Abstract
This study examined differential responses among partners who participated in a RCT designed to compare two social cognitive theory interventions, one designed for patients only (P-only) and one for patients and their intimate partners (P + P). The interventions were delivered following the patient receiving an initial ICD implant. Partner health outcomes were examined longitudinally from baseline at hospital discharge to 3, 6, and 12 months. Outcomes included 6 measures: partner physical and mental health status (Short-Form-36 PCS and MCS), depression (Patient Health Questionnaire-9), anxiety (State-Trait Anxiety Inventory), caregiver burden (Oberst Caregiver Burden Scale), and self-efficacy in ICD management (Sudden Cardiac Arrest Self-efficacy scale). Growth mixture and mixed effect modeling were used to identify and compare trajectories of 6 health outcomes within the P-only and P + P arms of the study. Partners (n = 301) were on average 62 years old, female (74.1%) and Caucasian (83.4%), with few co-morbidities (mean Charlson Co-morbidity index, 0.72 ± 1.1). Two types of profiles were observed for P-only and P + P, one profile where patterns of health outcomes were generally better across 12 months and one with outcome patterns that were generally worse across time. For PCS, no significant partner differences were observed between P-only or P + P in either the better (p = 0.067) or the worse (p = 0.129) profile types. Compared to P-only, partners in the worse profile improved significantly over 12 months in MCS (p = 0.006), caregiver burden P + P (p = 0.004) and self-efficacy P + P (p = 0.041). Compared to P-only, P + P partners in the low anxiety profile improved significantly (p = 0.001) at 3 months. Partners with more psychosocial distress at hospital discharge benefited most from the P + P intervention. Among partners with generally low levels of anxiety, those in the P + P intervention compared to P-only showed greater improvement in anxiety over 12 months.
Keywords: Caregivers, Implantable cardioverter defibrilator, Randomized-controlled trial, Outcomes
Introduction
Implanted cardioverter defibrillators (ICD) are commonly used to prevent sudden cardiac arrest for both primary and secondary prevention (Al-Khatib et al., 2018; Masoudi et al., 2017). Receiving an ICD significantly reduces mortality, but device implantation is also associated with adverse physical and psychological outcomes for patients (Cutitta et al., 2014; Kuck et al., 2000; Magyar-Russell et al., 2011; Palacios-Cena et al., 2011). Social support provided by partners (spouses or significant others) can buffer distress that patients experience as they learn to live successfully with an ICD (Allemann et al., 2018; Lache et al., 2007; van den Broek et al., 2007). Sears et al. (2005) identified social support as a significant predictor of patient health status after ICD implant. In addition, patients with heart failure and an ICD who live with a partner demonstrate better health status and fewer depressive symptoms compared to those living alone (Allemann et al., 2018). There is evidence, however, that partner outcomes can be negatively affected after a patient receives an ICD (Van Den Broek et al., 2010). Partners have been shown to have declines in physical health (Dougherty & Thompson, 2009; Jenkins et al., 2007), high levels of depressive symptoms, and higher levels of anxiety than the patient (Pedersen et al., 2004; Rottmann et al., 2018). Patients receiving an ICD shock have been shown to contribute to their partners’ distress (Pedersen et al., 2009; Rottmann et al., 2018). Rottmann et al., (2018), for example, demonstrated patients receiving at least one shock in the previous 12 months negatively affected partner mental health. Additionally, partners have fears and worries regarding their changing roles and relationships, as well as the potential sudden death of their loved one, which are seldom discussed with healthcare providers (Dougherty et al., 2004; Fluur et al., 2014).
Although partners are integral for the patient’s adaptation to living with an ICD, few interventions have specifically targeted partner needs and outcomes after an ICD implant. Recent studies exploring the health of ICD caregivers reveals substantial variability in partner health outcomes, particularly in physical and psychological symptoms (Dougherty & Thompson, 2009; Dougherty et al., 2012, 2019).
The Patient plus Partner intervention was developed to improve health outcomes for both the patient and partner after the patient receives an initial ICD (Dougherty et al., 2019). The Patient plus Partner randomized controlled trial (RCT) compared a patient-only intervention to a patient-and-partner intervention designed to enhance the self-efficacy of both the patient and partner in living with an ICD. The patient-and-partner intervention compared to the patient-only intervention resulted in improved depressive and physical symptoms for patients after 12 months (Dougherty et al., 2019). Furthermore, greater partner self-efficacy and less caregiver burden was observed for the patient-and-partner study arm (Dougherty et al., 2019).
In addition to demonstrating significant improvements in patient and partner outcomes, the patient-and-partner intervention also showed effects for sub-groups of patients based on ICD indication (primary vs secondary prevention). Patients who received an ICD for secondary prevention had a more robust response in physical health and ICD knowledge compared to patients who received an ICD for primary prevention (Auld et al., 2020; Dougherty et al., 2019). Of note, no intervention studies for partners of patients with ICDs have examined how sub-groups of partners respond to a partner-focused intervention. Thus, differentiation of how partners respond to the patient-and-partner intervention may provide new evidence of sub-group specific intervention effects. Better understanding how such partner heterogeneity may contribute to the overall effectiveness of the patient-and-partner intervention is needed to inform tailoring of future partner-focused interventions to optimize both partner and patient outcomes. To this end, the purpose of this study was to identify sub-groups of partners from the Patient plus Partner RCT based on trajectories of self-reported outcomes across 12 months. The primary goal was to determine if partner intervention effects differed for the patient-only versus patient-and-partner intervention conditions within sub-groups defined by their longitudinal response.
Methods
Study design and procedure
This is a secondary analysis of longitudinal partner data from the Patient plus Partner RCT (Dougherty et al., 2019). The Patient plus Partner intervention was developed to improve physical and mental health outcomes in patients receiving an initial ICD and their partners. The trial compared a patient-only intervention to another intervention that included components for both the patient and partner. Partners in the patient-only arm received no intervention. The interventions were delivered during the first 3 months following the patient’s hospital discharge for the initial ICD implant. Outcomes were measured from baseline (hospital discharge) to 3 months post-discharge. Follow-ups at 6 and 12 months were used to assess the longer-term intervention effects.
Sample and settings
A total of 301 patient-and-partner dyads participated in the study. Inclusion criteria were implant of first-time ICD in the patient, ability to speak English, and telephone access during the study for follow-up assessments. Exclusion criteria included co-morbidities of severe cognitive or physical impairment, being under 21 years old, and heavy alcohol or illicit drugs use. Details on recruitment and retention are described in Fig. 1. The Institutional Review Board of a major academic medical center in the Pacific Northwest approved the study.
Fig. 1.
