Abstract
Accurate determination of plasma human immunodeficiency virus type 1 (HIV-1) RNA levels is critical for the effective management of HIV-1 disease. The AMPLICOR HIV-1 MONITOR Test, a reverse transcription-PCR-based test for quantification of HIV-1 RNA in plasma, was developed when little sequence information on HIV-1 isolates from outside North America was available. It has since become apparent that many non-subtype B isolates, particularly subtypes A and E, are detected inefficiently by the test. We describe here the AMPLICOR HIV-1 MONITOR Test, version 1.5, an upgraded test developed to minimize subtype-related variation. We also developed a panel of HIV-1 standards containing 30 HIV-1 isolates of subtypes A through G. The virus particle concentration of each cultured viral stock was standardized by electron microscopic virus particle counting. We used this panel to determine the performance of the original AMPLICOR HIV-1 MONITOR Test and version 1.5 of the test with HIV-1 subtypes A through G. The original test underestimated the concentration of HIV-1 subtype A, E, F, and G RNA by 10-fold or more, whereas version of the 1.5 test yielded equivalent quantification of HIV-1 RNA regardless of the subtype. In light of the increasing intermixing of HIV-1 subtypes worldwide, standardization of PCR-based tests against well-characterized viral isolates representing the full range of HIV-1 diversity will be essential for the continued utility of these important clinical management tools.
The human immunodeficiency virus (HIV) type 1 (HIV-1) pandemic is associated with the geographic dispersal of genetically diverse viral strains. The prevalent group, group M (for major), is subdivided into at least nine subtypes (subtypes A through H plus J) on the basis of phylogenetic analysis of genomic or subgenomic proviral sequences (16). Two rare and highly divergent outlier groups, groups O and N, are also recognized (13, 36, 38). HIV-1 subtype B infection predominated in North American and European populations through the early 1990s, but evidence for entry of non-subtype B HIV-1 into these populations is increasing (1, 3, 20). HIV-1 genetic subtypes are also intermixed, although to varying degrees, in Africa, Asia, and South America. Attendant to this admixture is the growing recognition of intersubtype recombinants, which also contribute to the diversity of the pandemic (27, 34).
Molecular techniques that measure the plasma HIV RNA concentration (viral load) are increasingly used for the management of HIV-1 disease (6). The growing recognition that commercial viral load tests may underestimate the concentration of some non-subtype B HIV-1 isolates has introduced uncertainty into quantitation of circulating levels of viral RNA in populations in which non-subtype B isolates are prevalent (2, 10). Since plasma HIV-1 RNA levels are prognostic for disease progression and measure the efficacy of antiretroviral therapy (15, 29, 32), such uncertainty may result in the diminished ability to manage patients infected with non-subtype B HIV-1.
We developed a large panel of well-characterized, cultured HIV-1 standard isolates representing subtypes A through G in order to evaluate the performance of quantitative viral load platforms with different HIV-1 subtypes. Previous reports have shown that infectious viral titer, reverse transcriptase activity, and p24 antigen concentrations are imprecise and inaccurate surrogate markers for viral RNA concentration (7, 8); therefore, the viral stocks were calibrated by electron microscopic particle counting (19). We then used the calibrated standards to evaluate the performance of the AMPLICOR HIV-1 MONITOR Test and an upgraded test, the AMPLICOR HIV-1 MONITOR Test, version 1.5, with non-B subtypes of HIV-1.
MATERIALS AND METHODS
Viral stocks.
Cell-free viral stocks from 30 isolates representing HIV-1 subtypes A through G were prepared by infection of a common pool of four seronegative donor peripheral blood mononuclear cells (PBMCs) as described previously (24). Viral stocks were clarified by centrifugation at 980 × g, passed through a 0.22-μM-pore-size filter, and stored as 1-ml aliquots in the vapor phase of liquid nitrogen. Subtype assignments for the original isolates were established through sequence analysis of the gag and/or env genes derived from proviral DNA. The isolates used, subtype, country and date of isolation, references, and GenBank accession numbers are presented in Table 1.
TABLE 1.
