Abstract
V-domain immunoglobulin (Ig) suppressor of T cell activation (VISTA) is a novel negative checkpoint regulator that mediates T cell proliferation and cytokine production. The VISTA signaling pathway blockade has been proved as a promising strategy for cancer immunotherapy. Recent VISTA sequence analysis and crystal structure investigations have revealed its independent and unique function as compared with B7 family members, such as PD-1. This review will discuss VISTA binding partners and compare the structural differences between VISTA and other B7 family members, focusing on VISTA functions in immune activation and maintaining T cell quiescence. Recent progress and the therapeutic potential of biomacromolecules, such as monoclonal antibodies (mAbs) and small molecules targeting VISTA, are also discussed. Among these, a first-in-class small-molecule antagonist, CA-170, is highlighted.
This review reports the function of VISTA in modulating immune response, and recent advances in VISTA inhibitor development.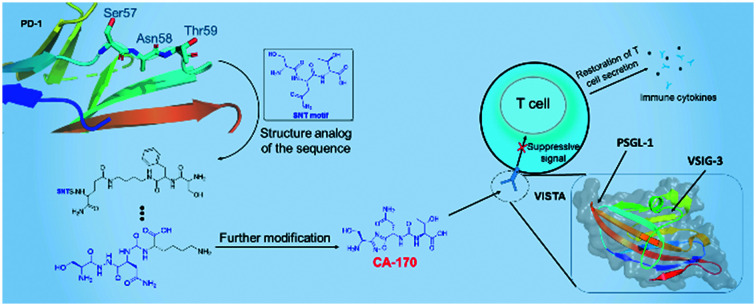
Introduction
Immune checkpoints (ICs) function as costimulatory or coinhibitory regulators of the immune response through interaction with their receptors or ligands.1 The costimulatory receptor cluster of differentiation (CD) 28, binds to CD80 (B7-1) and CD86 (B7-2) on antigen-presenting cells (APCs), competing with the coinhibitory receptor cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) in T cell activation.2 The existence of negative immune checkpoints is to maintain the immune tolerance and reduce the immune response that is unnecessary under normal conditions. However, suppression of the immune response also promotes cancer growth in the tumor microenvironment (TME).3 Thus, immune checkpoint inhibitors help to reduce immune escape in the TME and enhance the ability of the immune system to eliminate tumor cells. Blockade of the immune-modulatory pathway has proved to be a potential anticancer therapy. For example, the FDA-approved monoclonal antibody, nivolumab, an inhibitor of programmed death 1 (PD-1), has therapeutic benefits for patients with melanoma.4 With the discovery of novel checkpoints and the systematic investigation of immunomodulatory pathways, immunotherapy is a very promising strategy for cancer treatment.
V-domain immunoglobulin (Ig) suppressor of T cell activation (VISTA, also known as PD-1H, C10orf54, VSIR, B7-H5, DD1α, Gi24, or Dies1), is a coinhibitory immune checkpoint that is most highly expressed in hematopoietic cells or in tissues with large numbers of infiltrating leukocytes.5 As an essential mediator in T cell proliferation and cytokine production, VISTA shows limited homology with other B7 family members and has unique and conserved sequence motifs. Blockade of the VISTA signaling pathway leads to the increased risk of T cell-mediated autoimmune disease and an agonist of VISTA shows potential in preventing graft-versus-host disease (GVHD).6,7 Nevertheless, it has been reported that VISTA expression increases with tumor progression in a variety of cancers, including acute myeloid leukemia (AML), pancreatic cancer, non-small cell lung cancer (NSCLC), prostate cancer, melanoma, ovarian cancer, squamous cell carcinoma, gastric cancer, and colorectal cancer, commonly representing unfavorable survival for patients.8 Hence, inhibitors that target VISTA have become a novel strategy for cancer therapy, while VISTA agonists may also enable the control of T cell activation. To date, there is one monoclonal antibody (mAb) and one small molecule targeting VISTA in clinical trials.3
Herein, the latest progress relating to VISTA is explored, including its function, binding partners, and currently underdeveloped inhibitors. One key focus is on analyzing the recently determined crystal structure, and patents for CA-170, a molecule that is undergoing phase II clinical trials. This framework intends to attract more attention to this target and to share the authors' perspectives. Suggestions for the future development of chemical candidates are also provided.
The biological background of VISTA
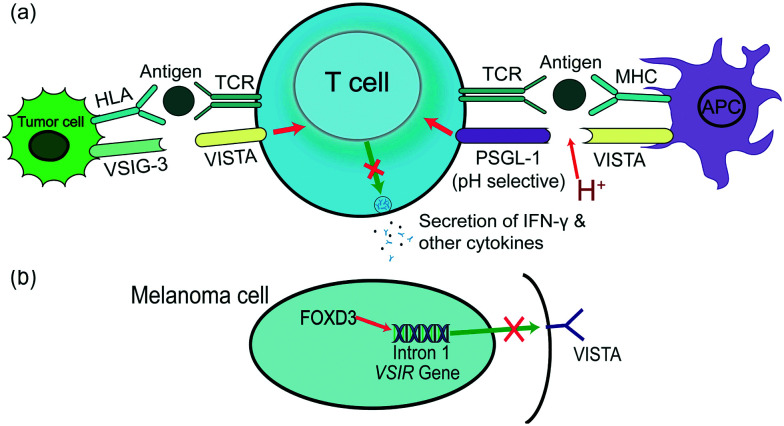
VISTA/PD-1H was determined by two research groups in 2011.6,7VSIR, the gene encoding VISTA, is located on the forward strand of murine chromosome 10, and its accurate location is 10qB4. Gene sequence analysis of VISTA in humans and mice shows a high level of conservation, especially of the cytoplasmic fragment.7 VISTA is a 311 amino acid type-I transmembrane protein composed of seven exons and expressed on the surface of a variety of cells, including CD4+ T cells, microglia, monocytes, macrophages, and other myeloid cell subsets, which reveals VISTA to be a critical member of the immunomodulatory family.6,8 In apoptotic cells, tumor suppression gene p53 induces VISTA to control signaling-mediated phagocytosis, ensuring cell corpse clearance and resulting in immune tolerance. The engulfment of the apoptotic cells was mediated by the homophilic intermolecular interaction of VISTA. Further investigations revealed that the interaction was abolished by the deletion of the VISTA IgV domain.9 Stemness factor Forkhead box D3 (FOXD3) selectively downregulates VISTA transcript levels in melanoma via directly binding with intron 1 of the VSIR gene (Fig. 1). Inhibition or knockdown of BRAF, a mediator of FOXD3, also achieves the same inhibition outcome at both protein and transcript levels.10
Fig. 1. The function and binding partners of VISTA in the tumor microenvironment. (a) General roles of VISTA in suppressing T cell activation and proliferation through binding with PSGL-1 at acidic pH or binding with VSIG-3. PSGL-1 is a pH-selective receptor that has lower efficacy at normal physiological pH 7.4. While the expression of VSIG-3 is not entirely understood, it may not exist in all types of tumor cells. (b) The VSIR gene is controlled by FOXD3, and the binding site is on intron 1. Their interaction leads to the blockade of VISTA expression in melanoma cells.