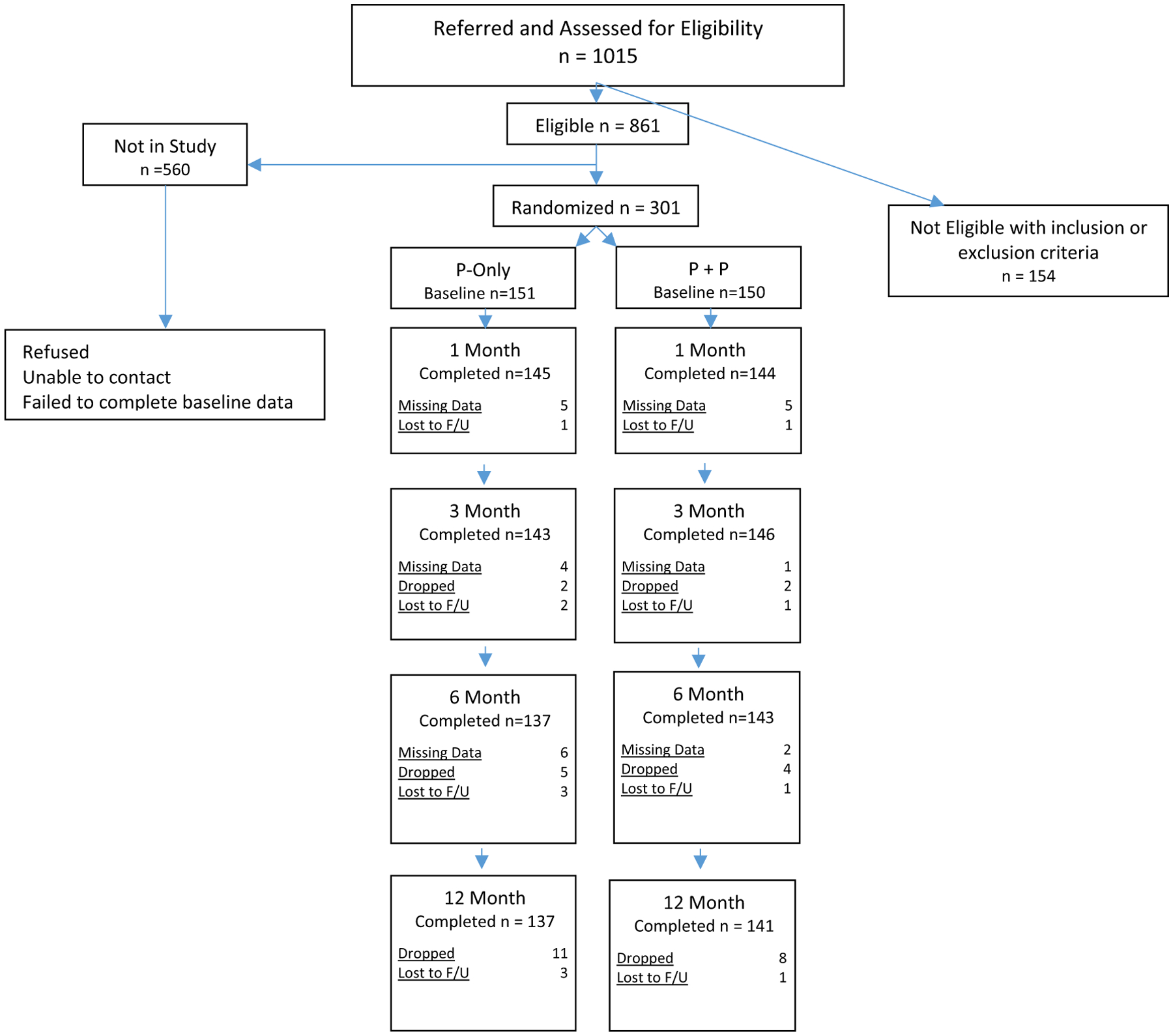
Partner consort
Interventions
Patients in both the P-only and the P + P intervention arms received the patient-focused intervention, which had four primary components: (1) An information booklet describing strategies for recovery after ICD implant; (2) Nurse telephone support with 10 telephone calls over 12 weeks that offered specific content and time for questions; (3) Access to a study nurse via pager 24 h/day, 7 days/weeks; and (4) an informational video developed by the device company. The intervention components were based on Social Cognitive Theory and Domains of Concern detailed by patients and their partners in previous research (Dougherty et al., 2004, 2019).
Partners in the P + P arm received the partner-focused component, whereas partners in P-only did not. Partners in P-only thus served as a control group for partner outcomes. The partner-focused component included a separate partner telephone group (PTG) and a partner-focused informational booklet with details regarding the PTG processes and topics. The PTG included a weekly telephone conference call for 6 weeks during the same time period patient’s received calls. The PTG elements included content regarding: (1) how partners could support the care of the ICD patient, (2) partner self-care, (3) how the patient-partner relationship might be influenced by the ICD, (4) ICD function and components, (5) planning for the future, and (6) other topics of interest and questions.
Measurement
Physical and Mental Health Status
Physical and Mental Health Status was measured using the SF-36 Physical Component Summary (PCS) and the SF-36 Mental Component Summary (MCS), based on responses to the Short Form Health Survey (SF-36). The SF-36 measures physical and mental health in 8 domains: physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, and mental health. The PCS and MCS scores are standardized, ranging from 0 to 100, with a score of 50 (sd = 10) representing the average score for the general US population (Ware et al., 1995), with higher scores meaning better physical or mental health status. Changes of 5–6 points across time have been interpreted as clinically meaningful (Witt et al., 2019).
Depression
Depression was evaluated using the 9-item Patient Health Questionnaire (PHQ-9). The PHQ-9 items are based on the Diagnostic and Statistical Manual, 4th Edition and use a scale from 0 (not at all) to 3 (nearly every day) with scores ranging from 0 to 27 (Spitzer et al., 1999). Higher values indicate more depression. Scores less than 5 indicate minimal depressive symptoms, scores from 5 to 9 indicate mild depression; from 10 to 19 indicate moderate depression, and scores ≥ 20 indicate severe depression (Kroenke et al., 2001). The PHQ-9 has been shown to have a sensitivity of 87% sensitive and specificity of 76% in detecting depression in the general population and excellent internal consistency (Kroenke et al., 2001).
Anxiety
Anxiety, measured with the State-Trait Anxiety Inventory (STAI) State scale that has 20 items and response options ranging from 1 to 4 (not at all—very much so). Higher scores indicate more anxiety at the time of assessment (Ramanaiah et al., 1983). The STAI has been used in multiple studies assessing caregiver anxiety, and has robust internal consistency (α = 0.92) (Ramanaiah et al., 1983). Scores > 40 suggest clinically significant anxiety (Knight et al., 1983).
Caregiver Burden
Caregiver Burden was measured with the Oberst Caregiving Burden Scale (OCBS). The OCBS evaluates 15 caregiver tasks in terms of time and difficulty on a scale of 1–5 (none—a great deal) (Bakas et al., 2004). Convergent validity for both the time and difficulty scales have been established with moderate and highly significant correlations with caregiver emotional distress and the Zarit Burden Interview, a measure of caregiver burden. The time and difficulty scales have demonstrated high internal consistency (α ≥ 0.90). The mean score was calculated by adding the sum of the time and difficulty scores then dividing by the number of items answered for both time and difficulty scales to evaluate overall caregiver burden, with higher scores reflecting greater burden.
Self-efficacy
Self-efficacy was measured with the Sudden Cardiac Arrest-Self-Efficacy (SCA-SE) scale. Sixteen items assess perception of one’s ability to engage in effective ICD self-management and one’s confidence to manage a cardiovascular illness (Dougherty et al., 2007). Items are rated on a 0–10 scale (not at all confident—very confident), and averaged for the SCA-SE total score, with higher scores meaning greater self-efficacy. The SCA-SE has demonstrated strong internal consistency (α = 0.98) (Dougherty et al., 2019).
