Abstract
Background:
The purpose of this study was to demonstrate the validity and efficiency of the Outcomes Management and Evaluation (OME) system, a prospectively designed electronic data collection tool, for collecting comprehensive and standardized surgical data in shoulder arthroplasty.
Methods:
Surgical data from the first 100 cases of shoulder arthroplasty that were collected into the OME database were analyzed. Surgeons completed a traditional narrative operative note and also an OME case report using an encrypted smartphone. A blinded reviewer extracted data from the operative notes and implant logs in the electronic medical records (EMR) by manual chart review. OME and EMR data were compared with regard to data counts and agreement between 39 variables related to preoperative pathology, including rotator cuff status and glenoid wear, and surgical procedures. Data counts were assessed using both raw percentages and with McNemar’s test (with continuity correction). Agreement of nominal variables was analyzed using Cohen’s unweighted kappa (κ) and of ordinal variables using the linearly weighted Cohen’s test. Efficiency was assessed by calculating the median time needed to complete OME.
Results:
Compared to the EMR, the OME database had significantly higher data counts for 56% (22 of 39) of the variables assessed. A high level of proportional and statistical agreement was demonstrated between the data in the two datasets. 10 of 39 variables had 100% agreement but could not be statistically compared because both datasets had the same single response under those variables. Among the 29 variables that were compared, 79% (23 of 29) of variables had >80% raw proportional agreement, and 69% (20 of 29) of variables showed at least substantial agreement (κ > 0.6). The median time for completing OME surgery data entry was 92 seconds (IQR 70 – 126).
Conclusion:
The prospectively designed, electronic data entry system (OME) is an efficient and valid tool for collecting comprehensive and standardized surgical data on shoulder arthroplasty.
Level of Evidence:
Level IV
Keywords: Shoulder, shoulder arthroplasty, electronic medical record, web-based operative report, implant documentation, data processing
As the United States healthcare system evolves from a volume-driven compensation model towards outcome-based and value-driven models, there is an increased importance in assessing the value associated with varied surgical interventions. In order to assess value, we must be able to determine the quality and cost of a particular procedure 22–24. Quality can be assessed by collecting surgical outcomes data while cost is attributed to preoperative workup, implants, hospitalization, and postoperative recovery. Reliable perioperative data are therefore required to better assess quality, cost, and thereby, the value of surgical procedures.
The number of shoulder arthroplasty procedures performed in the United States continues to rise, with current estimates ranging from 55,000–80,000 per year, and increases of 300% or more expected in the coming years 5; 16; 19. Surgical outcomes are influenced not only by type of implant used but by many preoperative and intraoperative measures including but not limited to rotator cuff status, presence and degree of glenoid and/or humeral bone loss, and implant design 13; 14; 33. In the past, these data have been collected by retrospective manual extraction from the patient medical record 9; 11. This creates many challenges to perform high-quality research due to the variability in documentation in operative reports and manual extraction errors from electronic medical records (EMR). Operative reports infrequently report quantitative data, thereby significantly limiting the precision of research and quality measurements 23; 27. Furthermore, error rates in the extraction of data from EMRs have been reported to range from 8–23%, and efforts to increase accuracy also increase cost 20. Such errors can be reduced by increasing quality assurance measures and with a more thorough data review, which can be both costly and time consuming.2; 20 Alternatively, large prospectively collected structured datasets can be developed to compile variables that potentially affect outcomes and/or costs. Such databases would permit the use of multivariate analysis to assess the primary outcome measures, particularly when these variables are uniformly defined and collected.
Many existing structured databases, such as Medicare, Nationwide Inpatient Sample (NIS), and ACS National Surgical Quality Improvement Program (ACS NSQIP), are not disease-specific, consistent or standardized, thus creating incomplete datasets and limiting the ability to conduct high quality studies. In regards to shoulder arthroplasty, existing outcomes databases include the Kaiser Permanente Shoulder Arthroplasty Registry, the Nordic Arthroplasty Register Association, and the Australian Orthopedic Association National Joint Replacement Registry. However, these databases have the limitations of not providing comprehensive, consistent, standardized, disease-specific data in order to undertake high-quality research 1; 7; 25. Currently, no prospectively collected, standardized, disease-specific database exists that gathers high-quality data after shoulder arthroplasty.
To address the need for high-quality, standardized data of common orthopedic procedures, the Cleveland Clinic Department of Orthopaedic Surgery developed the Outcomes Management and Evaluation (OME) system, a prospectively designed electronic data collection tool to allow for cost-effective, scientifically valid, and scalable data collection of patient- and surgeon-reported data surrounding a surgical episode for elective knee, hip, and shoulder surgery 4; 6; 12; 18; 26; 29. The purpose of this study was to evaluate the efficiency and validity of OME as a prospective data collection tool for surgical data in shoulder arthroplasty. We hypothesized that compared to a manually-extracted dataset from the narrative operative report within the EMR, the OME system would have higher completion rates with at least substantial agreement for clinically relevant surgical variables in patients undergoing shoulder arthroplasty, while avoiding excess time to complete the data collection tool.
