Abstract
Background:
Neuroinflammation and cerebral edema development following severe TBI affect subsequent cognitive recovery. Independent of its anticoagulant effects, antithrombin III (AT-III) has been shown to block neurovascular inflammation after severe TBI, reduce cerebral endothelial-leukocyte interactions and decrease blood brain barrier permeability. We hypothesized that AT-III administration after TBI would improve post-TBI cognitive recovery, specifically enhancing learning, and memory.
Methods:
Fifteen CD1 male mice were randomized to undergo severe TBI (controlled cortical impact, CCI: 6 m/sec velocity, 1 mm depth, 3 mm diameter) or sham craniotomy (SHAM) and received either intravenous AT-III (250 IU/kg) or vehicle (VEH:saline) 15 minutes and 24 hours post-TBI. Animals underwent Morris water maze testing from 6–14 days post-injury consisting of cued learning trials (platform visible), spatial learning trials (platform invisible, spatial cues present), and probe (memory) trials (platform removed, spatial cues present). Intergroup differences were assessed by the Kruskal-Wallis test (p < 0.05).
Results:
Morris water maze testing demonstrated that cumulative cued learning (overall mean time in seconds to reach the platform on days 6–8) was worst in CCI+VEH animals (26.1 ± 2.4s) compared to CCI+ATIII counterparts (20.3 ± 2.1s, p<0.01). Cumulative non-cued spatial learning was also worst in the CCI+VEH group (23.4 ± 1.8s) but improved with AT-III (17.6 ± 1.5s, p<0.01. In probe trials, AT-III failed to significantly improve memory ability. SHAM animals demonstrated preserved learning and memory compared to all CCI counterparts (p < 0.05).
Conclusions:
AT-III improves neurocognitive recovery weeks after TBI. This improvement is particularly related to improvement in learning but not memory function. Pharmacologic support of enhanced learning may support new skill acquisition or re-learning to improve outcomes after TBI.
INTRODUCTION
Traumatic brain injury (TBI) is a leading cause of mortality and morbidity in the developed world. An estimated 1.5–2 million Americans suffer from TBI each year, resulting in more than 50,000 annual TBI-related deaths(1). Although advanced basic and translational research has helped reduce mortality, TBI remains a leading cause of disability in young individuals as few post-injury therapeutic interventions are currently available to enhance functional recovery and reduce post-convalescence dependence(2)(3). Post-TBI lifetime care exerts a tremendous financial strain on both health systems and families. Important outcomes that permeate post-TBI recovery are related to both primary and secondary brain injury, the latter of which may be potentially addressed during inpatient acute care.
Ultimate outcomes are likely better defined by events and pathophysiological occurrences in the hours and days that follow TBI (secondary brain injury) than by the severity and nature of the initial impact resulting in TBI (primary brain injury)(4). Mechanisms underlying the impact of secondary injury are still subject to research but are known to include inflammatory, excitotoxic, and apoptotic processes(4), with neuroinflammatory events that activate glial cells and trafficking neutrophils, and upregulate both local and regional inflammatory mediators. Targeting these inflammatory cascades has been a promising pharmacological therapeutic pathway to alter the progression of the secondary injury following TBI(5)(6)(7). Though few therapeutics have been found successful in altering outcomes after TBI some do exist and have been recently reported such as the use of beta blockade post TBI, reducing mortality and improving outcomes (42).
Our previous work demonstrated that certain anticoagulants possess anti-inflammatory properties that can reduce leucocyte-mediated cerebral inflammation and swelling, both key processes that impact secondary brain injury. In particular heparin, but also antithrombin III (AT-III), demonstrate post-TBI neuroprotection by blunting neuroinflammation(8,9), through effects that appear to be independent of their potent anticoagulant properties(15). Antithrombin alters microvasculature cellular interactions and improves recovery from tissue injury in animal models of ischemia-reperfusion, acute lung injury, and sepsis(10–13)(14). Indeed, post-TBI AT-III therapy reduces in vivo penumbral neurovasculature recruitment of circulating leucocytes while mitigating both local microvascular permeability and cerebral edema(15). While this work offers the promise of acute functional recovery in injured animals, it is unknown if AT-III affects post-TBI cognitive recovery weeks after injury. Accordingly, we hypothesized that AT-III administered after blunt TBI would improve cognitive recovery weeks after injury as demonstrated by improved learning and memory.