Statistical analysis
Descriptive statistics and student’s t test or the Wilcoxon rank sum test (STATA v14, College Station, TX) were used respectively to characterize the full sample and to compare the partner characteristics between the patient-only and the patient-and-partner intervention groups. Growth mixture modeling (Mplus v7.4, Los Angeles, CA) was used to distinguish previously unknown and distinct patterns of change across 12 months for partners in the patient-only and partner-focused interventions for the six study outcomes. Growth mixture modeling is a longitudinal clustering method used to identify patterns of responses or trajectories across time (Ram and Grimm 2009). Alternative growth mixture models (e.g., 2-class vs. 3-class models) were compared using posterior probabilities (> 90%), model convergence (entropy near 1.0), and the size of the observed classes (> 5%) (Ram and Grimm 2009). Based on models with the highest posterior probabilities, entropy and adequate class sizes, two types of profiles were identified within the patient-only intervention (P-only) and the patient-and-partner intervention (P + P). Each of the 6 outcomes revealed a low profile characterized by patterns of consistently lower outcomes across time, and a high profile showing patterns of higher outcome values across time (Muthen et al., 2002). Mixed effects regression modeling, a form of hierarchical regression, was used to compare outcomes for P-only versus P + P from 3 to 12 months. That is, separate comparisons were made between the intervention groups within the low profile group and within the high profile group for all six outcomes. The mixed effect models included intervention group (P-only vs. P + P), time, and group × time interaction terms, using restricted maximum likelihood estimation to address missing data (Banks et al., 1985). Simple contrasts were used to examine change in outcomes for the P + P and P-only intervention groups from baseline to 3 months, and from baseline to 12 months. Using the z test, we also compared, within the high group and within the low profile group, differences between P-only and P + P at baseline, 3 and 12 months. All models were adjusted for baseline age, gender, co-morbidities, BMI, and prescription of anti-depressant medication. To compare P-only versus P + P intervention effects within each profile group (low, high), effect sizes (Cohen’s d) were calculated for change in outcomes between baseline and 3 months and between baseline and 12 months. Significance was set at p < 0.05.
Results
Sample description
The majority of partner participants (n = 301) were Caucasian (83.4%) women (74.1%), and average of 62 ± 12 years (Table 1). Partners had a mean Charlson Comorbidity Index of 0.72 ± 1.1 and an average BMI of 28.3 ± 6.4. The majority had more than a high school education (61%) and most were not currently employed (55%, endorsed either retired, disabled or homemaker). There were no significant differences between partners in patient-only and partner-focused groups for demographic (age, gender, education, employment, income) or clinical variables (Charlson Comorbidity Index, BMI). Significantly more partners in the P + P arm, however, were taking anti-depressant medications at study entry (p = 0.03). Finally, although, not significant, partners in P + P compared to P-only had fewer hospitalizations over the course of the study (p = 0.06) and were more likely to be employed (p = 0.08).
Table 1.
Partner characteristics on baseline
| Total sample n = 301 | P only n = 151 (%) | P + P n = 150 (%) | p value | |
|---|---|---|---|---|
| Age | 62.46 ± 12.39 | 63.46 ± 11.65 | 61.46 ± 13.06 | 0.163 |
| Gender (female) | 223 (74.1) | 106 (70.2) | 117 (78.0) | 0.122 |
| BMI | 28.26 ± 6.37 | 27.96 ± 5.98 | 28.56 ± 6.74 | 0.414 |
| Ethnicity | 0.874 | |||
| Caucasian | 266 (83.4) | 133 (88.1) | 133 (88.7) | |
| Education | 0.784 | |||
| High school or less | 117 (39.0) | 59 (39.1) | 58 (38.7) | |
| More than high school | 183 (61.0) | 91 (60.3) | 92 (61.3) | |
| Employment | 0.082 | |||
| Employed | 135 (45.0) | 60 (39.7) | 75 (50.0) | |
| Unemployed or retired | 165 (55.0) | 90 (59.6) | 75 (50.0) | |
| Hospitalized in last year | 46 (15.3) | 29 (19.2) | 17 (11.3) | 0.058 |
| Co-morbidity | 0.72 ± 1.07 | 0.73 ± 0.99 | 0.71 ± 1.16 | 0.458 |
| Anti-depressant medication | 76 (25.2) | 30 (19.9) | 46 (30.7) | 0.031 |
| Anti-anxiety medication | 57 (18.9) | 30 (19.9) | 27 (18.0) | 0.679 |
| Physical health (PCS) | 47.07 ± 10.63 | 46.12 ± 10.34 | 48.01 ± 10.87 | 0.125 |
| Mental health (MCS) | 50.45 ± 10.90 | 51.21 ± 10.40 | 49.69 ± 11.35 | 0.226 |
| Depression | 4.19 ± 4.53 | 4.17 ± 4.63 | 4.22 ± 4.44 | 0.860 |
| Anxiety | 33.12 ± 11.14 | 33.12 ± 11.63 | 33.13 ± 10.65 | 0.716 |
| Caregiver burden | 1.73 ± 0.43 | 1.75 ± 0.42 | 1.70 ± 0.43 | 0.136 |
| Self-efficacy | 8.05 ± 1.59 | 8.10 ± 1.53 | 8.01 ± 1.66 | 0.797 |
Partner outcomes for low and high profiles
For the six study outcomes, two distinct response trajectories were identified, that included a low profile trajectory with a pattern of relatively low outcome values over the 12 months, and a high profile trajectory showing a pattern of higher values over 12 months (Table 2). Our analyses focused on comparing the two intervention groups within the low and high profile types. Thus, effectiveness of the Patient plus Partner intervention was evaluated using separate comparisons of P-only versus P + P, one for the low profile group and one for the high profile group.
Table 2.
