Abstract
Neonatal hypoglycaemia is associated with adverse later development, particularly visuo-motor and executive function impairment. As neonatal hypoglycaemia is common and frequently asymptomatic in at-risk babies, it is recommended that these babies are screened for hypoglycaemia in the first 1–2 days after birth with frequent blood glucose measurements. Neonatal hypoglycaemia can be prevented and treated with buccal dextrose gel, and it is also common to treat hypoglycaemic babies with formula and intravenous dextrose. However, it is uncertain if screening, prophylaxis or treatment improves long-term outcomes of babies at risk of neonatal hypoglycaemia. This narrative review assesses the latest evidence for screening, prophylaxis and treatment of babies at risk to improve long-term neurodevelopmental outcomes.
Keywords: neonatal hypoglycaemia, executive function, developmental delay, buccal dextrose gel
Introduction
Severe, prolonged neonatal hypoglycaemia can cause seizures, brain damage and neurodevelopmental impairment.(1) Signs of neonatal hypoglycaemia are non-specific and hypoglycaemic babies are frequently asymptomatic.(2) Therefore, it is common to screen babies considered at higher risk for neonatal hypoglycaemia with repeated blood glucose tests.(3, 4) However, the majority of babies detected as having neonatal hypoglycaemia by screening of at-risk babies will have mild (<3 episodes of blood glucose concentrations 2.0–2.6 mmol/L)(5) or transient (resolved by 72 hours after birth)(6) rather than severe or prolonged hypoglycaemia, (7) and it is uncertain if mild transient hypoglycaemia has long-term effects on neurodevelopment.(5, 8) Therefore, it is unclear which blood glucose concentration threshold should be used to define hypoglycaemia requiring treatment to improve long-term neurodevelopment.(9) This narrative review explores the current literature on the optimal strategies to screen, prevent and treat neonatal hypoglycaemia to improve the neurodevelopmental outcome of children born at-risk.
Background
Postnatal blood glucose transition
During fetal life the pancreatic β-cell has a low glucose set-point for insulin secretion, and insulin plays a key role in regulating fetal growth, especially in matching growth velocity to substrate supply.(10) Insulin promotes protein synthesis, glycogenesis, and lipogenesis and inhibits proteolysis, gluconeogenesis and lipolysis. Although the hepatic enzymatic pathways for glycogenolysis and gluconeogenesis are expressed by late gestation, under normal conditions fetal hepatic glucose synthesis is negligible, likely due to relatively high circulating fetal insulin concentrations.(11)
At birth, the continuous supply of glucose from the mother via the placenta ceases and survival depends on activation of hepatic glucose output, initially via glycogenolysis and then gluconeogenesis. This requires a fall in the plasma insulin: glucagon ratio,(12) which in turn, depends on rapid postnatal adaptation of the β-cell, including an increase in the glucose set-point for suppression of insulin secretion.(13) This is necessary for the neonate to respond to bolus feeding and fasting. In addition, lipolysis must commence, releasing glycerol for gluconeogenesis and free fatty acids for ketogenesis, which also requires a fall in plasma insulin concentrations.
Previous reports about the patterns of blood glucose concentrations in healthy babies following birth have all been undertaken on populations of hospitalised babies. They have shown a prompt fall in glucose concentrations, reaching a nadir between 30 to 90 minutes after birth, followed by a spontaneous rise regardless of feeding.(1, 14–16) The recent GLOW Babies Study,(17) which used accurate glucose measurements and, uniquely, interstitial glucose monitoring in a population of 67 healthy largely breastfed babies in their own homes, reports the mean plasma glucose concentrations between 1 and 4 hours to be 3.2 (0.7) mmol/L and 3.3 (SD) 0.6 mmol/L for the first 48 hours. Mean glucose concentrations increased between 48 and 72 hours to reach values similar to adults of 4.6 (SD) 0.7 mmol/L by day 4 with a 3rd centile 3.3 mmol/L (Figure 1 and Table 1, online only). Importantly, there was considerable variation in glucose concentrations within and between babies, suggesting that the metabolic transition is a gradual process over the first four postnatal days. However, these data from appropriately grown, healthy babies should not be used as thresholds for babies with impaired metabolic transition.
Figure 1.

Glucose centiles in healthy term babies
From Harris D et al. Glucose profiles in healthy term infants in the first five days: The Glucose in Well Babies (GLOW) study. J Pediatr. 2020;223:34–41.(17) Permission to use to be sought from Elsevier
Table.
Ongoing randomised controlled trials investigating interventions to prevent or treat neonatal hypoglycaemia.
| Participants | Intervention | Comparator | Outcome | Clinical trial registry and number | |
|---|---|---|---|---|---|
| Prevention | |||||
| Groom et al (C*STEROID) | Women for whom caesarean section is planned pre-labour at 35+0 to 39+6 weeks gestation | Antenatal betamethasone (11.4mg IM, two doses 24 hours apart, within 7 days of planned delivery) |
Placebo | • Neonatal respiratory morbidity • Neonatal hypoglycaemia • (BGC <2.6 mmol.l−1) |
ANZCTR: ACTRN12620000914965 |
| Battarbee et al (E-ALPS) |
Women receiving antenatal steroids 34+0 to 36+5 weeks gestation due to a high probability of late preterm birth | Maternal euglycaemia (regular blood glucose screening and treatment of hyperglycaemia) |
Routine antenatal care | Umbilical cord blood C-peptide | ClinicalTrials: NCT03076775 |
| Kelleher et al (GEHPPI) | Very preterm neonates (≤ 32 weeks GA) | Buccal 40% dextrose gel (400 mg) | Placebo | Early neonatal hypoglycaemia (PGC <1.8 mmol.l−1, 30–60 minutes after birth) |
ClinicalTrials: NCT04353713 |
| Harding et al (hPOD) | Late preterm and term neonates at-risk of neonatal hypoglycaemia (infant of diabetic mother, small, large or late preterm) | Buccal 40% dextrose gel (200 mg.kg−1) at 1 hour after birth | Placebo | NICU admission | ANZCTR: ACTRN12614001263684 |
| De Bernando et al | Late preterm and term neonates at-risk of neonatal hypoglycaemia (small, large or late preterm) | Oral 40% Dextrogel (200 mg.kg−1 or 400 mg.kg−1) |
Placebo | Neonatal hypoglycaemia (BGC <2.6 mmol.l−1) | ClinicalTrials: NCT04185766 |
| Alallah et al (THIN) |
Late preterm and term neonates at risk of neonatal hypoglycaemia (infant of diabetic mother, small, large or late preterm) | Buccal date paste (glucose equivalent 200 mg.kg−1) | Standard care | • Neonatal hypoglycaemia (BGC <2.6 mmol.l−1) • NICU admission for hypoglycaemia |
ClinicalTrials: NCT03726697 |
| Treatment | |||||
| McKinlay et al (NeoGluCO) | Neonates with severe (BGC <1.2 mmol.l−1) or recurrent hypoglycaemia (3 or more episodes of BGC <2.6 mmol.l−1 or BGC 1.2–2.0 mmol.l−1 after two treatments with dextrose gel) | Oral diazoxide (loading dose 5 mg.kg−1 then 1.5 mg.kg−1 12-hourly) |
Placebo | Successful metabolic transition | ANZCTR: ACTRN12620000129987 |
| Thoene et al. | Neonates with hypoglycaemia (BGC < 40 mg/dl (mmol.l−1) first 4 hours after birth or BGC < 45 mg/dl (2.5 mmol.l−1) after 4 hours | Donor human milk (15 mL up to two times) |
Formula (15 mL up to two times) |
Resolution of hypoglycaemia | ClinicalTrials: NCT04030312 |
| Kennedy et al (GLAD) | Neonates of diabetic mothers with significant hypoglycaemia (BGC < 2.6 mmol.l−1 while receiving 90 ml.kg.−1day−1 of 10% dextrose) | Oral diazoxide (5 mg.kg.−1dose−1 8-hourly) |
Intravenous infusion of glucagon (10mcg.kg.−1hr−1) |
Treatment failure | ANZCTR: ACTRN12617001473358 |
PGC; plasma glucose concentration, BGC; blood glucose concentration, IM; intramuscular, ANZCTR; Australia New Zealand Clinical Trials Registry
Pathophysiology
Although central neurohormonal counterregulatory mechanisms are triggered in the neonate after birth by falling blood glucose concentrations, including secretion of glucagon, cortisol, catecholamines and increased sympathetic tone, if the normal postnatal adaptation in insulin secretion is impaired or delayed, insulin concentrations will remain inappropriately high. This results in ongoing suppression of hepatic glucose output and hypoglycaemia ensues. Thus, transitional neonatal hypoglycaemia is often described as hyperinsulinaemic hypoglycaemia, but persistent fetal metabolism may be a better term. Features of this impaired metabolic transition have yet to be fully characterised but are likely to include significant hypoglycaemia in the first 48 hours after birth with absent beta hydroxy butyrate and inappropriately high insulin concentrations.