Binding partners of VISTA
The widespread expression of VISTA leads to its correlation with several receptors and ligands. To date, at least two biomolecules have been revealed as ligand/receptor of VISTA, P-selectin glycoprotein ligand-1 (PSGL-1), and V-set and immunoglobulin domain containing 3 (VSIG-3/IGSF11). VISTA shows pH selectivity when interacting with co-inhibitory receptor PSGL-1 as a ligand, probably via its histidine residues in the loop region (Fig. 1). VISTA binds to leukocytes in an acidic environment, such as a TME, and its function is enhanced at acidic pH values as compared to the normal physiological pH of 7.4. The binding of VISTA to T cells decreases when PSGL-1 is lacking.11 VSIG-3 has been revealed as a unique ligand of VISTA in vitro (Fig. 1).12 The expression of VSIG-3, however, is not as widespread as that of VISTA, and the VISTA/VSIG-3 pathway may preferentially exist in the brain and testis.12 The binding of VISTA and VSIG-3 is independent of pH. Compared to PSGL-1, VSIG-3 shows a four-fold binding affinity at physiological pH.13
V-set and immunoglobulin domain containing 8 (VSIG-8) belongs to the VSIG family, same as VSIG-3, and is supposed to be a receptor of VISTA.14 However, no binding was observed between VSIG-8 and recombinant VISTA via ELISA.12,15 Whether VSIG-8 binds VISTA needs further investigation. Galectin-9, as a member of the β-galactoside-binding lectin family of galectins, is a ligand for T-cell immunoglobulin and mucin-domain containing-3 (TIM-3).16 The interaction of galectin-9 with VISTA was detected in ELISA-based assays. Soluble VISTA secreted by AML cells strengthens the immunosuppressive activity of galectin-9.17 Together with the affinity of TIM-3 and galectin-9, a complex interaction between the three proteins may exist, since the binding epitope of galectin-9 and VISTA is not determined.
Expression of VISTA in cancers
VISTA is detected in various tissues and participates in various kinds of cancers. Therefore, the factors that control its expression and the association between VISTA and other immune mediators may differ in different cancers, including microglia, AML, ovarian cancer, NSCLC, gastric carcinoma, and pancreatic cancer. VSIR expression in microglia is like that of microglia signature genes and decreases after TLR-stimulated microglial activation, which is a result of altered histone modification enrichment and chromatin accessibility.18 The upregulation of VISTA on MDSCs of AML patients impairs CD8+ T cell proliferation. A positive correlation was found between the cell numbers of MDSCs that express VISTA and T cells that express PD-1 from the same patient.19 DNA methylation or gene alteration such as NPM1 mutation may account for the enrichment of VISTA in AML.20 In contrast, VISTA expression decreased after methylation of its promoter in ovarian cancer.21 A study of the localized expression pattern and clinical significance of VISTA in NSCLC found the protein in 98% of stromal cells. The expression level was highest in cytotoxic T cells among T lymphocytes and macrophages and was associated with PD-1/PD-L1, CD8+ T cells, CD68+ macrophages, and epidermal growth factor receptor (EGFR) mutations.22 In gastric carcinoma, VISTA is expressed mainly in intratumoral cells and is positively associated with PD-L1 expression in tumor cells and with the Lauren phenotype in immune cells.23,24 Similarly, a high density of CD68+ macrophages has also been found in the pancreatic stromal area, as well as VISTA and its correlation with PD-1/PD-L1.25 Generally, VISTA is upregulated on TILs or cancer cells and shares a positive association with PD-1/PD-L1. The correlation between PD-1/PD-L1 and VISTA was confirmed to be independent when a monotherapy of blocking the PD-1/PD-L1 signaling pathway in patients with pancreatic cancer was applied.25 The same was observed in extranodal natural killer/T-cell lymphoma; poor response was observed in patients with high VISTA.26 Another study showed that the expression of VISTA, as well as PD-L1, expanded after an anti-CTLA-4 (ipilimumab) monotherapy in prostate cancer.27 The increasing expression of ICs may account for the poor response of patients to ipilimumab therapy. Due to the independent and high expression of VISTA and PD-L1, the suppressed immune response would not be restored unless the two ICs were blocked simultaneously. This suggests that combined immunotherapy should be considered if monotherapy resistance occurs.