Change in outcomes over 12 months for high and low profiles
| Outcome (standard error) | Baseline | 3 months | 12 months | BL-3 months | BL-12 months | ||||
|---|---|---|---|---|---|---|---|---|---|
| z value | p value | Cohen’s d | z value | p value | Cohen’s d | ||||
| PCS | |||||||||
| Low P only; n = 89 | 40.65 (1.02) | 41.45 (1.04) | 39.29 (1.06) | 0.76 | 0.447 | 0.08 | − 1.28 | 0.199 | 0.14 |
| Low P + P; n = 81 | 42.74 (10.7) | 43.06 (1.09) | 41.63 (1.10) | 0.29 | 0.768 | 0.01 | − 1.01 | 0.312 | 0.11 |
| High p only; n = 62 | 5.060 (0.43) | 54.88 (0.45) | 53.13 (0.45) | 2.82 | 0.005 | 0.30 | − 1.03 | 0.301 | 0.11 |
| High P + P; n = 69 | 54.75 (0.41) | 54.89 (0.42) | 54.26 (0.42) | 0.33 | 0.743 | 0.04 | − 1.15 | 0.250 | 0.13 |
| MCS | |||||||||
| Low P only; n = 64 | 42.49 (1.30) | 44.12 (1.44) | 45.21 (1.71) | 0.99 | 0.324 | 0.12 | 1.60 | 0.110 | 0.20 |
| Low P + P; n = 82 | 44.31 (1.14) | 47.58 (1.10) | 50.23 (1.29) | 2.27 | 0.023 | 0.25 | 4.08 | < 0.001 | 0.45 |
| High p only; n = 87 | 57.25 (0.43) | 58.32 (0.44) | 59.06 (0.44) | 2.22 | 0.026 | 0.28 | 3.74 | < 0.001 | 0.40 |
| High P + P; n = 68 | 56.72 (0.49) | 59.25 (0.49) | 58.63 (0.50) | 4.67 | < 0.001 | 0.52 | 3.50 | < 0.001 | 0.42 |
| Depression | |||||||||
| Low P only; n = 58 | 1.17 (0.15) | 0.56 (0.16) | 0.53 (0.16) | − 4.00 | < 0.001 | 0.52 | − 4.12 | < 0.001 | 0.54 |
| Low P + P; n = 84 | 1.60 (0.13) | 0.83 (0.13) | 1.36 (0.13) | − 6.18 | < 0.001 | 0.67 | − 1.94 | 0.052 | 0.21 |
| High p only; n = 93 | 6.36 (0.46) | 5.81 (0.47) | 5.57 (0.48) | − 1.12 | 0.263 | 0.12 | − 1.56 | 0.118 | 0.16 |
| High P + P; n = 66 | 6.99 (0.55) | 5.48 (0.56) | 5.04 (0.57) | − 2.59 | 0.010 | 0.32 | − 3.27 | 0.001 | 0.36 |
| Anxiety | |||||||||
| Low P only; n = 43 | 22.61 (0.50) | 21.52 (0.52) | 21.57 (0.52) | − 1.64 | 0.102 | 0.25 | − 1.56 | 0.119 | 0.24 |
| Low P + P; n = 50 | 25.88 (0.46) | 20.67 (0.47) | 20.37 (0.48) | − 8.59 | < 0.001 | 1.21 | − 8.98 | < 0.001 | 1.27 |
| High p only; n = 108 | 37.59 (1.02) | 34.27 (1.03) | 33.67 (1.06) | − 3.02 | 0.003 | 0.29 | − 3.48 | 0.001 | 0.33 |
| High P + P; n = 100 | 36.30 (1.06) | 33.89 (1.06) | 33.08 (1.09) | − 2.38 | 0.017 | 0.24 | − 2.79 | 0.005 | 0.28 |
| Caregiver burden | |||||||||
| Low P only; n = 108 | 1.60 (0.02) | 1.41 (0.02) | 1.41 (0.02) | − 10.76 | < 0.001 | 1.04 | − 10.17 | < 0.001 | 0.98 |
| Low P + P; n = 96 | 1.49 (0.02) | 1.30 (0.02) | 1.26 (0.02) | − 9.41 | < 0.001 | 0.97 | − 11.52 | < 0.001 | 1.18 |
| High p only; n = 43 | 2.12 (0.07) | 2.12 (0.08) | 2.10 (0.08) | − 0.05 | 0.959 | 0.01 | − 0.28 | 0.780 | 0.04 |
| High P + P; n = 54 | 2.08 (0.07) | 1.80 (0.07) | 1.79 (0.07) | − 4.38 | < 0.001 | 0.59 | − 4.34 | < 0.001 | 0.58 |
| Self-efficacy | |||||||||
| Low P only; n = 94 | 7.45 (0.15) | 7.84 (0.15) | 7.78 (0.15) | 2.64 | 0.008 | 0.27 | 2.18 | 0.029 | 0.22 |
| Low P + P; n = 78 | 7.07 (0.16) | 8.10 (0.16) | 8.24 (0.17) | 6.40 | < 0.001 | 0.72 | 7.12 | < 0.001 | 0.81 |
| High p only; n = 57 | 9.14 (0.06) | 9.71 (0.06) | 9.81 (0.07) | 7.02 | < 0.001 | 0.93 | 8.17 | < 0.001 | 1.18 |
| High P + P; n = 72 | 9.07 (0.06) | 9.71 (0.06) | 9.71 (0.06) | 8.95 | < 0.001 | 1.05 | 8.95 | < 0.001 | 1.05 |
Outcome values for high and low responders at baseline (BL), 3, and 12 months. The comparison of longitudinal outcomes from baseline to 3 months and from baseline to 12 months are simple contrasts from repeated measures mixed models. Differences between the high and low subgroups within the P + P and P-only for all outcomes are significantly different at a p value < 0.001. Bolded values indicate baseline and follow-up values are significantly different at a p value < 0.05
Physical Health Status.
For partners in the low physical health profile (i,e., meaning worse physical health), there were no differences in physical health status between P-only and P + P at any timepoint (Fig. 2). Also, no significant changes in PCS were found for P-only or for P + P across 12 months (Table 2).
Fig. 2.
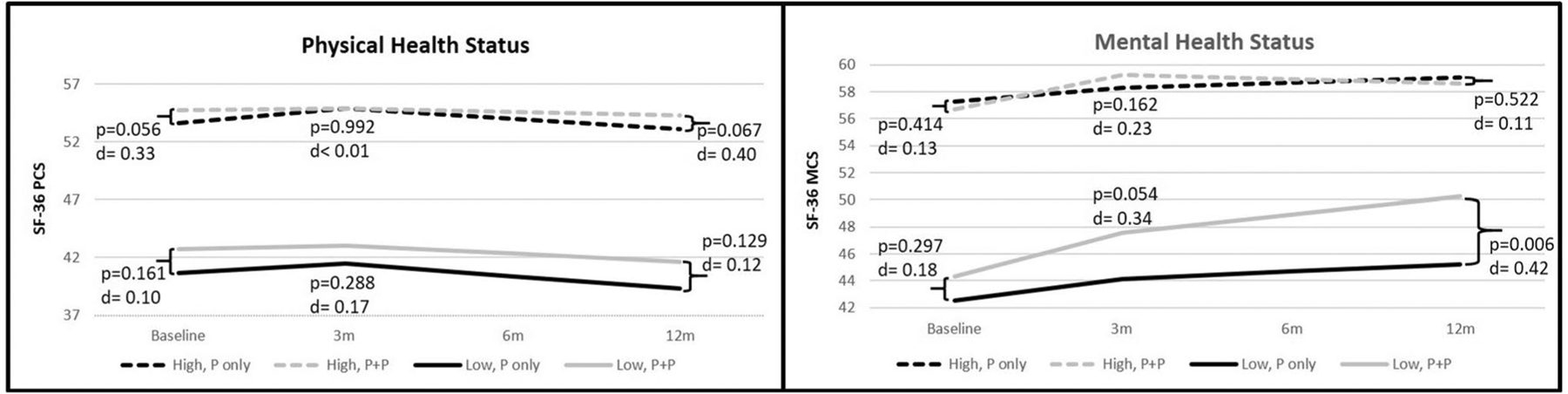
Physical and mental health status trajectories over 12 months by the high and low subgroups
For partners in the high physical health profile (better physical health), those in P-only demonstrated significant improvement in physical health from baseline to 3 months, while those in P + P did not change significantly during this interval (Table 2). Improvement in physical health for P-only, however, was not sustained over the 12 months (Table 2). That is, from 3 to 12 months, physical health for high profile P-only declined, nearing baseline levels. Although, P-only showed improved physical health at 3 months, there were no significant differences in physical health between the P-only and P + P at any timepoint (Fig. 2). In brief, being in the high profile P + P intervention had little impact on partner physical health, and was not, compared to P-only, associated with improved physical health.
Mental Health Status.
For the partners with low mental health profiles (worse mental health), those in P + P showed significant and clinically meaningful improvement in mental health by 12 months (> 5 points), whereas those in P-only experienced no significant change in mental health over the same period (Table 2). Additionally, P + P participants demonstrated significantly better mental health at 12 months compared to those in P-only (Fig. 2).