Maintenance of blood glucose concentrations after birth is of critical importance to the neonatal brain as glucose is the primary oxidative cerebral fuel and uptake is by facilitated diffusion, independent of insulin.(18) The capacity for cerebral glucose uptake after birth may be rate-limited, as the maximal expression of glucose transporter proteins at the blood-brain barrier (GLUT1 and GLUT3) does not occur for several days to weeks.(19) The availability of alternative fuels including ketones, lactate and some amino acids to maintain cerebral cellular metabolism has long been proposed as an important mechanism to prevent injury when glucose availability is reduced (14, 20, 21). However, the relationship between glucose, lactate and ketones concentrations and cerebral function in the neonate remains unclear. Higher circulating insulin concentrations suppress free fatty acid release and hepatic beta-oxidation, so that ketones are largely absent in babies with hypoglycaemia in the first 48 hours(22) and therefore unlikely to provide any neurological protection.(23, 24) It is possible that lactate provides a small but potentially useful energy source during this period.(25)
Large and small neonates appear to have similar risk of developing transitional hypoglycaemia(7) and although this is primarily due to dysregulated insulin secretion, underlying mechanisms may differ. Babies who are large for gestation (LGA) are typically born to mothers with obesity or diabetes, conditions which are associated with excess fetal supply of glucose and free fatty acids. This increases fetal insulin secretion, which in turn causes fetal pancreatic β-cell hypertrophy and hyperplasia, perturbations that persist for a period after birth.(26, 27) Conversely, in growth restriction and placental insufficiency, fetal supply of oxygen and nutrients is reduced. Fetal hypoxaemia raises plasma catecholamine concentrations, especially noradrenaline, which acts on β-cells to suppress in utero insulin secretion.(28, 29) At birth, loss of the sustained adrenergic signalling exposes β-cell hyper-responsiveness, resulting in increased insulin secretion.(28) Thus, both fetal over- and under-nutrition can result in dysregulated postnatal insulin secretion, leading to transitional neonatal hypoglycaemia.
Risk factors and incidence of neonatal hypoglycaemia
There are multiple risk factors for the development of neonatal hypoglycaemia, but those commonly recognised include maternal diabetes, preterm birth and being born small for gestational age.(7) Maternal drug use in pregnancy, including of beta-blockers, is a known risk factor for neonatal hypoglycaemia,(30) and antenatal corticosteroids prior to late preterm birth may also increase the risk of neonatal hypoglycaemia.(31, 32) Other risk factors include neonatal illness such as birth asphyxia or sepsis and poor feeding.
Babies who are born LGA after a non-diabetic pregnancy are also at risk of neonatal hypoglycaemia.(33) It is unknown if hypoglycaemia in these babies represents pathology, since there is little evidence that they are at increased risk of long-term neurodevelopmental impairment.(34) In a review of babies with severe brain injury secondary to hypoglycaemia resulting in litigious claims in the UK, most of the babies were low birth weight, several were babies of diabetic mothers but none were LGA babies of non-diabetic mothers.(35) Risks may vary depending on the centile chart and percentile threshold used to define LGA. For example, in one study neonatal morbidity (defined as a requirement for NICU admission for >48 hours for acute complications) was only increased in LGA babies who were > 90th percentile on both customised and population centiles.(36) While some guidelines recommend screening LGA babies for hypoglycaemia,(3, 37) others recommend higher birthweight percentile thresholds for screening(38) and some do not include them in risk groups to be screened.(4, 39) Further research is needed on whether screening and treatment of neonatal hypoglycaemia improves the long-term neurodevelopmental outcomes of LGA babies, and which birth weight centile should be used to define LGA for this purpose.
The proportion of all babies born at risk for neonatal hypoglycaemia is estimated to be as high as 30%.(40) Of those at risk, there is a wide variation in the reported incidence of hypoglycaemia, resulting from considerable variation in which babies are included, the screening method used and the blood glucose concentration threshold used for diagnosis.(41, 42) When babies born to diabetic mothers, late preterm, SGA and LGA babies were screened using an accurate gas analyser with a diagnostic threshold of a blood glucose concentration < 2.6 mmol/L, the incidence of hypoglycaemia was approximately 50% in all risk groups.(7) However, recently the GLOW Babies Study demonstrated that glucose concentrations below this threshold also occur in 39% of healthy, term normally grown babies.(17) This suggests that screening approaches need to focus more on identifying infants with impaired metabolic transition rather than simply detecting low blood glucose concentrations.
Long term neurodevelopmental outcomes
Perinatal insults can cause long-term neurodevelopmental impairment. In a cohort from Arkansas, where all newborns had blood glucose testing after birth, transient neonatal hypoglycaemia (a single glucose measurement < 2.5 mmol/l) was associated with poorer school performance at 10 years of age.(43) A systematic review of 10 observational studies reported that neonatal hypoglycaemia is associated with an increased risk of visual-motor impairment and executive dysfunction in early childhood and an increased risk of literacy and numeracy problems in later childhood (Table 2), but the evidence was of low quality based on GRADE criteria.(44),(45) A recent database study from Sweden has also demonstrated that moderate neonatal hypoglycaemia (blood glucose concentration < 2.2. mmol/L) in children who did not require NICU admission and whose mothers did not have diabetes was associated with an increased risk of cognitive or motor developmental delay in early childhood.(46)
Babies with severe neonatal hypoglycaemia causing symptoms or seizures had changes affecting the occipital lobe, white matter and basal ganglia on MRI soon after the event, which were associated with long-term neurodevelopmental impairment.(47, 48) However, there are few data on MRI changes after mild or transitional hypoglycaemia or in later childhood.
Screening for neonatal hypoglycaemia
As neonatal hypoglycaemia is often asymptomatic(41) it is standard practice to screen babies considered at risk with repeated, painful blood tests over the first 12–24 hours after birth.(3) There have been no studies that have compared the long-term neurodevelopmental outcomes of at-risk babies screened for neonatal hypoglycaemia and those not screened. However, screening babies at risk of hypoglycaemia and treating those with hypoglycaemic episodes to maintain the BGC ≥ 2.6 mmol/L appears to preserve cognitive function compared to at-risk babies who did not develop hypoglycaemic episodes.(5, 8) Nevertheless, interstitial glucose monitoring suggests that episodes of low glucose concentrations not detected and therefore not treated using this approach are associated with reduced executive function in later childhood. (8)
It is possible that screening at-risk babies for hypoglycaemia may be harmful. Babies with hypoglycaemia who subsequently develop neurodevelopmental impairment are more likely to have had a rapid rise of their interstitial glucose concentration after hypoglycaemia, potentially due to treatment.(5) Moreover, babies with risk factors for hypoglycaemia, such as babies of diabetic mothers and late preterm babies, are less likely to be exclusively breastfed on discharge.(49, 50) While lower breastfeeding rates may be partly explained by other factors such as maternal obesity and poor feeding, it may also be partially due to increased formula use in babies treated for neonatal hypoglycaemia,(51) loss of maternal confidence of their breastfeeding ability and poor adherence of neonatal hypoglycaemia guidelines to baby friendly hospital recommendations.(52)
Currently, selective screening of babies at risk for neonatal hypoglycaemia does not meet several screening test principles,(53) specifically, the long-term neurodevelopmental outcomes of transient neonatal hypoglycaemia are not well understood and there is no evidence that treatment improves these outcomes. As an estimated 30% of all babies may be screened for neonatal hypoglycaemia, further research is urgently needed to characterise babies with impaired metabolic transition and determine the natural history of transient neonatal hypoglycaemia and the effects of screening and treatment.
Detection of hypoglycaemia
Screening for neonatal hypoglycaemia is commonly performed by a heel-prick capillary blood sample. This simple test is beset with difficulties which influence the accuracy of the result including the warmth of the heel, the depth of the lance, flow of blood, how promptly the sample is analysed and, importantly, the analyser used.