Characteristics of viral standards in subtype panel
| Isolate | Subtypea | Country | Date (yr) of isolation | Reference(s) | GenBank accession no(s). | Reverse transcriptase activity (cpm/ml [105]) |
|---|---|---|---|---|---|---|
| UG273 | A | Uganda | 1991 | 22 | L22957 | 1.92 |
| DJ258 | A (IbNG) | Djibouti | 1991 | 23, 22 | L11763, L22939 | 0.86 |
| DJ263 | A (IbNG) | Djibouti | 1991 | 4, 22 | AF063223, L22941 | 1.16 |
| US1 | B | United States | 1991 | 24 | L14573 | 1.24 |
| US2 | B | United States | 1991 | 24 | L14574 | 1.66 |
| US3 | B | United States | 1991 | 24 | L14575 | 1.37 |
| US4 | B | United States | 1991 | 24 | L14576 | 1.43 |
| CM237 | B | Thailand | 1991 | 24 | L14570 | 1.06 |
| BK132 | B | Thailand | 1990 | 26 | L03696, L03697 | 2.95 |
| BZ167 | B | Brazil | 1990 | 23, 21 | L11752, L22087 | 1.97 |
| ZAM18 | C | Zambia | 1989 | 28, 22 | L03705, L22945 | 1.68 |
| UG268 | C | Uganda | 1991 | 23, 22 | L11799, L22948 | 2.62 |
| ETH2220 | C | Ethiopia | ? | 35 | U46016 | 3.00 |
| SE364 | C | Senegal | 1990 | 22 | L22944 | 1.54 |
| SM145 | C | Somalia | 1989 | 23, 22 | L11803, L22946 | 3.86 |
| SE365 | D | Senegal | 1990 | 23, 22 | L11797, L22945 | 0.66 |
| UG270 | D | Uganda | 1991 | 23 | L11800 | 1.01 |
| UG274 | D | Uganda | 1991 | 23, 22 | L11801, L22950 | 1.29 |
| CM235 | E | Thailand | 1991 | 26 | L03698 | 0.71 |
| CM238 | E | Thailand | 1991 | 23, 24 | L11760, L14571 | 0.75 |
| CM240 | E | Thailand | 1991 | 5, 23, 24 | U54771, L11761, L14572 | 1.56 |
| CM243 | E | Thailand | 1991 | 26 | L03702, L03703 | 0.98 |
| POC30506 | E | Thailand | 1994 | 25 | U48272 | 1.77 |
| ID12 | E | Indonesia | 1993 | 33 | U68193 | 0.73 |
| ID17 | E | Indonesia | 1993 | 33 | U68191 | 1.09 |
| NP1465 | E | Thailand | 1995 | 11 | Not in GenBank | 1.46 |
| BZ126 | F | Brazil | 1989 | 21 | L22083, L22082 | 3.39 |
| BZ162 | F | Brazil | 1990 | 23, 21 | L11751, L22084 | 0.93 |
| BZ163 | F | Brazil | 1990 | 21 | L22086, L22085 | 0.66 |
| HH8793 | G | Kenya | ? | 4 | AF061640, AF061641 | 1.99 |
| p24 concn (ng/ml) | TCID50/mlb | Particle count (no. of virions/ml [108]) | HIV-1 RNA concnc (log10 copies/ml)
|
|||
|---|---|---|---|---|---|---|
| Version 1.5 | Version 1.0 | Version 1.5 − version 1.0 | Mean difference | |||
| 83 | 14,115 | 4.5 | 5.60 | 4.00 | 1.60 | |
| 86 | 2,673 | 3.4 | 5.35 | 2.54 | 2.81 | 2.42 |
| 71 | 10,698 | 3.8 | 5.21 | 2.34 | 2.87 | |
| 82 | 6,144 | 2.9 | 5.37 | 5.23 | 0.14 | |
| 138 | 18,624 | 5.5 | 5.87 | 6.06 | −0.19 | |
| 59 | 14,115 | 6.0 | 5.34 | 5.24 | 0.10 | |
| 86 | 10,698 | 10 | 5.36 | 5.30 | 0.06 | 0.13 |
| 62 | 18,624 | 4.6 | 5.45 | 5.15 | 0.30 | |
| 130 | 42,789 | 4.7 | 5.35 | 5.19 | 0.16 | |
| 119 | 56,460 | 5.0 | 5.60 | 5.25 | 0.35 | |
| 52 | 24,576 | 5.0 | 5.48 | 5.06 | 0.42 | |
| 145 | 42,789 | 10 | 5.66 | 5.34 | 0.32 | |
| 88 | 10,698 | 9.4 | 5.76 | 5.37 | 0.39 | 0.30 |
| 78 | 18,624 | 8.9 | 5.44 | 5.03 | 0.41 | |
| 159 | 8,106 | 7.8 | 5.85 | 5.91 | −0.06 | |
| 98 | 74,502 | 6.9 | 5.49 | 5.33 | 0.16 | |
| 86 | 32,427 | 5.2 | 5.36 | 5.23 | 0.13 | 0.07 |
| 122 | 56,460 | 10 | 5.54 | 5.63 | −0.09 | |
| 47 | 6,144 | 5.1 | 5.22 | 4.33 | 0.89 | |
| 98 | 6,144 | 3.8 | 5.46 | 4.56 | 0.90 | |
| 133 | 8,106 | 13 | 5.59 | 4.65 | 0.94 | |
| 82 | 669 | 9.0 | 5.65 | 4.03 | 1.62 | 1.37 |
| 126 | 1,536 | 8.1 | 5.69 | 4.06 | 1.63 | |
| 101 | 1,536 | 4.7 | 5.28 | 3.31 | 1.97 | |
| 110 | 2,673 | 7.4 | 5.54 | 3.97 | 1.57 | |
| 97 | 6,144 | 6.5 | 5.39 | 3.90 | 1.49 | |
| 128 | 74,502 | 16 | 5.87 | 4.15 | 1.72 | |
| 49 | 6,144 | 4.0 | 5.49 | 4.65 | 0.84 | 1.28 |
| 48 | 14,115 | 3.6 | 5.39 | 4.10 | 1.29 | |
| 86 | 24,576 | 7.1 | 5.90 | 5.01 | 0.89 | 0.89 |
Subtype A (IbNG) viruses are A/G recombinant strains. Subtype E viruses are A/E recombinant strains.
TCID50, 50% tissue culture infective dose.
HIV-1 RNA concentration on 2.5 × 104 virus particles/ml determined with versions 1.0 and 1.5 of the AMPLICOR HIV-1 MONITOR Test.
Characterization of viral stocks.