For partners with high mental health profiles (better mental health), participants in P-only and P + P demonstrated similar improvements in mental health from baseline to 3 months, which was sustained at 12 months (P-only Cohen’s d = 0.40; P + P Cohen’s d = 0.42) (Table 2). Also, no differences were observed between P-only and P + P at any timepoint (Fig. 2). In sum, the P + P intervention was more effective than P-only in improving the mental health of partners in the low profile, but not for those in the high mental health profile.
Depressive Symptoms.
Average depressive symptoms in P-only and P + P were mild for partners in this study (Table 1). Nonetheless, for partners in the low depressive symptom profile (fewer depressive symptoms), those in both P-only and P + P showed significant declines in depressive symptoms at 3 months (Fig. 3). However, the reduction in depressive symptoms was sustained at 12 months for P-only, but not for P + P. That is, P + P participants reported an increase in depressive symptoms from 3 to 12 months (Fig. 3). Of note, the level of depressive symptoms was low (< 2 PHQ-9 points) for the P + P participants, and the subsequent change in symptoms from 3 to 12 months was small (~ 0.5 points), indicating that there was little meaningful change in depressive symptoms for P + P participants.
Fig. 3.
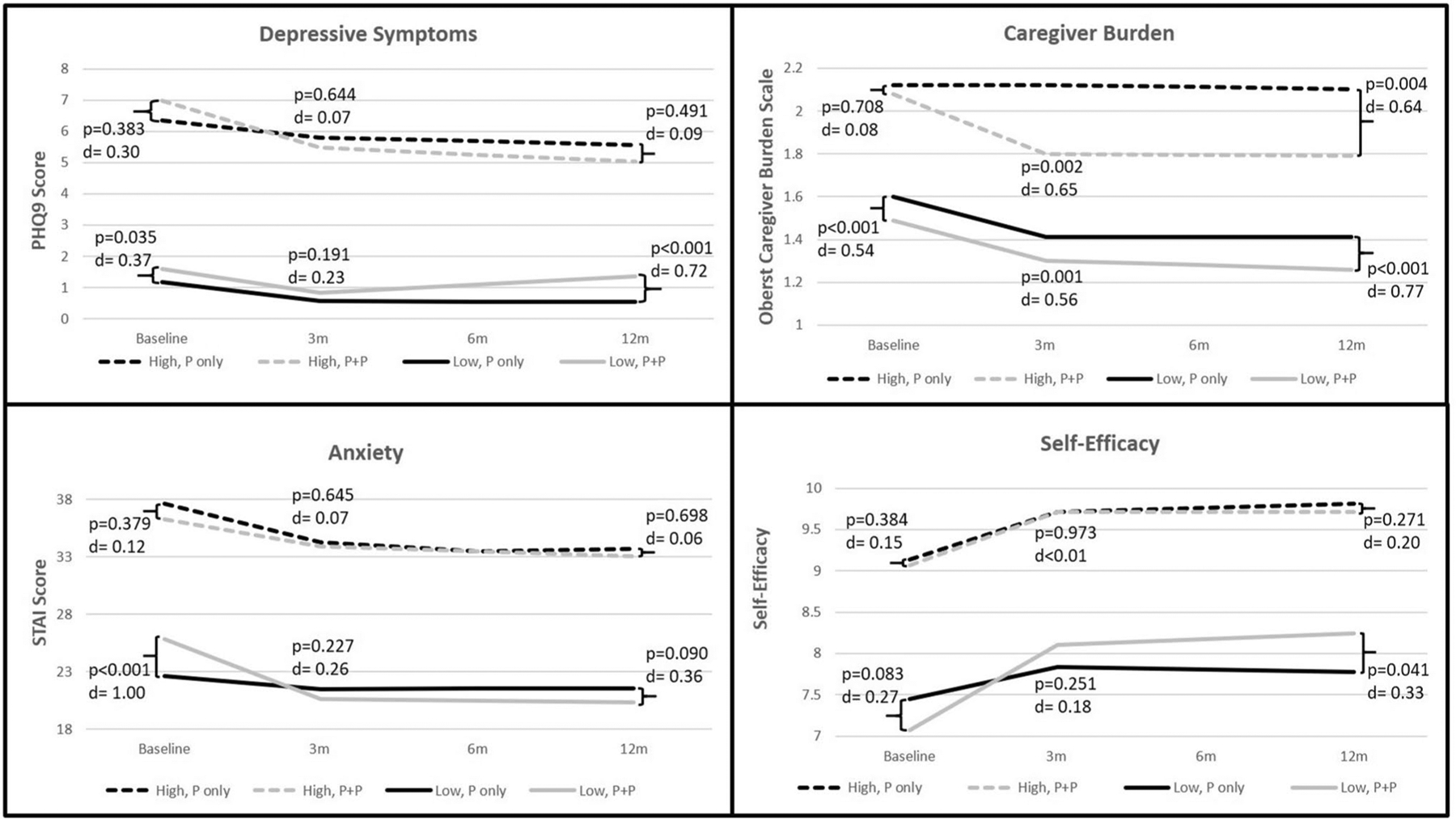
Psychosocial outcome trajectories over 12 months by high and low subgroups
For partners in the high depressive symptoms profile (more depressive symptoms), P + P participants showed a significant reduction in depressive symptoms from baseline to 3 months, which was sustained to 12 months (Table 2). Conversely, P-only showed no change in depressive symptoms over the 12 months. Although there was a significant decline in depressive symptoms for P + P, there were no statistically significant differences between P-only and P + P at any timepoint (Fig. 3). In sum, participants in P + P demonstrated a significant decline in depressive symptom from baseline to 3 months, but the improvements did not result in substantially different levels of depressive symptoms compared to P-only over 12 months.
Anxiety.
Average anxiety in partners in the P-only and P + P was less than 40 indicating mild anxiety in the sample as a whole. Yet, clinically meaningful levels of anxiety (STAI ≥ 40) were observed in 28.6% of the sample. In the low anxiety profile (less anxiety), partners in P + P compared to P-only showed more anxiety at baseline (Fig. 3) and greater reductions in anxiety from baseline to 3 months (P-only Cohen’s d = 0.25; P + P Cohen’s d = 1.21) (Table 2).
In the high anxiety profile (more anxiety), partners in both P-only and P + P demonstrated similar improvement over the 12 months (P-only Cohen’s d = 0.33; P + P Cohen’s d = 0.28) (Table 2). There were no significant differences between the P-only and P + P at any timepoint (Fig. 3). Overall, P + P was more effective than P-only in reducing anxiety for partners in the low anxiety profile, but not for partners in the high anxiety profile.
Caregiver Burden.
In the low caregiver burden profile (less caregiver burden), P + P partners reported significantly more burden at each time point than P-only (Fig. 3). Both P-only and P + P showed parallel (P-only Cohen’s d = 0.98; P + P Cohen’s d = 1.18) and significant reductions in caregiver burden over the course of the study (Table 2). That is, while the P + P partners reported consistently more caregiver burden, caregiver burden improved to similar degrees in both P + P and P-only.
In the high caregiver burden profile (more caregiver burden), partners in P + P showed a substantial reduction in burden from baseline to 3 months (Cohen’s d = 0.59) that was sustained to 12 months (Table 2). In addition, P-only demonstrated little change in burden from baseline to 12 months (Cohen’s d = 0.01). Notably, partners in P + P, compared to those in P-only, experienced significantly lower caregiver burden at 3 and 12 months (Fig. 3). These finding indicate the P + P was more effective than P-only in reducing caregiver burden for partners in the high burden profile, but not for those in the low caregiver burden profile.