Materials and Methods
OME Database Design
The design and development of the OME database has been described in detail previously. It is built upon the Research Electronic Data Capture (REDCap) platform to be able to collect scientifically valid, scalable data in a cost-effective manner 4; 6; 12; 18; 26; 29. Surgical data are entered prospectively using a web-based electronic data collection tool within 48 hours of surgery by the operating surgeon using a smartphone, laptop, or desktop computer to access an email link sent by the system immediately after procedure completion. As of April 2020, OME has baseline data (PROMs and surgeon-entered variables) on 97% of approximately 49,000 elective knee, hip, and shoulder surgeries, including nearly 3,000 cases of shoulder arthroplasty, from 73 orthopedists at 16 sites within the Cleveland Clinic Health System. OME’s design and implementation were approved by Cleveland Clinic’s Institutional Review Board and the system was vetted by the Information Security Department.
Patient Selection
For the present study, the surgical data of the first 100 shoulder arthroplasty records collected into the OME database starting in July 2015 were reviewed. The first 100 cases were chosen to represent the initial adaptation of this web-based electronic prospective data collection tool. There were no exclusion criteria.
OME Surgical Data Collection
In patients undergoing shoulder arthroplasty, OME collects surgical data on preoperative and pathologic details such as surgical history, diagnosis, rotator cuff status, presence and location of glenoid bone loss, and glenoid morphology, as well as procedural details such as type of subscapularis takedown/repair, performance of biceps tenodesis, need for glenoid bone grafting, implant type, and type of humeral fixation (Table 1, Supplementary Table). These variables were identified from the literature or by expert opinion as potential predictors of operative outcomes by the clinicians involved in the OME system design. Built-in branching logic is used to accelerate data entry, showing only fields applicable to each pathology and procedure, which prevents surgeons from having to answer irrelevant or unnecessary items; these un-encountered checkboxes and data-fields help streamline surgeon data entry. However, incomplete forms cannot be submitted within OME, thus ensuring that a standardized and complete dataset is obtained for every patient surgery. Total time required to complete the surgical data form is also collected to measure caregiver burden. For shoulder arthroplasty, a total of 39 variables related to preoperative pathology, including rotator cuff status and glenoid wear, and surgical procedures, were extracted from OME for comparison with data from the EMR (Table 1, Supplementary Table).
Table 1:
EMR vs. OME data completion rates.
| Measure | EMR completion count | OME completion count | Completion rate P value |
|---|---|---|---|
| Preoperative and pathologic details | |||
| Prior surgery, left shoulder | 11 | 100 | <.001 |
| Prior surgery, right shoulder | 11 | 100 | <.001 |
| History of joint infection | 1 | 100 | <.001 |
| Operative limb | 100 | 100 | >.99 |
| Indication for surgery | 100 | 100 | >.99 |
| Glenoid wear or bone loss | 52 | 100 | <.001 |
| Glenoid wear location | 31 | 37 | .307 |
| Presence of glenoid biconcavity | 10 | 28 | <.001 |
| Walch classification given | 4 | 58 | <.001 |
| Glenoid bone loss pattern (revision surgery) | 1 | 9 | .013 |
| Humeral head AVN involvement | 1 | 3 | .48 |
| RC subscapularis status | 39 | 100 | <.001 |
| RC subscapularis tear size | 0 | 10 | .004 |
| RC superior-posterior status | 70 | 100 | <.001 |
| RC superior-posterior rotator cuff tear tendon involvement | 27 | 36 | .016 |
| RC superior-posterior rotator cuff tear size | 3 | 36 | <.001 |
| Arthroplasty details | |||
| Type of subscapularis takedown/repair | 91 | 100 | .008 |
| Biceps tenodesis | 82 | 100 | <.001 |
| Rotator cuff repair | 6 | 100 | <.001 |
| Glenoid bone graft | 15 | 96 | <.001 |
| Glenoid bone graft location | 14 | 12 | .617 |
| Implant used | 96 | 96 | >.99 |
| Implant manufacturer | 95 | 96 | >.99 |
| Humeral fixation | 36 | 96 | <.001 |
| Antibiotic-containing cement | 8 | 9 | >.99 |
| Hemiarthroplasty details | |||
| Hemiarthroplasty type | 5 | 4 | >.99 |
| Glenoid reaming | 1 | 4 | .248 |
| Soft tissue interposition | 1 | 4 | .248 |
| Anatomic TSA details | |||
| Augmented glenoid component | 27 | 42 | <.001 |
| Glenoid component peg perforation | 25 | 42 | <.001 |
| Humeral neck prosthesis | 37 | 43 | .041 |
| Humeral head eccentricity | 37 | 43 | .041 |
| Reverse TSA details | |||
| Glenoid baseplate central peg/screw length | 43 | 50 | .023 |
| Revision arthroplasty details | |||
| Type of hardware revised or removed | 11 | 11 | >.99 |
| If anatomic or reverse TSA present, component side revised | 2 | 2 | >.99 |
| Humeral side revised (hemiarthroplasty or anatomic) | 4 | 4 | >.99 |
| Humeral side revised (reverse TSA) | 2 | 2 | >.99 |
| Glenoid side revised (reverse TSA) | 2 | 2 | >.99 |
| Antibiotic spacer placed | 11 | 11 | >.99 |
EMR Data Extraction
The surgeons’ narrative operative notes and the implant logs were obtained from the Epic EMR system (Epic Systems, Verona Wisconsin) for this patient cohort and queried for the same variables collected in OME by an examiner blinded to the OME data. When information about implants was not specifically stated in the operative note, the information was obtained from the implant log. When information on a variable was not present in the operative note, it was considered as “absent” in reporting data counts, but as an “implied negative” for agreement analysis (i.e., surgeons intentionally omitting non-applicable information with the implicit understanding that the item was negative or not present). Specifically, an “implied negative” was assumed when the following variables were not present in the operative note because it was felt that surgeons would, more likely than not, mention these points in the operative notes if they were applicable: past surgical history of the left/right shoulder, presence of glenoid biconcavity, presence of a tear in the subscapularis or superior-posterior rotator cuff, rotator cuff repair, presence of glenoid bone grafting, presence of glenoid reaming or soft tissue interposition for hemiarthroplasty, use of cemented humeral stem fixation, use of an augmented glenoid component, and use of a long glenoid baseplate central peg/screw.