Laboratory plasma chemical analysers using enzymatic (glucose oxidase or hexokinase) methods of analysis are the gold standard but whole blood laboratory gas analysers have similar accuracy and are calibrated to provide plasma equivalent values.(54) There are an increasing number of portable or mini-bench top gas analysers which have allowed for the more accurate laboratory methods to be used at the bedside in babies at-risk of hypoglcyaemia.(54).
Strip glucometers are not recommended in neonates as results may vary by as much as 30% compared to reference methods.(55, 56),(57) Consequently, screened babies may have episodes of hypoglycaemia left untreated, potentially resulting in long term neurosensory impairment or may receive unnecessary treatment, causing separation from their mothers, disturbing the establishment of breastfeeding, and increasing health care costs. Thus, if strip glucometers are used, low glucose concentrations should be confirmed by a laboratory or gas analyser sample. This increases the number of heel prick tests, and repetitive exposure to painful heel-pricks has been linked with neurosensory impairment in premature babies.(58)
Importantly, the less accurate strip glucometers are often used because they are perceived to be less expensive. However, formal cost analysis has shown that accurate analysers, such as the i-STAT® portable blood analyser (Abbott. Princeton NJ, USA) and the epoc® Blood Analysis system (Siemens, Erlangen, Germany) are actually more cost effective because they avoid the need for repeat confirmatory tests, as well as decreasing false positives and negatives, and allowing for prompt planning for appropriate treatment pathways.(59) Indeed, it could be argued that screening for neonatal hypoglycaemia to prevent potential brain injury using a method of analysis known to be unreliable, and then requiring positive tests to be repeated, causing more painful stimulation, is unethical when accurate analysis is available and less expensive.
Continuous glucose monitoring
Continuous glucose monitoring (CGM) may be performed in neonates using subcutaneous amperometric electrode sensors, although none of the available devices have been designed or approved for use in neonates.(60) CGM offers the possibility of adjusting treatment in real-time to improve glucose stability, avoid undetected episodes of hypoglycaemia, and reduce pain stress by decreasing the number heel pricks for blood glucose testing. However, the point accuracy of these devices is poor, especially at low glucose concentrations, with 95% limits of agreement that can exceed 1 mmol/L.(61, 62) Thus, the main potential use for CGM in neonates is to help guide the frequency of blood glucose testing. For example, one study in very low birthweight infants found that CGM-guided blood glucose measurements reduced the number of capillary blood tests by 25%.(63) However, this technology should be approached with caution until further outcome data are available, as both under- and over-treatment may occur, both of which may exacerbate brain injury. CGM trend data may be more accurate, but this is yet to be formally evaluated in neonates.
Definition of neonatal hypoglycaemia
The definition of neonatal hypoglycaemia has varied since its description by Cornblath in 1965, when hypoglycaemia was defined as a blood glucose concentration of < 30 mg/dl (1.6 mmol/L) in term babies and < 20 mg/dl (1.1 mmol/L) in low birthweight babies.(1) Over the subsequent 50 years the blood glucose concentration used to define neonatal hypoglycaemia has regularly increased, with the most recent guidance from the Pediatric Endocrine Society recommending < 3.3 mmol/L in 2015.(64) The most commonly used threshold for intervention is a blood glucose concentration < 2.6 mmol/L,(55) largely based on two studies published in 1988; evoked potentials during hypoglycaemia in 17 babies, only five of whom were neonates(65) and a sub-analysis of data from a randomised controlled nutrition trial in very preterm babies(66) which was not replicated in a small more recent study with longer follow up. (67) Clearly, data from these two studies are an inadequate basis upon which to base a widespread screening programme.
Prophylaxis
Given the findings that even a single, transient episode of neonatal hypoglycaemia is associated with poorer school performance(43) and that clinically undetected episodes are common and associated with impaired executive and visual motor function at 4.5 years,(8) prevention of neonatal hypoglycaemia is an important priority.
Feeding
It is generally recommended that early management of babies at risk of neonatal hypoglycaemia includes early skin-to-skin contact and attention to temperature control to minimise cold stress, and initiation of breastfeeding. Although these steps are appropriate, they are not different from those recommended for all newborns, and their contribution to prevention of hypoglycaemia is not clear. Early feeding has been reported to improve glucose concentrations in some studies(68) but not others.(69, 70)
Buccal dextrose gel
Since use of buccal dextrose gel is simple, non-invasive and inexpensive, it is attractive as a potential prophylactic strategy. A randomised trial (prehPOD) of different doses of prophylactic 40% dextrose gel given to babies at risk of hypoglycaemia at one hour after birth in combination with breastfeeding, with or without three additional doses, reported that any dose of dextrose gel reduced the incidence of hypoglycaemia.(71) For the most effective and practical dose (a single dose of 200mg/kg at one hour) the number needed to treat to prevent one case of hypoglycaemia was ten. Dextrose gel prophylaxis was also found to be acceptable, well tolerated and safe. There was no effect of prophylactic buccal dextrose gel on neurodevelopmental impairment at 2 years of age, although this study was underpowered to detect potentially clinically important effects on neurosensory outcome.(72)
A much larger multicenter trial of the most effective dose (hPOD) has recently completed recruitment(73) (ACTRN 12614001263684), and will provide more robust data about whether this approach reduces NICU admission (primary outcome). Importantly, planned long-term follow-up will provide much-needed information about the clinical utility of prophylaxis for prevention of the adverse later outcomes of neonatal hypoglycaemia.
In contrast, dextrose gel prophylaxis 200 mg/kg given after the initial feed was found not to reduce the incidence of hypoglycaemia in a study of 236 at-risk babies.(74) This finding is difficult to interpret, as the study was not randomised, glucose measurement was not using an accurate analysis method, and the gel preparation used (Insta-Glucose) contained a high concentration of carbohydrates (77%), some of which are likely to have competed with dextrose for uptake on the buccal mucosa.
Other approaches
Sucrose is commonly used for management of neonatal pain and is widely available. One trial of 425 babies at risk of hypoglycaemia randomised babies to a single dose of 24% sucrose solution (approximated 200 mg/kg) given by syringe into the cheek bulge with routine early breast or formula feeding, or to feeding alone.(75) There were no differences between groups in glucose concentrations at one, three or six hours of age, although the rate of hypoglycaemia was not reported. This is consistent with previous reports that sucrose treatment for neonatal pain does not affect blood glucose concentrations, perhaps because it requires digestion and is subject to first pass metabolism in the liver.
Treatment
Feeding
Treatment for neonatal hypoglycaemia is aimed at increasing the available cerebral fuels and the most common treatment is feeding. Yet, there is little evidence about the impact of feeding on glucose concentrations in hypoglycaemic babies. Most episodes of hypoglycaemia occur in the first 48 hours after birth(7) when breastfeeding is being established and the supply of milk is low. For this reason many hospitals have policies which encourage women to antenatally express breast milk, which is stored and later given to the baby. However, antenatal expressing has not been shown to reduce admission to NICU or the use of intravenous dextrose in infants of diabetic mothers.(76)
Breastfeeding alone has a minimal effect on blood glucose concentrations during episodes of hypoglycaemia.(51) However, there are many benefits of breastfeeding other than the transfer of milk. Oral stimulation and suckling increase release of gastrointestinal hormones such as gastrin and cholecystokinin which influence carbohydrate metabolism, although their effect on blood glucose concentrations and feeding behaviours remain unclear.(77) It is possible that this may contribute to the finding that breastfeeding reduces subsequent episodes of hypoglycaemia.(51)
Infant formula increases blood glucose concentrations in hypoglycaemic babies.(51) However, infant formula has adverse effects on the establishment of breastfeeding, and increases the risks of infection, allergies and altered microbiome. Further, there are no evidence-based guidelines for clinicians on optimal feeding for hypoglycaemic babies.
Buccal Dextrose gel
Buccal 40% dextrose gel 200 mg/kg is effective in reversing neonatal hypoglycaemia in late preterm and term babies.(78) It is also well tolerated, reduces the separation of mothers and babies soon after birth for hypoglycaemia, and no adverse effects have been reported up to 2 years of age.(78, 79) Observational studies of the introduction of dextrose gel for treatment of hypoglycaemia have consistently reported reduced NICU admission for treatment of hypoglycaemia and improved breastfeeding outcomes.(80–84) The combination of dextrose gel and formula increases the interstitial glucose concentration but also increases glycaemic instability, while the combination of dextrose gel and breastmilk appears to have an optimal effect on the glycaemic profile, including glycaemic stability, rate of rise and duration of hypoglycaemia.(85) Thus, dextrose gel and breastfeeding are considered first-line treatment of neonatal hypoglycaemia for at risk babies in middle and high income countries.(4, 37, 86, 87), and should be used in conjunction with an evidence based clinical practice guideline (Figure 2).(86)
Figure 2.