Self-efficacy.
The original parent study demonstrated that partners in the P + P arm showed significantly greater self-efficacy in ICD management over the course of the study. Similarly, with respect to self-efficacy in the current study, the P + P group in the low self-efficacy profile (worse self-efficacy) showed more improvement compared to P-only (P-only Cohen’s d = 0.22; P + P Cohen’s d = 0.81). Additionally, P + P participants reported significantly better self-efficacy at 12 months compared to P-only (Fig. 3).
In the high self-efficacy profile (better self-efficacy), P-only and P + P demonstrated similar improvements from baseline to 3 months (P-only Cohen’s d = 0.93; P + P Cohen’s d = 1.05), which were sustained at 12 months (Table 2). There were no significant differences between groups at any timepoint (Fig. 3). In summary, P + P was more effective than P-only in improving self-efficacy in partners in the low self-efficacy profile, but not those in the high self-efficacy profile.
Discussion
In this study, we characterized previously unknown trajectories of health outcomes over 12 months for partners participating in an intervention designed to improve partner outcomes after the patient counterpart had received an initial ICD. Comparing outcomes over 12 months between P-only and P + P for sub-groups of partners with higher and lower outcome profiles provided new insights into partners’ response to the Patient plus Partner intervention that were not evident in the original study. Our results demonstrate no significant intervention effect on partner physical health status. The intervention did not include elements that directly addressed partner physical health. Notably, previous partner-focused intervention studies, in the context of cancer and heart failure, that demonstrated improved physical function included elements of physical activity such as moderately strenuous walking or other exercise (Cuthbert et al., 2018; Gary et al., 2020). Future partner interventions with goals to improve partner physical health will likely need components that specifically address physical function.
Other outcomes demonstrated significant effects of the P + P over the P-only, defined by the level of the partner outcome. Specifically, compared to P-only, partners in P + P with poorer psychological health (mental health status, depression, caregiver burden, and self-efficacy) demonstrated more improvements over time and/or better outcomes at 3 and 12 months. On the other hand, partners in P + P and P-only with better psychological health responded similarly to the interventions with few significant differences observed between the P + P and P-only. These results suggest that partners experiencing poorer mental health, more severe caregiver burden, and lower self-efficacy benefited most from the P + P intervention. Thus, the P + P intervention improved health outcomes for partners who might have needed this assistance most.
The P + P intervention was designed to address previously identified domains of concern of intimate partners during the patients’ recovery after ICD implantation, including care of the ICD patient, partner self-care, the patient-partner relationship, ICD function and components, and planning for the future (Dougherty et al., 2004, 2019). Considering that partners with poorer mental health, greater caregiver burden, and lower self-efficacy responded more robustly to the P + P intervention, partners who were experiencing more psychological distress may have perceived more benefit from addressing the domains of concern. In other words, partners with more distress may have found the P + P intervention more effective in addressing their needs than partners in less distress (Webster & Heeley, 2010). A recent study by Hamama-Raz et al. (2020) demonstrated that perceived benefits were positively associated with psychological status in people with chronic illness. Comparably, Kim et al. (2020) showed patients who perceived more benefits of treatment after percutaneous coronary intervention were more likely to participate in a cardiac rehabilitation education program.
The findings that partners with worst psychosocial profiles responded more effectively to the intervention is important as poor mental health and self-efficacy have been shown to negatively affect self-management behaviors (Cramm & Nieboer, 2012; Irani et al., 2019; McClintock et al., 2020), which in turn can influence intervention outcomes. Our study indicates, however, that for the P + P intervention, worse mental health, more caregiver burden, and poorer self-efficacy were improved by the P + P intervention, and these factors may inform selection criteria for future intervention studies. For instance, developing cut-off scores and screening for mental health, caregiver burden, and self-efficacy prior to implementing an intervention may be key to identifying those in greatest need for an intervention post ICD implant. This approach would allow for intervention tailoring to the specific needs of partners as the patient is recovering from the ICD implant. Findings also suggest that some partners who were doing well at randomization continued to do well throughout 12 months, indicating that some did not need this intervention after the patient received an initial ICD.
Depression and anxiety are often co-morbid conditions in heart disease (Hollos et al., 2018). Since the participants receiving the partner-focused intervention with the low MCS profiles showed significant improvement over time and better MCS compared to the patient-only intervention, we anticipated partners with the P + P high anxiety profile would show significantly more improvement than P-only. Thus, it was notable that the P + P low anxiety profile demonstrated more improvement in anxiety compared to P-only, while partners with high anxiety profiles showed no differences in anxiety between the partner-focused and patient-only interventions. Our results suggest higher levels of anxiety may be a barrier to effective participation in the intervention. More severe anxiety is associated with poor adherence to medications and health behaviors (Alcántara et al., 2014; Husain et al., 2018; Müller-Tasch et al., 2018; Schweitzer et al., 2007). Additionally, anxiety has been observed to be more severe in partners than in patients with a recent ICD (Rottmann et al., 2018; Van Den Broek et al., 2013), and anxiety in partners has been shown to be associated with patient anxiety and depression (Van Den Broek et al., 2013). Therefore, developing programs to reduce partner anxiety may be particularly important with dyadic interventions to effectively improve outcomes in both partners and patients.
Of particular importance in this study was the large and sustained effect of the P + P intervention for partners with worse caregiver burden. Although the parent study also demonstrated a significant improvement in caregiver burden in the P + P group compared to the P-only group (Dougherty et al., 2019), our analysis suggests this effect was most pronounced in partners with more caregiver burden. The analyses demonstrated that elevated caregiver burden is prevalent in caregivers, affecting a substantial number of the participants (33%) in this study. Furthermore, caregiver burden is known to be associated with poorer physical and psychological health for both partners and patients (Chen et al., 2015; Pucciarelli et al., 2017). Thus, an intervention that can effectively reduce caregiver burden, particularly for whom the burden is high, holds promise to improve health for both partners and patients with an ICD.
Although RCTs to improve partner outcomes are more common in cancer and dementia populations, the Patient plus Partner intervention was the first trial to include an intervention specifically for intimate partners of patients with ICDs. The Patient plus Partner trial demonstrated a partner-focused social cognitive intervention could improve multiple psychosocial outcomes (caregiver burden, self-efficacy). Our current analysis of the partner data from the Patient plus Partner trial provides additional information to advance intervention science for partners of patients with ICDs. Future interventions aimed at improving patient outcomes after ICD implantation should consider including a partner-focused component to the intervention. Our study demonstrates that partners can benefit from a partner-focused intervention, particularly partners who may be most in need.
Strengths and limitations
Strengths of this study include the large sample size, use of growth mixture modeling to identify sub-groups with longitudinal data, a usual care control group, and multiple indicators of health outcomes. Study limitations included the use of secondary data that does not allow for the inclusion of other variables or time points not provided in the original study, and the fact that the sample was primarily female and Caucasian, which restricts generalizability of the findings. Furthermore, differences in hospitalizations, employment, and antidepressant use in the P + P versus P-only may reflect differences in functional ability and treatment of depression at baseline. Finally, due to the identification of high versus low profiles across 12 months, potential ceiling or floor effects may have limited detection of significant change for some outcomes in the partner sub-groups.