Statistical Analysis
Before the EMR and OME datasets were statistically compared, discrepancies between the two datasets were identified and rechecked and verified. Subsequently, the EMR and OME data were analyzed for completion counts and agreement. Comparison between surgeon operative reports and OME completion counts was made utilizing McNemar’s test with continuity correction. For assessment of agreement, variables were categorized as ordinal or nominal. Agreement between nominal variables was made using Cohen’s unweighted Kappa values while linearly weighted Cohen’s test was used for ordinal variables. Nominal variables received the same penalty for mismatches between the operative report and OME data regardless of the degree of disagreement. Ordinal variables received a variable amount of penalty depending on how different the mismatch was between the operative report data and the OME data. Four variables (superior-posterior rotator cuff status, superior-posterior rotator cuff tear size, subscapularis status, and subscapularis tear size) were considered ordinal. A kappa value of 1.00 indicates perfect agreement; 0.81–0.99 almost perfect agreement; 0.61–0.80 substantial agreement, 0.41–0.60 moderate agreement; 0.21–0.40 fair agreement; 0.00–0.20 slight agreement and <0.00 poor agreement 17. A 95% confidence interval (CI) was calculated for all values of agreement. In addition to these formal agreement metrics, the raw proportion of records for each variable showing agreement were also used to assess raw proportional agreement. Data were analyzed with R software (R version 3.3.3 (2017–03-06), Vienna, Austria)
Results
The first one hundred cases of shoulder arthroplasty (primary or revision) were entered into the Cleveland Clinic’s OME database by 5 surgeons between July 2015 and September 2015. The median time to complete OME surgical data entry for these 100 cases was 92 seconds (interquartile range, 70 – 126 seconds).
Completion rates
EMR and OME completion counts of the 39 variables assessed are listed in Table 1. Completion counts were significantly higher in OME compared to operative reports in 22 (56%) of the variables analyzed (p < 0.05). Notably, the presence/absence of glenoid wear or bone loss was mentioned in 52 out of 100 cases in the EMR compared to all 100 cases in OME (p<0.001), and the Walch classification was mentioned in only 4 cases in the EMR compared to the 58 cases in OME where it was appropriate to be assessed (p<0.001). Similarly, the status of the rotator cuff was noted significantly less in the narrative operative reports, with subscapularis tendon status recorded in 39 out of 100 cases in the EMR compared to all 100 cases in OME (p<0.001) and the superior-posterior rotator cuff status recorded in 70 out of 100 cases in the EMR compared to all 100 cases in OME (p<0.001). The remaining 17 variables showed no statistically significant differences in completion counts between EMR and OME, with the absolute completion counts being the same or higher in OME for all but 2 variables (Table 1). No variable had significantly higher completion counts in EMR than OME.
Data agreement
The agreement scores of data extracted from EMR and OME are listed in Table 2. Formal agreement statistics could not be calculated for 10 of the 39 variables for which the operative note and OME were in complete agreement, because only one option appeared in each data source despite multiple options being available. Among the 29 variables that were compared, agreement proportions exceeded 80% for 23 (79%) of the variables and only dipped below 50% for 1 (3%) of the variables (“Glenoid bone graft location”). The kappa values were perfect (κ = 1.00) for 5 (17%), almost perfect (κ: 0.81–0.99) for 9 (31%), substantial (κ: 0.61–0.80) for 6 (21%), moderate (κ: 0.41–0.60) for 3 (10%), fair (0.21–0.40) for 4 (14%), and slight (0.0–0.20) for 2 (7%) of variables. Therefore, there was at least substantial agreement between EMR and OME in 20 (69%) of the 29 compared variables.