MATERIALS AND METHODS
Experimental Design, TBI Model and Study Groups
Experimental procedures were conducted with the approval of the University of Pennsylvania Institutional Animal Care and Use Committee. CD1 adult male mice (25–30 g) (Charles River Laboratories, Wilmington, MA) were acclimatized in standard housing with water and chow ad libitum for seven days before experiments after which they underwent sham craniotomy or craniotomy accompanied by controlled cortical impact (CCI), a well-validated severe TBI rodent model(17)(18). Mice were anesthetized with intraperitoneal ketamine (Hospira, Lake Forest, IL), xylazine (Akorn, Decatur, IL), and acepromazine (Boehringer Ingelheim, St. Joseph, MO) (KXA: 100, 10, 1 mg/kg, respectively) followed by subcutaneous bupivacaine (0.5 mg/mL) for longer term analgesia. After creation of a left-sided, 4-mm craniotomy between bregma and lambda sutures, the exposed left parietotemporal cortex was injured via a controlled cortical impactor (AMS201, AmScien Instruments, Richmond, VA). CCI settings (3-mm-diameter impactor tip, impact velocity of 6 m/s, and cortical deformation depth of 1 mm) replicated severe TBI(18).
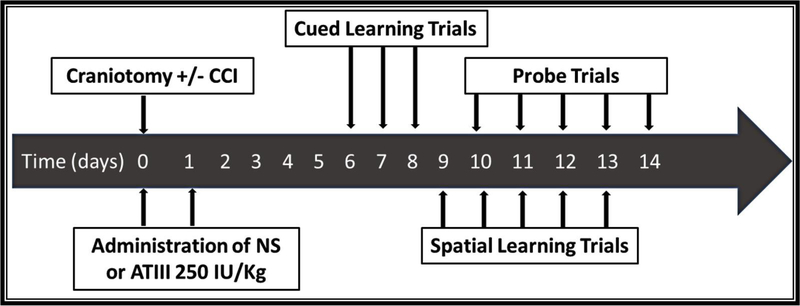
Rodents were then randomly allocated to receive either: 1) intravenous (via femoral vein) pharmaceutical grade AT-III (250 IU/kg, donated by Grifols S.A., Durham, NC) or 2) an equal volume of normal saline IV (0.9% NS; Baxter; Deerfield, IL) as a vehicle (VEH). Doses were administered 0.5 and 24 hours after CCI (Figure 1) as determined by previous published reports and the manufacturer’s recommendations(14–16).
Figure 1.
Timeline of experimental procedures. CCI: Controlled Cortical Impact, ATIII: Antithrombin III, NS: Normal Saline.
Fifteen (15) mice were randomized into three groups: (1) sham craniotomy (no CCI) plus vehicle (SHAM, n = 5); (2) TBI and vehicle (CCI+VEH, n =5); (3) TBI and AT-III (CCI+ATIII, n =5).
Morris Water Maze Exercises
Learning and memory were evaluated in a Morris water maze (MWM) using our previously published protocol(19)(20). After a recovery period of 6 days, each animal underwent daily MWM trials of learning and memory for 9 consecutive days. The apparatus consisted in a black circular pool (100 cm diameter, 50 cm height) filled with 21°C water and fashioned with a black cylindrical platform (10 cm in diameter, 23.5 cm height) differentially submerged below or at the water surface. Experiments consisted of three types of swimming trials to assess differential learning and memory: (1) cued learning trials (days 6–8 post-TBI), (2) spatial learning trials (days 9–13 post-TBI), and (3) probe memory trials (days 10–14 post-TBI) (Figure 1). All swimming trials were conducted and scored by the same operator, at the same time of day on all experiment days incorporating a computerized video-tracking and recording system over the pool to facilitate analysis (Ethovision, Noldus, Leesburg, VA). Mice received a 10-minute rest period between trials, passively being warmed by a heating lamp.
Cued Learning Trials
Four daily cued trials on days 6 to 8 post-TBI introduced animals to the water and pool. Cued trials served to reduce stress and to establish the MWM goal (navigation to the platform). The exposed platform was clearly visible to the swimming animal, adorned with a colorful marker and placed in one of four pool quadrants (southwest, southeast, northwest, northeast) randomly changed every day with no other wall visual cues provided. Animals were placed in the pool, in 1 of 4 randomly chosen locations relative to platform location. The video-tracking system collected performance parameters including time to reach the platform or the region around the platform (a predefined circular area around the platform), average swimming velocity, distance and duration of swimming in each region. Animals were allowed 60 seconds to locate, and then 15 seconds to stand on the platform prior to pool removal and passive warming. Animals that did not reach the platform within 60 seconds were gently guided to the platform and allowed to remain on it for 15 seconds before removal from the pool.
