimates for the trough serum concentrations were generated using the prespecified model.
Hereafter, we developed a model that also included the intestinal tissue concentrations. We thus used the mean of all measured biopsy concentrations that were taken per patient at one time point. The assessment of the serum-tissue PK models was based on plausible data estimations, physiological plausibility, goodness-of-fit plots, successful convergence of minimization, and precision of parameter estimates. The following structural models were evaluated: a 2-compartmental model or a 3-compartmental model with the compartments placed in parallel, serial, or circular. All models were tested with and without direct clearance from the intestinal tissue compartment. The calculated serum trough concentrations and estimated intestinal tissue trough concentrations were used for further statistical analyses.
Nonlinear mixed-effects modeling was performed using NONMEM (version 7.3.0, ICON Development Solutions, Ellicott City, MD), Perl-speaks-NONMEM (version 4.4.8), and Piraña (version 2.9.2). The first-order conditional estimation method with interaction was used for all models. Data preparation and evaluation were conducted using R (version 3.0.1).
Statistical Analysis
Normally distributed data were presented as mean ± SD and continuous data with a skewed distribution as median and first and third quartile (25th–75th). Categorical data were presented as numbers and percentages. After testing the data distribution by the D’Agostino and Pearson omnibus normality test, a 1-way analysis of variance, Mann-Whitney U test, or unpaired t test was used to evaluate the differences between the independent groups. The 1-way analysis of variance—a test for the linear trend between mean and column number—was used to determine the significance of the trend between ≥3 groups. Patients with IBD-U were included in the UC group for analyses. The correlation between VDZ tissue and serum levels was analyzed using a Pearson correlation for normally distributed data. Drug survival was assessed at week 54 by comparing patients still receiving VDZ at week 54 to patients who had ceased therapy before week 54. The optimal (the best discriminatory performance and clinically relevant) cutoff levels of VDZ for drug survival were assessed using receiver operating characteristic statistics. The sensitivity, specificity, positive predictive value, and negative predictive value of VDZ levels to predict drug survival were calculated by cross-tabulation. All analyses were performed in Graph Pad Prism version 5.0 and IBM SPS Statistics version 25.0 (IBM Corp., Armonk, NY). A 2-sided P value of < 0.05 was considered statistically significant.
RESULTS
Patient Cohort
A total of 37 patients were included: 12 with UC (32%), 3 with IBD-U (8%), and 22 with CD (60%), with a median age of 39 years (interquartile range, 26-50; Table 1). In total, 31/37 (84%) patients were exposed to ≥1 anti-TNFα drugs before starting VDZ, of whom 29/31 (94%) were anti-TNFα-refractory, defined as having primary nonresponse or secondary loss of response, and 2/31 (3%) stopped anti-TNFα because of adverse effects. In 22/37 patients (60%), VDZ induction was combined with corticosteroid induction therapy (6 on prednisone; 16 on budesonide), which was completely tapered at week 16 in 16/22 (73%) patients. No differences were observed in corticosteroid prescription rates between patients with UC and patients with CD (P = 0.51). In total, 7/37 patients (19%) were on concomitant immunomodulator therapy (2 on thiopurines, 5 on tacrolimus) and 3 patients were on 5-aminosalicylates during VDZ induction.
Table 1.