Table 2:
EMR vs. OME data agreement scores
| Measure | Records used in agreement comparison | Proportion with agreement | Agreement measure (kappa) | 95% confidence interval |
|---|---|---|---|---|
| Preoperative and pathologic details | ||||
| Prior surgery, left shoulder | 100 | 0.89 | 0.61 | (0.39, 0.83) |
| Prior surgery, right shoulder | 100 | 0.85 | 0.47 | (0.22, 0.72) |
| History of joint infection | 1 | NA | NA | NA |
| Operative limb | 100 | 0.96 | 0.92 | (0.84, 0.99) |
| Indication for surgery | 100 | 0.93 | 0.90 | (0.83, 0.97) |
| Glenoid wear or bone loss | 52 | 0.58 | 0.09 | (−0.2, 0.38) |
| Glenoid wear location | 22 | 0.82 | 0.63 | (0.30, 0.96) |
| Presence of glenoid biconcavity | 28 | 0.71 | 0.43 | (0.09, 0.76) |
| Walch classification given | 4 | 0.75 | 0.43 | (−0.54, 1.0) |
| Glenoid bone loss pattern (revision surgery) | 1 | NA | NA | NA |
| Humeral head AVN involvement | 1 | NA | NA | NA |
| RC subscapularis status | 100 | 0.83 | 0.33* | (0.21, 0.44) |
| RC subscapularis tear size | 0 | NA | NA | NA |
| RC superior-posterior status | 100 | 0.86 | 0.72* | (0.48, 0.97) |
| RC superior-posterior rotator cuff tear tendon involvement | 8 | 0.75 | 0.64 | (0.22, 1.0) |
| RC superior-posterior rotator cuff tear size | 2 | NA | NA | NA |
| Arthroplasty details | ||||
| Type of subscapularis takedown/repair | 91 | 0.88 | 0.80 | (0.69, 0.91) |
| Biceps tenodesis | 100 | 0.94 | 0.84 | (0.71, 0.96) |
| Rotator cuff repair | 100 | 0.95 | 0.26 | (−0.37, 0.89) |
| Glenoid bone graft | 96 | 0.96 | 0.83 | (0.67, 0.99) |
| Glenoid bone graft location | 11 | 0.45 | 0.36 | (0.02, 0.71) |
| Implant used | 96 | 0.98 | 0.96 | (0.91, 1.00) |
| Implant manufacturer | 95 | 0.97 | 0.91 | (0.81, 1.00) |
| Humeral fixation | 96 | 0.97 | 0.81 | (0.59, 1.00) |
| Antibiotic-containing cement | 7 | NA | NA | NA |
| Hemiarthroplasty details | ||||
| Hemiarthroplasty type | 4 | 1.00 | 1.00 | (1.00, 1.00) |
| Glenoid reaming | 4 | NA | NA | NA |
| Soft tissue interposition | 4 | 0.75 | 0.00 | (−1.00, 1.00) |
| Anatomic TSA details | ||||
| Augmented glenoid component | 42 | 0.91 | 0.72 | (0.45, 0.98) |
| Glenoid component peg perforation | 25 | 1.00 | 1.00 | (1.00, 1.00) |
| Humeral neck prosthesis | 37 | 0.81 | 0.30 | (−0.16, 0.77) |
| Humeral head eccentricity | 37 | 0.95 | 0.89 | (0.74, 1.00) |
| Reverse TSA details | ||||
| Glenoid baseplate central peg/screw length | 50 | 0.96 | 0.81 | (0.55, 1.00) |
| Revision arthroplasty details | ||||
| Type of hardware revised or removed | 11 | 1.00 | 1.00 | (1.00, 1.00) |
| If anatomic or reverse TSA present, component side revised | 2 | NA | NA | NA |
| Humeral side revised (hemiarthroplasty or anatomic) | 4 | 1.00 | 1.00 | (1.00, 1.00) |
| Humeral side revised (reverse TSA) | 2 | NA | NA | NA |
| Glenoid side revised (reverse TSA) | 2 | NA | NA | NA |
| Antibiotic spacer placed | 11 | 1.00 | 1.00 | (1.00, 1.00) |
NA, not analyzed; OME, Outcomes Management and Evalution.
Variables with only one value observed are unable to have agreement measure calculated despite the fact that they have perfect agreement in the colloquial sense.
kappa value based on linearly weighted Cohen’s test for ordinal variables
Discussion
The gap in overall quality between retrospective and prospective orthopedic research can be directly attributed to the limitations of extracting data from the medical record in retrospective studies 27; 28. With regards to surgical outcomes, the variability in quantitative data incorporated into operative reports by different surgeons creates inefficiencies ultimately hindering retrospective data collection and can also increase costs 20. The purpose of this study was to evaluate the validity and efficiency of OME as a prospective data collection tool for surgical data in shoulder arthroplasty. In the current study, we demonstrated that OME allows the prospective collection of relevant surgical data in a thorough and efficient manner. Data on preoperative pathology and surgical procedures performed were captured by the surgeon most commonly in less than two minutes per case. As hypothesized, compared to a manually extracted dataset from the narrative operative report within the EMR, the OME system had higher completion rates and at least substantial agreement with the majority of the relevant surgical variables in patients undergoing shoulder arthroplasty.