Spatial Learning Trials
On post-craniotomy days 9 to 13, daily spatial learning trials evaluated the animals’ ability to use environmental visual cues to locate and reach the platform. The MWM configuration was similar to that of the cued trials except that the platform remained in a fixed location (northwest) but hidden out of view as it was now submerged, thoughnimals reaching the platform could stand on it and not be submerged. Colored pictograms were mounted on the MWM walls (north, south, east, and west) for animals to use for localizing of the submerged platform. Animals were again placed in the water facing outwards from various points in relation to the platform so that they could see the wall pictograms but not the platform before entering the maze. Sixty seconds of swimming time and 15 seconds on the platform were again allowed before rest under the warming lamp. The same parameters as in the cued learning trials were collected using the video-tracking system.
Probe Trials (Long-Term Memory)
On post-craniotomy days 10 to 14, daily probe trials were conducted immediately preceding spatial learning trials if both trials were conducted on the same day. Probe trials assessed long-term memory in navigating to the platform’s prior location hinging on incorporating the prior days’ visual cues into memory. During 30-second intervals, animals were allowed to freely swim in the pool now devoid of a platform. The duration, frequency, and time to first reach the area where the platform had been previously located during spatial trials were recorded to assess animals’ cognitive recovery related to memory.
Statistical Analysis
All data are presented as mean ± SEM. Statistical analyses were performed using SPSS (SPSS, Chicago, IL, 2019). Differences between group means were compared using the Kruskal-Wallis test; significance was assumed for p < 0.05.
RESULTS
Cued Learning Trials
In the cued learning trials, swimming velocity was similar in CCI+VEH and CCI+ATIII groups (26.5 ± 1.2 cm/s and 28.4 ± 1.1 cm/s, respectively); SHAM animals swam more rapidly (30.5 ± 2.3, p < 0.05 vs. CCI+VEH). The overall swimming distance was similar in both injured groups confirming the persistent sensorimotor effects from the TBI injury model.
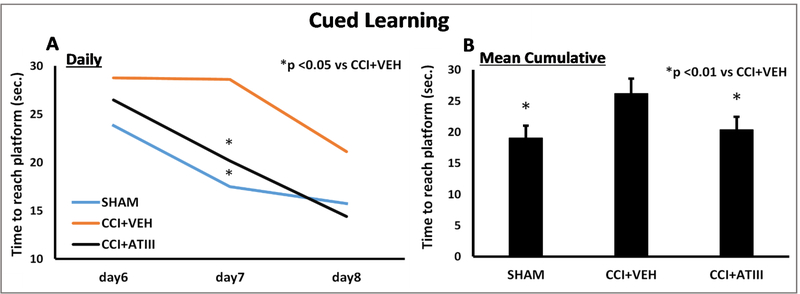
Learning patterns also differed between groups. Similar to SHAM, CCI+ATIII time to reach the exposed platform was less than the time for CCH+VEH counterparts (Figure 2A). CCI+ATIII animals began cued trials outperforming CCI+VEH and eventually reached SHAM times by day 7. CCI+VEH animals only began to improve time-to-platform performance at the time that CCI+ATIII had demonstrated performance similar to SHAM (day 8). Relatedly, mean time to reach the platform was greatest in CCI+VEH (28.6 ± 4.5 s). This time was nearly 30% greater than for either CCI+ATIII (20.1 ± 3.1s, p <0.05 vs. CCI+VEH) or SHAM (17.5 ± 3.5s, p <0.05 vs CCI+VEH) groups. By the end of cued learning trials, cumulative cued learning (overall mean group time to reach the platform throughout days 6 to 8) was worst in CCI+VEH (26.1 ± 2.4s) compared to both CCI+ATIII (20.3 ± 2.1s, p<0.01) and SHAM (19.0 ± 2.0s, p<0.01), without significant differences between the latter two groups.
Figure 2.
Cued Learning Trials (A) Daily assessment of the mean time needed for each group to reach the visible cued platform. Both SHAM and CCI+ATIII groups improved with time and took less time to reach the platform learning faster than the CCI+VEH group which was slower to improve and cut down their mean time needed to reach the platform. The difference was particularly manifest on the 7th day after injury (p<0.05).