Baseline Patient Characteristics
| N = 37 | |
|---|---|
| Female, n (%) | 20 (54) |
| Median age, y (25th-75th) | 39 (26-50) |
| Smoking, n (%) | 9 (24) |
| Median disease duration, y (25th-75th) | 11 (4-21) |
| Diagnosis, n (%) | |
| UC | 12 (32) |
| IBD-U | 3 (8) |
| CD | 22 (60) |
| CD disease location, n (%) | |
| L1 ileal | 1 (5) |
| L2 colonic | 4 (18) |
| L3 ileocolonic | 17 (77) |
| +L4 upper GI disease | 2 (9) |
| CD disease behavior, n (%) | |
| B1 | 7 (32) |
| B2 | 13 (59) |
| B3 | 2 (9) |
| Perianal disease | 3 (14) |
| UC disease location, n (%) | |
| E2 | 4 (27) |
| E3 | 11 (73) |
| Previous intestinal resection, n (%) | 13 (35) |
| Anti-TNFα-exposed, n (%) naive | 6 (16) |
| 1 | 14 (38) |
| ≥2 | 17 (46) |
| Anti-TNFα-refractory disease, n (%) | 29 (94) |
| Anti-TNFα cessation because of adverse effects, n (%) | 2 (6) |
| Concomitant steroid induction therapy,* n (%) | 22 (59) |
| Prednisolone | 6 (27) |
| Budesonide | 16 (73) |
| Concomitant IBD medication, n (%) | |
| Immunomodulator | 7 (19) |
| 5-ASA | 3 (8) |
| Median HBI score (25th-75th) | 7 (4-11) |
| Median SCCAI score (25th-75th) | 10 (8-12) |
| Median FC (25th-75th) | 934 (445-1800) |
| Median Mayo endoscopic score (25th-75th) | 2 (2-3) |
| Median SES-CD (25th-75th) | 12 (9-17) |
| Median Rutgeerts score (25th-75th) | 3 (3-3) |
5-ASA indicates 5-aminosalicylic acid; GI, gastrointestinal; L, location; B, behavior; E, extent.
At week 16, clinical response was observed in 20/37 patients (54%) and clinical remission in 16/37 patients (43%). In a subanalysis with only patients who had active clinical disease at baseline, the clinical response rate was 67% (20/30) and clinical remission was observed in 37% (11/30). Biochemical response was observed in 20/37 (54%) patients and biochemical remission in 19/37 (51%). In a subanalysis with only patients who had active biochemical disease at baseline, the biochemical response rate was 62% (18/29), and biochemical remission was observed in 52% of patients (15/29). The week 16 endoscopic response rate was 60% (22/37 patients), and the endoscopic remission rate was 32% (12/37 patients). Histologic remission was observed in 10/32 (31%) patients at week 16.
VDZ Levels in Serum Correlate to VDZ in Tissue Samples
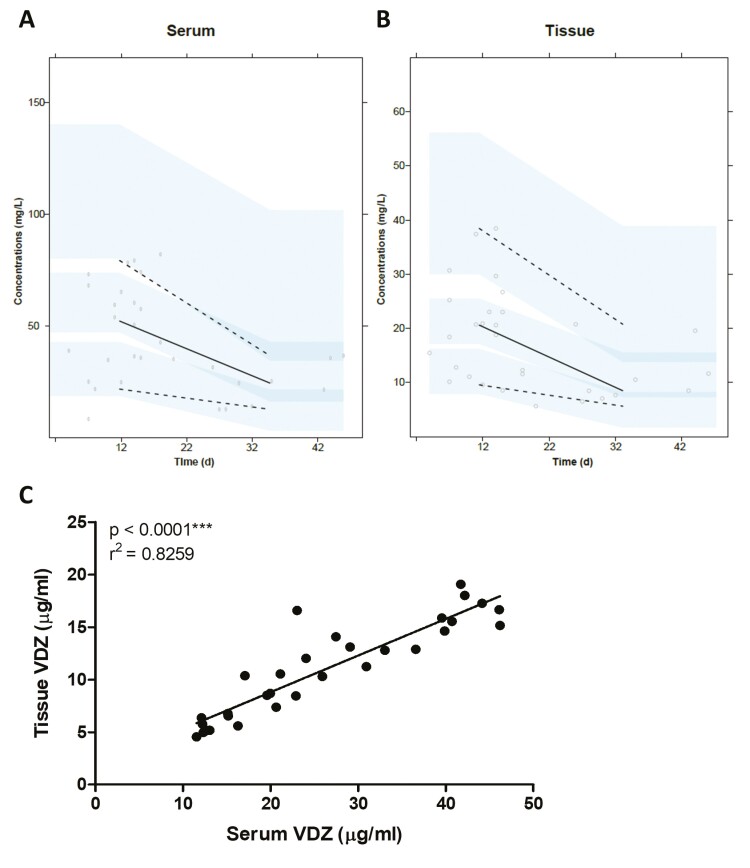