Completion rate was significantly higher in OME than EMR for 22 (56%) of the surgical variables relevant to shoulder arthroplasty. Notably, this occurred in the majority of preoperative pathologic variables collected in OME (12 of 16 variables), including the presence/absence of glenoid wear or bone loss, Walch classification, and the status of the rotator cuff. Similar significant discrepancies between the EMR and OME were also seen for the presence of a glenoid biconcavity, subscapularis tear size, and superior-posterior rotator cuff tear size. All of these are important variables measuring preoperative pathology which have been shown to impact outcomes in shoulder arthroplasty8,13; 14; 30; 33. The absence of important pathologic variables in the operative report for a large proportion of patients undergoing shoulder arthroplasty demonstrates some of the limitations in the use of the EMR for retrospective research and quality assessment and highlights the benefit of utilizing a prospectively designed and standardized database like OME. The branching logic implemented in OME also allows for efficient yet relevant and complete surgical documentation by streamlining the data capture process on a prospectively designed set of clinically relevant surgical variables. The discrete data collection format of OME also allows for efficient data extraction and retrieval because of its underlying REDCap platform, in contrast to manual data abstraction from operative notes that is associated with high error rates, and increased time and cost.20
The validity of the OME data is evidenced by the high level of proportional and statistical agreement between operative notes and OME data when the data for a given variable were available in both. Agreement proportions exceeded 80% for 79% of the 29 statistically compared variables (the other 10 studied variables had 100% agreement but could not be statistically compared because both data sets had the same single response under those variables), and 69% of these variables demonstrated at least substantial statistical agreement (Cohen’s unweighted κ > 0.6) between the two data sources. The Kappa-values were “fair” or worse (κ < 0.4) for 21% of the variables. This finding suggests situations where raw agreement was high because certain values were very common in both datasets, leading to substantial overlap at this value, but κ was low due to disagreements when the variable took less typical values. For example, data on humeral prosthesis neck type (standard/variable) in anatomic TSA showed high proportional agreement (30 of 37 cases, or 81%) because 28 cases in both operative notes and OME reported use of a standard neck prosthesis, but κ was low (0.3) because only 2 of the other 9 cases agreed on use of a variable neck prosthesis, with the remaining 7 cases marked as having a standard neck in OME and a variable neck in the operative notes.
Discrepancies between EMR and OME could arise for multiple reasons. For example, many of the discrepancies can be explained by lack of standardized content and verbiage in the surgeons’ dictated operative notes, whereas standardized responses were mandated in the prospectively-designed OME database. We also found several examples of inaccurate EMR data. One scenario was 17 cases where the operative report mentioned “glenoid wear” to indicate the presence of arthritis, but not necessarily indicating any type of glenoid bone loss. During EMR data extraction this can be confused as presence of glenoid wear/bone loss, when a case may be a typical Walch A1 glenoid. Another scenario was observed in a case where the operative report had no mention of glenoid bone loss, yet in OME it was entered by the surgeon as posterior glenoid bone loss with a B2 Walch classification. A review of this patient’s preoperative imaging (Figure 1) demonstrated posterior glenoid bone loss with biconcavity, consistent with a B2 Walch classification. Such important data omissions and errors in data entry and abstraction are well recognized issues in operative report data 31 and would compromise any retrospective outcome study data utilizing EMR. To address these shortcomings, computerized templates are being increasingly used to help increase consistency of data input and collection, improve the quality and completeness of surgical data, 10 and also reduce the need for transcription services thereby reducing costs 30. However, templates lack the streamlining features of branching logic of prospective disease-specific registries and may not require key variables to be entered into the dataset.21
Figure 1.