The viral stocks were characterized by using quantitative biological measures of p24 antigen concentration, reverse transcriptase activity, tissue culture infective dose, and viral particle counting. A commercial p24 antigen capture assay was performed with viral stocks and viral pellets (subjected to centrifugation at 100,000 × g for 1 h) according to the manufacturer’s instructions (Coulter Corporation, Hialeah, Fla.). Reverse transcriptase activity was measured from a lysate of viral proteins precipitated from culture supernatants with polyethylene glycol essentially as described previously (19). Briefly, 20 μl of lysate was mixed with 75 μl of a reverse transcriptase cocktail containing [3H]2′-deoxythymidine triphosphate and either poly(rA-dT) or poly(dA-dT) templates, and the mixture was incubated for 1 h at 37°C. The reaction products were precipitated with ice-cold 10% tricarboxylic acid, collected on glass fiber filters and washed with 5% trichloroacetic acid, and quantified by liquid scintillation counting. Infectious titers were determined (14) with the same donor leukocyte pool with which the stocks were propagated.
Viral particle counts by electron microscopy.
Coded samples of culture supernatants were subjected to viral particle counting by transmission electron microscopy essentially as described previously (19). Polystyrene spheres (4.9 × 1012 per ml; diameter, 155 ± 4 nm; Duke Scientific) were added to 1.0 ml of each culture supernatant to a final concentration of 1 × 109 spheres per ml and were cosedimented in a Heraeus 28RS Sepratech centrifuge with an HFA 22.1 rotor at 22,000 rpm (40,000 × g) for 50 min at 4°C. The resulting pellets were fixed with glutaraldehyde, embedded in Epon, postfixed with osmium tetroxide, and stained with uranyl acetate prior to thin sectioning. Thin sections were restained with uranyl acetate and lead hydroxide, and 7 to 10 fields from three to five sections were examined with a final magnification of ×60,000. Total viral particles and latex spheres in all fields were counted, and viral particle counts were derived by the following formula: [(total number of viral particles)/(total number of latex spheres)] × (109 spheres/ml).
Preparation of viral stocks for RT-PCR analysis.
HIV-1 stocks representing HIV-1 subtypes A to G were diluted in the same stock of normal human plasma (NABI, Boca Raton, Fla.) to generate stocks of 25,000 viral particles per ml. Aliquots of 0.8 ml were prepared and were stored frozen at −80°C. Vials of the viral isolate panel were thawed at room temperature. After mixing vigorously for 10 s, 200-μl aliquots of each specimen were analyzed with version 1.0 and version 1.5 of the kits according to kit specifications. Each panel member was analyzed in triplicate with versions 1.0 and 1.5 of the AMPLICOR HIV-1 MONITOR Test. Viral RNA concentrations were averaged for each panel member, and the mean difference in log10 HIV RNA copies per milliliter for version 1.5 versus that for version 1.0 was calculated for each subtype.
Primers in the AMPLICOR HIV-1 MONITOR Tests.
Version 1.0 of the test uses primers SK462 and SK431, which amplify a 142-nucleotide sequence in the HIV-1 gag gene (18). Reverse transcription (RT) and downstream (antisense) PCR primer SK431 (5′-TGCTATGTCAGTTCCCCTTGGTTCTCT-3′) is complementary to nucleotides 1473 to 1499 of HIV-1HXB2 (GenBank accession nos. K03455 and M38432). Upstream (sense) primer SK462 (5′-AGTTGGAGGACATCAAGCAGCCATGCAAAT-3′) is homologous to nucleotides 1358 to 1387 of HIV-1HXB2. In version 1.5 of the test, primers SK145 and SKCC1B are used to amplify a 155-nucleotide sequence of the HIV-1 gag gene. RT and downstream PCR primer SKCC1B (5′-TACTAGTAGTTCCTGCTATGTCACTTCC-3′) is complementary to nucleotides 1485 to 1512 of HIV-1HXB2. The differences from SK431 are indicated by the bold, underlined text. SKCC1B is 13 nucleotides downstream on the HIV-1 sequence compared to the location of SK431, and there is one base change in the overlapping region. Upstream primer SK145 (5′-AGTGGGGGGACATCAAGCAGCCATGCAAAT-3′) differs from SK462 at only two positions, as indicated by the bold, underlined text.
Statistical treatments.
All statistical analyses were performed with the StatView, version 4.5.1, software package (Abacus Concepts, Inc., Berkeley, Calif.).
RESULTS
Description of the AMPLICOR HIV-1 MONITOR Test.
The AMPLICOR HIV-1 MONITOR Test is a PCR-based test for quantitative measurement of HIV-1 RNA in plasma of HIV-1-infected individuals (viral load) (30). Version 1.0 of the test is the original commercial test (product codes 83088 [which is cleared by the U.S. Food and Drug Administration for use in the United States] and 83102 [which is distributed outside of the United States]). Version 1.5 of the test is a modification to version 1.0 of the test that is intended to perform equivalently with all group M subtypes of HIV-1 and differs from version 1.0 of the test in the primers used for RT and PCR, the composition of the RT-PCR mixture, the thermal cycling parameters, and the internal quantitation standard (QS) RNA.