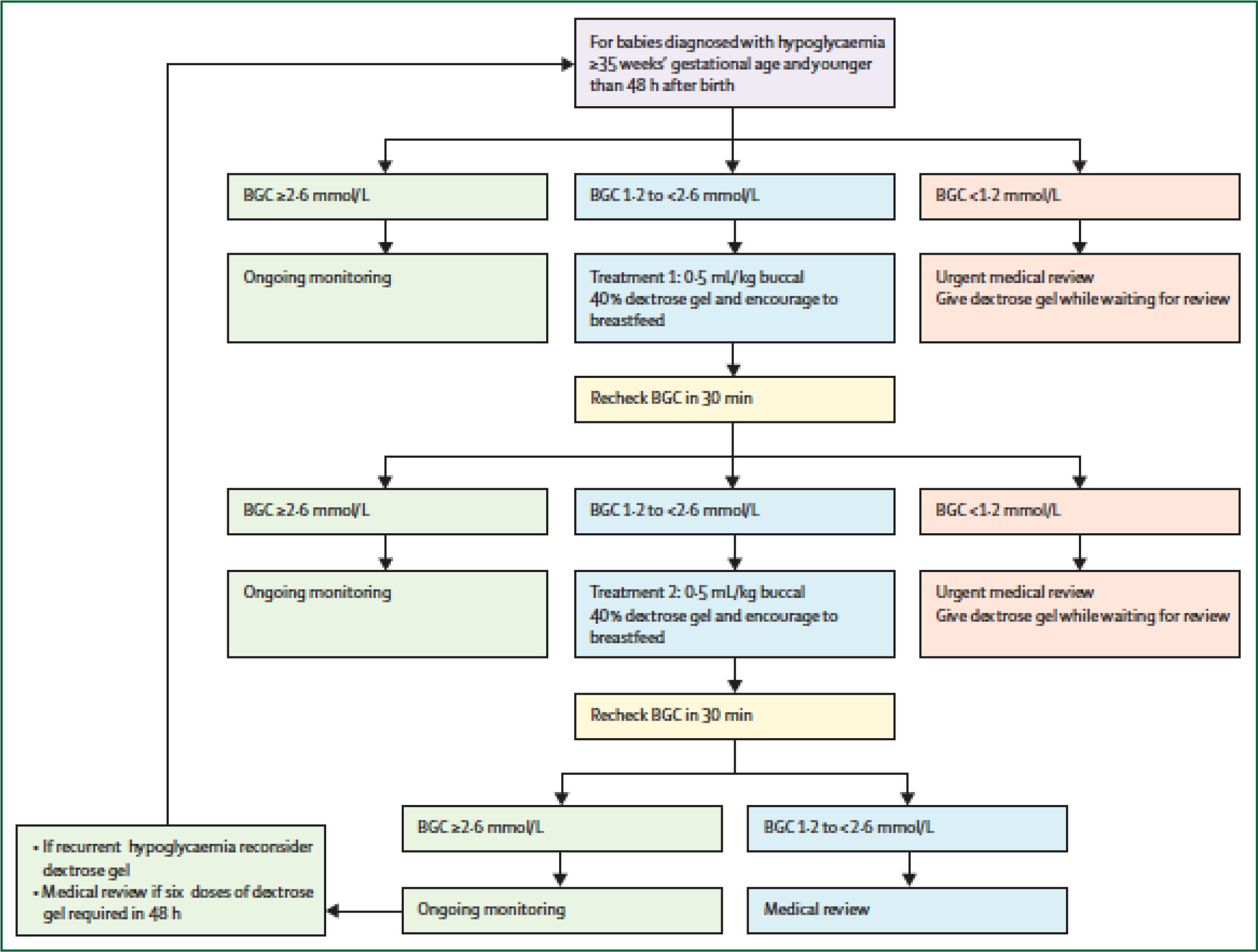
Buccal dextrose gel to treat neonatal hypoglycaemia algorithm.
From Alsweiler JM, Harding J, Crowther C, Woodall SM, “The Oral Dextrose Gel to Treat Neonatal Hypoglycaemia Clinical Practice Guidelines” Panel. Oral dextrose gel to treat neonatal hypoglycaemia: Clinical Practice Guidelines. Auckland: University of Auckland; 2015. http://hdl.handle.net/2292/26266. Accessed 5 Nov 2020.(86)
BGC, blood glucose concentration
Cost effectiveness of dextrose gel
There are few data on the economic implications of neonatal hypoglycaemia and its treatment. Treatment of hypoglycaemia with buccal dextrose gel has been estimated to save approximately US$950 in in-hospital costs per baby,(88) largely due to reductions in NICU stay. Others have also reported decreased hospital costs after implementation of dextrose gel treatment.(81) A recent estimate of the additional lifetime costs of neonatal hypoglycaemia to the healthcare system was US$46,000, excluding other costs such as education, welfare and employment.(89) The value to the healthcare system of preventing one additional case of neonatal hypoglycaemia was US$130,000. Thus, it is not surprising that, based on the effect estimates from the pre-hPOD trial,(71) a cost-utility analysis has estimated that dextrose gel prophylaxis both reduced healthcare costs (US$2,000 per child) and also improved quality of life.(90)
Intravenous dextrose
When first line management of hypoglycaemia with additional feeding and buccal dextrose gel fails, babies are usually commenced on intravenous dextrose. However, the evidence to guide use of intravenous dextrose for treatment of neonatal hypoglycaemia is limited to several small observational studies. A peripheral dextrose infusion of 8 mg.kg-1.min−1 was found to reverse hypoglycaemia in appropriate-for-gestational age neonates within 20 to 30 minutes, but in some small-for-gestational age neonates correction took longer, up to 50 to 60 minutes.(91) The addition of a 200 mg/kg intravenous dextrose bolus resulted in a rapid rise in glucose concentrations, reaching 3.3 to 5.6 mmol/L within 5 minutes. Glucose concentrations remained elevated for 20 minutes compared to a continuous dextrose infusion without bolus, though hyperglycaemia occurred in some neonates.(91) There are no studies that have directly compared different bolus doses or dextrose delivery rates for treatment of neonatal hypoglycaemia.
While prompt correction of hypoglycaemia may seem logical, there is increasing evidence to suggest that a rapid increase in blood glucose or to concentrations above the normal physiological range may exacerbate neuronal injury.(5) In a prospective study of late preterm and term neonates in the 6 hours after onset of hypoglycaemia, those who reached maximum interstitial glucose concentrations between 2 to 4 hours had lower likelihood of neurosensory impairment at 2 to 4 years of age than those who had a faster or slower rise to maximum glucose concentration.(85) These observations are supported by several studies in animals in which increased oxidative neuronal injury has been demonstrated after glucose reperfusion.(92, 93) Overall, these data indicate the need for caution with use of intravenous dextrose boluses, and a controlled, gradual increase in blood glucose concentrations after neonatal hypoglycaemia may be preferable to reduce the risk of metabolic brain injury.
There are also few data to guide the weaning of intravenous dextrose and when to introduce enteral feeds. A recent observational study of neonates on intravenous dextrose for hypoglycaemia found that those who continued enteral feeds had a lower maximum dextrose infusion rate and shorter duration of intravenous therapy compared with those whose feeds were ceased.(94) More research is needed to guide safe and effective use of intravenous dextrose therapy in neonatal hypoglycaemia to improve short- and long-term outcomes.
Adjunctive therapies
Glucagon acts in the liver to increase glucose output, both by glycogenolysis and gluconeogenesis.(12) Intramuscular glucagon can quickly treat neonatal hypoglycaemia, with three-quarters of neonates achieving euglycaemia 2 hours after a single dose of intramuscular glucagon.(95) In neonates who are refractory to intravenous dextrose therapy, including those with severe transitional hypoglycaemia and congenital hyperinsulinism, 85% to 90% will respond to an intravenous infusion of glucagon (0.5 to 1 mg per day).(96) Typically, blood glucose concentrations increase by 2 to 3 mmol/L within several hours and glucose infusion rates can be halved by 24 hours.(97) While raising circulating glucagon concentrations appears to be effective at increasing hepatic glucose output, infusions often have to be maintained for many days and rebound hypoglycaemia is common, occurring in 10% to 20% of neonates within 24 hours of ceasing treatment.(97, 98) This suggests that glucagon therapy has little effect on correcting the underlying dysregulated insulin secretion seen in most neonatal hypoglycaemia.