Both serum and tissue samples were available from 32 patients. One of these patients was sampled on the day of infusion and was considered to be an outlier, which resulted in a total of 31 evaluable patients for PK correlation analyses. As evaluated by a visual predictive check with n = 1000, as depicted in Fig. 1A, the serum PK data could be described by the previously established PK model, and serum trough concentrations were generated. The VDZ intestinal tissue concentrations (reported in Fig. 1B) were best described by a 3-compartmental model with direct clearance from the third compartment. Parameter estimates of this model are shown in Supplementary Table 2. The median-generated VDZ trough concentration was 23.02 µg/mL (interquartile ratio, 15-40) in serum and 10.54 µg/mL (interquartile ratio, 6-15) in intestinal tissue biopsy. Levels of VDZ in biopsies showed a positive correlation with VDZ concentrations in corresponding patient sera (r2 = 0.83; P < 0.001; Fig. 1C).
Figure 1.
Correlation between VDZ levels in serum and biopsies. (A-B) Visual predictive check plot with n = 1,000 of the VDZ serum (A) and tissue (B) PK model. Dots represent the observed concentrations, solid black line represents the observed median concentration, dashed lines represent the observed 5th and 95th percentiles, and light blue areas represent the 95% confidence intervals of the 5th percentile, median, and 95th percentile of the predictions. (C) Levels of VDZ detected in sera correlated to levels of VDZ detected in biopsies. The calculated serum trough concentrations and estimated intestinal tissue trough concentrations are used in the graph.
VDZ Levels in Biopsies Are Inversely Correlated With Mucosal Inflammation
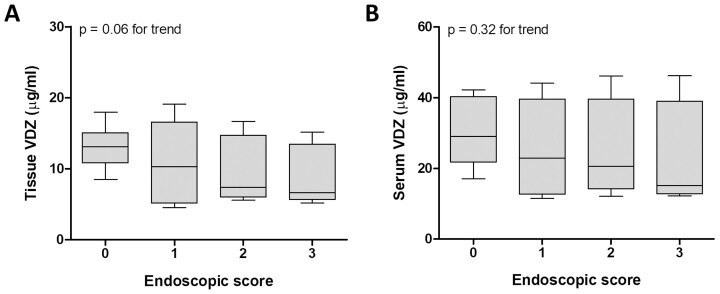
Looking at the total endoscopic score for each patient, we found that tissue levels of VDZ were highest in patients without inflammation (0) and decreased with the severity of inflammation. The median VDZ concentrations in patients with none, mild, moderate, and severe endoscopic inflammation were 13.10 µg/mL, 10.30 µg/mL, 7.37 µg/mL, and 6.65 µg/mL, respectively, with an inverse correlation between the tissue concentration of VDZ and the endoscopic score (P = 0.06 for the trend; Fig. 2A). In contrast, VDZ levels in serum were not affected by the severity of the endoscopic inflammation (P = 0.32; Fig. 2B).
Figure 2.
Endoscopic inflammation at week 16 was associated with reduced mucosal VDZ levels. Patients were stratified according to their endoscopic disease severity at week 16 of treatment: 0 = none (n = 9), 1 = mild (n = 11), 2 = moderate (n = 5), 3 = severe inflammation (n = 6). Median and range of VDZ levels measured in biopsies (A) or in serum (B) are shown. The boxplot represents the Q1-Q3 range and the whiskers correspond to the minimum and maximum value. The calculated serum trough concentrations and estimated intestinal tissue trough concentrations are used in the graphs.