Version 1.0 of the test uses primers SK462 and SK431 to amplify a highly conserved 142-base region of the HIV-1 gag gene. Since version 1.0 of the test was designed, numerous HIV-1 isolates from worldwide geographic locations have been sequenced, allowing the design of primers with more optimal primer-template homology to diverse HIV-1 isolates. For version 1.5 of the test, upstream primer SK462 was replaced with primer SK145, which binds to the same site and which differs from SK462 at only two positions (Fig. 1). This change increases the homology to nearly all HIV-1 isolates for which sequence information is available. Figure 1 shows the nucleotide sequence alignments of the viruses in our standard panel of subtypes (for which gag sequence information is available) to the primers in the HIV-1 MONITOR Test. The two nucleotides that were changed in the primer used in version 1.5 of the test are perfectly matched to all the viruses in our panel and nearly all sequences of group M HIV-1 isolates in GenBank, whereas nearly all HIV-1 isolates are mismatched at those positions to the primer used in version 1.0.
FIG. 1.
Primer and HIV-1 sequence alignments. The nucleotide sequences of the upstream (sense) primers and complement of the downstream (antisense) primers for HIV-1 MONITOR Test versions 1.0 and 1.5 are aligned with proviral DNA gag sequences from viral panel isolates. Dots indicate sequence positions where the bases match those in the primers used in version 1.5 of the test, while nucleotide assignments define primer template mismatches. a, sequence identity relative to sequences of primers used in version 1.0 of the test; b, the sense-strand sequence, which is complementary to primers SKCC1B and SK431, is shown.
Downstream primer SK431 was replaced with primer SKCC1B, which was shifted 13 bases downstream relative to the position of SK431 and which contains one base change in the overlapping region (Fig. 1). Examination of the sequences of diverse HIV-1 isolates revealed that there was considerable heterogeneity in the HIV sequence at the 3′ end of SK431 and that the sequence immediately downstream was more highly conserved. Shifting of the downstream primer 13 bases farther downstream eliminated nearly all of the HIV-1-to-primer mismatches near the 3′ end of the primer, where mismatches have a greater effect on the efficiency of PCR (17). The one base that was changed in the overlapping sequence of the version 1.0 and 1.5 primers was originally intended to increase the degree of homology to HIV-2; however, it was mismatched with all known HIV-1 sequences.
In addition, several minor changes to the composition of the RT-PCR mixture were made, including the final manganese concentration. To further improve the performance of the test with diverse HIV-1 isolates, the thermal cycling conditions were reoptimized for the new PCR primers and the annealing temperature was lowered to increase mismatch tolerance.
The AMPLICOR HIV-1 MONITOR Test quantifies viral load by coamplification of HIV RNA with a QS RNA introduced into each sample at a known concentration. The version 1.0 QS RNA is a 219-nucleotide in vitro transcript containing the primer binding sequences flanking an internal sequence unrelated to HIV-1, allowing independent detection of HIV and QS amplification products (amplicons) by hybridization to different probes. The QS RNA in version 1.0 contains no HIV sequence downstream of primer SK431 and therefore cannot be amplified with version 1.5 primers. For version 1.5 of the test the version 1.0 QS RNA was modified by the addition of 20 nucleotides of HIV sequence downstream of the SK431 binding site to include the binding site for SKCC1B, and by three base changes in the primer binding sites to make them perfectly homologous to primers SK145 and SKCC1B, yielding a 233-nucleotide RNA.
The changes described above are contained in three kit components, the master mixture (which contains the PCR primers and the RT-PCR mixture, except the manganese), the manganese solution, and the QS RNA. All other kit components in version 1.5 of the test kit are identical to those in version 1.0 of the test kit. No changes to the oligonucleotide probes for HIV-1 and QS RNAs were made.
All of these changes were intended to improve the performance of the AMPLICOR HIV-1 MONITOR Test with non-B subtypes of HIV-1 without altering the other performance characteristics of the test, including sensitivity, linear range, precision, and specificity. A series of studies with dilutions of a well-characterized, electron microscopically quantified stock of subtype B of HIV-1 and a correlation study with 254 clinical samples from HIV-1-infected patients from North America (presumed to be subtype B) demonstrated that version 1.5 of the test yields results and has performance equivalent to those of version 1.0 of the test with subtype B samples (data not shown).
Description of viral stocks.
Viruses collected worldwide from 1989 through 1995 were recovered by cocultivation with seronegative donor PBMCs (Table 1). All isolates were expanded and titers were determined with a common donor pool of seronegative PBMCs prior to characterization. None of these subjects were under therapy with antiretroviral drugs at the time of sampling. Subtype A isolates included representatives of subtype A and of representatives of subtype A (IbNG), an A/G intersubtype recombinant HIV-1 isolate (12). Subtype B isolates were included in samples from the United States, Thailand, and Brazil. Subtype C isolates were from Africa. Subtype E isolates were from Thailand and Indonesia; these are intersubtype recombinants whose gag genes and env genes are of subtypes A and E, respectively. Although our calibrated collection of viral particles contained isolates of subtypes A through G and some intersubtype recombinants from a broad geographic range, it does not yet include some strains that are prevalent in the pandemic, including strains of subtype C from India, strains of subtypes H and J, and the African variants of subtypes E and F.
Physicochemical correlations of viral isolates.