The prognostic value of VISTA
In addition to the functions of VISTA before and during immune activation, the prognostic value of VISTA also depends on the cancer type. A high expression of VISTA represents favorable overall survival in solid tumors, high-grade serous ovarian cancer, breast cancer, pancreatic cancer, testicular germ cell tumors, and esophageal adenocarcinoma.28–33 In contrast, the presence of VISTA is indicative of worse survival in melanoma, extranodal natural killer/T-cell lymphoma, bladder cancer, and colorectal cancer.26,34–37 All the findings are based on the statistical analysis of patients with the corresponding cancers. The expression of other ICs in cancers may account for the different outcomes. Among the cases of low prognostic value, a high density of PD-1/PD-L1, TIM-3, or LAG-3 was also detected. Compared with cases of favorable prognosis, the existence of several negative ICs represents a lower immune response, which is probably a risk factor for metastasis and recurrence.
Structural analysis of VISTA
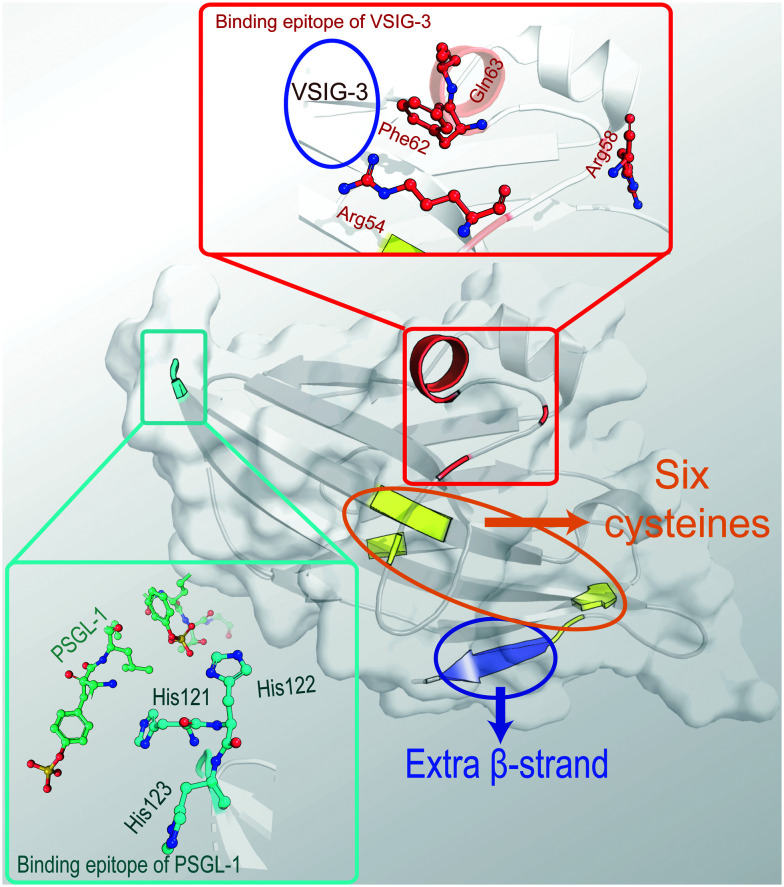
Recently, crystallographic studies have resolved the crystal structure of the extracellular domain of human VISTA and determined the potential binding epitope of VISTA/VSIG-3 or VISTA/anti-VISTA antibody.38,39 Based on the crystal structure (PDB: 6OIL), it is clear that VISTA contains three disulfide bonds that comprise six cysteine residues and an extended loop formed by 21 residues, including seven charged, surface-exposed residues (Fig. 2). In the loop area, there are 30 intramolecular hydrogen bonding interactions and a disulfide bond stabilizing the structure. A comparison of its crystal structure with other B7 family members revealed how VISTA is distinct, even from PD-L1, which shares the highest sequence similarity with VISTA. VISTA lacks a constant region domain but contains one more β-sandwich and an extra α-helix instead of a longer β-strand C′ located in a positively charged (six residue) area. These features contribute to the unique function and interactions of VISTA.
Fig. 2. The crystal structure of the extracellular domain of human VISTA (PDB ID: 6OIL) with several special residues highlighted. In the structure, there are six cysteines marked in yellow and an extra β-strand marked in blue. The supposed binding epitope with PSGL-1 consisting of three histidines is marked in cyan. The extra α-helix and suspected binding site with VSIG-3 consisting of four residues, two on the same helix and two on the loop, is marked in red. Among these, Arg54, Phe62, and Gln63 participate in the interaction while Arg58 is regarded as a conformation stabilizer.
Through screening the binding of VISTA mutants displayed on the surface of individual yeast cells and an anti-VISTA mAb, four residues (Arg54, Arg58, Phe62, and Gln63) were uncovered as potential binding epitopes of VISTA/anti-VISTA mAbs. These are all in the loop area, and Arg58 is suspected to be integral to the loop structure, while the other three residues are on the same surface and are highly exposed. They were also found to form the binding epitope of VISTA/VSIG-3.38 Another notable feature is that VISTA contains a high density of histidine. The mutation of conserved histidine clusters was found to partially abolish the suppression of anti-CD3-induced T cells activation, and the extra β-strand is functionally important, especially the clamping disulfide in this region.39 Together with PSGL-1, the acidic selective binding partner discussed above, it is reasonable to hypothesize that some of the histidine residues contribute to VISTA interactions, and changes to their protonation state at lower pH strengthen their binding.
Although no small molecule was revealed in the structure, the unique loop and extra strand are suitable targets for fragment-based drug design. The whole structure is valuable for aligning with small-molecule reagents and mAbs that are approved to interact with VISTA, especially those in clinical trials, to uncover more potential active sites. Due to the very recent elucidation of the crystal structure of VISTA, no research has been published that concentrated on this issue. It has raised considerable attention, however, and many investigations are underway.
mAbs targeting the VISTA signaling pathway
Currently, there are two anti-VISTA mAbs in clinical trials. Onvatilimab (JNJ-61610588, CI-8993), as mentioned before, is a mAb discovered by ImmuNext that entered phase I trial at Janssen in patients with advanced solid tumors in 2016 (Table 1). Janssen decided to terminate the study after the cytokine release syndrome-related side effect occurred (NCT02671955). Currently, Onvatilimab is developed by Curis and has entered phase I study (NCT04475523). VSIG-8 was identified as a receptor of VISTA in the patent of ImmuNext.14W0180, also named K01401-020, is a mAb developed by Pierre Fabre that recently commenced phase I clinical trials (Table 1). W0180 binds to VISTA at pH 6–7.4 and disrupts the interaction between PSGL-1 and VISTA in tumors.40
Small molecules and mAbs targeting VISTA currently in clinical and preclinical trialsa.