VDZ Tissue Levels Are Associated With Objective Treatment Response to VDZ
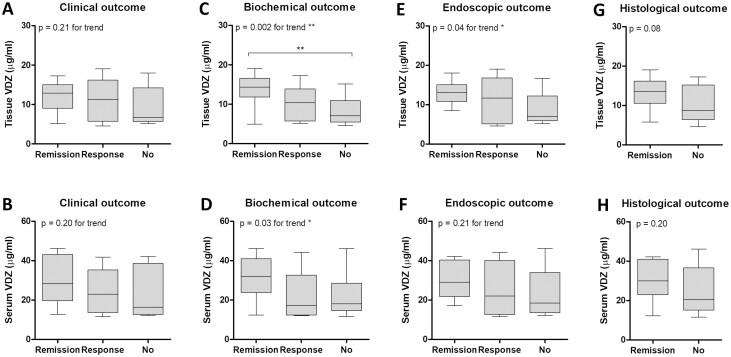
No significant association was observed between median VDZ levels in tissue or serum and clinical response or remission (Figs. 3A, B). Patients showing biochemical remission had significantly higher tissue levels in VDZ compared to those without any biochemical response (14.35 µg/mL vs 7.06 µg/mL; P < 0.01; Fig. 3C). Patients achieving a biochemical response but not complete remission showed an intermediate level of VDZ (10.37 µg/mL), resulting in a significant increasing trend between VDZ concentration and biochemical outcome (P = 0.002). A significant trend was also found in VDZ serum levels (P = 0.03; Fig. 3D).
Figure 3.
VDZ tissue levels were associated with biochemical and endoscopic outcome at week 16. (A-B) Comparison of VDZ tissue (A) and serum (B) levels between patients achieving complete clinical remission (n = 12) and patients showing a clinical response (n = 9) or those who did not show any clinical improvement (n = 9). (C-D) VDZ concentration in biopsies (C) or serum (D) in patients who achieved complete biochemical remission (n = 14), patients who showed some improvement (n = 5), and those who showed no improvement of biochemical activity (n = 10). (E-F) VDZ tissue (E) and serum (F) levels detected in patients stratified according to endoscopic outcome (remission: n = 9, response: n = 10, none: n = 12) (G-H) Boxplot of VDZ tissue (G) and serum (H) levels between patients achieving histologic remission (n = 10) and those who did not (n = 19). The boxplot represents the Q1-Q3 range and the whiskers correspond to the minimum and maximum value. The calculated serum trough concentrations and estimated intestinal tissue trough concentrations are used in the graphs. **P < 0.01.
The stratification of patients based on the endoscopic outcome showed incremental tissue levels of VDZ from patients showing no response to treatment to those who showed partial response to those who achieved complete remission. This trend was significant for VDZ tissue levels (P = 0.01) but not for VDZ serum levels (P = 0.34; Figs. 3E, F).
Because no biopsies were collected at baseline, it was not possible to define a histologic response. Patients achieving histologic remission showed higher VDZ tissue levels (13.58 µg/mL vs 8.69 µg/mL; P = 0.08; Fig. 3G) but comparable VDZ serum levels (30.01 µg/mL vs 20.63 µg/mL; P = 0.20; Fig. 3H) as compared to patients not achieving histologic remission.
A subanalysis including only patients with proven disease activity at baseline as based on endoscopy, biochemical (FC >250 µg/g) disease activity, and clinical (HBI score >5/SCCAI score >3) disease activity was also performed. This subanalysis showed that although VDZ serum and tissue levels were not different between clinical responders and nonresponders in this group (n = 26), VDZ tissue levels were significantly different between patients achieving biochemical or endoscopic remission and those with no response. In addition, both serum and tissue levels of VDZ were significantly different in patients achieving histologic remission and those who did not (Supplementary Fig. 2).