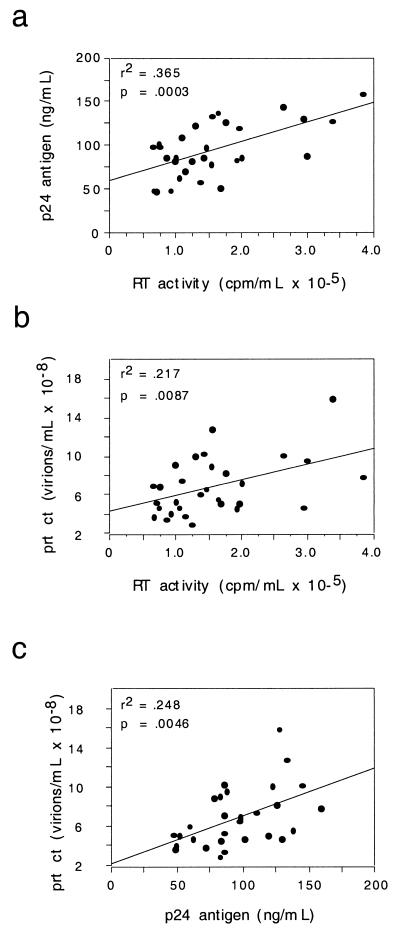
All isolates were characterized by measurement of reverse transcriptase activity, p24 antigen concentration in the viral stocks (data shown) and viral pellets (data not shown), infectious titer, and particle count (Table 1). Regression plots of these measurements are shown in Fig. 2. Reverse transcriptase activity correlated positively with supernatant p24 antigen concentration (Fig. 2a). Particle count correlated positively with both reverse transcriptase activity and supernatant p24 antigen concentration (Fig. 2b and 2c, respectively), with a higher degree of correlation shown for the latter. Neither reverse transcriptase activity nor supernatant p24 antigen concentration significantly correlated with viral infectious titer or viral pellet p24 antigen concentration (data not shown). Since measurements of HIV RNA concentration were performed with dilutions of viral supernatants normalized to particle count, regression analysis with particle counts was not performed.
FIG. 2.
Regression plots of viral isolate physicochemical characteristics. Bivariate regression plots of supernatant p24 antigen concentration, reverse transcriptase (RT) activity, and viral particle count (prt ct) are shown for the panel of viral isolates. The square of the correlation coefficient (r2) and its associated P value are given for each plot.
Viral load analysis of the subtype panel.
The performance of AMPLICOR HIV-1 MONITOR Test versions 1.0 and 1.5 with all 30 isolates in the subtype panel was assessed with panel aliquots containing 2.5 × 104 particles per ml. Each sample was tested in triplicate by both tests, and the mean log10 HIV RNA concentration was determined for each subtype and for the entire panel (Table 1). Version 1.5 of the test yielded a significantly higher mean log10 HIV-1 RNA for the panel and a lower standard deviation than version 1.0 of the test (mean ± standard deviation, 5.52 ± 0.19 and 4.66 ± 0.89, respectively; P < 0.0001 by the Wilcoxon signed-rank test). Although the particle count would predict quantitation of 5.0 × 104 RNA copies per ml (assuming that there are two genomic RNA copies per virion), the observed mean concentration with version 1.5 of the test was 6.6-fold higher than that predicted by the particle count, presumably due to conservative identification of viral particles by electron microscopy. The relationship between the predicted particle count and the observed viral RNA concentration with version 1.5 of the test was similar for all subtypes. The concentrations of specific HIV-1 subtypes showed wide ranges of disparity between the two versions of the test. Whereas the concentrations of HIV-1 subtype B, C, and D RNAs varied from 0.13 to 0.30 log10 RNA copies per ml, those of subtypes A and E through G varied from 0.89 to 2.42 log10 RNA copies per ml. Many, but not all, of these differential disparities between the two tests can be explained by differential primer-template homologies (Fig. 1). These data suggest that other unique characteristics of version 1.5 of the test, such as PCR cycling conditions, contribute to the enhanced accuracy of the assay with non-subtype B isolates.
DISCUSSION
We have shown that p24 antigen concentration, reverse transcriptase activity, and viral particle count are all positively correlated with each other and that the p24 antigen concentration may be a better surrogate for viral particle count than reverse transcriptase activity. However, we cannot say that viral particle count is superior to p24 antigen concentration or reverse transcriptase activity for prediction of the HIV-1 RNA concentration because we did not perform RNA quantitation for isolates normalized for p24 antigen concentration or reverse transcriptase activity. The relationships of these physicochemical parameters did not appear to be influenced by viral subtype, although such associations cannot be ruled out due to the relatively small sample size and narrow range of viral concentrations in this study.
Standardization of reference samples for assessment of HIV-1 RNA concentration has been controversial. Viral particle count standardization has been criticized by Nolte and coworkers (31) on the basis of disruption of viral particles during ultracentrifugation and the consequent underrepresentation of the true virion concentration. The data presented here are qualitatively consistent with this view, because the RNA concentration was 6.6-fold greater than that predicted by the particle count but was far less striking than the 2- to 3-log difference that Nolte et al. (31) reported. Nolte and coworkers (31) studied a single HIV-1 stock calibrated by cosedimentation of latex spheres and virions which were subsequently resuspended, layered onto copper grids, and counted by transmission electron microscopy. We initially used a similar technique (sedimentation of viral particles, resuspension and mixture with latex spheres, and layering onto copper grids). The particle counts determined by this method were 10-fold lower than the viral particle counts presented here (data not shown). Use of direct embedding and counting of cosedimented virion-latex spheres avoided potential loss of virus during resuspension and provided a much closer quantitative relationship between particle count and RNA concentration. Because the use of particle count standardization appeared to introduce a consistent bias across all subtypes tested in this study, we feel that it provided a useful benchmark for comparative studies of HIV-1 RNA concentration.