| Name | Description | Developed by | Phase | Target | Current indication |
|---|---|---|---|---|---|
| CA-170 | Small molecules | Aurigene & Curis | IIb | PD-L1 & VISTA | Non-squamous non-small cell lung cancer |
| Solid tumors | |||||
| Hodgkin lymphoma | |||||
| Onvatilimab | mAbs | ImmuNext & Curis | Ic | VISTA | Solid tumors |
| NCT02671955 | |||||
| NCT04475523 | |||||
| W0180 | mAbs | Pierre Fabre | I | VISTA | Locally advanced or metastatic solid tumors |
| NCT04564417 | |||||
| AUPM-493 | Small molecules | Aurigene | Preclinical | VISTA & VSIG-8 | — |
| IGN-381 | mAbs | Igenica Biotherapeutics | Preclinical | VISTA | Hematological cancer |
| Solid tumors | |||||
| HMBD-002 | mAbs | Hummingbird Bioscience | Preclinical | VISTA | Hematological cancer |
| Solid tumors | |||||
| PMC-309 | mAbs | PharmAbcine | Preclinical | VISTA | — |
| APX-201 | mAbs | Apexigen | Preclinical | VISTA | — |
All data were collected from Https://www.cortellis.com/drugdiscovery and www.clinicaltrials.gov.
The rights to fund and conduct the clinical study of CA-170 have been separated by Aurigene and Curis. Aurigene holds the rights to continue clinical trials in Russia and Asia and will launch a phase IIb/III study in patients with non-squamous non-small cell lung cancer. Curis has completed the phase I study in patients with advanced solid tumors or lymphomas in May 2020 (NCT02812875).
Onvatilimab had been in phase I trial at Janssen (NCT02671955). However, Janssen decided to close the trial after the cytokine release syndrome-related side effect occurred. The product is currently developed by Curis.
IGN-381, HMBD-002, 4C11, PMC-309, and APX-201 are all mAbs in the preclinical stage (Table 1). There have been few publications regarding these mAbs. Among these, HMBD-002 was developed by Hummingbird Bioscience and binds human and murine VISTA proteins with high affinity and specificity. In WT BALB/C mice inoculated with CT26 syngeneic colon cancer cells, HMBD-002 treatment resulted in significant inhibitory effects on tumor progression and >60% inhibition of tumor growth with no observable toxicity.41 Furthermore, the combination of HMBD-002 and anti-PD-L1 was found to be more effective than either antibody alone, especially in tumors that showed abundant myeloid-derived suppressor cell infiltration.42
Mehta et al. reported an engineered antibody named SG7 in 2020. SG7 is a cross-reactive anti-VISTA antibody that binds to murine and human VISTA with high affinity.13
Small molecule inhibitors
Small molecule inhibitors targeting VISTA, represented by compounds 1–3 (Fig. 3), have been released by two groups. Gabr et al. developed a fluorescence resonance energy transfer (FRET) assay to determine whether a small molecule could disrupt the interaction of VISTA and a mAb.43 The assay was then applied to screen about 4000 compounds via high-throughput screening, leading to the identification of compound 1. The affinity of compound 1 was determined by FRET and ELISA assay, and the IC50 values were 4.8 μM and 9.7 μM, respectively. Compound 1 was then docked with VISTA and compound 2 was designed based on the docking results. With the addition of two aryl groups, compound 2 displayed about 10-fold binding affinity in the FRET assay as compared to compound 1. Hu et al. developed compound 3 from virtual screening and its biological activities were determined by microscale thermophoresis with a KD value of 12.60 μM.44 Compound 4 was designed from virtual screening based on the protein–protein interaction of VSIG-3 and VISTA. As an inhibitor of VSIG-3, compound 4 interrupts the interaction of VSIG-3 and VISTA. The biological activities of compound 4 were determined by microscale thermophoresis with a KD value of 11.61 μM.45
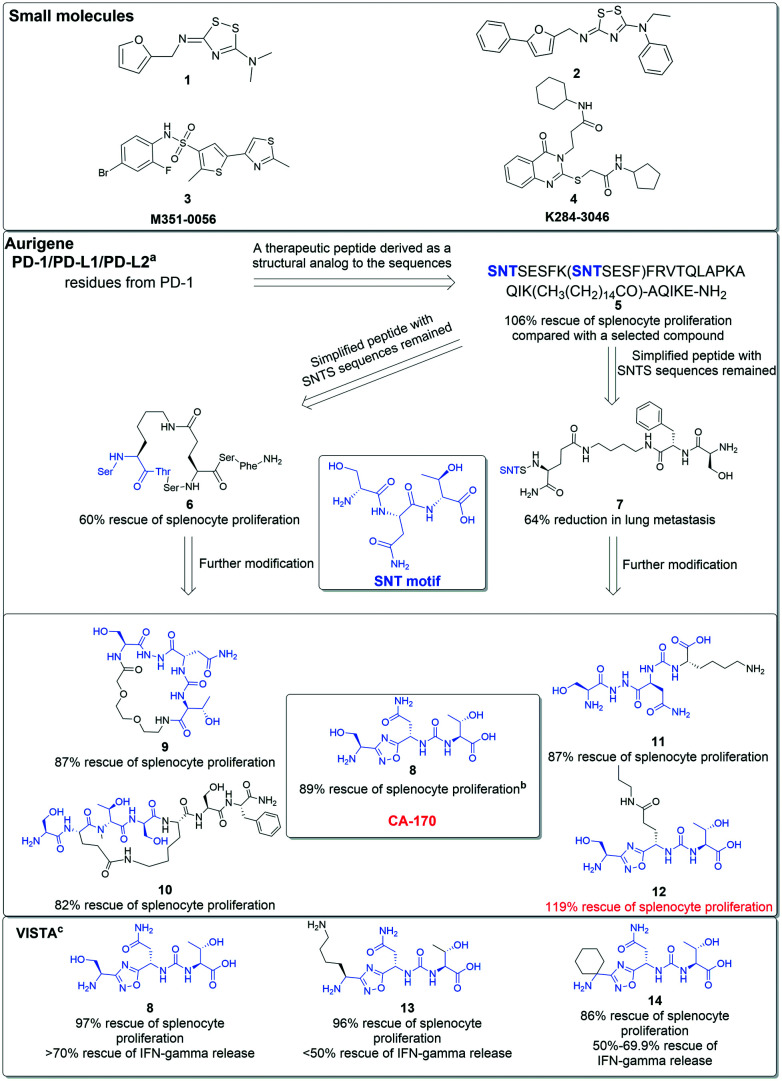
Fig. 3. Small molecule inhibitors of VISTA and development of the CA-170 series with chemical structures of selected representative regents as PD-1 or PD-1/VISTA inhibitors in patents of Aurigene Discovery Technologies. aThe biological efficacy was determined via an experiment testing the impact of compounds on mouse splenocyte proliferation in the presence of PD-1/PD-L1. T cells were stimulated by anti-CD3/CD28. All compounds were tested at 100 nM concentration and the results were compared to the group with no added compound. bFor compounds 4–8, the results were obtained from the experiment described above and were further normalized to 100% stimulated splenocyte proliferation. cThe biological efficacy results targeting VISTA were obtained from an experiment testing the impact of compounds on IFN-γ proliferation in the presence of VISTA. In this experiment, all compounds were tested at 100 nM concentration and the results were also normalized.