(B) Cumulative cued learning (mean group times across all daily cued learning trials) was greatest in the CCI+VEH group, significantly worse than in CCI+ATIII and SHAM counterparts (p<0.01).
Spatial Learning Trials
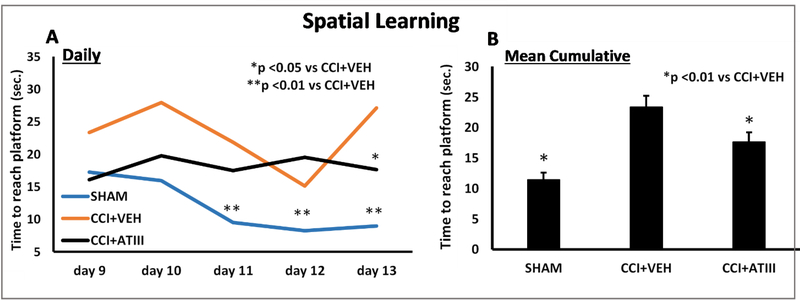
In the spatial learning trials occurring on post-craniotomy days 9–13, all groups now demonstrated similar swimming velocities. However, overall swim distance was greater in CCI+VEH (313.0 ± 25.7 cm) and CCI+ATIII (306.9 ± 27.9 cm) compared to SHAM (165.7 ± 18.2 cm, p<0.01 vs both CCI groups). CCI+VEH animals performed worst and demonstrated the slowest improvement. On, day 13 (the last day of spatial learning trials) CCI+VEH swam longer (27.1 ± 3.8 s) than CCI+AT-III (17.6 ± 3.9s, p<0.05) and SHAM (8.9 ± 1.6 seconds, p<0.01 vs. CCI+VEH; Figure 3A). Over the course of all 5 days’ spatial learning trials, mean group cumulative swimming time was longest in CCI+VEH (23.4 ± 1.8 seconds) compared to both CCI+ATIII (17.6 ± 1.5 seconds, p<0.01) and SHAM (11.4 ± 1.1 seconds, p<0.01) (Figure 3B).
Figure 3.
Spatial Learning Trials. (A) Of all groups, CCI + VEH animals showed the slowest improvement of reducing the time to reach the platform. The difference was most significant on the 13th after injury (p<0.05 vs CCI+ATIII and p<0.01 vs SHAM).
(B) Cumulative spatial learning. The CCI+VEH group’s performance was significantly worse than with AT-III treatment which resulted in times comparable to uninjured SHAM counterparts.
Probe Trials
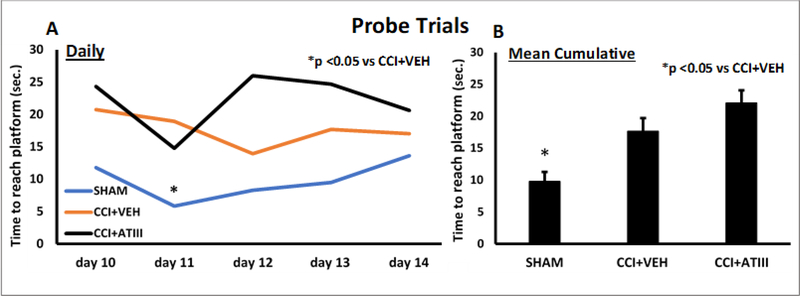
Probe trials disclosed no differences between the two injured groups in swimming velocity or distance, nor time to reach platform’s prior location (Figure 4). Uninjured animals consistently fared better and demonstrated faster times to reach the platform’s prior location.
Figure 4.
Probe Memory Trials. There were no significant differences in any of the parameters measured among the 2 injured groups. Uninjured animals consistenly fared better and demonstrated faster times to reach the platforms prior location.
DISCUSSION
This study demonstrates that post-TBI ATIII improves certain aspects of cognitive recovery - mainly cued and spatial learning - but not memory in a murine model of blunt brain injury.
Despite decades of clinical and basic science research initiatives, there is, as of yet, no therapy that is routinely utilized to improve cognitive outcomes following TBI, especially with respect to recovery of cognitive impairment. Therefore, this model and its underpinning mechanism illuminate a potential pathway to support enhanced cognitive performance following blunt TBI. Moreover, since AT-III has a clearly defined pharmacology and a newly appreciated interface with microvascular surfaces, its ability to impact outcomes after injury is appropriately framed by our burgeoning appreciation for endotheliopathy on either side of the blood-brain barrier(40).