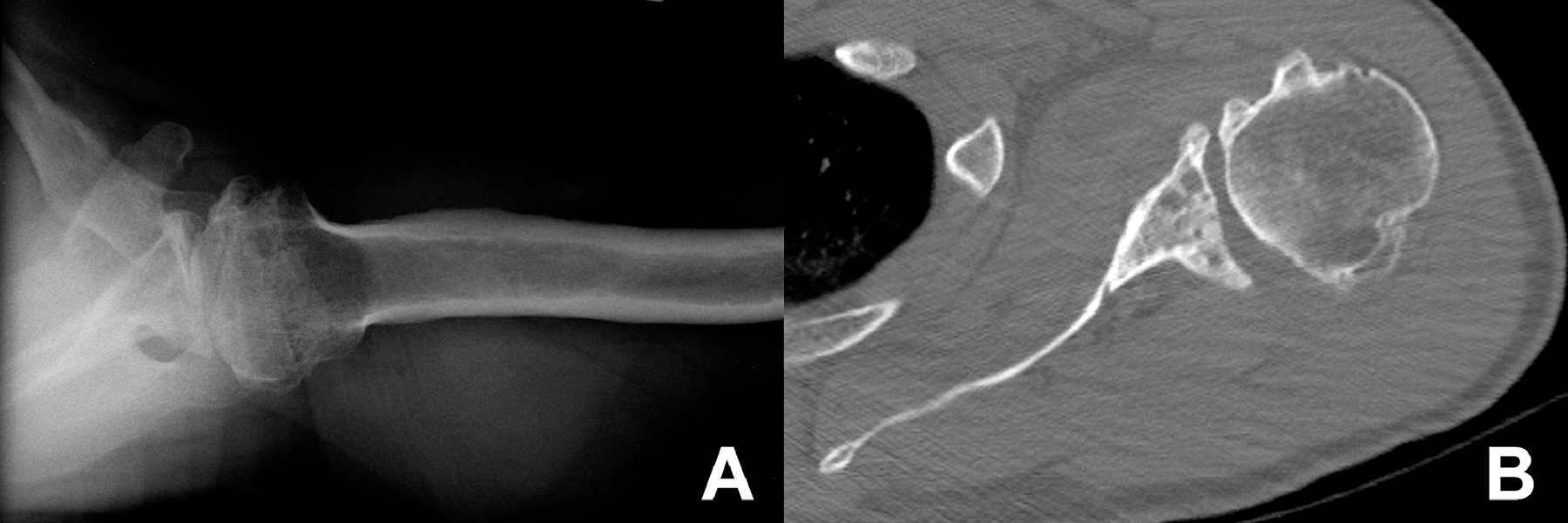
A case of posterior glenoid bone loss that was not mentioned in the operative report. (A) Axillary radiograph of the left shoulder showing arthritic findings, including posterior glenoid bone loss and glenoid biconcavity, as well as posterior subluxation of the humeral head. (B) Axial CT scan also showing posterior glenoid bone loss and glenoid biconcavity, consistent with the B2 Walch classification documented in OME.
The advantages of the OME system and its incorporated branching logic design include the ability to increase the consistency and completeness of reported data parameters, and increase efficiency of data entry and extraction 18. Data are collected in a time-efficient manner, with a median of only 92 seconds required to complete OME surgical data entry for a given shoulder arthroplasty case. This coupled with mandatory data entry and the accessibility and ease of use of the platform with surgeon smartphones and other portable devices increases completeness and compliance. Another advantage of OME is the ability to collect detailed implant data prospectively in a standardized manner and directly link the implant data to preoperative diagnosis to more easily create treatment groups for analysis. In contrast, many surgical databases use CPT or ICD-9/10 codes to try to sort patients for analysis. However, both CPT and ICD-9/10 codes can lack the necessary level of detail to correctly match up implant type with preoperative diagnoses across cases, leading to challenges with creating accurate treatment groups for analysis. In addition, while many surgical databases have the ability to record implant manufacturer, implant type, and size, OME also provides the ability to collect more specific implant related data such as whether cement was used, humeral and glenoid bone stock, and whether bone grafting was needed. Furthermore, OME is designed to allow for the incorporation of new variables (e.g., to include new implant types and models as they become available for use) and update or refine existing variables (e.g., to include the modified Walch classification system when introduced).
There are limitations to the current study and OME. First, there is currently no “gold standard” for surgical documentation to which to compare OME. The operative note is the most commonly used mode of recording surgical data, however, it is neither prospectively designed nor standardized. Consequently, it is difficult to fully gauge the accuracy of the OME database and discrepancies between the operative report and OME could be due to data entry errors in either source. Both sources rely on surgeons’ accurate data entry and errors can occur as a surgeon completes the operative report or inputs information into the OME system. Second, the reliability of OME is affected by the reliability of the data it collects. For example, the Walch classification has been shown to have only fair to moderate inter-observer reliability 32. Third, there is a potential for implicit bias in the study because the study variables and their available options were chosen based on the OME data dictionary and then looked for in the operative notes. Surgeons are required to enter a standardized dataset in OME, which though relatively comprehensive is not exhaustive and will be limited by its pre-defined classifications for certain variables. The operative notes may contain other important data that may not be present in OME, and some data that are absent in the operative notes may also be present in other locations in the EMR, such as in preoperative notes, implant logs, or preoperative imaging. Finally, for agreement analysis we assumed missing data in the operative notes as “implied negatives” for certain variables; however, it is possible that some other variables that were missing could also have been “implied negative” by the surgeon, but the retrospective nature of the study does not allow us to determine the accuracy of our assumption.
Future Directions
Collection of standardized prospective data captured in an electronic format in OME will allow investigation of the relationships between patient, disease, and surgical factors, and PROMs.3; 6; 34 The underlying structure of OME as a secure REDCap database also provides the ability to utilize this data collection tool across institutions for prospective multicenter cohort studies or clinical trials 18. Improvement in orthopedic outcomes research will become increasingly important as health care economics dictate justification of elective arthroplasty based on efficacy and outcomes.3; 15 Future areas of research include using OME data to investigate the relationship between patient, disease, and surgical factors, including implant type and PROMs, and outcomes following shoulder arthroplasty.
Conclusions
The OME data capture system demonstrated higher completion rates compared to operative notes for most variables examined after shoulder arthroplasty, and the validity of the OME system was evidenced by the high level of proportional and statistical agreement between OME data and operative notes when the data for a given variable were available in both. OME surgical data were captured by the surgeon most commonly in less than two minutes per case. This prospectively designed, electronic data entry system is a valid and efficient tool for collecting comprehensive and standardized data on shoulder arthroplasty.