Oral diazoxide is the main alternative therapy for refractory hypoglycaemia and it has the advantage of acting directly on the β-cell in a dose-dependent manner to diminish glucose-stimulated insulin secretion.(99) In neonates with prolonged transitional hypoglycaemia, diazoxide appears to be widely effective,(100, 101) and this may be partly due to a resetting of the set-point for insulin secretion. However, careful dose titration is sometimes required as hyperglycaemia can occur. In addition, diazoxide can cause congestive heart failure and pulmonary hypertension.(102) However, these side effects resolve on stopping diazoxide. In congenital hyperinsulinism, responsiveness to diazoxide is variable, depending on the underlying cause.(103)
Treatment targets
Ultimately the controversy about the blood glucose concentration at which treatment should be initiated can only be reliably addressed by randomised trials of treatment at different thresholds with assessment of long-term outcomes at least to school age. Recently van Kempen and colleagues reported the first such trial, randomising 689 healthy, asymptomatic, moderately hypoglycaemic babies to treatment at a lower threshold (<36 mg/dL, 2 mmol/l) or a more traditional threshold (< 47 mg/dL, 2.6 mmol/l).(9) Babies with early (< 3 hours) or severe hypoglycaemia (< 36 mg/dl, 2 mmol/l) were excluded, and not all glucose concentrations were determined using gold-standard methods. Babies randomised to the lower threshold experienced fewer interventions but more frequent and severe episodes of hypoglycaemia. There was no difference between groups for Bayley cognitive or motor scores at 18 months. However, since mild hypoglycaemia is not known to be associated with cognitive or motor impairment in preschool children,(45) differences in Bayley scores at 18 months would not be expected, even though there may be important consequences in older children. Long-term follow-up of this important trial will be essential before its findings should be used to influence clinical practice.
Guideline Adherence
While there are multiple international, state, and local guidelines on the screening, diagnosis and treatment of neonatal hypoglycaemia, there are wide variations between guidelines(104) and frequently poor adherence to guideline criteria.(105) Strategies to improve adherence are important as hypoglycaemia is more commonly detected with good guideline adherence.(106) Most guidelines require the identification of babies born small or large for gestational age based on weight centile criteria. These babies may not be identified as meeting the criteria for neonatal hypoglycaemia screening if the baby’s weight centile is not calculated soon after birth. Automatic calculation of the weight centile has been shown to improve adherence to guideline screening criteria.(106)
Future Directions
Currently, there are no evidence-based practices which have been shown to improve the neurodevelopmental outcomes of babies who develop neonatal hypoglycaemia. Future research should focus on determining which babies benefit from screening for neonatal hypoglycaemia, the appropriate blood glucose threshold to diagnose hypoglycaemia, and treatment strategies to improve neurodevelopmental outcomes. Ongoing randomised controlled trials to prevent and treat neonatal hypoglycaemia (Table 3) should report long-term neurodevelopmental outcomes into mid-childhood.
There is variation among international guidelines on which risk factors should be used as indicators for screening for neonatal hypoglycaemia in asymptomatic babies, and particularly whether LGA babies should be screened. Further investigation is urgently needed to understand the relationship between alternative fuels concentrations and long-term neurodevelopmental outcomes. This will require the development of methods to reliably measure these fuels at the bedside using small amounts of blood(107) or non-invasive techniques.
Current management of neonatal hypoglycaemia requires multiple, painful needle pricks to analyse the blood glucose concentration. Development of an accurate non-invasive glucose monitor, such as a pulse glucometer, which detects the glucose concentration in the blood in a similar fashion to pulse oximetry, would allow constant monitoring of the glucose concentration in at-risk babies. This technology could be used to determine the most effective blood glucose thresholds to improve neurodevelopmental outcomes, similar to the study design which used pulse oximetry to determine the safest oxygen concentrations in preterm babies.(108) The development of pulse glucometry technology has been attempted without success to date, but research to develop this technology continues.
There has been very little research on the effect of common treatments for neonatal hypoglycaemia, such as buccal dextrose gel, formula, intravenous dextrose, glucagon and diazoxide on long-term neurodevelopmental outcomes. Randomised controlled trials are urgently needed to determine the effects of these interventions on childhood outcomes.
Conclusion
Mild or transitional neonatal hypoglycaemia in at-risk babies is associated with neurodevelopmental impairment, specifically affecting visual-motor and executive function. Prophylactic buccal dextrose gel reduces the incidence of neonatal hypoglycaemia in at-risk babies. However, currently there are no strategies, including screening, prophylaxis or treatment, that have been proven to reduce the risk of neurodevelopmental impairment in babies at risk of neonatal hypoglycaemia. Further research is urgently needed on strategies to improve neurodevelopmental outcomes in babies at risk of neonatal hypoglycaemia.
Key Messages.
Neonatal hypoglycaemia is common, with half of those babies identified as being at-risk developing low blood glucose concentrations, but low blood glucose concentrations are also common in healthy term babies
Accurate enzymatic analysis of blood glucose concentrations is essential and more cost-effective than strip glucometers
Even mild or transient hypoglycaemia in at-risk babies is associated with later neurodevelopmental impairment
Prophylactic buccal dextrose gel reduces the risk of hypoglycaemia in at-risk babies
Screening, prophylaxis and treatment of neonatal hypoglycaemia have not been shown to improve neurodevelopmental outcomes
Search strategy and selection criteria.
We searched MEDLINE for articles published between Jan 1 2000 to Jun 1 2020, with the terms: (“hypoglycaemia”, “hypoglycemia”, “glucose”, “glycaemia”, “glycemia”, OR “dextrose gel”) AND (“neonate”, “postnatal”, “baby” OR “infant”). We selected more recent publications but did not exclude commonly referenced and highly regarded older publications. We searched only for articles published in English, or those translated into English. We also searched reference lists of articles identified by this strategy and selected those we judged relevant. We searched ClinicalTrials.gov, ISRCTN, ANZCTR and the EU Clinical Trials register for ongoing randomised controlled trials to prevent or treat neonatal hypoglycaemia. We included randomised controlled trials, observational studies, retrospective studies, meta-analyses, guidelines and review articles.
Acknowledgments
The authors are funded in part by the Health Research Council of New Zealand (15/216) and the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (R01HD091075). The funders had no role in the decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health. The authors accept responsibility to submit for publication.
Footnotes
Declaration of Interest
None of the authors have any conflicts of interest to declare.