Inflammation that arises as a result of the primary brain injury is a key event in understanding the patient’s ultimate trajectory. That such inflammation serves to prime the injured cerebral tissues for secondary injuriants appears to be plausible. This is especially true as a permeable blood-brain barrier coupled with neutrophil influx drive cerebral edema and intracranial hypertension, accelerating the risk of herniation if not successfully addressed(21). Lesser degrees of injury support survival but may do so at the risk of motor, sensory or cognitive compromise. It is in this patient population in particular, that early effective therapy may be singularly effective in reducing poor functional outcomes, including those related to the genesis of auto-antibodies (41).
Early after TBI, the BBB temporarily becomes more permeable, allowing extravasation of fluid and activated circulating leukocytes into the adjacent cerebral parenchyma with subsequent activation of resident microglia and astrocytes(22–24). Disruption to the hippocampal and cortical regions alters memory recovery in both human and animal TBI studies(25). The hippocampus receives spatial and non-spatial information about the environment via projections from the medial and lateral entorhinal cortex(26). Hippocampal injury would thus be anticipated to result in deficits of spatial, learning, and memory capabilities. The MWM reliably evaluates hippocampal-related spatial navigation and references memory abilities rendering it an ideal method to assess the role of pharmacologic therapies on potentially relevant long-term outcomes(19, 27, 28).
Controlled cortical impact (CCI) is one of the most frequently used TBI models to study both early and late cognitive impairment after brain injury. A CCI injury model, demonstrated a directly proportional relationship between the severity of the injury and MWM performance at 1–2 weeks post-TBI(28). Both learning and memory failure was noted regardless of injury severity and irrespective of the absence of grossly identifiable tissue damage. Local injury may lead to tissue damage or death but more importantly, can lead to integrative deficits in more remote domains as well. We previously demonstrated how CCI initiates specific neuroinflammatory changes that are immunohistochemically visible in the penumbral area of the injury and the hippocampus (29) cementing the relationship of injury remote from the site of impact. These observations underscore the need for a systemic approach to blunt TBI that complements local measures that may include hematoma evacuation as appropriate.
Antithrombin III is a plasma glycoprotein synthesized in the liver, and is the primary physiological inhibitor of thrombin and other serine proteases of the coagulation cascade. While the approved clinical use is limited to treating AT-III deficiency-related clotting, AT-III exerts potent anti-inflammatory effects(30–32). Inflammation reduction occurs most prominently at twice the normal plasma concentration of AT-III, appears to be endothelially mediated and is related to augmented prostacyclin production (33). Reduced reactive oxygen species and neutrophil-derived protease release are two described methods of inflammation reduction (33–34).
Antithrombin III has been demonstrated to improve outcome in a number of tissue injury models, not just following blunt TBI as in the current study. In a rodent sepsis model, AT-III reduced both organ failure and mortality (35). Using intravital microscopy of intestinal venules, AT-III similarly reduced microvascular neutrophil rolling and endothelial adhesion in a feline ischemia-reperfusion injury model(36). To wit, AT-III also reduces endothelial leakage, microvascular permeability and tissue edema by inhibiting neutrophil activation – observations that have been previously linked to high-mobility group box-1 (HMGB1) inhibition in acute lung injury and sepsis (37, 38). Incomplete spinal cord injury recovery may also benefit from AT-III as assessed by earlier recovery of spinal cord-evoked potentials and motor function in a rodent model (39). Finally, in the central nervous system, our group has recently demonstrated that early, post-TBI administration of AT-III reduces in vivo penumbral leucocyte recruitment and blunts local microvascular permeability, subsequently decreasing brain edema and resulting in greater animal weight loss recovery after blunt injury(15). Indeed, the correlation between post-injury tissue swelling and poor outcome is nowhere more relevant than in TBI where cerebral swelling can rapidly result in death through brain herniation to accommodate the increased intracranial tissue volume. Since these observations now cross species and models, they perhaps suggest a more ubiquitous mechanism and role for AT-III after tissue injury in support of organ recovery as a key step towards functional outcome enhancement. The results of the present study demonstrate the durability of the beneficial effects of AT-III on cognitive recovery following TBI as this inquiry spans weeks, and not the more common assessment measured in hours or a few days.