AUNP-12, co-developed by Aurigene Discovery Technologies and Pierre Fabre Laboratories, is a peptide regent that has shown impressive preclinical activity.46 Derived from the human and murine PD-1 BC loop, D strand, FG loop, and G strand, AUNP-12 is a 29-amino acid branched peptide with a structure like that of compound 5 (Fig. 3).47 In their patents, Aurigene identified various peptides or peptidomimetics, like compounds 6 and 7, as PD-1 inhibitors with promising biology efficacy.48 Compound 6 restored 60% splenocyte proliferation in a mouse model in which splenocyte proliferation had been inhibited by interactions of PD-1/PD-L1 in MDAMB231 overexpressing PD-L1, as compared to background proliferation (13%) and stimulated proliferation (72%), for example. AUNP-12 has a half-life of more than 90 minutes and shows medium clearance to overcome the pharmacokinetic limitations of mAbs.47 Although AUNP-12 is a PD-1/PD-L1 inhibitor and there has been no claim from Aurigene that this peptide targets VISTA, a small molecule derived from residues of the PD-1 BC loop SNTSESF, CA-170, has been found to highly affect the immunomodulating function of VISTA.
CA-170 is the only small molecule antagonist that has entered phase II clinical trials targeting VISTA as well as PD-1/PD-L1 and other ICs. Developed by Aurigene, CA-170 functions as a modulator in two immune signaling pathways, PD-L1 and VISTA.49,50 Preclinical data from patents demonstrate that CA-170 rescues the release of human PBMC IFN-γ in the presence of recombinant VISTA. Another experiment determined the co-inhibitory function of CA-170 targeting PD-L1 (EC50: 66.1 + 23.2 nM) and VISTA (EC50: 82.9 + 37.1 nM).49 The basic structural form of CA-170 (compound 8) is a derivative of 1,2,4-oxadiazole. Based on the structure shown in Fig. 3, the origin of CA-170 is the SNT motif from PD-1 BC loop sequences, a peptide consisting of the three amino acids serine, asparagine, and threonine. Compounds 9 and 10 continue the cognition by developing circular peptidomimetics and show comparable efficacy to compounds 11 and 12 listed on the right.
In terms of targeting VISTA and PD-1/PD-L1, compound 8 shows the potential to be a dual inhibitor of two pathways.49 Its biological efficacy in targeting VISTA was determined by a protocol testing the secretion ability of T cells in the presence of VISTA. Compared with compound 8, compounds 13 and 14 had a similar outcome in targeting PD-1/PD-L1 but had lower efficacy in targeting VISTA. It is noted that compound 12 showed a 119% rescue of splenocyte proliferation as compared to stimulation groups. In the patents, the protocol testing the rescue ability of candidate compounds in mouse splenocytes was developed to determine the efficacy of targeting PD-1/PD-L1. All molecules were inspected in the presence of PD-L1 and the results were compared with groups that were fully stimulated; a result of over 100% indicated the compound not only entirely restored the suppression of T cells, but also exceeded the stimulation of anti-CD3/28 antibody.
Despite the possibility that these compounds act as agonists of T cells, there is also the possibility that the target of these molecules may not be any of the ICs mentioned above. Biophysical examinations support this suspicion for CA-170. Studies screening the expected binding area of CA-170 and human PD-L1 via nuclear magnetic resonance and surface plasmon resonance detected no direct binding.51,52 The FRET assay discussed above was also applied to investigate the binding of CA-170 to VISTA and negligible change was observed.43 This seems to be a controversial finding since both preclinical and clinical trials indicate the high efficacy of CA-170. There are multiple potential reasons for this result, but it confirms that the splenocyte screening assay does not have to be hPD-1/hPD-L1 specific due to its complex nature.51 It also accounts for the various targets of CA-170. A recent study on CA-170 using a two-point HSQC method disclosed the special mechanism of CA-170 binding to PD-L1. Based on the different relaxation rates of small molecules and cells, the interaction of CA-170 with PD-L1 was confirmed in the cellular context.49 The method may also be applied to determine the interaction of CA-170 and VISTA. Further investigation of the mechanism of action, especially to identify the binding receptor, is highly recommended.