Discussion
Serum levels of VDZ are not yet implemented in daily clinical practice for TDM during VDZ therapy because serologic VDZ levels have shown an exposure-response relationship but are not sufficiently accurate correlated to mucosal inflammation and response.14 This real-world prospective cohort study, with a high percentage of patients with IBD exposed to anti-TNFα and with simultaneous assessment of both serum and tissue concentrations of patients treated using VDZ, showed a high (positive) correlation between VDZ concentrations in tissue and in serum. We showed that despite the correlation between VDZ tissue and serum levels, high VDZ levels in intestinal mucosa, rather than in serum, showed the strongest correlation to mucosal inflammation and were associated with objective response rates. Taken together, these results suggest that VDZ tissue levels show a better correlation with objective treatment outcome at week 16 and may supplement the serum drug concentration for TDM, implying that VDZ tissue levels (compared to serum) may be a new target for VDZ treatment optimization in patients with no response and loss of response to VDZ.
In a recently published study by Van den Berghe et al,29 a positive trend between VDZ serum and tissue levels was observed along with higher tissue VDZ levels in patients with endoscopic response or remission. A subanalysis of their data showed that this difference between responders and nonresponders was no longer present when only patients with adequate serum levels were included, suggesting that nonresponse in the presence of adequate serum levels is not caused by inadequate tissue levels. In our study, 6 (18%) patients had inadequate serum levels according to the used cutoff of 14 µg/µL. After excluding these patients from analysis, we consistently observed a significantly reduced tissue level in VDZ responders compared to nonresponders.
In addition, note that methodological differences exist between our studies. Van den Berghe et al29 employed an in-house developed ELISA and reported measured values, whereas we reported trough levels (PK modeled for serum and biopsies separately) from data obtained with commercially available VDZ ELISA kits. Furthermore, although Van den Berghe et al included only patients with UC and evaluated only 1 biopsy from the rectosigmoid area, our cohort was extended to both patients with UC and patients with CD, and paired samples of both inflamed and noninflamed tissue from the terminal ileum and all colon segments were collected within 1 patient, allowing more generalizable results. Finally, in Van den Berghe et al, no clinical, biochemical, or histologic endpoints were used, whereas in our study data collection included all endpoints to allow for more robust observations with regard to (mucosal) disease activity.
As previously established, the elimination of VDZ from serum decreases bi-exponentially as described by a 2-compartmental model. In our study, a third compartment was added to describe the distribution to and elimination from the intestinal tissue. Although the impact of IBD type seemed minor but significant, this covariate was added to confirm the findings by Rosario, Dirks, et al.28 In addition, the effects of albumin level and body weight seemed minor and may only reach clinical relevance at extreme values, eg, albumin levels <32 g/L and weight >120 kg. Although none of the patients in the dataset had an albumin level of <32 g/L, this covariate was taken into account to confirm the findings by Rosario, Dirks, et al. Because no data on body weight were available in the dataset, this covariate was not included in PK analysis. Because previous data supported flat VDZ dosing regardless of a patient’s weight, it is not expected that differences in weight have a clinically relevant impact on VDZ PK in this dataset.
Mechanistically, it is challenging to explain why high VDZ tissue levels in patients with IBD are associated with response. Although anti-TNFα and anti-interleukin-12/interleukin-23 antibodies may be expected to play a mucosal role—their targets are expressed there—VDZ is expected to perform its actions mainly in the peripheral blood, preventing the recruitment of α4β7-expressing cells to the mucosa.17 Because VDZ is a monoclonal antibody, the distribution of VDZ from the blood into the tissue occurs mainly via convective transport, determined by the flux of fluid from the vascular space to the tissue and transcytosis through vascular epithelial cells mediated by the neonatal Fc receptor.30 Although increased blood flow and permeability in inflamed regions may result in higher levels of blood-borne antibodies, in our study reduced rather than increased tissue levels of VDZ were associated with increased mucosal inflammation. Thus, whereas the rationale for the development of α4β7 integrin blockers has been to reduce inflammatory T-cell homing to the inflamed mucosa, sufficient evidence from human data is lacking, and the actual mechanism of the action of VDZ may be more complicated than the anti-integrin mechanism alone.