The counts obtained with version 1.0 of the AMPLICOR HIV-1 MONITOR Test with subtype B, C, and D isolates were in much closer agreement to the viral particle counts than the counts obtained with subtype A, E, F, and G isolates owing partly to a lesser degree of primer-template mismatches with subtype B, C, and D isolates. Similar results have been reported by others in studies of HIV-1 stocks whose input was not normalized by direct particle counting (2, 9, 31). This observation is consistent with both the original design of the test primers for hybridization with subtype B gag sequences and the relative degree of homology between gag sequences from subtypes B, C, and D compared with the degree of homology between gag sequences from other HIV-1 subtypes. HIV-1 MONITOR version 1.5 accurately quantified the concentration of HIV-1 subtype A through G RNA. A similar conclusion was reached in a separate study with plasma samples from 96 patients infected with HIV-1 subtypes A through E and G and aliquots of the particle count-standardized isolates in this report (37). Although version 1.5 of the test also used lower-stringency amplification conditions than version 1.0 of the test, this modification alone was insufficient to impart performance equivalent to that of version 1.0 of the test with non-subtype B isolates in control experiments (data not shown). Compared with U.S. Food and Drug Administration-cleared version 1.0 of the HIV-1 MONITOR Test, version 1.5 of the test should provide clinicians more reliable viral load data because the test is substantially less influenced by viral subtype.
Given the increasing evidence for global migration of and recombination between all known HIV-1 group M subtypes, the paradigm of static geographic localization of subtypes must be abandoned, and nucleic acid-based assays for the detection and quantitation of HIV-1 must be designed or modified to account for this fact. As new variants are discovered, it will be necessary to reevaluate the performance of viral load assays with these novel HIV-1 variants and to update the assay as required. Modification of the viral load assays, establishment of performance characteristics of an updated assay, conduct of clinical trials, and approval from regulatory agencies require considerable time and resources. Research on methods that can increase the tolerance of PCRs for sequence diversity is ongoing at Roche Molecular Systems.
The panel of viruses used in the experiments described here underrepresented subtypes A, D, F, and G and contained no members of subtypes H and J. We are expanding the panel to address this situation, and we intend to continue adding new isolates to the panel as new variants are discovered. Ongoing evaluation of both established and new quantitative viral load tests with such increasingly refined panels of physicochemical-normalized viral isolates that reflect the global diversity of the isolates causing the HIV-1 pandemic will be critical to the ability to use viral load as an accurate tool for the management of HIV-1 disease.
ACKNOWLEDGMENTS
We thank D. Wiggins for technical support, D. Birx and A. Brown for helpful discussions, M. Vahey for manuscript review, M. Peeters for sharing unpublished data, and members of the U.S. Military HIV Research Program for providing viral isolates.
This work was supported in part by Cooperative Agreement DAMD17-93-V-3004 between the U.S. Army Medical Research and Materiel Command and the Henry M. Jackson Foundation for the Advancement of Military Medicine.
REFERENCES
- 1.Alaeus A, Leitner T, Lidman K, Albert J. Most HIV-1 genetic subtypes have entered Sweden. AIDS. 1997;11:199–202. doi: 10.1097/00002030-199702000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Alaeus A, Lidman K, Sonnerborg A, Albert J. Subtype-specific problems with quantification of plasma HIV-1 RNA. AIDS. 1997;11:859–865. doi: 10.1097/00002030-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Brodine S K, Mascola J R, Weiss P J, Ito S I, Porter K R, Artenstein A W, Garland F C, McCutchan F E, Burke D S. Detection of diverse HIV-1 genetic subtypes in the USA. Lancet. 1995;346:1198–1199. doi: 10.1016/s0140-6736(95)92901-0. [DOI] [PubMed] [Google Scholar]
- 4.Carr J K, Salminen M O, Albert J, Sanders-Buell E, Gotte D, Birx D L, McCutchan F E. Full genome sequences of human immunodeficiency virus type 1 subtypes G and A/G intersubtype recombinants. Virology. 1998;247:22–31. doi: 10.1006/viro.1998.9211. [DOI] [PubMed] [Google Scholar]
- 5.Carr J K, Salminen M O, Koch C, Gotte D, Artenstein A W, Hegerich P A, St. Louis D, Burke D S, McCutchan F E. Full-length sequence and mosaic structure of a human immunodeficiency virus type 1 isolate from Thailand. J Virol. 1996;70:5935–5943. doi: 10.1128/jvi.70.9.5935-5943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. Morbid Mortal Weekly Rep. 1998;47:43–82. . (Erratum, 47:619.) [Google Scholar]
- 7.Coombs R W, Henrard D R, Mehaffey W F, Gibson J, Eggert E, Quinn T C, Phillips J. Cell-free plasma human immunodeficiency virus type 1 titer assessed by culture and immunocapture-reverse transcription-polymerase chain reaction. J Clin Microbiol. 1993;31:1980–1986. doi: 10.1128/jcm.31.8.1980-1986.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corrigan G E, Al-Khalili L, Malmsten A, Thorstensson R, Fenyo E M, Kallander C F, Gronowitz J S. Differences in reverse transcriptase activity versus p24 antigen detection in cell culture, when comparing a homogeneous group of HIV type 1 subtype B viruses with a heterogeneous group of divergent strains. AIDS Res Hum Retroviruses. 1998;14:347–352. doi: 10.1089/aid.1998.14.347. [DOI] [PubMed] [Google Scholar]
- 9.Coste J, Montes B, Reynes J, Peeters M, Segarra C, Vendrell J P, Delaporte E, Segondy M. Comparative evaluation of three assays for the quantitation of human immunodeficiency virus type 1 RNA in plasma. J Med Virol. 1996;50:293–302. doi: 10.1002/(SICI)1096-9071(199612)50:4<293::AID-JMV3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Debyser Z, Van Wijngaerden E, Van Laethem K, Beuselinck K, Reynders M, De Clercq E, Desmyter J, Vandamme A M. Failure to quantify viral load with two of the three commercial methods in a pregnant woman harboring an HIV type 1 subtype G strain. AIDS Res Hum Retroviruses. 1998;14:453–459. doi: 10.1089/aid.1998.14.453. [DOI] [PubMed] [Google Scholar]
- 11.DeSouza, M. 1998. Personal communication.