Conclusion
The P + P intervention was more effective than the P-only intervention at improving health outcomes for partners with initially lower mental health, higher caregiver burden, and lower self-efficacy and less effective for partners with higher anxiety. The P + P intervention had little impact on physical health status of partners. Future applications of the P + P intervention could consider targeting partners with lower psychological health, caregiver burden, and self-efficacy.
Acknowledgements
This manuscript is not under review at any other journal, and the investigation conforms to the principles outlined in the Declaration of Helsinki. The Institutional Review Board of a major academic medical center in the Pacific Northwest approved the study. This work was supported by the National Institute of Nursing Research (T32NR016913) and the parent study was funded by the National Heart, Lung, and Blood Institute (R01HL086580-01A2). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The Institutional Review Board of a major academic medical center in the Pacific Northwest approved the study.
Footnotes
Conflict of interest Jonathan P. Auld, Elaine A. Thompson and Cynthia M. Dougherty declare that they have no conflict of interest.
Human and animal rights All procedures followed were in accordance with ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Informed consent Informed consent was obtained from all patients for being included in the study.
References
- Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, & Page RL (2018). 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart rhythm society. Heart Rhythm, 15, e73–e189. 10.1016/j.hrthm.2017.10.036 [DOI] [PubMed] [Google Scholar]
- Alcántara C, Edmondson D, Moise N, Oyola D, Hiti D, & Kronish IM (2014). Anxiety sensitivity and medication nonadherence in patients with uncontrolled hypertension. Journal of Psychosomatic Research, 77, 283–286. 10.1016/j.jpsychores.2014.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allemann H, Stromberg A, & Thylen I (2018). Perceived Social support in persons with heart failure living with an implantable cardioverter defibrillator: A cross-sectional explorative study. Journal of Cardiovascular Nursing, 33, E1–E8. 10.1097/JCN.0000000000000523 [DOI] [PubMed] [Google Scholar]
- Auld JP, Thompson EA, & Dougherty CM (2020). Social cognitive intervention following an initial implantable cardioverter defibrillator: Better treatment response for secondary vs. primary prevention. Pacing and Clinical Electrophysiology. 10.1111/pace.13929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakas T, Austin JK, Jessup SL, Williams LS, & Oberst MT (2004). Time and difficulty of tasks provided by family caregivers of stroke survivors. Journal of Neuroscience Nursing, 36, 95. [DOI] [PubMed] [Google Scholar]
- Banks BD, Mao IL, & Walter JP (1985). Robustness of the restricted maximum likelihood estimator derived under normality as applied to data with skewed distributions. Journal of Dairy Science, 68, 1785–1792. [Google Scholar]
- Chen MC, Chen KM, & Chu TP (2015). Caregiver burden, health status, and learned resourcefulness of older caregivers. Western Journal of Nursing Research, 37, 767–780. 10.1177/0193945914525280 [DOI] [PubMed] [Google Scholar]
- Cramm JM, & Nieboer AP (2012). Self-management abilities, physical health and depressive symptoms among patients with cardiovascular diseases, chronic obstructive pulmonary disease, and diabetes. Patient Education and Counseling, 87, 411–415. 10.1016/j.pec.2011.12.006 [DOI] [PubMed] [Google Scholar]
- Cuthbert CA, King-Shier KM, Ruether JD, Tapp DM, Wytsma-Fisher K, Fung TS, & Culos-Reed SN (2018). The effects of exercise on physical and psychological outcomes in cancer caregivers: Results from the RECHARGE randomized controlled trial. Annals of Behavioral Medicine, 52, 645–661. 10.1093/abm/kax040 [DOI] [PubMed] [Google Scholar]
- Cutitta KE, Woodrow LK, Ford J, Shea J, Fischer A, Hazelton G, & Sears SF (2014). Shocktivity: Ability and avoidance of daily activity behaviors in ICD patients. Journal of Cardiopulmonary Rehabilitation and Prevention, 34, 241–247. 10.1097/HCR.0000000000000055 [DOI] [PubMed] [Google Scholar]
- Dougherty CM, Johnston SK, & Thompson EA (2007). Reliability and validity of the self-efficacy expectations and outcome expectations after implantable cardioverter defibrillator implantation scales. Applied Nursing Research, 20, 116–124. 10.1016/j.apnr.2007.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty CM, Pyper GP, & Benoliel JQ (2004). Domains of concern of intimate partners of sudden cardiac arrest survivors after ICD implantation. Journal of Cardiovascular Nursing, 19, 21–31 [DOI] [PubMed] [Google Scholar]
- Dougherty CM, & Thompson EA (2009). Intimate partner physical and mental health after sudden cardiac arrest and receipt of an implantable cardioverter defibrillator. Research in Nursing & Health, 32, 432–442. 10.1002/nur.20330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty CM, Thompson EA, & Kudenchuk PJ (2012). Development and testing of an intervention to improve outcomes for partners following receipt of an implantable cardioverter defibrillator in the patient. ANS Advances in Nursing Science, 35, 359–377. 10.1097/ANS.0b013e318271d2e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty CM, Thompson EA, & Kudenchuk PJ (2019). Patient plus partner trial: A randomized controlled trial of 2 interventions to improve outcomes after an initial implantable cardioverter-defibrillator. Heart Rhythm, 16, 453–459. 10.1016/j.hrthm.2018.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluur C, Bolse K, Strömberg A, & Thylén I (2014). Spouses’ reflections on Implantable Cardioverter Defibrillator treatment with focus on the future and the end-of-life: A qualitative content analysis. Journal of Advanced Nursing, 70, 1758–1769. 10.1111/jan.12330 [DOI] [PubMed] [Google Scholar]
- Gary R, Dunbar SB, Higgins M, Butts B, Corwin E, Hepburn K, & Miller AH (2020). An intervention to improve physical function and caregiver perceptions in family caregivers of persons with heart failure. Journal of Applied Gerontology, 39, 181–191. 10.1177/0733464817746757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamama-Raz Y, Nativ S, & Hamama L (2020). Posttraumatic growth in inflammatory bowel disease patients: The role of illness cognitions and physical quality in life. Journal of Crohn’s and Colitis. 10.1093/ecco-jcc/jjaa247 [DOI] [PubMed] [Google Scholar]
- Hollos P, Marchisella F, & Coffey ET (2018). JNK Regulation of depression and anxiety. Brain Plast, 3, 145–155. 10.3233/BPL-170062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain SA, Edmondson D, Kautz M, Umland R, & Kronish IM (2018). Posttraumatic stress disorder due to acute cardiac events and aversive cognitions towards cardiovascular medications. Journal of Behavioral Medicine, 41, 261–268. 10.1007/s10865-017-9906-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani E, Moore SE, Hickman RL, Dolansky MA, Josephson RA, & Hughes JW (2019). The contribution of living arrangements, social support, and self-efficacy to self-management behaviors among individuals with heart failure: A path analysis. Journal of Cardiovascular Nursing, 34, 319–326. 10.1097/JCN.0000000000000581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins LS, Powell JL, Schron EB, McBurnie MA, Bosworth-Farrell S, Moore R, & Investigators A (2007). Partner quality of life in the antiarrhythmics versus implantable defibrillators trial. Journal of Cardiovascular Nursing, 22, 472–479. 10.1097/01.jcn.0000297378.98754.06 [DOI] [PubMed] [Google Scholar]