Recently, it has been suggested that innate immune responses rather than T-cell migration underlie the beneficial effects of VDZ, with VDZ treatment associated with a skewing toward wound-healing M2 macrophages.31 Furthermore, atypical monocytes expressing the α4β7 integrin, required for wound healing, are also recruited to the inflamed mucosa, suggesting that the net effect of VDZ on the negative actions of T-cell homing and the positive actions of monocyte homing may affect treatment outcome. Notably, an increasing amount of evidence suggests that the method of action of anti-TNFα is mediated by its Fc-tail.32 Furthermore, the intravenous administration of immunoglobulins also seems to be efficacious in patients with IBD, suggesting that idiotype-independent effects of antibodies may play a role in VDZ treatment.33
Together, these findings suggest not only that the mechanism of action of VDZ is related to its ability to bind the α4β7 integrin present in the bloodstream but also that the effectiveness of the treatment can be attributed to a complex set of other interactions that take place in the intestinal mucosa. A better understanding of the molecular mechanisms of action of VDZ may allow a better-tailored approach in the use of these medications.
An important strength of our study is the prospective inclusion of a real-world cohort of patients exposed to anti-TNFα with follow-up according to a standardized protocol with endoscopy after induction. We also acknowledge several limitations. First, endoscopists were not blinded to the treatment of patients, nor were the endoscopies read centrally. However, it is unlikely that endoscopy scoring influenced our results because the primary analysis in this study focuses on the relationship of VDZ levels and disease activity instead of response rates. Second, the sample size of our study is relatively small and includes a heterogeneous population of patients. In addition, this cohort concerns a therapy-refractory IBD population, which may limit the external validity of the results to patients who are anti-TNFα-refractory. Finally, further investigation is required to determine whether dose intensification increases VDZ tissue levels and correspondingly reduces mucosal inflammation, and whether patients with secondary loss of response show a decline in VDZ tissue levels. To answer these questions, a clinical trial with a longer follow-up period is required.
Conclusions
This prospective study shows a correlation between serum and tissue levels of VDZ. In addition, our data indicate that VDZ tissue levels but not VDZ serum levels alone are associated with mucosal inflammation and the therapy outcome at week 16. Our findings suggest that the additional measurement of tissue VDZ may be considered for therapeutic drug monitoring strategies during VDZ therapy.
Supplementary Material
Glossary
Abbreviations
- CD
Crohn disease
- ELISA
enzyme-linked immunosorbent assay
- FC
fecal calprotectin
- HBI
Harvey-Bradshaw Index
- IBD
inflammatory bowel disease
- IBD-U
IBD-unclassified
- PK
pharmacokinetic
- SCCAI
Simple Clinical Colitis Activity Index
- SES-CD
Simplified Endoscopic Score for CD
- TDM
therapeutic drug monitoring
- TNF
tumor necrosis factor
- UC
ulcerative colitis
- VDZ
vedolizumab
Author contributions: ACV, GMF: study concept and design, critical revisions of the manuscript for important intellectual content. RWMP: patient recruitment, prospective follow-up of patients, acquisition of data, statistical analysis and interpretation of the data, drafting of the manuscript. EP: laboratory analysis, acquisition of data, statistical analysis and interpretation of the data, drafting of the manuscript. MBSC: statistical analysis and interpretation of the pharmacokinetics data. MPP, UG, CJW, MBSC: critical revisions and final approval of the version to be submitted.