- 12.Gao F, Robertson D L, Carruthers C D, Morrison S G, Jian B, Chen Y, Barre-Sinoussi F, Girard M, Srinivasan A, Abimiku A G, Shaw G M, Sharp P M, Hahn B H. A comprehensive panel of near-full-length clones and reference sequences for non-subtype B isolates of human immunodeficiency virus type 1. J Virol. 1998;72:5680–5698. doi: 10.1128/jvi.72.7.5680-5698.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurtler L G, Hauser P H, Eberle J, von Brunn A, Knapp S, Zekeng L, Tsague J M, Kaptue L. A new subtype of human immunodeficiency virus type 1 (MVP-5180) from Cameroon. J Virol. 1994;68:1581–1585. doi: 10.1128/jvi.68.3.1581-1585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Japour A J, Fiscus S A, Arduino J M, Mayers D L, Reichelderfer P S, Kuritzkes D R. Standardized microtiter assay for determination of syncytium-inducing phenotypes of clinical human immunodeficiency virus type 1 isolates. J Clin Microbiol. 1994;32:2291–2294. doi: 10.1128/jcm.32.9.2291-2294.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katzenstein D A, Hammer S M, Hughes M D, Gundacker H, Jackson J B, Fiscus S, Rasheed S, Elbeik T, Reichman R, Japour A, Merigan T C, Hirsch M S. The relation of virologic and immunologic markers to clinical outcomes after nucleoside therapy in HIV-infected adults with 200 to 500 CD4 cells per cubic millimeter. AIDS Clinical Trials Group Study 175 Virology Study Team. N Engl J Med. 1996;335:1091–1098. doi: 10.1056/NEJM199610103351502. . (Erratum, 337:1097, 1997.) [DOI] [PubMed] [Google Scholar]
- 16.Korber B, Foley B, Leitner T, McCutchan F, Hahn B, Mellors J W, Myers G, Kuiken C. Human retroviruses and AIDS. Los Alamos, N.M: Los Alamos National Laboratory; 1997. [Google Scholar]
- 17.Kwok S, Kellogg D E, McKinney N, Spasic D, Goda L, Levenson C, Sninsky J J. Effects of primer-template mismatches on the polymerase chain reaction: human immunodeficiency virus type 1 model studies. Nucleic Acids Res. 1990;18:999–1005. doi: 10.1093/nar/18.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwok S, Sninsky J J. PCR detection of human immunodeficiency virus type 1 proviral DNA sequences. In: Persing D H, Smith T F, Tenover F C, White T J, editors. Diagnostic molecular microbiology: principles and applications. Washington, D.C: ASM Press; 1993. pp. 309–315. [Google Scholar]
- 19.Layne S P, Merges M J, Dembo M, Spouge J L, Conley S R, Moore J P, Raina J L, Renz H, Gelderblom H R, Nara P L. Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus. Virology. 1992;189:695–714. doi: 10.1016/0042-6822(92)90593-e. [DOI] [PubMed] [Google Scholar]
- 20.Leitner T, Escanilla D, Marquina S, Wahlberg J, Brostrom C, Hansson H B, Uhlen M, Albert J. Biological and molecular characterization of subtype D, G, and A/D recombinant HIV-1 transmissions in Sweden. Virology. 1995;209:136–146. doi: 10.1006/viro.1995.1237. [DOI] [PubMed] [Google Scholar]
- 21.Louwagie J, Delwart E L, Mullins J I, McCutchan F E, Eddy G, Burke D S. Genetic analysis of HIV-1 isolates from Brazil reveals presence of two distinct genetic subtypes. AIDS Res Hum Retroviruses. 1994;10:561–567. doi: 10.1089/aid.1994.10.561. [DOI] [PubMed] [Google Scholar]
- 22.Louwagie J, Janssens W, Mascola J, Heyndrickx L, Hegerich P, van der Groen G, McCutchan F E, Burke D S. Genetic diversity of the envelope glycoprotein from human immunodeficiency virus type 1 isolates of African origin. J Virol. 1995;69:263–271. doi: 10.1128/jvi.69.1.263-271.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louwagie J, McCutchan F E, Peeters M, Brennan T P, Sanders-Buell E, Eddy G A, van der Groen G, Fransen K, Gershy-Damet G M, Deleys R, et al. Phylogenetic analysis of gag genes from 70 international HIV-1 isolates provides evidence for multiple genotypes. AIDS. 1993;7:769–780. doi: 10.1097/00002030-199306000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Mascola J R, Louwagie J, McCutchan F E, Fischer C L, Hegerich P A, Wagner K F, Fowler A K, McNeil J G, Burke D S. Two antigenically distinct subtypes of human immunodeficiency virus type 1: viral genotype predicts neutralization serotype. J Infect Dis. 1994;169:48–54. doi: 10.1093/infdis/169.1.48. [DOI] [PubMed] [Google Scholar]