- Kim JS, Kim GS, Kang SM, & Chu SH (2020). Symptom experience as a predictor of cardiac rehabilitation education programme attendance after percutaneous coronary intervention: A prospective questionnaire survey. European Journal of Cardiovascular Nursing. 10.1177/1474515120940534 [DOI] [PubMed] [Google Scholar]
- Knight RG, Waal-Manning HJ, & Spears GF (1983). Some norms and reliability data for the State-Trait Anxiety Inventory and the Zung Self-Rating Depression scale. British Journal of Clinical Psychology, 22, 245–249. 10.1111/j.2044-8260.1983.tb00610.x [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Willaims JB (2001). The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine, 16, 606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuck K-H, Cappato R, Siebels J, & Rüppel R (2000). Randomized Comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest. Circulation, 102, 748–754. 10.1161/01.CIR.102.7.748 [DOI] [PubMed] [Google Scholar]
- Lache B, Meyer T, & Herrmann-Lingen C (2007). Social support predicts hemodynamic recovery from mental stress in patients with implanted defibrillators. Journal of Psychosomatic Research, 63, 515–523. 10.1016/j.jpsychores.2007.06.024 [DOI] [PubMed] [Google Scholar]
- Magyar-Russell G, Thombs BD, Cai JX, Baveja T, Kuhl EA, Singh PP, & Ziegelstein RC (2011). The prevalence of anxiety and depression in adults with implantable cardioverter defibrillators: A systematic review. Journal of Psychosomatic Research, 71, 223–231. 10.1016/j.jpsychores.2011.02.014 [DOI] [PubMed] [Google Scholar]
- Masoudi FA, Ponirakis A, de Lemos JA, Jollis JG, Kremers M, Messenger JC, & Spertus JA (2017). Trends in U.S. cardiovascular care: 2016 report from 4 ACC national cardiovascular data registries. Journal of American College of Cardiology, 69, 1427–1450. 10.1016/j.jacc.2016.12.005 [DOI] [PubMed] [Google Scholar]
- McClintock HF, BeKampis AN, Hartmann E, & Bogner HR (2020). Adherence to antidepressants in underserved communities: A comparison of electronic monitoring and self-report measures. Community Mental Health Journal. 10.1007/s10597-019-00533-2 [DOI] [PubMed] [Google Scholar]
- Müller-Tasch T, Löwe B, Lossnitzer N, Frankenstein L, Täger T, Haass M, & Herzog W (2018). Anxiety and self-care behaviour in patients with chronic systolic heart failure: A multivariate model. European Journal of Cardiovascular Nursing, 17, 170–177. 10.1177/1474515117722255 [DOI] [PubMed] [Google Scholar]
- Muthen B, Brown CH, Masyn K, Jo B, Khoo ST, Yang CC, & Liao J (2002). General growth mixture modeling for randomized preventive interventions. Biostatistics, 3, 459–475. 10.1093/biostatistics/3.4.459 [DOI] [PubMed] [Google Scholar]
- Palacios-Cena D, Losa-Iglesias ME, Alvarez-Lopez C, Cachon-Perez M, Reyes RA, Salvadores-Fuentes P, & Fernandezde-Las-Penas C (2011). Patients, intimate partners and family experiences of implantable cardioverter defibrillators: Qualitative systematic review. Journal of Advanced Nursing, 67, 2537–2550. 10.1111/j.1365-2648.2011.05694.x [DOI] [PubMed] [Google Scholar]
- Pedersen SS, Van Den Berg M, Erdman RAM, Van Son J, Jordeans L, & Thues DAMJ (2009). Increased anxiety in partners of patients with a cardioverter-defibrillator: The role of indication for ICD therapy, shocks, and personality. Pacing and Clinical Electrophysiology, 32, 184–192. 10.1111/j.1540-8159.2008.02201.x [DOI] [PubMed] [Google Scholar]
- Pedersen SS, van Domburg RT, Theuns DA, Jordaens L, & Erdman RA (2004). Type D personality is associated with increased anxiety and depressive symptoms in patients with an implantable cardioverter defibrillator and their partners. Psychosomatic Medicine, 66, 714–719. 10.1097/01.psy.0000132874.52202.21 [DOI] [PubMed] [Google Scholar]
- Pucciarelli G, Vellone E, Savini S, Simeone S, Ausili D, Alvaro R, & Lyons KS (2017). Roles of Changing physical function and caregiver burden on quality of life in stroke: A longitudinal dyadic analysis. Stroke, 48, 733–739. 10.1161/STROKEAHA.116.014989 [DOI] [PubMed] [Google Scholar]
- Ram N, & Grimm KJ (2009). Methods and measures: Growth mixture modeling: A method for identifying differences in longitudinal change among unobserved groups. International Journal of Behavioral Development, 33(6), 565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanaiah NV, Franzen M, & Schill T (1983). A Psychometric study of the state-trait anxiety inventory. Journal of Personality Assessment, 47, 531. 10.1207/s15327752jpa4705_14 [DOI] [PubMed] [Google Scholar]
- Rottmann N, Skov O, Andersen CM, Theuns D, & Pedersen SS (2018). Psychological distress in patients with an implantable cardioverter defibrillator and their partners. Journal of Psychosomatic Research, 113, 16–21. 10.1016/j.jpsychores.2018.07.010 [DOI] [PubMed] [Google Scholar]
- Schweitzer RD, Head K, & Dwyer JW (2007). Psychological factors and treatment adherence behavior in patients with chronic heart failure. Journal of Cardiovascular Nursing, 22, 76–83 [DOI] [PubMed] [Google Scholar]
- Sears SF, Lewis TS, Kuhl EA, & Conti JB (2005). Predictors of quality of life in patients with implantable cardioverter defibrillators. Psychosomatics, 46, 451–457. 10.1176/appi.psy.46.5.451 [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, & Williams JDW (1999). Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. JAMA, 282, 1737–1744 [DOI] [PubMed] [Google Scholar]
- Van Den Broek K, Heijmans N, & Van Assen M (2013). Anxiety and depression in patients with an implantable cardioverter defibrillator and their partners: A longitudinal study. Pacing and Clinical Electrophysiology, 36, 362–371. 10.1111/pace.12055 [DOI] [PubMed] [Google Scholar]
- Van Den Broek KC, Habibovic M, & Pedersen SS (2010). Emotional distress in partners of patients with an implantable cardioverter defibrillator: A systematic review and recommendations for future research. Pacing and Clinical Electrophysiology, 33, 1442–1450. 10.1111/j.1540-8159.2010.02885.x [DOI] [PubMed] [Google Scholar]
- van den Broek KC, Martens EJ, Nyklicek I, van der Voort PH, & Pedersen SS (2007). Increased emotional distress in type-D cardiac patients without a partner. Journal of Psychosomatic Research, 63, 41–49. 10.1016/j.jpsychores.2007.03.014 [DOI] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, & Raczek A (1995). Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: Summary of results from the medical outcomes study. Medical care, 33, AS264–AS279 [PubMed] [Google Scholar]
- Webster R, & Heeley E (2010). Perceptions of risk: Understanding cardiovascular disease. Risk Management and Healthcare Policy, 3, 49–60. 10.2147/RMHP.S8288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt S, Krauss E, Barbero MAN, Muller V, Bonniaud P, Vancheri C, & Guenther A (2019). Psychometric properties and minimal important differences of SF-36 in Idiopathic Pulmonary Fibrosis. Respiratory Research, 20, 47. 10.1186/s12931-019-1010-5 [DOI] [PMC free article] [PubMed] [Google Scholar]