Nevertheless, our study is small and incorporates important limitations. First, it was conducted in a murine model, and the generalizability of findings to humans with TBI is unmerited. However, the use of anticoagulants is common in both prophylactic and therapeutic doses in humans, suggesting viable routes of inquiry. Second the MWM operator was not blinded to animal treatment groups. This was mitigated in large part by deriving data from objective digital recordings and not subjective assessments. Third, AT-III plasma levels were not measured at the early and late intervals and therefore, the correlation between AT-III activity and cognitive function cannot be ascertained. In particular, restoration of physiologic levels of circulating AT-III is the norm when treating familial ATIII deficiency with this reagent and the current study is unable to answer this important question in rodent TBI. Furthermore, no true guidance exists on the dose, frequency and route of administration of ATIII in trauma and thus currently dosing regimens may have been suboptimal. Nonetheless, we based the choice of once-a-day dosing on extensive discussions with the manufacturer and review of the considerable literature in familial AT-III deficiency. Fourth, no cerebral parenchymal microscopy was performed to assess cellular integrity. However, since this study was performed using the same techniques as an earlier one in which both intravital microscopy and cerebral histology were performed documenting enhanced acute organism and organ outcomes, this seem less impactful than could be asserted (15). Fifth, while the MWM is a useful tool, it cannot serve as a surrogate for life-sustaining activities such as food foraging, predator evasion, nor litter guidance – all activities relevant for rodent survival but impractical to assess in their totality within a laboratory environment. Finally, greater group sample sizes may have revealed subtler differences with AT-III treatment though MWM studies generally use similar animal numbers as were used in this study.
CONCLUSION
The current study demonstrates that early, post-TBI administration of AT-III improves neurocognitive recovery weeks after injury. This improvement appears specifically related to recovering learning abilities but not long-term memory. Additional exploration of AT-III’s safety and efficacy is warranted to determine if AT-III can also enhance human cognitive recovery after blunt TBI. Given AT-III’s anti-inflammatory properties, it is poised to be a powerful tool that augments the armamentarium of neurocritical care management in the care of injured patients.
Acknowledgments
This work was supported in part by an investigator-sponsored research grant from Grifols S.A International
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Gardner AJ, Zafonte R. Neuroepidemiology of traumatic brain injury. In: Rosano C, Ikram MA, Ganguli M. eds. Handbook of Clinical Neurology, Vol. 138: Neuroepidemiology. New York: Elsevier; 2016. [DOI] [PubMed] [Google Scholar]
- 2.Campbell-Sills L, Stein MB, Liu H, Agtarap S, Heeringa SG, Nock MK, Ursano RJ, Kessler RC. Associations of lifetime traumatic brain injury characteristics with prospective suicide attempt among deployed US army soldiers. J Head Trauma Rehabil. 2020. January; 35: 14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison-Felix C, Whiteneck G, DeVivo M, Hammond FM, Jha A. Mortality following rehabilitation in the Traumatic Brain Injury Model Systems of Care. NeuroRehabilitation. 2004. January;19(1):45–54. [PubMed] [Google Scholar]
- 4.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007. July;99(1):4–9. [DOI] [PubMed] [Google Scholar]
- 5.Morganti-Kossmann MC, Rancan M, Otto VI, Stahel PF, Kossmann T. Role of cerebral inflammation after traumatic brain injury: a revisited concept. Shock. 2001. September;16(3):165–77. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A, Loane DJ. Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav Immun. 2012. November;26(8):1191–201. [DOI] [PubMed] [Google Scholar]
- 7.Morganti-Kossmann MC, Semple BD, Hellewell SC, Bye N, Ziebell JM. The complexity of neuroinflammation consequent to traumatic brain injury: from research evidence to potential treatments. Acta Neuropathol (Berl). 2019. May;137(5):731–55. [DOI] [PubMed] [Google Scholar]