References
- 1.Cornblath M, Reisner SH. Blood glucose in the neonate and its clinical significance. N Engl J Med. 1965;273(7):378–81. [DOI] [PubMed] [Google Scholar]
- 2.Stenninger E, Flink R, Eriksson B, Sahlen C. Long-term neurological dysfunction and neonatal hypoglycaemia after diabetic pregnancy. Arch Dis Child Fetal Neonatal Ed. 1998;79(3):F174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamkin DH, Committee on Fetus and Newborn. Postnatal glucose homeostasis in late-preterm and term infants. Pediatrics. 2011;127(3):575–9. [DOI] [PubMed] [Google Scholar]
- 4.British Association of Perinatal Medicine. Identification and management of neonatal hypocycaemia in the full term infant: framework for practice. 2017. [Available from: https://www.bapm.org/sites/default/files/files/Identification%20and%20Management%20of%20Neonatal%20Hypoglycaemia%20in%20the%20%20full%20term%20infant%20-%20A%20Framework%20for%20Practice%20revised%20Oct%202017.pdf. [DOI] [PubMed]
- 5.Mckinlay CJD, Alsweiler JM, Ansell JM, Anstice NS, Chase JG, Gamble GD, et al. Neonatal glycemia and neurodevelopmental outcomes at two years. N Engl J Med. 2015;373:1507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey MJ, Rout A, Harding JE, Alsweiler JM, Cutfield WS, CJD M. Prolonged transitional neonatal hypoglycaemia: characterisation of a clinical syndrome. J Perinatol. 2020;In Press. [DOI] [PubMed] [Google Scholar]
- 7.Harris DL, Weston PJ, Harding JE. Incidence of neonatal hypoglycemia in babies identified as at risk. J Pediatr. 2012;161(5):787–91. [DOI] [PubMed] [Google Scholar]
- 8.McKinlay CJD, Alsweiler JM, Anstice NS, Burakevych N, Chakraborty A, Chase JG, et al. Association of neonatal glycemia with neurodevelopmental outcomes at 4.5 Years. JAMA Peds. 2017;07:07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Kempen AAMW, Eskes PF, Nuytemans DHGM, van der Lee JH, Dijksman LM, van Veenendaal NR, et al. Lower versus traditional treatment threshold for neonatal hypoglycemia. N Engl J Med. 2020;382(6):534–44. [DOI] [PubMed] [Google Scholar]
- 10.Aldoretta PW, Carver TD, Hay WW Jr. Maturation of glucose-stimulated insulin secretion in fetal sheep. Biol Neonate. 1998;73(6):375–86. [DOI] [PubMed] [Google Scholar]
- 11.Hay WW Jr., Sparks JW. Placental, fetal, and neonatal carbohydrate metabolism. Clin Obstet Gynecol. 1985;28(3):473–85. [DOI] [PubMed] [Google Scholar]
- 12.Cherrington AD, Chiasson JL, Liljenquist JE, Lacy WW, Park CR. Control of hepatic glucose output by glucagon and insulin in the intact dog. Biochemical Society Symposium. 1978;43:31–45. [PubMed] [Google Scholar]
- 13.Helman A, Cangelosi AL, Davis JC, Pham Q, Rothman A, Faust AL, et al. A nutrient-sensing transition at birth triggers glucose-responsive insulin secretion. Cell Metabolism. 2020;31(5):1004–16.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawdon JM, Ward-Platt MP, Aynsley-Green A. Patterns of metabolic adaption for preterm and term infants in the first neonatal week. Arch Dis Child. 1992;67:357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srinivasan G, Pildes RS, Cattamanchi G, Voora S, Lilien LD. Plasma glucose values in normal neonates: a new look. J Pediatr. 1986;109(1):114–7. [DOI] [PubMed] [Google Scholar]
- 16.Kaiser Jeffrey R., Bai Shasha, Rozance Paul J.. Newborn plasma glucose concentration nadirs by gestational-age group. Neonatology. 2018;113:353–9. [DOI] [PubMed] [Google Scholar]
- 17.Harris D, Weston PJ, Gamble G, Harding JE. Glucose profiles in healthy term infants in the first five days: The Glucose in Well Babies (GLOW) study. J Pediatr. 2020;223:34–41. [DOI] [PubMed] [Google Scholar]
- 18.Denne SC, Kalhan SC. Glucose carbon recycling and oxidation in human newborns. Am J Physiol. 1986;251:E71–7. [DOI] [PubMed] [Google Scholar]
- 19.Vannucci RC, Vannucci SJ. Glucose metabolism in the developing brain. Semin Perinatol. 2000;24(2):107–15. [DOI] [PubMed] [Google Scholar]
- 20.de L Costello AM, Par DK, Manandhar DS, Rajbhandari S, Land JM, Patel N. Neonatal hypoglycaemia in Nepal 2. Availability of alternative fuels. 2000;82:F52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Rooy L, Hawdon J. Nutritional factors that affect the postnatal metabolic adaptation of full-term small- and large-for-gestational-age infants. Pediatrics. 2002;109(3):e42–e. [DOI] [PubMed] [Google Scholar]
- 22.Harris DL, Weston PJ, Harding JE. Lactate, rather than ketones, may provide alternative cerebral fuel in hypoglycaemic newborns. Arch Dis Child Fetal Neonatal Ed. 2014;100(2):F161–4. [DOI] [PubMed] [Google Scholar]
- 23.Stanley CA, Anday EK, Baker L, Delivoria-Papadopolous M. Metabolic fuel and hormone responses to fasting in newborn infants. Pediatrics. 1979;64(5):613–9. [PubMed] [Google Scholar]
- 24.Harris DL, Weston PJ, Williams CE, Pleasants AB, Battin MR, Spooner CG, et al. Cot-side electroencephalography monitoring is not clinically useful in the detection of mild neonatal hypoglycemia. J Pediatr. 2011;159(5):755–60.e1. [DOI] [PubMed] [Google Scholar]
- 25.Harris DL, Weston PJ, Harding JE. Lactate, rather than ketones, may provide alternative cerebral fuel in hypoglycaemic newborns. Archives of Disease in Childhood Fetal and neonatal edition. 2015;100(2):F161–F4. [DOI] [PubMed] [Google Scholar]
- 26.Avagliano L, Mascherpa M, Massa V, Doi P, Bulfamante GP. Fetal pancreatic Langerhans islets size in pregnancies with metabolic disorders. J Matern Fetal Neonatal Med. 2018:1–6. [DOI] [PubMed] [Google Scholar]
- 27.Ford SP, Zhang L, Zhu M, Miller MM, Smith DT, Hess BW, et al. Maternal obesity accelerates fetal pancreatic beta-cell but not alpha-cell development in sheep: prenatal consequences. Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Limesand SW, Rozance PJ. Fetal adaptations in insulin secretion result from high catecholamines during placental insufficiency. J Physiol. 2017;595(15):5103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boehmer BH, Limesand SW, Rozance PJ. The impact of IUGR on pancreatic islet development and beta-cell function. J Endocrinol. 2017;235(2):R63–r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bateman BT, Patorno E, Desai RJ, Seely EW, Mogun H, Maeda A, et al. Late pregnancy beta blocker exposure and risks of neonatal hypoglycemia and bradycardia. Pediatrics. 2016;138(3):09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gyamfi-Bannerman C, Thom EA, Blackwell SC, Tita ATN, Reddy UM, Saade GR, et al. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uquillas KR, Lee RH, Sardesai S, Chen E, Ihenacho U, Cortessis VK, et al. Neonatal hypoglycemia after initiation of late preterm antenatal corticosteroids. J Perinatol. 2020;14:14. [DOI] [PubMed] [Google Scholar]
- 33.Schaefer-Graf UM, Rossi R, Buhrer C, Siebert G, Kjos SL, Dudenhausen JW, et al. Rate and risk factors of hypoglycemia in large-for-gestational-age newborn infants of nondiabetic mothers. Am J Obstet Gynecol. 2002;187(4):913–7. [DOI] [PubMed] [Google Scholar]
- 34.Brand PLP, Molenaar NLD, Kaaijk C, Wierenga WS. Neurodevelopmental outcome of hypoglycaemia in healthy, large for gestational age, term newborns. Arch Dis Child. 2005;90(1):78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hawdon JM, Beer J, Sharp D, Upton M, N. H. S. Improvement Patient Safety Programme ‘Reducing Term Admissions to Neonatal Units’. Neonatal hypoglycaemia: learning from claims. Arch Dis Child Fetal Neonatal Ed. 2017;102(2):F110–F5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cartwright RD, Anderson NH, Sadler LC, Harding JE, McCowan LME, McKinlay CJD. Neonatal morbidity and small and large size for gestation: a comparison of birthweight centiles. J Perinatol. 2020;40(5):732–42. [DOI] [PubMed] [Google Scholar]
- 37.Narvey MR, Marks SD. The screening and management of newborns at risk for low blood glucose. Paed Child Health. 2019;24:536–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Danish Paediatric Society. Neonatal hypogycaemia, national guideline 2014. [Available from: http://paediatri.dk.web14.redhost.dk/neonatologi-vejl.