AUPM-493, also developed by Aurigene, is a small molecule targeting VISTA and VSIG-8 with desirable drug-like properties including good oral bioavailability.53 However, the structure of AUPM-493 is still undisclosed.
As for PSGL-1, the receptor of VISTA mentioned above, no anti-PSGL1 antibodies are currently in clinical trials. However, the potential of targeting this pathway has been recognized: Verseau Therapeutics launched the development of VTX-0811, a PSGL-1 antibody, aiming to reprogram tumor-associated macrophages (TAMs) to a more proinflammatory antitumor phenotype.54
Conclusions and perspectives
In this review, we have revealed the VISTA modulator as a promising target for immunotherapy. The widespread presence of VISTA in the immune system and involvement in cancer, inflammatory, and auto-immune disease has proved its critical status. VISTA blockade partially restores T cell activation and proliferation while deducing the tolerance of naive T cells, ultimately leading to an elevated immune response. As both a receptor and a ligand, VISTA can interact with distinct partners and have a strong effect on immune regulation, although details of its receptors or ligands have not been determined. Structural analysis has revealed the distinctive features of VISTA, indicating its unique and independent effect. Additionally, expression relationships with other modulators also provide possibilities for combined therapy, especially when monotherapy resistance of other ICs appears. Modulators targeting the VISTA signaling pathway have proved to be of clinical benefit.
With the entry of CA-170 into phase II clinical trials for solid tumors and Hodgkin lymphoma, a new hit in the pharmaceutical market seems imminent. If the compound is indeed a simultaneous multi-IC antagonist, it would be suitable in certain conditions where the expression of several ICs is not positively correlated. Furthermore, more potential candidates are under biological efficacy assessment. However, there are still multiple unknowns and uncertainties that remain to be solved and unique features to be uncovered. Additional studies are needed to fill these gaps and maximize the role of VISTA targeting as an effective immunotherapeutic strategy.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Grants 81773571) (X. Z.), Natural Science Foundation of Shanghai (Grant 20ZR1410400) (X. C.). All figures showing binding modes were generated using PyMol (www.pymol.org).
Biographies
Biography
Chenyang Wu.

Chenyang Wu received his B.S. degree in Medicinal Chemistry from Shenyang Pharmaceutical University in 2019. Currently, he is a Master's student majoring in Medicinal Chemistry under the supervision of professor Xiaojin Zhang, China Pharmaceutical University. His research is focused on small-molecule drug design and discovery for cancer treatment.
Biography
Xin Cao.

Xin Cao completed his B.S. degree at the China Pharmaceutical University in 2003 and obtained his Ph.D. degree from the China Pharmaceutical University in 2008. He worked as a post-doctoral fellow at the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences from 2009 to 2011. He was promoted to associate professor at the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences in 2011. He worked as a post-doctoral fellow at Harvard University from 2013 to 2014, and then he moved back to Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences in 2014. He became a Principal Investigator at the Zhongshan Hospital Institute of Clinical Science, Fudan University Shanghai Medical College in 2018.
Biography
Xiaojin Zhang.

Xiaojin Zhang received his B.S. degree from the China Pharmaceutical University, where he also earned his Ph.D. in Medicinal Chemistry in 2012. He worked as a visiting scholar in the Department of Chemistry at the University of Oxford from 2019 to 2020. He is currently a professor of Medicinal Chemistry and serves as the deputy director of the Department of Chemistry at the China Pharmaceutical University. His main research interests include small-molecule drug design and discovery. He is devoted to the design and discovery of drug candidates in clinical trials for the treatment of anemia.
Notes and references
- Collins M. Ling V. Carreno B. M. Genome Biol. 2005;6:223. doi: 10.1186/gb-2005-6-6-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torphy R. J. Schulick R. D. Zhu Y. Int. J. Mol. Sci. 2017;18:2642. doi: 10.3390/ijms18122642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X. Zhang X. Li E. Zhang G. Wang X. Tang T. Bai X. Liang T. J. Hematol. Oncol. 2020;13:83. doi: 10.1186/s13045-020-00917-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott P. A. Hodi F. S. Robert C. Clin. Cancer Res. 2013;19:5300–5309. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- Lines J. L. Pantazi E. Mak J. Sempere L. F. Wang L. O'Connell S. Ceeraz S. Suriawinata A. A. Yan S. Ernstoff M. S. Noelle R. Cancer Res. 2014;74:1924–1932. doi: 10.1158/0008-5472.CAN-13-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Rubinstein R. Lines J. L. Wasiuk A. Ahonen C. Guo Y. Lu L. F. Gondek D. Wang Y. Fava R. A. Fiser A. Almo S. Noelle R. J. J. Exp. Med. 2011;208:577–592. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flies D. B. Wang S. Xu H. Chen L. J. Immunol. 2011;187:1537–1541. doi: 10.4049/jimmunol.1100660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElTanbouly M. A. Croteau W. Noelle R. J. Lines J. L. Semin. Immunol. 2019;42:101308. doi: 10.1016/j.smim.2019.101308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K. W. Byun S. Kwon E. Hwang S. Y. Chu K. Hiraki M. Jo S. H. Weins A. Hakroush S. Cebulla A. Sykes D. B. Greka A. Mundel P. Fisher D. E. Mandinova A. Lee S. W. Science. 2015;349:1261669. doi: 10.1126/science.1261669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum S. R. Knecht M. Mollaee M. Zhong Z. Erkes D. A. McCue P. A. Chervoneva I. Berger A. C. Lo J. A. Fisher D. E. Gershenwald J. E. Davies M. A. Purwin T. J. Aplin A. E. Cell Rep. 2020;30:510–524. doi: 10.1016/j.celrep.2019.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]; , e516
- Johnston R. J. Su L. J. Pinckney J. Critton D. Boyer E. Krishnakumar A. Corbett M. Rankin A. L. Dibella R. Campbell L. Martin G. H. Lemar H. Cayton T. Huang R. Y. Deng X. Nayeem A. Chen H. Ergel B. Rizzo J. M. Yamniuk A. P. Dutta S. Ngo J. Shorts A. O. Ramakrishnan R. Kozhich A. Holloway J. Fang H. Wang Y. K. Yang Z. Thiam K. Rakestraw G. Rajpal A. Sheppard P. Quigley M. Bahjat K. S. Korman A. J. Nature. 2019;574:565–570. doi: 10.1038/s41586-019-1674-5. [DOI] [PubMed] [Google Scholar]
- Wang J. Wu G. Manick B. Hernandez V. Renelt M. Erickson C. Guan J. Singh R. Rollins S. Solorz A. Bi M. Li J. Grabowski D. Dirkx J. Tracy C. Stuart T. Ellinghuysen C. Desmond D. Foster C. Kalabokis V. Immunology. 2019;156:74–85. doi: 10.1111/imm.13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta N. Maddineni S. Kelly R. L. Lee R. B. Hunter S. A. Silberstein J. L. Parra Sperberg R. A. Miller C. L. Rabe A. Labanieh L. Cochran J. R. Sci. Rep. 2020;10:15171. doi: 10.1038/s41598-020-71519-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy M., Guo Y. and Rothstein J., WO Pat., 2016090347A1, 2015
- Fromm G. Silva S. D. Johannes K. Patel A. Hornblower J. Schreiber T. H. Cancer Res. 2018;78:5564. [Google Scholar]
- Merani S. Chen W. Elahi S. Rev. Med. Virol. 2015;25:175–186. doi: 10.1002/rmv.1832. [DOI] [PubMed] [Google Scholar]
- Yasinska I. M. Meyer N. H. Schlichtner S. Hussain R. Siligardi G. Casely-Hayford M. Fiedler W. Wellbrock J. Desmet C. Calzolai L. Varani L. Berger S. M. Raap U. Gibbs B. F. Fasler-Kan E. Sumbayev V. V. Front. Immunol. 2020;11:580557. doi: 10.3389/fimmu.2020.580557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borggrewe M. Grit C. Den Dunnen W. F. A. Burm S. M. Bajramovic J. J. Noelle R. J. Eggen B. J. L. Laman J. D. Glia. 2018;66:2645–2658. doi: 10.1002/glia.23517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Jia B. Claxton D. F. Ehmann W. C. Rybka W. B. Mineishi S. Naik S. Khawaja M. R. Sivik J. Han J. Hohl R. J. Zheng H. OncoImmunology. 2018;7:e1469594. doi: 10.1080/2162402X.2018.1469594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufva O. Pölönen P. Brück O. Keränen M. A. I. Klievink J. Mehtonen J. Huuhtanen J. Kumar A. Malani D. Siitonen S. Kankainen M. Ghimire B. Lahtela J. Mattila P. Vähä-Koskela M. Wennerberg K. Granberg K. Leivonen S.-K. Meriranta L. Heckman C. Leppä S. Nykter M. Lohi O. Heinäniemi M. Mustjoki S. Cancer Cell. 2020;38:380–399. doi: 10.1016/j.ccell.2020.06.002. [DOI] [PubMed] [Google Scholar]; , e313
- Mulati K. Hamanishi J. Matsumura N. Chamoto K. Mise N. Abiko K. Baba T. Yamaguchi K. Horikawa N. Murakami R. Taki M. Budiman K. Zeng X. Hosoe Y. Azuma M. Konishi I. Mandai M. Br. J. Cancer. 2019;120:115–127. doi: 10.1038/s41416-018-0313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroel-Espindola F. Yu X. Datar I. Mani N. Sanmamed M. Velcheti V. Syrigos K. Toki M. Zhao H. Chen L. Herbst R. S. Schalper K. A. Clin. Cancer Res. 2018;24:1562–1573. doi: 10.1158/1078-0432.CCR-17-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boger C. Behrens H. M. Kruger S. Rocken C. OncoImmunology. 2017;6:e1293215. doi: 10.1080/2162402X.2017.1293215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z. Peng Z. Liu C. Wang Z. Wang Y. Jiao X. Li J. Shen L. Innovation. 2020;1:100041. doi: 10.1016/j.xinn.2020.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blando J. Sharma A. Higa M. G. Zhao H. Vence L. Yadav S. S. Kim J. Sepulveda A. M. Sharp M. Maitra A. Wargo J. Tetzlaff M. Broaddus R. Katz M. H. G. Varadhachary G. R. Overman M. Wang H. Yee C. Bernatchez C. Iacobuzio-Donahue C. Basu S. Allison J. P. Sharma P. Proc. Natl. Acad. Sci. U. S. A. 2019;116:1692–1697. doi: 10.1073/pnas.1811067116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H. X. Gao Y. Fu J. C. Zhou Q. H. Wang X. X. Bai B. Li P. F. Huang C. Rong Q. X. Ping L. Q. He Y. X. Mao J. Y. Chen X. Huang H. Q. OncoImmunology. 2021;10:1907059. doi: 10.1080/2162402X.2021.1907059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J. Ward J. F. Pettaway C. A. Shi L. Z. Subudhi S. K. Vence L. M. Zhao H. Chen J. Chen H. Efstathiou E. Troncoso P. Allison J. P. Logothetis C. J. Wistuba I. I. Sepulveda M. A. Sun J. Wargo J. Blando J. Sharma P. Nat. Med. 2017;23:551–555. doi: 10.1038/nm.