- 8.Nagata K, Kumasaka K, Browne KD, Li S, St-Pierre J, Cognetti J, Marks J, Johnson VE, Smith DH, Pascual JL. Unfractionated heparin after TBI reduces in vivo cerebrovascular inflammation, brain edema and accelerates cognitive recovery. J Trauma Acute Care Surg. 2016. December;81(6):1088–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li S, Marks JA, Eisenstadt R, Kumasaka K, Samadi D, Johnson VE, Holena DN, Allen SR, Browne KD, Smith DH, Pascual JL. Enoxaparin ameliorates post-traumatic brain injury edema and neurologic recovery, reducing cerebral leukocyte endothelial interactions and vessel permeability in vivo. J Trauma Acute Care Surg. 2015. July;79(1):78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeda A, Ohta K, Ohta K, Nakayama Y, Hashida Y, Toma T, Saito T, Maruhashi K, Yachie A. Effects of antithrombin III treatment in vascular injury model of mice. Pediatr Int. 2011. October;53(5):747–53. [DOI] [PubMed] [Google Scholar]
- 11.Rehberg S, Yamamoto Y, Sousse LE, Jonkam C, Zhu Y, Traber LD, Cox RA, Prough DS, Traber DL, Enkhbaatar P. Antithrombin attenuates vascular leakage via inhibiting neutrophil activation in acute lung injury. Crit Care Med. 2013. December;41(12):439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iba T, Levy JH, Hirota T, Hiki M, Sato K, Murakami T, Nagaoka I. Protection of the endothelial glycocalyx by antithrombin in an endotoxin-induced rat model of sepsis. Thromb Res. 2018. November;171:1–6. [DOI] [PubMed] [Google Scholar]
- 13.Mizutani A, Okajima K, Uchiba M, Isobe H, Harada N, Mizutani S, Noguchi T. Antithrombin reduces ischemia/reperfusion-induced renal injury in rats by inhibiting leukocyte activation through promotion of prostacyclin production. Blood. 2003. April;101(8):3029–36. [DOI] [PubMed] [Google Scholar]
- 14.Uchiba M, Okajima K, Murakami K. Effects of various doses of antithrombin III on endotoxin-induced endothelial cell injury and coagulation abnormalities in rats. Thromb Res. 1998. March;89(5):233–41. [DOI] [PubMed] [Google Scholar]
- 15.ElSaadani M, Ahmed SM, Jacovides C, Lopez A, Johnson VE, Kaplan LJ, Schwab CW, Smith DH, P. J. Antithrombin-III Ameliorates Post-TBI Cerebral Leukocyte Mobilization Enhancing Recovery Of Blood Brain Barrier Integrity. J Trauma Acute Care Surg.2020. October, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uchiba M, Okajima K. Antithrombin III (AT III) prevents LPS-induced pulmonary vascular injury: novel biological activity of AT III. Semin Thromb Hemost. 1997. December; 23(6):583–90. [DOI] [PubMed] [Google Scholar]
- 17.Pascual JL, Murcy MA, Li S, Gong W, Eisenstadt R, Kumasaka K, Sims C, Smith DH, Browne K, Allen S, Baren J. Neuroprotective effects of progesterone in traumatic brain injury: blunted in vivo neutrophil activation at the blood-brain barrier. Am J Surg. 2013. December;206(6):840–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith DH, Soares HD, Pierce JS, Perlman KG, Saatman KE, Meaney DF, Dixon CE, Mcintosh TK. A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J Neurotrauma. 1995. April;12(2):169–78. [DOI] [PubMed] [Google Scholar]
- 19.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2): 848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacovides CL, Ahmed S, Suto Y, Paris AJ, Leone R, McCarry J, Christofidou-Solomidou M, Kaplan LJ, Smith DH, Holena DN, Schwab CW, Pascual JL. An inflammatory pulmonary insult post-traumatic brain injury worsens subsequent spatial learning and neurological outcomes. J Trauma Acute Care Surg. 2019. September;87(3):552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010. July;6(7):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lukaszewicz AC, Soyer B, Payen D. Water, water, everywhere: sodium and water balance and the injured brain. Curr Opin Anaesthesiol. 2011. April;24(2):138–43. [DOI] [PubMed] [Google Scholar]