Financial disclosures and conflicts of interest: CJW received grant support from Falk Benelux and Pfizer; received speaker fees from AbbVie, Takeda, Ferring, Dr. Falk Pharma, Hospira, and Pfizer; and served as a consultant for AbbVie, MSD, Takeda, Celgene, Mundipharma, and Janssen. MPP received grant support from Pfizer and Janssen. GMF received speaker fees from Janssen. ACV has participated on advisory boards and/or received financial compensation from Janssen, Takeda, AbbVie, and Tramedico.
References
- 1. Feagan BG, Rutgeerts P, Sands BE, et al. ; GEMINI 1 Study Group . Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 2. Sandborn WJ, Feagan BG, Rutgeerts P, et al. ; GEMINI 2 Study Group . Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–721. [DOI] [PubMed] [Google Scholar]
- 3. Schreiber S, Dignass A, Peyrin-Biroulet L, et al. Systematic review with meta-analysis: real-world effectiveness and safety of vedolizumab in patients with inflammatory bowel disease. J Gastroenterol. 2018;53:1048–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ricciuto A, Dhaliwal J, Walters TD, et al. Clinical outcomes with therapeutic drug monitoring in inflammatory bowel disease: a systematic review with meta-analysis. J Crohns Colitis. 2018;12:1302–1315. [DOI] [PubMed] [Google Scholar]
- 5. Mitrev N, Vande Casteele N, Seow CH, et al. ; IBD Sydney Organisation and the Australian Inflammatory Bowel Diseases Consensus Working Group . Review article: consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;46:1037–1053. [DOI] [PubMed] [Google Scholar]
- 6. Rosario M, French JL, Dirks NL, et al. Exposure-efficacy relationships for vedolizumab induction therapy in patients with ulcerative colitis or Crohn’s disease. J Crohns Colitis. 2017;11:921–929. [DOI] [PubMed] [Google Scholar]
- 7. Ungaro RC, Yarur A, Jossen J, et al. Higher trough vedolizumab concentrations during maintenance therapy are associated with corticosteroid-free remission in inflammatory bowel disease. J Crohns Colitis. 2019;13:963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guidi L, Pugliese D, Panici Tonucci T, et al. Early vedolizumab trough levels predict treatment persistence over the first year in inflammatory bowel disease. United European Gastroenterol J. 2019;7:1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dreesen E, Verstockt B, Bian S, et al. Evidence to support monitoring of vedolizumab trough concentrations in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2018;16:1937–1946.e8. [DOI] [PubMed] [Google Scholar]
- 10. Williet N, Boschetti G, Fovet M, et al. Association between low trough levels of vedolizumab during induction therapy for inflammatory bowel diseases and need for additional doses within 6 months. Clin Gastroenterol Hepatol. 2017;15:1750–1757.e3. [DOI] [PubMed] [Google Scholar]
- 11. Pouillon L, Vermeire S, Bossuyt P. Vedolizumab trough level monitoring in inflammatory bowel disease: a state-of-the-art overview. BMC Med. 2019;17:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yacoub W, Williet N, Pouillon L, et al. Early vedolizumab trough levels predict mucosal healing in inflammatory bowel disease: a multicentre prospective observational study. Aliment Pharmacol Ther. 2018;47:906–912. [DOI] [PubMed] [Google Scholar]
- 13. Plevris N, Jenkinson PW, Chuah CS, et al. Association of trough vedolizumab levels with clinical, biological and endoscopic outcomes during maintenance therapy in inflammatory bowel disease. Frontline Gastroenterol. 2020;11:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ward MG, Sparrow MP, Roblin X. Therapeutic drug monitoring of vedolizumab in inflammatory bowel disease: current data and future directions. Therap Adv Gastroenterol. 2018;11:1756284818772786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dreesen E, Gils A. Blocking α4β7 integrin through vedolizumab: necessary but not sufficient? J Crohns Colitis. 2017;11:903–904. [DOI] [PubMed] [Google Scholar]