Supplementary Material
Acknowledgments:
The authors acknowledge William Messner, MS, Alexander Zajichek, MS, Yuxuan Jin, MS for statistical analysis. The following orthopaedic surgeons contributed the cases analyzed in this study: Eric T. Ricchetti, Joseph P. Iannotti, Gregory J. Gilot, Anthony Miniaci, and Mark S. Schickendantz.
This study was supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH) under award R01 AR075286. We also acknowledge the Orthopaedic and Rheumatologic Institute at Cleveland Clinic for support of the OME database and related infrastructure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Greg Strnad and Kurt Spindler are inventors with rights to royalties from nPhase/REDCap Cloud for technology related to the subject of this article.
The other authors, their immediate family, and any research foundation with which they are affiliated did not receive any financial payments or other benefits from any commercial entity related to the subject of this article.
References
- 1.Brown JS, Gordon RJ, Peng Y, Hatton A, Page RS, Macgroarty KA. Lower operating volume in shoulder arthroplasty is associated with increased revision rates in the early postoperative period: long-term analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Shoulder Elbow Surg 2020; 29:1104–14. doi: 10.1016/j.jse.2019.10.026 [DOI] [PubMed] [Google Scholar]
- 2.Capurro D, Yetisgen M, van Eaton E, Black R, Tarczy-Hornoch P. Availability of structured and unstructured clinical data for comparative effectiveness research and quality improvement: a multisite assessment. EGEMS (Wash DC) 2014; 2:1079. doi: 10.13063/2327-9214.1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cleveland Clinic Orthopaedic A. The Association Between Readmission and Patient Experience in a Total Hip Arthroplasty Population. The Journal of arthroplasty 2018; 33:1668–74. doi: 10.1016/j.arth.2017.12.023 [DOI] [PubMed] [Google Scholar]
- 4.Cleveland O, Piuzzi NS, Strnad G, Brooks P, Hettrich CM, Higuera-Rueda C et al. Implementing a Scientifically Valid, Cost-Effective, and Scalable Data Collection System at Point of Care: The Cleveland Clinic OME Cohort. J Bone Joint Surg Am 2019; 101:458–64. doi: 10.2106/JBJS.18.00767 [DOI] [PubMed] [Google Scholar]
- 5.Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg 2010; 19:1115–20. 10.1016/j.jse.2010.02.009 [DOI] [PubMed] [Google Scholar]
- 6.Derwin KA, Sahoo S, Zajichek A, Strnad G, Spindler KP, Iannotti JP et al. Tear characteristics and surgeon influence repair technique and suture anchor use in repair of superior-posterior rotator cuff tendon tears. J Shoulder Elbow Surg 2018. doi: 10.1016/j.jse.2018.07.028 [DOI] [PMC free article] [PubMed]
- 7.Dillon MT, Ake CF, Burke MF, Singh A, Yian EH, Paxton EW et al. The Kaiser Permanente shoulder arthroplasty registry: results from 6,336 primary shoulder arthroplasties. Acta Orthop 2015; 86:286–92. doi: 10.3109/17453674.2015.1024565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson BJ, Ling D, Wong A, Eno JJ, Dines JS, Dines DM et al. Does having a rotator cuff repair prior to reverse total shoulder arthroplasty influence the outcome? The bone & joint journal 2019; 101-B:63–67. doi: 10.1302/0301-620X.101B1.BJJ-2018-0874.R1 [DOI] [PubMed] [Google Scholar]
- 9.Ghani Y, Thakrar R, Kosuge D, Bates P. ‘Smart’ electronic operation notes in surgery: an innovative way to improve patient care. Int J Surg 2014; 12:30–2. doi: 10.1016/j.ijsu.2013.10.017 [DOI] [PubMed] [Google Scholar]
- 10.Godeneche A, Boulahia A, Noel E, Boileau P, Walch G. Total shoulder arthroplasty in chronic inflammatory and degenerative disease. Rev Rhum Engl Ed 1999; 66:560–70. [PubMed] [Google Scholar]
- 11.Gur I, Gur D, Recabaren JA. The computerized synoptic operative report: a novel tool in surgical residency education. Arch Surg 2012; 147:71–4. doi: 10.1001/archsurg.2011.228 [DOI] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho JC, Amini MH, Entezari V, Jun BJ, Alolabi B, Ricchetti ET et al. Clinical and Radiographic Outcomes of a Posteriorly Augmented Glenoid Component in Anatomic Total Shoulder Arthroplasty for Primary Osteoarthritis with Posterior Glenoid Bone Loss. J Bone Joint Surg Am 2018; 100:1934–48. doi: 10.2106/JBJS.17.01282 [DOI] [PubMed] [Google Scholar]
- 14.Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am 2013; 95:e82. doi: 10.2106/JBJS.L.00336 [DOI] [PubMed] [Google Scholar]