- 23.Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006. January;147(1):232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubes P, Ward PA. Leukocyte Recruitment and the Acute Inflammatory Response. Brain Pathol. 2006. April 5;10(1):127–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paterno R, Folweiler KA, Cohen AS. Pathophysiology and Treatment of Memory Dysfunction After Traumatic Brain Injury. Curr Neurol Neurosci Rep. 2017. July;17(7):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carbonell WS, Grady MS. Regional and temporal characterization of neuronal, glial, and axonal response after traumatic brain injury in the mouse. Acta Neuropathol. 1999;98:396–406). [DOI] [PubMed] [Google Scholar]
- 27.Brody DL, Holtzman DM. Morris water maze search strategy analysis in PDAPP mice before and after experimental traumatic brain injury. Exp Neurol. 2006;197(2):330–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheff SW, Baldwin SA, Brown RW, Kraemer PJ. Morris water maze deficits in rats following traumatic brain injury: lateral controlled cortical impact. J Neurotrauma. 1997;14(9):615–627. [DOI] [PubMed] [Google Scholar]
- 29.Suto Y, Nagata K, Ahmed SM, et al. A concomitant bone fracture delays cognitive recovery from traumatic brain injury. J TraumaAcute Care Surg. 2018;85(2):275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allingstrup M, Wetterslev J, Ravn FB, Møller AM, Afshari A. Antithrombin III for critically ill patients. Cochrane Database Syst Rev. 2016. February; 2(2):CD005370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiedermann CJ, Römisch J. The anti- inflammatory actions of antithrombin–a review. Acta Med Austriaca. 2002. July;29(3):89–92 [DOI] [PubMed] [Google Scholar]
- 32.Uchiba MI, Okajima KI, Murakami KA, Okabe HI, Takatsuki KI. Attenuation of endotoxin-induced pulmonary vascular injury by antithrombin III. Am J Physiol. 1996. June;270(6): L921–30. [DOI] [PubMed] [Google Scholar]
- 33.Hoffmann JN, Vollmar B, Römisch J, Inthorn D, Schildberg FW, Menger MD. Antithrombin effects on endotoxin-induced microcirculatory disorders are mediated mainly by its interaction with microvascular endothelium. Crit Care Med. 2002. January;30(1):218–25. [DOI] [PubMed] [Google Scholar]
- 34.Nevière R, Tournoys A, Mordon S, Maréchal X, Song FL, Jourdain M, Fourrier F. Antithrombin reduces mesenteric venular leukocyte interactions and small intestine injury in endotoxemic rats. Shock. 2001. March;15(3):220–5. [DOI] [PubMed] [Google Scholar]
- 35.Iba T, Kidokoro A, Fukunaga M, Nagakari K, Suda M, Yoshikawa S, Ida Y. Antithrombin ameliorates endotoxin-induced organ dysfunction more efficiently when combined with danaparoid sodium than with unfractionated heparin. Intensive Care Med. 2005. August;31(8):1101–8. [DOI] [PubMed] [Google Scholar]
- 36.Ostrovsky L, Woodman RC, Payne D, Teoh D, Kubes P. Antithrombin III prevents and rapidly reverses leukocyte recruitment in ischemia/reperfusion. Circulation. 1997. October;96(7):2302–10 [DOI] [PubMed] [Google Scholar]
- 37.Rehberg S, Yamamoto Y, Sousse LE, Jonkam C, Zhu Y, Traber LD, Cox RA, Prough DS, Traber DL, Enkhbaatar P. Antithrombin attenuates vascular leakage via inhibiting neutrophil activation in acute lung injury. Crit Care Med. 2013. December;41(12):e439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hagiwara S, Iwasaka H, Matsumoto S, Noguchi T. High dose antithrombin III inhibits HMGB1 and improves endotoxin-induced acute lung injury in rats. Intensive Care Med. 2008. February;34(2):361–7. [DOI] [PubMed] [Google Scholar]
- 39.Arai M, Goto T, Seichi A, Nakamura K. Effects of antithrombin III on spinal cord-evoked potentials and functional recovery after spinal cord injury in rats. Spine. 2004. February;29(4):405–12. [DOI] [PubMed] [Google Scholar]
- 40.Wu F, Chipman A, Pati S, Miyasawa B, Corash L, Kozar RA. Resuscitative strategies to modulate the Endotheliopathy of trauma: from cell to patient. Shock. 2020. May 1;53(5):575–84). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Needham EJ, Helmy A, Zanier ER, Jones JL, Coles AJ, Menon DK. The immunological response to traumatic brain injury. Journal of Neuroimmunology. 2019. July 15;332:112–25 [DOI] [PubMed] [Google Scholar]
- 42.Ley EJ, Leonard SD, et al. (2018). “Beta blockers in critically ill patients with traumatic brain injury: Results from a multicenter, prospective, observational American Association for the Surgery of Trauma study.” J Trauma Acute Care Surg 84(2): 234–244. [DOI] [PubMed] [Google Scholar]