- 16. Gouynou C, Pouillon L, Rousseau H, et al. Early changes in the pharmacokinetic profile of vedolizumab-treated patients with IBD may predict response after dose optimisation. Gut. 2019;68:178–179. [DOI] [PubMed] [Google Scholar]
- 17. Wyant T, Fedyk E, Abhyankar B. An overview of the mechanism of action of the monoclonal antibody vedolizumab. J Crohns Colitis. 2016;10:1437–1444. [DOI] [PubMed] [Google Scholar]
- 18. Schleier L, Wiendl M, Heidbreder K, et al. Non-classical monocyte homing to the gut via α4β7 integrin mediates macrophage-dependent intestinal wound healing. Gut. 2020;69:252–263. [DOI] [PubMed] [Google Scholar]
- 19. Kempster SL, Kaser A. α4β7 integrin: beyond T cell trafficking. Gut. 2014;63:1377–1379. [DOI] [PubMed] [Google Scholar]
- 20. Xu B, Cook RE, Michie SA. Alpha4beta7 integrin/MAdCAM-1 adhesion pathway is crucial for B cell migration into pancreatic lymph nodes in nonobese diabetic mice. J Autoimmun. 2010;35:124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Soler D, Chapman T, Yang LL, et al. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330:864–875. [DOI] [PubMed] [Google Scholar]
- 22. Yarur AJ, Jain A, Sussman DA, et al. The association of tissue anti-TNF drug levels with serological and endoscopic disease activity in inflammatory bowel disease: the ATLAS study. Gut. 2016;65:249–255. [DOI] [PubMed] [Google Scholar]
- 23. Novak G, Parker CE, Pai RK, et al. Histologic scoring indices for evaluation of disease activity in Crohn’s disease. Cochrane Database Syst Rev. 2017;7:CD012351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marchal-Bressenot A, Scherl A, Salleron J, et al. A practical guide to assess the Nancy histological index for UC. Gut. 2016;65:1919–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vermeire S, Schreiber S, Sandborn WJ, et al. Correlation between the Crohn’s disease activity and Harvey-Bradshaw indices in assessing Crohn’s disease severity. Clin Gastroenterol Hepatol. 2010;8:357–363. [DOI] [PubMed] [Google Scholar]
- 26. Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505–512. [DOI] [PubMed] [Google Scholar]
- 27. Moskovitz DN, Daperno M, Assche GA. Defining and validating cut-offs for the simple endocopic score for Crohn’s disease. Gastroenterology. 2007;132:S1097. [Google Scholar]
- 28. Rosario M, Dirks NL, Gastonguay MR, et al. Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn’s disease. Aliment Pharmacol Ther. 2015;42:188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Van den Berghe N, Verstockt B. Gils A, et al. Tissue exposure does not explain non-response in ulcerative colitis patients with adequate serum vedolizumab concentrations. J Crohns Colitis. Published online November 27, 2020. doi: 10.1093/ecco-jcc/ jjaa239. [DOI] [PubMed] [Google Scholar]
- 30. Ryman JT, Meibohm B. Pharmacokinetics of monoclonal antibodies. CPT Pharmacometrics Syst Pharmacol. 2017;6:576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zeissig S, Rosati E, Dowds CM, et al. Vedolizumab is associated with changes in innate rather than adaptive immunity in patients with inflammatory bowel disease. Gut. 2019;68:25–39. [DOI] [PubMed] [Google Scholar]
- 32. McRae BL, Levin AD, Wildenberg ME, et al. Fc receptor-mediated effector function contributes to the therapeutic response of anti-TNF monoclonal antibodies in a mouse model of inflammatory bowel disease. J Crohns Colitis. 2016;10:69–76. [DOI] [PubMed] [Google Scholar]
- 33. Horton N, Kochhar G, Patel K, et al. Efficacy and factors associated with treatment response of intravenous immunoglobulin in inpatients with refractory inflammatory bowel diseases. Inflamm Bowel Dis. 2017;23:1080–1087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.