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser H. Kraemer M. Gebauer F. Bruns C. Schroder W. Zander T. Persa O. D. Alakus H. Hoelscher A. Buettner R. Lohneis P. Quaas A. OncoImmunology. 2019;8:e1581546. doi: 10.1080/2162402X.2019.1581546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X.-L. Zhou Y. Lu H.-Z. Li Q.-X. Wang Z. Sci. Rep. 2020;10:2662. doi: 10.1038/s41598-020-59608-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong L. Zhou Y. Zhang M. Chen J. Xiang Y. Cancer Immunol. Immunother. 2020;69:33–42. doi: 10.1007/s00262-019-02434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X. Ren X. Zhou Y. Mao F. Lin Y. Wu H. Sun Q. Front. Oncol. 2020;10:583966. doi: 10.3389/fonc.2020.583966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z. Pan Y. Fei Q. Lin Y. Zhou Y. Liu Y. Guan H. Yu X. Lin X. Lu F. Huang H. J. Cancer Res. Clin. Oncol. 2021;147:517–531. doi: 10.1007/s00432-020-03463-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peksa R. Kunc M. Popeda M. Piatek M. Bienkowski M. Zok J. Starzynska A. Perdyan A. Sowa M. Duchnowska R. Biernat W. Cancers. 2021;13:1750. doi: 10.3390/cancers13081750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh R. Taha R. Z. Toor S. M. Nair V. S. Murshed K. Khawar M. Al-Dhaheri M. Petkar M. A. Nada M. A. Elkord E. Cancer Immunol. Immunother. 2020;69:1989–1999. doi: 10.1007/s00262-020-02593-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuklinski L. F. Yan S. Li Z. Fisher J. L. Cheng C. Noelle R. J. Angeles C. V. Turk M. J. Ernstoff M. S. Cancer Immunol. Immunother. 2018;67:1113–1121. doi: 10.1007/s00262-018-2169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo W. I. Lee C. H. Jung S. J. Lee D. S. Park H. Y. Jeong D. H. Kim W. Chung J. I. Choi I. Cancer Immunol. Immunother. 2021 doi: 10.1007/s00262-021-02906-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. W. Kim Y. J. Yun K. A. Won C. H. Lee M. W. Choi J. H. Chang S. E. Lee W. J. Sci. Rep. 2020;10:14372. doi: 10.1038/s41598-020-71216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta N. Maddineni S. Mathews I. I. Sperberg R. A. P. Huang P. S. Cochran J. R. Cell Rep. 2019;28:2509–2516. doi: 10.1016/j.celrep.2019.07.073. [DOI] [PubMed] [Google Scholar]
- Slater B. T. Han X. Chen L. Xiong Y. Proc. Natl. Acad. Sci. U. S. A. 2020;117:1648–1657. doi: 10.1073/pnas.1908711117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzalegui F. Loukili N. Delfour O. Boute N. Thuilliez C. Champion T. Malissard M. Launay P. Chetaille E. Passioukov A. Corvaïa N. Ferré P. Cancer Res. 2020;80:3372. doi: 10.1158/0008-5472.CAN-20-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd-Kirkup J. D. Thakkar D. Paszkiewicz K. Ingram P. J. Cancer Res. 2018;78:1729. [Google Scholar]
- Ingram P. J. Thakkar D. Boyd-Kirkup J. D. Cancer Res. 2017;77:587. [Google Scholar]
- Gabr M. T. Gambhir S. S. J. Am. Chem. Soc. 2020;142:16194–16198. doi: 10.1021/jacs.0c07276. [DOI] [PubMed] [Google Scholar]
- Hu X. Qie C. Jiang J. Xie X. Chen W. Liu W. Liu J. Br. J. Pharmacol. 2021;178:1445–1458. doi: 10.1111/bph.15357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X. Chen C. Chen W. Jiang J. Wang L. Li T. Sun H. Liu J. Front. Immunol. 2021;12:625808. doi: 10.3389/fimmu.2021.625808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan M. M. Hu X. Q. Liu X. X. Ruan B. F. Xu J. Liao C. Drug Discovery Today. 2016;21:1027–1036. doi: 10.1016/j.drudis.2016.04.011. [DOI] [PubMed] [Google Scholar]
- Sasikumar P. G. Ramachandra R. K. Adurthi S. Dhudashiya A. A. Vadlamani S. Vemula K. Vunnum S. Satyam L. K. Samiulla D. S. Subbarao K. Nair R. Shrimali R. Gowda N. Ramachandra M. Mol. Cancer Ther. 2019;18:1081–1091. doi: 10.1158/1535-7163.MCT-18-0737. [DOI] [PubMed] [Google Scholar]
- Sasikumar P. G. and Ramachandra M., WO Pat., 2013144704A1, 2013
- Sasikumar P. G. Sudarshan N. S. Adurthi S. Ramachandra R. K. Samiulla D. S. Lakshminarasimhan A. Ramanathan A. Chandrasekhar T. Dhudashiya A. A. Talapati S. R. Gowda N. Palakolanu S. Mani J. Srinivasrao B. Joseph D. Kumar N. Nair R. Atreya H. S. Gowda N. Ramachandra M. Commun. Biol. 2021;4:699. doi: 10.1038/s42003-021-02191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasikumar P. G., Ramachandra M. and Naremaddepalli S. S., WO Pat., 2018073754A1, 2017
- Blevins D. J. Hanley R. Bolduc T. Powell D. A. Gignac M. Walker K. Carr M. D. Hof F. Wulff J. E. ACS Med. Chem. Lett. 2019;10:1187–1192. doi: 10.1021/acsmedchemlett.9b00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musielak B. Kocik J. Skalniak L. Magiera-Mularz K. Sala D. Czub M. Stec M. Siedlar M. Holak T. A. Plewka J. Molecules. 2019;24:2804. doi: 10.3390/molecules24152804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasikumar P. G. Naremaddepalli S. S. Ramachandra R. K. Gowda N. Yerramsetti M. R. Bandireddy S. R. Adurthi S. Mani J. Nair R. Dhudashia A. A. Dodheri S. S. Gowda N. M. Ramachandra M. Mol. Cancer Ther. 2018;17:B006. [Google Scholar]
- ElTanbouly M. A. Schaafsma E. Noelle R. J. Lines J. L. Clin. Exp. Immunol. 2020;200:120–130. doi: 10.1111/cei.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]