- 39.The Baby Friendly Initiative. Guidance on the development of policies and guidelines for the prevention and management of hypoglycaemia of the newborn. United Kingdom: UNICEF; 2013. [Available from: https://www.unicef.org.uk/babyfriendly/wp-content/uploads/sites/2/2010/10/hypo_policy.pdf. [Google Scholar]
- 40.Nagy T, Hegarty JE, Alsweiler JM. Audit of neonatal hypoglycaemia screening in at-risk babies. Paediatric Society of New Zealand; Palmerston North: 2012. [Google Scholar]
- 41.Maayan-Metzger A, Lubin D, Kuint J. Hypoglycemia rates in the first days of life among term infants born to diabetic mothers. Neonatology. 2009;96(2):80–5. [DOI] [PubMed] [Google Scholar]
- 42.Pildes R, Forbes AE, O’Connor SM, Cornblath M. The incidence of neonatal hypoglycemia—a completed survey. J Pediatr. 1967;70(1):76–80. [DOI] [PubMed] [Google Scholar]
- 43.Kaiser JR, Bai S, Gibson N, Holland G, Lin T, Swearingen CJ, et al. Association between transient newborn hypoglycemia and fourth-grade achievement test proficiency: a population-based study. JAMA Peds. 2015;169(10):913–21. [DOI] [PubMed] [Google Scholar]
- 44.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6. [DOI] [PubMed] [Google Scholar]
- 45.Shah R, Harding J, Brown J, McKinlay C. Neonatal glycaemia and neurodevelopmental outcomes: a systematic review and meta-analysis. Neonatology. 2019;115(2):116–26. [DOI] [PubMed] [Google Scholar]
- 46.Wickstrom R, Skiold B, Petersson G, Stephansson O, Altman M. Moderate neonatal hypoglycemia and adverse neurological development at 2–6 years of age. Eur J Epidemiol.33(10):1011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Filan PM, Inder TE, Cameron FJ, Kean MJ, Hunt RW. Neonatal hypoglycemia and occipital cerebral injury. J Pediatr. 2006;148(4):552–5. [DOI] [PubMed] [Google Scholar]
- 48.Burns CM, Rutherford MA, Boardman JP, Cowan FM. Patterns of cerebral injury and neurodevelopmental outcomes after symptomatic neonatal hypoglycemia. Pediatrics. 2008;122(1):65–74. [DOI] [PubMed] [Google Scholar]
- 49.Longmore DK, Barr ELM, Wilson AN, Barzi F, Kirkwood M, Simmonds A, et al. Associations of gestational diabetes and type 2 diabetes during pregnancy with breastfeeding at hospital discharge and up to 6 months: the PANDORA study. Diabetologia. 2020;10:10. [DOI] [PubMed] [Google Scholar]
- 50.Jonsdottir RB, Jonsdottir H, Skuladottir A, Thorkelsson T, Flacking R. Breastfeeding progression in late preterm infants from birth to one month. Matern Child Nutr. 2020;16(1):e12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harris DL, Gamble GD, Weston PJ, Harding JE. What happens to blood glucose concentrations after oral treatment for neonatal hypoglycemia? J Pediatr. 2017;190:136–41. [DOI] [PubMed] [Google Scholar]
- 52.Sundercombe SL, Raynes-Greenow CH, Turner RM, Jeffery HE. Do neonatal hypoglycaemia guidelines in Australia and New Zealand facilitate breast feeding? Midwifery. 2014;30(12):1179–86. [DOI] [PubMed] [Google Scholar]
- 53.Wilson JMG, Jungner G. Principles and practice of screening for disease. WHO Chron. 1968;22(11):281–393. [PubMed] [Google Scholar]
- 54.Papadea C, Foster J, Grant S, SA B, JC C, WM S, et al. Evaluation of the i-STAT portable clinical analyzer for point-of-care blood testing in the intensive care units of a University Children’s Hospital. Ann Clin Lab Sci. 2002;32(3):231–43. [PubMed] [Google Scholar]
- 55.Harris DL, Weston PJ, Battin MR, Harding JE. A survey of the management of neonatal hypoglycaemia within the Australian and New Zealand Neonatal Network. J Paediatr Child Health. 2014;50(10):E55–62. [DOI] [PubMed] [Google Scholar]
- 56.Dixon KC, Ferris RL, Marikar D, Chong M, Mittal A, Manikam L, et al. Definition and monitoring of neonatal hypoglycaemia: A nationwide survey of NHS England Neonatal Units. Arch Dis Child Fetal Neonatal Ed. 2017;102(1):F92–F3. [DOI] [PubMed] [Google Scholar]
- 57.Inman M, Parker K, Strueby L, Lyon AW, Lyon ME. A simulation study to assess the effect of analytic error on neonatal glucose measurements using the Canadian Pediatric Society Position Statement action thresholds. J Diabetes Sci Technol. 2020;14(3):519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ranger M, Chau CM, Garg A, Woodward TS, Beg MF, Bjornson B, et al. Neonatal pain-related stress predicts cortical thickness at age 7 years in children born very preterm. PLOS ONE. 2013;8:e76702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glasgow MJ, Harding JE, Edlin R, For the CHYLD Study Team. Cost analysis of cot-side screening methods for neonatal hypoglycaemia. Neonatology. 2018;114(2):155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McKinlay CJD, Chase JG, Dickson J, Harris DL, Alsweiler JM, Harding JE. Continuous glucose monitoring in neonates: a review. Matern Health Neonatol Perinatol. 2017(3):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tiberi E, Cota F, Barone G, Perri A, Romano V, Iannotta R, et al. Continuous glucose monitoring in preterm infants: evaluation by a modified Clarke error grid. Ital J Peds. 2016;42:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harris DL, Battin MR, Weston PJ, Harding JE. Continuous glucose monitoring in newborn babies at risk of hypoglycemia. J Pediatr. 2010;157(2):198–202. [DOI] [PubMed] [Google Scholar]
- 63.Uettwiller F, Chemin A, Bonnemaison E, Favrais G, Saliba E, Labarthe F. Real-time continuous glucose monitoring reduces the duration of hypoglycemia episodes: a randomized trial in very low birth weight neonates. PLoS ONE. 2015;10(1):e0116255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thornton PS, Stanley CA, De Leon DD, Harris D, Haymond MW, Hussain K, et al. Recommendations from the Pediatric Endocrine Society for evaluation and management of persistent hypoglycemia in neonates, infants, and children. J Pediatr. 2015;167(2):238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koh THHG, Aynsley-Green A, Tarbit M, Eyre JA. Neural dysfunction during hypoglycemia. Arch Dis Child. 1988;63:1353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lucas A, Morley R, Cole TJ. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. BMJ. 1988;297(6659):1304–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tin W, Brunskill G, Kelly T, Fritz S. 15-year follow-up of recurrent “hypoglycemia” in preterm infants. Pediatrics. 2012;130(6):e1497–503. [DOI] [PubMed] [Google Scholar]
- 68.Chertok IR, Raz I, Shoham I, Haddad H, A W. Effects of early breastfeeding on neonatal glucose levels of term infants born to women with gestational diabetes. J Hum Nutr Diet. 2009;22(2):166–9. [DOI] [PubMed] [Google Scholar]
- 69.Bragg JJ, Green R, Holzman IR. Does early enteral feeding prevent hypoglycemia in small for gestational age neonates? J Neonatal Perinatal Med. 2013;6(2):131–5. [DOI] [PubMed] [Google Scholar]
- 70.Zhou Y, Bai S, Bornhorst JA, Elhassan NO, Kaiser JR. The effect of early feeding on initial glucose concentrations in term newborns. J Pediatr. 2017;181:112–5. [DOI] [PubMed] [Google Scholar]
- 71.Hegarty JE, Harding JE, Gamble GD, Crowther CA, Edlin R, Alsweiler JM. Prophylactic oral dextrose gel for newborn babies at risk of neonatal hypoglycaemia: a randomised controlled dose-finding trial (the Pre-hPOD Study). PLoS Med. 2016;13(10):e1002155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Griffith R, Hegarty JE, Alsweiler JM, Gamble GD, May R, McKinlay CJD, et al. Two-year outcomes after dextrose gel prophylaxis for neonatal hypoglycaemia. Archives of Disease in Childhood - Fetal and Neonatal Edition. 2020:fetalneonatal-2020–320305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harding JE, Hegarty JE, Crowther CA, Edlin R, Gamble G, Alsweiler JM. Randomised trial of neonatal hypoglycaemia prevention with oral dextrose gel (hPOD): study protocol. BMC Pediatr. 2015;15:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coors SM, Cousin JJ, Hagan JL, Kaiser JR. Prophylactic dextrose gel does not prevent neonatal hypoglycemia: a quasi-experimental pilot study. J Pediatr. 2018;198:156–61. [DOI] [PubMed] [Google Scholar]