- 15.Iyengar JJ, Samagh SP, Schairer W, Singh G, Valone FH 3rd, Feeley BT. Current trends in rotator cuff repair: surgical technique, setting, and cost. Arthroscopy 2014; 30:284–8. doi: 10.1016/j.arthro.2013.11.018 [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am 2011; 93:2249–54. doi: 10.2106/JBJS.J.01994 [DOI] [PubMed] [Google Scholar]
- 17.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–74. [PubMed] [Google Scholar]
- 18.OME Cleveland Clinic Orthopaedics. Value in Research: Achieving Validated Outcome Measurements While Mitigating Follow-up Cost. The Journal of bone and joint surgery. American volume 2020; 102:419–427. doi: 10.2106/JBJS.19.00531. [DOI] [PubMed] [Google Scholar]
- 19.Palsis JA, Simpson KN, Matthews JH, Traven S, Eichinger JK, Friedman RJ. Current Trends in the Use of Shoulder Arthroplasty in the United States. Orthopedics 2018; 41:e416–e23. doi: 10.3928/01477447-20180409-05 [DOI] [PubMed] [Google Scholar]
- 20.Pan L, Fergusson D, Schweitzer I, Hebert PC. Ensuring high accuracy of data abstracted from patient charts: the use of a standardized medical record as a training tool. Journal of clinical epidemiology 2005; 58:918–23. doi: 10.1016/j.jclinepi.2005.02.004 [DOI] [PubMed] [Google Scholar]
- 21.Paxton EW, Inacio MC, Khatod M, Yue EJ, Namba RS. Kaiser Permanente National Total Joint Replacement Registry: aligning operations with information technology. Clin Orthop Relat Res 2010; 468:2646–63. doi: 10.1007/s11999-010-1463-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porter ME. What is value in health care? N Engl J Med 2010; 363:2477–81. doi: 10.1056/NEJMp1011024 [DOI] [PubMed] [Google Scholar]
- 23.Porter ME, Larsson S, Lee TH. Standardizing Patient Outcomes Measurement. N Engl J Med 2016; 374:504–6. doi: 10.1056/NEJMp1511701 [DOI] [PubMed] [Google Scholar]
- 24.Porter ME, Lee TH. From Volume to Value in Health Care: The Work Begins. JAMA 2016; 316:1047–8. doi: 10.1001/jama.2016.11698 [DOI] [PubMed] [Google Scholar]
- 25.Rasmussen JV, Brorson S, Hallan G, Dale H, Aarimaa V, Mokka J et al. Is it feasible to merge data from national shoulder registries? A new collaboration within the Nordic Arthroplasty Register Association. J Shoulder Elbow Surg 2016; 25:e369–e77. doi: 10.1016/j.jse.2016.02.034 [DOI] [PubMed] [Google Scholar]
- 26.Sahoo S, Mohr J, Strnad GJ, Vega J, Jones M, Schickendantz MS et al. Validity and efficiency of a smartphone-based electronic data collection tool for operative data in rotator cuff repair. J Shoulder Elbow Surg 2019; 28:1249–56. doi: 10.1016/j.jse.2018.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scherer R, Zhu Q, Langenberg P, Feldon S, Kelman S, Dickersin K et al. Comparison of information obtained by operative note abstraction with that recorded on a standardized data collection form. Surgery 2003; 133:324–30. doi: 10.1067/msy.2003.74 [DOI] [PubMed] [Google Scholar]
- 28.Severn A, Research Collaborative in O. Assessing the quality of operation notes: a review of 1092 operation notes in 9 UK hospitals. Patient Saf Surg 2016; 10:5. doi: 10.1186/s13037-016-0093-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah J, Rajgor D, Pradhan S, McCready M, Zaveri A, Pietrobon R. Electronic data capture for registries and clinical trials in orthopaedic surgery: open source versus commercial systems. Clin Orthop Relat Res 2010; 468:2664–71. doi: 10.1007/s11999-010-1469-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shields E, Ho A, Wiater JM. Management of the subscapularis tendon during total shoulder arthroplasty. J Shoulder Elbow Surg 2017; 26:723–31. doi: 10.1016/j.jse.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 31.ten Broek RP, van den Beukel BA, van Goor H. Comparison of operative notes with real-time observation of adhesiolysis-related complications during surgery. Br J Surg 2013; 100:426–32. doi: 10.1002/bjs.8994 [DOI] [PubMed] [Google Scholar]
- 32.Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. The Journal of arthroplasty 1999; 14:756–60. [DOI] [PubMed] [Google Scholar]
- 33.Walch G, Moraga C, Young A, Castellanos-Rosas J. Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. J Shoulder Elbow Surg 2012; 21:1526–33. doi: 10.1016/j.jse.2011.11.030 [DOI] [PubMed] [Google Scholar]
- 34.Westermann RW, Lynch TS, Jones MH, Spindler KP, Messner W, Strnad G et al. Predictors of Hip Pain and Function in Femoroacetabular Impingement: A Prospective Cohort Analysis. Orthop J Sports Med 2017; 5:2325967117726521. doi: 10.1177/2325967117726521 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