- 25.McCutchan F E, Artenstein A W, Sanders-Buell E, Salminen M O, Carr J K, Mascola J R, Yu X F, Nelson K E, Khamboonruang C, Schmitt D, Kieny M P, McNeil J G, Burke D S. Diversity of the envelope glycoprotein among human immunodeficiency virus type 1 isolates of clade E from Asia and Africa. J Virol. 1996;70:3331–3338. doi: 10.1128/jvi.70.6.3331-3338.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCutchan F E, Hegerich P A, Brennan T P, Phanuphak P, Singharaj P, Jugsudee A, Berman P W, Gray A M, Fowler A K, Burke D S. Genetic variants of HIV-1 in Thailand. AIDS Res Hum Retroviruses. 1992;8:1887–1895. doi: 10.1089/aid.1992.8.1887. [DOI] [PubMed] [Google Scholar]
- 27.McCutchan F E, Salminen M O, Carr J K, Burke D S. HIV-1 genetic diversity. AIDS. 1996;10(Suppl. 3):S13–S20. [PubMed] [Google Scholar]
- 28.McCutchan F E, Ungar B L, Hegerich P, Roberts C R, Fowler A K, Hira S K, Perine P L, Burke D S. Genetic analysis of HIV-1 isolates from Zambia and an expanded phylogenetic tree for HIV-1. J Acquired Immune Defic Syndr. 1992;5:441–449. [PubMed] [Google Scholar]
- 29.Mellors J W, Rinaldo C R, Jr, Gupta P, White R M, Todd J A, Kingsley L A. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. . (Erratum, 275:14, 1997). [DOI] [PubMed] [Google Scholar]
- 30.Mulder J, McKinney N, Christopherson C, Sninsky J, Greenfield L, Kwok S. Rapid and simple PCR assay for quantitation of human immunodeficiency virus type 1 RNA in plasma: application to acute retroviral infection. J Clin Microbiol. 1994;32:292–300. doi: 10.1128/jcm.32.2.292-300.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nolte F S, Boysza J, Thurmond C, Clark W S, Lennox J L. Clinical comparison of an enhanced-sensitivity branched-DNA assay and reverse transcription-PCR for quantitation of human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 1998;36:716–720. doi: 10.1128/jcm.36.3.716-720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Brien W A, Hartigan P M, Martin D, Esinhart J, Hill A, Benoit S, Rubin M, Simberkoff M S, Hamilton J D. Changes in plasma HIV-1 RNA and CD4+ lymphocyte counts and the risk of progression to AIDS. Veterans Affairs Cooperative Study Group on AIDS. N Engl J Med. 1996;334:426–431. doi: 10.1056/NEJM199602153340703. [DOI] [PubMed] [Google Scholar]
- 33.Porter K R, Mascola J R, Hupudio H, Ewing D, VanCott T C, Anthony R L, Corwin A L, Widodo S, Ertono S, McCutchan F E, Burke D S, Hayes C G, Wignall F S, Graham R R. Genetic, antigenic and serologic characterization of human immunodeficiency virus type 1 from Indonesia. J Acquired Immune Defic Syndr Hum Retrovirol. 1997;14:1–6. doi: 10.1097/00042560-199701010-00001. [DOI] [PubMed] [Google Scholar]
- 34.Robertson D L, Sharp P M, McCutchan F E, Hahn B H. Recombination in HIV-1. Nature. 1995;374:124–126. doi: 10.1038/374124b0. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 35.Salminen M O, Johansson B, Sonnerborg A, Ayehunie S, Gotte D, Leinikki P, Burke D S, McCutchan F E. Full-length sequence of an Ethiopian human immunodeficiency virus type 1 (HIV-1) isolate of genetic subtype C. AIDS Res Hum Retroviruses. 1996;12:1329–1339. doi: 10.1089/aid.1996.12.1329. [DOI] [PubMed] [Google Scholar]
- 36.Simon F, Mauclere P, Roques P, Loussert-Ajaka I, Muller-Trutwin M C, Saragosti S, Georges-Courbot M C, Barre-Sinoussi F, Brun-Vezinet F. Identification of a new human immunodeficiency virus type 1 distinct from group M and group O. Nat Med. 1998;4:1032–1037. doi: 10.1038/2017. [DOI] [PubMed] [Google Scholar]
- 37.Triques K, Coste J, Petter J L, Segarra C, Mpoudi E, Reynes J, Delaporte E, Butcher A, Dreyer K, Herman S, Spadoro J, Peeters M. Efficiencies of four versions of the Amplicor HIV-1 Monitor test for quantification of different subtypes of human immunodeficiency virus type 1. J Clin Microbiol. 1999;37:110–116. doi: 10.1128/jcm.37.1.110-116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanden Haesevelde M, Decourt J L, De Leys R J, Vanderborght B, van der Groen G, van Heuverswijn H, Saman E. Genomic cloning and complete sequence analysis of a highly divergent African human immunodeficiency virus isolate. J Virol. 1994;68:1586–1596. doi: 10.1128/jvi.68.3.1586-1596.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]