- 75.Surachaidungtavil S, Chanvorachote P, Suksumek N. A randomized control trial of oral sucrose solution for prevention of hypoglycemia in high risk infants. In Vivo. 2020;34(3):1493–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Forster DA, Moorhead AM, Jacobs SE, Davis PG, Walker SP, McEgan KM, et al. Advising women with diabetes in pregnancy to express breastmilk in late pregnancy (Diabetes and Antenatal Milk Expressing [DAME]): a multicentre, unblinded, randomised controlled trial. Lancet.389(10085):2204–13. [DOI] [PubMed] [Google Scholar]
- 77.Uvnas-Moberg K, Widstrom AM, Marchini G, Winberg J. Release of GI hormones in mother and infant by sensory stimulation. Acta Paediatrica Scandinavica. 1987;76(6):851–60. [DOI] [PubMed] [Google Scholar]
- 78.Harris DL, Weston PJ, Signal M, Chase JG, Harding JE. Dextrose gel for neonatal hypoglycaemia (the Sugar Babies Study): a randomised, double-blind, placebo-controlled trial. The Lancet. 2013;382(9910):2077–83. [DOI] [PubMed] [Google Scholar]
- 79.Harris DL, Alsweiler JM, Ansell JM, Gamble GD, Thompson B, Wouldes TA, et al. Outcome at 2 years after dextrose gel treatment for neonatal hypoglycemia: Follow-Up of a randomized trial. J Pediatr. 2016;170:54–9 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gregory K, Turner D, Benjamin CN, Monthe-Dreze C, Johnson L, Hurwitz S, et al. Incorporating dextrose gel and feeding in the treatment of neonatal hypoglycaemia. Archives of Disease in Childhood Fetal and neonatal edition. 2019. [DOI] [PubMed] [Google Scholar]
- 81.Rawat M, Chandrasekharan P, Turkovich S, Barclay N, Perry K, Schroeder E, et al. Oral dextrose gel reduces the need for intravenous dextrose therapy in neonatal hypoglycemia. Biomed. 2016;1(3):Sep–Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ter M, Halibullah I, Leung L, Jacobs S. Implementation of dextrose gel in the management of neonatal hypoglycaemia. J Paediatr Child Health. 2017;53(4):408–11. [DOI] [PubMed] [Google Scholar]
- 83.LeBlanc S, Haushalter J, Seashore C, Wood KS, Steiner MJ, Sutton AG. A quality-improvement initiative to reduce NICU transfers for neonates at risk for hypoglycemia. Pediatrics. 2018;141(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Makker K, Alissa R, Dudek C, Travers L, Smotherman C, Hudak ML. Glucose gel in infants at risk for transitional neonatal hypoglycemia. Am J Perinatol. 2018;35(11):1050–6. [DOI] [PubMed] [Google Scholar]
- 85.Burakevych N, McKinlay CJD, Harris DL, Alsweiler JM, Harding JE. Factors influencing glycaemic stability after neonatal hypoglycaemia and relationship to neurodevelopmental outcome. Sci Rep. 2019;9(1):8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alsweiler JM, Harding J, Crowther C, Woodall SM, “The Oral Dextrose Gel to Treat Neonatal Hypoglycaemia Clinical Practice Guidelines” Panel. Oral dextrose gel to treat neonatal hypoglycaemia: Clinical Practice Guidelines. Auckland: University of Auckland; 2015. Report No.: http://hdl.handle.net/2292/26266. [Google Scholar]
- 87.Wackernagel D, Gustafsson A, Edstedt Bonamy AK, Reims A, Ahlsson F, Elfving M, et al. Swedish national guideline for prevention and treatment of neonatal hypoglycaemia in newborn infants with gestational age >/=35 weeks. Acta Paediatr. 2020;109(1):31–44. [DOI] [PubMed] [Google Scholar]
- 88.Glasgow MJ, Harding JE, Edlin R, Children with H, Their Later Development Study T. Cost analysis of treating neonatal hypoglycemia with dextrose gel. J Pediatr. 2018;198:151–5.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Glasgow M, Edlin R, JE H. Cost burden and net monetary benefit loss of neonatal hypoglycaemia. BMC Health Services Research. 2020. In revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Glasgow M, Edlin R, Harding JE. Cost-utility analysis of prophylactic dextrose gel vs standard care for neonatal hypoglycemia in at-risk infants. J Pediatr. 2020(Available online 4 July):Available online 4 July. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lilien LD, Grajwer LA, Pildes RS. Treatment of neonatal hypoglycemia with continuous intravenous glucose infusion. J Pediatr. 1977;91(5):779–82. [DOI] [PubMed] [Google Scholar]
- 92.Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest. 2007;117(4):910–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ennis K, Dotterman H, Stein A, Rao R. Hyperglycemia accentuates and ketonemia attenuates hypoglycemia-induced neuronal injury in the developing rat brain. Pediatr Res. 2015;77(1–1):84–90. [DOI] [PubMed] [Google Scholar]
- 94.Alsaleem M, Saadeh L, Kumar VHS, Wilding GE, Miller L, Mathew B. Continued enteral feeding is beneficial in hypoglycemic infants admitted to intensive care for parenteral dextrose therapy. Global pediatric health. 2019;6:2333794×. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Smolkin T, Makhoul JS, Elias R, Farah F, Kugelman A, Dallashi M, et al. Experience with intramuscular glucagon for infants with early neonatal hypoglycemia. J Pediatr Endocrinol Metab. 2019;32(9):1023–6. [DOI] [PubMed] [Google Scholar]
- 96.Miralles RE, Lodha A, Perlman M, Moore AM. Experience with intravenous glucagon infusions as a treatment for resistant neonatal hypoglycemia. Arch Pediatr Adolesc Med. 2002;156(10):999–1004. [DOI] [PubMed] [Google Scholar]
- 97.Hawkes CP, Lado JJ, Givler S, De Leon DD. The effect of continuous intravenous glucagon on glucose requirements in infants with congenital hyperinsulinism. JIMD reports. 2019;45:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miralles RE, Lodha A, Perlman M, Moore AM. Experience with intravenous glucagon infusions as a treatment for resistant neonatal hypoglycemia. Arch Pediatr Adolesc Med. 2002;156(10):999–1004. [DOI] [PubMed] [Google Scholar]
- 99.Hirose H, Maruyama H, Ito K, Kido K, Koyama K, Saruta T. Effects of diazoxide on alpha- and beta-cell function in isolated perfused rat pancreas. Diabetes Res Clin Pract. 1994;25(2):77–82. [DOI] [PubMed] [Google Scholar]
- 100.Hoe FM, Thornton PS, Wanner LA, Steinkrauss L, Simmons RA, Stanley CA. Clinical features and insulin regulation in infants with a syndrome of prolonged neonatal hyperinsulinism. J Pediatr. 2006;148(2):207–12. [DOI] [PubMed] [Google Scholar]
- 101.Balachandran B, Mukhopadhyay K, Sachdeva N, Walia R, Attri SV. Randomised controlled trial of diazoxide for small for gestational age neonates with hyperinsulinaemic hypoglycaemia provided early hypoglycaemic control without adverse effects. Acta Paediatr. 2018;107(6):990–5. [DOI] [PubMed] [Google Scholar]
- 102.Timlin MR, Black AB, Delaney HM, Matos RI, CS P. Development of pulmonary hypertension during treatment with diazoxide: a case series and literature review. Pediatr Cardiol. 2017;38(6):1247–50. [DOI] [PubMed] [Google Scholar]
- 103.Hussain K. Diagnosis and management of hyperinsulinaemic hypoglycaemia of infancy. Horm Res. 2008;69(1):2–13. [DOI] [PubMed] [Google Scholar]
- 104.Rajay A, JE H. Variations in New Zealand and Australian guidelines for the management of neonatal hypoglycaemia – a secondary analysis of data from the hPOD trial. J Paediatr Child Health. 2020. submitted. [DOI] [PubMed] [Google Scholar]
- 105.Sundercombe SL, Raynes-Greenow CH, Carberry AE, Turner RM, Jeffery HE. Audit of a clinical guideline for neonatal hypoglycaemia screening. J Paediatr Child Health. 2013;49(10):833–8. [DOI] [PubMed] [Google Scholar]
- 106.Alsweiler JM, Gomes L, Nagy T, Gilchrist CA, Hegarty JE. Adherence to neonatal hypoglycaemia guidelines: A retrospective cohort study. J Paediatr Child Health. 2019;22:22. [DOI] [PubMed] [Google Scholar]
- 107.Harris DL, Weston PJ, Harding JE. Point-of-care measurements of blood ketones in newborns. Archives of Disease in Childhood Fetal and neonatal edition. 2019;104(5):F544–F6. [DOI] [PubMed] [Google Scholar]
- 108.Askie LM, Darlow BA, Finer N, Schmidt B, Stenson B, Tarnow-Mordi W, et al. Association between oxygen saturation targeting and death or disability in extremely preterm infants in the Neonatal Oxygenation Prospective Meta-analysis Collaboration. JAMA. 2018;319(21):2190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]


